Abstract
Malignant brain tumors constitute nearly one-third of cancer diagnoses in children and have recently surpassed hematologic malignancies as the most lethal neoplasm in the pediatric population. Outcomes for children with brain tumors are unacceptably poor and current standards of care—surgical resection, chemotherapy, and radiation—are associated with significant long-term morbidity. Oncolytic virotherapy has emerged as a promising immunotherapy for the treatment of brain tumors. While the majority of brain tumor clinical trials utilizing oncolytic virotherapy have been in adults, five viruses are being tested in pediatric brain tumor clinical trials: herpes simplex virus (G207), reovirus (pelareorep/Reolysin), measles virus (MV-NIS), poliovirus (PVSRIPO), and adenovirus (DNX-2401, AloCELYVIR). Herein, we review past and current pediatric immunovirotherapy brain tumor trials including the relevant preclinical and clinical research that contributed to their development. We describe mechanisms by which the viruses may overcome barriers in treating pediatric brain tumors, examine challenges associated with achieving effective, durable responses, highlight unique aspects and successes of the trials, and discuss future directions of immunovirotherapy research for the treatment of pediatric brain tumors.
Keywords: pediatric, oncolytic, virotherapy, immunotherapy, brain tumors, glioma
1. Introduction
Primary central nervous system (CNS) tumors account for nearly one-third of all childhood and adolescent cancer diagnoses (Siegel, et al., 2021). Roughly 30% of primary CNS tumors are malignant and, as such, have recently surpassed leukemia as the most common cause of childhood cancer-related deaths (Ostrom, et al., 2020). Treatment regimens for malignant brain tumors centered on surgical resection, chemotherapy, and radiation have only modestly improved outcomes for decades. Additionally, current treatments are associated with significant adverse effects and long-term treatment sequelae including neurocognitive and neurosensory impairment and endocrine dysfunction, which are of particular concern for the pediatric patient population (Plant-Fox, et al., 2021). Immunotherapies, including oncolytic virotherapy, offer novel, targeted approaches for adult and pediatric patients with brain tumors and may be beneficial as adjuvant therapy, allowing for lower doses and reduced toxicity from traditional therapies (Cripe, et al., 2015; Friedman, et al., 2015).
Most oncolytic viruses (OVs) are engineered to selectively lyse and replicate in cancer cells, sparing healthy cells from the adverse effects of antitumor treatments. These OVs may be inoculated intratumorally or intracranially to bypass the blood-brain barrier which allows them to overcome this significant challenge in the successful treatment of pediatric brain tumors. Following infection and lysis of tumor cells, released viral progeny spread to adjacent tumor cells propagating oncolysis throughout heterogeneous tumor tissue. Accompanying the release of viral progeny, tumor-associated antigens, which are typically limited in pediatric brain tumors due to low somatic mutational burden, are released and made available for immune recognition (Mackay, et al., 2017). Furthermore, molecules containing pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are also released, leading to inflammation and innate immune responses that increase tumor antigen presentation and result in T cell priming and activation (Zhang, et al., 2021). OVs can be further engineered or combined with other therapies to amplify the release of tumor antigens and stimulate an antitumor immune response, turning immunologically inert tumor microenvironments into antitumor immune hotspots (Jones & Baker, 2014).
While OVs differ in their mechanisms of tumor specificity and extent of immune modulation, they are all used with the intent to selectively kill tumor cells and/or trigger durable antitumor immune responses while minimizing toxicities. Of the many viruses being studied as potential oncolytic virotherapy agents, five have been used in children with brain tumors. Herein, we review pediatric brain tumor clinical trials using OVs. The timeline in which these therapies have been tested in adults and subsequently children is detailed in Figure 1. In discussing selected adult clinical trials and preclinical evidence, we aim to provide context and rationale for the design of pediatric brain tumor trials. Additionally, we highlight unique features and successes of the trials and discuss implications for the future of oncolytic virotherapy for the treatment of pediatric brain tumors.
Figure 1: Timeline of clinical trials contributing evidence to the design and rationale of current clinical trials of oncolytic viruses for the treatment of pediatric brain tumors.
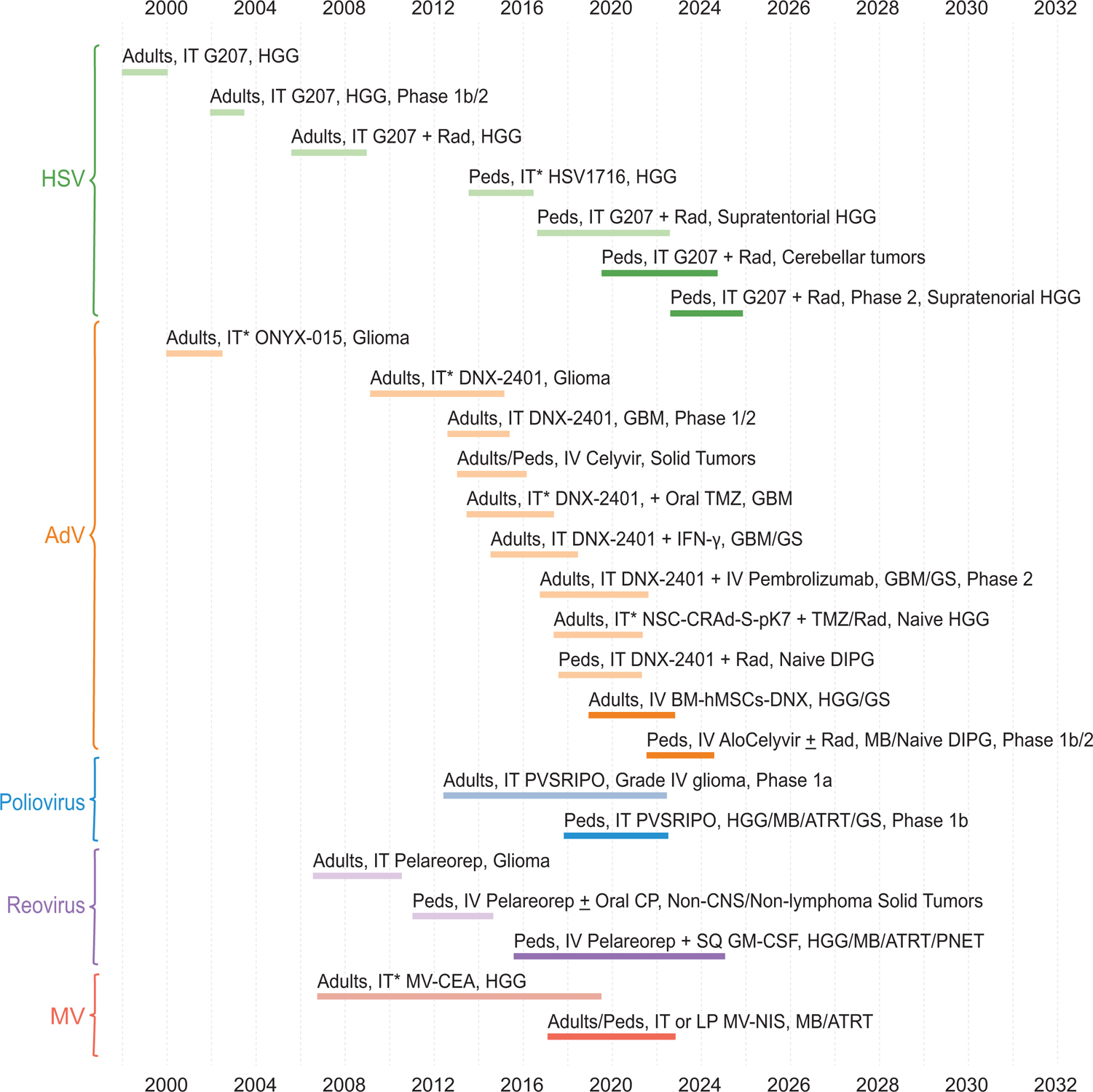
Each block represents a clinical trial. Lighter colored blocks represent trials that have been completed or terminated. Darker colored sections indicate active trials with estimated start and end dates as reflected on Clinicaltrials.gov, Gene Therapy Clinical Trials Worldwide (http://www.abedia.com/wiley/index.html), and (Chiocca, et al., 2004)as of January 2022. Unless otherwise noted, all trials are Phase 1. Age groups up to 21 years are designated pediatric. AdV adenovirus; ATRT atypical teratoid/rhabdoid tumor; CNS central nervous system; CP cyclophosphamide; DIPG diffuse intrinsic pontine glioma; GBM glioblastoma; GM-CSF granulocyte-macrophage colony-stimulating factor; GS gliosarcoma; HGG high-grade glioma; HSV herpes simplex virus; IFN-γ interferon-gamma; IT intratumoral (*indicates treatment may have been delivered peritumorally or into resection cavity); IV intravenous; LP lumbar puncture; MB medulloblastoma; MV measles virus; Peds pediatric; PNET primitive neuroectodermal tumor; Rad radiation; SQ subcutaneous; TMZ temozolomide. Created with BioRender.com
2. Herpes Simplex Virus-1 (HSV-1)
HSV-1 is an enveloped double-stranded DNA virus and member of the Herpesviridae family. The first OV to receive U.S. Food and Drug Administration (FDA) approval as an immunotherapeutic agent was talimogene laherparepvec (T-Vec), an engineered oncolytic HSV-1 (oHSV) that produces granulocyte-macrophage colony-stimulating factor (GM-CSF) during replication, for the treatment of advanced melanoma (Andtbacka, et al., 2015); see Table 1 for a summary of viruses discussed in the text. Currently, there are six oHSVs (G207, HSV1716, G47Delta, M032, C134, and rQNestin) that have been used in clinical trials for brain tumors (Cassady, et al., 2017; Chiocca, et al., 2020; Friedman, et al., 2021; Markert, et al., 2000; Patel, et al., 2016; Rampling, et al., 2000; Todo, et al., 2001). These clinically relevant oHSVs are engineered to include deletions of the γ134.5 neurovirulence gene (Mineta, et al., 1995; Whitley, et al., 1993), thereby preventing productive infection in normal cells through protein kinase R (PKR)-mediated translational arrest (Liu, et al., 2003). Tumor cells often contain signaling alterations such as Ras overactivity that prevent effective shutdown of translation, allowing for viral replication and lysis of tumor cells (Farassati, et al., 2001).
Table 1.
Summary of viruses
| Virus | Deletion/Mutation | Foreign gene/promoter insertion | Reference |
|---|---|---|---|
| HSV-1 | |||
| C134 | Deletion in both copies of γ134.5 gene | IRS1 gene under control of an HCMV immediate early promoter | (Cassady, et al., 2017) |
| G207 | Deletion in both copies of γ134.5 gene and disabling lacZ insertion in UL39 | None | (Mineta, et al., 1995) |
| G47Delta | Deletion of the γ134.5 and α47 genes and a disabling lacZ insertion within UL39 | None | (Todo, et al., 2001) |
| HSV1716 | Deletion in both copies of γ134.5 gene | None | (Streby, et al., 2017) |
| M032 | Deletion in both copies of γ134.5 gene | Human IL-12 gene insertion | (Patel, et al., 2016) |
| rQNestin | Deletion in γ134.5 gene and UL39 | ICP-34.5 under control of synthetic nestin promoter | (Chiocca, et al., 2020) |
| T-Vec | Deletions of the ICP34.5 and ICP47 genes | Granulocyte-macrophage colony-stimulating factor, CMV promoter | (Andtbacka, et al., 2015) |
| Adenovirus | |||
| Ad-TD-nsIL12 | Deletions in E1A, E1B, E3gp-19K genes | Non-secretory IL-12 gene under control of E3gp-19K promoter | (Wang, et al., 2017) |
| CRAd-S-pk7 | Deletion of native E1 promoter | E1A expression under control of human survivin; pk7 encoding polylysine | (Ulasov, et al., 2007) |
| DNX-2401 | Deletion of Rb-binding region from E1A | RGD peptide motif insertion | (Fueyo, et al., 2003) |
| DNX-2440 | Deletion of Rb-binding region from E1A | RGD peptide motif insertion; OX40 ligand expression cassette replacing E3 region | (Jiang, et al., 2017) |
| ICOVIR-5 | Deletion of Rb-binding region from E1A | RGD peptide motif insertion; Substitution of the E1A promoter for E2F1-responsive elements | (Majem, et al., 2006) |
| ONYX-015 | E1B gene deletion preventing production of E1B-55kDa protein | none | (Bischoff, et al., 1996) |
| Poliovirus | |||
| PVSRIPO | Native IRES | Native IRES substituted with IRES from human rhinovirus type 2 | (Gromeier, et al., 2000) |
| Reovirus | |||
| Pelareorep | None relative to wild type reovirus Type 3 Dearing strain | None relative to wild type reovirus Type 3 Dearing strain | (Kicielinski, et al., 2014) |
| Measles Virus | |||
| MV-CEA | None relative to Edmonston vaccine strain | Expresses soluble extracellular domain of human CEA | (Peng, et al., 2002) |
| MV-NIS | None relative to Edmonston vaccine strain | Expresses human thyroidal NIS | (Dingli, et al., 2004) |
Most oHSVs used in clinical trials have taken advantage of the large HSV genome to include additional modifications. For example, to further reduce the risk of infection in normal cells and enhance safety, G207 and G47Delta contain a disabling lacZ insertion within UL39, the viral gene that encodes the large subunit of ribonucleotide reductase (Mineta, et al., 1995; Todo, et al., 2001). Additionally, oHSVs have an intact or reinserted thymidine kinase gene and, therefore, maintain sensitivity to anti-viral agents already in clinical use such as acyclovir, which serves as an additional safety mechanism should viral replication cause unexpected toxicity and need to be halted (Coen, et al., 1989; Mineta, et al., 1994). oHSVs use a variety of mechanisms to increase viral replication in tumors. G47Delta has an additional deletion in the α47 gene which results in immediate early expression of US11 (Todo, et al., 2001); C134 expresses the human cytomegalovirus (HCMV) IRS1 gene (Cassady, et al., 2017); and rQNestin restores one copy of ICP34.5 under transcriptional control of the promoter/enhancer element of nestin, which is overexpressed in many cancers including high-grade glioma (HGG) (Chiocca, et al., 2020). Each of these alterations allow improved viral replication in tumor cells without compromising safety in normal tissues. To enhance antitumor immune responses, G47Delta contains the α47 gene deletion that downregulates the transporter associated with antigen presentation (TAP) in host cells and sequesters major histocompatibility complex I (MHC I) in the endoplasmic reticulum thereby increasing antigen presentation (Todo, et al., 2001); and M032 contains the human interleukin-12 (IL-12) gene which results in physiologically relevant amounts of IL-12 during replication (Patel, et al., 2016) and produces a Th1 immune response and activation of natural killer (NK) cells (Gately, et al., 1998).
oHSV works through the dual mechanism of action described above, which involves an initial virus-induced direct oncolysis followed by stimulation of a consequent antitumor immune response. The virus attaches to and enters cells through several cell surface receptors shown to be expressed on pediatric brain tumors including the cell adhesion molecule nectin-1 (CD111) (Friedman, et al., 2018; Krummenacher, et al., 2004). In preclinical studies, oHSV has been shown to infect and kill a heterogeneous population of pediatric brain tumor histotypes including HGG, ependymoma, medulloblastoma, primitive neuroectodermal tumors (PNET), and atypical teratoid/rhabdoid tumors (AT/RT) (Bernstock, et al., 2020b; Friedman, et al., 2018; Friedman, et al., 2009; Friedman, et al., 2016; Studebaker, et al., 2017). Therefore, oHSV is an attractive therapeutic for overcoming intratumoral and intertumoral cell heterogeneity found in brain tumors. In addition, oHSV has been shown to kill chemotherapy- and radiotherapy-resistant cancer stem cells in HGG and medulloblastoma (Friedman, et al., 2009; Friedman, et al., 2016). Interestingly, as a whole, pediatric brain tumor patient-derived xenograft (PDX) cells express more nectin-1 than adult HGG PDX cells and are 11- to 37-fold more sensitive to oHSVs suggesting that pediatric brain tumors may be an ideal target for oHSV (Friedman, et al., 2018).
The time required for an antitumor immune response to occur following oHSV administration is an important consideration, particularly in aggressive recurrent malignant brain tumors like HGG for which median survival in children is less than 6 months (Jakacki, et al., 2016). While data from brain tumor trials are limited and additional data are needed, a study utilizing GM-CSF-producing oHSV T-Vec (Imlygic®) in melanoma patients highlights the importance of accounting for late responses to oncolytic immunovirotherapy even after an apparent initial tumor progression; the researchers demonstrated that delayed regional and systemic antitumor responses may occur after treatment, ranging from approximately 18 weeks for directly injected lesions to 23 or more weeks for uninjected lesions (Kaufman, et al., 2016). Therefore, when evaluating response to OVs in brain tumor clinical trials, investigators may need to consider additional parameters beyond the standard criteria defined by immunotherapy Response Assessment in Neuro-Oncology (iRANO) or Response Assessment in Pediatric Neuro-Oncology (RAPNO) (Erker, et al., 2020; Okada, et al., 2015). In addition, testing OVs upfront when patients have more time to respond and a more functional immune system prior to receiving myelosuppressive chemotherapy is likely important to fully evaluate the clinical efficacy of OVs.
The first oHSV tested in a pediatric brain tumor clinical trial was HSV1716, but the trial only enrolled two patients and was subsequently terminated in 2016 (NCT02031965). Outcomes from the trial have not been published, and HSV1716 has not been tested in any additional pediatric brain tumor clinical trials. G207 has been the most extensively studied in humans and is the only oHSV currently being studied and advanced in pediatrics. The design of the G207 pediatric brain tumor trials, including a completed trial in supratentorial tumors (NCT02457845) and an ongoing trial in cerebellar tumors (NCT03911388) (Bernstock, et al., 2020a; Waters, et al., 2017), is largely based on the results from three early-phase clinical trials (NCT00157703, US0235, NCT00157703) of G207 in adults with recurrent HGG (Markert, et al., 2009; Markert, et al., 2000; Markert, et al., 2014). G207 was safely inoculated into adults with up to 3 × 109 plaque-forming units (PFU) in up to two doses. Three methods were used for these intracranial inoculations: directly into the tumor and by controlled-rate infusion via intratumoral catheters, into the brain surrounding a resection cavity, and combined with a single 5 Gy dose of radiation within 24 hours of virus inoculation. The use of radiation was based on preclinical studies that demonstrated a 5 Gy dose within 24 hours of virus optimally increased viral replication and spread throughout the tumor (Advani, et al., 2011; Mezhir, et al., 2005). These adult trials demonstrated the safety of G207, and a maximum tolerated dose (MTD) was not reached. Furthermore, evidence of radiographic and neuropathologic responses was seen in approximately half of the patients. Interestingly, while doses up to 3 × 109 PFU were safely administered without dose-limiting toxicities, responses were not dose-dependent and occurred at doses as low as 1 × 106 PFU, suggesting that the direct oncolytic effect, which depends on virus dose, is likely not as important as the secondary immune response engendered against the tumor to achieve clinical responses.
The Phase 1 trial of G207 alone and combined with a 5 Gy dose of radiation in children with recurrent or progressive supratentorial HGG (NCT02457845) was the first completed oncolytic virotherapy brain tumor trial in children; see Table 2 for summary of pediatric oncolytic virotherapy brain tumor clinical trials. The trial included 12 children with an age range of 7–18 years old and demonstrated the safety of G207 at a maximum planned dose of 1 × 108 PFU delivered by controlled rate infusion through up to four intratumoral catheters alone and when combined with radiation dose (Friedman, et al., 2021). All patients had isocitrate dehydrogenase (IDH)-wild type HGG without known favorable mutations or H3.3 mutations. There were no serious adverse events (AEs) attributable to G207. Related AEs were mild, grade 1 and infrequent with a total of 20 events reported. Fever, which was seen in 33% of patients, was the most common AE attributable to G207. There was no evidence of G207 shedding in the blood, conjunctiva secretions or saliva in any patient.
Table 2.
Pediatric Oncolytic Virotherapy Clinical Trials
| Class | Virus (Dose) | Phase; Status | Delivery/ Location | Combinations | Age (Years) | Disease | Novel Aspects; Key findings | NCT |
|---|---|---|---|---|---|---|---|---|
| Herpes Simplex Virus | HSV1716 (105 PFU) | 1; terminated | Peri- and intratumoral after tumor resection | IV dexamethasone prior to and 6 and 12 hrs post-surgery | 12–21 | Refractory/recurrent HGG | Well tolerated but trial retracted after 2 pts enrolled | NCT02031965 |
| HSV G207 (107 or 108 PFU) | 1; completed | IT infusion over 6 hrs via 3–4 catheters | 5 Gy radiation to tumor within 24 hrs after virus | 3–18 | Progressive/recurrent supratentorial malignant glioma | First completed trial of OV for pediatric brain tumor; 12.2 month median overall survival. 5/12 patients lived ≥18 months post treatment. 108 PFU safe and tolerable. Marked increase in TILs | NCT02457845 | |
| HSV G207 | 1; recruiting | IT via catheter | 5 Gy radiation to tumor within 24 hrs of virus | 3–18 | Refractory/recurrent malignant cerebellar tumors; includes LMD | First OV delivered via catheter to the cerebellum; first trial of oHSV for infratentorial tumors | NCT03911388 | |
| HSV G207 (108 PFU) | 2; not yet recruiting | IT infusion over 6 hrs via ≤4 catheters | 5 Gy radiation to tumor | 3–21 | Progressive/recurrent malignant HGG | First phase 2 trial of OV in children | NCT04482933 | |
| Adenovirus | DNX-2401 (≤ 5×1010 viral particles in 1mL) | 1; completed | IT infusion via catheter in cerebellar peduncle | Standard radiation and/or chemotherapy 3–4 wks after virus | 1 −18 | Naïve DIPG | Including OV as upfront therapy; Safe and tolerable. All patients showed reduced tumor volume |
NCT03178032 |
| AloCELYVIR (500 cells/kg) | 1b/2; recruiting | IV infusion; weekly × 8 | Radiotherapy for naïve DIPG | 1 – 21 | Naïve DIPG; Relapsed/refractory MB | Cellular therapy | NCT04758533 | |
| Poliovirus | PVSRIPO (5 × 107 TCID50) | 1b; active, not recruiting | IT infusion via intracerebral CED | 12 – 21 | Recurrent HGG/MB/ATRT | Disease involving cerebellum, pituitary, leptomeninges, brainstem, spinal cord, or requiring ventricular access can be included at discretion of neurosurgeon | NCT03043391 | |
| Reovirus | Pelareorep | 1; active, not recruiting | IV infusion over 60 min on days 3–5 of 28-day cycle × ≤ 12 | SQ GM-CSF on days 1 and 2 of 28 day cycle. Total cycles ≤ 12 | 10–21 | Refractory/relapsed HGG/MB/ATRT/PNET | IV virus delivery | NCT02444546 |
| Measles Virus | MV-NIS | 1; recruiting | Locally for recurrent tumors; Lumbar puncture for disseminated disease |
1 – 39 | Disseminated or locally recurrent MB; refractory ATRT | NIS allows noninvasive spatial and temporal virus tracking Lumbar puncture delivery |
NCT02962167 |
ATRT, atypical teratoid/rhabdoid tumor; CED, convection enhanced delivery; DIPG, diffuse intrinsic pontine glioma; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGG, high-grade glioma; hrs, hours; IT, intratumoral; IV, intravenous; LMD, leptomeningeal disease MB, medulloblastoma; oHSV, oncolytic herpes simplex virus therapy; OV, oncolytic virotherapy; PNET, primitive neuroectodermal tumor; SQ, subcutaneous; wks, weeks
Matched pre-treatment and 2–9 months post-treatment resection tissue from several patients conclusively demonstrated that G207 created an inflammatory tumor microenvironment and resulted in a shift from immunologically ‘cold’ to ‘hot’ with a significant increase in tumor-infiltrating lymphocytes (CD4+ and CD8+ T cells) within tumor areas both adjacent to and several centimeters away from the site of virus inoculation. Radiographic changes consistent with antitumor activity, including pseudoprogression or the development of progressively enlarging benign-appearing intratumoral cystic spaces were seen in most patients. The median overall survival of 12.2 months (95% confidence interval, 8.0 to 16.4) compared favorably to historical survival of 5.6 months in children with recurrent HGG (Jakacki, et al., 2016; Kline, et al., 2018). Of note, four of five patients without pre-existing HSV-1 antibodies seroconverted between 1–5 months after receiving G207 at the 1 × 108 PFU dose. Those that seroconverted survived on average longer than patients with pre-existing neutralizing antibodies; however, the number of patients was small and thus no definitive conclusion could be drawn. Whether the presence of pre-existing neutralizing antibodies or seroconversion after receiving an OV affects outcomes is unclear and may ultimately depend on tumor location, route of administration, and dosing regimen (e.g. single dose versus multiple doses). Based on these promising results, a Phase 2 multi-institutional trial in children with recurrent HGG at first relapse is expected to open in 2022 (NCT04482933). The trial will use the Recommended Phase 2 Dose (RP2D) of 1 × 108 PFU with a 5 Gy dose of radiation and the primary objective of the trial will be efficacy as evaluated by post-progression survival. Planned secondary objectives include safety, virologic shedding, radiographic response, and changes in lymphocyte infiltration within tumor sites.
Based on preclinical data suggesting that embryonal tumors, such as medulloblastoma that arises in the cerebellum, may be even more sensitive to oHSV than pediatric HGG (Friedman, et al., 2018), a second Phase 1 trial with G207 was initiated and is ongoing in children 3–18 years old with recurrent or refractory malignant cerebellar brain tumors (NCT03911388) (Bernstock, et al., 2020a). Similar to the supratentorial pediatric trial, the study is testing G207 alone and then combined with a 5 Gy dose of radiation. The starting dose chosen was lower (1 × 106 PFU) than the supratentorial trial secondary to unique challenges of inoculating tumors in the cerebellum: the posterior fossa is less amendable to placement of catheters and swelling from pseudoprogression; and the cerebellum is adjacent to the brainstem, which is responsible for central autonomic functions and potentially vulnerable to smaller degrees of inflammation (Bernstock, et al., 2020a). If lower doses are proven safe, the trial will test a maximum planned dose of 1 × 108 with 5 Gy dose of radiation, similar to the completed Phase 1 trial.
3. Adenovirus
Adenovirus (AdV) is a nonenveloped, double-stranded DNA virus that causes mild upper respiratory symptoms in healthy individuals. AdVs have been exploited both as vectors for gene transfer therapies and as OVs in the treatment of brain tumors (Castro, et al., 2014; Kiyokawa & Wakimoto, 2019). While virally-delivered gene therapies are beyond the scope of this review, they have shown some promise in clinical trials. To date, six oncolytic AdVs derived from human AdV serotype 5 (ONYX-015, DNX-2401, DNX-2440, CRAd-S-pk7, ICOVIR-5, and Ad-TD-nsIL12) have been used in HGG clinical trials. Some of these trials use neural stem cell (NSC) or mesenchymal stem cell (MSC)-based carrier systems to deliver the AdVs. Oncolytic AdVs have been engineered to conditionally replicate in tumor cells through various mechanisms. For example, ONYX-015 lacks the E1B gene, leading to loss of E1B-mediated late viral RNA export and restriction of replication in normal cells, while replication is supported in tumor cells that provide the RNA export function of E1B (Bischoff, et al., 1996; O’Shea, et al., 2004). A 24-base pair deletion in the E1A gene of DNX-2401, DNX-2440, and ICOVIR-5 limits viral replication to malignant cells that have defective Rb tumor suppressor protein (Whyte, et al., 1989). To further increase tumor tropism and bioavailability while limiting potential damage to surrounding brain parenchyma or off-target tissues, DNX-2401, DNX-2440, and ICOVIR-5 are further modified to include the arginine-glycine-aspartate (RGD) peptide. RGD directs the engineered virus to bind integrins, which are expressed at much higher levels than the natural AdV receptor on glioma cell surfaces (Fueyo, et al., 2003). In addition, DNX-2440 expresses the immune modulator OX40 ligand, which is designed to enhance antitumor T cell responses (Jiang, et al., 2017). NSC-CRAd-S-pk7 is a conditionally replicative AdV delivered by NSCs that contains the human survivin promoter to drive E1 expression and a polylysine modification of the fiber knob to enhance the ability to transduce glioma (Ulasov, et al., 2007). Ad-TD-nsIL12 harbors protective deletions in both E1A and E1B as well as a deletion in E3gp-19K and an insertion of non-secreting IL-12 gene driven by the endogenous E3gp-19K promoter to enhance antitumor immune responses (Wang, et al., 2017; Wang, et al., 2003).
In two completed Phase 1 adult trials for recurrent malignant gliomas, oncolytic AdVs were safe with evidence of efficacy when administered up to 1010 PFU directly into 10 sites of the tumor resection cavity (ONYX-015) (Chiocca, et al., 2004) and as a single dose up to 3 × 1010 intratumorally (DNX-2401; NCT00805376) (Lang, et al., 2018). Five of 25 patients (20%) that received DNX-2401 survived over three years after treatment and three patients (12%) had a ≥ 95% reduction in enhancing tumor (Lang, et al., 2018). The latter trial included an additional group of patients that received intratumoral DNX-2401 via an implanted catheter followed by en bloc resection of the tumor and catheter, and second dose of virus 14 days later. Viral replication and spread within the tumor was documented and histopathology demonstrated tumor infiltration by CD8+ and T-bet+ cells suggesting that responses were likely due to direct oncolysis and generation of an antitumor immune response. A single Phase 1/2 dose-escalation study in the Netherlands designed to test up to 1011 viral particles of DNX-2401 in adults with recurrent brain tumors was completed in 2014 (NCT0158251648), but results have not yet been published. Additionally, combining therapies with OVs to maximize an immune response is of great interest, and several drugs including temozolomide (NCT01956734), pembrolizumab (NCT02798406), and interferon gamma (IFN-γ) (NCT02197169) have been tested in combination with DNX-2401 in early phase trials for adults with recurrent HGG. These trials have completed enrollment, and results are forthcoming.
Another important area of oncolytic virotherapy research is novel delivery approaches. Delivery of virus via infected NSCs, or allogeneic or patient-derived bone marrow-derived MSC carriers is of interest due to their tumor tropism, and ability to evade neutralizing antibodies, cross the blood-brain barrier, distribute throughout a tumor and its margins, and migrate in brain parenchyma to target tumor cells (Fares, et al., 2021; Ruano, et al., 2020; Shimizu, et al., 2021). Recently, a first-in-human Phase 1 trial using NSC-CRAd-S-pk7 was conducted in adults with newly diagnosed HGG (NCT03072134) (Fares, et al., 2021). Patients received the virus into the walls of the resection cavity at a maximum planned dose of 1.875 × 1011 viral particles administered by 1.5 × 108 NSCs, and subsequently received standard of care with temozolomide and radiation. The therapy was deemed safe, with a single grade 3 event, viral meningitis due to inadvertent injection in the lateral ventricle. Immunological and histopathological evidence of responses was seen to support further investigation of the virus and delivery technique.
Additionally, a first-in-human trial of autologous bone marrow-derived MSCs as carriers for oncolytic AdV ICOVIR-5 (CELYVIR) in children and adults with relapsed/refractory solid tumors (NCT01844661), including a child with medulloblastoma and three adults with HGG, demonstrated safety of six weekly intravenous (IV) infusions of 2 × 106 cells/kg in children and 0.5–1 × 106 cells/kg in adults at a dose of 2 × 104 viral particles/MSC (Ruano, et al., 2020). Adenoviral replication was detected by PCR in 7 of 9 pediatric patients but none of the six adult patients. There are two ongoing clinical trials of allogenic MSCs infected with AdVs: a Phase 1b/2 trial with ICOVIR-5 (AloCELYVIR) in children and young adults with newly diagnosed diffuse intrinsic pontine glioma (DIPG) combined with radiation or in relapsed medulloblastoma as monotherapy (NCT04758533); and a Phase 1 trial with DNX-2401 administered via intra-arterial injection in adults with recurrent HGG (NCT03896568).
Based on preclinical data demonstrating efficacy of DNX-2401 in models of pediatric HGG and DIPG, the most deadly form of pediatric HGG (Martinez-Velez, et al., 2019a; Martinez-Velez, et al., 2019b), and the established safe doses of intratumoral DNX-2401 from the adult trials, a first-in-human Phase 1 trial was initiated for children 1–18 years old with newly diagnosed DIPG that have not received previous treatment (NCT03178032) (Tejada, et al., 2018a; Tejada, et al., 2018b). After stereotactic biopsy, patients received intratumoral infusion of 1–5 × 1010 viral particles in 1 mL delivered over approximately 70 minutes through a cannula, and subsequently, received standard radiotherapy within 1 month of DNX-2401 administration. While final study results have not been reported, 12 patients received the virus and no dose-limiting toxicities were observed (Garcia-Moure, et al., 2021). Efficacy evaluations and correlative analyses of tumor biopsy and blood samples including pre- and post-treatment titers of neutralizing antibodies are ongoing; however, the investigators recently reported an increased clonal T cell diversity following treatment with virus when comparing paired pre- and post-treatment samples (Iñigo-Marco, et al., 2022). Since patients also received standard radiation and the timing of the post-treatment samples in relation to DNX-2401 and radiation was not provided, it is unclear if these changes are due specifically to the virus. Nevertheless, this proof-of-principle study demonstrates the ability to directly treat a tumor in a very challenging location with an OV as part of initial therapy, which is an important paradigm shift towards upfront immunovirotherapy.
4. Poliovirus
Poliovirus (PV) is a non-enveloped, single-stranded positive-sense RNA virus of the Picornaviridae family. PV has an inherent tropism for lower motor neurons and can cause paralytic poliomyelitis. Its neuropathogenicity and CNS tropism, while not entirely understood, depend on tissue-specific function of its internal ribosomal entry site (IRES) element and differential expression of integral membrane protein CD155, which is the viral attachment and entry receptor. CD155 is known to play a role in cell adhesion and immune response (Takai, et al., 2008) and is upregulated in malignant cells and stromal components of many solid tumors including gliomas (Merrill, et al., 2004). Recombination with human rhinovirus type 2 ablates the inherent neuropathogenicity of PV while preserving its oncolytic properties, and preclinical studies have demonstrated that HGG were highly susceptible to oncolysis by the recombinant PV (Gromeier, et al., 1996; Gromeier, et al., 2000; Merrill, et al., 2004).
The first Phase 1 trial of an attenuated polio-rhinovirus chimera, PV (Sabin)-Rhinovirus IRES PV Open reading frame (PVSRIPO) was in 61 adults with recurrent glioblastoma who received a dose between 107-1010 median tissue culture infectious dose (TCID50) infused via intratumoral convection-enhanced delivery (CED) (NCT01491893) (Desjardins, et al., 2018). Pre-clinical studies using tagged viruses have demonstrated that CED has the potential to improve volume distribution and tissue penetration of viral doses in animal models (White, et al., 2011a; White, et al., 2011b). Seven of the tumors were IDH-mutant and 9 had unknown IDH status. Patients were given a boost immunization with trivalent inactivated PV vaccine at least one week prior to PVSRIPO in an effort to generate a robust immune response against PVSRIPO and further limit the possibility of neurovirulence in normal brain cells. In the dose expansion phase, 19% of the participants had a ≥ grade 3 AE. Due to locoregional inflammation after treatment resulting in prolonged glucocorticoid use, the dose was deescalated to the RP2D of 5 × 107 TCID50. Overall survival was reported at 21% at 24 months (95% confidence interval, 11 to 33); based on the results, the therapy was granted Breakthrough Therapy designation by the FDA in 2016. When patients were stratified by median tumor mutational burden (TMB; 1.3 mutations/megabase), those with a lower TMB had a significantly longer survival (Gromeier, et al., 2021), which may be attributable to an inverse relationship between TMB and enrichment of inflammatory gene signatures discovered in cohorts of recurrent but not newly diagnosed glioblastoma. These findings may have important implications for treating pediatric brain tumors with OVs since most pediatric brain tumors have a very low TMB and generally have a lower TMB than adult brain tumors (Jones & Baker, 2014).
The promising results of the Phase 1 trial of PVSRIPO in adults prompted the initiation of a Phase 1b trial for recurrent pediatric HGG in 2017 designed to determine safety of the approach in children 12–17 years of age (NCT03043391) (Ashley, et al., 2018). Based on preclinical data demonstrating susceptibility of both pediatric glial and embryonal brain tumors to PVSRIPO, the pediatric trial was subsequently amended to include any recurrent malignant glial tumor (e.g. malignant glioma, anaplastic oligodendroglioma, ependymoma), medulloblastoma, or AT/RT, and to include patients up to 21 years of age (Thompson, et al., 2018). Similar to the adult study design, patients receive a boost immunization with inactivated PV vaccine at least one week prior to PVSRIPO delivery. Immediately following stereotactic biopsy and histopathologic confirmation of recurrent tumor, patients then receive 5 × 107 TCID50 of PVSRIPO via catheters over 6.5 hours. The study is no longer recruiting and results are forthcoming.
5. Reovirus
Reovirus is a non-enveloped double-stranded RNA (dsRNA) virus that typically results in asymptomatic or mild symptoms including fever, vomiting or diarrhea in healthy individuals, as the PKR pathway in non-malignant cells recognizes the presence of dsRNA and blocks active viral translation (Muller, et al., 2020). In many tumor types, including gliomas, Ras upregulation inhibits PKR activation and renders wild-type reovirus naturally oncoselective in a manner similar to engineered oHSV; sensitivity of tumor cells to reovirus, however, is likely dependent on both molecular and cellular determinants that are still being elucidated (Gong & Mita, 2014; Muller, et al., 2020). In the first U.S. clinical trial utilizing reovirus for brain tumors, escalating doses of 108 to 1010 TCID50 of pelareorep (Reolysin), a live, replication-competent wild-type reovirus serotype-3-Dearing strain, were infused intratumorally over a 72-hour period in 15 adults with recurrent HGG (NCT00528684) (Kicielinski, et al., 2014). Pelareorep was safe without dose-limiting toxicities, and while the trial was the first to show that the presumed approach of viral delivery by CED was safe in recurrent HGG, the volume of distribution of the virus was not assessed secondary to financial limitations and difficulty of demonstrating the distribution of the viral dose versus that of the vehicle in humans. Similar to the completed adult and pediatric oHSV G207 trials, a MTD was not reached. In addition, evidence of antitumor activity was seen in some patients but did not appear to be dose-dependent.
Prior to testing pelareorep in pediatric brain tumors, safety of the virus alone and combined with low-dose oral cyclophosphamide was confirmed in children and young adults with relapsed or refractory non-CNS, non-lymphoma solid tumors in a multi-institutional Children’s Oncology Group Phase 1 trial (NCT01240538) (Kolb, et al., 2015). Twenty-four evaluable patients 3–21 years of age received IV reovirus at 5 × 108 TCID50/kg daily for 5 days every 28 days, and five of those patients also received low-dose oral cyclophosphamide at 50 mg/m2 × 21 days (Kolb, et al., 2015). Cyclophosphamide was used to test the hypothesis that reovirus could be safely administered with an immunosuppressive agent, as this combination was shown to inhibit both NK cells and T-regulatory cells and facilitate antitumor efficacy in murine models (Ghiringhelli, et al., 2007; Qiao, et al., 2008). The trial demonstrated the virus was rapidly cleared from the serum with a median time of 6.5 days, and there was no difference in the duration of viremia or peak viremia in nine patients without pre-existing reovirus antibodies compared to the 14 patients with baseline antibodies. In addition, there was no shedding of virus in the saliva or stool. Cyclophosphamide did not appear to affect viral clearance or peak anti-reovirus antibody level. While there were no objective responses, three patients had stable disease and received a second cycle.
Similar to the pediatric non-CNS trial, the ongoing Phase 1 trial of pelareorep in patients 10–21 years of age with high-grade relapsed or refractory brain tumors (NCT02444546) utilizes an IV delivery route; however, the virus is given on days 3–5 of a 28-day cycle after subcutaneous GM-CSF daily on days 1 and 2 of each cycle for up to 12 cycles in the absence of disease progression or unacceptable toxicity. The use of GM-CSF for this trial was based on preclinical murine melanoma model data demonstrating that IV reovirus is rapidly neutralized by pre-existing reovirus antibodies but functional virus bound by antibody was present in CD11b+ monocytes/macrophages, which effectively chaperoned the virus to tumor cells (Ilett, et al., 2014). Mobilization of monocytes/macrophages with GM-CSF prior to reovirus treatment resulted in improved tumor control in the melanoma model, and conditioning with GM-CSF was most efficacious when there were pre-existing neutralizing antibodies. The primary objective of the pediatric Phase 1 study is to determine the MTD and associated toxicities of the combination therapy, and secondary objectives are to assess median progression-free survival and overall survival. Exploratory objectives are to determine whether there is a correlation between antibody responsiveness to the virus or an increase in number of circulating monocytes with tumor response. This study completed accrual with 6 patients, and results are pending.
6. Measles Virus
Measles virus (MV) belongs to the Paramyxoviridae family of single-stranded negative-sense RNA viruses. Oncolytic modified MV, derived from the attenuated Edmonston B vaccine strain, displays tropism for CD46, a cofactor of complement inactivation (Dorig, et al., 1993). Tumor cells, which express higher densities of CD46 surface receptors than normal surrounding tissue as a possible mechanism to overcome complement-dependent cytotoxicity, are selectively infected by MV (Anderson, et al., 2004; Fishelson, et al., 2003). There are two Edmonston lineage MVs in clinical trials that have been genetically engineered to express trackable proteins: MV-CEA expresses human carcinoembryonic antigen (CEA), an inert peptide that is widely used as a tumor marker (Peng, et al., 2002); and MV-NIS expresses a sodium iodide symporter (NIS), which enables non-invasive monitoring of viral accumulation and propagation (Dingli, et al., 2004). MV-CEA, showed no dose limiting toxicities following direct intratumoral or resection cavity administration of up to 107 TCID50 in a Phase 1 clinical trial of adults with HGG (NCT00390299). Full results have not been published to date, and this virus has not been used in pediatric trials.
Safety of MV-NIS has been demonstrated in adult extracranial solid tumors (Galanis, et al., 2015), and while the virus has not been used in clinical trials for adult brain tumors, there is an active trial of MV-NIS (NCT02962167) for children and young adults (12 months to 39 years old) with recurrent medulloblastoma or AT/RT. The trial design was based on preclinical studies showing expression of CD46 in medulloblastoma tissue specimens and cells lines, and the effectiveness of intratumoral, IV, or intraventricular MV in prolonging survival in mice with localized or disseminated medulloblastoma or AT/RT (Hutzen, et al., 2012; Studebaker, et al., 2012; Studebaker, et al., 2015; Studebaker, et al., 2010). The primary objective of this ongoing Phase 1 trial is to determine AE frequency and a RP2D. Secondary and exploratory objectives include objective response rate and progression-free survival, and distribution of MV-NIS via single-photon emission computed tomography (SPECT) imaging after technetium-99m. Patients with locally recurrent tumors receive MV-NIS directly into the tumor bed following local resection. Those with disseminated disease will receive either one or two doses of MV-NIS via lumbar puncture into the subarachnoid space. This study is the first and only OV trial in pediatric brain tumors to utilize inoculation of virus into the cerebrospinal fluid via this route, which enables the direct targeting of leptomeningeal disease and spinal metastases.
7. Future Perspectives
Undoubtedly, significant progress has been made in the clinical use of OVs for pediatric brain tumors. Based on the results of completed clinical trials, OVs generally have favorable safety profiles, particularly in comparison to standard-of-care chemotherapy and radiation. Despite the predominance of infratentorial tumors amongst children with brain tumors, the majority of pediatric clinical trials to date have enrolled children with supratentorial brain tumors, mirroring the patient populations enrolled in counterpart adult trials. With increasing evidence of the safety of OVs in both adult and pediatric patients, trials focused on infratentorial tumors have been initiated. oHSV, MV, and AdV-based therapies are being evaluated in Phase 1 clinical trials specifically enrolling children with infratentorial tumors. Notably, the latter therapies are being explored as upfront therapy, potentially foreshadowing the implementation of immunovirotherapy as a frontline adjuvant treatment.
Several challenges exist and must be overcome to maximize immunovirotherapy and achieve durable responses in brain tumor patients. Improved preclinical models of disease are needed to gain a better understanding of therapeutic mechanisms in order to optimize oncolytic virotherapy. PDX models of brain tumors recapitulate many of the histological and genetic features of patient tumors, but the necessity to inoculate PDX lines into immunocompromised mice precludes comprehensive evaluation of immune responses. Alternatively, syngeneic murine models of brain tumors enable study of immune responses in immunocompetent mice, but there is significant heterogeneity in the infectivity and replicative capacity of OVs to various murine tumor models. Defining biomarkers to predict response from oncolytic virotherapy is critical so that patients most likely to benefit are included in trials and those unlikely to benefit can receive an alternative therapy. In addition, delivery of OVs to the intracranial compartment typically necessitates a neurosurgical procedure. While intracranially inoculated OVs can minimize concerns of systemic toxicity and ensure adequate delivery to the tumor, these procedures can be technically challenging and not without risk. Developing increasingly novel methods of viral delivery, such as the use of nanoparticle or cell-based carriers, may eliminate the need for a neurosurgical procedure and open new therapeutic avenues for patients with lesions in locations that are not surgically accessible or pose significant operative risks.
Currently, there are inadequate response assessment tools available after a patient receives immunovirotherapy, which makes it challenging to make subsequent treatment decisions. Improved imaging modalities to define responses from immunovirotherapy are critical. OVs can cause inflammation within brain tumors and surrounding brain parenchyma, which can lead to neurologic symptoms from increased edema. Corticosteroids are typically used in brain tumor patients to control neurologic symptoms; however, corticosteroids can cause immunosuppression. Bevacizumab has been used off-label as an alternative anti-inflammatory agent, but the ideal approach to managing inflammation is unknown. Lastly, an improved understanding of the tumor microenvironment and mechanisms of resistance to oncolytic virotherapy is needed to design next generation OVs and rational combination therapies to maximize antitumor immune responses. In addition to the combination approaches currently being tested in clinical trials, future immunovirotherapy brain tumor trials will likely include small molecule inhibitors, monoclonal antibodies, adoptive cellular therapies, radioimmunotherapy, cancer vaccines, and checkpoint inhibitors in conjunction with OVs. Oncolytic immunovirotherapy clinical trial results to date have been very promising in children, who may be ideal candidates for the therapy based on both their immune responsiveness and tumor biology. If the challenges described above can be overcome, oncolytic virotherapy has the potential to change the clinical landscape for the treatment of pediatric brain tumors.
Acknowledgements:
GKF supported by U.S. Food and Drug Administration (R01FD006368 and R01FD005379), Cannonball Kids cancer Foundation, the Rally Foundation for Childhood Cancer Research, CureSearch for Children’s Cancer, The V Foundation for Cancer Research, Hyundai Hope on Wheels, Andrew McDonough B+ Foundation, the National Pediatric Cancer Foundation, the Pediatric Cancer Research Foundation, and the Kaul Pediatric Research Institute. JMM supported by NCI (R01CA222903 and R01CA217179 and U19CA264512), Gateway for Cancer Research and DOD W81XWH1810315.
Conflict of Interest Statement:
JDB has an equity position in Avidea Technologies, Inc., which is commercializing polymer-based drug delivery technologies for immunotherapeutic applications. JDB has an equity position in Treovir LLC, an oHSV clinical stage company and is a member of the POCKiT Diagnostics Board of Scientific Advisors. JMM received payments from a structured buyout of Catherex, Inc., completed March 2021 (Catherex no longer exists); he has an equity position in and has received royalties from Aettis, Inc., which holds frozen oncolytic viral stocks, and in Treovir, Inc., which holds a small business innovation research fund to execute a clinical trial of G207 in pediatric patients; he has served as a consultant for Imugene; finally, he holds intellectual property for an oncolytic virus which has been licensed to Mustang Bio, Inc. but is blinded to the specifics of the relationship. GKF receives support from Eli Lilly and Company and Pfizer through contracts to UAB for clinical trials unrelated to OVs.
The remaining authors (GG, KK, SKT, SG, AR, SB, KK, MRC, RL, EB, AB, and JMJ) declare no conflict of interest exists.
Abbreviations:
- AdV
Adenovirus
- AE
adverse event
- AloCELYVIR
allogenic mesenchymal stem cells loaded with ICOVIR-5
- AT/RT
atypical teratoid/rhabdoid tumor
- CEA
carcinoembryonic antigen
- CED
convection-enhanced delivery
- CELYVIR
autologous bone marrow-derived mesenchymal stem cells loaded with ICOVIR-5
- CNS
central nervous system
- DIPG
diffuse intrinsic pontine glioma
- GBM
glioblastoma
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HGG
high-grade glioma
- HSV
Herpes Simplex Virus-1
- IDH
isocitrate dehydrogenase
- IFN-γ
interferon gamma
- IL-12
interleukin-12
- IRES
internal ribosomal entry site
- IT
intratumoral
- IV
intravenous
- LP
lumbar puncture
- MB
medulloblastoma
- MSC
mesenchymal stem cell
- MTD
maximum tolerated dose
- MV-CEA
engineered oncolytic measles virus expressing carcinoembryonic antigen
- MV-NIS
engineered oncolytic measles virus expressing sodium iodide symporter
- MV
Measles virus
- NIS
sodium iodide symporter
- NSC
neural stem cell
- oHSV
engineered oncolytic HSV-1
- OV
oncolytic virus
- Peds
pediatric
- PFU
plaque-forming units
- PKR
protein kinase R
- PNET
primitive neuroectodermal tumors
- PV
Poliovirus
- PVSRIPO
PV (Sabin)-Rhinovirus IRES PV Open reading frame
- Rad
radiation
- RGD
arginine-glycine-aspartate
- RP2D
Recommended Phase 2 Dose
- SQ
subcutaneous
- T-Vec
talimogene laherparepvec
- TCID50
median tissue culture infectious dose
- TMB
tumor mutational burden
Citations:
- Advani SJ, Markert JM, Sood RF, Samuel S, Gillespie GY, Shao MY, Roizman B, & Weichselbaum RR (2011). Increased oncolytic efficacy for high-grade gliomas by optimal integration of ionizing radiation into the replicative cycle of HSV-1. Gene Ther, 18, 1098–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BD, Nakamura T, Russell SJ, & Peng KW (2004). High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res, 64, 4919–4926. [DOI] [PubMed] [Google Scholar]
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH Jr., Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, & Coffin RS (2015). Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol, 33, 2780–2788. [DOI] [PubMed] [Google Scholar]
- Ashley DM, Thompson EM, Landi D, Desjardins A, Friedman AH, Threatt S, Herndon I, James E, Boulton S, McSherry F, Lipp ES, Sampson JH, Friedman HS, Bigner DD, & Gromeier M (2018). HGG-22. PHASE 1b STUDY POLIO VACCINE SABIN-RHINOVIRUS POLIOVIRUS (PVSRIPO) FOR RECURRENT MALIGNANT GLIOMA IN CHILDREN. Neuro-Oncology, 20, i93–i93. [Google Scholar]
- Bernstock JD, Bag AK, Fiveash J, Kachurak K, Elsayed G, Chagoya G, Gessler F, Valdes PA, Madan-Swain A, Whitley R, Markert JM, Gillespie GY, Johnston JM, & Friedman GK (2020a). Design and Rationale for First-in-Human Phase 1 Immunovirotherapy Clinical Trial of Oncolytic HSV G207 to Treat Malignant Pediatric Cerebellar Brain Tumors. Hum Gene Ther, 31, 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstock JD, Vicario N, Li R, Nan L, Totsch SK, Schlappi C, Gessler F, Han X, Parenti R, Beierle EA, Whitley RJ, Aban I, Gillespie GY, Markert JM, & Friedman GK (2020b). Safety and efficacy of oncolytic HSV-1 G207 inoculated into the cerebellum of mice. Cancer Gene Ther, 27, 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, & McCormick F (1996). An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science, 274, 373–376. [DOI] [PubMed] [Google Scholar]
- Cassady KA, Bauer DF, Roth J, Chambers MR, Shoeb T, Coleman J, Prichard M, Gillespie GY, & Markert JM (2017). Pre-clinical Assessment of C134, a Chimeric Oncolytic Herpes Simplex Virus, in Mice and Non-human Primates. Mol Ther Oncolytics, 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro MG, Candolfi M, Wilson TJ, Calinescu A, Paran C, Kamran N, Koschmann C, Moreno-Ayala MA, Assi H, & Lowenstein PR (2014). Adenoviral vector-mediated gene therapy for gliomas: coming of age. Expert Opin Biol Ther, 14, 1241–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, Kracher J, Grossman SA, Fisher JD, Carson K, Rosenblum M, Mikkelsen T, Olson J, Markert J, Rosenfeld S, Nabors LB, Brem S, Phuphanich S, Freeman S, Kaplan R, & Zwiebel J (2004). A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther, 10, 958–966. [DOI] [PubMed] [Google Scholar]
- Chiocca EA, Nakashima H, Kasai K, Fernandez SA, & Oglesbee M (2020). Preclinical Toxicology of rQNestin34.5v.2: An Oncolytic Herpes Virus with Transcriptional Regulation of the ICP34.5 Neurovirulence Gene. Mol Ther Methods Clin Dev, 17, 871–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D, Goldstein D, & Weller S (1989). Herpes simplex virus ribonucleotide reductase mutants are hypersensitive to acyclovir. Antimicrobial agents and chemotherapy, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripe TP, Chen CY, Denton NL, Haworth KB, Hutzen B, Leddon JL, Streby KA, Wang PY, Markert JM, Waters AM, Gillespie GY, Beierle EA, & Friedman GK (2015). Pediatric cancer gone viral. Part I: Strategies for utilizing oncolytic herpes simplex virus-1 in children. Molecular Therapy - Oncolytics, 2, 15015–15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins A, Gromeier M, Herndon JE 2nd, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, & Bigner DD (2018). Recurrent Glioblastoma Treated with Recombinant Poliovirus. N Engl J Med, 379, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingli D, Peng KW, Harvey ME, Greipp PR, O’Connor MK, Cattaneo R, Morris JC, & Russell SJ (2004). Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood, 103, 1641–1646. [DOI] [PubMed] [Google Scholar]
- Dorig RE, Marcil A, Chopra A, & Richardson CD (1993). The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell, 75, 295–305. [DOI] [PubMed] [Google Scholar]
- Erker C, Tamrazi B, Poussaint TY, Mueller S, Mata-Mbemba D, Franceschi E, Brandes AA, Rao A, Haworth KB, Wen PY, Goldman S, Vezina G, MacDonald TJ, Dunkel IJ, Morgan PS, Jaspan T, Prados MD, & Warren KE (2020). Response assessment in paediatric high-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol, 21, e317–e329. [DOI] [PubMed] [Google Scholar]
- Farassati F, Yang AD, & Lee PW (2001). Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol, 3, 745–750. [DOI] [PubMed] [Google Scholar]
- Fares J, Ahmed AU, Ulasov IV, Sonabend AM, Miska J, Lee-Chang C, Balyasnikova IV, Chandler JP, Portnow J, Tate MC, Kumthekar P, Lukas RV, Grimm SA, Adams AK, Hebert CD, Strong TV, Amidei C, Arrieta VA, Zannikou M, Horbinski C, Zhang H, Burdett KB, Curiel DT, Sachdev S, Aboody KS, Stupp R, & Lesniak MS (2021). Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol, 22, 1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishelson Z, Donin N, Zell S, Schultz S, & Kirschfink M (2003). Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol, 40, 109–123. [DOI] [PubMed] [Google Scholar]
- Friedman GK, Beierle EA, Gillespie GY, Markert JM, Waters AM, Chen CY, Denton NL, Haworth KB, Hutzen B, Leddon JL, Streby KA, Wang PY, & Cripe TP (2015). Pediatric cancer gone viral. Part II: potential clinical application of oncolytic herpes simplex virus-1 in children. Mol Ther Oncolytics, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GK, Bernstock JD, Chen D, Nan L, Moore BP, Kelly VM, Youngblood SL, Langford CP, Han X, Ring EK, Beierle EA, Gillespie GY, & Markert JM (2018). Enhanced Sensitivity of Patient-Derived Pediatric High-Grade Brain Tumor Xenografts to Oncolytic HSV-1 Virotherapy Correlates with Nectin-1 Expression. Sci Rep, 8, 13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GK, Johnston JM, Bag AK, Bernstock JD, Li R, Aban I, Kachurak K, Nan L, Kang KD, Totsch S, Schlappi C, Martin AM, Pastakia D, McNall-Knapp R, Farouk Sait S, Khakoo Y, Karajannis MA, Woodling K, Palmer JD, Osorio DS, Leonard J, Abdelbaki MS, Madan-Swain A, Atkinson TP, Whitley RJ, Fiveash JB, Markert JM, & Gillespie GY (2021). Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N Engl J Med, 384, 1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GK, Langford CP, Coleman JM, Cassady KA, Parker JN, Markert JM, & Yancey Gillespie G (2009). Engineered herpes simplex viruses efficiently infect and kill CD133+ human glioma xenograft cells that express CD111. J Neurooncol, 95, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GK, Moore BP, Nan L, Kelly VM, Etminan T, Langford CP, Xu H, Han X, Markert JM, Beierle EA, & Gillespie GY (2016). Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro Oncol, 18, 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, Liu TJ, Jiang H, Lemoine MG, Suzuki K, Sawaya R, Curiel DT, Yung WK, & Lang FF (2003). Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst, 95, 652–660. [DOI] [PubMed] [Google Scholar]
- Galanis E, Atherton PJ, Maurer MJ, Knutson KL, Dowdy SC, Cliby WA, Haluska P Jr., Long HJ, Oberg A, Aderca I, Block MS, Bakkum-Gamez J, Federspiel MJ, Russell SJ, Kalli KR, Keeney G, Peng KW, & Hartmann LC (2015). Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res, 75, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Moure M, Pérez-Larraya JG, Patiño A, Gonzalez-Huarriz M, Jones C, MacKay A, Van der Lugt J, Hulleman E, de Andrea C, Astigarraga I, García-Ariza M, Lopez-Ibor B, Villalba M, Lang FF, Fueyo J, Gomez-Manzano C, Dobbs J, Diez-Valle R, Alonso MM, & Tejada S (2021). EPCT-04. RESULTS OF A PHASE 1 STUDY OF THE ONCOLYTIC ADENOVIRUS DNX-2401 WITH RADIOTHERAPY FOR NEWLY DIAGNOSED DIFFUSE INTRINSIC PONTINE GLIOMA (DIPG). Neuro-Oncology, 23, i47–i47. [Google Scholar]
- Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, & Presky DH (1998). The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol, 16, 495–521. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, & Chauffert B (2007). Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother, 56, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, & Mita MM (2014). Activated ras signaling pathways and reovirus oncolysis: an update on the mechanism of preferential reovirus replication in cancer cells. Front Oncol, 4, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromeier M, Alexander L, & Wimmer E (1996). Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci U S A, 93, 2370–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromeier M, Brown MC, Zhang G, Lin X, Chen Y, Wei Z, Beaubier N, Yan H, He Y, Desjardins A, Herndon JE 2nd, Varn FS, Verhaak RG, Zhao J, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Lipp ES, Nair SK, Khasraw M, Peters KB, Randazzo D, Sampson JH, McLendon RE, Bigner DD, & Ashley DM (2021). Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat Commun, 12, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, & Wimmer E (2000). Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A, 97, 6803–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzen B, Pierson CR, Russell SJ, Galanis E, Raffel C, & Studebaker AW (2012). Treatment of medulloblastoma using an oncolytic measles virus encoding the thyroidal sodium iodide symporter shows enhanced efficacy with radioiodine. BMC Cancer, 12, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilett E, Kottke T, Donnelly O, Thompson J, Willmon C, Diaz R, Zaidi S, Coffey M, Selby P, Harrington K, Pandha H, Melcher A, & Vile R (2014). Cytokine conditioning enhances systemic delivery and therapy of an oncolytic virus. Mol Ther, 22, 1851–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñigo-Marco I, Gonzalez-Huarriz M, García-Moure M, Tamayo I, Hervas S, Hernandez R, Buñales M, DeAndrea C, Villalba M, Jones C, MacKay A, Hulleman E, Van der Lugt J, Aldave G, Lopez-Ibor B, Patiño-Garcia A, Diez-Valle R, Fueyo J, Gomez-Manzano C, de Larraya JGP, Tejada S, & Alonso MM (2022). THER-09. Oncolytic adenovirus, DNX-2401, for naive diffuse intrinsic pontine gliomas: A phase 1 clinical trial. Neuro-Oncology, 22. [Google Scholar]
- Jakacki RI, Cohen KJ, Buxton A, Krailo MD, Burger PC, Rosenblum MK, Brat DJ, Hamilton RL, Eckel SP, Zhou T, Lavey RS, & Pollack IF (2016). Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol, 18, 1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Rivera-Molina Y, Gomez-Manzano C, Clise-Dwyer K, Bover L, Vence LM, Yuan Y, Lang FF, Toniatti C, Hossain MB, & Fueyo J (2017). Oncolytic Adenovirus and Tumor-Targeting Immune Modulatory Therapy Improve Autologous Cancer Vaccination. Cancer Research, 77, 3894–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, & Baker SJ (2014). Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat Rev Cancer, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HL, Amatruda T, Reid T, Gonzalez R, Glaspy J, Whitman E, Harrington K, Nemunaitis J, Zloza A, Wolf M, & Senzer NN (2016). Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J Immunother Cancer, 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicielinski KP, Chiocca EA, Yu JS, Gill GM, Coffey M, & Markert JM (2014). Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol Ther, 22, 1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa J, & Wakimoto H (2019). Preclinical And Clinical Development Of Oncolytic Adenovirus For The Treatment Of Malignant Glioma. Oncolytic Virother, 8, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline C, Felton E, Allen IE, Tahir P, & Mueller S (2018). Survival outcomes in pediatric recurrent high-grade glioma: results of a 20-year systematic review and meta-analysis. J Neurooncol, 137, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb EA, Sampson V, Stabley D, Walter A, Sol-Church K, Cripe T, Hingorani P, Ahern CH, Weigel BJ, Zwiebel J, & Blaney SM (2015). A phase I trial and viral clearance study of reovirus (Reolysin) in children with relapsed or refractory extra-cranial solid tumors: a Children’s Oncology Group Phase I Consortium report. Pediatr Blood Cancer, 62, 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Baribaud F, Ponce de Leon M, Baribaud I, Whitbeck JC, Xu R, Cohen GH, & Eisenberg RJ (2004). Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology, 322, 286–299. [DOI] [PubMed] [Google Scholar]
- Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD, Gumin J, Vence LM, Wistuba I, Rodriguez-Canales J, Villalobos PA, Dirven CMF, Tejada S, Valle RD, Alonso MM, Ewald B, Peterkin JJ, Tufaro F, & Fueyo J (2018). Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J Clin Oncol, 36, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Robinson M, Han Z, Branston R, English C, Reay P, McGrath Y, Thomas S, Thornton M, Bullock P, CA L, & Coffin R (2003). ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene therapy, 10. [DOI] [PubMed] [Google Scholar]
- Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, Bjerke L, Clarke M, Vinci M, Nandhabalan M, Temelso S, Popov S, Molinari V, Raman P, Waanders AJ, Han HJ, Gupta S, Marshall L, Zacharoulis S, Vaidya S, Mandeville HC, Bridges LR, Martin AJ, Al-Sarraj S, Chandler C, Ng HK, Li X, Mu K, Trabelsi S, Brahim DH, Kisljakov AN, Konovalov DM, Moore AS, Carcaboso AM, Sunol M, de Torres C, Cruz O, Mora J, Shats LI, Stavale JN, Bidinotto LT, Reis RM, Entz-Werle N, Farrell M, Cryan J, Crimmins D, Caird J, Pears J, Monje M, Debily MA, Castel D, Grill J, Hawkins C, Nikbakht H, Jabado N, Baker SJ, Pfister SM, Jones DTW, Fouladi M, von Bueren AO, Baudis M, Resnick A, & Jones C (2017). Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell, 32, 520–537 e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majem M, Cascallo M, Bayo-Puxan N, Mesia R, Germa JR, & Alemany R (2006). Control of E1A under an E2F-1 promoter insulated with the myotonic dystrophy locus insulator reduces the toxicity of oncolytic adenovirus Ad-Delta24RGD. Cancer Gene Ther, 13, 696–705. [DOI] [PubMed] [Google Scholar]
- Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, Nabors LB, Markiewicz M, Lakeman AD, Palmer CA, Parker JN, Whitley RJ, & Gillespie GY (2009). Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther, 17, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, & Martuza RL (2000). Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther, 7, 867–874. [DOI] [PubMed] [Google Scholar]
- Markert JM, Razdan SN, Kuo HC, Cantor A, Knoll A, Karrasch M, Nabors LB, Markiewicz M, Agee BS, Coleman JM, Lakeman AD, Palmer CA, Parker JN, Whitley RJ, Weichselbaum RR, Fiveash JB, & Gillespie GY (2014). A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther, 22, 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Velez N, Garcia-Moure M, Marigil M, Gonzalez-Huarriz M, Puigdelloses M, Gallego Perez-Larraya J, Zalacain M, Marrodan L, Varela-Guruceaga M, Laspidea V, Aristu JJ, Ramos LI, Tejada-Solis S, Diez-Valle R, Jones C, Mackay A, Martinez-Climent JA, Garcia-Barchino MJ, Raabe E, Monje M, Becher OJ, Junier MP, El-Habr EA, Chneiweiss H, Aldave G, Jiang H, Fueyo J, Patino-Garcia A, Gomez-Manzano C, & Alonso MM (2019a). The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models. Nat Commun, 10, 2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Velez N, Marigil M, Garcia-Moure M, Gonzalez-Huarriz M, Aristu JJ, Ramos-Garcia LI, Tejada S, Diez-Valle R, Patino-Garcia A, Becher OJ, Gomez-Manzano C, Fueyo J, & Alonso MM (2019b). Delta-24-RGD combined with radiotherapy exerts a potent antitumor effect in diffuse intrinsic pontine glioma and pediatric high grade glioma models. Acta Neuropathol Commun, 7, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill MK, Bernhardt G, Sampson JH, Wikstrand CJ, Bigner DD, & Gromeier M (2004). Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro Oncol, 6, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezhir JJ, Advani SJ, Smith KD, Darga TE, Poon AP, Schmidt H, Posner MC, Roizman B, & Weichselbaum RR (2005). Ionizing radiation activates late herpes simplex virus 1 promoters via the p38 pathway in tumors treated with oncolytic viruses. Cancer Research, 65, 9479–9484. [DOI] [PubMed] [Google Scholar]
- Mineta T, Rabkin S, & Martuza R (1994). Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer research, 54. [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Yazaki T, Hunter WD, & Martuza RL (1995). Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med, 1, 938–943. [DOI] [PubMed] [Google Scholar]
- Muller L, Berkeley R, Barr T, Ilett E, & Errington-Mais F (2020). Past, Present and Future of Oncolytic Reovirus. Cancers (Basel), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, Boyle L, Pandey K, Soria C, Kunich J, Shen Y, Habets G, Ginzinger D, & McCormick F (2004). Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell, 6, 611–623. [DOI] [PubMed] [Google Scholar]
- Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, Franceschi E, Herold-Mende C, Nayak L, Panigrahy A, Pope WB, Prins R, Sampson JH, Wen PY, & Reardon DA (2015). Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol, 16, e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, & Barnholtz-Sloan JS (2020). CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol, 22, iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DM, Foreman PM, Nabors LB, Riley KO, Gillespie GY, & Markert JM (2016). Design of a Phase I Clinical Trial to Evaluate M032, a Genetically Engineered HSV-1 Expressing IL-12, in Patients with Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma. Hum Gene Ther Clin Dev, 27, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, & Russell SJ (2002). Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Research, 62, 4656–4662. [PubMed] [Google Scholar]
- Plant-Fox AS, O’Halloran K, & Goldman S (2021). Pediatric brain tumors: the era of molecular diagnostics, targeted and immune-based therapeutics, and a focus on long term neurologic sequelae. Curr Probl Cancer, 45, 100777. [DOI] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, Thompson J, Selby P, de Bono J, Melcher A, Pandha H, Coffey M, Vile R, & Harrington K (2008). Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res, 14, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, Petty R, MacLean A, Harland J, McKie E, Mabbs R, & Brown M (2000). Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther, 7, 859–866. [DOI] [PubMed] [Google Scholar]
- Ruano D, Lopez-Martin JA, Moreno L, Lassaletta A, Bautista F, Andion M, Hernandez C, Gonzalez-Murillo A, Melen G, Alemany R, Madero L, Garcia-Castro J, & Ramirez M (2020). First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol Ther, 28, 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Gumin J, Gao F, Hossain A, Shpall EJ, Kondo A, Parker Kerrigan BC, Yang J, Ledbetter D, Fueyo J, Gomez-Manzano C, & Lang FF (2021). Characterization of patient-derived bone marrow human mesenchymal stem cells as oncolytic virus carriers for the treatment of glioblastoma. J Neurosurg, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, & Jemal A (2021). Cancer Statistics, 2021. CA Cancer J Clin, 71, 7–33. [DOI] [PubMed] [Google Scholar]
- Streby KA, Geller JI, Currier MA, Warren PS, Racadio JM, Towbin AJ, Vaughan MR, Triplet M, Ott-Napier K, Dishman DJ, Backus LR, Stockman B, Brunner M, Simpson K, Spavin R, Conner J, & Cripe TP (2017). Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients. Clin Cancer Res, 23, 3566–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studebaker AW, Hutzen B, Pierson CR, Russell SJ, Galanis E, & Raffel C (2012). Oncolytic measles virus prolongs survival in a murine model of cerebral spinal fluid-disseminated medulloblastoma. Neuro Oncol, 14, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studebaker AW, Hutzen B, Pierson CR, Shaffer TA, Raffel C, & Jackson EM (2015). Oncolytic measles virus efficacy in murine xenograft models of atypical teratoid rhabdoid tumors. Neuro Oncol, 17, 1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studebaker AW, Hutzen BJ, Pierson CR, Haworth KB, Cripe TP, Jackson EM, & Leonard JR (2017). Oncolytic Herpes Virus rRp450 Shows Efficacy in Orthotopic Xenograft Group 3/4 Medulloblastomas and Atypical Teratoid/Rhabdoid Tumors. Mol Ther Oncolytics, 6, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studebaker AW, Kreofsky CR, Pierson CR, Russell SJ, Galanis E, & Raffel C (2010). Treatment of medulloblastoma with a modified measles virus. Neuro Oncol, 12, 1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Miyoshi J, Ikeda W, & Ogita H (2008). Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol, 9, 603–615. [DOI] [PubMed] [Google Scholar]
- Tejada S, Alonso M, Patino A, Fueyo J, Gomez-Manzano C, & Diez-Valle R (2018a). Phase I Trial of DNX-2401 for Diffuse Intrinsic Pontine Glioma Newly Diagnosed in Pediatric Patients. Neurosurgery, 83, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Tejada S, Diez-Valle R, Dominguez PD, Patino-Garcia A, Gonzalez-Huarriz M, Fueyo J, Gomez-Manzano C, Idoate MA, Peterkin J, & Alonso MM (2018b). DNX-2401, an Oncolytic Virus, for the Treatment of Newly Diagnosed Diffuse Intrinsic Pontine Gliomas: A Case Report. Front Oncol, 8, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EM, Brown M, Dobrikova E, Ramaswamy V, Taylor MD, McLendon R, Sanks J, Chandramohan V, Bigner D, & Gromeier M (2018). Poliovirus Receptor (CD155) Expression in Pediatric Brain Tumors Mediates Oncolysis of Medulloblastoma and Pleomorphic Xanthoastrocytoma. J Neuropathol Exp Neurol, 77, 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo T, Martuza RL, Rabkin SD, & Johnson PA (2001). Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A, 98, 6396–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulasov IV, Zhu ZB, Tyler MA, Han Y, Rivera AA, Khramtsov A, Curiel DT, & Lesniak MS (2007). Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther, 18, 589–602. [DOI] [PubMed] [Google Scholar]
- Wang P, Li X, Wang J, Gao D, Li Y, Li H, Chu Y, Zhang Z, Liu H, Jiang G, Cheng Z, Wang S, Dong J, Feng B, Chard LS, Lemoine NR, & Wang Y (2017). Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat Commun, 8, 1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hallden G, Hill R, Anand A, Liu TC, Francis J, Brooks G, Lemoine N, & Kirn D (2003). E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nature Biotechnology, 21, 1328–1335. [DOI] [PubMed] [Google Scholar]
- Waters AM, Johnston JM, Reddy AT, Fiveash J, Madan-Swain A, Kachurak K, Bag AK, Gillespie GY, Markert JM, & Friedman GK (2017). Rationale and Design of a Phase 1 Clinical Trial to Evaluate HSV G207 Alone or with a Single Radiation Dose in Children with Progressive or Recurrent Malignant Supratentorial Brain Tumors. Hum Gene Ther Clin Dev, 28, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E, Bienemann A, Sena-Esteves M, Taylor H, Bunnun C, Castrique E, & Gill S (2011a). Evaluation and optimization of the administration of recombinant adeno-associated viral vectors (serotypes 2/1, 2/2, 2/rh8, 2/9, and 2/rh10) by convection-enhanced delivery to the striatum. Hum Gene Ther, 22, 237–251. [DOI] [PubMed] [Google Scholar]
- White E, Bienemann A, Taylor H, Castrique E, Bunnun C, Wyatt M, & Gill S (2011b). An evaluation of site-specific immune responses directed against first-generation adenoviral vectors administered by convection-enhanced delivery. J Gene Med, 13, 269–282. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Kern ER, Chatterjee S, Chou J, & Roizman B (1993). Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Invest, 91, 2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P, Williamson NM, & Harlow E (1989). Cellular targets for transformation by the adenovirus E1A proteins. Cell, 56, 67–75. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li Y, Chen K, Qian L, & Wang P (2021). Oncolytic virotherapy reverses the immunosuppressive tumor microenvironment and its potential in combination with immunotherapy. Cancer Cell Int, 21, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]


