Abstract
Background
Cardiac surgery is performed worldwide. Most types of cardiac surgery are performed using cardiopulmonary bypass (CPB). Cardiac surgery performed with CPB is associated with morbidities. CPB needs an extracorporeal circulation that replaces the heart and lungs, and performs circulation, ventilation, and oxygenation of the blood. The lower limit of mean blood pressure to maintain blood flow to vital organs increases in people with chronic hypertension. Because people undergoing cardiac surgery commonly have chronic hypertension, we hypothesised that maintaining a relatively high blood pressure improves desirable outcomes among the people undergoing cardiac surgery with CPB.
Objectives
To evaluate the benefits and harms of higher versus lower blood pressure targets during cardiac surgery with CPB.
Search methods
We used standard, extensive Cochrane search methods. The latest search of databases was November 2021 and trials registries in January 2020.
Selection criteria
We included randomised controlled trials (RCTs) comparing a higher blood pressure target (mean arterial pressure 65 mmHg or greater) with a lower blood pressure target (mean arterial pressure less than 65 mmHg) in adults undergoing cardiac surgery with CPB.
Data collection and analysis
We used standard Cochrane methods. Primary outcomes were 1. acute kidney injury, 2. cognitive deterioration, and 3. all‐cause mortality. Secondary outcomes were 4. quality of life, 5. acute ischaemic stroke, 6. haemorrhagic stroke, 7. length of hospital stay, 8. renal replacement therapy, 9. delirium, 10. perioperative transfusion of blood products, and 11. perioperative myocardial infarction. We used GRADE to assess certainty of evidence.
Main results
We included three RCTs with 737 people compared a higher blood pressure target with a lower blood pressure target during cardiac surgery with CPB. A high blood pressure target may result in little to no difference in acute kidney injury (risk ratio (RR) 1.30, 95% confidence interval (CI) 0.81 to 2.08; I² = 72%; 2 studies, 487 participants; low‐certainty evidence), cognitive deterioration (RR 0.82, 95% CI 0.45 to 1.50; I² = 0%; 2 studies, 389 participants; low‐certainty evidence), and all‐cause mortality (RR 1.33, 95% CI 0.30 to 5.90; I² = 49%; 3 studies, 737 participants; low‐certainty evidence). No study reported haemorrhagic stroke. Although a high blood pressure target may increase the length of hospital stay slightly, we found no differences between a higher and a lower blood pressure target for the other secondary outcomes.
We also identified one ongoing RCT which is comparing a higher versus a lower blood pressure target among the people who undergo cardiac surgery with CPB.
Authors' conclusions
A high blood pressure target may result in little to no difference in patient outcomes including acute kidney injury and mortality. Given the wide CIs, further studies are needed to confirm the efficacy of a higher blood pressure target among those who undergo cardiac surgery with CPB.
Keywords: Adult, Humans, Acute Kidney Injury, Acute Kidney Injury/epidemiology, Cardiac Surgical Procedures, Cardiac Surgical Procedures/adverse effects, Cardiopulmonary Bypass, Cardiopulmonary Bypass/adverse effects, Hemorrhagic Stroke, Hypertension, Hypotension, Randomized Controlled Trials as Topic
Plain language summary
Blood pressure targets for people undergoing heart surgery
Review question
What effect does a high blood pressure target compared with a low blood pressure target have in people undergoing heart surgery while on cardiopulmonary bypass (CPB).
Key messages
A high blood pressure target compared with a lower target may result in little to no difference in kidney injury, cognition (ability to learn and understand) damage, or survival.
A high blood pressure target may increase the length of hospital stay slightly.
What is heart surgery?
Heart surgery is a common type of surgery throughout the world. Most types of heart surgery are performed with CPB. CPB is a medical device that replaces the work of the heart and lungs by pumping the blood, and taking oxygen into and removing carbon dioxide from the blood. People undergoing heart surgery usually have high blood pressure (called hypertension). People with hypertension need a higher blood pressure to keep the blood flow to important organs such as the brain and kidneys. However, the evidence about the best blood pressure targets to use during heart surgery is scarce.
What did we want to find out?
We wanted to assess the effects of a higher blood pressure target compared with a lower blood pressure target on the kidneys, brain, quality of life, and complications occurring while in hospital.
What did we do?
We searched medical databases for clinical trials comparing high versus low blood pressure targets during heart surgery while on CPB.
What did we find?
We found three studies including 737 people undergoing heart surgery. The duration of the studies varied from two to three years. The average age of included participants ranged between 65.8 and 76 years and about 72% were men.
There was little to no difference between a high and low blood pressure target in injury to the kidneys, cognition damage, or deaths. Although a high blood pressure target may increase the length of hospital stay slightly, there may be little to no differences in quality of life or complications during hospitalisation.
What are the limitations of the evidence
Our confidence in the evidence for kidney injury and death was very limited as the studies were small, did not provide data about everything that we were interested in, and included different types of people. We are also less confident in the evidence for cognition damage as the studies were small and did not provide data about everything that we were interested in.
How up to date is this evidence?
The evidence is current to November 2021.
Summary of findings
Summary of findings 1. High versus low blood pressure target for cardiac surgery with cardiopulmonary bypass.
| High versus low blood pressure target for cardiac surgery with cardiopulmonary bypass | ||||||
| Patient or population: adults undergoing cardiac surgery with cardiopulmonary bypass Setting: hospital Experimental: high blood pressure target with mean arterial pressure ≥ 65 mmHg Comparison: low blood pressure target with mean arterial pressure < 65 mmHg | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with low blood pressure target | Risk with high blood pressure target | |||||
|
Acute kidney injury Follow‐up: until discharge from the surgical department or 6 months after the surgery |
Study population | RR 1.30 (0.81 to 2.08) | 487 (2 RCTs) | ⊕⊝⊝⊝ Very lowa | — | |
| 107 per 1000 | 139 per 1000 (86 to 222) | |||||
|
Cognitive deterioration Follow‐up: 90 days to 6 months |
Study population | RR 0.82 (0.45 to 1.50) | 389 (2 RCTs) | ⊕⊕⊝⊝ Lowb | Definition of cognitive deterioration of each study was: Vedel 2018: change from baseline neuropsychological test performance; ISPOCD test (Moller 1998) 90 days after surgery. Gold 1995: deterioration on ≥ 3 cognitive tests at 6 months after surgery defined as a cognitive complication. For each test, assessment was based on within‐patient change in test performance from preoperative baseline. Since we could not obtain the study protocol, details of each test were unclear. Minimally important difference of cognitive deterioration was defined as a minimal difference in frequency of cognitive deterioration required to have a clinical significance. |
|
| 104 per 1000 | 89 per 1000 (48 to 162) | |||||
|
All‐cause mortality Follow‐up: 30 days to 6 months |
Study population | RR 1.33 (0.30 to 5.90) | 737 (3 RCTs) | ⊕⊝⊝⊝ Very lowc | — | |
| 22 per 1000 | 29 per 1000 (7 to 128) | |||||
|
Quality of life Follow‐up: 6 months |
Study population | RR 0.78 (0.30 to 2.01) | 218 (1 RCT) | ⊕⊝⊝⊝ Very lowd | Since Gold 1995 counted quality of life as a dichotomous outcome defined as a decline of > 5 points on the Physical Component Summary score of the SF‐36 (Stewart 1989), we used this definition in this review. | |
| 83 per 1000 | 64 per 1000 (24 to 161) | |||||
|
Acute ischaemic stroke Follow‐up: 30 days to 6 months |
Study population | RR 1.29 (0.07 to 23.63) | 426 (2 RCTs) | ⊕⊝⊝⊝ Very lowe | — | |
| 46 per 1000 | 43 per 1000 (18 to 100) | |||||
|
Length of hospital stay Follow‐up: 6 months |
The mean length of stay in the low blood pressure target group was 12 days | MD 1.25 days longer (0.78 longer to 1.73 longer) | — | 540 (2 RCTs) | ⊕⊝⊝⊝ Very lowf | — |
|
Perioperative transfusion of blood products Follow‐up: not reported |
The mean perioperative transfusion of blood products was 2.0 units. | MD 0.1 units higher (0.13 lower to 0.34 higher) | — | 540 (2 RCTs) |
⊕⊝⊝⊝ Very lowg | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ISPOCD: International Study of Post‐Operative Cognitive Dysfunction; MD: mean difference; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio; SF‐36: 36‐item Short Form. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision because the OIS of 33,954 was over 10 times larger than the number of participants; one level for indirectness because the definitions used in the included studies were inconsistent with each other, causing quite different occurrences; one level for risk of bias since Azau 2014 was not of overall low risk of bias; and one level for inconsistency with large heterogeneity (I² = 72%). bDowngraded one level for risk of bias because the number of follow‐ups was not balanced in one study; and one level for imprecision because the OIS of 910 was not met. cDowngraded one level for risk of bias because the two studies were not at overall low risk of bias; one level for inconsistency with large heterogeneity I² = 49%; and one level for imprecision because the OIS of 5948 was not met. dDowngraded one level for risk of bias because the study was not at overall low risk of bias; and two levels for imprecision because the sample size was small and the CIs around the RR included 1.0. eDowngraded one level for risk of bias because the number of follow‐ups was not balanced in one study; one level for imprecision because the OIS of 4670 was not met; one level because the CI spanned potential benefit, no benefit, and possible harm; and one level for inconsistency for large heterogeneity (I² = 82%). fDowngraded one level for risk of bias because it was unclear whether physicians decided the date of discharge could know the allocation of the patients in one study; one level for imprecision because the OIS (1540 or 4906 depending on standard deviation used) was not met; and one level for inconsistency with large heterogeneity (I² = 76%). gDowngraded one level for risk of bias because the study was not at overall low risk of bias; one level for performance bias because the allocation of the participants could affect the strategy of transfusion; and one level for imprecision because the CI spanned potential benefit, no benefit, and possible harm. The OIS was met (174 or 230 depending on standard deviation used).
Background
Description of the condition
Cardiac surgery is performed worldwide. The annual number of cardiac surgeries was over 200,000 in North America in 2016, over 40,000 in Japan in 2015, over 20,000 in China in 2013, over 100,000 in Europe in 2008, and over 35,000 in Brazil in 2005 (D'Agostino 2018; Gurfinkel 2007; Head 2013; Masuda 2018; Rao 2016). The costs associated with the procedure are enormous. In the USA, for example, the mean cost per person for a cardiac surgery between 2005 and 2008 was US dollars (USD) 40,000; the total cost was more than USD 20 billion, which accounted for 1% to 2% of total national healthcare costs (Kilic 2014). Most types of cardiac surgery are commonly performed using cardiopulmonary bypass (CPB) (D'Agostino 2018; Hillis 2011; Masuda 2018). CPB is an extracorporeal circulation that replaces the heart and lungs, includes circulation of blood, oxygenation, and ventilation by draining venous blood from the body, oxygenating the blood and sending the oxygenated blood back to the body so that other end organs remain adequately oxygenated and perfused. The CPB circuit consists of pumps, cannulae, reservoir, oxygenator, heat exchanger, and arterial line filter. The right atrium or both superior and inferior vena cavae are cannulated to drain blood through the venous line of the CPB circuit into a venous reservoir. The arterial pump moves blood from the venous reservoir to the oxygenator through a heat exchanger and finally to an arterial line filter. The blood is then returned to the body via an arterial cannula located in the ascending aorta or other major arteries. During CPB, the ascending aorta is usually cross‐clamped and cardioplegia solution is administered to allow surgeons to operate safely on a heart without beating in a bloodless field. Modern CPB machines also have systems for monitoring circuit pressure, temperature, oxygen saturation, haemoglobin, blood gases and electrolytes, as well as safety features, such as air detectors (Wahba 2020).
Cardiac surgery performed with CPB is associated with morbidities. For example, cardiac surgery‐associated acute kidney injury (CSA‐AKI) is one of the major complications of cardiac surgery. Acute kidney injury (AKI) is an abrupt kidney dysfunction, defined as a relative increase of serum creatinine level within seven days or oliguria (KDIGO 2012). CSA‐AKI occurs in 20% to 40% of people after cardiac surgery and is the second most common cause of AKI among critically ill people (Englberger 2011; Machado 2014; Mao 2013). CSA‐AKI is associated with worse mortality even at 10 years after the surgery (Hobson 2009). About 1% to 5% of people with CSA‐AKI received renal replacement therapy (Conlon 1999). Another important complication of cardiac surgery with CPB is perioperative delirium. Delirium after cardiac surgery is common and is associated with mortality and long‐term cognitive decline (Rudolph 2010; Saczynski 2012).
Effective strategies backed by robust evidence to prevent or treat the CSA‐AKI are lacking. Studies evaluating statins, remote ischaemic preconditioning, and fenoldopam have found no benefits for the kidneys (Bove 2014; Lewicki 2015; Menting 2017). One single‐centre trial showed that an AKI care bundle guideline reduced the incidence of severe AKI among the people undergoing cardiac surgery. However, because this was a phase II trial, we could not draw any definitive conclusion from this result (Meersch 2017). There is no proven effective strategy to prevent or treat delirium among people undergoing cardiac surgery, either. Therefore, finding a treatment strategy to improve the desirable outcomes of cardiac surgery remains a challenge.
Description of the intervention
Blood pressure is an important determinant for blood supply to vital organs. The concept of autoregulation is important when considering perfusion to the brain and kidney (Palmer 2002; Strandgaard 1973). Autoregulation maintains a constant blood flow to organs, even if the blood pressure varies within a specific range. Because it is well known that the lower limit of autoregulation blood pressure shifts in people with chronic hypertension, maintaining a relatively high blood pressure may be beneficial for this population. One randomised controlled trial (RCT) evaluating a higher versus lower blood pressure target in septic shock showed that a higher blood pressure target did not result in survival benefit, but was associated with higher incidence of atrial fibrillation and a requirement for higher doses of noradrenaline (Asfar 2014). However, the prespecified subgroup analysis of the RCT showed that a higher blood pressure target led to less renal replacement therapy among the people with chronic hypertension. In addition, one animal experiment showed that the lower limit of renal autoregulation can be higher than that of cerebral autoregulation (Rhee 2012), which might suggest that a high blood pressure target has different effects on renal and cerebral outcomes.
Several RCTs have evaluated different blood pressure targets during non‐cardiac surgery, but they did not reach a clinically meaningful result (Carrick 2016; Williams‐Russo 1999). One guideline on perioperative care in non‐cardiac surgery suggested individualising care in people with associated conditions and comorbidities (Fleisher 2014). One RCT showed that individualised blood pressure management, close to the preoperative value, led to less organ dysfunction compared to standard management (Futier 2017).
Among the people undergoing cardiac surgery, the optimal blood pressure target is controversial. Several observational studies have suggested an association between blood pressure abnormality and adverse outcomes. Hypotension during cardiac surgery can lead to decreased organ perfusion and is associated with organ dysfunction and mortality after the surgery (Ono 2013; Ono 2014). On the contrary, excessive hypertension is also associated with postoperative delirium (Hori 2014), or may result in excess haemorrhage.
How the intervention might work
A higher blood pressure target might be beneficial for organ perfusion given that most people who undergo a cardiac surgery have hypertension (Gillinov 2016; Landoni 2019; Mazer 2017). However, maintaining a higher blood pressure target might require an increased level of fluid intake as well as higher doses of vasoactive agents (drugs that increase blood pressure by increasing vascular resistance, e.g. noradrenaline), which may lead to adverse outcomes (Asfar 2014).
Why it is important to do this review
Several Cochrane Reviews have compared a higher versus a lower blood pressure target in chronic management amongst various populations (Arguedas 2013; Garrison 2017; Saiz 2018). An international guideline for AKI stated that the optimal blood pressure target may vary according to the characteristics of people, such as comorbidities or premorbid blood pressure (KDIGO 2012). However, the question of how one determines the optimal blood pressure target in the acute settings, especially for people undergoing cardiac surgery with CPB, remains unanswered. Consequently, this review is our attempt to determine the optimal blood pressure target for cardiac surgery requiring CPB.
Objectives
To evaluate the benefits and harms of higher versus lower blood pressure targets during cardiac surgery with CPB.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs irrespective of their publication type, publication status, publication date, or language. We included all individual RCTs and only those cluster‐RCTs that reported the intracluster correlation coefficient (ICC) because the ICC is necessary for an approximately correct analysis of cluster‐RCTs by reducing the size of a cluster‐RCT to the effective sample size (Rao 1992).
We excluded cross‐over studies because it is unlikely that the participants in this review underwent the same surgery twice. We excluded quasi‐randomised studies for which the applied randomisation methods are inadequate and susceptible to selection bias.
Types of participants
We included adults (aged 18 years or older) undergoing cardiac surgery with CPB. For this review, we defined cardiac surgery as coronary artery bypass graft (CABG) or heart valve surgery. We also included isolated aortic surgery because we believe such procedural differences hardly affect blood pressure management, although we planned to exclude isolated aortic surgery in the protocol (Kotani 2019). We excluded adults undergoing surgical procedures for congenital heart diseases or cardiac tumours because these surgical procedures are quite different from a coronary or a valve surgery and these conditions are rare.
We defined CPB as all CPB irrespective of how it was performed in terms of site of cannulation, non‐pulsatile or pulsatile flow, body temperature, or with or without cardioplegic arrest.
Types of interventions
Experimental intervention: high blood pressure target (defined as mean arterial pressure (MAP) 65 mmHg or greater during CPB).
Comparator: low blood pressure target (defined as MAP less than 65 mmHg during CPB).
Although there is currently a lack of consensus on the optimum blood pressure target in cardiac surgery, we used the threshold of MAP based on the evidence in other populations: four large cohort studies in non‐cardiac surgery showed that intraoperative MAP less than 65 mmHg or less than 60 mmHg was associated with mortality or morbidities (Mascha 2015; Salmasi 2017; Sun 2015; van Waes 2016). In addition, the latest clinical guidelines for sepsis and AKI recommends maintaining MAP at 65 mmHg or greater (KDIGO 2012; Rhodes 2017). We defined experimental intervention and comparator by blood pressure targets during CPB. We allowed any blood pressure targets intraoperatively without CPB because most studies on blood pressure targets in cardiac surgery have investigated blood pressure during CPB (Gottesman 2007; Haase 2012; Hori 2014; Kanji 2010; Ono 2013; Ono 2014; Reich 1999; Sickeler 2014). We performed a sensitivity analysis that excluded studies defining blood pressure targets without CPB.
We regarded any co‐interventions that were not part of the randomised treatment as equally delivered in the intervention and comparator groups. We assessed the risk of bias of co‐interventions.
Types of outcome measures
The reporting of outcomes was not an inclusion criterion in the review.
Primary outcomes
We referred to a core outcome set for adult cardiac surgery trials to identify our outcome measures of interest (Benstoem 2017).
AKI within seven days after the surgery. AKI was defined as an abrupt kidney dysfunction within seven days after the surgery. If a study used the Risk, Injury, Failure, Loss and End‐stage kidney disease (RIFLE) criteria (Bellomo 2004), AKI was defined as 'R' or worse within seven days after the surgery. If a study used the Acute Kidney Injury Network (AKIN) (Mehta 2007), or Kidney Disease Improving Global Outcomes (KDIGO) criteria (KDIGO 2012), AKI was defined as stage I or worse of each criterion within seven days after the surgery. If a study did not use RIFLE, AKIN, or KDIGO criteria, AKI was defined by trial authors within seven days after the surgery. We defined the minimally important difference for AKI as a 20% decrease in relative risk (Azau 2014).
Cognitive deterioration was defined as a decrease in cognition in any validated assessment scale, such as the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph 1998), from three to six months after the surgery (Annane 2018; Pandharipande 2013). If a study reported cognitive deterioration between three and six months after the surgery more than once, we included only the last timing as the outcome because a longer‐term cognitive deterioration has a more negative impact. We defined the minimally important difference for cognitive deterioration as a 50% decrease in relative risk (Cheng 2019).
All‐cause mortality during the longest study period. We defined the minimally important difference for mortality as 1% in absolute risk (Landoni 2019).
Secondary outcomes
Quality of life was defined as physical functioning and mental health measured on any validated scale, such as the 36‐item Short Form (SF‐36) Survey, during the longest study period. We defined quality of life as a dichotomous outcome and the minimally important difference for quality of life as a decline of more than 5 points on the Physical Component Summary score in the SF‐36 (Busija 2008).
Acute ischaemic stroke during hospitalisation, defined by trial authors. We defined the minimally important difference for acute ischaemic stroke as a 35% decrease in relative risk (Mack 2017).
Haemorrhagic stroke during hospitalisation, defined by trial authors. We defined the minimally important difference for haemorrhagic stroke as a 10% decrease in relative risk (Li 2013).
Length of hospital stay during the longest study period. We defined the minimally important difference for length of stay in hospital as two days between the two groups (Gillinov 2016).
Renal replacement therapy during hospitalisation, defined by trial authors. We did not restrict modality of renal replacement therapy, such as intermittent or continuous; haemofiltration, haemodialysis, or haemodiafiltration. We defined the minimally important difference for renal replacement therapy as a 50% decrease in relative risk (Bove 2014).
Delirium at any time during hospitalisation. Delirium was diagnosed with the Confusion Assessment Method (CAM; Inouye 1990), Confusion Assessment Method for Intensive Care Unit (CAM‐ICU; Ely 2001), Intensive Care Delirium Screening Checklist (ICDSC; Bergeron 2001), International Classification of Diseases the 11th Revision (ICD‐11; WHO 2018), or Diagnostic and Statistical Manual of Mental Disorders, the Fifth Edition (DSM‐V; APA 2013). We allowed all the previous versions of these criteria. We included only the earliest timing as the outcome when a study reported delirium during hospitalisation more than once. We defined the minimally important difference for delirium as a 50% decrease in relative risk (Subramaniam 2019).
Perioperative transfusion of blood products at any time from the cardiac surgery to hospital discharge. We defined the minimally important difference for the perioperative transfusion of blood products as two units between the two groups (Zhang 2018). We defined a unit of blood product as the minimal unit of packed red blood cells, which is equivalent to nearly 300 mL (Carson 2016).
Perioperative myocardial infarction during hospitalisation, defined by trial authors. We defined the minimally important difference for perioperative myocardial infarction as a 50% decrease in relative risk (Briguori 2009).
Reporting one or more of the outcomes listed here in the trial was not an inclusion criterion in this review. Where a published article did not appear to report one of these outcomes, we accessed the trial protocol and contacted the trial authors to ascertain whether the outcomes were measured but not reported. We included relevant trials that measured these outcomes but did not report the data at all, or not reported them in a usable format, in the review as part of the narrative.
Search methods for identification of studies
Electronic searches
We identified RCTs through systematic searches of the following bibliographic databases on 28 November 2021:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 11, 2021);
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 24 November 2021);
Embase (Ovid, 1980 to 2021 week 46);
Web of Science Core Collection (Clarivate Analytics, 1900 to 28 November 2021).
We adapted the preliminary search strategy for MEDLINE (Ovid), as illustrated in Appendix 1, for use in the other databases. We applied the Cochrane sensitivity‐ and precision‐maximising RCT filter to MEDLINE (Ovid) and adaptations of it to the other databases (Lefebvre 2011), with the exception of CENTRAL.
We also conducted a search of ClinicalTrials.gov (www.clinicaltrials.gov) on 20 July 2021 and the World Health Organization International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch) for ongoing or unpublished trials on 21 July 2021.
We searched all databases from their inception, and imposed no restriction on language of publication or publication status. We did not perform a separate search for adverse events but we considered adverse events described in included studies.
Searching other resources
We checked the reference lists of all included studies and any identified relevant systematic reviews for additional references to trials. We also examined any relevant retraction statements and errata for included studies.
Data collection and analysis
Selection of studies
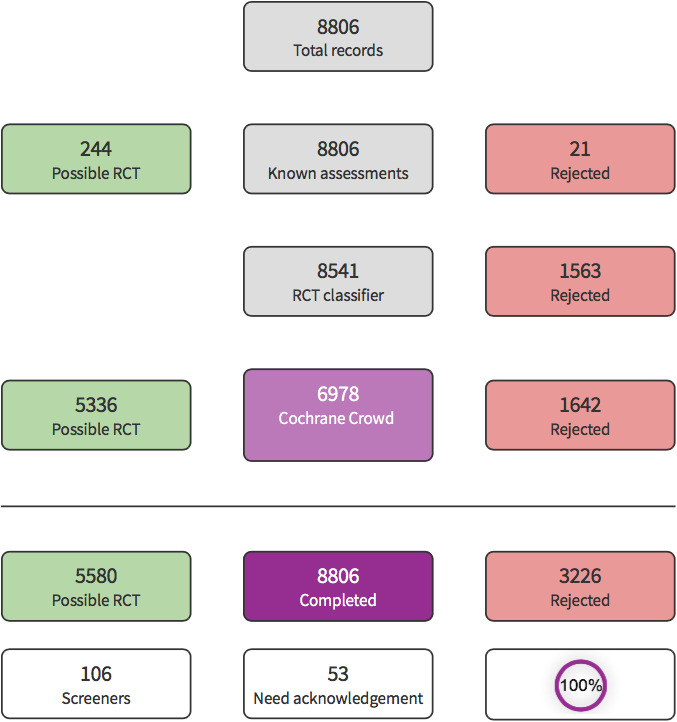
We used the Cochrane's Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as an RCT or as not an RCT; the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs, and if appropriate, Cochrane Crowd – Cochrane's citizen science platform where the crowd help to identify and describe health evidence.
More information about Screen4Me and the evaluations that have been done are available on the Screen4Me webpage on the Cochrane Information Specialist's portal (community.cochrane.org/organizational-info/resources/resources-groups/information-specialists-portal). In addition, more detailed information regarding evaluations of the Screen4Me components can be found in Marshall 2018, McDonald 2017, Noel‐Storr 2018, and Thomas 2017.
Following Screen4Me, three review authors (YKo, SF, TY) independently screened the titles and abstracts of all the potential studies identified and coded them as 'to retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. If there were any disagreements, a fourth review author arbitrated (JK or JI or JSK or YKa). We retrieved the full‐text study reports/publications and three review authors (YKo and SF and TY) independently screened the full‐text, identified studies for inclusion, and recorded the reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, we consulted a fourth review author (JK or JI or JSWK or YKa). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of analysis in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009), and we provided our reasons for excluding studies in the Characteristics of excluded studies table.
Data extraction and management
We used a prestandardised data collection form to extract study characteristics and outcome data. We piloted this data extraction sheet on at least one included study before using it on the remaining studies. Two review authors (SF and TY) performed data extraction and collected the following study characteristics.
Methods: total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, and date of study.
Participants: number randomised, number lost to follow‐up/withdrawn, number analysed, mean age, gender, inclusion criteria, and exclusion criteria.
Interventions: experimental intervention, comparison, concomitant medications, and excluded medications.
Outcomes: specified and collected primary and secondary outcomes, and their reported time points.
Notes: funding source for trial, and notable conflict of interests among trial authors.
Two review authors (SF and TY) independently extracted outcome data from the included studies. We resolved disagreements by consensus or by involving a third review author (YKo or JK or JI or JSWK or YKa). One review author (YKo) input data into Review Manager 2020.
Assessment of risk of bias in included studies
Two review authors (SF and TY) independently assessed risk of bias for each study using the Cochrane RoB 1 tool for assessing risk of bias, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third review author (JK or JI or JSWK or YKa). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessor.
Incomplete outcome data.
Selective outcome reporting.
Other biases.
We assessed and categorised each potential source of bias as 'low risk', 'high risk', or 'unclear risk', and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias was related to unpublished data or to correspondence with trial authors, we noted this in the risk of bias table.
When considering treatment effects, we considered the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
We analysed dichotomous variables, that is, variable in two mutually exclusive categories (AKI, delirium, all‐cause mortality, acute ischaemic stroke, renal replacement therapy, haemorrhagic stroke, and perioperative myocardial infarction) as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous outcomes (length of stay in hospital, perioperative transfusion of blood products), we presented mean differences (MDs) with 95% CIs. We planned to pool quality of life as a continuous variable and present MDs with 95% CIs when studies used the same scale or standardised mean differences with 95% CIs when studies used different scales. However, since Gold 1995 was the only study to report quality of life and they reported it as a dichotomous outcome, we counted quality of life as a dichotomous variable.
Unit of analysis issues
When analysing multiple‐armed trials, we combined all relevant experimental intervention groups of the study into a single group and all relevant control groups into a single control group. If we could not classify one of the arms into either of the experimental or comparator intervention, we excluded it from the analysis.
When incorporating the result of cluster‐RCTs with ICCs with that of individual RCTs, we obtained the effective sample size of cluster‐RCTs using the design effect calculated from the number of clusters and ICC. After reducing cluster‐RCTs to the effective sample size, we combined the result of both individual and cluster‐RCTs in a meta‐analysis.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where possible, we used the Review Manager 5 calculator to calculate missing standard deviations (SDs) using other data from the trial (Review Manager 2020), such as CIs. When sample sizes were large and the distribution of the outcome was similar to the normal distribution, we regarded the width of the interquartile range as 1.35 SDs. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results using a sensitivity analysis. We analysed on an intention‐to‐treat basis for all outcomes whenever possible.
Assessment of heterogeneity
We inspected forest plots visually to consider the direction and magnitude of effects and the degree of overlap between CIs. We used the I² statistic to measure heterogeneity among the trials in each analysis, but acknowledged that there is substantial uncertainty in the value of the I² statistic when there is a small number of studies; we also considered the P value from the Chi² test, for which a value less than 0.1 defined statistical significance (Higgins 2002).
We followed the recommendations for heterogeneity threshold of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: may represent considerable heterogeneity.
If we identified substantial and considerable heterogeneity, we reported it and explored possible causes by prespecified subgroup analysis.
Assessment of reporting biases
To assess reporting biases, we used funnel plots and assessed its asymmetry by visual inspection. If 10 or more studies were included in the meta‐analysis, we performed Egger's test to assess the small‐study effects (Egger 1997).
Data synthesis
We undertook meta‐analyses only where this was meaningful, that is, if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
As we expected heterogeneity in the blood pressure target of experimental and comparator interventions across included studies, we used a random‐effects model (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses for all outcomes.
Preoperative chronic hypertension: participants with preoperative chronic hypertension versus participants without preoperative chronic hypertension. Trial authors defined preoperative chronic hypertension.
Age: participants aged 65 years or older versus participants aged less than 65 years.
Gender: men versus women.
Type of cardiac surgery: CABG alone, valve surgery alone, or CABG plus valve surgery.
We planned to use the formal test for subgroup differences in Review Manager 2020 and based our interpretation on this.
Sensitivity analysis
We planned to carry out the following sensitivity analyses, to test whether key methodological factors or decisions had affected the effect size.
Only including studies with overall low risk of bias. We classified the outcome result as overall low risk of bias if we classified the bias for random sequence generation, allocation concealment, incomplete outcome data, and selective reporting as low risk.
Only including studies evaluating AKI within seven days after the surgery using the RIFLE (Bellomo 2004), AKIN (Mehta 2007), or KDIGO (KDIGO 2012) classifications.
We replaced any kidney dysfunction defined by trial authors within 90 days after the surgery or during the hospitalisation with AKI to explore for any effect of outcome definition on effect size.
Excluding studies that define target blood pressure not on CPB.
-
Where we were unable to obtain missing numerical outcome data, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis. To assess the effect of the missing data for dichotomous outcomes, we performed the following sensitivity analyses and reported the results from both scenarios in the review.
Best‐case scenario: we assumed that all individuals lost to follow‐up in the experimental group survived, did not have AKI, cognitive deterioration, acute ischaemic stroke, haemorrhagic stroke, renal replacement therapy, delirium, or perioperative myocardial infarction; and all those with missing outcomes in the control group did not survive, had AKI, cognitive deterioration, acute ischaemic stroke, haemorrhagic stroke, renal replacement therapy, delirium, or perioperative myocardial infarction.
Worst‐case scenario: we assumed that all individuals lost to follow‐up in the experimental group did not survive, had AKI, cognitive deterioration, acute ischaemic stroke, haemorrhagic stroke, renal replacement therapy, delirium, or perioperative myocardial infarction; and all those with missing outcomes in the control group survived, did not have AKI, cognitive deterioration, acute ischaemic stroke, haemorrhagic stroke, renal replacement therapy, delirium, or perioperative myocardial infarction.
To assess the effect of missing SDs for continuous outcomes, we performed a sensitivity analysis where we excluded studies that were imputed.
We performed a sensitivity analysis excluding studies that included participants undergoing isolated aortic surgery to investigate whether the procedural difference could affect the effect size.
We performed sensitivity analyses excluding studies published before 2000 because the practice and outcomes of cardiac surgery have evolved significantly over time.
Cognitive deterioration was defined as a relative decrease of cognitive score from the baseline. This definition does not consider learning effects caused by repeated examination of the same test. In contrast, neuropsychological tests such as the International Study of Postoperative Cognitive Dysfunction (ISPOCD) (Moller 1998) subtract the mean learning effect from the postoperative changes from the baseline, which can provide more appropriate criteria. Thus, we conducted a sensitivity analysis for cognitive deterioration, including only studies that considered learning effects caused by multiple testings.
We limited the first sensitivity analysis to the primary outcomes (AKI, cognitive deterioration, and all‐cause mortality) and the second and third sensitivity analysis to AKI. We planned to conduct the fourth to seventh sensitivity analyses for all the outcomes. However, we could not perform the following sensitivity analyses.
Only including studies evaluating AKI within seven days after the surgery using the RIFLE (Bellomo 2004), AKIN (Mehta 2007), or KDIGO (KDIGO 2012) because there was no such study using the AKI criteria.
Replacing any kidney dysfunction with AKI as no study reported kidney dysfunction.
Excluding studies that defined target blood pressure not on CPB because there was no study defining target blood pressure not on CPB.
The best‐case and worst‐case scenarios for AKI, all‐cause mortality, and delirium because there were no missing outcome data in the included studies.
Excluding studies that were imputed for missing SDs of perioperative because there was no such study.
Excluding studies that included participants undergoing isolated aortic surgery for cognitive deterioration, quality of life, acute ischaemic stroke, delirium because only Gold 1995 included participants with isolated aortic surgery.
Excluding studies published before 2000 for AKI, renal replacement therapy, delirium, because there was no study reporting such outcomes and for quality of life because Gold 1995 was the only study reporting quality of life.
Summary of findings and assessment of the certainty of the evidence
We created Table 1 using the following outcomes (AKI, cognitive deterioration, all‐cause mortality, quality of life, acute ischaemic stroke, length of stay in hospital, and perioperative transfusion of blood products). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro GDT software (GRADEpro GDT).
Two review authors (SF and TY) independently assessed certainty of the evidence, with disagreements resolved by discussion or by involving a third review author (YKo or JK or JI or JSWK or YKa). We justified all decisions to downgrade the certainty of the evidence using footnotes, and we made comments to aid the readers' understanding of the review where necessary.
As planned in the protocol, we extracted study data, formatted our comparisons in data tables, and prepared a summary of findings table before writing the results and conclusions of our review.
For the purposes of assessing imprecision in dichotomous outcomes, the optimal information size (OIS) was determined to provide 80% power to detect a minimally important difference in each outcome on the basis of the outcome occurrence in the low blood pressure target group with a two‐sided P value of less than 0.05 indicating statistical significance. For continuous outcomes, the OIS was determined to provide 80% power to detect a minimally important difference in each outcome on the basis of the SD of the outcome in the low blood pressure target group with a two‐sided P value of less than 0.05 indicating statistical significance.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies tables.
Results of the search
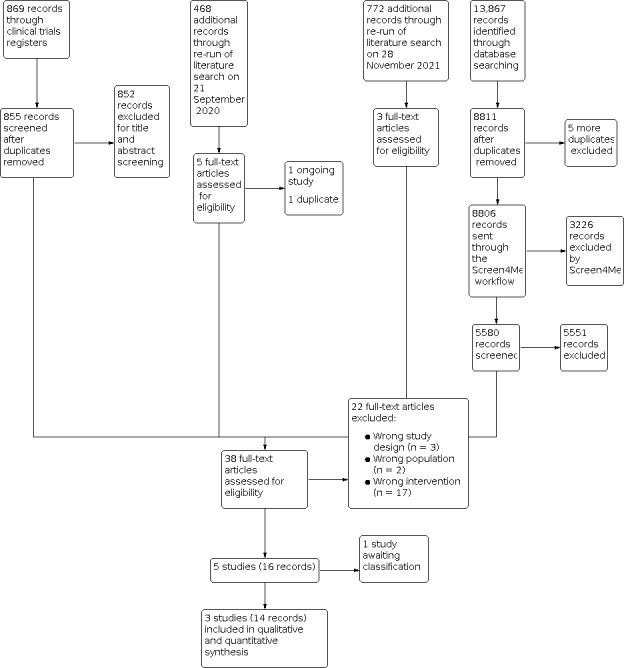
Our study selection process is illustrated in Figure 1. The comprehensive literature search identified a total of 13,867 results. After deduplication, we screened 8806 titles and abstracts. We used Cochrane's Screen4Me workflow to help identify potential reports of randomised trials. The results of the assessment process with the Screen4Me assessment process can be seen in Figure 2.
1.
Study flow diagram.
2.
Screen4Me summary diagram.
A total of 5580 records remained after the assessment process with Screen4Me. We excluded 5551 irrelevant records from which 29 study reports remained for full‐text review. After excluding 21 full texts (Characteristics of excluded studies table), we identified one study awaiting assessment and we contacted the corresponding author for further details in order to determine its eligibility but we received no response (Characteristics of studies awaiting classification table). Therefore, we eventually included three studies (Azau 2014; Gold 1995; Vedel 2018).
Considering the time lapse since the first literature search was run, we conducted updated literature searches on 21 September 2020 and 28 November 2021 and screened an additional 468 and 772 records, respectively. For the second literature search, we excluded 463 irrelevant records. One was identified as an ongoing study (Characteristics of ongoing studies table). We eventually included three articles from this updated search, for which all were publications of a single study (Vedel 2018). For the third literature search, we excluded 769 records. Among the remaining three records, one was excluded for wrong intervention (Damén 2021), while the other two records were publications of a single study (Vedel 2018).
Overall we included three studies, excluded 22 studies, one study is awaiting classification, and one study is ongoing.
Included studies
See Characteristics of included studies table.
In total, we included three RCTs (Azau 2014; Gold 1995; Vedel 2018). All were single‐centre, open‐label, parallel‐group trials. The sample size and the inclusion period for each study were: 300 people between January 2008 and June 2010 for Azau 2014, 251 people between October 1991 and February 1994 for Gold 1995, and 197 people between July 2014 and January 2016 for Vedel 2018. The mean age of the included participants ranged from 65.8 to 76 years. Of the 745 study participants, 72.1% (537/745) were men. Azau 2014 included people undergoing elective cardiac surgery, including CABG, valvular surgery, or reconstructive surgery of the ascending aorta under normothermic CPB with known risk factors for AKI. Gold 1995 included people receiving elective CABG. Vedel 2018 included people undergoing elective or subacute CABG or left heart valve surgery, or both. The MAP targets for intervention versus comparison in Azau 2014 were 75 mmHg to 85 mmHg versus 50 mmHg to 60 mmHg, in Gold 1995 were 80 mmHg to 100 mmHg versus 50 mmHg to 60 mmHg, and in Vedel 2018 were 70 mmHg to 80 mmHg versus 40 mmHg to 50 mmHg. Azau 2014 was supported by a grant from the "Programme Hospitalier pour la Recherche Clinique" (PHRC Inter‐Régional, 2007) from the French Health Ministry. Gold 1995 received grant HL44719 from the National Institutes of Health, National Heart, Lung, and Blood Institute. Vedel 2018 received grants from the Danish Heart Foundation (14‐R97‐A5179‐22868 and 15‐R99‐A6034‐22905) and the Research Foundations at Rigshospitalet (E‐22329‐01), University of Copenhagen, Denmark.
Excluded studies
We excluded 22 full texts and summarised the reasons for exclusion in Characteristics of excluded studies table.
Studies awaiting classification
We contacted the corresponding author of one study for further details to determine its eligibility but we received no response (von Knobelsdorff 1996).
Ongoing studies
One study is ongoing (ChiCTR2000028941).
Risk of bias in included studies
We summarised the results of our assessment of risk of bias for included studies in Figure 3 and Figure 4.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All three included studies were RCTs. Two studies described the details of the randomisation processes. We considered the random sequence generation of both studies at low risk of bias (Gold 1995; Vedel 2018). Azau 2014 did not report the randomisation processes, so the random sequence generation of this study was unclear.
Allocation concealment was unclear in Azau 2014 and Gold 1995 because the details of the allocation process were not described in the manuscript and a protocol was not available. Allocation concealment was at low risk of bias in Vedel 2018, where the allocation sequence was computer‐generated with a varying block size of four to eight.
Blinding
Performance bias in Azau 2014 was unclear because study personnel was not blinded. Detection bias was unclear in Azau 2014 because the definition and assessor for several outcomes were unclear. Performance bias was unclear in Gold 1995. Because not all treatment strategies were predetermined, some of the outcomes could be affected by treatment allocation. For example, some physicians could use more blood products to a patient in a higher blood pressure target arm. In Vedel 2018, the protocol reported that participants and healthcare providers were blinded from the group allocation. Detection bias was at low risk of bias in both studies because the outcome assessors were blinded to the intraoperative management (Gold 1995; Vedel 2018).
Incomplete outcome data
Attrition bias was low in all three studies. They reported complete outcome data for all‐cause mortality. Although Vedel 2018 reported a difference in follow‐up of cognitive deterioration between high and low blood pressure target groups, we considered that the missing outcome data did not lead to a significant bias because the missingness in the outcome was unrelated to its true value in each intervention group.
Selective reporting
Reporting bias was unclear in Azau 2014 and Gold 1995 because the protocols were not available. Vedel 2018 reported all outcome data.
Other potential sources of bias
There was no other identified bias in all three studies.
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
Acute kidney injury
The evidence is very uncertain about the effect of a high blood pressure target on AKI, but the wide CIs were consistent with possible benefit and possible harm (RR 1.30, 95% CI 0.81 to 2.08; I² = 72%; 2 studies, 487 participants; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: High versus low blood pressure target, Outcome 1: Acute kidney injury
Cognitive deterioration
A high blood pressure target may result in little to no difference in cognitive deterioration, but the wide CIs were consistent with possible benefit and possible harm (RR 0.82, 95% CI 0.45 to 1.50; I² = 0%; 2 studies, 389 participants; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: High versus low blood pressure target, Outcome 2: Cognitive deterioration
All‐cause mortality
The evidence is very uncertain about the effect of a high blood pressure target on all‐cause mortality, but the wide CIs were consistent with possible benefit and possible harm (RR 1.33, 95% CI 0.30 to 5.90; I² = 49%; 3 studies, 737 participants; very low‐certainty evidence; Analysis 1.3). However, the worst absolute effect of the high blood pressure target compared with the low blood pressure target was 106 more deaths per 1000 participants, which could be judged as appreciable harm.
1.3. Analysis.

Comparison 1: High versus low blood pressure target, Outcome 3: All‐cause mortality
Secondary outcomes
Quality of life
The evidence is very uncertain about the effect of a high blood pressure target on quality of life, but the wide CIs were consistent with possible benefit and possible harm (RR 0.78, 95% CI 0.30 to 2.01; 1 study, 218 participants; very low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: High versus low blood pressure target, Outcome 5: Quality of life
Acute ischaemic stroke
The evidence is very uncertain about the effect of a high blood pressure target on acute ischaemic stroke (RR 1.29, 95% CI 0.07 to 23.73; I² = 82%; 2 studies, 437 participants; very low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: High versus low blood pressure target, Outcome 6: Acute ischaemic stroke
Haemorrhagic stroke
None of the three studies reported the number of haemorrhagic strokes.
Length of hospital stay
Vedel 2018 reported the length of stay in intensive care unit (ICU) and cardiac surgery ward separately. In addition, the length of ICU stay was reported in hours (mean 21 hours in high and low blood pressure groups) while the length of stay in cardiac surgery ward was reported in days (mean six days in both groups). However, it was unclear whether the length of stay in cardiac surgery ward included that in the ICU or not. Therefore, we excluded Vedel 2018 from this analysis.
The evidence is very uncertain about the effect of a high blood pressure target on length of hospital stay (MD 1.25 days, 95% CI 0.78 days to 1.73 days; I² = 76%; 2 studies, 540 participants; very low‐certainty evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1: High versus low blood pressure target, Outcome 7: Length of hospital stay
Renal replacement therapy
A high blood pressure target may result in little to no difference in renal replacement therapy but the wide CIs were consistent with possible benefit and possible harm (RR 1.33, 95% CI 0.47 to 3.77; I² = 0%; 2 studies, 486 participants; Analysis 1.8).
1.8. Analysis.

Comparison 1: High versus low blood pressure target, Outcome 8: Renal replacement therapy
Delirium
A high blood pressure target may result in little to no difference in delirium but the wide CIs were consistent with possible benefit and possible harm (RR 1.44, 95% CI 0.57 to 3.64; 1 study, 197 participants; Analysis 1.9). Since there were no missing data for delirium, we did not perform sensitivity analyses using best‐case scenario or worst‐case scenario.
1.9. Analysis.

Comparison 1: High versus low blood pressure target, Outcome 9: Delirium
Perioperative transfusion of blood products
The evidence is very uncertain about the effect of a high blood pressure target on perioperative transfusion of blood products (MD 0.10 units, 95% CI ‒0.13 to 0.34; I² = 0%; 2 studies, 540 participants; very low‐certainty evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1: High versus low blood pressure target, Outcome 10: Perioperative transfusion of blood products
Perioperative myocardial infarction
To diagnose perioperative myocardial infarction, the protocol of Vedel 2018 stated that they used European Society of Cardiology (ESC) classification. Although we contacted the author to request which version of ESC classification they used, we received no reply. Gold 1995 used the agreement of two cardiologists. Since we could not obtain the protocol of Azau 2014, it is uncertain how they diagnosed perioperative myocardial infarction.
A high blood pressure target may result in little to no difference in perioperative myocardial infarction but the wide CIs were consistent with possible benefit and possible harm (RR 0.91, 95% CI 0.26 to 3.16; I² = 18%; 3 studies, 734 participants; Analysis 1.11).
1.11. Analysis.

Comparison 1: High versus low blood pressure target, Outcome 11: Perioperative myocardial infarction
Sensitivity analysis
Sensitivity analyses for all the outcomes were consistent with their primary analyses except for AKI (Table 2). When studies at high risk of bias and those including participants undergoing isolated aortic surgery were excluded, the results appeared to be inconsistent with the primary analysis for the AKI outcome. However, given the sparsity of data, these results should be interpreted with caution.
1. Sensitivity analyses.
| Outcome | Studies with overall low risk of bias | Best‐case scenario | Worst‐case scenario | Exclusion of studies with SD imputed | Exclusion of studies including participants undergoing isolated aortic surgery | Exclusion of studies published before 2000 | Including only studies that considered learning effects caused by multiple testings |
| Acute kidney injury | RR 4.64 (95% CI 1.03 to 20.93) | — | — | — | RR 4.64 (95% CI 1.03 to 20.93) | — | — |
| Cognitive deterioration | RR 0.74 (95% CI 0.25 to 2.37) | RR 0.41 (95% CI 0.24 to 0.69) | RR 2.39 (95% CI 1.18 to 4.86) | — | — | RR 0.74 (95% CI 0.25 to 2.17) | RR 0.74 (95% CI 0.25 to 2.17) |
| All‐cause mortality | RR 9.09 (95% CI 0.50 to 166.63) | — | — | — | RR 1.52 (95% CI 0.07 to 34.75) | RR 0.62 (95% CI 0.16 to 2.34) | — |
| Acute ischaemic stroke | — | RR 0.81 (95% CI 0.14 to 4.74) | RR 1.85 (95% CI 0.05 to 72.46) | — | — | RR 6.33 (95% CI 0.78 to 51.54) | — |
| Length of hospital stay, day | — | — | — | MD ‒4.00 (95% CI ‒9.04 to 1.04) | MD ‒4.00 (95% CI ‒9.04 to 1.04) | MD 1.30 (95% CI 0.82 to 1.78) | — |
| Renal replacement therapy | — | RR 1.32 (95% CI 0.46 to 3.75) | RR 1.79 (95% CI 0.67 to 4.80) | — | RR 1.02 (95% CI 0.15 to 7.10) | — | — |
| Perioperative transfusion of blood products, unit | — | — | — | — | MD 0.20 (95% CI ‒1.06 to 1.46) | MD 0.10 (95% CI ‒0.14 to 0.34) | — |
| Perioperative myocardial infarction | — | RR 0.79 (95% CI 0.24 to 2.63) | RR 1.17 (95% CI 0.32 to 4.26) | — | RR 0.62 (95% CI 0.16 to 2.34) | RR 1.45 (95% CI 0.40 to 5.27) | — |
CI: confidence interval; MD: mean difference; RR: risk ratio; SD: standard deviation.
Discussion
Summary of main results
This systematic review identified three prospective RCTs evaluating adults undergoing cardiac surgery with CPB who were randomised to a high (MAP 65 mmHg or greater) or a low (MAP less than 65 mmHg) blood pressure target. We found that the evidence is very uncertain about the effect of a high blood pressure target on AKI and all‐cause mortality compared with a low blood pressure target and that a high blood pressure target may result in little to no difference in cognitive deterioration. Due to the low to very low certainty of the evidence and wide CIs, the effects of interventions were unclear in the primary and secondary outcomes reported. No studies reported haemorrhagic stroke. The sensitivity analyses excluding the study enroling participants undergoing isolated aortic surgery showed similar results with the main analysis. Based on the published data, we were unable to conduct any predetermined subgroup analyses.
Overall completeness and applicability of evidence
There are several limitations to the evidence identified in this systematic review. The number of studies that met the inclusion criteria for the review was low and all the included studies were conducted in a single‐centre setting, which may limit the external validity of the results. The number of participants included in the meta‐analysis was lower than the OIS for most outcomes. Not all the outcomes predefined in our protocol for the review were included in all the trials and some were not reported in any included trial (Kotani 2019). Since we used the MAP of 65 mmHg as the cut‐off value between high and low blood pressure targets, we excluded several studies that used different definitions of high and low blood pressure targets.
Quality of the evidence
See Table 1.
Although we found that two included studies reported AKI, the two studies used different definitions. Vedel 2018 reported the proportion of people with doubling of serum creatinine from the baseline. We considered this as AKI in this study because such a definition is consistent with stage 2 AKI according to the creatinine criteria given in the latest AKI classification (KDIGO 2012). Azau 2014 reported a 30% rise in serum creatinine as a surrogate for AKI, which is consistent with stage 1 AKI. These differences in the definitions of AKI between the studies could lead to indirectness of the outcome, which might be illustrated in the different incidence between the studies (2% to 9% in Vedel 2018 and 17% in Azau 2014). Therefore, we downgraded the certainty of the evidence of AKI for indirectness. In addition, due to serious concern about imprecision, risk of bias, and inconsistency, we concluded that the certainty of evidence for AKI was very low.
The evidence for cognitive deterioration was downgraded to low certainty due to imprecision from small sample size and study limitations.
The evidence for all‐cause mortality was downgraded to very low certainty. There was serious concern about imprecision in the small sample size, and the wide CIs suggested potential harm that was not neglectable. There was also serious concern about inconsistency and risk of bias.
The evidence of certainty was very low for the secondary outcomes, mainly due to study limitations, inconsistency, imprecision from the small sample size, and wide CIs, including the possibility of benefit and harm. Therefore, our review could not evaluate the effect of a high blood pressure target on these outcomes. No study reported haemorrhagic stroke.
Potential biases in the review process
Due to the relatively small sample size of the included studies, this review did not reach the OIS in most outcomes. Since most of the participants in the included studies were men, the included studies under‐represented women in their study populations. Because of the nature of the intervention, the attending physicians were not blinded to the allocation of the participants to the study groups. However, it was unlikely that a lack of blinding had an impact on primary outcomes of AKI, cognitive deterioration, and all‐cause mortality because the assessors of the primary outcomes were blinded to treatment allocation.
Agreements and disagreements with other studies or reviews
In this review, we investigated the benefits and harms of a high blood pressure target in comparison to a low blood pressure target during cardiac surgery with CPB. The result of our review found no beneficial effect of a high blood pressure target (MAP 65 mmHg or greater) during CPB. Our review was consistent with one narrative review on blood pressure management in perioperative care (Meng 2018), which stated that maintaining a higher blood pressure target, compared with a lower one, during CPB has no detrimental effect in people undergoing cardiac surgery based on the results of previous RCTs (Azau 2014; Charlson 2007; Gold 1995; Siepe 2011; Vedel 2018). Our review was also consistent with one guideline on CPB, which stated that the MAP of 50 mmHg to 80 mmHg is acceptable (Wahba 2020).
We found that the evidence is very uncertain about the effect of a high blood pressure target on AKI and that a high blood pressure target may result in little to no difference in renal replacement therapy. One review article on cardiac surgery‐associated AKI suggested that although clinicians seek to avoid low MAP during cardiac surgery on the basis of the conventional physiological assumption, there is a lack of evidence obtained from RCTs to support this clinicians' attitude (Wang 2017). The small number of participants included in our review led to a wide CIs. Thus, we are uncertain about the benefits or harms of a higher blood pressure target in terms of AKI.
We found that the evidence is very uncertain about the effect of a high blood pressure target on all‐cause mortality. Another RCT enroling people undergoing elective or urgent CABG found no deaths among the population (Siepe 2011). Since the mortality rate is low in elective cardiac surgery (Landoni 2019; Subramaniam 2019; Turan 2020), it would be difficult to find a significant difference in mortality between groups. Thus, we are uncertain about the benefits or harms of a higher blood pressure target in terms of mortality.
We found that a high blood pressure target may result in little to no difference in cognitive deterioration and delirium. In contrast, Siepe 2011 tested a hypothesis that keeping MAP of 80 mmHg to 90 mmHg compared with 60 mmHg to 70 mmHg during CPB would decrease early cognitive dysfunction and delirium after coronary bypass surgery. Siepe 2011 included people undergoing elective or urgent CABG and reported that cognitive deterioration was significantly less in the higher blood pressure target and that significantly fewer people in the higher blood pressure target developed postoperative delirium. However, there are two major limitations of Siepe 2011. One is the small sample size (92 people) and the other is that Siepe 2011 used the Mini‐Mental Status Examination as a cognitive outcome. Therefore, we are uncertain about the effect of a higher blood pressure target on cognitive deterioration and delirium. In addition, the evidence is very uncertain about the effect of a high blood pressure target on acute ischaemic stroke. None of the included studies in our review reported haemorrhagic stroke. Thus, we are uncertain about the benefits or harms of a higher blood pressure target in terms of neural outcomes.
Our review excluded two studies (Charlson 2007; Siepe 2011) due to a wrong intervention that were included in an existing review article (Meng 2018). Although the definition of a higher or lower blood pressure is still under investigation, it was essential to define the cut‐off value between high and low blood pressure targets for this review. We chose the cut‐off value of 65 mmHg because this value was adopted in sepsis and AKI guidelines (KDIGO 2012; Rhodes 2017). In addition, observational studies have suggested the association of hypotension during and after CPB with end‐organ dysfunction and mortality among people undergoing cardiac surgery with CPB. One large cohort study (6523 participants) showed that MAP less than 65 mmHg after CPB was associated with AKI and renal replacement therapy (Ngu 2020). One cohort study enroling 7457 people showed that MAP less than 65 mmHg during CPB was associated with stroke (Sun 2018). Another cohort study (6627 participants) showed the association between MAP less than 65 mmHg after CPB and mortality in people at intermediate risk of mortality (Ristovic 2020). These findings support that MAP 65 mmHg is appropriate for the cut‐off value of high and low blood pressure targets during cardiac surgery and warrant future trials to investigate the optimal blood pressure targets to reduce mortality or morbidities in the population. In contrast, one existing review article examining blood pressure targets in a perioperative setting did not set specific cut‐off values for blood pressure targets for literature search (Meng 2018). As a result, we excluded the two studies (Charlson 2007; Siepe 2011) that were included in the Meng 2018 review. Since the definition of a higher or lower blood pressure is still under investigation, we chose the cut‐off value of 65 mmHg because this value was adopted in a sepsis and AKI guideline (KDIGO 2012; Rhodes 2017).
Different patterns of CPB flow among studies might influence the effect of a high blood pressure target. All the included studies prespecified the flow rate during CPB. Vedel 2018 set 2.4 L/minute/m² in normothermia. Azau 2014 set CPB flow at 2.4 L/minute/m² in normothermia and further adjusted to maintain venous oxygen saturation above 70%. Gold 1995 used 1.6 L/minute/m² during cooling and 2.4 L/minute/m² during warming. A high blood pressure target showed a negative effect on several outcomes (mortality, acute ischaemic stroke, length of hospital stay, perioperative myocardial infarction) in Vedel 2018 and Azau 2014, while Gold 1995 revealed that a high blood pressure target was beneficial in these outcomes. This discrepancy can be explained by different CPB flows. During adequate CPB flow, a high blood pressure target can require more vasopressors or fluids, leading to adverse effects of these drugs, such as increased tissue oxygen demand. During low CPB flow, a high blood pressure can help to restore organ perfusion, reducing adverse outcomes.
Authors' conclusions
Implications for practice.
The findings of our review showed that a higher blood pressure target may make little or no difference to any outcomes including acute kidney injury (AKI), cognitive deterioration, or mortality among people undergoing cardiac surgery with cardiopulmonary bypass (CPB).
Implications for research.
The small number of participants included in our review and the very low quality of evidence indicate that future research is highly likely to change the estimated effect. Further research is needed to evaluate the effect of a higher blood pressure, to investigate the best cut‐off value for higher or lower mean arterial pressure (MAP) considering preoperative blood pressure in the CPB as well as post‐CPB periods among people undergoing cardiac surgery with CPB.
Conducting these studies will help to clarify the definition and effects of a higher blood pressure target for cardiac surgery with CPB.
History
Protocol first published: Issue 11, 2019
Acknowledgements
Cochrane Heart supported the authors in the development of this systematic review. The following people conducted the editorial process for this review.
Co‐ordinating Editor/Sign‐off Editor (final editorial decision): Professor Rui Providencia, Cochrane Heart, University College London.
Managing Editors (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the review): Ghazaleh Aali and Nicole Martin, Cochrane Heart, University College London.
Copy Editor (copy‐editing and production): Anne Lawson, Central Production Service, Cochrane.
Information Specialist: Farhad Shokraneh, Cochrane Heart, University College London and Charlene Bridges, Cochrane Heart, University College London.
We would like to acknowledge and thank the following people for the support of peer review: Adrian V Hernandez, Louise Sun, Charles W Hogue, David R McIlroy, Balachundhar Subramaniam, and one additional peer reviewer who wishes to remain anonymous.
We would like to acknowledge and thank the following people for their help in assessing the search results for this review via the Cochrane's Screen4Me workflow: Ben Ridley, Nicole Edworthy, Kathryn Vela, Stella Maria O'Brien, Abhijit Dutta, Tarig Fadalla, Ricky Ravindra Fajar Adi Putra, Tatjana Schuetz, Monika Buszko, Thidar Aung, Amanda Qiao Ying Yap, Nikolaos Sideris, Matias Mean, Dorothy Halfhide, Artem Oganesyan, Basem Emad Ali, Peter Edward Penson, Moustafa ElBadry Ahmed, Nuno Fernandes, Roberto Altamirano, Abhijna Vithal Yergolkar, Basavaraj Poojar, Abdul Shakoor, Amr Elsareih, Mohammad Shahbaz, Chet Chaulagai, Hao‐Wen Sim, Abdul Rehman Mustafa, SarahJane Moll, Brian Duncan, Nicole Askin, Vahid Reisi‐vanani, Mohammad Aloulou, Prasenjit Mitra, Gesiane Pajarinen, Martin Robausch, Constance Stegbauer, Bernardo Costa, Karen Ma, Diana Laura De la Torre, Sunu Alice Cherian, Ahmad Ozair, Igor Svintsitskyi, Fazal Ghani, Emma Branch, Therese Dalsbø, Ned Chalmers, Ilona Kovalova, Leire Leache, Ivan Belitsky, and Mohammad Nour Kitaz.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor: [Blood Pressure] explode all trees
#2 (blood pressure or bloodpressure)
#3 MeSH descriptor: [Hypertension] explode all trees
#4 hypertens*
#5 ((target* or strict* or goal* or tight* or intensive* or below or control or lowering) NEAR/3 (systolic or diastolic or bp or dbp or sbp or antihypertensive* or anti hypertensive*))
#6 ((bp or blood pressure) NEAR/2 lowering)
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Cardiac Surgical Procedures] explode all trees
#9 ((heart or cardiac or aortic or mitral or pulmonary or tricuspid or valv*) NEAR/4 (surg* or replace* or repair* or reconstruc* or operat*))
#10 MeSH descriptor: [Coronary Artery Bypass] explode all trees
#11 ((coronary or heart or cardio* or cardiac* or valve) NEAR/5 (surg* or graft* or bypass or plasty or replacement))
#12 CABG
#13 #8 or #9 or #10 or #11 or #12
#14 MeSH descriptor: [Cardiopulmonary Bypass] this term only
#15 ((coronary or heart or cardio* or cardiac* or valve) NEAR/5 (surg* or graft* or bypass or plasty or replacement))
#16 CPB
#17 #14 or #15 or #16
#18 #13 and #17
#19 #7 and #18
MEDLINE Ovid
1 exp blood pressure/
2 (blood pressure or bloodpressure).tw.
3 exp hypertension/
4 hypertens*.tw.
5 ((target* or strict* or goal* or tight* or intensive* or below or control or lowering) adj3 (systolic or diastolic or bp or dbp or sbp or antihypertensive* or anti hypertensive*)).tw.
6 ((bp or blood pressure) adj2 lowering).tw.
7 1 or 2 or 3 or 4 or 5 or 6
8 exp Cardiac surgical procedures/
9 ((heart or cardiac or aortic or mitral or pulmonary or tricuspid or valv*) adj4 (surg* or replace* or repair* or reconstruc* or operat*)).tw.
10 exp Coronary Artery Bypass/
11 ((coronary or heart or cardio* or cardiac* or valve) adj5 (surg* or graft* or bypass or plasty or replacement)).tw.
12 CABG.tw.
13 8 or 9 or 10 or 11 or 12
14 Cardiopulmonary Bypass/
15 ((coronary or heart or cardio* or cardiac* or valve) adj5 (surg* or graft* or bypass or plasty or replacement)).tw.
16 CPB.tw.
17 14 or 15 or 16
18 13 and 17
19 7 and 18
20 randomized controlled trial.pt.
21 controlled clinical trial.pt.
22 randomized.ab.
23 placebo.ab.
24 clinical trials as topic.sh.
25 randomly.ab.
26 trial.ti.
27 20 or 21 or 22 or 23 or 24 or 25 or 26
28 exp animals/ not humans.sh.
29 27 not 28
30 19 and 29
Embase Ovid
1 exp blood pressure/
2 (blood pressure or bloodpressure).tw.
3 exp hypertension/
4 hypertens*.tw.
5 ((target* or strict* or goal* or tight* or intensive* or below or control or lowering) adj3 (systolic or diastolic or bp or dbp or sbp or antihypertensive* or anti hypertensive*)).tw.
6 ((bp or blood pressure) adj2 lowering).tw.
7 1 or 2 or 3 or 4 or 5 or 6
8 exp heart surgery/
9 ((heart or cardiac or aortic or mitral or pulmonary or tricuspid or valv*) adj4 (surg* or replace* or repair* or reconstruc* or operat*)).tw.
10 exp coronary artery bypass graft/
11 ((coronary or heart or cardio* or cardiac* or valve) adj5 (surg* or graft* or bypass or plasty or replacement)).tw.
12 CABG.tw.
13 8 or 9 or 10 or 11 or 12
14 cardiopulmonary bypass/
15 ((coronary or heart or cardio* or cardiac* or valve) adj5 (surg* or graft* or bypass or plasty or replacement)).tw.
16 CPB.tw.
17 14 or 15 or 16
18 13 and 17
19 7 and 18
20 random$.tw.
21 factorial$.tw.
22 crossover$.tw.
23 cross over$.tw.
24 cross‐over$.tw.
25 placebo$.tw.
26 (doubl$ adj blind$).tw.
27 (singl$ adj blind$).tw.
28 assign$.tw.
29 allocat$.tw.
30 volunteer$.tw.
31 crossover procedure/
32 double blind procedure/
33 randomized controlled trial/
34 single blind procedure/
35 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34
36 (animal/ or nonhuman/) not human/
37 35 not 36
38 19 and 37
39 limit 38 to embase
Web of Science
# 16 #15 AND #14
# 15 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)
# 14 #13 AND #5
# 13 #12 AND #9
# 12 #11 OR #10
# 11 TS=CPB
# 10 TS=((coronary or heart or cardio* or cardiac* or valve) NEAR/5 (surg* or graft* or bypass or plasty or replacement))
# 9 #8 OR #7 OR #6
# 8 TS=CABG
# 7 TS=((coronary or heart or cardio* or cardiac* or valve) NEAR/5 (surg* or graft* or bypass or plasty or replacement))
# 6 TS=((heart or cardiac or aortic or mitral or pulmonary or tricuspid or valv*) NEAR/4 (surg* or replace* or repair* or reconstruc* or operat*))
# 5 #4 OR #3 OR #2 OR #1
# 4 TS= ((bp or "blood pressure") NEAR/2 lowering)
# 3 TS=((target* or strict* or goal* or tight* or intensive* or below or control or lowering) NEAR/3 (systolic or diastolic or bp or dbp or sbp or antihypertensive* or "anti hypertensive*"))
# 2 TS=hypertens*
# 1 TS=(blood pressure or bloodpressure)
ClinicalTrials.gov
Condition or disease: cardiac surgery
Study type: Interventional Studies (Clinical Trials)
Other terms: cardiopulmonary bypass
WHO ICTRP
"cardiac surgery" AND "cardiopulmonary bypass"
Data and analyses
Comparison 1. High versus low blood pressure target.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Acute kidney injury | 2 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.81, 2.08] |
| 1.2 Cognitive deterioration | 2 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.45, 1.50] |
| 1.3 All‐cause mortality | 3 | 737 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.30, 5.90] |
| 1.5 Quality of life | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6 Acute ischaemic stroke | 2 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.07, 23.63] |
| 1.7 Length of hospital stay | 2 | 540 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [0.78, 1.73] |
| 1.8 Renal replacement therapy | 2 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.47, 3.77] |
| 1.9 Delirium | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.10 Perioperative transfusion of blood products | 2 | 540 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.13, 0.34] |
| 1.11 Perioperative myocardial infarction | 3 | 734 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.26, 3.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azau 2014.
| Study characteristics | ||
| Methods | Study design: randomised, open‐label controlled clinical trial Study duration: 30 months between January 2008 and June 2010 'Run‐in' period: none Number of study centres and location: 1 in France |
|
| Participants | Total number of study participants: 300 Number of randomised participants: 300 Number lost to follow‐up: 0 Number withdrawn: 8 Number analysed: 292 Mean age: 76 years Gender: 200 men Inclusion criteria: elective cardiac surgery, including CABG, valvular surgery or reconstructive surgery of the ascending aorta performed under normothermic CPB in people with known risk factors for AKI. These risk factors were serum creatinine clearance 30–60 mL/minute/1.73 m² or 2 factors among the following: aged > 60 years, diabetes mellitus, and diffuse atherosclerosis. Exclusion criteria: infusion of a radiocontrast agent 1 week before surgery or treatment with a nephrotoxic agent 3 weeks before surgery; chemotherapy within last 3 months; liver cirrhosis; heart failure (left ventricular ejection fraction < 30%); renal artery stenosis; pulmonary hypertension (systolic pulmonary pressure > 60 mmHg); endocarditis; surgery requiring hypothermic CPB. Patients who eventually had a major perioperative complication (shock, emergent reoperation) identified as AKI cause disclosed a major perioperative complication (shock, emergent reoperation) |
|
| Interventions | Experimental: maintaining MAP during CPB at 75–85 mmHg by infusing noradrenaline Comparison: maintaining MAP during CPB at 50–60 mmHg. Vasopressors were administered when MAP < 50 mmHg. Concomitant treatment: following anaesthesia induction, a systematic 12 mL/kg saline infusion load was administered in all participants. CPB was performed with a roller pump. Before and after CPB, and in both groups, the MAP endpoint was 70–90 mmHg, with venous oxygen saturation > 70% until the end of surgery. CPB flow was set at 2.4 L/minute/m2 and further adjusted to maintain SvO2 > 70%. |
|
| Outcomes | Primary endpoint: 30% rise in serum creatinine was the primary endpoint and surrogate for AKI. Renal function assessed at day 28 and 6 months after surgery. There were additional surrogate endpoints for AKI: RIFLE "risk" category (50% rise in serum creatinine or glomerular filtration rate decrease > 25%, urine output < 0.5 mL/kg/hour × 6 hours); RIFLE 'injury' category (100% rise in serum creatinine or glomerular filtration rate decrease > 50%, urine output < 0.5 mL/kg/hour × 12 hours); > 50% postoperative rise of serum creatinine; and need for haemodialysis. Neurological complications of surgery (stroke, seizure, transient mental confusion/agitation) were also recorded. Death date was identified either by medical records received from doctors who cared for the patients after discharge from hospital or, in the absence of such records, the authors asked the national registry of deaths. |
|
| Notes | Supported by a grant from the "Programme Hospitalier pour la Recherche Clinique" (PHRC Inter‐Régional, 2007) from the French Health Ministry. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not provided. |
| Allocation concealment (selection bias) | Unclear risk | Although this study used opaque sealed envelopes, their random sequence generation was unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | In the methods section, the authors reported that participants were blinded but study personnel were not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The definitions and assessors for several outcomes, i.e. cognitive deterioration, acute ischaemic stroke, haemorrhagic stroke, delirium, and perioperative myocardial infarction, were unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up 2.7% (8/300). |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. |
| Other bias | Low risk | Review authors believed the study free of other sources of bias. |
Gold 1995.
| Study characteristics | ||
| Methods | Study design: randomised, open‐label controlled clinical trial Study duration: 30 months between October 1991 and February 1994 'Run‐in' period: none Number of study centre and location: 1 in the US |
|
| Participants | Total number of study participants: 251 Number of randomised participants: 248 Number lost to follow‐up: 0 Number withdrawn: 0 Number analysed: 248 Mean age: 65.8 years Gender: 160 men Inclusion criteria: people undergoing primary elective multivessel CABG for left main or multivessel coronary artery disease Exclusion criteria: inability to complete the neuropsychological tests (blindness, deafness, language difficulties), participation in other studies, and inability to return for follow‐up. |
|
| Interventions | Experimental: maintaining MAP during CPB at 80–100 mm Hg Comparison: maintaining MAP during CPB at 50–60 mm Hg Concomitant medications: CPB flow by body surface area and temperature were held constant, and vasoactive drugs were used to maintain MAP in the desired range. Anaesthesia was induced with thiopental (1–2 mg/kg), fentanyl (25 μg/kg), and pancuronium, and was maintained with a fentanyl bolus (1–5 μg/kg, to a total of 50–70 μg/kg), midazolam, or isoflurane (pre‐CPB and post‐CPB periods only). After sternotomy and pericardial incision, heparin was administered to maintain an activated clotting time > 480 seconds. After cannulation of the aorta and right atrium, non‐pulsatile CPB was instituted. Flow rates were set at 1.6 L/minute/m² during cooling and 2.4 L/minute/m² during warming. If the MAP increased above the target level and was unresponsive to fentanyl or midazolam, sodium nitroprusside infusion was administered. If the MAP fell below the target level, phenylephrine was used. If necessary, noradrenaline or metaraminol was added. Intraoperative ischaemia was managed by an identical algorithm in both groups. Excluded medications: none |
|
| Outcomes | Mortality, cardiac morbidity, neurological morbidity, deterioration in cognitive status, and deterioration in quality of life reported at 6 months. Cardiac complications were myocardial infarction, pulmonary oedema, adult respiratory distress syndrome, low flow state/cardiogenic shock, and cardiopulmonary arrest. Definite stroke was the principal neurological complication determined by the neurologist. Stroke included the new onset of a localised and persistent neurological deficit (e.g. paresis, plegia, aphasia, hemianopsia, cortical blindness). Deterioration on ≥ 3 cognitive tests was defined as a cognitive complication. For each test, the assessment was based on within‐patient change in test performance from preoperative baseline. Changes from preoperative to postoperative function that would be considered clinically important were determined a priori by a panel of experts. Deterioration in quality of life was defined as a decline of > 5 points on the Physical Component Summary score of the SF‐36. |
|
| Notes | Funding source: grant HL44719 from the National Institutes of Health, National Heart, Lung, and Blood Institute. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation based on a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Information not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information not provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study cardiologist and neurologist, blinded to the intraoperative management, performed standardised examinations at 1, 2, and 7 days, and 6 months after the operation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up among the randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | Information not provided. |
| Other bias | Low risk | Review authors believed the study to be free of other sources of bias. |
Vedel 2018.
| Study characteristics | ||
| Methods | Study design: randomised, open‐label controlled clinical trial Study duration: 19 months between July 2014 and January 2016 'Run‐in' period: none Number of study centre and location: 1 in Denmark |
|
| Participants | Total number of study participants: 197 Number of randomised participants: 197 Number lost to follow‐up: 0 Number withdrawn: 3 Number analysed: 197 Mean age: 67.2 years Gender: 177 men Inclusion criteria: aged ≥ 18 years and in need of elective or subacute CABG or left heart valve surgery (or both) using CPB Exclusion criteria: history of stroke or intracranial bleeding, history of reversible ischaemic deficits (duration of symptoms 24–72 hours), history of transient ischaemic attacks (duration of symptoms < 24 hours), diagnosis of neurodegenerative disorders such as Alzheimer's disease and multiple sclerosis, or contraindications to magnetic resonance imaging |
|
| Interventions | Experimental: maintaining MAP at 70–80 mmHg during CPB Comparison: maintaining MAP at 40–50 mmHg during CPB Concomitant medications: an intended fixed, equal, and non‐pulsatile blood flow of 2.4 L/minute/m² body surface area plus 10–20% was applied in both groups, and assigned MAP levels were targeted with intermittent intravenous doses of phenylephrine to a total maximum dose of 2.0 mg followed by continuous intravenous infusion of noradrenaline up to 0.4 μg/kg/minute. Concomitant treatment interventions were at the treating clinicians' discretion. According to departmental guidelines, CPB was performed with arterial oxygen saturation > 96% (and partial pressure of oxygen in the arterial blood > 13.0 kPa), normocapnia (partial pressure of carbon dioxide in the arterial blood 4.5–6.0 kPa), normothermia (body temperature > 36.5 °C), α‐stat pH management, and transfusion of packed red blood cells if haematocrit was < 24% (or at higher haematocrit levels in case of lactic acidosis or low mixed venous saturation). Excluded medications: no vasodilatory drugs were accepted in the low‐target group, and MAP levels above the low‐target window were accepted. |
|
| Outcomes | Primary outcome: total volume of new ischaemic lesions (sum in millimetres cubed), expressed as the difference between DWI conducted preoperatively and again between days 3 and 6. Secondary outcomes: total number of new ischaemic cerebral lesions, POCD, new focal neurological deficits, both evaluated as a change from baseline neuropsychological and neurological test performance to the result at 1 week, at discharge from the hospital, or at healthcare relocation from the cardiac surgery ward to a local hospital, whichever came first. Cognitive function was re‐evaluated at 2–4 months postoperatively. Used the International Study of Postoperative Cognitive Dysfunction test battery, 14–16 which was developed for people with an intact cognitive capability. Also conducted a Mini‐Mental State Examination to screen for potential signs of dementia. If a participant had a baseline Mini‐Mental State Examination score ≤ 24, there was no further cognitive testing. |
|
| Notes | Dr Vedel received grants from the Danish Heart Foundation (14‐R97‐ A5179‐22868 and 15‐R99‐A6034‐22905) and the Research Foundations at Rigshospitalet (E‐22329‐01), University of Copenhagen, Denmark. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised web‐based randomisation was performed with a computer‐generated allocation sequence with varying block size of 4–8. |
| Allocation concealment (selection bias) | Low risk | Centralised web‐based randomisation was performed with a computer‐generated allocation sequence with varying block size of 4–8. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were blinded from the group allocation. Healthcare providers in the intensive care unit and ward were unaware of the assigned MAP strategy unless they specifically consulted the handwritten anaesthesia report and guessed to which group the participant was allocated based on the continuous registration of MAP data. Throughout the trial, the study authors stressed that the staff involved in the experimental setup in the operating room at no point disclosed group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | According to the protocol, assessors of the primary and selected secondary endpoints (DWI scans and POCD) were blinded to treatment allocation. Furthermore, blood samples were labelled with a unique participant number, and personnel carrying out blood sample analysis did not know the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up 3.0% (6/197). |
| Selective reporting (reporting bias) | Low risk | All outcome data but MRS described in the protocol (Vedel 2016 – see under Vedel 2018) were reported. |
| Other bias | Low risk | Review authors believed the study free of other sources of bias. |
AKI: acute kidney injury; CABG: coronary artery bypass graft; CPB: cardiopulmonary bypass; DWI: diffusion‐weighted imaging; MAP: mean arterial pressure; MRS: magnetic resonance spectroscopy; POCD: postoperative cognitive dysfunction; SF‐36: 36‐item Short Form.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aghadavoudi Jolfaei 2012 | Wrong intervention |
| Aronson 2011 | Wrong study design |
| Bagheri 2012 | Wrong intervention |
| Bertolissi 1996 | Wrong intervention |
| Brown 2019 | Wrong population |
| Charlson 2007 | Wrong intervention |
| CTRI/2018/01/011487 | Wrong intervention |
| Damén 2021 | Wrong intervention |
| Ge 2018 | Wrong study design |
| Getsios 2013 | Wrong intervention |
| Goepfert 2013 | Wrong intervention |
| Hamada 2004 | Wrong intervention |
| IRCT2015112916151N4 | Wrong population |
| Kapoor 2008 | Wrong intervention |
| Kapoor 2016 | Wrong intervention |
| Mölström 2017 | Wrong intervention |
| NCT01408420 | Wrong intervention |
| NCT04005105 | Wrong intervention |
| Paulson 2019 | Wrong study design |
| Siepe 2011 | Wrong intervention |
| Sirvinskas 2008 | Wrong intervention |
| Urzua 1992 | Wrong intervention |
Characteristics of studies awaiting classification [ordered by study ID]
von Knobelsdorff 1996.
| Methods | Randomised, parallel group trial |
| Participants | ASA III patients undergoing CABG |
| Interventions | Experimental: mean arterial pressure > 70 mmHg during rewarming of cardiopulmonary bypass. Comparator: mean arterial pressure 55–65 mmHg during rewarming of cardiopulmonary bypass. |
| Outcomes | Jugular bulb oxygen saturation |
| Notes | No response to email requests for the full‐text article. |
ASA: American Society of Anesthesiologists; CABG: coronary artery bypass graft.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR2000028941.
| Study name | Target blood pressure management during cardiopulmonary bypass improves postoperative lactic acid levels: a randomised controlled clinical study |
| Methods | Design: single‐centre parallel‐group double‐blind randomised controlled trial |
| Participants | Inclusion criteria: selective heart valve surgery; aged > 18 years; New York Heart Association class II–III; ejection fraction > 50%; blood lactate level < 1.6 mmol/L; alanine transferase < 50 U/L; brain natriuretic peptide < 100 pg/mL; stay overnight in intensive care unit after surgery Exclusion criteria: secondary aortic occlusion during operation; emergency surgery again within 24 hours after the operation. |
| Interventions | Experimental: mean arterial pressure 70–80 mmHg with noradrenaline Control: mean arterial pressure 50–60 mmHg with or without vasoactive drugs |
| Outcomes | Primary outcome: postoperative blood lactate value |
| Starting date | Date of approved by ethic committee: 31 October 2019 |
| Contact information | Miao Qing Tel: +86 15026635802 E‐mail: miaoqmz@163.com Affiliation: Department of Anesthesiology, Shanghai Chest Hospital, Shanghai Jiaotong University, Shanghai, China |
| Notes | Prospective registration Status: recruiting |
Differences between protocol and review
We made the following changes from the protocol (Kotani 2019).
In the protocol, we excluded isolated aortic surgery because the surgical procedure itself can affect peripheral organ perfusion differently from CABG or heart valve surgery. However, we included isolated aortic surgery because we believe such a procedural difference hardly affects blood pressure management. Besides, we added a sensitivity analysis excluding studies including participants undergoing isolated aortic surgery to investigate the procedural difference can affect the effect size.
Since Gold 1995 was the only study to report quality of life and they reported quality of life as a dichotomous outcome, we also counted quality of life as a dichotomous variable.
Since the amount of intraoperative haemorrhage is easily influenced by multiple factors such as intraoperative blood salvage and cardioplegic solution, we removed the outcome and added perioperative transfusion of blood products. Since we did not replace the outcome of the amount of intraoperative haemorrhage with perioperative transfusion of blood products in the summary of findings table in the protocol, we revised the outcome.
We defined the measurement period to the outcome of perioperative transfusion of blood products as at any time from the cardiac surgery to hospital discharge.
We added the sensitivity analysis excluding studies published before 2000 because the practice and outcomes of cardiac surgery have evolved significantly over time.
We conducted a sensitivity analysis for cognitive deterioration, including only studies which considered learning effects caused by multiple testings for the following reason. Cognitive deterioration was defined as a relative decrease of cognitive score from the baseline, which does not consider learning effects caused by repeated examination of the same test. In contrast, neuropsychological tests such as the International Study of Postoperative Cognitive Dysfunction (ISPOCD; Moller 1998) subtract the mean learning effect from the postoperative changes from the baseline, which can provide more appropriate criteria.
Contributions of authors
YKo: conception of the review, design of the review, search and selection of studies for inclusion in the review, assessment of the risk of bias in the included studies, analysis of data, assessment of certainty of evidence, interpretation of data, writing of the review, and approval of the final version of the manuscript. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
YKa: design of the review, search and selection of studies for inclusion in the review, assessment of the risk of bias in the included studies, analysis of data, assessment of certainty of evidence, interpretation of data, writing of the review, and approval of the final version of the manuscript. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
JI: conception of the review, design of the review, search and selection of studies for inclusion in the review, interpretation of data, writing of the review, and approval of the final version of the manuscript. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
SF: conception of the review, design of the review, search and selection of studies for inclusion in the review, collection of data for the review, assessment of the risk of bias in the included studies, interpretation of data, writing of the review, and approval of the final version of the manuscript. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
TY: conception of the review, design of the review, search and selection of studies for inclusion in the review, collection of data for the review, assessment of the risk of bias in the included studies, interpretation of data, writing of the review, and approval of the final version of the manuscript. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
JK: conception of the review, design of the review, search and selection of studies for inclusion in the review, interpretation of data, writing of the review, and approval of the final version of the manuscript. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
JSWK: design of the review, co‐ordination of the review, writing of the review, and approval of the final version of the manuscript. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sources of support
Internal sources
-
New Source of support, Other
None
External sources
-
NIHR, UK
This project was supported by the NIHR via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health and Social Care.
Declarations of interest
YKo: none.
YKa: none.
JI: none.
SF: none.
TY: none.
JK: none.
JSWK: none.
New
References
References to studies included in this review
Azau 2014 {published data only}
- Azau A, Markowicz P, Corbeau JJ, Cottineau C, Moreau X, Baufreton C, et al. Increasing mean arterial pressure during cardiac surgery does not reduce the rate of postoperative acute kidney injury. Perfusion 2014;29(6):496-504. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gold 1995 {published data only}
- Gold JP, Charlson ME, Williams-Russo P, Szatrowski TP, Peterson JC, Pirraglia PA, et al. Improvement of outcomes after coronary artery bypass. A randomized trial comparing intraoperative high versus low mean arterial pressure. Journal of Thoracic and Cardiovascular Surgery 1995;110(5):1302-11. [DOI] [PubMed] [Google Scholar]
- NCT00248885. Peri-operative morbidity and quality of life after coronary artery bypass graft (CABG). clinicaltrials.gov/ct2/show/NCT00248885 (first received 4 November 2005).
- Pirraglia PA, Peterson JC, Hartman GS, Yao FS, Thomas SJ, Charlson ME. The efficacy and safety of a pharmacologic protocol for maintaining coronary artery bypass patients at a higher mean arterial pressure during cardiopulmonary bypass. Journal of Extra-Corporeal Technology 1998;30(2):64-72. [PubMed] [Google Scholar]
Vedel 2018 {published data only}
- Holmgaard F, Vedel AG, Lange T, Nilsson JC, Ravn HB. Impact of 2 distinct levels of mean arterial pressure on near-infrared spectroscopy during cardiac surgery: secondary outcome from a randomized clinical trial. Anesthesia & Analgesia 2019;128(6):1081-8. [DOI] [PubMed] [Google Scholar]
- Holmgaard F, Vedel AG, Ravn HB, Nilsson JC, Rasmussen LS. Impact of mean arterial pressure on sublingual microcirculation during cardiopulmonary bypass-secondary outcome from a randomized clinical trial. Microcirculation 2018;25(5):e12459. [DOI] [PubMed] [Google Scholar]
- Larsen MH, Draegert C, Vedel AG, Holmgaard F, Siersma V, Nilsson JC, et al. Long-term survival and cognitive function according to blood pressure management during cardiac surgery. A follow-up. Acta Anaesthesiologica Scandinavica 2020;64(7):936-44. [DOI] [PubMed] [Google Scholar]
- NCT02185885. Perfusion Pressure Cerebral Infarction trial (PPCI) [The importance of mean arterial pressure during cardiopulmonary bypass to prevent cerebral complications after cardiac surgery – a randomised clinical trial]. clinicaltrials.gov/ct2/show/NCT02185885 (first received 10 July 2014).
- Vedel AG, Holmgaard F, Danielsen ER, Langkilde A, Paulson OB, Ravn HB, et al. Blood pressure and brain injury in cardiac surgery: a secondary analysis of a randomized trial. European Journal of Cardiothoracic Surgery 2020;58(5):1035-44. [DOI] [PubMed] [Google Scholar]
- Vedel AG, Holmgaard F, Rasmussen LS, Langkilde A, Paulson OB, Lange T, et al. High-target versus low-target blood pressure management during cardiopulmonary bypass to prevent cerebral injury in cardiac surgery patients: a randomized controlled trial. Circulation 2018;137(17):1770-80. [DOI] [PubMed] [Google Scholar]
- Vedel AG, Holmgaard F, Rasmussen LS, Paulson OB, Thomsen C, Danielsen ER, et al. Perfusion Pressure Cerebral Infarct (PPCI) trial – the importance of mean arterial pressure during cardiopulmonary bypass to prevent cerebral complications after cardiac surgery: study protocol for a randomised controlled trial. Trials 2016;17(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedel AG, Holmgaard F, Siersma V, Langkilde A, Paulson OB, Ravn HB, et al. Domain-specific cognitive dysfunction after cardiac surgery. A secondary analysis of a randomized trial. Acta Anaesthesiologica Scandinavica 2019;63(6):730-8. [DOI] [PubMed] [Google Scholar]
- Wiberg S, Holmgaard F, Blennow K, Nilsson JC, Kjaergaard J, Wanscher M, et al. Associations between mean arterial pressure during cardiopulmonary bypass and biomarkers of cerebral injury in patients undergoing cardiac surgery: secondary results from a randomized controlled trial. Interactive Cardiovascular and Thoracic Surgery 2021;32(2):229-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg S, Vedel AG, Holmgaard F, Kjaergaard J, Langkilde AR, Hassager C, et al. Lack of association between gaseous microembolisms assessed by a single detection device and cerebral complications in cardiac surgery patients. Journal of Cardiothoracic and Vascular Anesthesia 2020;34(6):1496-503. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aghadavoudi Jolfaei 2012 {published data only}
- Aghadavoudi Jolfaei O, Bagheri K, Motamedi O, Akbari M. The effect of mean arterial pressure during cardiopulmonary bypass on clinical and para clinical parameters during and after coronary artery bypass graft surgery: 4AP7-2. European Journal of Anaesthesiology 2012;29:69-70. [Google Scholar]
Aronson 2011 {published data only}
- Aronson S, Varon J. Hemodynamic control and clinical outcomes in the perioperative setting. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(3):509-25. [DOI] [PubMed] [Google Scholar]
Bagheri 2012 {published data only}
- Bagheri K, Motamedi O, Aghadavoudi O, Akbari M. The effects of mean arterial pressure during cardiopulmonary bypass on clinical and paraclinical parameters during and after coronary artery bypass graft surgery. Journal of Isfahan Medical School 2012;29(169):1-10. [Google Scholar]
Bertolissi 1996 {published data only}
- Bertolissi M, Antonucci F, De Monte A, Padovani R, Giordano F. Effects on renal function of a continuous infusion of nifedipine during cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 1996;10(2):238-42. [DOI] [PubMed] [Google Scholar]
Brown 2019 {published data only}
- Brown CH 4th, Neufeld KJ, Tian J, Probert J, LaFlam A, Max L, et al. Effect of targeting mean arterial pressure during cardiopulmonary bypass by monitoring cerebral autoregulation on postsurgical delirium among older patients: a nested randomized clinical trial. JAMA Surgery 2019;154(9):819-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charlson 2007 {published data only}
- Charlson ME, Peterson JC, Krieger KH, Hartman GS, Hollenberg JP, Briggs WM, et al. Improvement of outcomes after coronary artery bypass II: a randomized trial comparing intraoperative high versus customized mean arterial pressure. Journal of Cardiac Surgery 2007;22(6):465-72. [DOI] [PubMed] [Google Scholar]
CTRI/2018/01/011487 {published data only}
- CTRI/2018/01/011487. Comparison of blood pressure control by CLAPS with manual control in patients undergoing elective cardiac surgery. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=22127 (first received 23 January 2018).
Damén 2021 {published data only}
- Damén T, Saadati S, Forssell-Aronsson E, Hesse C, Bentzer P, Ricksten SE, et al. Effects of different mean arterial pressure targets on plasma volume, ANP and glycocalyx – a randomized trial. Acta Anaesthesiologica Scandinavica 2021;65(2):220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ge 2018 {published data only}
- Ge HW, Kong F. Letter by Ge and Kong regarding article, "High-target versus low-target blood pressure management during cardiopulmonary bypass to prevent cerebral injury in cardiac surgery patients: a randomized controlled trial". Circulation 2018;138(21):2445-6. [DOI] [PubMed] [Google Scholar]
Getsios 2013 {published data only}
- Getsios D, Wang Y, Stolar M, Williams G, Ishak KJ, Hu MY, et al. Improved perioperative blood pressure control leads to reduced hospital costs. Expert Opinion on Pharmacotherapy 2013;14(10):1285-93. [DOI] [PubMed] [Google Scholar]
Goepfert 2013 {published data only}
- Goepfert MS, Richter HP, Zu Eulenburg C, Gruetzmacher J, Rafflenbeul E, Roeher K, et al. Individually optimized hemodynamic therapy reduces complications and length of stay in the intensive care unit: a prospective, randomized controlled trial. Anesthesiology 2013;119(4):824-36. [DOI] [PubMed] [Google Scholar]
Hamada 2004 {published data only}
- Hamada H, Nakagawa I, Uesugi F, Kubo T, Hiramatsu T, Kai T, et al. Effects of perfusion pressure on cerebral blood flow and oxygenation during normothermic cardiopulmonary bypass. Masui 2004;53(7):744-52. [PubMed] [Google Scholar]
IRCT2015112916151N4 {published data only}
- IRCT2015112916151N4. Effect of intraoperative hypertension on pain after surgery. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015112916151N4 (accessed 24 April 2020).
Kapoor 2008 {published data only}
- Kapoor PM, Kakani M, Chowdhury U, Choudhury M, Lakshmy, Kiran U. Early goal-directed therapy in moderate to high-risk cardiac surgery patients. Annals of Cardiac Anaesthesia 2008;11(1):27-34. [DOI] [PubMed] [Google Scholar]
Kapoor 2016 {published data only}
- Kapoor PM, Magoon R, Rawat R, Mehta Y. Perioperative utility of goal-directed therapy in high-risk cardiac patients undergoing coronary artery bypass grafting: "A clinical outcome and biomarker-based study". Annals of Cardiac Anaesthesia 2016;19(4):638-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mölström 2017 {published data only}
- Mölström S, Nielsen TH, Andersen C, Nordström CH, Toft P. Bedside monitoring of cerebral energy state during cardiac surgery – a novel approach utilizing intravenous microdialysis. Journal of Cardiothoracic and Vascular Anesthesia 2017;31(4):1166-73. [DOI] [PubMed] [Google Scholar]
NCT01408420 {published data only}
- NCT01408420. Perfusion – Pressure – Creatinine trial (PPC). clinicaltrials.gov/ct2/show/NCT01408420 (first received 3 August 2011).
NCT04005105 {published data only}
- NCT04005105. Acute post-cardiac surgery renal failure: prevention through individualized intensive hemodynamic management (PrevHemAKI). clinicaltrials.gov/ct2/show/NCT04005105 (first received 2 July 2019).
Paulson 2019 {published data only}
- Paulson OB, Vedel AG, Holmgaard F, Rasmussen LS, Danielsen ER, Langkilde A, et al. Hemodynamic or thromboembolic stroke – what have we learned from cardiac surgery? Journal of Cerebral Blood Flow and Metabolism 2019;39(1 Suppl):PB01-V07. [Google Scholar]
Siepe 2011 {published data only}
- Siepe M, Pfeiffer T, Gieringer A, Zemann S, Benk C, Schlensak C, et al. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. European Journal of Cardio-Thoracic Surgery 2011;40(1):200-7. [DOI] [PubMed] [Google Scholar]
Sirvinskas 2008 {published data only}
- Sirvinskas E, Andrejaitiene J, Raliene L, Nasvytis L, Karbonskiene A, Pilvinis V, et al. Cardiopulmonary bypass management and acute renal failure: risk factors and prognosis. Perfusion 2008;23(6):323-7. [DOI] [PubMed] [Google Scholar]
Urzua 1992 {published data only}
- Urzua J, Troncoso S, Bugedo G, Canessa R, Muñoz H, Lema G, et al. Renal function and cardiopulmonary bypass: effect of perfusion pressure. Journal of Cardiothoracic and Vascular Anesthesia 1992;6(3):299-303. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
von Knobelsdorff 1996 {published data only}
- Knobelsdorff G, Hänel F, Werner C, Schulte am Esch J. Effect of arterial blood pressure on cerebral vein oxygen saturation in the rewarming phase of extracorporeal circulation. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 1996;31(5):298-303. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR2000028941 {published data only}
- ChiCTR2000028941. Target blood pressure management during cardiopulmonary bypass improves postoperative lactic acid levels: a randomized controlled clinical study [体外循环期间目标血压管理改善术后乳酸水平:一项随机对照的临床研究]. www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2000028941 (first received 31 October 2019).
Additional references
Annane 2018
- Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. New England Journal of Medicine 2018;378(9):809-18. [DOI] [PubMed] [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington (DC): American Psychiatric Publishing, 2013. [Google Scholar]
Arguedas 2013
- Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD008277. [DOI: 10.1002/14651858.CD008277.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asfar 2014
- Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. New England Journal of Medicine 2014;370(17):1583-93. [DOI] [PubMed] [Google Scholar]
Bellomo 2004
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care 2004;8(4):R204-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benstoem 2017
- Benstoem C, Moza A, Meybohm P, Stoppe C, Autschbach R, Devane D, et al. A core outcome set for adult cardiac surgery trials: a consensus study. PLOS One 2017;12(11):e0186772. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergeron 2001
- Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Medicine 2001;27(5):859-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bove 2014
- Bove T, Zangrillo A, Guarracino F, Alvaro G, Persi B, Maglioni E, et al. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. JAMA 2014;312(21):2244-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Briguori 2009
- Briguori C, Visconti G, Focaccio A, Golia B, Chieffo A, Castelli A, et al. Novel approaches for preventing or limiting events (Naples) II trial: impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction. Journal of the American College of Cardiology 2009;54(23):2157-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Busija 2008
- Busija L, Osborne RH, Nilsdotter A, Buchbinder R, Roos EM. Magnitude and meaningfulness of change in SF-36 scores in four types of orthopedic surgery. Health and Quality of Life Outcomes 2008;6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrick 2016
- Carrick MM, Morrison CA, Tapia NM, Leonard J, Suliburk JW, Norman MA, et al. Intraoperative hypotensive resuscitation for patients undergoing laparotomy or thoracotomy for trauma: early termination of a randomized prospective clinical trial. Journal of Trauma and Acute Care Surgery 2016;80(6):886-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carson 2016
- Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD002042. [DOI: 10.1002/14651858.CD002042.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2019
- Cheng XQ, Mei B, Zuo YM, Wu H, Peng XH, Zhao Q, et al. A multicentre randomised controlled trial of the effect of intra-operative dexmedetomidine on cognitive decline after surgery. Anaesthesia 2019;74(6):741-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Conlon 1999
- Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, et al. Acute renal failure following cardiac surgery. Nephrology, Dialysis, Transplantation 1999;14(5):1158-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
D'Agostino 2018
- D'Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 update on outcomes and quality. Annals of Thoracic Surgery 2018;105(1):15-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Christoph M. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ely 2001
- Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA 2001;286(21):2703-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Englberger 2011
- Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Critical Care 2011;15(1):R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleisher 2014
- Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130(24):e278-333. [PMID: ] [DOI] [PubMed] [Google Scholar]
Futier 2017
- Futier E, Lefrant JY, Guinot PG, Godet T, Lorne E, Cuvillon P, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017;318(14):1346-57. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garrison 2017
- Garrison SR, Kolber MR, Korownyk CS, McCracken RK, Heran BS, Allan GM. Blood pressure targets for hypertension in older adults. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD011575. [DOI: 10.1002/14651858.CD011575.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillinov 2016
- Gillinov AM, Bagiella E, Moskowitz AJ, Raiten JM, Groh MA, Bowdish ME, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. New England Journal of Medicine 2016;374(20):1911-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottesman 2007
- Gottesman RF, Hillis AE, Grega MA, Borowicz LM Jr, Selnes OA, Baumgartner WA, et al. Early postoperative cognitive dysfunction and blood pressure during coronary artery bypass graft operation. Archives of Neurology 2007;64(8):1111-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 21 September 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gurfinkel 2007
- Gurfinkel EP, Lernoud VS, Laguens RP, Favaloro RR. Advances in coronary heart disease surgery in Latin America. Circulation 2007;115(9):1147-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Haase 2012
- Haase M, Bellomo R, Story D, Letis A, Klemz K, Matalanis G, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrology, Dialysis, Transplantation 2012;27(1):153-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Head 2013
- Head SJ, Howell NJ, Osnabrugge RL, Bridgewater B, Keogh BE, Kinsman R, et al. The European Association for Cardio-Thoracic Surgery (EACTS) database: an introduction. European Journal of Cardio-Thoracic Surgery 2013;44(3):e175-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hillis 2011
- Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. Journal of the American College of Cardiology 2011;58(24):e123-210. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hobson 2009
- Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119(18):2444-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hori 2014
- Hori D, Brown C, Ono M, Rappold T, Sieber F, Gottschalk A, et al. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. British Journal of Anaesthesia 2014;113(6):1009-17. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Inouye 1990
- Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine 1990;113(12):941-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kanji 2010
- Kanji HD, Schulze CJ, Hervas-Malo M, Wang P, Ross DB, Zibdawi M, et al. Difference between pre-operative and cardiopulmonary bypass mean arterial pressure is independently associated with early cardiac surgery-associated acute kidney injury. Journal of Cardiothoracic Surgery 2010;5:71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
KDIGO 2012
- Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements 2012;2:1-138. [Google Scholar]
Kilic 2014
- Kilic A, Shah AS, Conte JV, Mandal K, Baumgartner WA, Cameron DE, et al. Understanding variability in hospital-specific costs of coronary artery bypass grafting represents an opportunity for standardizing care and improving resource use. Journal of Thoracic and Cardiovascular Surgery 2014;147(1):109-15. [DOI] [PubMed] [Google Scholar]
Landoni 2019
- Landoni G, Lomivorotov VV, Nigro Neto C, Monaco F, Pasyuga VV, Bradic N, et al. Volatile anesthetics versus total intravenous anesthesia for cardiac surgery. New England Journal of Medicine 2019;380(13):1214-25. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Lewicki 2015
- Lewicki M, Ng I, Schneider AG. HMG CoA reductase inhibitors (statins) for preventing acute kidney injury after surgical procedures requiring cardiac bypass. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD010480. [DOI: 10.1002/14651858.CD010480.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2013
- Li X, Sun Z, Zhao W, Zhang J, Chen J, Li Y, et al. Effect of acetylsalicylic acid usage and platelet transfusion on postoperative hemorrhage and activities of daily living in patients with acute intracerebral hemorrhage. Journal of Neurosurgery 2013;118(1):94-103. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Machado 2014
- Machado MN, Nakazone MA, Maia LN. Prognostic value of acute kidney injury after cardiac surgery according to Kidney Disease: Improving Global Outcomes definition and staging (KDIGO) criteria. PLOS One 2014;9(5):e98028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mack 2017
- Mack MJ, Acker MA, Gelijns AC, Overbey JR, Parides MK, Browndyke JN, et al. Effect of cerebral embolic protection devices on CNS infarction in surgical aortic valve replacement: a randomized clinical trial. JAMA 2017;318(6):536-47. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2013
- Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adybelli Z, Giuliani A, et al. Cardiac surgery-associated acute kidney injury. Cardiorenal Medicine 2013;3(3):178-99. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mascha 2015
- Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology 2015;123(1):79-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Masuda 2018
- Masuda M, Endo S, Natsugoe S, Shimizu H, Doki Y, Hirata Y, et al. Thoracic and cardiovascular surgery in Japan during 2015: annual report by the Japanese Association for Thoracic Surgery. General Thoracic and Cardiovascular Surgery 2018;66(10):581-615. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mazer 2017
- Mazer CD, Whitlock RP, Fergusson DA, Hall J, Belley-Cote E, Connolly K, et al. Restrictive or liberal red-cell transfusion for cardiac surgery. New England Journal of Medicine 2017;377(22):2133-44. [DOI] [PubMed] [Google Scholar]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane Reviews. Global Evidence Summit; 2017 Sept 13-16; Cape Town, South Africa.
Meersch 2017
- Meersch M, Schmidt C, Hoffmeier A, Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Medicine 2017;43(11):1551-61. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 2007
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care 2007;11(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meng 2018
- Meng L, Yu W, Wang T, Zhang L, Heerdt PM, Gelb AW. Blood pressure targets in perioperative care. Hypertension 2018;72(4):806-17. [DOI] [PubMed] [Google Scholar]
Menting 2017
- Menting TP, Wever KE, Ozdemir-van Brunschot DM, Vliet DJ, Rovers MM, Warle MC. Ischaemic preconditioning for the reduction of renal ischaemia reperfusion injury. Cochrane Database of Systematic Reviews 2017;3:CD010777. [DOI: 10.1002/14651858.CD010777.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moller 1998
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998;351(9106):857-61. [DOI] [PubMed] [Google Scholar]
Ngu 2020
- Ngu JM, Jabagi H, Chung AM, Boodhwani M, Ruel M, Bourke M, et al. Defining an intraoperative hypotension threshold in association with de novo renal replacement therapy after cardiac surgery. Anesthesiology 2020;132(6):1447-57. [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2018
- Noel-Storr AH and the Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. Evidence Live 2018; 2018 Jun 18-20; Oxford, UK.
Ono 2013
- Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, Zheng Y, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Critical Care Medicine 2013;41(2):464-71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ono 2014
- Ono M, Brady K, Easley RB, Brown C, Kraut M, Gottesman RF, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. Journal of Thoracic and Cardiovascular Surgery 2014;147(1):483-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2002
- Palmer BF. Renal dysfunction complicating the treatment of hypertension. New England Journal of Medicine 2002;347(16):1256-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pandharipande 2013
- Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. New England Journal of Medicine 2013;369(14):1306-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Randolph 1998
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology 1998;20(3):310-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rao 1992
- Rao JN, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics 1992;48(2):577-85. [PMID: ] [PubMed] [Google Scholar]
Rao 2016
- Rao C, Zhang H, Gao H, Zhao Y, Yuan X, Hua K, et al. The Chinese cardiac surgery registry: design and data audit. Annals of Thoracic Surgery 2016;101(4):1514-20. [DOI] [PubMed] [Google Scholar]
Reich 1999
- Reich DL, Bodian CA, Krol M, Kuroda M, Osinski T, Thys DM. Intraoperative hemodynamic predictors of mortality, stroke, and myocardial infarction after coronary artery bypass surgery. Anesthesia and Analgesia 1999;89(4):814-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rhee 2012
- Rhee CJ, Kibler KK, Easley RB, Andropoulos DB, Czosnyka M, Smielewski P, et al. Renovascular reactivity measured by near-infrared spectroscopy. Journal of Applied Physiology (1985) 2012;113(2):307-14. [DOI] [PubMed] [Google Scholar]
Rhodes 2017
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Medicine 2017;43(3):304-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ristovic 2020
- Ristovic V, Roock S, Mesana TG, Diepen S, Sun LY. The impact of preoperative risk on the association between hypotension and mortality after cardiac surgery: an observational study. Journal of Clinical Medicine 2020;9(7):2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rudolph 2010
- Rudolph JL, Inouye SK, Jones RN, Yang FM, Fong TG, Levkoff SE, et al. Delirium: an independent predictor of functional decline after cardiac surgery. Journal of the American Geriatrics Society 2010;58(4):643-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saczynski 2012
- Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. New England Journal of Medicine 2012;367(1):30-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saiz 2018
- Saiz LC, Gorricho J, Garjon J, Celaya MC, Erviti J, Leache L. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database of Systematic Reviews 2018, Issue 7. Art. No: CD010315. [DOI: 10.1002/14651858.CD010315.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salmasi 2017
- Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017;126(1):47-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sickeler 2014
- Sickeler R, Phillips-Bute B, Kertai MD, Schroder J, Mathew JP, Swaminathan M, et al. The risk of acute kidney injury with co-occurrence of anemia and hypotension during cardiopulmonary bypass relative to anemia alone. Annals of Thoracic Surgery 2014;97(3):865-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stewart 1989
- Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, et al. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA 1989;262(7):907-13. [PubMed] [Google Scholar]
Strandgaard 1973
- Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. British Medical Journal 1973;1(5852):507-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Subramaniam 2019
- Subramaniam B, Shankar P, Shaefi S, Mueller A, O'Gara B, Banner-Goodspeed V, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA 2019;321(7):686-96. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2015
- Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology 2015;123(3):515-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sun 2018
- Sun LY, Chung AM, Farkouh ME, Diepen S, Weinberger J, Bourke M, et al. Defining an intraoperative hypotension threshold in association with stroke in cardiac surgery. Anesthesiology 2018;129(3):440-7. [DOI] [PubMed] [Google Scholar]
Thomas 2017
- Thomas J, Noel-Storr A, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. [DOI] [PubMed] [Google Scholar]
Turan 2020
- Turan A, Duncan A, Leung S, Karimi N, Fang J, Mao G, et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet 2020;396(10245):177-85. [DOI] [PubMed] [Google Scholar]
van Waes 2016
- Waes JA, Klei WA, Wijeysundera DN, Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology 2016;124(1):35-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wahba 2020
- Wahba A, Milojevic M, Boer C, De Somer FM, Gudbjartsson T, den Goor J, et al, EACTS/EACTA/EBCP Committee Reviewers. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. European Journal of Cardio-Thoracic Surgery 2020;57(2):210-51. [DOI] [PubMed] [Google Scholar]
Wang 2017
- Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nature Reviews Nephrology 2017;13(11):697-711. [DOI] [PubMed] [Google Scholar]
WHO 2018
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics – 11th Revision. icd.who.int/browse11/l-m/en (accessed 14 November 2019).
Williams‐Russo 1999
- Williams-Russo P, Sharrock NE, Mattis S, Liguori GA, Mancuso C, Peterson MG, et al. Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology 1999;91(4):926-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2018
- Zhang Y, Gao X, Yuan S, Guo J, Lv H, Zhou Y, et al. Effects of tranexamic acid on short-term and long-term outcomes of on-pump coronary artery bypass grafting: randomized trial and 7-year follow-up. Cardiovascular Therapeutics 2018;36(6):e12472. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kotani 2019
- Kotani Y, Kataoka Y, Izawa J, Fujioka S, Yoshida T, Kumasawa J, et al. High versus low blood pressure targets for cardiac surgery with cardiopulmonary bypass. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD013494. [DOI: 10.1002/14651858.CD013494] [DOI] [PMC free article] [PubMed] [Google Scholar]


