Abstract
Background
Identifying characteristics associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA shedding may be useful to understand viral compartmentalization, disease pathogenesis, and risks for viral transmission.
Methods
Participants were enrolled August 2020 to February 2021 in ACTIV-2/A5401, a placebo-controlled platform trial evaluating investigational therapies for mild-to-moderate coronavirus disease 2019 (COVID-19), and underwent quantitative SARS-CoV-2 RNA testing on nasopharyngeal and anterior nasal swabs, oral wash/saliva, and plasma at entry (day 0, pretreatment) and days 3, 7, 14, and 28. Concordance of RNA levels (copies/mL) across compartments and predictors of nasopharyngeal RNA levels were assessed at entry (n = 537). Predictors of changes over time were evaluated among placebo recipients (n = 265) with censored linear regression models.
Results
Nasopharyngeal and anterior nasal RNA levels at study entry were highly correlated (r = 0.84); higher levels of both were associated with greater detection of RNA in plasma and oral wash/saliva. Older age, White non-Hispanic race/ethnicity, lower body mass index (BMI), SARS-CoV-2 immunoglobulin G seronegativity, and shorter prior symptom duration were associated with higher nasopharyngeal RNA at entry. In adjusted models, body mass index and race/ethnicity associations were attenuated, but the association with age remained (for every 10 years older, mean nasopharyngeal RNA was 0.27 log10 copies/mL higher; P < .001). Examining longitudinal viral RNA levels among placebo recipients, women had faster declines in nasopharyngeal RNA than men (mean change, −2.0 vs −1.3 log10 copies/mL, entry to day 3; P < .001).
Conclusions
SARS-CoV-2 RNA shedding was concordant across compartments. Age was strongly associated with viral shedding, and men had slower viral clearance than women, which could explain sex differences in acute COVID-19 outcomes.
Keywords: SARS-CoV-2 RNA, COVID-19, nasal swabs, nasopharyngeal swabs, predictors, serostatus, sex differences
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), is primarily transmitted through viral shedding from the upper respiratory tract. SARS-CoV-2 RNA can be found across upper respiratory tract compartments that can be readily sampled, such as the anterior nose, nasopharynx, and oropharynx [1]. High levels of viral RNA sampled from the respiratory tract have been linked to an increased risk of disease severity, detection of culturable virus, and increased risk of viral transmission [2–4]. The diagnosis and monitoring of SARS-CoV-2 infection has thus relied on viral RNA or antigen detection through upper respiratory tract sampling, though the detection of SARS-CoV-2 is not limited to the respiratory tract as viral RNA can also be detected in plasma RNAemia, and levels of plasma RNAemia are associated with risk of disease progression [5, 6].
There remains uncertainty over differences in the levels and duration of viral shedding across different respiratory and blood compartments. Rigorous characterization across compartments has been limited by the logistical challenges of performing intensive, longitudinal sampling from multiple sites at standardized time points. In addition, although the vast majority of studies to date have relied on qPCR cycle threshold (Ct) values as a surrogate for viral load, it is well known that Ct value interpretation and comparison can be unreliable due to variation between runs, instruments, and methodologies [7].
In this study, we analyzed quantitative SARS-CoV-2 RNA results obtained from longitudinal nasopharyngeal (NP), anterior nasal (AN), oral wash/saliva, and plasma samples collected from outpatients with mild-to-moderate COVID-19 in the ACTIV-2/A5401 study, enrolled during the pre-Omicron era. We assessed the relationship between RNA levels in these compartments at study entry and across follow-up in those receiving placebo. We also evaluated the impact of symptom duration and other baseline factors on SARS-CoV-2 RNA kinetics. Identifying characteristics that are associated with levels of upper respiratory tract viral shedding may be useful in understanding viral compartmentalization, disease pathogenesis, and viral transmission [8–10].
METHODS
Study Design and Population
ACTIV-2/A5401 is a phase II/III randomized controlled platform trial designed to evaluate the safety and efficacy of investigational agents for treatment of nonhospitalized adults with mild or moderate COVID-19 (NCT04518410). The population for this report included participants enrolled in the placebo-controlled phase II evaluations of the first 2 agents studied in ACTIV-2, bamlanivimab and amubarvimab + romlusevimab [11, 12]. Participants were randomized to bamlanivimab or placebo between August and November 2020 and to amubarvimab + romlusevimab or placebo between January and February 2021. Enrollment to amubarvimab + romlusevimab or placebo was restricted to persons at higher risk for severe COVID-19 (see the Supplementary Data for protocol definition of higher risk). All participants were enrolled in the United States within 10 days of symptom onset, had documented SARS-CoV-2 infection by viral RNA or antigen testing, and had symptoms present within 24 (protocol version 2.0) or 48 (protocol version 1.0) hours of study entry.
Patient Consent
The protocol was approved by a central institutional review board (IRB), Advarra (Pro00045266), with additional local IRB review and approval as required by participating sites. All participants provided written informed consent.
Virology
Dry NP swabs, dry AN swabs, oral wash/saliva (on a subset), and plasma samples were collected using standardized procedures at study entry/day 0 (before initiating treatment) and days 3, 7, 14, and 28. NP swabs, oral wash/saliva, and plasma were collected by site staff, and AN swabs were self-collected by the participants. The collection methods are described in the Supplementary Data.
All samples were frozen and stored at −80°C (−65°C to −95°C) and shipped on dry ice to the central laboratory (University of Washington) for quantitative SARS-CoV-2 RNA testing using the qPCR Abbott m2000sp/rt platform with a validated internal standard. The collection, storage, processing, and assay methods have previously been validated [13].
The SARS-CoV-2 RNA qPCR assay has a limit of detection (LoD) of 25 copies/mL (1.4 log10 copies/mL), a lower limit of quantification (LLoQ) of 100 copies/mL (2 log10 copies/mL), and an upper limit of quantification (ULoQ) of 10 million copies/mL (7 log10 copies/mL). Assays were rerun with dilution for results above the ULoQ to obtain a quantifiable result that was rescaled by the dilution factor.
Serology
Serum binding antibody assays were performed at day 0 to evaluate immunoglobulin (Ig)G responses to SARS-CoV-2 nucleocapsid (N), spike S1 and S2 domains, and receptor binding domain (RBD) using the Bio-Plex multiplex assay, per the manufacturer's protocols (Bio-Rad Laboratories, Inc, Hercules, CA, USA). IgG seropositivity was defined as detectable IgG to any of N, RBD, S1, and S2 antigens, where “detectable” was defined using manufacturer-specified thresholds or determined in-house following manufacturer recommendations for values above the limit of detection [14].
Statistical Analyses
SARS-CoV-2 RNA values (copies/mL) were transformed to log10 scale for all analyses. Baseline associations were evaluated among all participants. For samples that resulted in unquantifiable RNA levels above the ULoQ (4 of the 537 participants), an imputed value of 8 log10 copies/mL was used, which was close to the mean NP RNA value (7.8 log10 copies/mL) among the 87 samples that had initial results >ULoQ and underwent dilution and retesting. Concordance of SARS-CoV-2 RNA from AN vs NP swabs at day 0 was evaluated using descriptive plots and Spearman's correlation. Concordance of SARS-CoV-2 RNA from plasma and oral wash/saliva with NP swabs was limited to descriptive summaries due to a large proportion of plasma and oral wash/saliva results being below the LLoQ. To assess whether AN swabs, which are potentially easier to obtain in clinical trials, might be an alternative to the gold standard NP swab for quantifying RNA, regression analysis was conducted. For participants with quantifiable NP RNA levels at day 0, the magnitude of the difference between AN and NP RNA was evaluated using linear regression models for censored data; AN RNA values below the LLoQ were censored. Associations between day 0 SARS-CoV-2 RNA and baseline clinical/sociodemographic characteristics were evaluated with linear regression models for censored data (RNA values < LLoQ were left-censored), with and without adjustment for prior symptom duration and serostatus (seropositive vs seronegative) at day 0. Effect modification of associations between clinical/sociodemographic characteristics and serostatus was also considered in the models.
To evaluate predictors of viral RNA decay over time in the absence of therapeutic intervention, the study population was further restricted to participants who received placebo. The mean change from day 0 to day 3 was modeled using linear regression models for censored data, with and without adjustment for duration of symptoms, serostatus, and NP RNA at day 0. This analysis was restricted to those who had NP SARS-CoV-2 RNA above the LLoQ at day 0 as the large majority (80%, 36/45) of participants with RNA values below the LLoQ at day 0 remained below the LLoQ at day 3, so changes could not be reliably quantified. Reference groups for categorical variables in regression models were specified as the largest subgroup, or as “no” for variables categorized as “yes” vs “no.” All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
The analysis of baseline (pretreatment) measures included 537 participants, including 265 who subsequently received placebo and contributed to the analysis of untreated changes in RNA from day 0 to day 3. The median (interquartile range [IQR]) age was 48 (37–57) years, 49% were female sex, and 2% self-identified as Black Hispanic, 7% as Black non-Hispanic, 24% as White Hispanic, 59% as White non-Hispanic, and 9% as other races/ethnicities (Table 1). Two participants had a history of SARS-CoV-2 vaccination, and 50% were IgG seropositive at enrollment. The median (IQR) symptom duration at study entry was 6 (4–8) days, with 39% enrolling within 5 days of symptom onset. The majority (67%) were considered at higher risk for severe COVID-19. See Supplementary Table 1 for prevalence of high-risk comorbidities as defined by the protocol.
Table 1.
Baseline Characteristics of Study Participants
| Total (n = 537) |
|
|---|---|
| Age, median (IQR), y | 48 (37–57) |
| Sex, No. (%) | |
| Female | 264 (49) |
| Male | 273 (51) |
| Gender identity, No. (%) | |
| Cis-gender | 535 (100) |
| Transgender | 1 (0) |
| Not reported | 1 (0) |
| Race, No. (%) | |
| American Indian or Alaska Native | 1 (0) |
| Asian | 18 (3) |
| Black or African American | 49 (9) |
| Multiple | 9 (2) |
| Native Hawaiian or other Pacific Islander | 1 (0) |
| Other | 17 (3) |
| White | 444 (83) |
| Not reported | 1 |
| Race/ethnicity, No. (%) | |
| Black Hispanic/Latino | 9 (2) |
| Black not Hispanic/Latino | 37 (7) |
| Hispanic/Latino other racea | 16 (3) |
| Not Hispanic/Latino other racea | 30 (6) |
| White Hispanic/Latino | 129 (24) |
| White not Hispanic/Latino | 313 (59) |
| Not reported | 3 |
| Country, No. (%) | |
| United States | 537 (100) |
| Body mass index, median (IQR), kg/m2 | 28.7 (25.4–33.5) |
| >35 kg/m2, No. (%) | 107 (21) |
| ≤35 kg/m2, No. (%) | 401 (79) |
| Not reported | 50 |
| Diabetes, No. (%) | |
| Yes | 61 (12) |
| No | 447 (88) |
| Not reported | 29 |
| Hypertension, No. (%) | |
| Yes | 174 (34) |
| No | 334 (66) |
| Not reported | 29 |
| High risk, No. (%) | |
| Yes | 359 (67) |
| No | 178 (33) |
| Symptom duration at study entry, median (IQR), d | 6 (4–8) |
| History of SARS-CoV-2 vaccination, No. (%) | |
| Yes | 2 (0) |
| No | 535 (100) |
| SARS-CoV-2 IgG serostatus, No. (%) | |
| Positive | 259 (50) |
| Negative | 259 (50) |
| Not reported | 19 |
Abbreviations: IgG, immunoglobulin G; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Other race defined as self-reporting a race as not Black or White.
Concordance Across Compartments
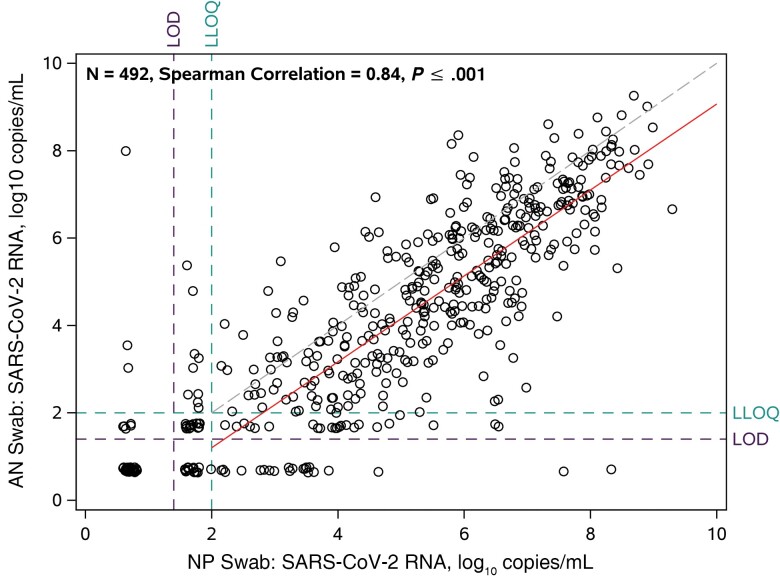
At day 0, the median (IQR) NP RNA and AN RNA were 5.21 (3.24–6.69) and 4.25 (2.11–6.09) log10 copies/mL, respectively, with 17% and 23% below the LLoQ, including 9% and 15% undetectable (Figure 1). NP and AN RNA were highly correlated (Spearman r = 0.84; P < .001). The mean AN RNA was about 1 log10 copies/mL lower than NP RNA across the range of quantifiable NP RNA values estimated from regression analysis (Figure 2). Most oral wash/saliva (75%) and plasma (99%) samples had RNA <LLoQ (Figure 1). NP RNA increased with higher ordered categories of oral wash/saliva and plasma RNA. For oral wash/saliva, the median (IQR) NP RNA was 4.27 (2.74–5.87) log10 copies/mL for undetectable oral wash/saliva RNA, 5.80 (4.88–6.74) log10 copies/mL for detectable <LLoQ, and 6.90 (5.94–7.79) log10 copies/mL for ≥LLoQ (P < .001); for plasma, median (IQR) NP RNA values were 5.00 (2.91–6.48) log10 copies/mL for undetectable plasma RNA and 6.15 (4.84–7.18) log10 copies/mL for detectable RNA (either <LLoQ or ≥LLoQ; P < .001). Similar associations were observed comparing AN RNA with ordered categories of oral wash/saliva and plasma (Supplementary Table 2). Among those with NP RNA <LLoQ at day 0, all plasma RNA and all but 1 oral wash/saliva RNA were <LLoQ.
Figure 1.
Distribution of RNA by compartment at day 0. A, Levels of RNA (log10 copies/mL), with horizontal line = median, box = interquartile range, whiskers = minimum/maximum. B, Proportion with quantitative RNA, detectable but not quantifiable RNA, and undetectable. For (A), results below the LoD were imputed as 0.7 log10 copies/mL (half the distance from 0 to the LoD), results above the LoD but below the LLoQ were imputed as 1.7 log10 copies/mL (half the distance between the LoD and LLoQ), and values above the ULoQ that were not able to be quantified with dilution were imputed as 8 log10 copies/mL. Abbreviations: LLoQ, lower limit of quantification; LoD, limit of detection; NP, nasopharyngeal; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ULoQ, upper limit of quantification.
Figure 2.
Scatterplot of nasopharyngeal RNA vs anterior nasal RNA at day 0. Dashed line represents perfect concordance, where NP RNA = AN RNA; solid line represents association from linear regression model among participants with NP RNA >LLoQ (the model was fit using methods for censored data with AN RNA values <LLoQ considered left-censored at the LLoQ). For graphical presentation, results below the LoD were imputed as 0.7 log10 copies/mL (half the distance from 0 to the LoD), results above the LoD but below the LLoQ were imputed as 1.7 log10 copies/mL (half the distance between the LoD and LLoQ), and values above the ULoQ that were not able to be quantified with dilution were imputed as 8 log10 copies/mL. Participants had their values jittered for presentation when AN RNA was equal to NP RNA. Abbreviations: AN, anterior nasal; LLoQ, lower limit of quantification; LoD, limit of detection; NP, nasopharyngeal; ULoQ, upper limit of quantification.
Baseline Associations
Across all compartments, the median day 0 RNA was lower for those who were seropositive (vs seronegative) and for those with >5 days of prior symptoms (vs ≤5 days) (Supplementary Tables 3 and 4, Supplementary Figure 1). Univariable analysis also identified that older age and lower BMI were associated with higher day 0 NP RNA, and Black non-Hispanic race/ethnicity (vs White non-Hispanic) but not other race/ethnicity groups was associated with lower day 0 NP RNA (Supplementary Table 5). Examining these factors together in multivariable models with and without adjustment for prior symptom duration and serostatus (Table 2), the associations between age and day 0 NP RNA were stronger than in the univariable models (for every 10 years older age, the adjusted mean NP RNA was 0.27 log10 copies/mL higher; P < .001). Black non-Hispanic race/ethnicity remained associated with lower NP RNA levels, though the effects were attenuated (the mean NP RNA was 0.85 log10 copies/mL lower for Black non-Hispanic than White non-Hispanic participants; P = .036). Associations between BMI and NP RNA were no longer significant.
Table 2.
Multivariable Models Evaluating Associations of Nasopharyngeal RNA at Day 0 With Age, BMI, and Race/Ethnicity
| Model | Variable | Estimate | 95% CI | P Value |
|---|---|---|---|---|
| Model 1 | Age (per 10 y) | 0.329 | 0.18, 0.48 | <.001 |
| BMI (per 5 kg/m2) | −0.128 | −0.29, 0.03 | .12 | |
| Race/ethnicitya | … | … | ||
| (Black not Hispanic/Latino) | −1.662 | −2.58, −0.75 | <.001 | |
| (Hispanic/Latino, any race) | −0.254 | −0.74, 0.23 | .30 | |
| (Multiracial/others) | −0.318 | −1.27, 0.63 | .51 | |
| Model 2: adjusted also for symptom duration and serostatus | Age (per 10 y) | 0.274 | 0.15, 0.40 | <.001 |
| BMI (per 5 kg/m2) | −0.125 | −0.26, 0.01 | .07 | |
| Race/ethnicitya | … | … | ||
| (Black not Hispanic/Latino) | −0.848 | −1.64, −0.06 | .036 | |
| (Hispanic/Latino, any race) | −0.353 | −0.77, 0.06 | .09 | |
| (Multiracial/others) | −0.290 | −1.08, 0.50 | .47 |
Estimates, 95% CIs, and P values were obtained from linear regression models for censored data.
Abbreviation: BMI, body mass index.
White not Hispanic/Latino is the reference group for race/ethnicity comparisons.
Longitudinal Changes in RNA
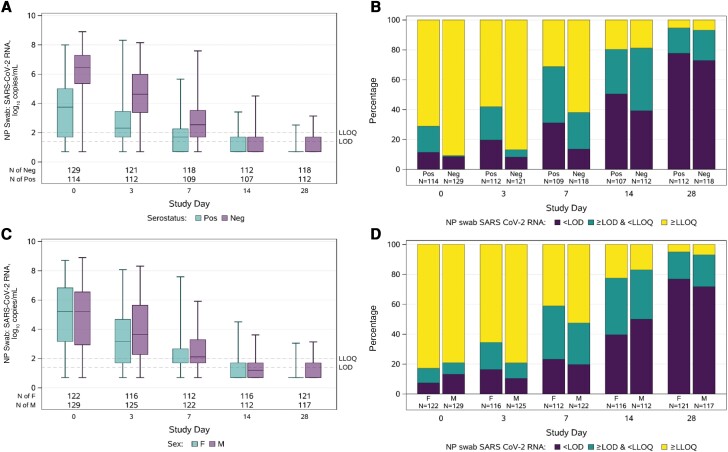
Among 265 participants who received placebo, RNA decreased over time in all compartments (Supplementary Figure 2 and 3). At both day 3 and day 7, NP RNA levels were higher for participants who were seronegative vs seropositive at entry, but by day 14 >80% of participants in both serostatus groups had NP RNA <LLoQ (Figure 3AandB). Similar differences by serostatus were observed for AN swabs, oral wash/saliva, and plasma (Supplementary Figures 4 and 5). However, among participants with NP RNA >LLoQ at day 0, the change in NP RNA from day 0 to day 3 did not differ by serostatus (mean change, −1.67 log10 copies/mL for seropositive and −1.65 log10 copies/mL for seronegative; difference, −0.02; 95% CI, −0.45 to 0.42; P = .94) or by symptom duration (mean change, −1.70 for >5 days and −1.57 for ≤5 days; difference, −0.14; 95% CI, −0.28 to 0.56; P = .52).
Figure 3.
Distributions of nasopharyngeal RNA over time by serostatus (A and B) and sex (C and D) among the 265 participants who received placebo. Levels of RNA (log10 copies/mL), with horizontal line = median, box = interquartile range, whiskers = minimum/maximum (A and C). Proportion with quantitative RNA, detectable but not quantifiable RNA, and undetectable (B and D). Results below the LoD were imputed as 0.7 log10 copies/mL (half the distance from 0 to the LoD), results above the LoD but below the LLoQ were imputed as 1.7 log10 copies/mL (half the distance between the LoD and LLoQ), and values above the ULoQ that were not able to be quantified with dilution were imputed as 8 log10 copies/mL. Abbreviations: AN, anterior nasal; LLoQ, lower limit of quantification; LoD, limit of detection; neg, seronegative; NP, nasopharyngeal; pos, seropositive; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ULoQ, upper limit of quantification.
Women had faster declines in NP RNA from day 0 to day 3 compared with men (mean change, −2.00 log10 copies/mL for women and −1.32 log10 copies/mL for men; difference, −0.68; 95% CI, −1.08 to −0.28; P < .001) (Figure 3C and D). This result persisted with adjustment for day 0 RNA level, symptom duration at day 0, and serostatus at day 0, none of which differed by sex. This association did not depend on serostatus (Pinteraction = .55). Change in NP RNA levels was not associated with other participant characteristics, including age, race/ethnicity, risk for severe COVID-19, BMI, diabetes status, obesity, or hypertension (Supplementary Table 6).
DISCUSSION
This study analyzed SARS-CoV-2 RNA, measured in multiple types of upper respiratory and plasma samples that were collected as part of a large randomized trial to evaluate concordance of viral levels across anatomic compartments and predictors of measured viral levels. Concerning concordance of viral levels across compartments, SARS-CoV-2 RNA levels from NP swabs and AN swabs were highly correlated (r = 0.84), consistent with other reports [15, 16]. Although NP swabs are viewed as the “gold standard,” many studies have demonstrated good diagnostic performance of AN swabs (or combined oropharynx/AN) compared with NP swabs for SARS-CoV-2 RT-PCR testing [17–19]. Thus, self-collected AN swabs can be considered to assess early SARS-CoV-2 infection status and response to interventions in research and clinical settings as AN swabs are easier to collect and are better tolerated than NP swabs and show concordant results with NP swabs. However, as RNA levels from AN swabs were consistently 10-fold lower than RNA levels from NP swabs, their utility for assessing response to antiviral medications is likely most valuable in early SARS-CoV-2 infection, when nasal RNA levels are highest [11]. This study also found that when NP swabs demonstrated high levels of SARS-CoV-2 RNA, oral wash/saliva and plasma samples were more likely to show detectable viral RNA. While only a subset of oral wash/saliva and plasma samples had RNA levels above the lower limit of quantification, these results highlight that while SARS-CoV-2 RNA levels are higher in certain anatomic compartments, higher overall viral burden is reflected across multiple anatomic compartments.
Concerning predictors of viral RNA levels, we found in this population enrolled in the first year of the pandemic (ie, prior to widespread exposure to SARS-CoV-2 or vaccination), shorter prior symptom duration and seronegativity at the time of RNA measurement and older age were all associated with higher NP RNA levels. The association of prior symptom duration and serostatus with NP RNA levels has been observed in other large COVID-19 therapeutics trials conducted early in the pandemic [20–22], but analyses of associations with age have shown conflicting results [21, 23–26]. The association of older age with higher NP RNA even among seropositive participants may reflect unmeasured immune deficiencies with aging such as decreased mucosal interferon responses [27–30] or less robust or functional humoral responses not captured by examining serostatus as a blunt qualitative measure.
Of interest, we found that Black non-Hispanic participants had lower NP RNA at day 0 compared with White non-Hispanic participants, though there was some attenuation of this difference when adjusting for prior symptom duration and serostatus. Black non-Hispanic participants (compared with White non-Hispanic participants) were more likely to be seropositive (71% vs 46%) and to have longer symptom duration at study entry (median, 8 days vs 6 days), suggesting that these individuals were more likely to enter the study later in the course of their infections. Our findings may reflect differences in access to testing and trial research sites for minoritized groups rather than true differences in viral levels by race/ethnicity, as we do not suspect that SARS-CoV-2 RNA kinetics differ biologically by race/ethnic identity [31, 32].
We also found that women had faster declines in NP RNA between day 0 and day 3 compared with men, which persisted in analyses adjusting for serostatus, prior symptom duration, and NP RNA level at day 0. This is a novel finding in the outpatient setting, but is consistent with reports of hospitalized patients with COVID-19 that found male sex to be associated with prolonged duration of SARS-CoV-2 RNA shedding [33–36] as well as a population surveillance study in Italy, which reported higher proportions of women achieving early viral clearance [37]. These data are consistent with a large body of literature that suggests that females are able to clear pathogens faster than males, potentially due to greater innate and adaptive immune responses to infections, including SARS-CoV-2 [38–42]. Whether these differences in SARS-CoV-2 clearance rates contribute to sex-based differences in COVID-19 outcomes that have been observed, including increased risk for COVID-19 hospitalization and mortality among men, is not known [43, 44].
This study has limitations, including the small number of participants in some racial/ethnic subgroups, which limited our ability to make inferences for these populations. Another important limitation is that this study enrolled earlier in the pandemic, so it does not allow us to evaluate associations among participants infected with later variants, like Omicron, or persons who were vaccinated. Additionally, serology data were limited to day 0, preventing us from making inferences about changes in viral load and prospective seroconversion. Although duration of symptoms is often used as a surrogate for duration of infection, we cannot be certain when infection occurred, which could also influence serostatus. In addition, there was low sensitivity of oral wash–collected saliva for SARS-CoV-2 RNA detection, which was likely due to the method of specimen collection, as other approaches to saliva sampling have had higher yields [1, 45, 46].
In summary, in this analysis of viral levels in plasma and multiple upper airway compartments early in symptomatic SARS-CoV-2 infection, we observed concordant viral loads across compartments and that older age, seronegativity, and shorter prior symptom duration at the time of measurement were all associated with higher viral RNA levels from NP and AN swabs. Faster declines in RNA levels were seen for women compared with men, but not by serostatus or other clinical or demographic factors. These findings should be explored further to understand the potential mediators of sex-based differences in SARS-CoV-2 clearance and their relationship to important clinical outcomes.
Supplementary Material
Acknowledgments
We thank the study participants, site staff, site investigators, and the entire ACTIV-2/A5401 study team; the AIDS Clinical Trials Group, including Lara Hosey, Jhoanna Roa, and Nilam Patel; the UW Virology Specialty Laboratory staff, including Emily Degli-Angeli, Erin Goecker, Glenda Daza, Socorro Harb, and Joan Dragavon; the ACTG Laboratory Center, including Grace Aldrovandi and William Murtaugh; Frontier Science, including Marlene Cooper, Howard Gutzman, Kevin Knowles, and Rachel Bowman; the Harvard Center for Biostatistics in AIDS Research (CBAR) and ACTG Statistical and Data Analysis Center (SDAC); the ACTIV-2/A5401 Community Advisory Board; the National Institute of Allergy and Infectious Diseases (NIAID)/Division of AIDS (DAIDS); Bill Erhardt; the Foundation for the National Institutes of Health and the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership, including Stacey Adams; and the PPD clinical research business of Thermo Fisher Scientific.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Carlee Moser, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Jonathan Z Li, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Joseph J Eron, Department of Medicine, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, North Carolina, USA.
Evgenia Aga, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Eric S Daar, Lundquist Institute at Harbor-UCLA Medical Center, Torrance, California, USA.
David A Wohl, Department of Medicine, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, North Carolina, USA.
Robert W Coombs, Department of Laboratory Medicine and Pathology, Department of Medicine, University of Washington, Seattle, Washington, USA.
Arzhang Cyrus Javan, National Institutes of Health, Rockville, Maryland, USA.
Rachel A Bender Ignacio, Department of Medicine, University of Washington, Seattle, Washington, USA; Vaccine and Infectious Disease Division, Fred Hutch Cancer Center, Seattle, Washington, USA.
Prasanna Jagannathan, Department of Medicine, Stanford University, Palo Alto, California, USA.
Justin Ritz, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Scott F Sieg, Department of Medicine, Case Western University, Cleveland, Ohio, USA.
Urvi M Parikh, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Michael D Hughes, Department of Biostatistics and Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Judith S Currier, Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles, Los Angeles, California, USA.
Davey M Smith, Department of Medicine, University of California, San Diego, La Jolla, California, USA.
Kara W Chew, Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles, Los Angeles, California, USA.
ACTIV-2/A5401 Study Team:
Lara Hosey, Jhoanna Roa, Nilam Patel, Emily Degli-Angeli, Erin Goecker, Glenda Daza, Socorro Harb, Joan Dragavon, Grace Aldrovandi, William Murtaugh, Marlene Cooper, Howard Gutzman, Kevin Knowles, Rachel Bowman, Bill Erhardt, and Stacey Adams
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Mohammadi A, Esmaeilzadeh E, Li Y, Bosch RJ, Li JZ. SARS-CoV-2 detection in different respiratory sites: a systematic review and meta-analysis. eBioMedicine 2020; 59:102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020; 11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020; 25:2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marks M, Millat-Martinez P, Ouchi D, et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis 2021; 21:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobs JL, Bain W, Naqvi A, et al. Severe acute respiratory syndrome coronavirus 2 viremia is associated with coronavirus disease 2019 severity and predicts clinical outcomes. Clin Infect Dis 2022; 74:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Schneider AM, Mehta A, et al. SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. J Clin Invest 2021; 131:148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rhoads D, Peaper DR, She RC, et al. College of American Pathologists (CAP) Microbiology Committee perspective: caution must be used in interpreting the cycle threshold (Ct) value. Clin Infect Dis 2021; 72:e685–6. [DOI] [PubMed] [Google Scholar]
- 8. Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med 2021; 385:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magleby R, Westblade LF, Trzebucki A, et al. Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis 2021; 73:e4197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dougan M, Azizad M, Mocherla B, et al. A randomized, placebo-controlled clinical trial of bamlanivimab and etesevimab together in high-risk ambulatory patients with COVID-19 and validation of the prognostic value of persistently high viral load. Clin Infect Dis 2022; 75:e440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chew KW, Moser C, Daar ES, et al. Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19. Nat Commun 2022; 13:4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evering TH, Giganti M, Chew KW, et al. LB2. Safety and efficacy of combination SARS-CoV-2 monoclonal neutralizing antibodies (mAb) BRII-196 and BRII-198 in non-hospitalized COVID-19 patients. Open Forum Infect Dis 2021; 8:S807–8. [Google Scholar]
- 13. Berg MG, Zhen W, Lucic D, et al. Development of the RealTime SARS-CoV-2 quantitative laboratory developed test and correlation with viral culture as a measure of infectivity. J Clin Virol 2021; 143:104945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bio-Rad Laboratories Inc . Bio-Plex Pro Human IgG SARS-CoV-2 Serology Assays product data sheet. Accessed 24 October 2022.
- 15. McCulloch DJ, Kim AE, Wilcox NC, et al. Comparison of unsupervised home self-collected midnasal swabs with clinician-collected nasopharyngeal swabs for detection of SARS-CoV-2 infection. JAMA Netw Open 2020; 3:e2016382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alemany A, Millat-Martinez P, Ouchi D, et al. Self-collected mid-nasal swabs and saliva specimens, compared with nasopharyngeal swabs, for SARS-CoV-2 detection in mild COVID-19 patients. J Infect Elsevier 2021; 83:709–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsang NNY, So HC, Ng KY, Cowling BJ, Leung GM, Ip DKM. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis. Lancet Infect Dis 2021; 21:1233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsujimoto Y, Terada J, Kimura M, et al. Diagnostic accuracy of nasopharyngeal swab, nasal swab and saliva swab samples for the detection of SARS-CoV-2 using RT-PCR. Infect Dis 2021; 53:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. LeBlanc JJ, Pettipas J, Di Quinzio M, Hatchette TF, Patriquin G. Reliable detection of SARS-CoV-2 with patient-collected swabs and saline gargles: a three-headed comparison on multiple molecular platforms. J Virol Methods 2021; 295:114184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med 2021; 385:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mollan KR, Eron JJ, Krajewski TJ, et al. Infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus in symptomatic coronavirus disease 2019 (COVID-19) outpatients: host, disease, and viral correlates. Clin Infect Dis 2022; 75:e1028–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knudtzen FC, Jensen TG, Lindvig SO, et al. SARS-CoV-2 viral load as a predictor for disease severity in outpatients and hospitalised patients with COVID-19: a prospective cohort study. PLoS One 2021; 16:e0258421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liao T, Yin Z, Xu J, et al. The correlation between clinical features and viral RNA shedding in outpatients with COVID-19. Open Forum Infect Dis 2020; 7:ofaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leeds JS, Raviprakash V, Jacques T, Scanlon N, Cundall J, Leeds CM. Risk factors for detection of SARS-CoV-2 in healthcare workers during April 2020 in a UK hospital testing programme. EClinicalMedicine 2020; 26:100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molony RD, Nguyen JT, Kong Y, Montgomery RR, Shaw AC, Iwasaki A. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci Signal 2017; 10:eaan2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol 2002; 56:518–21. [DOI] [PubMed] [Google Scholar]
- 29. Pérez-Cabezas B, Naranjo-Gómez M, Fernández MA, Grífols JR, Pujol-Borrell R, Borràs FE. Reduced numbers of plasmacytoid dendritic cells in aged blood donors. Exp Gerontol 2007; 42:1033–8. [DOI] [PubMed] [Google Scholar]
- 30. Feng E, Balint E, Poznanski SM, Ashkar AA, Loeb M. Aging and interferons: impacts on inflammation and viral disease outcomes. Cells 2021; 10:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khazanchi R, Evans CT, Racism MJ, Race N. Drives inequity across the COVID-19 continuum. JAMA Netw Open 2020; 3:e2019933. [DOI] [PubMed] [Google Scholar]
- 32. Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis 2020; 222:890–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi D, Wu W, Wang Q, et al. Clinical characteristics and factors associated with long-term viral excretion in patients with severe acute respiratory syndrome coronavirus 2 infection: a single-center 28-day study. J Infect Dis 2020; 222:910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dezza F C, Oliva A, Cancelli F, et al. Determinants of prolonged viral RNA shedding in hospitalized patients with SARS-CoV-2 infection. Diagn Microbiol Infect Dis 2021; 100:115347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19). Clin Infect Dis 2020; 71:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 2020; 369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fortunato F, Martinelli D, Lo Caputo S, et al. Sex and gender differences in COVID-19: an Italian local register-based study. BMJ Open 2021; 11:e051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020; 588:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays 2012; 34:1050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghosh S, Klein RS. Sex drives dimorphic immune responses to viral infections. J Immunol 2017; 198:1782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Lunzen J, Altfeld M. Sex differences in infectious diseases-common but neglected. J Infect Dis 2014; 209:S79–80. [DOI] [PubMed] [Google Scholar]
- 42. Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008; 8:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scully EP, Schumock G, Fu M, et al. Sex and gender differences in testing, hospital admission, clinical presentation, and drivers of severe outcomes from COVID-19. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Miguel-Diez J, Lopez-de-Andres A, Jimenez-Garcia R, et al. Sex differences in COVID-19 hospitalization and hospital mortality among patients with COPD in Spain: a retrospective cohort study. Viruses 2022; 14:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beyene GT, Alemu F, Kebede ES, et al. Saliva is superior over nasopharyngeal swab for detecting SARS-CoV2 in COVID-19 patients. Sci Rep 2021; 11:22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med 2021; 181:353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.