Abstract
Background and Objectives
Multiple sclerosis (MS) is a neuroinflammatory and neurodegenerative disease characterized by infiltration of immune cells in multifocal areas of the CNS. The specific molecular processes allowing autoreactive immune cells to enter the CNS compartment through the blood-brain barrier remain elusive.
Methods
Using endothelial cell (EC) enrichment and single-cell RNA sequencing, we characterized the cells implicated in the neuroinflammatory processes in experimental autoimmune encephalomyelitis, an animal model of MS. Validations on human MS brain sections of the most differentially expressed genes in venous ECs were performed using immunohistochemistry and confocal microscopy.
Results
We found an upregulation of genes associated with antigen presentation and interferon in most populations of CNS-resident cells, including ECs. Interestingly, instead of transcriptionally distinct profiles, a continuous gradient of gene expression separated the arteriovenous zonation of the brain vasculature. However, differential gene expression analysis presented more transcriptomic alterations on the venous side of the axis, suggesting a prominent role of venous ECs in neuroinflammation. Furthermore, analysis of ligand-receptor interactions identified important potential molecular communications between venous ECs and infiltrated immune populations. To confirm the relevance of our observation in the context of human disease, we validated the protein expression of the most upregulated genes (Ackr1 and Lcn2) in MS lesions.
Discussion
In this study, we provide a landscape of the cellular heterogeneity associated with neuroinflammation. We also present important molecular insights for further exploration of specific cell processes that promote infiltration of immune cells inside the brain of experimental autoimmune encephalomyelitis mice.
The cellular landscape of the CNS is highly heterogeneous. The CNS contains multiple populations of cells including neurons, astrocytes, oligodendrocytes, microglia, and ependymal cells. Under homeostatic conditions, the presence of a semipermeable barrier, called the blood-brain barrier (BBB), maintains a stable CNS environment. This structure tightly regulates molecule and cell trafficking between the blood and the CNS parenchyma.1 In certain neuroinflammatory disorders, BBB dysfunction promotes infiltration of immune cells and proinflammatory molecules from the blood into the brain parenchyma.2,3
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS. The main characteristics of the disease include immune cell infiltration, demyelination, gliosis, and axonal damage. Experimental autoimmune encephalomyelitis (EAE) is the most common animal model used to study different neuroinflammatory processes reminiscent of MS. Both MS and EAE are characterized by the migration of various immune cell populations across the BBB and by the subsequent interplay occurring between the different cell subsets present within the CNS. This process is coordinated by an array of secreted and surface molecules, including cytokines and chemokines. The infiltration of immune cells across the BBB is also mediated by interactions between trafficking molecules such as cell adhesion molecules (CAMs) expressed on the surface of barrier endothelial cells (ECs) and their ligands on the migrating immune cells.4,5 MS lesions are associated with the presence of a “central vein sign” visible in MRI, suggesting that immune cells invade the brain though the veins.3,6 However, the reason why veins are the main site of immune cell infiltration remain unclear.
Among the different populations of immune cells involved in MS and EAE, T lymphocytes were first considered to be central drivers of neuroinflammation.7 Myelin-reactive Th1 and Th17 cells are both documented as sufficient to transfer disease to naïve mice.8,9 Moreover, a pathogenic role of CD8+ T lymphocytes was proven by the development of a CD8+ T-lymphocyte–mediated model of EAE.10 By contrast, regulatory T lymphocytes (Treg) are described as a population that inhibits the function of proinflammatory cells, and EAE severity is decreased by the transfer of Treg.11,12 Natural killer (NK) cells were also described as playing a protective effect because their depletion resulted in a more severe EAE,13,14 but a study supported that NK cells may negatively affect the reparative efficacy of the CNS.15 Finally, B lymphocytes also play a key role in neuroinflammation, as shown by the efficacy of B-cell depletion by anti-CD20 therapy in the treatment of EAE and remitting-relapsing MS.16,17
Myeloid cells are also known to play a substantial role in the formation of CNS lesions in MS and EAE. Monocyte-derived dendritic cells (DCs), which are professional antigen-presenting cells, have an important role in EAE and MS pathophysiology because they are known to orchestrate the activation and polarization of T lymphocytes in both the periphery and the brain.18,19 Moreover, several studies support a dual role of monocyte-derived macrophages in MS. Indeed, they can not only promote neuroinflammation but also be neuroprotective and promote tissue repair.20 In response to the cytokines from their environment, macrophages can polarize in a spectrum of phenotypes going from proinflammatory (M1) to anti-inflammatory (M2) phenotypes. Furthermore, CNS-resident cells such as microglia and astrocytes are believed to contribute to MS and EAE pathogenesis by recruiting immune cells and adopting proinflammatory and neurotoxic phenotypes. However, they can also promote neuroprotection and tissue repair.21-23
The complexity of the immune and CNS cellular environment highlights the need for unbiased experimental approaches to capture a more comprehensive landscape of the cellular interactions at play. The introduction of single-cell RNA sequencing (scRNA-seq) technologies has facilitated detailed characterization of the heterogeneity of oligodendrocytes, neurons, microglia, and astrocytes in MS and EAE.24-29 However, these studies focused on a specific cell type and have not analyzed the transcriptome of brain ECs.
In this study, we used scRNA-seq to provide a comprehensive data set of transcriptomic changes associated with neuroinflammation in different cell populations in the brain during EAE, with a focus on ECs. We based our analysis on 2 studies that characterized murine ECs at single-cell level.30,31 Vanlandewijck et al. demonstrated a continuum of the different brain EC populations in healthy mice, and Kalucka et al. provided an atlas of major organ vasculature in mice and identified a population of interferon-activated ECs. Interestingly, we were also able to identify this population of ECs that was, after venous ECs, the cell type with the most transcriptional changes during neuroinflammation. Thus, these 2 studies laid the foundation for this article, which characterizes how the cerebral vascular tree responds to neuroinflammation in a murine model of MS. Finally, we used a database of interactions32 to identify novel inflammatory networks potentially involved in neuroinflammation. The single-cell transcriptomic atlas provided in our study expands the understanding of molecular mechanism leading to neuroinflammatory diseases.
Data Availability
The data is freely shared via two interactive R shiny applications available at https://pratlab.shinyapps.io/EAEbrain_10X (total cells) and https://pratlab.shinyapps.io/EAEbrain_CD31 (CD31-selected cells). ScRNA-seq raw data (FASTQ files) are available to investigators via the GEO accession number GSE199460 (Tables 1–8, links.lww.com/NXI/A772, links.lww.com/NXI/A773, links.lww.com/NXI/A774, links.lww.com/NXI/A775, links.lww.com/NXI/A776, links.lww.com/NXI/A777, links.lww.com/NXI/A778, links.lww.com/NXI/A779, respectively).
Results
scRNA-Seq Unravels the Cellular Heterogeneity of the Murine Brain
We extracted brains from myelin oligodendrocyte glycoprotein (MOG)–induced EAE at peak of the disease and control mice to isolate single cells using an optimized protocol adapted from the Miltenyi Brain Dissociation Kit. Cells from each brain were subjected to scRNA-seq using a microdroplet-based method from 10X genomics (Figure 1A). After the removal of cells with high mitochondrial gene content (>25%) and containing less than 750 genes (or more than 5,500), 9,728 cells were sequenced from the control samples (CTL1 = 5,063, CTL2 = 2,989, and CTL3 = 1,676) and 17,093 cells from the EAE samples (EAE1 = 4,847, EAE2 = 5,665, and EAE3 = 6,581) (eFigure 1, links.lww.com/NXI/A780).
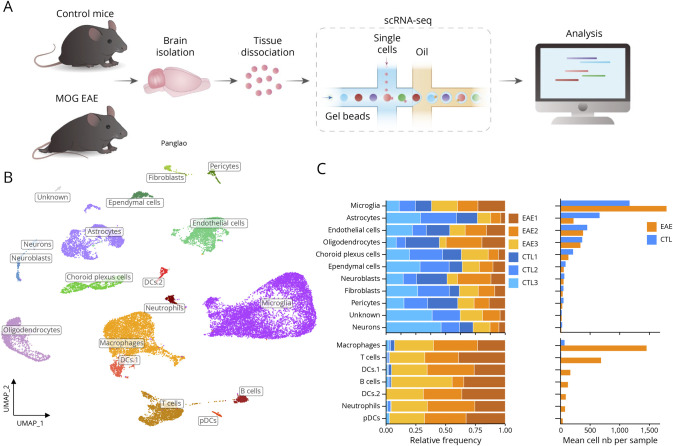
Figure 1. Single-Cell Landscape of CNS Tissue.
(A) Experimental design. (B) Uniform manifold approximation and projection (UMAP) showing the cell clusters on control and EAE brains. Cell types were annotated with the 2021 Panglao cell types gene sets from enrichR. (C) Relative proportions of each identified cell type. Upper graphs show proportions among CNS-resident cells and lower graphs show proportions among all cells. Abbreviation: EAE = experimental autoimmune encephalomyelitis.
We first characterized cell populations in homeostatic and inflammatory conditions using an unbiased clustering approach, visualized by uniform manifold approximation and projection (Uniform Manifold Approximation and Projection [UMAP]) for dimension reduction41 (Figure 1B). Unbiased clustering with the Louvain algorithm from Seurat33 revealed 18 clusters of cells, which were validated by previously reported canonical cellular markers (Figure 1C and eFigure 2A, links.lww.com/NXI/A780). Across the samples, most of the cells were microglia (35.9% in controls and 31.4% in EAE), followed by immune cells (2.8% in controls and 46.2% in EAE), astrocytes (20.3% in controls and 3.8% in EAE), ECs (ECs; 13.9% in controls and 6.7% in EAE), and mature oligodendrocytes (MOLs11.3% in controls and 5.9% in EAE). We were also able to detect neurons, pericytes, choroid plexus epithelial cells, neuroblasts, and fibroblasts, but in smaller proportions (<5%) (Figure 1, B and C). Compared with controls, EAE mice displayed a lower proportion of astrocytes, while we observed an increased proportion of microglia and infiltrated immune cells (Figure 1C). Using MAST,36 we produced a list of expression markers associated with each of the identified cell types (eFigure 2B). Overall, our protocol allowed a broad view of the cellular heterogeneity of the mouse brain under control and neuroinflammatory conditions. We identified the main expected CNS cell types, and the gene markers for each cellular cluster were coherent with public annotation of cell types.
scRNA-Seq Reveals Disease-Specific Subpopulations of Glial Cells
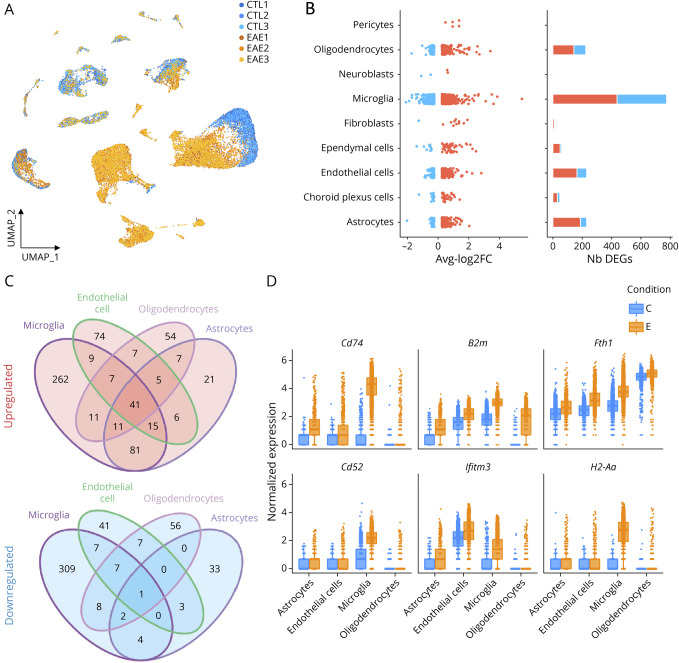
To investigate the molecular pathways associated with neuroinflammation, a differential expression analysis was performed on each of the labeled CNS-resident cell types using MAST.36 While differentially expressed genes (DEGs) with an average log fold change higher than 0.5 and with an adjusted p value lower than 0.01 were found in all cell types, microglia, oligodendrocytes, ECs, and astrocytes showed the highest number of DEGs (Figure 2B). We found substantial overlap in the significantly upregulated genes (Figure 2C), suggesting a common transcriptional response to immune signaling. However, the overlap in the significantly downregulated genes was very low. To better describe the biological pathways represented by the sets of DEGs, we used a gene set enrichment approach using the gene ontology databases. Most cell types showed an enrichment for antigen processing and response to cytokines, suggesting a global, nonspecific state of activation within the CNS in EAE mice (eFigure 2C, links.lww.com/NXI/A780). Among the 41 shared upregulated genes, the top 6 ranking genes were Cd74, H2-Aa, Fth1, Ifitm3, B2m, and Cd52 (eFigure 2D). These results demonstrate that microglia, oligodendrocytes, ECs, and astrocytes are among the CNS-resident cell types that show the greatest transcriptional modifications during EAE.
Figure 2. Single-Cell RNA Sequencing Reveals Transcriptional Changes of Resident Cells in the Mouse Brain During EAE.
(A) Uniform manifold approximation and projection (UMAP) of all cell clusters, colored by disease condition (control vs EAE). (B) On the left, distribution of log2 fold changes for significant DEGs (adjusted p < 0.01 and |log2FC|>0.25) between CTL and EAE mice across CNS-resident cell populations. Bar plot showing the total number of DEGs both downregulated (blue) and upregulated (red). (C) Venn diagrams of upregulated and downregulated genes in endothelial cells, microglia, mature oligodendrocytes, and astrocytes showing overlaps between sets of DEGs. (D) Boxplot of the significantly upregulated genes shared by ECs, microglia, mature oligodendrocytes, and astrocytes that showed the highest fold changes in at least 1 of the 4 cell types. Abbreviations: DEG = differentially expressed gene; EAE = experimental autoimmune encephalomyelitis.
To better define myeloid transcriptional changes associated with neuroinflammation, we reclustered the microglial cells (eFigure 3A.a, links.lww.com/NXI/A780), MOLs (eFigure 3B.a), and astrocytes (eFigure 3C.a) to ignore the effect of gene expression variation associated with other cell types. Six different clusters of microglia were identified, with clusters 0, 2, and 4 exclusively found in EAE mice, while clusters 1 and 3 were mainly found in control mice (eFigure 3A.b). We then produced the list of expression markers associated with each of the myeloid population (eFigure 3A.c). The EAE-specific clusters are characterized by the expression of Ccl12, Ccl4, Ccl2, Ccl3, and Il1b (cluster 0); Ccl5, Apoe, Cst7, Cxcl9, and Spp1 (cluster 2); or Stmn1, H2afz, Hmgb2, Ube2c, and Tubb5 (cluster 4). The clusters found only in control mice were characterized by the expression of Fcrls, P2ry12, Gpr34, Selplg, and Fscn1 (cluster 1) and Jun, Egr1, Jund, Fos, and Junb (cluster 3). With the same strategy, we identified disease-specific populations of MOLs (clusters 1 and 3; eFigure 3B.a and 3B.b), characterized by the high expression of Sgk1, H2-D1, B2m, H2-K1, and Igtp (cluster 1); Serpina3n, Apod, klk6, Ifi27l2a, and Ifit3 (cluster 3; eFigure 3B.c). On the contrary, a population of MOLs was mainly found in control mice (cluster 0; eFigure 3B.a and 3B.b). This population was differentiated from the others by the higher expression of Qdpr, Cryab, Pex5l, Hsp90aa1, and Slc38a2 (eFigure 3B.c).
Finally, we also identified a disease-specific population of astrocytes (cluster 2; eFigure 3, C.a and C.b, links.lww.com/NXI/A780), characterized by the expression of C1qa, C1qb, C1qc, CD74, and Lyz2 (eFigure 3C.c). Of interest, this population also expressed C3, which is a known marker of the A1 phenotype found in astrocytes.42 The transcriptomic signatures associated with these populations were in line with previously reported findings in scRNA-seq studies.25,28,43
Identification of Endothelial Cell Heterogeneity and Transcriptional Changes During EAE
At the transcriptomic level, brain ECs have been shown to span an arteriovenal axis that recapitulates the classical vascular tree.30,31 In EAE, our data demonstrated that ECs are greatly modulated at the peak of disease. To assess the effect of neuroinflammation at higher resolution, we performed scRNA-seq on CD31-enriched brain cells. The representation of ECs within the sequenced cells thus increased from 9.3% to 71.6%, and our new data set featured 28,573 ECs (CTL1 = 4,457, CTL2 = 8,949, CTL3 = 7,342, EAE1 = 3,852, and EAE2 = 3,020) (eFigure 4, A and B, links.lww.com/NXI/A780). ECs were the only cell type in which the relative proportion increased in the CD31-selected samples, suggesting the technique is suited to enrich for ECs (eFigure 4C). Even if we were still able to detect other cell types following the CD31-based cell selection, we focused on labeled ECs to avoid bias introduced by the selection process.
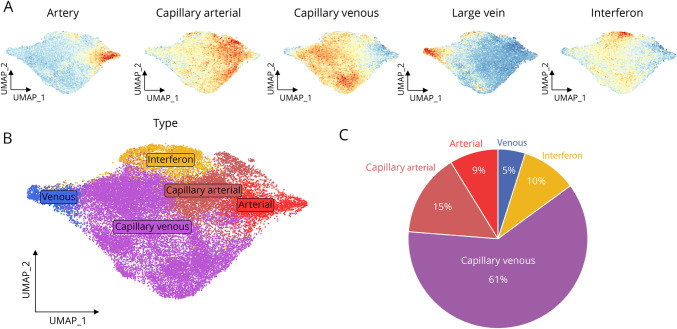
Because we were able to successfully enrich the number of ECs sequenced, we took advantage of this data set to characterize the heterogeneity of ECs in neuroinflammation. To identify EC subtypes, we integrated ECs from the control and EAE mice across both batch and condition to allow overlay of homologous structures despite condition-wise transcriptional differences (eFigure 5A.a and 5A.b, links.lww.com/NXI/A780). Integration across samples allowed an overlay of vascular structures despite significant transcriptional differences between EAE and control mice. First, we analyzed the expression of markers of the vascular bed provided by previous studies.30,31 While the genes Bmx, Gkn3, Efnb2, Vegfc, and Sema3g were mostly expressed in arterial ECs, venous ECs were characterized by the expression of Nr2f2, Slc38a5, Vcam1, and Vwf (eFigure 5, B.a and C). To characterize more ECs subtypes, we used a cell-based enrichment score analysis (Figure 3A), which was validated using a cluster-level analysis (eFigure 5A.c). As previously described by Kalucka et al. (2020), we also identified an EC population that exhibited an interferon response (cluster 3, Figure 3A, 3B, 3C and eFigure 5A.a, 5A.b, 5A.c). Thereby, this transcriptional profile differentiates venous ECs from interferon, capillary venous, capillary arterial, and arterial ECs (Figure 3B). However, the absence of isolated clusters of cells suggests a gradient of gene expression along arteriovenous structures. Most of the cells were identified as capillary-venous ECs (61.33%), followed by capillary arterial ECs (14.97%), interferon ECs (10.01%), arterial ECs (8.78%), and venous ECs (4.91%) (Figure 3C). However, the proportions of EC subpopulations were similar in control and EAE mice (eFigure 5D). With MAST, we produced a list of expression markers associated with each of the identified EC subtypes (eFigure 6B). When integrating samples only at the batch level, dimensional reduction showed a clear separation between control and EAE ECs, reflecting important modifications of transcriptomic profiles under inflammatory conditions (eFigure 6, A.a and A.b). Relative proportions of cluster representation showed a disease-specific population (cluster 2, eFigure 6, A.c and A.e). Of interest, all the venous ECs from EAE mice were included in the disease-specific cluster 2 (eFigure 6A.d), indicating that neuroinflammatory processes affect all venous ECs.
Figure 3. Characterization of EC Phenotypes.
(A) Mapping of module score generated from distinct murine brain EC signatures onto the integrated uniform manifold approximation and projection (UMAP). (B) Integrated UMAP projection of ECs colored by subtypes. (C) Pie chart of the proportions of each identified EC subpopulation. Abbreviation: EC = endothelial cell.
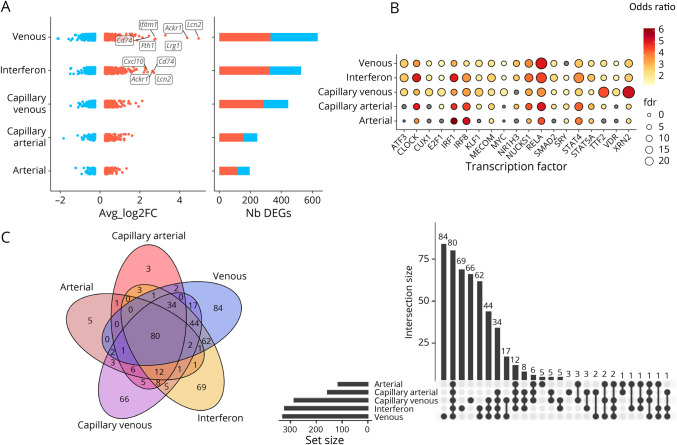
To identify the genes associated with vascular response to neuroinflammation, a differential expression analysis was performed across EC subtypes between controls and EAE mice. DEGs were found in all EC populations, but venous ECs harbored the largest transcriptional modifications (Figure 4A, 4C). Lcn2, Ackr1, Lrg1, CD74, Fth1, and Ifitm1 were among the most significantly upregulated genes associated with neuroinflammation in venous ECs (Figure 4A), and the largest transcriptional downregulation in venous ECs included Car4, Ptn, Ttr, Slc6a6, and Cst3. Transcription factors associated with upregulated DEGs were similar for venous, capillary-venous, and interferon ECs, suggesting a common response of these EC subtypes to neuroinflammation (Figure 4B). We used a gene set enrichment approach to describe biological pathways represented by significant transcriptional shifts shared in all ECs and those specific to venous ECs. The most significant pathways shared by all ECs were associated with immune response to cytokine, such as type 1 interferon and interferon gamma (eFigure 6C.a, links.lww.com/NXI/A780). Furthermore, the main enriched pathways associated with venous-specific DEGs included regulation of cellular component movement, biological adhesion, cell motility, and tissue migration, in line with the role of BBB ECs in regulating cell trafficking (eFigure 6C.b).
Figure 4. Characterization of EC Phenotypes and Their Transcriptional Changes During EAE.
(A) DEGs fold changes across EC subtypes. Labels are shown for the overall top 10 DEGs. (B) Dot plot showing the enrichment for transcription factor targets among the sets of upregulated DEGs in EC subtypes. The color gradient corresponds to odds ratio, and the size of the dots shows the significance. (C) Description of DEG overlaps between the identified EC subtypes. Abbreviations: DEG = differentially expressed gene; EAE = experimental autoimmune encephalomyelitis; EC = endothelial cell.
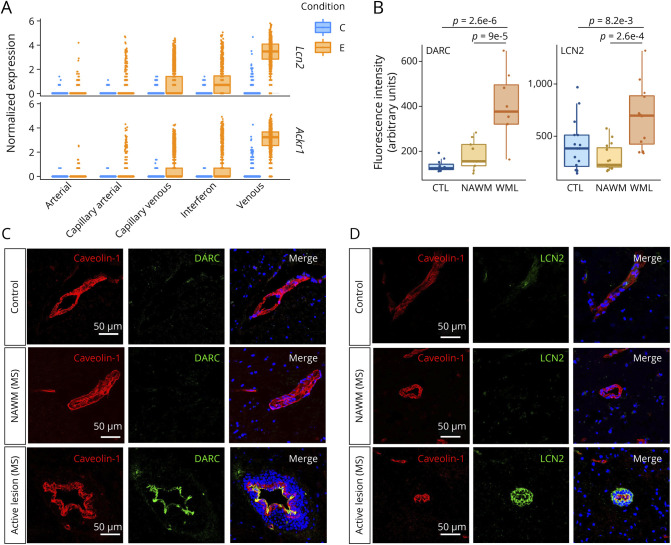
To verify the relevance of our observations in the context of MS, we validated protein levels of the top 2 DEGs (highest fold changes, Figure 5A) by immunofluorescence, comparing expression in human control brains and active MS brain lesions. We confirmed the elevated expression of ACKR1 (coding for the protein DARC, Figure 5, B and C) and LCN2 (coding for the protein lipocalin 2, Figure 5, B and D) on blood vessels in active MS lesions, compared with normal appearing white matter (NAWM) and with control brains. Thereby, this highlights that our results obtained in mice are relevant in the context of MS.
Figure 5. DARC and LCN2 Are Upregulated in Multiple Sclerosis (MS).
(A) Boxplots of the most significantly upregulated genes in venous ECs. C = control; E = EAE. (B) Quantification of LCN2 and DARC expression in human control and MS brains by confocal microscopy; n = 28–45 area from 3 MS and 3 control brains. One-way ANOVA with the Tukey post hoc test. (C) Immunofluorescence images of the protein expression of ACKR1 (DARC) in vessels from human healthy control, from normal appearing white matter (NAWM), and active lesion of patients with MS. Costainings for caveolin-1 (endothelial cell marker, red) and DARC (green). Merged images are presented in the right panels. Scale bar = 50 µm. (D) Immunohistochemistry with confocal microscopy of the duffy antigen/receptor for chemokines (DARC) and lipocalin-2 (LCN2, green) in vessels (caveolin-1, red) and nuclei (blue) of control brains, MS NAWM, and MS active lesions. Scale bar = 50 μm; data shown are representative of n = 3. Abbreviation: EAE = experimental autoimmune encephalomyelitis.
Expression of CAMs and tight junctions (TJs) is altered in MS and its animal models. While the expression of the TJs is generally reduced,44,45 the CAMs are mainly upregulated.1,5,46 However, it is still unclear where these modifications occur in the vascular bed. Thereby, we analyzed the gene expression of CAMs and TJs across the vascular bed. Using slingshot,47 we performed pseudotime analysis of the ECs that revealed gradual transition in the cells based on transcriptional variation (eFigure 7, A and B, links.lww.com/NXI/A780). The trajectory unraveled an arteriovenous axis going from arterial ECs to venous ECs. In accordance with the observation that venous ECs are the most affected subtype in neuroinflammation, venous ECs displayed the highest absolute fold changes for selected CAMs and TJs (eFigure 8, A and B). While CAMs were significantly upregulated in EAE compared with controls, the gene expression of TJs was generally downregulated. These results suggest that transmigration toward the CNS compartment would be particularly increased across venous ECs because under neuroinflammatory conditions, they exhibit a greater potential to mediate adhesion of other cell types such as infiltrating immune cells while their barrier properties are compromised.
scRNA-Seq Identifies Interactions Between Infiltrating Immune Cell Subsets and ECs
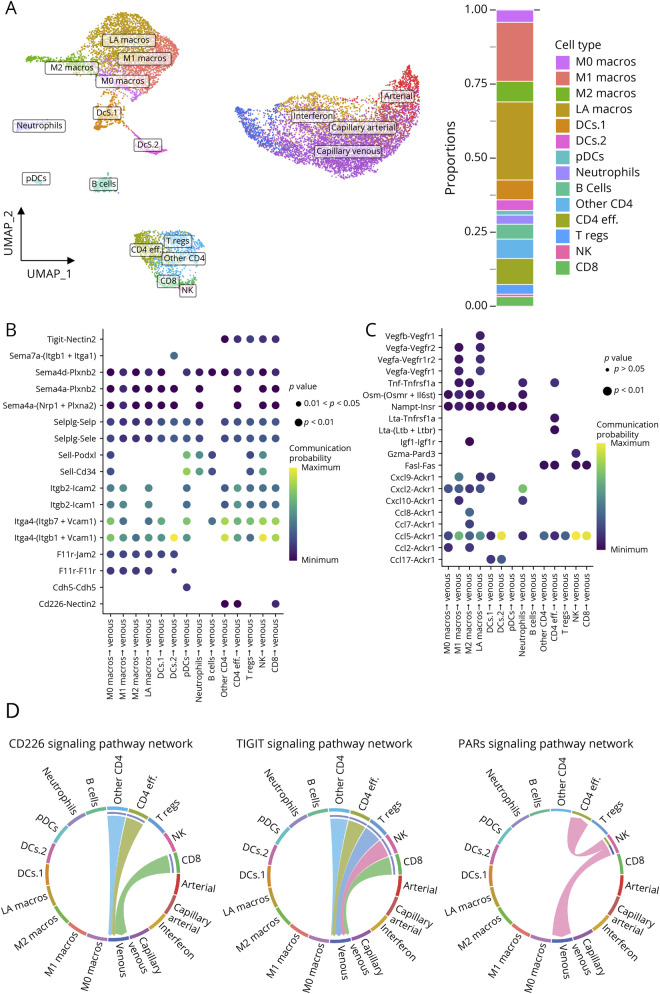
CD4 T lymphocytes are considered to play a pivotal role in MS and EAE, but the contribution of other innate and adaptive immune cells is crucial to the pathobiology of both. The scRNA-seq data we generated shows that at the peak of disease, brain infiltration consists of a mix of T and B lymphocytes, DCs, macrophages, neutrophils, and natural killer cells. To characterize in depth the immune cell types and their potential interactions with the endothelium, we merged the CD31-selected ECs and the infiltrated cells from EAE mice (Figure 6A). Most of the immune cells were found in EAE samples and were thus identified as infiltrated immune cells. They composed 2.34% (CTRL1), 1.97% (CTRL2), 2.68% (CTRL3), 41.78% (EAE1), 48.88% (EAE2), and 47.15% (EAE3) of all cells found in each sample. To define the cell types, we used previously described key signature genes: Ms4a1 for B lymphocytes, S100a8 for neutrophils, Nos2 and Il12 for M1 macrophages (M1 macros), Fabp5 and Trem2 for lipid-associated macrophages (LA macros), Cd163 and Mrc1 for M2 macrophages (M2 macros), Clec9a and Ccr7 for DCs, Ly6d for plasmacytoid DCs (pDCs), Cd8a for CD8+ cytotoxic T lymphocytes, and CD4 for the T-helper lymphocytes. To discriminate Th1/Th17 CD4 effector lymphocytes (CD4 eff), we used Il17a, Rorc, Tnf, and Ifng. The T lymphocytes negative for S100a4, Il17a, Rorc, Tnf, and Ifng were identified as other CD4 lymphocytes (other CD4). The IL2Ra+/Foxp3+ T lymphocytes were recognized as regulatory T lymphocytes (T regs). Finally, NK cells were defined by the expression of Ncr1 (eFigures 9 and 10A, links.lww.com/NXI/A780).
Figure 6. Identification of Brain Inflammatory Networks During EAE.
(A) Left: Uniform manifold approximation and projection (UMAP) from merged data of CD31-selected cells and infiltrated cells from EAE mice. Right: Proportions of immune subsets in EAE mice. (B) Dot plot summarizing probability of interaction for molecular pairs involved in potential cell-cell contact interactions. (C) Dot plot summarizing probability of interaction for molecular pairs involved in potential cell interactions from immune subsets targeting venous ECs through secreted signaling. (D) Circos plot showing the specific cell type and the directionality of the interaction pathways involving adhesion molecules and secreted cytokines used by immune cells and EC subtypes. Abbreviation: EAE = experimental autoimmune encephalomyelitis.
To infer specific cell-cell communication networks between immune cell types and ECs, we used CellChat,32 a set of tools that leverage a database describing known ligand-receptor interactions. Because venous ECs showed the greatest transcriptional alterations during neuroinflammation (635 DEGs found in venous ECs; 527 DEGs in interferon ECs), we focused our analysis on interactions involving venous ECs and immune cells (Figure 6 and eFigure 11, links.lww.com/NXI/A780). Because vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and P-selectin ligands are expressed by most immune cells, our analysis showed that these CAMs are potentially involved in the interaction between ECs and many of the immune subsets (Figure 6B). Interestingly, the molecular pair CD226-Nectin2 could potentially be involved in the specific interaction of CD8 lymphocytes and CD4 lymphocytes with the venous ECs. Moreover, the analysis of the CAMs revealed the Tigit-Nectin2 pair as a potential player in the interactions of CD4 T lymphocytes, CD8 lymphocytes, and NK cells with venous ECs (Figure 6, B and D and eFigure 10C).
Using the same strategy, we analyzed cytokine signaling between immune cell types and ECs. CCL chemokines were involved in the communications of immune cells and venous ECs through Ackr1 (Figure 6C, eFigure 11B.a, 11B.b, 11C, links.lww.com/NXI/A780). Ackr1, which is expressed specifically in the venous ECs (eFigures 10D and 11B.a, 11B.b), is known to bind to a broad range of inflammatory chemokines of the CCL family. Of interest, Ccl7 and Ccl8 are mainly expressed by the M2 macrophages and could be involved in their communication with venous ECs (Figure 6C and eFigure 11B.a). Furthermore, the analysis revealed the couple granzyme-A (Gzma)-Pard3 as a potential vector of interaction between NK cells and veinous ECs (Figure 6, C and D). Overall, our results will help to identify potential pathways underlying interactions involved in the development of neuroinflammation. Notably, cell types of the same lineage (ECs, lymphocytes, and myeloid cells) displayed specific patterns of outgoing and incoming interactions, concordant with their known biological functions (eFigure 12).
Because we detected immune cells in control mice, we performed our analysis to identify possible interactions involved in immune surveillance (eFigure 13, links.lww.com/NXI/A780). Most of the immune cells found in control mice were macrophages (eFigure 13A.a) and were identified as perivascular macrophages because they express Lyve1, Mrc1, and Cd163 (eFigure 13A.b). Of interest, Cd163+/Mrc1+ macrophages found in EAE mice were transcriptionally different from those found in control mice because they upregulate genes associated to antigen presentation and immune response (eFigure 13B.a and B.b). Analysis of cell-cell interaction revealed that resident macrophages are susceptible to interact with all EC subtypes (eFigure 13C). Leveraging the highly heterogeneous nature of single-cell RNA-Seq data, we were able to identify specific brain inflammatory networks of cell communication based on the expression of referenced genes across distinct cell types.
Discussion
This study reports single-cell profiling of the CNS in EAE. CNS-resident cells associated with neuroinflammation showed a global state of activation characterized by upregulation of genes involved in antigen presentation. Among the various resident cells, the largest transcriptional changes were found in microglia, MOLs, ECs, and astrocytes. This observation is coherent with the vascular activation characteristic of EAE, leading to immune cell infiltration, glial activation, and oligodendrocyte damage.
Using CD31-positive cell selection, we were able to investigate ECs at a high resolution, allowing the annotation of arteriovenous zonation based on known molecular markers.30,31 The use of EC enrichment based on CD31 expression allowed to increase the proportion of ECs, but a substantial number of immune cells was still present in our sample after the selection. Our hypothesis is that these immune cells were adherent to ECs or have been captured by the column during the selection. Indeed, although ECs are the cell type expressing the highest level of CD31, some immune cells can also express it. To investigate the arteriovenous axis, we removed the immune cells to keep only the cells identified as ECs. As previously described in the murine brain, a population of ECs expressing interferon-signaling genes was found in all mice. Of interest, the ECs from control and EAE mice displayed significant transcriptional differences. The largest transcriptional changes were found in venous ECs, followed by interferon ECs. Moreover, we demonstrated that the main CAMs involved in immune cell infiltration across the BBB displayed larger upregulation in venous ECs when compared with other arteriovenous subtypes. This finding suggests that the enhanced adhesive properties enabling immune transendothelial migration are more prominent on the venous side of the arteriovenous zonation. However, it is important to note that we were also able to detect an upregulation of the main CAMs in interferon ECs, suggesting that they could also support immune cell trafficking into the brain parenchyma. In addition, TJs were downregulated in EAE and mostly associated with venous ECs. Thus, these results provide molecular features underlying the fact that MS lesions are often characterized by the presence of a central vein inside white matter lesions.48,49
To confirm that our findings are relevant in the context of MS, we validated protein expression in human brain tissue of the 2 most DEGs identified in mouse venous ECs. Thus, upregulation of DARC and LCN2 in human vasculature were validated by immunostaining in human control, NAWM, and active lesions of patients with MS. Of interest, the staining for DARC was restricted to the vessel, while LCN2 was also present in perivascular cells in MS lesions. This observation is in accordance with our scRNA-seq data showing that Ackr1 expression is almost restricted to ECs, while Lcn2 expression can also be found in immune cells. These results confirm the relevance of these 2 molecules, not only in EAE, but also in the context of MS.
Immune cell migration from peripheral blood into the brain parenchyma is crucial in the development of MS. Natalizumab and efalizumab, 2 clinically effective monoclonal antibodies against the integrin α4 and αL (the ligands of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, respectively), act through the reduction of immune cell infiltration across the vascular wall. Efalizumab was used to treat psoriasis, and natalizumab is still used to treat relapsing-remitting MS. However, these 2 antibodies can impair brain immunosurveillance.50 Finding new mediators of infiltration that might be more specific to pathogenic immune subtypes could be promising in the development of safer treatments by reducing off-target effects. We identified CD226, an adhesion molecule expressed by CD8+ and CD4+ lymphocytes. Our analysis shows that nectin-2, one of the ligands of CD226, is detected only in the venules. Because venules are known to be the main site of lesion formation in MS,49 we hypothesize that the study of nectin-2 could be useful in understanding the molecular mechanisms leading to MS progression. Contrarily to CD226, we found that T-cell immunoglobulin and ITIM domain (TIGIT), another ligand for nectin-2, is rather expressed on regulatory T lymphocytes, in line with previous reports showing that TIGIT is expressed on regulatory T lymphocytes and competes with CD226 for their shared ligands.51,52
Taken together, these results support the role of venous ECs to be deeply involved in EAE pathophysiology. Venous ECs not only control immune cell trafficking through the expression of CAMs but can also act as immune stimulators or inhibitors through the expression of cell-specific cytokines/chemokines and receptors. Moreover, our study demonstrates, in accordance with previous studies, that ECs upregulate genes associated with antigen presentation,53-55 confirming observations that ECs are capable of enhancing leukocyte recruitment through antigen presentation. This observation is in accordance with the emerging evidence that ECs, by their pivotal location (border between blood and brain parenchyma), are involved in the regulation of neuroinflammation.56-58
We chose to use the MOG-induced EAE model to understand the alterations in gene expression in the CNS during neuroinflammation. This model recapitulates the inflammatory component of MS and has been used to develop treatments for MS.59 A limitation of this study is the use of only 1 specific time point during EAE, e.g., peak of disease. We chose this time point because the vascular activation and the immune cell infiltration in the CNS are maximal at the peak of the disease.5,60 The use of the MOG(35-55)–induced EAE, which is a very stable model, allowed us to use fewer animals in this study. However, despite the use of a limited number of mice, we were able to achieve a sufficient number of cells for a thorough analysis of the transcriptomic changes occurring during neuroinflammation. In addition, while EAE is a good model to explore inflammatory mechanisms underlying neuroinflammation, the relevance of the identified candidates for MS requires further validation using human samples, as we and other studies have previously demonstrated for DARC and LCN2.61,62 Moreover, we cannot exclude that some brain cell subtypes are more sensitive than others to tissue dissociation protocols, which could induce immediate stress responses, thus affecting gene expression or introducing a cell capture bias. We speculate that, due to their morphology with numerous branches and ramifications, neurons and astrocytes are more sensitive to tissue dissociation and therefore that most are lost before sequencing. Furthermore, we cannot rule out that inflammation in EAE animals may increase the sensitivity of these cells to the digestion protocol compared with control animals. Finally, given their scarcity in control animals, differential expression analysis within all infiltrating immune cell types was not possible. Future studies investigating the gene expression differences between control peripheral immune cells and these infiltrating populations would allow more insight into the profile of immune CNS infiltrates in diseased animals.
In conclusion, this study successfully leveraged single-cell transcriptomics to explore the inflammatory interplay across brain and immune cells involved in neuroinflammation. This atlas of gene expression signatures and interaction networks provides useful information by which the resident cells of the brain react to inflammation and how the ECs and immune cells interact with each other to modulate their activation. This will be a valuable resource for the investigation of new targets to modulate neuroinflammation in the context treatments for neurologic diseases such as MS.
Acknowledgment
The authors are grateful to the CRCHUM's animal facility led by H. Héon, imaging platform led by A. Cleret-Buhot, and their entire staff for their expertise and enthusiastic support throughout this project.
Glossary
- BBB
blood-brain barrier
- CAM
cell adhesion molecule
- DC
dendritic cell
- DEG
differentially expressed gene
- EAE
experimental autoimmune encephalomyelitis
- EC
endothelial cell
- MOG
myelin oligodendrocyte glycoprotein
- NAWM
normal appearing white matter
- NK
natural killer
- scRNA-seq
single-cell RNA sequencing
- TJ
tight junction
- UMAP
Uniform Manifold Approximation and Projection
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
No disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011;585(23):3770-3780. doi: 10.1016/j.febslet.2011.04.066 [DOI] [PubMed] [Google Scholar]
- 3.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12(9):623-635. doi: 10.1038/nri3265 [DOI] [PubMed] [Google Scholar]
- 4.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol. 2012;33(12):579-589. doi: 10.1016/j.it.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 5.Fournier AP, Quenault A, Martinez de Lizarrondo S, et al. Prediction of disease activity in models of multiple sclerosis by molecular magnetic resonance imaging of P- selectin. Proc Natl Acad Sci USA. 2017;114(23):6116-6121. doi: 10.1073/pnas.1619424114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Louzi O, Letchuman V, Manukyan S, et al. Central vein sign profile of newly developing lesions in multiple sclerosis: a 3-year longitudinal study. Neurol Neuroimmunol Neuroinflammation. 2022;9(2):e1120. doi: 10.1212/NXI.0000000000001120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legroux L, Arbour N. Multiple sclerosis and T lymphocytes: an entangled story. J Neuroimmune Pharmacol. 2015;10(4):528-546. doi: 10.1007/s11481-015-9614-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233-240. doi: 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KHG. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162(1):1-11. doi: 10.1111/j.1365-2249.2010.04143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlén C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194(5):669-676. doi: 10.1084/jem.194.5.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu P, Gregg RK, Bell JJ, et al. Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. J Immunol. 2005;174(11):6772-6780. doi: 10.4049/jimmunol.174.11.6772 [DOI] [PubMed] [Google Scholar]
- 12.Stephens LA, Malpass KH, Anderton SM. Curing CNS autoimmune disease with myelin-reactive Foxp3+ Treg. Eur J Immunol. 2009;39(4):1108-1117. doi: 10.1002/eji.200839073 [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto Y, Kohyama K, Aikawa Y, et al. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28(5):1681-1688. doi: [DOI] [PubMed] [Google Scholar]
- 14.Høglund RA, Maghazachi AA. Multiple sclerosis and the role of immune cells. World J Exp Med. 2014;4(3):27-37. doi: 10.5493/wjem.v4.i3.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Sanai N, Jin WN, La Cava A, Van Kaer L, Shi FD. Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat Neurosci. 2016;19(2):243-252. doi: 10.1038/nn.4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676-688. doi: 10.1056/NEJMoa0706383 [DOI] [PubMed] [Google Scholar]
- 17.Al-Ani MR, Raju TK, Hachim MY, et al. Rituximab prevents the development of experimental autoimmune encephalomyelitis (EAE): comparison with prophylactic, therapeutic or combinational regimens. J Inflamm Res. 2020;13:151-164. doi: 10.2147/JIR.S243514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ifergan I, Kébir H, Bernard M, et al. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain. 2008;131(Pt 3):785-799. doi: 10.1093/brain/awm295 [DOI] [PubMed] [Google Scholar]
- 19.Nuyts AH, Lee WP, Bashir-Dar R, Berneman ZN, Cools N. Dendritic cells in multiple sclerosis: key players in the immunopathogenesis, key players for new cellular immunotherapies?. Mult Scler Houndmills Basingstoke Engl. 2013;19(8):995-1002. doi: 10.1177/1352458512473189 [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Wang J, Wang J, Yang B, Weng Q, He Q. Targeting microglia and macrophages: a potential treatment strategy for multiple sclerosis. Front Pharmacol. 2019;10:286. doi: 10.3389/fphar.2019.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponath G, Park C, Pitt D. The role of astrocytes in multiple sclerosis. Front Immunol. 2018;9:217. doi: 10.3389/fimmu.2018.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voet S, Prinz M, van Loo G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol Med. 2019;25(2):112-123. doi: 10.1016/j.molmed.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 23.Wheeler MA, Quintana FJ. Regulation of astrocyte functions in multiple sclerosis. Cold Spring Harb Perspect Med. 2019;9(1):a029009. doi: 10.1101/cshperspect.a029009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jäkel S, Agirre E, Mendanha Falcão A, et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature. 2019;566(7745):543-547. doi: 10.1038/s41586-019-0903-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falcão AM, van Bruggen D, Marques S, et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat Med. 2018;24(12):1837-1844. doi: 10.1038/s41591-018-0236-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler MA, Clark IC, Tjon EC, et al. MAFG-driven astrocytes promote CNS inflammation. Nature. 2020;578(7796):593-599. doi: 10.1038/s41586-020-1999-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schirmer L, Velmeshev D, Holmqvist S, et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature. 2019;573(7772):75-82. doi: 10.1038/s41586-019-1404-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordão MJC, Sankowski R, Brendecke SM, et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363(6425):eaat7554. doi: 10.1126/science.aat7554 [DOI] [PubMed] [Google Scholar]
- 29.Masuda T, Sankowski R, Staszewski O, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566(7744):388-392. doi: 10.1038/s41586-019-0924-x [DOI] [PubMed] [Google Scholar]
- 30.Vanlandewijck M, He L, Mäe MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554(7693):475-480. doi: 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- 31.Kalucka J, de Rooij LPMH, Goveia J, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180(4):764-779.e20. doi: 10.1016/j.cell.2020.01.015 [DOI] [PubMed] [Google Scholar]
- 32.Jin S, Guerrero-Juarez CF, Zhang L, et al. Inference and analysis of cell-cell communication using CellChat. Nat Commun. 2021;12(1):1088. doi: 10.1038/s41467-021-21246-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888-1902.e21. doi: 10.1016/j.cell.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T, Li B, Nelson CE, Nabavi S. Comparative analysis of differential gene expression analysis tools for single-cell RNA sequencing data. BMC Bioinformatics. 2019;20(1):40. doi: 10.1186/s12859-019-2599-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bindea G, Mlecnik B, Hackl H, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinforma Oxf Engl. 2009;25(8):1091-1093. doi: 10.1093/bioinformatics/btp101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finak G, McDavid A, Yajima M, et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 2015;16:278. doi: 10.1186/s13059-015-0844-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangiola S, Doyle MA, Papenfuss AT. Interfacing Seurat with the R tidy universe. Bioinforma Oxf Engl. 2021;37(2):4100-4107. doi: 10.1093/bioinformatics/btab404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol (Berl). 2017;133(1):13-24. doi: 10.1007/s00401-016-1653-y [DOI] [PubMed] [Google Scholar]
- 40.Dhaeze T, Tremblay L, Lachance C, et al. CD70 defines a subset of proinflammatory and CNS-pathogenic TH1/TH17 lymphocytes and is overexpressed in multiple sclerosis. Cell Mol Immunol. 2019;16(7):652-665. doi: 10.1038/s41423-018-0198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becht E, McInnes L, Healy J, et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. 2019;37:38-44. 10.1038/nbt.4314 [DOI] [PubMed] [Google Scholar]
- 42.Li T, Chen X, Zhang C, Zhang Y, Yao W. An update on reactive astrocytes in chronic pain. J Neuroinflammation. 2019;16(1):140. doi: 10.1186/s12974-019-1524-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasel P, Rose IVL, Sadick JS, Kim RD, Liddelow SA. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat Neurosci. 2021;24(10):1475-1487. doi: 10.1038/s41593-021-00905-6 [DOI] [PubMed] [Google Scholar]
- 44.Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol. 2015;209(4):493-506. doi: 10.1083/jcb.201412147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsson A, Gustavsen S, Langkilde AR, et al. Circulating levels of tight junction proteins in multiple sclerosis: association with inflammation and disease activity before and after disease modifying therapy. Mult Scler Relat Disord. 2021;54:103136. doi: 10.1016/j.msard.2021.103136 [DOI] [PubMed] [Google Scholar]
- 46.Rossi B, Angiari S, Zenaro E, Budui SL, Constantin G. Vascular inflammation in central nervous system diseases: adhesion receptors controlling leukocyte-endothelial interactions. J Leukoc Biol. 2011;89(4):539-556. doi: 10.1189/jlb.0710432 [DOI] [PubMed] [Google Scholar]
- 47.Street K, Risso D, Fletcher RB, et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics. 2018;19(1):477. doi: 10.1186/s12864-018-4772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12(12):714-722. doi: 10.1038/nrneurol.2016.166 [DOI] [PubMed] [Google Scholar]
- 49.Maggi P, Absinta M, Grammatico M, et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies: central Vein Sign. Ann Neurol. 2018;83(2):283-294. doi: 10.1002/ana.25146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carson KR, Focosi D, Major EO, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009;10(8):816-824. doi: 10.1016/S1470-2045(09)70161-5 [DOI] [PubMed] [Google Scholar]
- 51.Gagliani N, Magnani CF, Huber S, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19(6):739-746. doi: 10.1038/nm.3179 [DOI] [PubMed] [Google Scholar]
- 52.Piédavent-Salomon M, Willing A, Engler JB, et al. Multiple sclerosis associated genetic variants of CD226 impair regulatory T cell function. Brain. 2015;138(11):3263-3274. doi: 10.1093/brain/awv256 [DOI] [PubMed] [Google Scholar]
- 53.Lopes Pinheiro MA, Kamermans A, Garcia-Vallejo JJ, et al. Internalization and presentation of myelin antigens by the brain endothelium guides antigen-specific T cell migration. eLife. 2016;5:e13149. doi: 10.7554/eLife.13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bagai R, Valujskikh A, Canaday DH, et al. Mouse endothelial cells cross-present lymphocyte-derived antigen on class I MHC via a TAP1- and proteasome-dependent pathway. J Immunol. 19502005;174(12):7711-7715. doi: 10.4049/jimmunol.174.12.7711 [DOI] [PubMed] [Google Scholar]
- 55.Howland SW, Poh CM, Rénia L. Activated brain endothelial cells cross-present malaria antigen. PLoS Pathog. 2015;11(6):e1004963. doi: 10.1371/journal.ppat.1004963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young MR. Endothelial cells in the eyes of an immunologist. Cancer Immunol Immunother CII. 2012;61(10):1609-1616. doi: 10.1007/s00262-012-1335-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carman CV, Martinelli RT. Lymphocyte-endothelial interactions: emerging understanding of trafficking and antigen-specific immunity. Front Immunol. 2015;6:603. doi: 10.3389/fimmu.2015.00603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shao Y, Saredy J, Yang WY, et al. Vascular endothelial cells and innate immunity. Arterioscler Thromb Vasc Biol. 2020;40(6):e138–e152. doi: 10.1161/ATVBAHA.120.314330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011;164(4):1079-1106. doi: 10.1111/j.1476-5381.2011.01302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montagne A, Gauberti M, Macrez R, et al. Ultra-sensitive molecular MRI of cerebrovascular cell activation enables early detection of chronic central nervous system disorders. NeuroImage. 2012;63(2):760-770. doi: 10.1016/j.neuroimage.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 61.Minten C, Alt C, Gentner M, et al. DARC shuttles inflammatory chemokines across the blood–brain barrier during autoimmune central nervous system inflammation. Brain. 2014;137(5):1454-1469. doi: 10.1093/brain/awu045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berard JL, Zarruk JG, Arbour N, et al. Lipocalin 2 is a novel immune mediator of experimental autoimmune encephalomyelitis pathogenesis and is modulated in multiple sclerosis. Glia. 2012;60(7):1145-1159. doi: 10.1002/glia.22342 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is freely shared via two interactive R shiny applications available at https://pratlab.shinyapps.io/EAEbrain_10X (total cells) and https://pratlab.shinyapps.io/EAEbrain_CD31 (CD31-selected cells). ScRNA-seq raw data (FASTQ files) are available to investigators via the GEO accession number GSE199460 (Tables 1–8, links.lww.com/NXI/A772, links.lww.com/NXI/A773, links.lww.com/NXI/A774, links.lww.com/NXI/A775, links.lww.com/NXI/A776, links.lww.com/NXI/A777, links.lww.com/NXI/A778, links.lww.com/NXI/A779, respectively).