Abstract
Background
Persons who inject drugs are at increased risk for acquiring hepatitis C virus (HCV). Medications for opioid use disorder (MOUD) are associated with reduced injection drug use (IDU) frequency among persons with opioid use disorder (OUD). However, whether HCV treatment uptake or changes in IDU frequency differ by HIV serostatus among persons receiving MOUD is incompletely understood.
Methods
A secondary analysis was performed of data collected from 2 prospective cohort studies of participants with (PWH) or without HIV with Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition–diagnosed OUD who were initiated on methadone, buprenorphine, or naltrexone.
Results
Of 129 participants, 78 (60.5%) were HCV antibody positive. PWH underwent increased HCV viral load testing (76.7% vs 43.3%; P = .028), but HCV treatment rates did not differ (17.6% vs 10.0%; P = .45) by HIV status. Participants without HIV reported a greater reduction in mean opioid IDU at 90 days (10.7 vs 2.0 fewer days out of 30; P < .001), but there were no group differences at 90 days. Stimulant use did not differ between groups. Urine opioid positivity declined from baseline to 90 days among the entire cohort (61.4% to 38.0%; P < .001) but did not differ by HIV serostatus.
Conclusions
PWH who received MOUD underwent higher rates of follow-up HCV testing, but HCV treatment rates did not significantly differ by HIV serostatus. Participants without HIV on MOUD reported a greater reduction in opioid IDU. Improved integration of concomitant OUD with HCV and HIV screening, linkage to care, and treatment are needed for persons without HIV.
Keywords: opioid use disorder, HIV, hepatitis c virus, injection drug use, medications for opioid use disorder
Hepatitis C virus (HCV) is the most common bloodborne pathogen in the United States, affecting 4.1 million people, and is associated with cirrhosis, liver failure, and hepatocellular carcinoma [1]. Persons who inject drugs (PWID) are at increased risk for acquisition and transmission of HCV [2] as well as HIV [3]. Of note, the prevalence of injection drug use (IDU) has increased, particularly among younger individuals in US suburban settings [4], as well as psycho-stimulant use, in combination with opioids, leading to twin epidemics [5].
Direct-acting antivirals (DAAs) are highly effective evidence-based HCV treatments that lead to cure of HCV [6]. Yet, in spite of their availability, uptake and initiation are low among PWID [7] and those with HIV/HCV coinfection [8–11]; the latter are at risk for more rapid progression to liver cirrhosis [12]. Treatment of acute HCV among PWID has the potential to serve as a preventative measure for HCV transmission, and increased uptake has been associated with reduction in prevalence of chronic HCV [13].
Medications for opioid use disorder (MOUD; eg, buprenorphine, methadone, and extended-release naltrexone) are an evidence-based strategy to address opioid addiction and are associated with reduced opioid IDU [14–20]. However, MOUD retention and opioid abstinence may be affected by co-stimulant use [21]. For PWID, MOUD is also associated with increased self-reported retention and uptake of DAA treatment; however, the HCV cascade of care highlights important gaps [22], and successful HCV treatment strategies involve a multidisciplinary effort in care coordination and case management, particularly for those with psychiatric illness or substance use disorder [23, 24].
To our knowledge, no previous studies have examined the HCV care cascade among HCV/HIV-coinfected persons in the context of receiving MOUD. Thus, our primary aim was to assess the HCV treatment cascade among PWID with opioid use disorder (OUD) who were initiated on MOUD, specifically comparing persons with HIV (PWH) with those without HIV infection. Secondarily, we aimed to assess whether receipt of MOUD would modulate opioid IDU and stimulant use frequency, which are 2 risk factors that are associated with increased risk of HCV transmission.
METHODS
Data Collection and Sample Size
Our secondary analysis stems from data that were obtained from 2 National Institutes of Health–sponsored prospective observational cohort studies, Project MAT BIO (The Impact of HIV Infection on Immunologic, Transcriptomic, and Metabolomic Signatures of Medication-Assisted Therapy for Opioid Addiction) [25] and Persistence (Evaluating the Role of Medication Treatments for OUD in HIV-1 Persistence for PWH and OUD) [26].
As previously published, the aim of MAT BIO was to assess for differences in immunologic markers between PWH and those at risk for HIV with OUD who were initiated on MOUD [25]. Participants with Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)–diagnosed OUD with and without HIV were screened and enrolled on the day they were to initiate treatment with methadone or buprenorphine in the community. Participants with a previous diagnosis of HIV were eligible if they were virally suppressed (HIV viral load <200 copies/mL) on antiretroviral treatment (ART). After informed consent was obtained, participants completed baseline interview assessments and blood draws for the parent grant immunobiological assessments before initiating MOUD; follow-up interviews and blood draws were also completed on day 7, day 14, and months 1, 3, and 6. Participants were not excluded from follow-up interviews or blood draws if they stopped MOUD treatment. A total of 112 participants were enrolled in the MAT BIO study (n = 31 with HIV and n = 81 without HIV) between April 2018 and March 2020.
The aim of Project Persistence was to assess the role of MOUD in HIV-1 persistence and reactivation, as previously described [26]. PWH with DSM-5 moderate to severe OUD who were virally suppressed through ART were screened and enrolled on the day they were to be initiated on methadone, buprenorphine, or extended-release naltrexone in the community [26]. After informed consent was obtained, participants completed baseline interview assessments and blood draws for the parent grant immunobiological assessments before starting MOUD, as well as interviews and blood draws at the month 1 and 3 study visits. Seventeen participants with HIV were enrolled in Persistence [26]. No intervention or study medications were delivered during either longitudinal cohort study. Participants were enrolled from December 2018 through June 2022. More detailed methods regarding recruitment, enrollment, and ethical oversight in each study have been previously published [25, 26].
Interview questions were similar for both the MAT BIO and Persistence research projects, including baseline (before MOUD initiation) demographics, medical history, current medications, HIV medication type (if PWH), and mental health and substance use disorder diagnoses via the Mini International Neuropsychiatric Interview (MINI) [27]. Retention on MOUD was collected via self-report, and medical record extraction was used for persons lost to project follow-up. Self-reported days of opioid use per month via the timeline follow back (TLFB) method [28] was examined at each study visit, and self-reported stimulant use, including cocaine and methamphetamine, was assessed via the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST, version 3.0) [29] at baseline, month 3 (MAT BIO and Persistence), and month 6 (MAT BIO). Eleven-panel urine toxicology screens (Abbott), including methadone, opiates, oxycodone, fentanyl, buprenorphine, amphetamine, cocaine, methamphetamine, and alcohol, were obtained at baseline and follow-up study visits for both parent projects [25, 26].
Upon enrollment in the parent studies, participants underwent rapid HCV and HIV testing. Participants with a newly reactive rapid HIV (OraQuick ADVANCE Rapid HIV-1/2 Antibody Test) or HCV (OraQUICK HCV test) test were referred by research study staff for confirmatory blood testing (HIV VL and HCV antibody with reflex RNA, Quest Diagnostics). Participants (MAT BIO or Persistence) who endorsed a previous positive HIV or HCV diagnosis were directly referred to Quest Diagnostics to undergo a fourth-generation HIV antigen-antibody test and HIV VL or an HCV antibody test with reflex confirmatory testing by research staff. Only participants who had confirmed HIV VL suppression were eligible for parent study participation. HIV VL was obtained at baseline and at months 3 (MAT BIO and Persistence) and 6 (MAT BIO). HCV VL was repeated at months 3 (Persistence) and 6 (MAT BIO). A rapid HIV and HCV test was repeated at month 6 (MAT BIO) study visits for those who tested negative at baseline [25, 26]. Information regarding HCV DAA treatment initiation and completion was collected via self-report during patient interviews and entered into a secure database (REDCap, Vanderbilt University). Data were retrospectively extracted for our analysis.
Analysis Plan
Primary Outcome
Our primary outcome was the proportion of participants who completed the HCV care cascade. Baseline and follow-up HCV testing (HCV ab and VL) and treatment data were collected from both longitudinal parent cohort studies and evaluated in this retrospective analysis. From these data, HCV care cascades were created for the entire cohort as well as by HIV serostatus. Participants who endorsed previous HCV treatment were excluded from the HCV care cascades.
Secondary Outcomes
Our secondary outcomes were change in opioid IDU and stimulant use among the whole cohort and by HIV serostatus from baseline to 90 days after initiation of MOUD. Self-reported injection opioid use was assessed from TLFB data collected at baseline and at 90-day (month 3) follow-up after initiating MOUD and defined as mean proportion of opioid IDU over 30 days. Opioid use was also assessed via urine toxicological data obtained at baseline and 90 days. Stimulant use (any route) was assessed for the cohort and by HIV serostatus at baseline and 90 days, defined as proportion of participants who utilized stimulants through either self-report (ASSIST self-reported use—yes/no) or biological (urine toxicology detection—yes/no) data. Participants who did not complete either TLFB or ASSIST for the 90-day period were excluded from the analysis.
Statistical Analysis
To assess whether stepwise progression through the HCV care cascade was dependent on HIV status (primary outcome), a series of chi-square tests were used. Generalized linear regression models were utilized to assess for changes in self-reported opioid IDU and stimulant use from baseline to 90 days after initiation of MOUD (secondary outcomes). Mean (number of days injecting opioids over 30 days) self-reported opioid IDU was modeled with a Poisson distribution and log link function. Stimulant use was modeled with a binary distribution with a log link function. All models included a compound symmetry covariance structure to account for intraparticipant correlations. In addition to the primary variables of HIV status, time, and a time-by-HIV status interaction, some models also included the following covariates: gender, age, race, educational attainment, homeless status, and form of MOUD. Models of dichotomous urine toxicology results for stimulants and opioids (heroin, fentanyl, nonprescribed pharmaceutical opioids, fentanyl) were run using predictors for observation, HIV status, and an observation-by-HIV status interaction. Data were analyzed using SAS software, version 9.4 (Cary, NC, USA).
RESULTS
Baseline Characteristics
Participants were predominately male, White, and non-Hispanic (Table 1). All participants with HIV were on ART and virally suppressed at baseline and 3-month follow-up. Approximately one-third (n = 40, 31%) of participants were homeless in the 30 days before study enrollment (Table 1). Buprenorphine (n = 68, 52.7%) and methadone (n = 55, 42.6%) were the 2 most frequent forms of MOUD, followed by extended-release naltrexone (n = 6, 4.7%). HCV antibody serostatus was high (n = 78, 60.5%), particularly among PWH (n = 40, 83.3%). A significant proportion of participants had utilized opioids in the 3 months before study enrollment (n = 112, 86.8%), predominantly with intravenous heroin (n = 49, 38.3%). Eighty-two (63.6%) participants had a urine toxicology screen that was positive for heroin, while 27 (20.9%) participants were positive for fentanyl. Sixty-one (47.3%) participants endorsed cocaine use in the past 3 months, while 5 (3.9%) participants, all persons without HIV, endorsed methamphetamine use. Fifty-six (46.7%) participants had a DSM-5 diagnosis of cocaine use disorder via the MINI. There was a statistically significant difference between gender, age, race, educational attainment, homeless status, MOUD form, HCV antibody serostatus, and a diagnosis of bipolar disorder between PWH and participants without HIV (Table 1). Participants without HIV were more likely to carry a diagnosis of bipolar disorder. Retention in the parent cohort study project interviews was 66.7% at month 3 follow-up, and retention on MOUD for those who had an interview at each time point was 91%, 80%, and 78% at months 1, 3, and 6, respectively, for MAT BIO [25], as well as 89% and 72% at months 1 and 3 for Persistence [26].
Table 1.
Baseline Characteristics of the Entire Cohort and Subdivided by HIV Serostatus
| Variable | Total (n = 129) |
% | HIV Positive (n = 48) |
% | HIV Negative (n = 81) |
% | P Value |
|---|---|---|---|---|---|---|---|
| Gender | .003 | ||||||
| Male | 93 | 72.1 | 40 | 83.3 | 53 | 65.4 | |
| Female | 34 | 26.4 | 6 | 12.5 | 28 | 34.6 | |
| Transgender (male to female) | 2 | 1.6 | 2 | 4.2 | 0 | 0 | |
| Age, median (SD), y | 41 | 31 (53) | 53 | 47 (59) | 35 | 29 (45) | <.001 |
| Race | <.001 | ||||||
| White | 76 | 58.9 | 60 | 74.1 | 16 | 33.3 | |
| Black | 31 | 24.0 | 12 | 14.8 | 19 | 39.6 | |
| >1 race or other race | 8 | 6.2 | 2 | 2.5 | 6 | 12.5 | |
| Hispanic | 39 | 30.2 | 15 | 31.3 | 24 | 29.6 | .85 |
| Homeless—past 30 d | 40 | 31.0 | 22 | 45.8 | 18 | 22.2 | .005 |
| Educational attainment (HS equivalent or greater) | 102 | 79.1 | 31 | 64.6 | 71 | 87.7 | .004 |
| Controlled environment | .066 | ||||||
| Jail or prison | 19 | 14.7 | 10 | 20.8 | 9 | 11.1 | |
| Alcohol or drug treatment | 9 | 7.0 | 1 | 2.1 | 8 | 9.9 | |
| MOUD prescribed | <.001 | ||||||
| Buprenorphine | 68 | 52.7 | 33 | 68.8 | 35 | 43.2 | |
| Methadone | 55 | 42.6 | 12 | 25.0 | 43 | 53.1 | |
| Extended-release naltrexone | 6 | 4.7 | 3 | 6.3 | 3 | 3.7 | |
| Prescribed HIV ART | 48 | – | 48 | 100 | – | – | – |
| MOUD retention through 90 d | 86 | 66.7 | 32 | 66.67 | 54 | 66.67 | 1.00 |
| HIV VL <200 copies/mL | 48 | – | 48 | 100 | – | – | – |
| CD4 count, mean (SD) | 555.5 | 373.5 (949.5) | 555.5 | 373.5 (949.5) | – | – | – |
| Hepatitis C ab positive | 78 | 60.5 | 40 | 83.3 | 38 | 46.9 | <.001 |
| Urine toxicology screen positive at baseline | |||||||
| All opioids | 83 | 64.3 | 24 | 50.0 | 59 | 72.8 | .38 |
| Heroin | 82 | 63.6 | 24 | 50.0 | 58 | 71.6 | .01 |
| Fentanyl | 27 | 20.9 | 14 | 29.2 | 13 | 16.0 | .08 |
| Fentanyl and heroin | 21 | 16.3 | 9 | 18.8 | 12 | 14.8 | .56 |
| Stimulants | 51 | 39.5 | 17 | 35.4 | 34 | 42.0 | .46 |
| ASSIST any opioid use past 3 mo | 112 | 86.8 | 36 | 75.0 | 76 | 93.8 | .002 |
| ASSIST any stimulant use past 3 mo | 62 | 48.1 | 19 | 39.6 | 43 | 53.1 | .14 |
| Cocaine | 61 | 47.3 | 19 | 39.6 | 42 | 51.9 | .18 |
| Methamphetamine | 5 | 3.9 | 0 | 0 | 5 | 6.2 | .09 |
| TLFB reported use (not as prescribed) at baseline | |||||||
| Heroin use, intranasal | 46 | 36.0 | 16 | 33.3 | 30 | 37.5 | .63 |
| Heroin use, injection | 49 | 38.3 | 14 | 29.7 | 35 | 43.8 | .10 |
| Fentanyl use, injection | 1 | 0.8 | 1 | 2.1 | 0 | 0 | .38 |
| Oxycodone use | 3 | 2.3 | 0 | 0 | 3 | 3.75 | .29 |
| Morphine use | 2 | 1.6 | 2 | 4.2 | 0 | 0 | .14 |
| Methadone use | 5 | 3.9 | 3 | 6.3 | 2 | 2.5 | .36 |
| MINI substance use disorder | |||||||
| Cannabis | 28 | 23.3 | 7 | 15.2 | 21 | 28.4 | .10 |
| Cocaine | 56 | 46.7 | 21 | 45.7 | 35 | 47.3 | .86 |
| MINI disorder | |||||||
| Major depressive | 46 | 38.3 | 13 | 28.3 | 33 | 44.6 | .07 |
| Bipolar | 24 | 20.0 | 4 | 8.7 | 20 | 27.0 | .02 |
| Generalized anxiety | 10 | 8.3 | 1 | 2.2 | 9 | 12.2 | .09 |
| PTSD | 21 | 17.5 | 5 | 10.9 | 16 | 21.6 | .13 |
Abbreviations: ART, antiretroviral therapy; ASSIST, Alcohol, Smoking and Substance Involvement Screening Test; HS, high school; MINI, Mini International Neuropsychiatric Interview; MOUD, medication for opioid use disorder; PTSD, post-traumatic stress disorder ; TLFB, timeline follow back; VL, viral load. Bolded data mean statistically significant at p < 0.05.
HCV Care Cascade
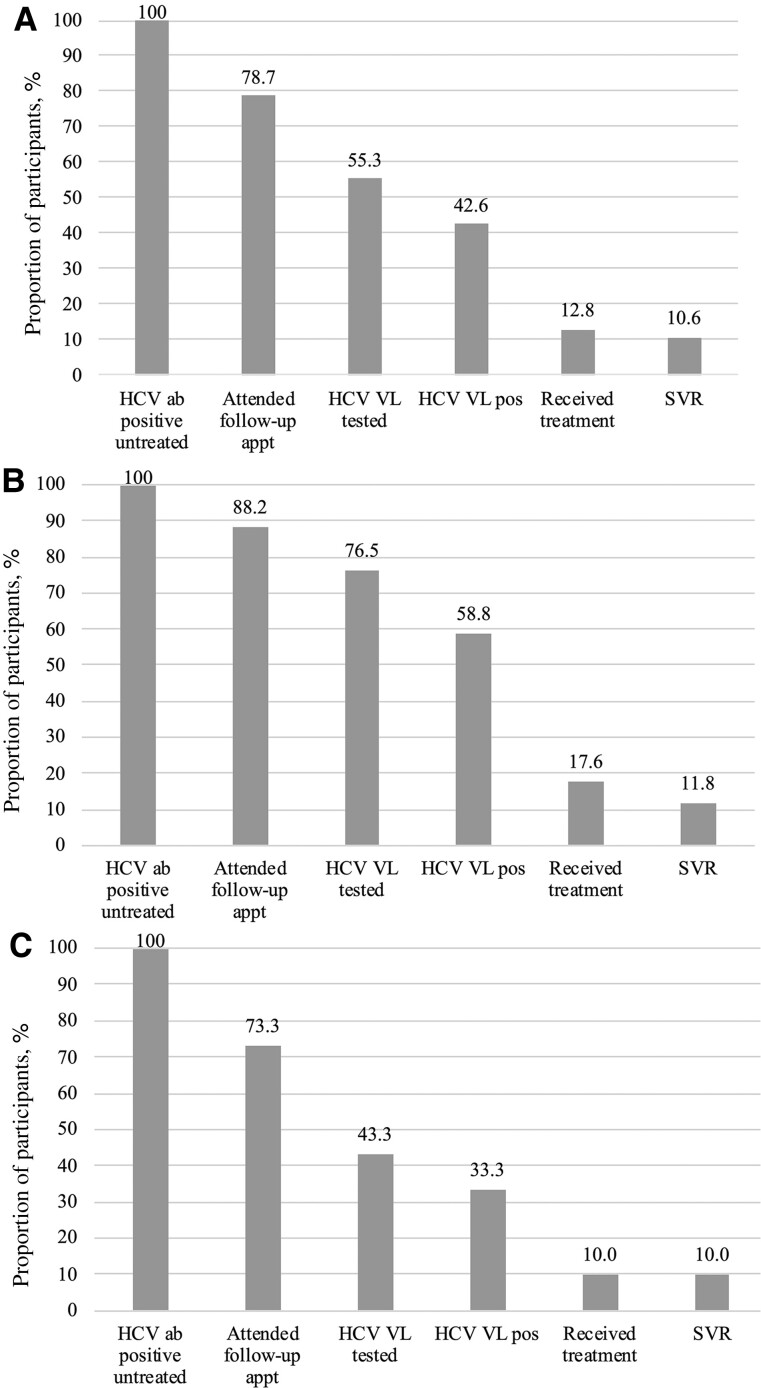
Among the 129 subjects, 78 (60.5%) were HCV antibody positive, of whom 61 self-reported a positive HCV status before baseline testing and 31 (39.7%) self-reported receipt of previous treatment, with 28 (87.5%) endorsing a sustained virologic response (SVR). SVR was confirmed with HCV VL testing by study staff. One previously treated participant without HIV was reinfected with HCV. Forty-seven participants were previously untreated, of whom 37 (78.7%) attended a follow-up appointment, 26 (55.3%) underwent follow-up HCV VL testing, 20 (42.6%) were HCV VL positive, 6 (12.8%) were started on DAA therapy, and 5 (10.6%) achieved SVR (Figure 1A). When grouped by HIV serostatus, participants without HIV underwent a significantly lower rate of follow-up HCV VL confirmatory testing (43.3% vs 76.5%; P = .03) (Figure 1B and C). There was no significant difference with respect to initiation onto (17.6% PWH vs persons without HIV 10%; P = .45) or completion of DAA therapy (11.8% PWH vs persons without HIV 10%; P = .85). Twenty-four PWH self-reported previous receipt of HCV treatment (83.3% endorsing SVR) at enrollment compared with 8 participants (100% endorsed SVR) without HIV.
Figure 1.
A, HCV care cascade among persons with OUD who are receiving MOUD. B, HCV care cascade of PWH with OUD who are receiving MOUD. C, HCV care cascade of persons without HIV with OUD who are receiving MOUD. Abbreviations: Ab pos, antibody positive; Appt, appointment; DAA, direct-acting antiviral therapy; HCV, hepatitis C virus; MOUD, medications for opioid use disorder; OUD, opioid use disorder; PWH, persons with HIV; SVR, sustained virologic response; VL, viral load.
Injection Opioid Use
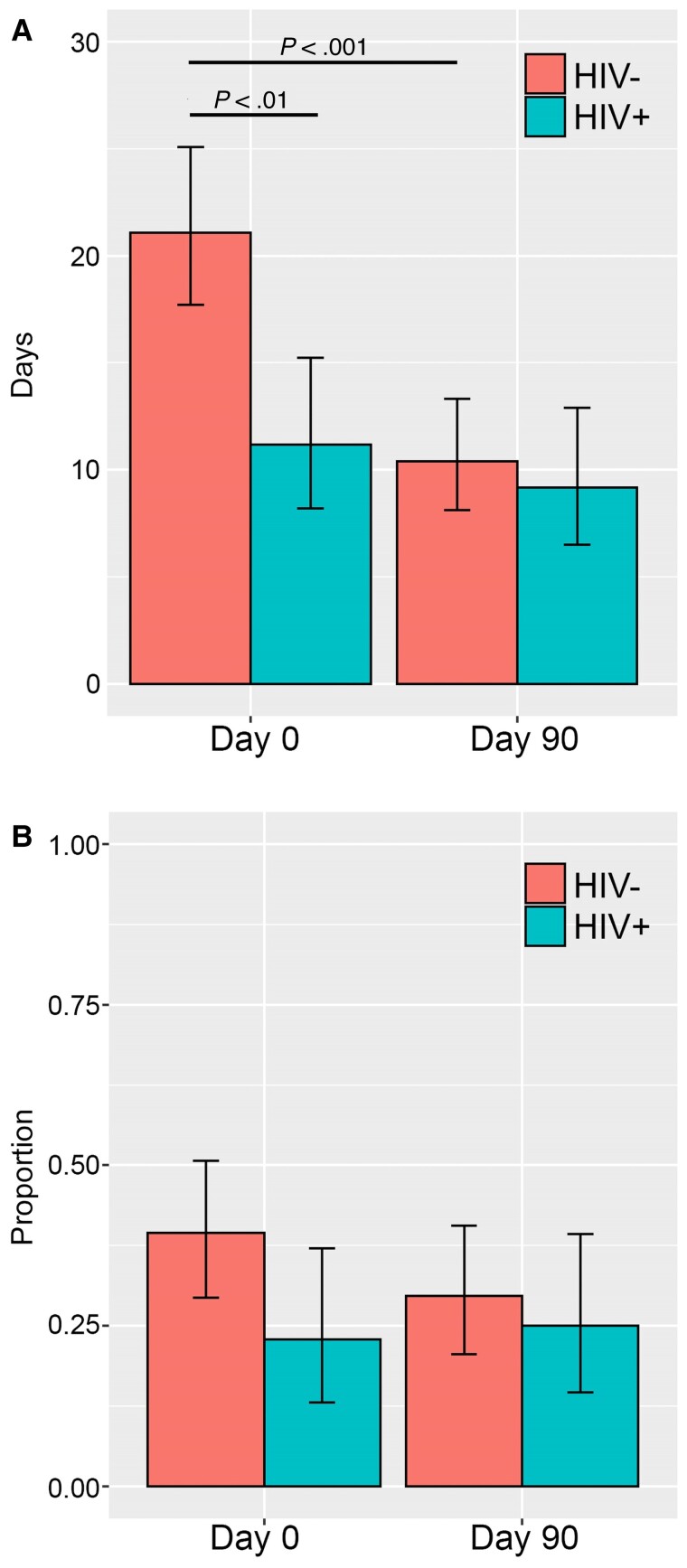
In an unadjusted model of injection opioid use, we observed a significant reduction in use over observations (P < .001), with a significant main effect of HIV status (P < .001), and a significant HIV*observation interaction (P < .001) (Figure 2A). Most participants endorsed injection heroin use, as reflected in the IDU variable. Participants without HIV notably self-reported a higher proportion of opioid IDU at baseline compared with PWH (21.0 days vs 11.2 days out of 30; P < .001) and experienced a much larger decline in self-reported opioid IDU from day 0 to day 90 after initiating MOUD (10.7 fewer days vs 2.0 fewer days out of 30; P < .001). Among the entire cohort, the number of positive opioid urine toxicology samples was statistically significantly reduced from baseline to 90 days (61.4% to 38.0%; P < .001); however, there was no significant difference when stratified by HIV serostatus (48.9% to 32.9% PWH vs 72.5% to 43.2% persons without HIV; P = .22). Next, when adjusting for baseline differences in gender, age, race, educational attainment, housing status, and MOUD, the only covariate that was significant was the form of MOUD, with participants receiving buprenorphine endorsing lower opioid IDU from baseline to 90 days (P < .05), compared with participants receiving methadone.
Figure 2.
A, Unadjusted self-reported injection opioid use from baseline to 90 days, grouped by HIV serostatus. B, Unadjusted self-reported stimulant use from baseline to 90 days, grouped by HIV serostatus.
Stimulant Use
In an unadjusted model, there was no significant decline in any route of stimulant use among the cohort from baseline to 90 days after initiation of MOUD (39.5% to 30.2%; P = .75). Proportion of stimulant use was noted to decrease from baseline to 90 days (40.0% vs 30.0%; P = .51) among participants without HIV and slightly increased among PWH (23.0% vs 25.0%; P = .13); however, this was not significant (Figure 2B). Additionally, we observed no significant effect of time, HIV status, or their interaction. Inclusion of the previously mentioned covariates did not change this result.
DISCUSSION
To our knowledge, this is the first study to assess for differences in HCV testing and treatment uptake by HIV serostatus among a cohort of persons with OUD who were receiving MOUD in the community. This retrospective analysis is further strengthened by its utilization of prospective longitudinal data from 2 cohorts of participants with and without HIV with OUD who were initiating MOUD in community settings. This analysis examined completion of HCV testing as well as follow-up appointment attendance and receipt of care in the community in the 2 parent cohort studies. Further, there was no direct intervention or treatment for HIV, HCV, or OUD by study staff in the parent cohort studies. As such, our analysis provides a real-world assessment of the HCV care continuum. The HCV care cascade reflects a high rate of HCV antibody testing and follow-up appointment attendance; however, there was diminished HCV VL testing and initiation onto DAAs. Most notably, in this evaluation there was a significantly lower number of participants without HIV who completed follow-up HCV VL testing compared with PWH after receiving the same referral to a commercial laboratory. This discrepancy may be related to lower contact with health care providers among participants without HIV at baseline or lower health literacy regarding the importance of HCV screening and treatment, and thus lower motivation to undergo follow-up HCV VL testing. For instance, PWH who are uninsured or underinsured may receive Ryan White HIV/AIDS services for primary care, including HCV and OUD assessment and treatment, or essential support services [30] and undergo more frequent laboratory monitoring of HIV VL. Nevertheless, low rates of initiation onto DAA therapy were observed regardless of HIV serostatus. This finding is concerning, particularly given the abundant health care resources available to PWH and in light of high attendance at follow-up appointments and high adherence to MOUD among enrolled participants. As such, our HCV care cascade indicates continued missed opportunities to engage persons with OUD who are receiving MOUD to evaluate HCV infection and initiate HCV treatment. It is vital to integrate both infectious diseases screening and therapy with concomitant treatment for OUD, which has been recommended by the National Academies of Science, Engineering, and Medicine (NASEM) [31–33]. This evaluation of the HCV care cascade among a contemporaneous cohort of persons with OUD receiving MOUD in the community reflects a significant need to improve screening with follow-up HCV VL testing, initiation of DAA treatment, and completion of therapy among persons regardless of HIV serostatus. HCV is now curative, and any opportunity to be able to increase cure of a potentially fatal disease is paramount. A previously published study supports high retention in care and initiation of and adherence to DAAs among persons with OUD while on MOUD [34]. Given that the state in which this study was conducted, Connecticut, is a Medicaid expansion state [35], where DAA [36] and MOUD are covered [37], as well as the availability of hands-on HCV and HIV treatment through multidisciplinary services [38], there are many missed opportunities to intervene early to ensure that patients who would be eligible for treatment are not overlooked.
Our analysis further examined whether receipt of MOUD would modulate opioid IDU frequency among those with HCV and whether this would differ by HIV serostatus. We found that receipt of MOUD reduced self-reported IDU frequency in both groups from baseline to 90 days after receipt of MOUD. Further, participants without HIV had a higher baseline mean proportion of self-reported opioid IDU and a greater reduction in self-reported opioid IDU at 90 days. To our knowledge, this is the first study to examine opioid IDU behavior in persons on MOUD with HCV by HIV serostatus. Given that both groups were started on MOUD in the parent cohort trials and PWH were virally suppressed at baseline per study inclusion criteria, perhaps PWH had greater prior contact with the health care system at baseline compared with participants without HIV, thus providing increased opportunity to receive counseling regarding transmission of bloodborne pathogens. The biological data (urine toxicology) confirmed a reduction in opioid use among the entire cohort from baseline to 90 days after receipt of MOUD, which is supported by previous studies [14–20]. Further, though there was a decline in proportion of opioid-positive urine samples in both groups from baseline to 90 days, there was no significant difference when stratified by HIV serostatus. These findings would have been further strengthened by comparison to a group of participants who were not on MOUD; however, the parent cohort studies were not powered to assess for this difference.
We also examined whether receipt of MOUD would modulate stimulant use among participants with OUD and if there was a difference between PWH and participants without HIV. Our analysis found that the use of stimulants was high in this cohort (48.1%), primarily of cocaine. However, there was no statistically significant change in self-reported or biologically confirmed stimulant use from baseline to 90 days among PWH or participants without HIV following initiation of MOUD. Previous studies of the effect of MOUD on stimulant use are conflicting, with some evidence that higher-dose buprenorphine (16 mg) may reduce cocaine use [39], while it has also been found that persons who use methamphetamine or amphetamine may have lower opioid abstinence and receipt of or retention on MOUD [21]. In our cohort, methamphetamine use was very low. In the parent studies, MAT BIO and Persistence, there was high retention on MOUD among all participants, even among those who endorsed stimulant use, though we did not specifically examine for opioid abstinence.
In our adjusted analysis, receipt of buprenorphine did appear to be significantly associated with a greater self-reported reduction in opioid IDU compared with receipt of methadone. This relationship also does not appear to differ by HIV serostatus. Importantly, a recent publication from the same cohort found that high-dose methadone (>85 mg) or buprenorphine (≥16 mg) was significantly associated with retention on MOUD; however, differences in IDU by MOUD dose were not compared [40]. Our results differ from a Cochrane Review of 31 trials and 5430 participants that found that there was no difference between high-dose buprenorphine and methadone with respect to suppression of heroin use [41].
Lastly, participants in both cohort studies endorsed a significantly higher proportion of heroin use at baseline than fentanyl, which was unexpected given the rising rates of fentanyl-associated overdose deaths seen throughout the United States [42]. Upon review of urine toxicology at baseline, fentanyl was found in the urine of 20.9% of participants, but the proportion of positive fentanyl urine samples did not differ by HIV serostatus. It is likely that participants who self-endorsed injection heroin use were unaware of the increased supply of fentanyl and fentanyl contamination of heroin that was available in Connecticut and were likely injecting both substances.
Our analysis had a few limitations. First, while we assessed data from 2 prospective cohorts of persons with OUD initiated on MOUD, the parent trials were not designed to include a comparison group not on MOUD. Thus, we were unable to assess how much of a treatment effect MOUD had on reducing opioid IDU or stimulant use compared with an untreated group. Next, our analysis examined 2 groups, PWH with confirmed HIV VL suppression who were likely highly engaged with care and a second group (participants without HIV) that likely had limited health care engagement at baseline, which may limit interpretation of our results. Further, follow-up durations in the parent trials for participants enrolled in MAT BIO (6 months) and Persistence (3 months) were comparatively short; thus it is possible that longer follow-up would have led to a more pronounced effect of MOUD on the HCV care cascade as well as on opioid IDU or stimulant use. Previous studies have found a positive effect of MOUD on HIV VL suppression [43–45] after longer durations of receipt of MOUD. In the parent trials, following a new diagnosis of HCV, participants were referred by study staff to their clinicians for evaluation for treatment. All participants with a positive HCV antibody on rapid testing were referred for confirmatory HCV antibody and HCV VL testing at a commercial laboratory, and results were shared with clinicians if the patients approved via a signed medical release of information form. HCV VL testing was again repeated at 3 (Persistence) and 6 months (MAT BIO) for those with newly diagnosed HCV to assess for SVR and those who were HCV negative to assess for new infection. Our HCV testing strategy was dependent on participants attending an outside laboratory rather than receiving a 1-time on-site HCV antibody test with confirmatory assessment. HCV rapid antibody screening with reflex confirmatory testing is an important component of the HCV care cascade in terms of reducing loss to follow-up [46]. Furthermore, this vulnerable population, particularly those without HIV, would have greatly benefited from interaction with patient navigators, which is an evidence-based strategy to improve HCV testing, appointment attendance, and HCV treatment [47]. Our sample size was also small, which limits the strength of our conclusions or the generalizability regarding differences in HCV treatment follow-up as well as opioid IDU or stimulant use by HIV serostatus. Lastly, given that this population demonstrated strong engagement via high adherence to MOUD and VL suppression among PWH, our results may not be generalizable to populations with more heterogeneous VL suppression.
CONCLUSIONS
Our analysis suggests that while initiation and maintenance on MOUD were high in both parent cohort studies, subsequent confirmatory HCV testing was poor among participants without HIV, indicating that this population may need improved integration of OUD and HCV screening and linkage to care. This is particularly valid in a Medicaid expansion state like Connecticut. Additionally, we found that MOUD may modulate opioid IDU, especially among participants at risk for HIV. Nevertheless, improved strategies to integrate care for both OUD and infectious diseases regardless of HIV status are needed [32, 48].
Acknowledgments
The authors would like to thank Mark Sanchez and the MAT BIO and Persistence research teams for their efforts on this study.
Financial support. This work was supported by the National Institutes on Drug Abuse (NIDA) for author Springer:R01 DA043337; R61DA047037; R33 DA047037; DP1 DA056106; and K02 DA032322. We would like to acknowledge additional support from the Claude D. Pepper Older Americans Independence Center from the NIH/NIA (P30AG021342).
Disclaimer. The funders were not involved in the research design, analysis or interpretation of the data, or the decision to publish the manuscript.
Author contributions. A.L. was involved with data analysis, manuscript writing, and editing; B.V.W. was involved with statistical analysis and manuscript writing; A.D. was involved with manuscript writing and editing; S.S. was involved with study funding acquisition, concept of manuscript, manuscript writing and editing.
Contributor Information
Audun J Lier, Division of Infectious Diseases, Department of Medicine, Northport VA Medical Center, Northport, New York, USA.
Brent Vander Wyk, Section of Geriatrics, Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Angela Di Paola, AIDS Program, Section of Infectious Diseases, Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Sandra A Springer, AIDS Program, Section of Infectious Diseases, Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
References
- 1. Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology 2019; 69:1020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeuzem S, Teuber G, Lee JH, Ruster B, Roth WK. Risk factors for the transmission of hepatitis C. J Hepatol 1996; 24:3–10. [PubMed] [Google Scholar]
- 3. Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 2008; 372:1733–45. [DOI] [PubMed] [Google Scholar]
- 4. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep 2015; 64:453–8. [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer B, O'Keefe-Markman C, Lee AM, Daldegan-Bueno D. ‘Resurgent’, ‘twin’ or ‘silent’ epidemic? A select data overview and observations on increasing psycho-stimulant use and harms in North America. Subst Abuse Treat Prev Policy 2021; 16:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017; 166:637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin NK, Foster GR, Vilar J, et al. HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. J Viral Hepat 2015; 22:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma J, Non L, Amornsawadwattana S, Olsen MA, Garavaglia Wilson A, Presti RM. Hepatitis C care cascade in HIV patients at an urban clinic in the early direct-acting antiviral era. Int J STD AIDS 2019; 30:834–42. [DOI] [PubMed] [Google Scholar]
- 9. Busschots D, Kremer C, Koc OM, et al. The hepatitis C cascade of care in the Belgian HIV population: one step closer to elimination. Int J Infect Dis 2021; 105:217–23. [DOI] [PubMed] [Google Scholar]
- 10. Cachay ER, Hill L, Wyles D, et al. The hepatitis C cascade of care among HIV infected patients: a call to address ongoing barriers to care. PLoS One 2014; 9:e102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saeed S, Strumpf EC, Moodie EE, et al. Disparities in direct acting antivirals uptake in HIV-hepatitis C co-infected populations in Canada. J Int AIDS Soc 2017; 20:e25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:a:D study. Arch Intern Med 2006; 166:1632–41. [DOI] [PubMed] [Google Scholar]
- 13. Palmateer NE, McAuley A, Dillon JF, et al. Reduction in the population prevalence of hepatitis C virus viraemia among people who inject drugs associated with scale-up of direct-acting anti-viral therapy in community drug services: real-world data. Addiction 2021; 116:2893–907. [DOI] [PubMed] [Google Scholar]
- 14. Woody GE, Bruce D, Korthuis PT, et al. HIV risk reduction with buprenorphine-naloxone or methadone: findings from a randomized trial. J Acquir Immune Defic Syndr 2014; 66:288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ball J, Corty E, Bond H, Myers C, Tommasello A. The reduction of intravenous heroin use, non-opiate abuse and crime during methadone maintenance treatment: further findings. NIDA Res Monogr 1988; 81:224–30. [PubMed] [Google Scholar]
- 16. Lott DC, Strain EC, Brooner RK, Bigelow GE, Johnson RE. HIV risk behaviors during pharmacologic treatment for opioid dependence: a comparison of levomethadyl acetate [corrected] buprenorphine, and methadone. J Subst Abuse Treat 2006; 31:187–94. [DOI] [PubMed] [Google Scholar]
- 17. Marsch LA, Bickel WK, Badger GJ, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch Gen Psychiatry 2005; 62:1157–64. [DOI] [PubMed] [Google Scholar]
- 18. Meade CS, Weiss RD, Fitzmaurice GM, et al. HIV risk behavior in treatment-seeking opioid-dependent youth: results from a NIDA clinical trials network multisite study. J Acquir Immune Defic Syndr 2010; 55:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schottenfeld RS, Chawarski MC, Mazlan M. Maintenance treatment with buprenorphine and naltrexone for heroin dependence in Malaysia: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sullivan LE, Moore BA, Chawarski MC, et al. Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. J Subst Abuse Treat 2008; 35:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frost MC, Lampert H, Tsui JI, Iles-Shih MD, Williams EC. The impact of methamphetamine/amphetamine use on receipt and outcomes of medications for opioid use disorder: a systematic review. Addict Sci Clin Pract 2021; 16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown JL, Gause NK, Lewis D, Winhusen T. Examination of the hepatitis C virus care continuum among individuals with an opioid use disorder in substance use treatment. J Subst Abuse Treat 2017; 76:77–80. [DOI] [PubMed] [Google Scholar]
- 23. Ho SB, Bräu N, Cheung R, et al. Integrated care increases treatment and improves outcomes of patients with chronic hepatitis C virus infection and psychiatric illness or substance abuse. Clin Gastroenterol Hepatol 2015; 13:2005–14.e2001–3. [DOI] [PubMed] [Google Scholar]
- 24. Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis 2013; 57:S56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biondi BE, Mohanty S, Wyk BV, Montgomery RR, Shaw AC, Springer SA. Design and implementation of a prospective cohort study of persons living with and without HIV infection who are initiating medication treatment for opioid use disorder. Contemp Clin Trials Commun 2021; 21:100704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schultheis A, Sanchez M, Pedersen S, et al. Design and implementation of a cohort study of persons living with HIV infection who are initiating medication treatment for opioid use disorder to evaluate HIV-1 persistence. Contemp Clin Trials Commun 2021; 24:100866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59:22–33; quiz 34–57. [PubMed] [Google Scholar]
- 28. Sobell LC, Cunningham JA, Sobell MB. Recovery from alcohol problems with and without treatment: prevalence in two population surveys. Am J Public Health 1996; 86:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. WHO ASSIST Working Group . The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction 2002; 97:1183–94. [DOI] [PubMed] [Google Scholar]
- 30. Klein PW, Geiger T, Chavis NS, et al. The Health Resources and Services Administration's Ryan White HIV/AIDS Program in rural areas of the United States: geographic distribution, provider characteristics, and clinical outcomes. PLoS One 2020; 15:e0230121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Springer SA, Barocas JA, Wurcel A, et al. Federal and state action needed to end the infectious complications of illicit drug use in the United States: IDSA and HIVMA's Advocacy Agenda. J Infect Dis 2020; 222:S230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Springer SA, Merluzzi AP, Del Rio C. Integrating responses to the opioid use disorder and infectious disease epidemics: a report from the National Academies of Sciences, Engineering, and Medicine. JAMA 2020; 324:37–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. The National Academies of Sciences, Engineering, Medicine . Examination of the integration of opioid and infectious disease prevention efforts in select programs. Available at: https://www.nationalacademies.org/our-work/examination-of-the-integration-of-opioid-and-infectious-disease-prevention-efforts-in-select-programs. Accessed August 2, 2022.
- 34. Norton BL, Beitin A, Glenn M, DeLuca J, Litwin AH, Cunningham CO. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat 2017; 75:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sommers BD, Kenney GM, Epstein AM. New evidence on the Affordable Care Act: coverage impacts of early Medicaid expansions. Health Aff (Millwood) 2014; 33:78–87. [DOI] [PubMed] [Google Scholar]
- 36. The National Academies of Sciences, Engineering, Medicine . A National Strategy for the Elimination of Hepatitis B and C: A Phase Two Report. The National Academies Press; 2017. [PubMed] [Google Scholar]
- 37. The Centers for Medicaid and CHIP Services . Medication assisted treatment for substance use disorders, CMCS information bulletin. Available at: http://www.medicaid.gov/federal-policy-guidance/downloads/cib-07-11-2014.pdf. Accessed August 2, 2022.
- 38. APT Foundation . Our mission. Available at: https://aptfoundation.org/our-mission. Accessed August 2, 2022.
- 39. Montoya ID, Gorelick DA, Preston KL, et al. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther 2004; 75:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Biondi BE, Vander Wyk B, Schlossberg EF, Shaw A, Springer SA. Factors associated with retention on medications for opioid use disorder among a cohort of adults seeking treatment in the community. Addict Sci Clin Pract 2022; 17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; 2:CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. National Institute on Drug Abuse . Overdose death rates. Available at: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates. Accessed October 14, 2022.
- 43. Springer SA, Di Paola A, Azar MM, et al. Extended-release naltrexone improves viral suppression among incarcerated persons living with HIV with opioid use disorders transitioning to the community: results of a double-blind, placebo-controlled randomized trial. J Acquir Immune Defic Syndr 2018; 78:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Springer SA, Qiu J, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PLoS One 2012; 7:e38335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McNamara KF, Biondi BE, Hernández-Ramírez RU, Taweh N, Grimshaw AA, Springer SA. A systematic review and meta-analysis of studies evaluating the effect of medication treatment for opioid use disorder on infectious disease outcomes. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sivakumar A, Madden L, DiDomizio E, Eller A, Villanueva M, Altice FL. Treatment of hepatitis C virus among people who inject drugs at a syringe service program during the COVID-19 response: the potential role of telehealth, medications for opioid use disorder and minimal demands on patients. Int J Drug Policy 2022; 101:103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hunt BR, Ahmed C, Ramirez-Mercado K, Patron C, Glick NR. Routine screening and linkage to care for hepatitis C virus in an urban safety-net health system 2017–2019. Public Health Rep 2021; 136:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Springer SA, Korthuis PT, Del Rio C. Integrating treatment at the intersection of opioid use disorder and infectious disease epidemics in medical settings: a call for action after a National Academies of Sciences, Engineering, and Medicine workshop. Ann Intern Med 2018; 169:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]