Abstract
Background and Objectives
To clinically characterize post–immune checkpoint inhibitor (ICI) Hu antibody (Ab) neurologic disorders, we analyzed Hu-Ab–positive patients with neurologic immune-related adverse events (n-irAEs) and compared them with patients with other n-irAEs, ICI-naive patients with Hu-Ab paraneoplastic neurologic syndromes (PNSs) identified in the same study center, and those with Hu-Ab n-irAEs reported elsewhere.
Methods
Patients whose samples were sent to the French reference center for a suspicion of n-irAE (2015–2021) were identified; those with a final diagnosis of n-irAE and Hu-Ab were included. Control groups included patients with a final diagnosis of n-irAE occurring during the same period as the patients included (2018–2021) but without Hu-Ab, and ICI-naive patients with Hu-Ab PNS diagnosed during the same period; a systematic review was performed to identify previous reports.
Results
Eleven patients with Hu-Ab and n-irAEs were included (median age, 66 years, range 44–76 years; 73% men). Ten patients had small cell lung cancer, and 1 had lung adenocarcinoma. The median follow-up from onset was 3 months (range 0.5–18 months). Compared with those with other n-irAEs (n = 63), Hu-Ab–positive patients had more frequently co-occurring involvement of both central and peripheral nervous systems (36% vs 8%, p = 0.02) and limbic (54% vs 14%, p < 0.01), brainstem (27% vs 5%, p = 0.02), and dorsal root ganglia (45% vs 5%, p < 0.01) involvement. The proportion of patients with severe disability (modified Rankin Scale score >3) at diagnosis was higher among Hu-Ab n-irAEs (91% vs 52%, p = 0.02). Patients with Hu-Ab had also poorer outcome (100% vs 28%, p < 0.01) and higher mortality (91% vs 46%, p < 0.01). There was no significant difference in terms of clinical features between Hu-Ab n-irAEs and ICI-naive Hu-Ab PNS (n = 92), but there was a poorer outcome (56/78, 71%, p < 0.01) and higher mortality (26%, p < 0.01) among the former. No significant difference was found between the patients reported herein and those in the literature.
Discussion
The presence of Hu-Ab identifies a subgroup of n-irAEs that consistently reproduce the phenotypes of Hu-Ab-related PNS, supporting the hypothesis of ICI triggering or unmasking PNS. As these patients show high disability and mortality, further studies are required to investigate the underlying immunopathogenic mechanisms and to improve the outcome of Hu-Ab n-irAEs.
Immune checkpoint inhibitors (ICIs) target crucial regulators of the immune system that provide self-tolerance under physiologic conditions but are exploited by cancer cells to escape immune surveillance.1 Although ICIs have been shown to be effective for the treatment of certain cancer types, their expanding use has resulted in an increasing number of reports of immune-related adverse events (irAEs),2 and neurologic manifestations represent a rare yet relevant part of this spectrum.3 Neurologic syndromes observed in irAEs (n-irAEs) are extremely heterogeneous, probably reflecting different underlying pathogenic mechanisms.4-6 Among these, the antitumor immune response stimulated by ICI might cross-react with neural autoantigens, resembling the classical mechanism of paraneoplastic autoimmunity.5,7 Hu antibodies (Abs) are the most frequent neural Abs in paraneoplastic neurologic syndromes (PNSs)8,9 and well-recognized biomarkers of an underlying small cell lung cancer (SCLC).10 Although all these tumors overexpress the Hu antigen, only 16–22.5% of patients with SCLC harbor low titers of Hu-Ab,11-13 and a minority of 1–3% develop PNS.14,15 The clinical picture of PNS associated with Hu-Ab is highly pleomorphic, with subacute sensory neuronopathy (SNN) being the most frequent presentation at diagnosis.10 Of interest, Hu-Abs have recently been reported in a few case reports of n-irAEs, but the neurologic disorders of patients with Hu-Ab post-ICI have not thoroughly been described. To clinically characterize post-ICI Hu-Ab neurologic disorders, we analyzed a cohort of patients with n-irAEs who tested positive for Hu-Ab at the French reference center for autoimmune encephalitis and PNS, and we compared their characteristics with those of patients with other n-irAEs and ICI-naive patients with Hu-Ab PNS identified in the same study center. We also performed a systematic review of the literature to analyze previously reported patients with Hu-Ab n-irAEs, and we compared their features with those of patients identified in the study center.
Methods
Patients
All consecutive patients whose samples were sent to the French reference center for autoimmune encephalitis and PNS for a suspicion of n-irAE that occurred between January 1, 2015, and December 31, 2021, were identified; those with a final diagnosis of n-irAE and Hu-Ab positivity in serum and/or CSF were included. Two distinct control groups were identified. The first was composed of patients with a final diagnosis of n-irAE occurring during the same period as the patients included (2018–2021) but without Hu-Ab. The second was composed of ICI-naive patients with Hu-Ab PNS diagnosed during the same period. Patients with worsening of a previously known Hu-Ab-associated PNS after ICI therapy were also included but analyzed separately.
Hu-Abs were tested using an indirect immunofluorescence assay on rat brain sections and further confirmed by dot blot analysis on recombinant proteins (EUROLINE PNS 12 Ag; Euroimmun, Lübeck, Germany) and an in-house cell-based assay and/or Western blot. The diagnosis of n-irAE was established for cases with neurologic symptoms occurring no later than the 12 months after the last ICI dose and a comprehensive exclusion of alternative diagnoses.3
Clinical and paraclinical data (CSF analysis, nerve conduction studies [NCSs], and MRI of the brain and/or spinal cord) were retrospectively extracted from medical reports; treating physicians were contacted in case of missing information. The clinical syndromes were classified according to the updated PNS criteria10 and the anatomic area of involvement (limbic, cerebellar, brainstem, diencephalic, basal ganglia, meningeal, spinal cord, cranial nerves, dorsal root ganglia [DRG], roots and peripheral nerves, neuromuscular junction, muscle, and/or myenteric plexus). The definition of definite, probable, possible, and non-PNS was based on the PNS-Care score.16 Inflammatory CSF was defined as the presence of pleocytosis (>5/mm3), high protein content (>0.6 g/L), and/or CSF-restricted oligoclonal bands. The neurologic disability was evaluated using the modified Rankin Scale (mRS) at the time of diagnosis and at last visit. Severe neurologic disability was defined as mRS score >3. Poor outcome was defined as residual severe neurologic disability at last follow-up. Cancer response to ICI treatment was based on oncologic reports.
Literature Review
We performed a systematic review of the literature and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Individual Patient Data systematic reviews reporting guidelines (eFigure 1, links.lww.com/NXI/A768). We searched MEDLINE (PubMed) for records published in the English language between January 1, 2011 (year of the first approval of ICI), and April 1, 2022, using the following search terms (alone or joined in logical combinations): “Hu antibodies,” “paraneoplastic neurological syndrome,” “ANNA-1,” “neurologic immune related adverse event,” “neurological irAE,” “neurological complication,” “toxicity,” “immune checkpoint inhibitor,” “anti-CTLA4,” “anti-PD1,” “anti-PDL1,” “atezolizumab,” “nivolumab,” “pembrolizumab,” “avelumab,” “durvalumab,” “ipilimumab,” “tremelimumab,” “cemiplimab,” “encephalitis,” “neuropathy,” and “encephalomyelitis.” We also manually examined the reference lists of included studies to identify possibly missed articles. Only patients in whom clinical information was assessable at an individual patient level were included. The following data were collected: demographic characteristics, type of cancer, ICI used, neurologic presentation, as well as CSF, NCS, and MRI findings, and treatment of the neurologic syndrome, and outcome.
Statistical Analysis
Data are presented as frequencies and percentages for qualitative variables and as median and range for quantitative variables. Comparisons between groups were made using the Fisher exact test (2 tailed) for qualitative variables and the Mann-Whitney U test for quantitative variables. Statistical analyses were performed using R, version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria). All p values were 2 tailed, and p values <0.05 were considered statistically significant.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Institutional Review Board of Université Claude Bernard Lyon 1 and Hospices Civils of Lyon (69HCL21-474) and the National Data Protection Commission (Commission Nationale de l’Informatique et des Libertés; CNIL, 21-5474). Written information was sent to all patients, and none of them explicitly opposed their participation in the study.
Data Availability
Data reported in this study are available within the article and/or its supplementary material. More information regarding the data will be shared by the corresponding author on reasonable request.
Results
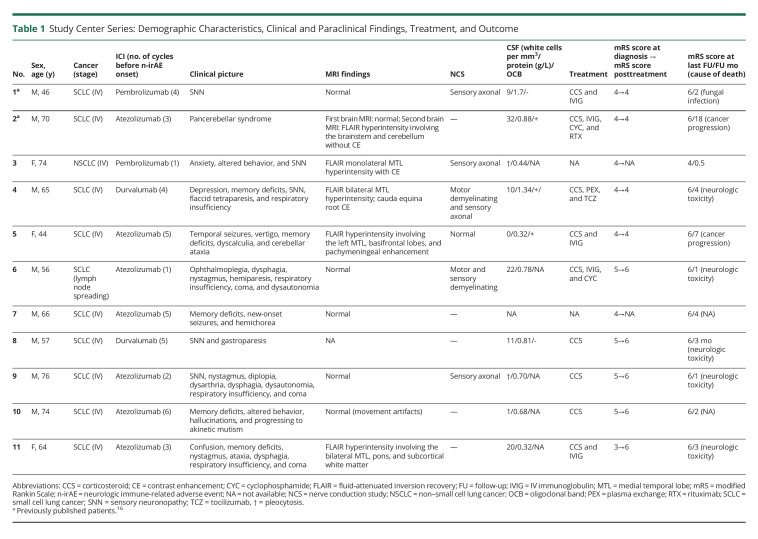
Among the 86 patients with an n-irAE during the study period, 12 patients (14%) had Hu-Ab (Figure 1). Among the latter, 11 developed the neurologic syndrome after ICI administration; 1 patient was already diagnosed with a Hu-Ab–associated PNS before ICI introduction and therefore analyzed separately (see below). All patients were diagnosed in 2018 (1/12, 8%) or thereafter (11/12, 92%). Two of the 11 patients (patient 1 and patient 2) were previously published,17 but new follow-up information is provided for 1 of them (Table 1).
Figure 1. Flowchart Presenting the Selection Process of Patients From the Study Center Database.
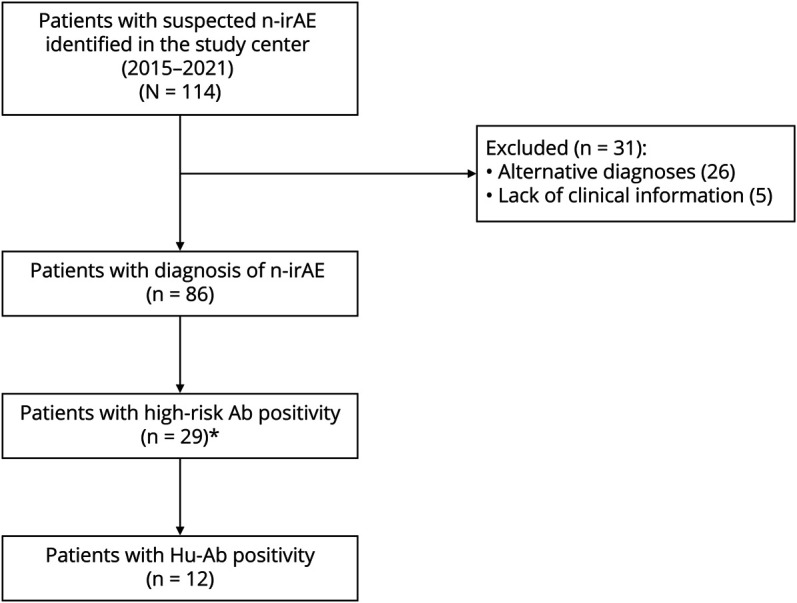
*High-risk Abs other than Hu included Ma2 (n = 9), Yo (n = 3), SOX1 (n = 2), Ri (n = 1), and CV2/CRMP5 (n = 1). In 2 patients, coexistent Hu-Ab and, respectively, SOX1 and CRMP5/CV2 were present. Ab = antibody; N-irAE = neurologic immune-related adverse event.
Table 1.
Study Center Series: Demographic Characteristics, Clinical and Paraclinical Findings, Treatment, and Outcome
Demographic Characteristics and Clinical Presentation of Hu Antibody n-irAEs
The median age at clinical onset was 66 years (range 44–76 years), and 8/11 (73%) were men. Eight of the 9 (89%) patients with available information were heavy smokers. Ten (10/11, 91%) patients had SCLC, and 1 had lung adenocarcinoma. In all but 1 patient, the cancer was metastatic at the onset of neurologic symptoms. All patients were treated with anti-PD1 or anti-PDL1 monoclonal antibodies, and none with anti-CTLA4 antibodies. ICI was the first-line treatment in all but 1 patient (91%); 10/11 (91%) patients also received chemotherapy, 1/11 (9%) prophylactic cranial irradiation, and 1/11 (9%) brain metastasis surgery; the frequency of neurotoxic treatments did not significantly differ from ICI-naive Hu-PNS patients with onset of neurologic symptoms after cancer diagnosis (n = 10, eTable 1, links.lww.com/NXI/A768). Cancer response to ICI was observed in 8/9 (89%) patients with available data. Neurologic manifestations appeared after a median of 62 days (range 10–147 days) and 4 cycles (range 1–6 cycles) from ICI initiation. Concomitant non-neurologic irAEs were reported in 2/11 (18%, including pneumonitis and thyroiditis, 1 case each). Neurologic dysfunction was confined to 1 area of the nervous system in 3/11 (27%) patients (SNN; rapidly progressive cerebellar ataxia; limbic encephalitis [LE]), and there was evidence of multifocal involvement in 8/11 (73%) patients (LE and SNN; LE and cerebellar ataxia; LE, brainstem encephalitis, and cerebellar ataxia; LE, demyelinating polyradiculoneuropathy, and SNN; LE and hemichorea; brainstem encephalitis and SNN; brainstem encephalitis and demyelinating polyradiculoneuropathy; and SNN and enteric neuropathy). Three of the 4 patients (75%) with SNN received platinum compounds (median number of cycles 3, range 2–4) before the onset of symptoms; all of them had CSF pleocytosis. All (3/3) patients with brainstem encephalitis developed respiratory insufficiency and coma, and 2 of them had also dysautonomia. MRI showed inflammatory lesions in 5/10 patients (50%), mainly in those with LE (4/6, 67%). Brain and/or spine MRI detected lesions in 5/10 (50%) patients, including T2/FLAIR hyperintensity of medial temporal lobes (4/10, 40%), brainstem (2/10, 20%), cerebellum (1/10, 10%), and/or subcortical white matter (1/10, 10%), pachymeningeal enhancement (1/10, 10%), and cauda equina nerve root contrast enhancement (1/10, 10%; Figure 2). One patient with LE had additional SOX1 antibodies. CSF was inflammatory in all (10/10) patients with available data.
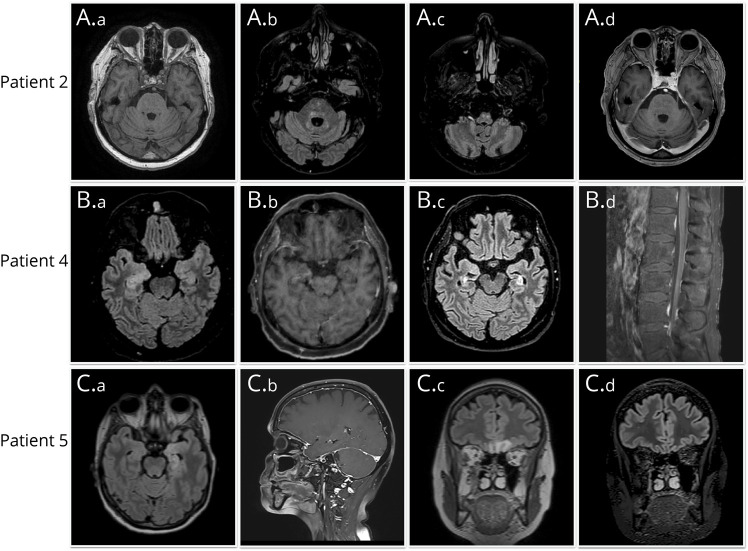
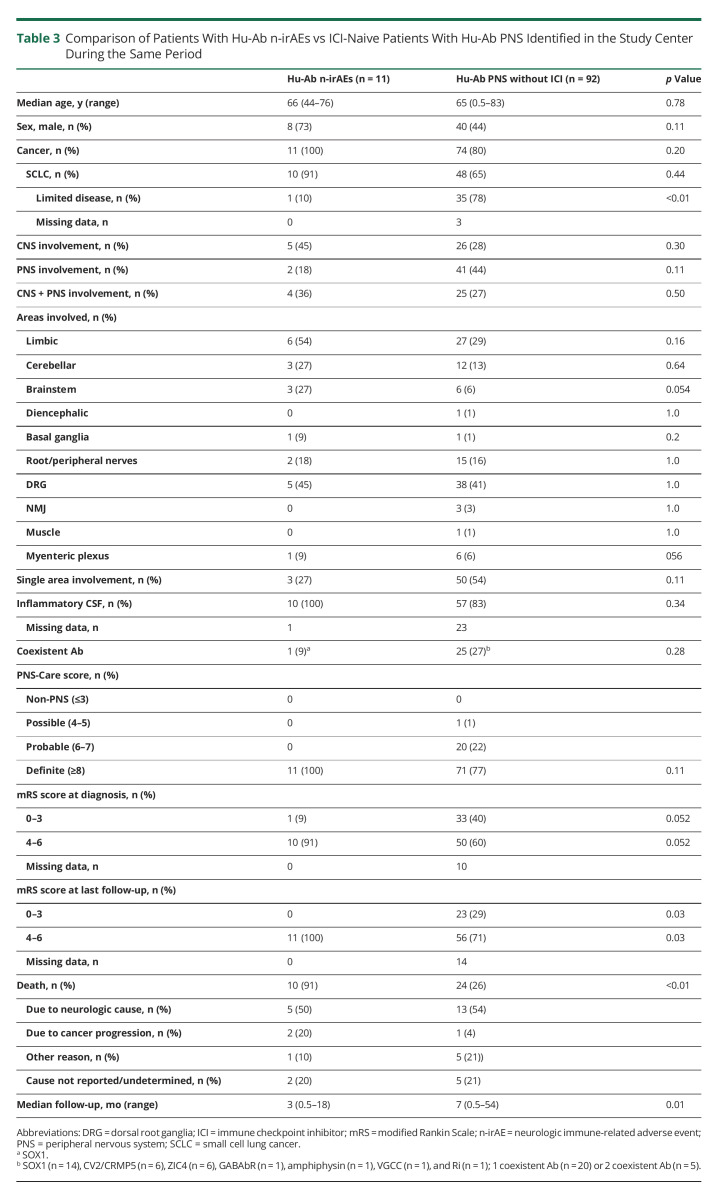
Figure 2. Representative Neuroimaging Findings.
(A) Patient 2: onset, a) mild cerebellar atrophy. Follow-up, b) hyperintense signal involving the dentate nuclei and periventricular brainstem regions; c) hyperintense signal involving the anterior medulla; d) moderate cerebellar atrophy. (B) Patient 4: onset, a) T2-hyperintense signal involving the bilateral medial temporal lobes; b) contrast enhancement involving the right medial temporal lobe. Follow-up, c) moderate atrophy of the medial temporal lobes; d) cauda equina root contrast enhancement. (C) Patients 5: onset, a) T2-hyperintense signal involving the left medial temporal lobe; b) diffuse pachymeningeal enhancement; c) T2-hyperintense signal involving both orbitofrontal lobes. Follow-up, d) regression of the orbitofrontal lobe T2-hyperintense signal.
Treatment and Outcome of Hu Antibody n-irAEs
ICI was permanently withdrawn in all patients. Treatment for n-irAEs included corticosteroids (7/9, 78%), IV immunoglobulins (IVIGs; 4/9, 44%), cyclophosphamide (2/9, 22%), plasma exchange (1/8, 12%), rituximab (1/8, 12%), and tocilizumab (1/8, 12%), alone or in combination. At diagnosis, all but 1 patient had severe neurologic disability (mRS score >3). No patient improved after treatment, despite evidence of biological effect in the patient treated with tocilizumab (CSF interleukin 6 [IL6]; pre-tocilizumab 56 pg/mL, CSF IL6 post-tocilizumab 15.1 pg/mL, normal values 1–4 pg/mL). Only 1 patient (patient 11) experienced a transitory improvement after IVIG administration, but soon after, she rapidly worsened with a fatal outcome due to central apnea. The median follow-up from clinical onset was 3 months (range 0.5–18 months). At last known status, death was reported in 10/11 (91%) patients. The median time to death was 7 months (range 2–20 months) from cancer diagnosis and 3 months (range 1–18 months) from neurologic toxicity. Death was attributed to neurologic toxicity (5/10, 50%), cancer progression (2/10, 20%), and other reasons (1/10, 10%); the cause of death was unknown or undetermined in 2/10 patients (20%). The only patient alive had severe neurologic disability; the last known status was 15 days after neurologic symptoms onset.
Hu Antibody n-irAEs vs Other n-irAEs
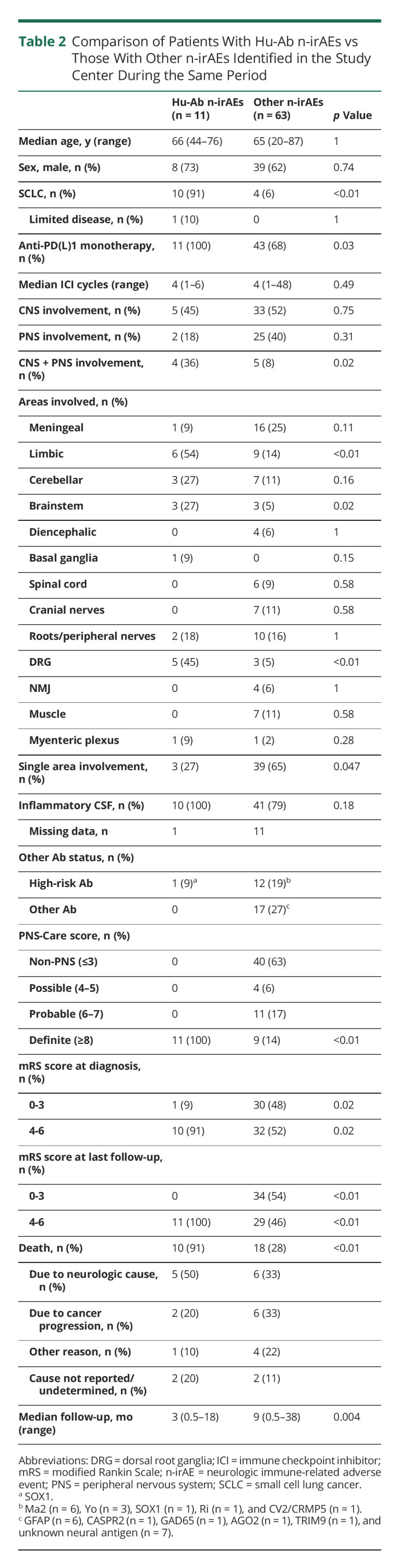
Compared with patients with Hu-Ab–negative n-irAEs (n = 63), the frequency of SCLC was higher among those with Hu-Ab n-irAEs (p < 0.01; Table 2). Hu-Ab–positive patients had more frequently co-occurring involvement of both central and peripheral nervous systems (p = 0.02). They had more frequently limbic (p < 0.01), brainstem (p = 0.02), and DRG (p < 0.01) involvement. In addition, involvement of a single anatomic area was less frequent in this group (p = 0.047). In patients with Hu-Ab, the clinical presentation was consistent with high-risk PNS phenotypes in all patients, and, accordingly, all patients fulfilled the criteria for definite PNS; 9/63 (14%) Hu-Ab–negative n-irAEs fulfilled the criteria for definite PNS (p < 0.01). The proportion of patients with severe neurologic disability at diagnosis was higher among Hu-Ab n-irAEs (p = 0.02). Patients with Hu-Ab had also poorer outcome and higher mortality rate (both p < 0.01).
Table 2.
Comparison of Patients With Hu-Ab n-irAEs vs Those With Other n-irAEs Identified in the Study Center During the Same Period
Hu Antibody n-irAEs vs ICI-naive Hu Antibody PNS
Compared with ICI-naive patients (n = 92), extensive SCLC disease was more frequent among those ICI treated (p < 0.01; Table 3). There was no significant difference in the clinical features between Hu-Ab n-irAEs and ICI-naive Hu-Ab PNS, but there was a poorer outcome at last visit (p < 0.01) and higher mortality (p < 0.01) among the former.
Table 3.
Comparison of Patients With Hu-Ab n-irAEs vs ICI-Naive Patients With Hu-Ab PNS Identified in the Study Center During the Same Period
Comparison of the Study Cohort to the Patients Identified by the Literature Review
A total of 16 previously reported patients were included for further analysis.18-29 In 4/16 patients, the Hu-Ab–associated PNS was present before ICI introduction and therefore analyzed separately. Detailed individual information of the 12 patients who developed the neurologic syndrome after ICI administration is presented in eTable 1 (links.lww.com/NXI/A768); in 3 of them, Hu-Abs were retrospectively detected in samples collected before ICI administration.18,21,27 No significant difference in terms of demographic characteristics, oncologic association, or clinical features was found between the patients reported herein and those from the literature. Among those identified from the literature, the criteria for definite PNS were fulfilled in all but 1 patient (eTable 2, links.lww.com/NXI/A768), who presented with right arm myoclonus, mild dysarthria, apraxia, and recurrent dysphasia, a clinical presentation atypical for PNS.19
Preexistent Hu Antibody PNS Worsened by ICI
One patient in the study cohort had Hu-Ab–associated SNN diagnosed before ICI initiation, and she worsened after 3 cycles of pembrolizumab (mRS score pretreatment 4; mRS score posttreatment 5). Four additional patients with PNS present before ICI exposure22,24-26 were previously reported in the literature (eTable 3, links.lww.com/NXI/A768); in 3 of them, the antibody positivity was known before ICI treatment, and in the remaining patient, Hu-Abs were tested in a previous sample only after the ICI-related neurologic worsening. All of them had peripheral involvement, either SNN (3/4) or polyradiculoneuropathy (1/4); 1 of them also had cerebellar ataxia. In all patients, the PNS worsened or relapsed after ICI exposure, leading to severe neurologic disability in 3/4 (75%) patients and death (due to neurologic toxicity) in the remaining 1.
Discussion
The present study shows a striking similarity between Hu-Ab n-irAEs and Hu-Ab PNS, which suggests similar pathogenic mechanisms in both conditions. Among all n-irAEs diagnosed at the study center, Hu-Abs were found in 1 in 7 patients, and the vast majority occurred after the introduction of ICI treatment for SCLC,30 the most frequent tumor found in Hu-Ab PNS.10 Furthermore, all the patients reported herein, including both those identified from the literature and the study center, had either SCLC or a cancer previously known to express Hu antigens,31 suggesting a major role of tumor characteristics for the development of these disorders. However, Hu-Ab n-irAEs likely occur in a small number of patients with SCLC exposed to ICI. In the absence of specific epidemiologic studies, this presumption is supported by the modest frequency (0–3%) of all types of n-irAEs reported by clinical trials in patients with SCLC and ICI therapy.32-34 In addition, our own experience also suggests this, as Hu-Ab n-irAEs were registered in less than 1% of patients with SCLC who received ICI in the Lyon University Hospitals during the study period (unpublished data). However, the rare occurrence of Hu-Ab n-irAEs should not overshadow the associated morbidity and mortality reported herein.
As typically reported in Hu-Ab PNS,10,16,35 post-ICI patients with Hu Ab presented with LE, SNN, rapidly progressive cerebellar ataxia, and/or gastroparesis. Such neurologic manifestations could not be explained by other causes, including other oncologic treatments. Although platinum compounds could have contributed to the occurrence of neuropathic symptoms in some patients, the relatively low cumulative dose received, the presence of CSF pleocytosis, and the broader involvement of the nervous system supported the diagnosis of n-irAE and argued against a major role for chemotherapy-induced toxicity.36 No significant difference regarding clinical presentation or PNS-Care score was found between post-ICI Hu-Ab–positive patients and their naive counterparts. By contrast, other n-irAEs without Hu-Ab had different profiles in terms of cancer associations and clinical manifestations, and only a minority of them fulfilled the criteria for definite PNS. These findings suggest that the detection of Hu-Ab identifies a subgroup of n-irAEs with distinctive paraneoplastic-like features, highlighting the importance of neural antibody testing in patients experiencing neurologic symptoms after ICI exposure. However, a positive antibody test should always be interpreted coupled with the clinical picture, as patients with SCLC asymptomatically harbor low Hu-Ab titers in 16–22.5% of cases11-13 and can be susceptible of developing other kind of n-irAEs.19 It is also of note that this latent antitumor immune response to Hu antigens in a subset of patients with SCLC could serve as a substrate for the occurrence of PNS after the ICI boosting effect,37 as suggested by previous reports where Hu-Abs were retrospectively detected in pre-ICI serum samples.18,21,27 To clarify this, prospective unbiased studies are needed to assess whether the presence of Hu-Ab in patients with SCLC increases the risk of PNS after ICI administration.
Identifying patients at risk of Hu-Ab syndromes occurring after ICI is of major importance because, as shown in the present study, they have universally severe presentations and poor outcomes. Likewise, worsening of the symptoms and unfavorable outcomes were observed following ICI treatment in all patients with a previously known PNS with Hu-Ab,22,24-26 as well as previously reported in PNS with other Ab.25 Consistently with the worse prognosis, the mortality among patients with Hu-Ab syndromes after ICI was significantly higher compared with ICI-naive patients with Hu-Ab PNS, suggesting a more aggressive neurologic condition among the former. However, this finding could be partly related to the extensive stage of SCLC in most ICI-treated patients, as opposed to the chiefly limited-stage disease in Hu-Ab ICI-naive patients found both herein and elsewhere.15 In addition, most of the present ICI-treated patients were elderly, severely disabled at diagnosis, and with evidence of multifocal nervous system involvement, factors previously reported as predictors of death in Hu-Ab–associated PNS.10 Therefore, age, advanced cancer-related frailty, and severe neurologic presentation seem to be relevant in determining the fatality of post-ICI Hu-Ab syndromes.
As opposed to other n-irAEs,38 most of the Hu-Ab–positive patients did not improve after ICI discontinuation and immunosuppressive treatment. This almost universal lack of treatment response in such patients parallels the experience of those with Hu-Ab–associated PNS outside the ICI context.39 Of note, following the positive experience in the context of other severe n-irAEs,40 1 of the included patients was treated with anti-IL6 therapy (tocilizumab), with biological (reduction of IL6) effects but without clinical benefit. The therapeutic failure could be explained by differences in the pathogenic mechanisms between Hu-Ab and other n-irAEs6 or by the already extensive and irreversible neuronal loss at the time of tocilizumab administration. In this setting, there is an urgent need to study the exact immunologic mechanisms underlying these conditions to define more effective therapeutic strategies. At the moment, prompt ICI discontinuation is essential to prevent further deterioration before neuronal damage is too advanced, and some authors suggest accelerating ICI clearance by the use of plasma exchange, although its schedule still needs to be defined.41 In addition, even in case of neurologic stabilization, reintroducing ICI is not recommendable, as the risk of neurologic worsening may overcome the modest survival benefit gained by adding ICI to chemotherapy in extensive-stage SCLC.32 Likewise, extreme caution and reconsideration of oncologic treatment alternatives are advisable when facing a patient with PNS and Hu-Ab before initiation of ICI. However, a case-by-case discussion involving an experienced multidisciplinary team, as well as the patients and their caregivers, is necessary to evaluate the best approach in these challenging scenarios.
The present study is limited by its retrospective nature, the unavailability of pre-ICI serum samples, the limited number of patients included, and possibly by a referral bias toward patients with more severe presentations. Nevertheless, it reflects the wide experience of a national reference center and offers a large descriptive series of the infrequent yet increasing and challenging Hu-Ab PNS occurring after ICI.
In conclusion, the presence of Hu-Ab identifies a subgroup of n-irAEs that consistently reproduce the phenotypes of Hu-Ab–related PNS, supporting the hypothesis of ICI triggering or unmasking a latent PNS. Because patients show high disability and mortality, further studies are required to investigate the immunopathology underlying these disorders and to improve their management.
Acknowledgment
The authors thank NeuroBioTec Hospices Civils de Lyon BRC (France, AC-2013-1867, NFS96-900) for banking sera and CSF samples. The authors express their grateful thanks to Dr. Luca Servan, who sent them clinical data and biological samples for the study. They thank Philip Robinson for help in manuscript preparation (Service de relecture scientifique, Hospices Civils de Lyon) and Dr. Pierre-Jean Souquet (Service de pneumologie, Hospices Civils de Lyon) who shared data regarding the number of patients with small cell lung cancer receiving immune checkpoint inhibitors.
Glossary
- CTLA4
cytotoxic T-lymphocyte-associated protein 4
- DRG
dorsal root ganglia
- Hu-ab
Hu antibody
- ICI
immune checkpoint inhibitor
- LE
limbic encephalitis
- mRS
modified Rankin Scale
- n-irAE
neurologic immune-related adverse event
- NCS
nerve conduction study
- PD1
programmed cell death protein 1
- PDL1
programmed death-ligand 1
- PNS
paraneoplastic neurologic syndrome
- SNN
sensory neuronopathy
Appendix. Authors
Study Funding
This work is supported by a public grant overseen by the Agence nationale de la recherche (ANR; French research agency) as part of the “Investissements d'Avenir” program (ANR-18-RHUS-0012). This study was performed within the framework of the LABEX CORTEX (ANR-11-LABX-0042) of the Université Claude Bernard Lyon 1, within the program “Investissements d'Avenir” (ANR-11-LABX-0042) operated by the ANR.
Disclosure
Antonio Farina received a research fellowship grant from the European Academy of Neurology. Go to Neurology.org/NN for full disclosure.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-1355. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primer. 2020;6(1):38. doi: 10.1038/s41572-020-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidon AC, Burton LB, Chwalisz BK, et al. Consensus disease definitions for neurologic immune-related adverse events of immune checkpoint inhibitors. J Immunother Cancer. 2021;9(7):e002890. doi: 10.1136/jitc-2021-002890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marini A, Bernardini A, Gigli GL, et al. Neurologic adverse events of immune checkpoint inhibitors: a systematic review. Neurology. 2021;96(16):754-766. doi: 10.1212/WNL.0000000000011795 [DOI] [PubMed] [Google Scholar]
- 5.Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol. 2017;13(12):755-763. doi: 10.1038/nrneurol.2017.144 [DOI] [PubMed] [Google Scholar]
- 6.Vogrig A, Muñiz-Castrillo S, Desestret V, Joubert B, Honnorat J. Pathophysiology of paraneoplastic and autoimmune encephalitis: genes, infections, and checkpoint inhibitors. Ther Adv Neurol Disord. 2020;13:1756286420932797. doi: 10.1177/1756286420932797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pignolet BS, Gebauer CM, Liblau RS. Immunopathogenesis of paraneoplastic neurological syndromes associated with anti-Hu antibodies. OncoImmunology. 2013;2(12):e27384. doi: 10.4161/onci.27384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hébert J, Riche B, Vogrig A, et al. Epidemiology of paraneoplastic neurologic syndromes and autoimmune encephalitides in France. Neurol Neuroimmunol Neuroinflammation. 2020;7(6):e883. doi: 10.1212/NXI.0000000000000883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogrig A, Gigli GL, Segatti S, et al. Epidemiology of paraneoplastic neurological syndromes: a population-based study. J Neurol. 2020;267(1):26-35. doi: 10.1007/s00415-019-09544-1 [DOI] [PubMed] [Google Scholar]
- 10.Graus F, Keime-Guibert F, Reñe R, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain J Neurol. 2001;124(Pt 6):1138-1148. doi: 10.1093/brain/124.6.1138 [DOI] [PubMed] [Google Scholar]
- 11.Verschuuren J, Perquin M, ten V, De Baets M, Vriesman Pv, Twijnstra A. Anti-Hu antibody titre and brain metastases before and after treatment for small cell lung cancer. J Neurol Neurosurg Psychiatry. 1999;67(3):353-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalmau J, Furneaux HM, Gralla RJ, Kris MG, Posner JB. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer—a quantitative Western blot analysis. Ann Neurol. 1990;27(5):544-552. doi: 10.1002/ana.410270515 [DOI] [PubMed] [Google Scholar]
- 13.Monstad SE, Knudsen A, Salvesen HB, Aarseth JH, Vedeler CA. Onconeural antibodies in sera from patients with various types of tumours. Cancer Immunol Immunother CII. 2009;58(11):1795-1800. doi: 10.1007/s00262-009-0690-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seute T, Leffers P, ten Velde GPM, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer. 2004;100(4):801-806. doi: 10.1002/cncr.20043 [DOI] [PubMed] [Google Scholar]
- 15.Maddison P, Lang B, Thomsen S, et al. Prospective study of cancer survival in patients with HuD-antibody-associated paraneoplastic neurological disorders. J Neurol Neurosurg Psychiatry. 2021;92(12):1350-1351. doi: 10.1136/jnnp-2021-326067 [DOI] [PubMed] [Google Scholar]
- 16.Graus F, Vogrig A, Muñiz-Castrillo S, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflammation. 2021;8(4):e1014. doi: 10.1212/NXI.0000000000001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mongay-Ochoa N, Vogrig A, Muñiz-Castrillo S, Honnorat J. Anti-Hu-associated paraneoplastic syndromes triggered by immune-checkpoint inhibitor treatment. J Neurol. 2020;267(7):2154-2156. doi: 10.1007/s00415-020-09940-y [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos KP, Romero RS, Gonzalez G, Dix JE, Lowy I, Fury M. Anti-Hu-associated autoimmune limbic encephalitis in a patient with PD-1 inhibitor-responsive myxoid chondrosarcoma. Oncologist. 2018;23(1):118-120. doi: 10.1634/theoncologist.2017-0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raskin J, Masrori P, Cant A, et al. Recurrent dysphasia due to nivolumab-induced encephalopathy with presence of Hu autoantibody. Lung Cancer. 2017;109:74-77. doi: 10.1016/j.lungcan.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 20.Hottinger AF, de Micheli R, Guido V, Karampera A, Hagmann P, Du Pasquier R. Natalizumab may control immune checkpoint inhibitor–induced limbic encephalitis. Neurol Neuroimmunol Neuroinflammation. 2018;5(2):e439. doi: 10.1212/NXI.0000000000000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuoka H, Kimura H, Koba H, et al. Nivolumab-induced limbic encephalitis with anti-Hu antibody in a patient with advanced pleomorphic carcinoma of the lung. Clin Lung Cancer. 2018;19(5):e597–e599. doi: 10.1016/j.cllc.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 22.Gill A, Perez MA, Perrone CM, Bae CJ, Pruitt AA, Lancaster E. A case series of PD-1 inhibitor-associated paraneoplastic neurologic syndromes. J Neuroimmunol. 2019;334:576980. doi: 10.1016/j.jneuroim.2019.576980 [DOI] [PubMed] [Google Scholar]
- 23.Kang K, Zheng K, Zhang Y. Paraneoplastic encephalitis and enteric neuropathy associated with anti-Hu antibody in a patient following immune-checkpoint inhibitor therapy. J Immunother. 19972020;43(5):165-168. doi: 10.1097/CJI.0000000000000314 [DOI] [PubMed] [Google Scholar]
- 24.Raibagkar P, Ho D, Gunturu KS, Srinivasan J. Worsening of anti-Hu paraneoplastic neurological syndrome related to anti-PD-1 treatment: case report and review of literature. J Neuroimmunol. 2020;341:577184. doi: 10.1016/j.jneuroim.2020.577184 [DOI] [PubMed] [Google Scholar]
- 25.Sechi E, Markovic SN, McKeon A, et al. Neurologic autoimmunity and immune checkpoint inhibitors: autoantibody profiles and outcomes. Neurology. 2020;95(17):e2442–e2452. doi: 10.1212/WNL.0000000000010632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chompoopong P, Zekeridou A, Shelly S, et al. Comparison of immune checkpoint inhibitor-related neuropathies among patients with neuroendocrine and non-neuroendocrine tumours. J Neurol Neurosurg Psychiatry. 2022;93(1):112–114. [DOI] [PubMed] [Google Scholar]
- 27.Morimoto T, Orihashi T, Yamasaki K, Tahara M, Kato K, Yatera K. Paraneoplastic sensory polyneuropathy related to anti-PD-L1-including anticancer treatment in a patient with lung cancer. Intern Med. 2021;60(10):1577-1581. doi: 10.2169/internalmedicine.5629-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakashima K, Demura Y, Kurokawa K, et al. Immune checkpoint inhibitor-induced limbic encephalitis during treatment with atezolizumab in a patient with small-cell lung cancer: a case report and review of the literature. Case Rep Immunol. 2022;2022:9290922. doi: 10.1155/2022/9290922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arai H, Utsu Y, Horio J, Furukawa S, Kikkawa Y. Paraneoplastic opsoclonus-myoclonus syndrome with anti-Hu and anti-SOX-1 antibodies after immune-checkpoint inhibitor treatment combined with chemotherapy in a patient with small-cell lung cancer. Intern Med. 2022;61(1):71-74. doi: 10.2169/internalmedicine.7167-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12(3):E738. doi: 10.3390/cancers12030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalmau J, Furneaux HM, Cordon-Cardo C, Posner JB. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141(4):881-886. [PMC free article] [PubMed] [Google Scholar]
- 32.Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-2229. doi: 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 33.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883-895. doi: 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 34.Ready NE, Ott PA, Hellmann MD, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol. 2020;15(3):426-435. doi: 10.1016/j.jtho.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 35.Dalmau J, Graus F, Rosenblum MK, Posner JB. Anti-Hu--associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine (Baltimore). 1992;71(2):59-72. doi: 10.1097/00005792-199203000-00001 [DOI] [PubMed] [Google Scholar]
- 36.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82(1):51-77. doi: 10.1016/j.critrevonc.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 37.Graus F, Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2019;16(9):535-548. doi: 10.1038/s41571-019-0194-4 [DOI] [PubMed] [Google Scholar]
- 38.Plaçais L, Michot JM, Champiat S, et al. Neurological complications induced by immune checkpoint inhibitors: a comprehensive descriptive case-series unraveling high risk of long-term sequelae. Brain Commun. 2021;16(4):fcab220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keime-Guibert F, Graus F, Fleury A, et al. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (Anti-Hu, anti-Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J Neurol Neurosurg Psychiatry. 2000;68(4):479-482. doi: 10.1136/jnnp.68.4.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picca A, Valyraki N, Birzu C, et al. Anti-Interleukin-6 and Janus kinase inhibitors for severe neurologic toxicity of checkpoint inhibitors. Neurol Neuroimmunol Neuroinflammation. 2021;8(6):e1073. doi: 10.1212/NXI.0000000000001073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsumoto TR, Wilson KL, Giri VK, et al. Plasma exchange for severe immune-related adverse events from checkpoint inhibitors: an early window of opportunity? Immunother Adv. 2022:ltac012. doi: 10.1093/immadv/ltac012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data reported in this study are available within the article and/or its supplementary material. More information regarding the data will be shared by the corresponding author on reasonable request.