Abstract
Burkholderia cepacia has emerged as an important pulmonary pathogen in immunocompromised patients and in patients with cystic fibrosis (CF). Little is known about the virulence factors and pathogenesis of B. cepacia, although the persistent and sometimes invasive infections caused by B. cepacia suggest that the organism possesses mechanisms for both cellular invasion and evasion of the host immune response. In this study, cultured human cells were used to analyze the invasion and intracellular survival of B. cepacia J2315, a highly transmissible clinical isolate responsible for morbidity and mortality in CF patients. Quantitative invasion and intracellular growth assays demonstrated that B. cepacia J2315 was able to enter, survive, and replicate intracellularly in U937-derived macrophages and A549 pulmonary epithelial cells. Transmission electron microscopy of infected macrophages confirmed the presence of intracellular B. cepacia and showed that intracellular bacteria were contained within membrane-bound vacuoles. An environmental isolate of B. cepacia, strain J2540, was also examined for its ability to invade and survive intracellularly in cultured human cells. J2540 entered cultured macrophages with an invasion frequency similar to that of the clinical strain, but it was less invasive than the clinical strain in epithelial cells. In marked contrast to the clinical strain, the environmental isolate was unable to survive or replicate intracellularly in either cultured macrophages or epithelial cells. Invasion and intracellular survival may play important roles in the ability of virulent strains of B. cepacia to evade the host immune response and cause persistent infections in CF patients.
The gram-negative bacterium Burkholderia cepacia causes serious opportunistic infections in humans and has recently emerged as an important pulmonary pathogen in patients with cystic fibrosis (CF) (7, 8, 11, 24). In CF patients the clinical outcome of B. cepacia colonization can vary from maintenance of a normal respiratory function to a rapid and ultimately fatal clinical decline (11, 22). This latter condition, referred to as “B. cepacia syndrome,” occurs in approximately 25% of CF patients and is characterized by fever, acute necrotizing pneumonia and, in some cases, bacteremia (7). The specific mechanisms by which B. cepacia is able to subvert host defense mechanisms, invade deeper tissues of the lung, and ultimately become blood-borne are poorly understood. Compounding this lack of knowledge is the inherent resistance of B. cepacia to multiple antibiotics, which has made treatment of B. cepacia infections especially difficult (14, 21). Once a CF patient is colonized with B. cepacia, the organism is rarely eradicated.
There is growing evidence that the persistent infections caused by B. cepacia may be due, in part, to the ability of the organism to invade and survive intracellularly in human cells. Two of the main cell types encountered by B. cepacia infecting the CF lung are respiratory epithelial cells and pulmonary macrophages. B. cepacia organisms have been observed in tracheal epithelial cells harvested at the time of autopsy from a CF patient (J. L. Burns, D. K. Clark, and C. D. Wadsworth, Proc. 6th Annu. N. Am. Cystic Fibrosis Conf., abstr. 201, 1992). B. cepacia has also been shown to invade and survive in cultured respiratory epithelial cells (2). In contrast to epithelial cells, the interaction between B. cepacia and macrophages has received little attention (7). Since pulmonary macrophages represent a first line of defense within the CF lung, the ability of B. cepacia to enter and survive within macrophages could provide a mechanism for evasion of the host immune response and may help to explain the reported ability of B. cepacia to achieve prolonged pulmonary colonization despite a pronounced antibody response (17). Moreover, an intracellular niche may also explain the persistence of B. cepacia in the CF lung despite the use of antibiotics with demonstrated activity against the organism in vitro (5).
B. cepacia can be cultured from a range of natural environments, including soil, water, and plants (3). The pathogenic potential of environmental isolates and their genetic relationship to clinical strains responsible for severe and sometimes fatal pulmonary infections is an important, yet unresolved issue. One clinical strain in particular, J2315, has been responsible for epidemic outbreaks and increased mortality in CF patients (12, 20, 25). Strain J2315 expresses an unusual cable-like pilus that has been shown to play a role in adherence to CF mucin and airway respiratory epithelial cells (25). Other studies have demonstrated that J2315 exoproducts stimulate interleukin-8 (IL-8) release from cultured lung epithelial cells and peripheral blood monocytes (18). More recently, it has been shown that strain J2315 produces a hemolytic toxin that induces apoptosis (programmed cell death) in cultured macrophages (9). Taken together, these findings suggest that strain J2315 possesses mechanisms for both host cell invasion and evasion of the host immune response. A cell culture model for both invasion and intracellular survival would be a valuable tool to further define these processes and determine their role in the pathogenesis of B. cepacia.
In this study, we established a macrophage model of invasion and intracellular survival for B. cepacia. We examined the ability of B. cepacia strain J2315, as well as an environmental isolate of B. cepacia, to enter and survive intracellularly in cultured human macrophages, as well as in respiratory epithelial cells. Our findings suggest that invasion and intracellular survival may play important roles in the ability of virulent strains of B. cepacia to evade the host immune response and cause persistent and sometimes fatal infections in CF patients.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Two strains of B. cepacia were used in this study. The clinical strain, J2315, is a representative of the Edinburgh/Toronto (ET)/12 lineage and belongs to the genomovar III group of B. cepacia (12, 20). Strain J2540 is an environmental isolate belonging to genomovar II (3). B. cepacia and Escherichia coli were grown aerobically at 37°C in Luria-Bertani (LB) broth or on LB agar plates.
Cell invasion assays.
The ability of B. cepacia to invade U937-derived macrophages and A549 epithelial cells was examined. The U937 line (American Type Culture Collection) is a human monocytic cell line which differentiates into macrophage-like cells when treated with phorbol 12-myristate 13-acetate (PMA; Sigma). The A549 line (American Type Culture Collection) is a human alveolar epithelial carcinoma cell line. U937-derived macrophages and A549 epithelial cells were grown in RPMI tissue culture medium containing 10% fetal calf serum (Gibco-BRL). Invasion assays were performed by a modification of the gentamicin protection assay originally described by Isberg and Falkow (10). B. cepacia is resistant to gentamicin, the drug of choice for killing extracellular bacteria in assays of invasion. However, ceftazidime, in combination with other antibiotics, has been shown to efficiently kill B. cepacia (2). We found that a combination of ceftazidime (1 mg/ml) and amikacin (1 mg/ml) incubated with B. cepacia for 2 h resulted in greater than 99.99% killing (fewer than 20 CFU were recovered with an initial inoculum of 5.6 × 107 CFU). Bacterial cells, grown to mid-log phase (optical density at 600 nm of 0.6) and washed with tissue culture medium, were used to infect confluent monolayers of eucaryotic cells (5 × 105 cells per well) in 24-well tissue culture plates. The infected monolayers were centrifuged (165 × g for 5 min) and incubated at 37°C in 5% CO2 for 30 min to allow bacterial entry. After 30 min of incubation, the monolayers were washed with phosphate-buffered saline (PBS; pH 7.0), and tissue culture medium containing a combination of amikacin and ceftazidime was added. The monolayers were then incubated for 2 h to kill the extracellular bacteria. After 2 h of incubation, the cell monolayers were washed with PBS, released by treatment with 10 mM EDTA, and lysed with 0.25% Triton X-100. The intracellular bacteria were quantitated by plating serial dilutions of the lysate. All quantitative invasion assays were performed in triplicate. A noninvasive strain of E. coli, HB101, was routinely used as a negative control.
Assay of intracellular growth.
Bacterial cell suspensions of the strains J2315 and J2540 were inoculated in parallel onto macrophage and epithelial cell monolayers at a multiplicity of infection (MOI) of 10:1. After a 30-min incubation, the infected monolayers were washed with PBS, and tissue culture medium containing a combination of amikacin (1 mg/ml) and ceftazidime (1 mg/ml) was added; the monolayers were then incubated for 2 h to kill the extracellular bacteria. Following 2 h of incubation, the medium was replaced with antibiotic-free tissue culture medium, and the intracellular bacteria were quantitated over time. At each time point, the infected monolayers were lysed as described above and the bacterial titers were determined by serial dilution and plating.
Transmission electron microscopy.
Monolayers of U937-derived macrophages were grown on glass coverslips and infected with B. cepacia. Infected cells were prepared for microscopy as previously described (19). Briefly, at different time points postinfection, the infected cell monolayers were gently rinsed with PBS and fixed with 2% glutaraldehyde. Specimens were postfixed in 1% OSO4, dehydrated stepwise in ethanol, and embedded in Polybed 812 (Polysciences, Inc.). Ultrathin sections were prepared by using a diamond knife (Diatome). Transmission electron microscopy (TEM) studies were carried out with a Phillips Model CM12 electron microscope operating at 80 keV.
RESULTS
Invasion of U937 macrophages and A549 pulmonary epithelial cells by B. cepacia.
The ability of B. cepacia to invade cultured U937 macrophages and A549 pulmonary epithelial cells was examined. We first assayed the ability of B. cepacia to invade U937 macrophages. The B. cepacia clinical strain J2315 entered cultured macrophages with an invasion frequency of 5.82% and was more than 1,000-fold more invasive than the noninvasive E. coli control strain HB101 (Table 1). The B. cepacia environmental isolate, J2540, entered cultured macrophages with an invasion frequency of 5.49% and was also more than a 1,000-fold more invasive than the noninvasive E. coli strain. Thus, both the clinical and environmental isolates of B. cepacia entered U937-derived macrophages with statistically indistinguishable invasion frequencies. When the invasion assays were repeated with a higher MOI, the invasion frequencies were, again, statistically equivalent (Table 1). Interestingly, the invasion frequency for both strains was reduced at the higher MOI, suggesting that invasion of macrophages is saturable. It has previously been shown that B. cepacia entry into epithelial cells can be saturated (2).
TABLE 1.
Entry of B. cepacia J2315 and J2540 into cultured U937 macrophages and A549 respiratory epithelial cellsa
| Strain | U937 Macrophages
|
A549 Epithelial cells
|
||
|---|---|---|---|---|
| % Invasion (MOI 10:1) | % Invasion (MOI 70:1) | % Invasion (MOI 10:1) | % Invasion (MOI 100:1) | |
| J2315 | 5.82 ± 0.225 | 3.30 ± 0.19 | 0.15 ± 0.0063 | 0.10 ± 0.0019 |
| J2540 | 5.49 ± 0.056 | 3.16 ± 0.35 | 0.024 ± 0.0015 | 0.034 ± 0.0017 |
| E. coli HB101 | 0.0028 ± 0.0005 | 0.011 ± 0.00002 | 0.0058 ± 0.000035 | 0.0082 ± 0.0005 |
Invasion assays were performed as described in Materials and Methods. Values represent the percentage of the bacterial inoculum that survived 2 h antibiotic treatment and are the means ± the standard deviation of six independent determinations with a total of three wells.
The ability of J2315 and J2540 to enter A549 pulmonary epithelial cells was also examined. The clinical strain, J2315, entered cultured epithelial cells with an invasion frequency of 0.15% (MOI 10:1) and was 25-fold more invasive than the noninvasive E. coli strain (Table 1). The environmental isolate, J2540 entered cultured epithelial cells with an invasion frequency of 0.024% (MOI 10:1) and was fourfold more invasive than the noninvasive E. coli control strain (Table 1). When epithelial cells were infected at a higher MOI, there was a slight decrease in the invasion frequency for J2315 and a small increase in the invasion frequency for J2540 (Table 1). Our findings are consistent with those of Burns et al. (2), who previously described the ability of another clinical isolate of B. cepacia to enter A549 cells.
Intracellular growth of B. cepacia.
The ability of both the clinical and the environmental isolates of B. cepacia to enter cultured human cells with similar invasion frequencies prompted us to examine their intracellular fates. Intracellular growth assays were performed by using B. cepacia-infected U937-derived macrophages and A549 respiratory epithelial cells. Bacterial cell suspensions of the clinical isolate J2315 and the environmental isolate J2540 were inoculated onto cell monolayers at an MOI of 10:1. After 30 min of incubation, the infected monolayers were washed and tissue culture medium containing antibiotics was added to kill the extracellular bacteria. Following a 2-h incubation, the medium was replaced with antibiotic-free tissue culture medium, and the ability of B. cepacia to survive and replicate intracellularly was determined by quantitating intracellular bacteria at 6-h time points for a 24-h period.
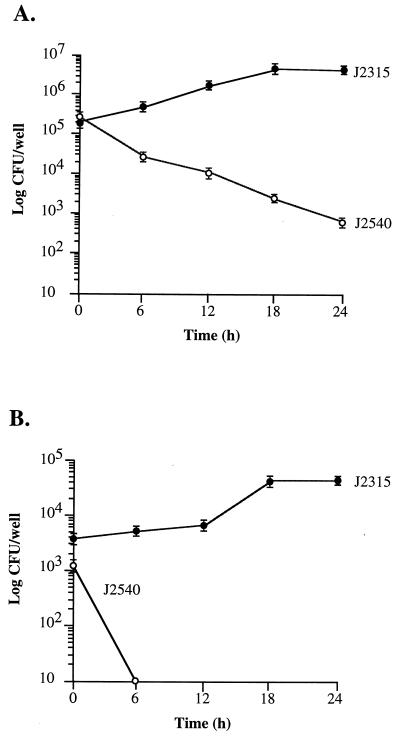
Shown in Fig. 1A are the results of intracellular growth assays with cultured macrophages. At time zero the number of intracellular bacteria was similar when macrophages were infected with either J2315 or J2540, a finding consistent with the similar invasion frequencies of the two strains for macrophages (Table 1). Following entry, the clinical strain survived and replicated, with a log unit increase over 24 h in the number of organisms located intracellularly. In contrast, the environmental strain, J2540, was killed by macrophages, with a >100-fold decrease in the number of intracellular bacteria over 24 h. A similar result was obtained when intracellular growth was assessed on infected A549 epithelial cells (Fig. 1B). Following entry, the clinical strain survived and replicated, while the environmental isolate was readily killed.
FIG. 1.
Intracellular growth of B. cepacia in U937 macrophages (A) and A549 respiratory epithelial cells (B). Intracellular growth assays were performed as described in Materials and Methods. Brackets represent standard errors. Time zero is 2 h and 30 min postinfection.
The integrity of the B. cepacia-infected monolayers was examined by light microscopy throughout the 24-h period (data not shown). By 24 h postinfection, visible disruption of the J2315-infected macrophage and epithelial cell monolayers was observed, suggesting that J2315 may be cytotoxic to cultured human cells. Due to the possible loss of host cell viability, time points beyond 24 h were not measured.
Electron microscopy.
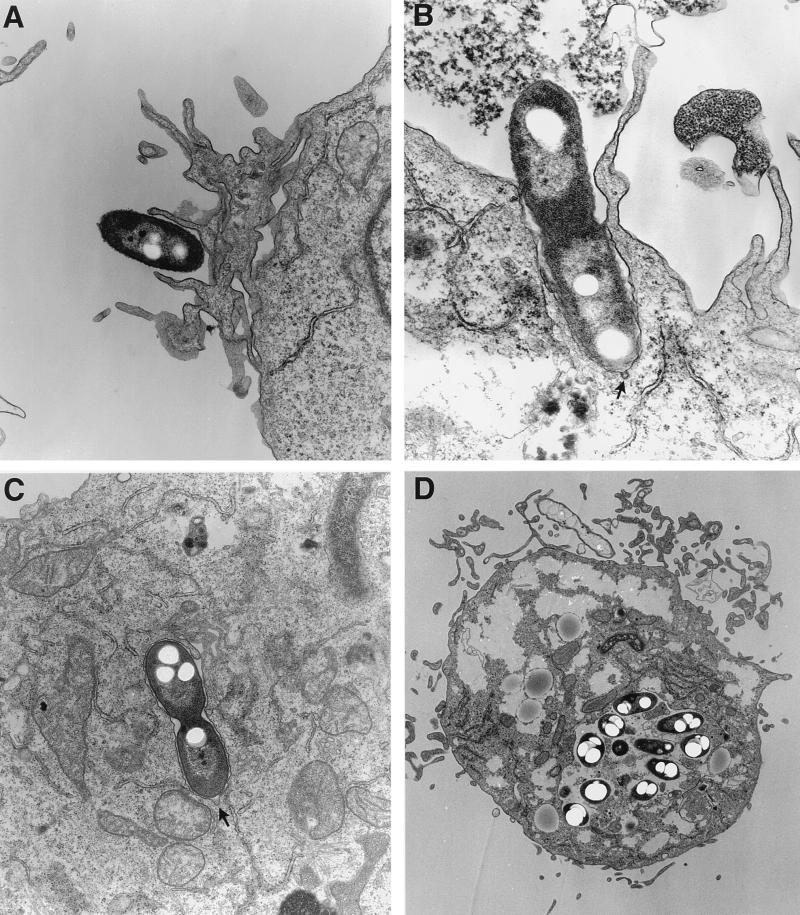
TEM confirmed the presence of intracellular B. cepacia. The interaction between the clinical strain J2315 and U937 macrophages is shown in Fig. 2. At the site of initial contact between the bacterium and macrophage, a thickening of the cell membrane was visible (Fig. 2A and B). Microvilli were observed in close association with the invading bacteria. Invaginations, resembling coated pits (6), were also visible at the site of contact between the bacterium and the macrophage (Fig. 2B and C). Intracellular B. cepacia were observed both singly and in groups and were enclosed in membrane-bound vacuoles (Fig. 2C and D). Many of the bacterial cells observed by TEM had electron transparent regions that are likely poly-β-hydroxyalkanoate granules, which are known to accumulate in B. cepacia (26). By 12 h postinfection there was significant rounding up of the mitochondria, as well as vacuolization of the cytosol of infected macrophages (Fig. 2D). These cytotoxic effects are consistent with the disruption of J2315-infected monolayers that we observed by light microscopy.
FIG. 2.
Transmission electron micrographs of U937 macrophages infected with B. cepacia J2315. Monolayers were infected with bacteria at an MOI of 10:1. The infected monolayers were then prepared for electron microscopy at different time points postinfection. (A and B) Initial contact of bacterial cells with the macrophage surface. (C) Intracellular B. cepacia within a membrane-bound vacuole (20-min postinfection). (D) B. cepacia-infected macrophage containing numerous intracellular bacteria within a single membrane-bound vacuole (12 h postinfection). The arrows denote invaginations resembling coated pits.
Few intracellular bacteria were observed in macrophages infected with the environmental isolate J2540 (data not shown). The J2540 cells that were observed intracellularly appeared to be electron dense, suggesting that they were nonviable, a finding consistent with the inability of strain J2540 to survive intracellularly, as determined by intracellular growth assays.
DISCUSSION
In this study we established a macrophage model of invasion and intracellular survival for B. cepacia. We have shown that a virulent and highly transmissible clinical isolate of B. cepacia, strain J2315, is able to enter, survive, and replicate intracellularly in cultured human macrophages and pulmonary epithelial cells. We have also shown that an environmental isolate of B. cepacia, strain J2540, is able to enter cultured macrophages and epithelial cells with invasion frequencies similar to those of the clinical strain. However, in contrast to the clinical strain, the environmental isolate of B. cepacia is unable to survive or replicate intracellularly. Our findings provide further evidence that B. cepacia is a facultative intracellular pathogen with the capacity to invade and persist in pulmonary epithelial cells and macrophages. Our data clearly suggest that intracellular survival and replication contribute to the virulence potential of pathogenic strains of B. cepacia.
To our knowledge, this is the first report describing the ability of B. cepacia to enter and survive intracellularly in cultured human macrophages. Intracellular survival is a key component in the pathogenic cycle of a number of bacterial pathogens, including Mycobacteria spp., Shigella flexneri, Salmonella spp., and Legionella pneumophila. The mechanisms by which intracellular pathogens resist killing by macrophages include inhibition of phagosome-lysosome fusion, escape into the cytoplasmic compartment, and resistance to reactive oxygen intermediates and lysosomal enzymes (4, 13). The intracytoplasmic B. cepacia cells we observed in infected macrophages were all enclosed in membrane-bound vacuoles. Interestingly, in respiratory epithelial cells, B. cepacia cells are also found enclosed in membrane-bound vacuoles (2). This may indicate that B. cepacia intracellular survival does not require escape into the cytoplasmic compartment but rather that B. cepacia is able to survive or inhibit the antimicrobial response of macrophages. It has recently been shown that the melanin-like pigment produced by epidemic strains of B. cepacia is able to scavenge superoxide anion, which may allow B. cepacia to survive the respiratory burst response of phagocytic cells (27). Resistance to nonoxidative killing has been suggested by the ability of B. cepacia to cause invasive infections in patients with chronic granulomatous disease, an inherited disorder of phagocytes in which polymorphonuclear leukocytes are unable to generate microbicidal oxygen radicals (24).
Intracellular survival does not appear to be a function common to all strains of B. cepacia. We have shown that the environmental isolate J2540 is able to enter cultured epithelial cells and macrophages but is unable to survive intracellularly. Thus, both the clinical and the environmental isolates of B. cepacia are able to gain entry into cultured human cells, but they differ in their abilities to survive intracellularly. B. cepacia CF isolates have been demonstrated to be clonally distinct from environmental isolates based on both phenotypic and genetic typing methods (12, 15). However, the pathogenic potential of environmental isolates of B. cepacia has not been extensively addressed. Our findings suggest that a phenotypic distinction between strains of B. cepacia may be in their ability to survive intracellularly in human cells. Differences in the ability to survive intracellularly may have implications for our understanding of the varied disease progressions associated with B. cepacia infections in CF.
We found that the invasion frequency of B. cepacia is significantly greater for cultured macrophages than for epithelial cells, suggesting that distinct or additional mechanisms mediate B. cepacia entry into cultured macrophages. One of the principal uptake mechanisms of macrophages is phagocytosis, which involves the uptake of particles coated with complement and/or antibodies. Binding of complement to the intracellular pathogens L. pneumophila and Mycobacterium spp. facilitates their entry into phagocytic cells (1, 23). While we have no direct evidence that complement-mediated phagocytosis is involved in the internalization of B. cepacia, we did find that when heat-inactivated serum was used in the invasion assay, entry of B. cepacia was significantly impaired, suggesting that macrophage entry may involve complement deposition (unpublished). Another principle uptake mechanism of phagocytic cells is receptor-mediated endocytosis, which involves specialized regions of the eucaryotic surface membrane, known as coated pits (6). Receptors cluster in these pits which, upon binding to small particles, invaginate into the cell during endocytosis. We observed, by TEM, structures resembling coated pits at the site of contact between invading bacteria and macrophages (Fig. 2B and C). Their association with invading B. cepacia suggests they may play a role in the entry process. Further studies are necessary to confirm the identity of these structures and to determine their relationship with B. cepacia entry into macrophages.
TEM of J2315-infected macrophages revealed considerable vacuolization of the macrophage cytosol at 12-h postinfection (Fig. 2D). Similar morphological changes have been observed in macrophages infected with the intracellular pathogens Salmonella typhimurium and S. flexneri (16, 28). These morphological changes result from bacterium-mediated induction of apoptosis (programmed cell death). Induction of programmed cell death by bacterial pathogens is believed to play a role in both immune system evasion and the initiation of inflammation (29). In the case of S. typhimurium, apoptosis is triggered upon macrophage entry, while S. flexneri induces apoptosis upon escape from the phagosome into the macrophage cytoplasm (16, 28). Extensive vacuolization of the macrophage cytosol was not observed at early stages of B. cepacia infection (Fig. 2A to C), indicating that the observed morphological changes may occur at stages during or subsequent to entry. It is noteworthy that J2315 produces a hemolytic toxin that has been shown to induce apoptosis in cultured macrophages (9). It has been proposed that the induction of apoptosis may enhance the establishment of B. cepacia infection by blocking release of the bacteriocidal contents of phagocytes. The possible role of apoptosis in the pathogenesis of B. cepacia is currently under investigation.
The ability of B. cepacia to invade and survive intracellularly in cultured macrophages and pulmonary epithelial cells clearly suggests that B. cepacia may have an intracellular phase during pulmonary infections in CF. Intracellular survival in macrophages may play a role in immune evasion. Alternatively, macrophages may serve as a vehicle for translocation and systemic dissemination of B. cepacia outside of the CF lung. With an established cell culture model for invasion and intracellular survival, studies are now possible to further characterize the mechanisms of pathogenesis and the genetic elements required for these processes.
ACKNOWLEDGMENTS
We thank John Govan, Department of Medical Microbiology, University of Edinburgh, for kindly providing B. cepacia J2315 and J2540. We also thank Nafisa Ghori, Stanford University, for TEM preparation and sectioning. We are grateful to Stanley Falkow, Lucy Thompkins, and Lucy Shapiro, in whose laboratories the initial stages of this study were conducted.
C.D.M. was supported by University of Minnesota Grant-in-Aid grant 17929. D.W.M. was supported by a Fellowship Award from the Center for Indoor Air Research. Lucy Thompkins and Lucy Shapiro were supported by NIH grants AI30618 and GM32506/5120MZ, respectively.
REFERENCES
- 1.Bellinger-Kawahara C, Horwitz M A. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J Exp Med. 1990;172:1201–1210. doi: 10.1084/jem.172.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns J, Jonas M, Chi E, Clark D, Berger A, Griffith A. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect Immun. 1996;64:4054–4059. doi: 10.1128/iai.64.10.4054-4059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler S, Doherty C, Hughes J, Nelson J, Govan J. Burkholderia cepacia and cystic fibrosis: do natural environments present a potential hazard? J Clin Microbiol. 1995;33:1001–1004. doi: 10.1128/jcm.33.4.1001-1004.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finlay B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold R, Jin E, Levinson H, Isles A, Fleming P C. Ceftazidime alone and in combination in patients with cystic fibrosis: lack of efficacy in treatment of severe respiratory infections caused by Pseudomonas cepacia. J Antimicrob Chemother. 1983;12:331–336. doi: 10.1093/jac/12.suppl_a.331. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein J L, Anderson R G W, Brown M S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979;279:679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- 7.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govan J R W, Nelson J. Microbiology of lung infection in cystic fibrosis. Br Med Bull. 1992;48:912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- 9.Hutchison M, Poxton I, Govan J. Burkholderia cepacia produces a hemolysin that is capable of inducing apoptosis and degranulation of mammalian phagocytes. Infect Immun. 1998;66:2033–2039. doi: 10.1128/iai.66.5.2033-2039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isberg R R, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature (London) 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 11.Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 12.Johnson W, Tyler S, Rozee K. Linkage analysis of geographic and clinical clusters in Pseudomonas cepacia infections by multilocus enzyme electrophoresis and ribotyping. J Clin Microbiol. 1994;32:924–930. doi: 10.1128/jcm.32.4.924-930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 14.Lewin C, Doherty C, Govan J W R. In vitro activities of meropenem, PD 127391, PD 131628, ceftazidime, chloramphenicol, cotrimoxazole, and ciprofloxacin against Pseudomonas cepacia. Antimicrob Agents Chemother. 1993;37:123–125. doi: 10.1128/aac.37.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahenthiralingam E, Campbell M, Henry D, Speert D. Epidemiology of Burkholderia cepacia infection in patients with cystic fibrosis: analysis by randomly amplified polymorphic DNA fingerprinting. J Clin Microbiol. 1996;34:2914–2920. doi: 10.1128/jcm.34.12.2914-2920.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monack D, Raupach B, Hromockyj A, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson J W, Butler S L, Brown P H, Greening A P, Govan J R W. Serum IgG and sputum IgA antibody to core lipopolysaccharide antigen from Pseudomonas cepacia in patients with cystic fibrosis. J Med Microbiol. 1993;39:39–47. doi: 10.1099/00222615-39-1-39. [DOI] [PubMed] [Google Scholar]
- 18.Palfreyman R, Watson M, Eden C, Smith A. Induction of biologically active interleukin-8 from lung epithelial cells by Burkholderia (Pseudomonas) cepacia products. Infect Immun. 1997;65:617–622. doi: 10.1128/iai.65.2.617-622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascopella L, Raupach B, Ghori N, Monack D, Falkow S, Small P L C. Host restriction phenotypes of Salmonella typhi and Salmonella gallinarum. Infect Immun. 1995;63:4329–4335. doi: 10.1128/iai.63.11.4329-4335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitt T, Kaufmann M, Patel P, Benge L, Gaskin S, Livermore D. Type characterisation and antibiotic susceptibility of Burkholderia (Pseudomonas) cepacia isolates from patients with cystic fibrosis in the United Kingdom and the Republic of Ireland. J Med Microbiol. 1996;44:203–210. doi: 10.1099/00222615-44-3-203. [DOI] [PubMed] [Google Scholar]
- 21.Prince A. Antibiotic resistance of Pseudomonas species. J Pediatr. 1986;108:830–834. doi: 10.1016/s0022-3476(86)80753-3. [DOI] [PubMed] [Google Scholar]
- 22.Rosenstein B J, Hall D E. Pneumonia and septicemia due to Pseudomonas cepacia in a patient with cystic fibrosis. John Hopkins Med J. 1980;147:188–189. [PubMed] [Google Scholar]
- 23.Schlesinger L S, Horwitz M A. A role for natural antibody in the pathogenesis of leprosy: antibody in nonimmune serum mediates C3 fixation to the Mycobacterium leprae surface and hence phagocytosis by human mononuclear phagocytes. Infect Immun. 1994;62:280–289. doi: 10.1128/iai.62.1.280-289.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speert D, Bond M, Woodman R, Curnutte J. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J Infect Dis. 1994;170:1524–1531. doi: 10.1093/infdis/170.6.1524. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Jiang R, Steinbach S, Holmes A, Campanelli C, Forstner J, Sajjan U, Tan Y, Riley M, Goldstein R. The emergence of a highly transmissible lineage of cbl+ Pseudomonas (Burkholderia) cepacia causing CF centre epidemics in North America and Britain. Nature Medicine. 1995;1:661–666. doi: 10.1038/nm0795-661. [DOI] [PubMed] [Google Scholar]
- 26.Wieczorek R, Steinbuchel A, Schmidt B. Occurrence of polyhydroxyalkanoic acid granule-associated proteins related to the Alcaligenes eutrophus H16 GA24 protein in other bacteria. FEMS Microbiol Lett. 1996;135:23–30. doi: 10.1111/j.1574-6968.1996.tb07961.x. [DOI] [PubMed] [Google Scholar]
- 27.Zughaier S M, Ryley H C, Jackson S K. A melanin pigment purified from an epidemic strain of Burkholderia cepacia attenuates monocyte respiratory burst activity by scavenging superoxide anion. Infect Immun. 1999;67:908–913. doi: 10.1128/iai.67.2.908-913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zychlinsky A, Kenny B, Menard R, Prevost M, Holland I, Sansonetti P. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 29.Zychlinsky A, Sansonetti P. Apoptosis in bacterial pathogenesis. J Clin Investig. 1997;100:S63–S65. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]