Abstract
We have studied the influence of periplasmic Cu,Zn superoxide dismutase on the intracellular survival of Escherichia coli strains able to invade epithelial cells by the expression of the inv gene from Yersinia pseudotuberculosis but unable to multiply intracellularly. Intracellular viability assays, confirmed by electron microscopy observations, showed that invasive strains of E. coli engineered to increase Cu,Zn superoxide dismutase production are much more resistant to intracellular killing than strains containing only the chromosomal sodC copy. However, we have found only a slight difference in survival within HeLa cells between a sodC-null mutant and its isogenic wild-type strain. Such a small difference in survival correlates with the very low expression of this enzyme in the wild-type strain. We have also observed that acid- and oxidative stress-sensitive E. coli HB101(pRI203) is more rapidly killed in epithelial cells than E. coli GC4468(pRI203). The high mortality of E. coli HB101(pRI203), independent of the acidification of the endosome, is abolished by the overexpression of sodC. Our data suggest that oxyradicals are involved in the mechanisms of bacterial killing within epithelial cells and that high-level production of periplasmic Cu,Zn superoxide dismutase provides bacteria with an effective protection against oxidative damage. We propose that Cu,Zn superoxide dismutase could offer an important selective advantage in survival within host cells to bacteria expressing high levels of this enzyme.
Until a few years ago Cu,Zn superoxide dismutase (Cu,ZnSOD) was considered almost exclusively a eukaryotic enzyme, protecting the cytosol and the extracellular environment of higher organisms from damage by oxygen free radicals (1). Recently, Cu,ZnSOD has been identified in the periplasmic space of a wide range of gram-negative bacteria, including Brucella abortus (6), Haemophilus spp., Actinobacillus spp., Pasteurella spp., Neisseria meningitidis (24–26), Escherichia coli K-12 (7), Legionella pneumophila (40), Salmonella spp. (9), and Mycobacterium tuberculosis (45). This enzyme is thought to protect bacteria from toxic oxygen-free radicals generated outside the cell or in the periplasm itself, since superoxide is unable to cross the cytoplasmic membrane (21). Therefore, Cu,ZnSOD has been proposed to be a determinant of virulence in bacteria potentially exposed to toxic free radicals produced by the host in response to bacterial infection. In vivo experiments have demonstrated the role of bacterial Cu,ZnSOD in the virulence and pathogenicity of infecting microorganisms (15, 18, 19, 36, 42, 43), while in vitro models have provided conflicting data concerning Cu,ZnSOD involvement in bacterial resistance to macrophage killing (19, 42) or survival within nonprofessional phagocytes (42). However, more recent results have shown that this enzyme protects Salmonella enterica serovar Typhimurium (15) and an overproducing strain of E. coli (4) from macrophage killing and that neutropenia restores virulence to an attenuated Cu,ZnSOD-deficient strain of Haemophilus ducreyi in a swine model of chancroid (36).
It is well known that some bacteria, defined as facultative intracellular pathogens, are able to survive within host professional or nonprofessional phagocytes and that this ability plays a pivotal role in infection and disease (20). While it has been clearly demonstrated that the oxidative burst contributes to bacterial killing in phagocytic cells, it is unknown whether nonphagocytic cells are able to kill bacteria by an oxidative pathway.
Considering the wide occurrence of Cu,ZnSOD in facultative intracellular bacteria, we decided to investigate whether this enzyme could offer a selective advantage in survival within epithelial cells. To test this hypothesis, we have used E. coli strains bearing the inv gene from Yersinia pseudotuberculosis in the pRI203 plasmid (23). The expression of invasin, the product of the inv gene, renders noninvasive E. coli strains able to enter cultured mammalian cells but unable to replicate intracellularly. In fact, recombinant invasive E. coli HB101 resides in endocytic vesicles (23) and the number of intracellular viable bacteria significantly diminishes some hours after infection (13). However, this strain carries mutations in the recA and rpoS (39) genes, which are both expected to increase bacterial sensitivity towards oxidative stress. In particular, rpoS encodes a stationary-phase sigma factor, RpoS, which controls a regulon of over 30 genes required for survival in the stationary phase, including several genes providing protection against oxidative stress and resistance to low pH (17, 27). As rpoS is believed to play an important role in bacterial survival within phagocytes (12, 33, 41, 44) and has been shown to modulate sodC expression in E. coli (20a), in this work we have compared the intracellular survival of E. coli HB101(pRI203) with that of a different E. coli strain, GC4468(pRI203), expressing functional rpoS and recA genes.
We have found significantly higher intracellular survival in all invasive strains of E. coli bearing the sodC gene on a multicopy plasmid than in those containing the chromosomal copy or an inactivated sodC gene. These results suggest that bacteria encounter an oxidative stress upon invasion of epithelial cells and show that overproduction of Cu,ZnSOD offers a selective advantage.
MATERIALS AND METHODS
Reagents.
Ampicillin, bafilomycin A1, bovine serum albumin, diethyldithiocarbamate, cytochrome c, gentamicin, kanamycin, penicillin, pyrogallol, streptomycin, trypsin, xanthine, and xanthine oxidase were purchased from Sigma. Restriction endonucleases, DNA-modifying enzymes, and catalase were obtained from Boehringer Mannheim. All other chemicals were purchased from BDH and were of the highest grade available. Oligonucleotides were synthesized by Genset. Culture tissue media were obtained from Seromed.
Plasmids, bacterial strains, and culture conditions.
The plasmids, E. coli strains, and oligonucleotides used in this work are listed in Tables 1 and 2. Plasmid pRI203 was kindly provided by S. Falkow. This plasmid, which carries the inv gene of Y. pseudotuberculosis, renders E. coli cells able to invade animal cells (23). A DNA fragment containing the whole E. coli sodC gene (22) was obtained by PCR amplification of E. coli chromosomal DNA carried out with the oligonucleotides prom5′ and prom3′. The approximately 920-bp amplified DNA was digested with EcoRI and HindIII and subcloned in the corresponding sites of pRI203 to obtain pRIEcSOD. In order to study the transcriptional regulation of sodC, its promoter region (corresponding to the 223 bp before the translation start site) was amplified with the oligonucleotides prom5′ and lacZ. The amplified DNA fragment was digested with EcoRI and BamHI and inserted into promoter probe plasmid pMC1403 (11) to obtain pMCPromEcSOD. In this vector, the sodC promoter is cloned upstream of the lacZ coding region, allowing the possibility of analyzing sodC expression following the accumulation of β-galactosidase (β-Gal).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| E. coli K-12 | ||
| HB101 | F−leuB6 supE44 hsdS20(rB− mB−) recA13 ara-14 proA2 galK2 lacY1 rpsL20 xyl-5 mtl-1 | 8 |
| GC4468 | F− Δ(lac-argF)U169 rpsL179 | 10 |
| QC2725 | GC4468 sodC::Kanr | This work |
| QC1110 | GC4468 katF::Tn10 Tetr | This work |
| Plasmids | ||
| PCR 2-1-topo | Cloning vector | Invitrogen |
| pTP223 | gam bet exo-expressing plasmid under Ptac control | 32 |
| pRI203 | Y. pseudotuberculosis invA cloned in pBR325 | 23 |
| pRIEcSOD | E. coli sodC cloned in pRI203 | This work |
| pMC1403 | Promoter probe vector | 11 |
| pMCPromEcSOD | sodC promoter cloned upstream of lacZ in pMC1403 | This work |
TABLE 2.
Oligonucleotides used
| Oligonucleotide | Sequence |
|---|---|
| prom5′ | 5′-ATGAATTCGTTTACCATGGCAGCCGC-3′ |
| prom3′ | 5′-GCAAGCTTGGCGTTCAGCAAAAATCAC-3′ |
| lacZ | 5′-TTTGGATCCATAGGACCTCCGTTCAT-3′ |
| sodCa1 | 5′-CGACGTCGACGTTGGCGGCGATAATATGTCCG-3′ |
| sodCa2 | 5′-GCGATATCGCCTTCTGGCAGCGACTACG-3′ |
| sodCb1 | 5′-CGAGTCGACGTGACGAGGTTCATCTCGAC-3′ |
| sodCb2 | 5′-CGTCGATATCAGACGAACCGGAGCAACCATCACTC-3′ |
| sodC1 | 5′-CGCGTTCCGATCCGTTATCGC-3′ |
| sodC2 | 5′-GAACTGGCTGTGGTGGCAGAGGAG-3′ |
| sodC3 | 5′-CTACGCCCGACCAACGTCGCCGACTATC-3′ |
| sodC4 | 5′-GAGGTGCTGCGCCTTTGTCGGCAGC-3′ |
| kan1 | 5′-GATTCAGGCCTGGTATGAGTCAGC-3′ |
| kan2 | 5′-CCCGTTGAATATGGCTCATAACACC-3′ |
E. coli HB101 (8) and E. coli GC4468 (10) were from our laboratory collection. The rpoS mutant E. coli QC1110 was obtained by P1 transduction of the katF13::Tn10 allele (28) in the parental strain GC4468. Regions (about 1,150 bp) of DNA flanking and entering into the sodC structural gene were amplified by PCR and ligated in order to generate a 350-bp internal deletion of the sodC gene. The primers used to amplify the upstream fragment (ending at position 93 of the structural sodC gene sequence) were sodCb2, generating an EcoRV restriction site at its 5′ end, and sodCb1, generating a SalI restriction site at its 5′ end. The primers used to amplify the downstream fragment (starting at position 438 of the structural sodC gene) were sodCa1, generating a SalI site at its 5′ end, and sodCa2, generating an EcoRV restriction site at its 5′ end. The PCR products were cloned into the pCR2-1-topo cloning vector (Invitrogen) to create pDT32 and pDT31. After appropriate digestion, the fragments were successively transferred into pUC19 and ligated at the SalI restriction site, generating a deleted sodC gene (pDT34). The deleted region was substituted by a kanamycin resistance gene block (Pharmacia) inserted at the SalI site. The construction was verified by sequencing. The resulting plasmid (pDT35) was digested with EcoRV and the 3.6-kb linear fragment containing the sodC mutated region was used to transfer the mutation to the chromosome, transforming a wild-type strain bearing plasmid pTP223, which avoids nucleolytic digestion of linear DNA (32). A Kanr Amps transformant was selected. The strain was further tested for correct integration of the kanamycin-containing sodC gene by PCR with different primers, including oligonucleotides internal to both the kanamycin and sodC genes, and by bracketing the insertion fragment. Amplifications with sodC3-sodC4, sodC1-sodC4, sodC2-sodC3, sodC4-kan1, and sodC3-kan2 oligonucleotide pairs (see Table 2) were carried out. In all cases, a band of the expected molecular weight was obtained. The sodC mutation was P1, transduced into GC4468 and selected for Kanr (QC2725). The sodC mutation was 56% cotransducible with sodB, in good agreement with the distance between the two genes (∼10 kb). Activity assays carried out on a periplasmic extract of QC2725 confirmed the lack of Cu,ZnSOD in this strain.
E. coli strains were grown on Luria-Bertani (LB) medium containing 100 μg of ampicillin per ml. E. coli cells bearing pRI203 and pRIEcSOD exhibited identical growth rates and viability. Bacterial cells were grown overnight until the stationary phase was reached; a bacterial suspension in exponential phase was obtained by subculturing the overnight cultures in fresh medium at 37°C. The cultures, in different growth phases, were pelleted and washed with phosphate-buffered saline (PBS) without Ca2+ and Mg2+ and then resuspended in minimal essential medium (MEM) for the invasion assay at a concentration of about 107 cells/ml. The minimal bactericidal concentration of gentamicin for each strain was determined by counting the number of CFU after incubation of 107 bacterial cells/ml in gentamicin-containing MEM for 2 h at 37°C.
Host cells.
HeLa S3 cells (from an epithelioid carcinoma of the human cervix) and Caco-2 cells (from a human colonic carcinoma) were grown as monolayers at 37°C in MEM supplemented with 1.2 g of NaHCO3 per liter, 2 mM glutamine, 100 U of penicillin per ml, 0.1 mg of streptomycin per ml, and 10% heat-inactivated fetal calf serum (FCS) in a 5% CO2 incubator. During the infection experiments, FCS was added at a concentration of 2%.
Invasion assay.
Invasion of cultured cells was assayed by a modification of the technique of Isberg and Falkow (23). Briefly, semiconfluent monolayers of HeLa S3 or Caco-2 cells grown without antibiotics in 12-well plates (Costar) were infected with invasive E. coli strains, in either exponential or stationary phase, at a multiplicity of infection (MOI) of 100 (defined as 100 bacteria per cell). The infection was performed for 1 h at 37°C. Then, cells were washed extensively with PBS without Ca2+ and Mg2+, and 1 ml of fresh medium containing 200 or 100 μg of gentamicin per ml was added to each well, which was infected with E. coli GC4468(pRI203) or GC4468(pRIEcSOD), QC2725(pRI203) or QC2725(pRIEcSOD), and HB101(pRI203) or HB101(pRIEcSOD). After a further 2-h incubation period at 37°C, infected cells were washed extensively and subsequently treated with trypsin-EDTA (a mixture of 0.05% trypsin [1/250] and 0.02% EDTA) for 5 min at 37°C and lysed by the addition of 1.0 ml of cold 0.1% Triton X-100. Cell lysates were diluted in PBS and plated on LB medium containing 100 μg of ampicillin per ml to quantify the number of viable intracellular bacteria.
Intracellular survival assay.
After bacterial infection, HeLa or Caco-2 cells were washed and fresh medium containing 50 μg of gentamicin per ml, 2% FCS, 1.2 g of NaHCO3 per liter, and 2 mM glutamine was added. The monolayers were then incubated for 4, 6, 24, and 48 h at 37°C. At these times, cells were washed and lysed and the number of viable intracellular bacteria was evaluated by CFU counts.
Effect of ammonium chloride and bafilomycin on bacterial invasion and survival.
Prior to the invasion assays, HeLa cell monolayers were preincubated for 30 min with or without 20 mM NH4Cl or 100 nM bafilomycin A1, either of which is known to inhibit vacuolar acidification (34). After bacterial infection, gentamicin- and NH4Cl- or bafilomycin-containing medium was added to the cell monolayers. At different times, cultured cells were washed with PBS and lysed, and viable intracellular bacteria were counted, as described above.
Electron microscopy.
Twenty-four-well tissue culture plates were seeded with 1 × 106 HeLa cells/well and pulsed with ferritin (0.2 mg/ml) for labeling of lysosomes as described by D'Arcy et al. (14). After 3 h of incubation at 37°C, monolayers were washed five times with MEM to remove free ferritin; then, they were infected with E. coli strains bearing pRI203 and pRIEcSOD at an MOI of 100 (see “Invasion assay,” above). At different time intervals (2, 24, and 48 h), cells were incubated with a 0.05 trypsin–0.02% EDTA solution, washed gently with PBS, and pelleted at 600 × g for 10 min. Pellets were fixed with 2.5 mM glutaraldehyde in cacodylate buffer for 1 h at room temperature and postfixed in 1% unbuffered OsO4. Cells were then dehydrated with increasing concentrations of ethyl alcohol and embedded in Agar 100. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Philips 208S transmission electron microscope.
Activity assays.
Cu,ZnSOD activity was assayed by the pyrogallol method (30). The periplasmic fraction was obtained by a procedure described previously (3), with the only difference that cells were resuspended at an optical density at 600 nm (OD600) of 100. The low expression level of Cu,ZnSOD and the presence of small amounts of cytoplasmic FeSOD and MnSOD in the periplasmic extracts prevent accurate measurements of Cu,ZnSOD activity in E. coli. Therefore, to characterize our model we have determined the Cu,ZnSOD activity in periplasmic extracts before and after a 15-min incubation with 2 mM diethyldithiocarbamate, a copper chelator which inactivates the Cu,ZnSOD enzyme without affecting the activity of MnSOD and FeSOD (7). Protein content was determined by the method of Lowry et al. (29). β-Gal activity was measured by a previously described procedure (35).
Characterization of the sodC strain.
Susceptibility to extracellular superoxide was evaluated by monitoring bacterial survival upon exposure to superoxide generated by the action of xanthine oxidase on xanthine. Bacteria grown until stationary phase were washed and suspended at a density of about 105 cells/ml in PBS containing 0.1 to 1 mM xanthine and 1 U of catalase (Boehringer Mannheim) to remove hydrogen peroxide generated by xanthine oxidase and ensure that bacterial death was due to superoxide and not to the hydrogen peroxide formed by the spontaneous dismutation of the superoxide anion under assay conditions (37). Superoxide generation was initiated by the addition of xanthine oxidase to a final concentration of 0.1 to 0.5 U/ml. Effective superoxide formation under the above-mentioned conditions was checked by monitoring the rate of reduction of cytochrome c (previously purified by gel filtration) at 550 nm (31). Aliquots of the reaction mixture were diluted and plated at different times to determine the number of CFU per milliliter.
To detect the bacterial survival in stationary phase, E. coli GC4468 and E. coli QC2725 cells were grown in a 250-ml Erlenmeyer flask containing 50 ml of LB medium at 37°C. Each day, the OD of the cultures was checked and cell survival was evaluated by plating serial dilutions of the bacterial suspensions.
RESULTS
Cu,ZnSOD expression in E. coli.
Measurements of Cu,ZnSOD activity in periplasmic extracts of E. coli HB101 and GC4468 cells bearing pRI203 or pRIEcSOD are reported in Table 3. Cu,ZnSOD was detectable in all strains only in the stationary phase and the enzyme activity was two- to threefold higher in strains bearing the sodC gene on the multicopy plasmid than in strains bearing the control vector. Strain GC4468 showed higher Cu,ZnSOD activity than HB101. It is worth noting that the Cu,ZnSOD activity values we have found in E. coli strains overexpressing sodC are well below those previously measured in some bacterial pathogens (22, 40). We have also studied sodC expression with a fusion between the sodC promoter and the nucleotide sequence which codifies for β-Gal (Table 4). Our results confirm previously reported data showing that rpoS controls sodC (20a, 22) but in addition indicate that a low level of transcription from the sodC promoter may also occur in the absence of this specific sigma factor.
TABLE 3.
Cu,ZnSOD activity in E. coli periplasmic extractsa
| E. coli strain | Activity (U/mg) at:
|
|
|---|---|---|
| 24 h | 48 h | |
| HB101(pRI203) | 6.1 ± 2.7 | 14.5 ± 4.5 |
| HB101(pRIEcSOD) | 13.9 ± 3.9 | 32.6 ± 8.8 |
| GC4468(pRI203) | 11.1 ± 2.3 | 22.8 ± 5.9 |
| GC4468(pRIEcSOD) | 34.5 ± 8.3 | 71.7 ± 11.0 |
Cu,ZnSOD activity was determined by subtracting diethyldithiocarbamate-resistant activity from the total SOD activity present in periplasmic extracts in the absence of such a copper-chelating agent. Cu,ZnSOD activity was not detectable in mid-log-phase E. coli cells. Values represent means ± standard deviations.
TABLE 4.
rpoS-dependent transcriptional activity of E. coli sodC promotera
| E. coli strain | β-Gal activity (U) at:
|
||
|---|---|---|---|
| 3 h | 24 h | 48 h | |
| GC4468(pMC1403) | 0.40 ± 0.27 | 0.2 ± 0.17 | 0.1 ± 0.2 |
| GC4468(pMCPromEcSOD) | 58.75 ± 18.50 | 258.7 ± 63.3 | 420 ± 32.6 |
| QC1110(pMC1403) | 0.1 ± 0.05 | 0.11 ± 0.1 | 0.14 ± 0.03 |
| QC1110(pMCPromEcSOD) | 3.05 ± 0.4 | 31.5 ± 7.54 | 39.4 ± 1.8 |
Overnight E. coli cultures were diluted 1:100 in LB medium and grown up to 2 days at 37°C. Samples were withdrawn at different times, and activity values were standardized to cell concentrations. Cells collected at 3 h after subculturing were still in mid-log phase. The values reported here are the means ± standard deviations of four different experiments.
Invasiveness and intracellular survival of different invasive E. coli strains.
The capacity of E. coli HB101 or E. coli GC4468 harboring pRI203 and pRIEcSOD to invade epithelial HeLa cells or enterocyte-like Caco-2 cells was assayed. Cell monolayers were infected at 37°C for 1 h with an MOI of 100 at different growth phases. Data of intracellular CFU counts, reported in Table 5, showed a different invasion efficiency (assayed at 2 h postinfection) of E. coli strains depending on the bacterial growth phase: as previously reported (13, 38), the highest level of invasiveness was obtained with logarithmically grown bacteria, which synthesized the greatest amount of invasin. Intracellular bacterial survival at 24 and 48 h after infection was also determined. The results showed that E. coli HB101(pRI203) and E. coli GC4468(pRI203) were susceptible to intracellular killing, whereas E. coli HB101(pRIEcSOD) and E. coli GC4468(pRIEcSOD) were resistant. When cell monolayers were infected with bacteria in the stationary growth phase, in addition to a significant decrease of invasive efficiency for all the strains tested, E. coli HB101(pRI203) displayed the lowest intracellular viability (no viable bacterial cell was recovered within HeLa cells after 24 h of infection). Gentamicin incubation times longer than 48 h of infected monolayers were excluded owing to antibiotic entry into cell monolayers and the consequent killing of intracellular bacteria. Similar results were obtained from the infection of Caco-2 cell monolayers, although the resulting invasive efficiency was at least 50-fold lower (data not shown).
TABLE 5.
Invasiveness and intracellular survival of invasive E. coli strainsa
| E. coli strain | Growth phase | No. of intracellular bacteria at 2 h | Intracellular survival at:
|
|
|---|---|---|---|---|
| 24 h | 48 h | |||
| HB101(pRI203) | Logarithmic | (1.8 ± 1.3) × 106 | (5.9 ± 3.3) × 103 | 0 |
| HB101(pRIEcSOD) | (2.3 ± 1.5) × 106 | (4.0 ± 1.7) × 105 | (1.5 ± 0.6) × 105 | |
| HB101(pRI203) | Stationary | (2.0 ± 0.6) × 105 | 0 | 0 |
| HB101(pRIEcSOD) | (9.2 ± 0.5) × 104 | (6.3 ± 0.6) × 104 | (1.0 ± 0.5) × 104 | |
| GC4468(pRI203) | Logarithmic | (1.3 ± 0.5) × 106 | (1.4 ± 0.7) × 104 | (1.0 ± 0.6) × 103 |
| GC4468(pRIEcSOD) | (2.1 ± 0.8) × 106 | (1.8 ± 0.6) × 106 | (3.6 ± 1.9) × 105 | |
| GC4468(pRI203) | Stationary | (3.0 ± 0.4) × 105 | (3.8 ± 0.6) × 104 | (1.0 ± 0.8) × 103 |
| GC4468(pRIEcSOD) | (2.8 ± 0.3) × 105 | (1.3 ± 0.9) × 105 | (1.2 ± 1.0) × 105 | |
| QC2725(pRI203) | Logarithmic | (1.0 ± 0.4) × 106 | (1.6 ± 0.5) × 104 | (2.0 ± 0.5) × 102 |
| QC2725(pRIEcSOD) | (1.1 ± 0.3) × 106 | (0.8 ± 0.4) × 106 | (3.6 ± 0.4) × 105 | |
| QC2725(pRI203) | Stationary | (3.3 ± 0.6) × 105 | (2.7 ± 0.6) × 104 | (2.0 ± 0.5) × 102 |
| QC2725(pRIEcSOD) | (5.1 ± 0.5) × 105 | (1.8 ± 0.7) × 105 | (1.6 ± 0.4) × 105 | |
Invasiveness (measured as the number of viable intracellular bacteria 2 h postinfection) and survival of E. coli strains within HeLa cells at 24 and 48 h postinfection were determined by infecting cell monolayers with bacteria (MOI, 100) in different growth phases, as described in Materials and Methods. Values are the means ± standard deviations of at least four independent experiments.
To further understand the role of sodC in determining the intracellular survival of E. coli, we also constructed a strain with sodC-deleted. Wild-type and mutant E. coli strains exhibited identical morphology, growth rates, and survival in the stationary phase. In full agreement with the results recently reported by Gort and coworkers (20a), we were unable to observe a significant mortality either in GC4468 (wild type) or in QC2725 (sodC-null mutant) E. coli cells exposed to an extracellular superoxide challenge obtained by incubation of cells in the presence of xanthine and xanthine oxidase. Furthermore, the invasion efficiency and intracellular survival of mutant E. coli QC2725 harboring pRI203 or pRIEcSOD were tested; the results are reported in Table 5. Twenty-four hours after invasion, the intracellular survival of logarithmically grown and stationary E. coli QC2725 (pRI203) was very close to that of E. coli GC4468(pRI203). However, at 48 h after infection, the intracellular survival of E. coli QC2725(pRI203) was lower than that of E. coli GC4468(pRI203), thus suggesting that physiological levels of Cu,ZnSOD production by E. coli is functionally important for long-term survival within the endosome. E. coli QC2725(pRIEcSOD) resistance to intracellular killing was found to be similar to that of the parental strain, E. coli GC4468(pRIEcSOD).
Influence of endosome pH on bacterial survival.
Acidification is an important event in several endocytic pathways. E. coli HB101 is known to be highly sensitive to low pH, due to a mutation in the rpoS gene (39). To verify if such a pH sensitivity was responsible for the higher mortality of E. coli HB101(pRI203) than of E. coli GC4468(pRI203), the effects of two different inhibitors of vacuolar acidification, the lipophilic weak base ammonium chloride, and the antibiotic bafilomycin A1, were tested. The invasion efficiency and intracellular survival of E. coli HB101 were measured in cell monolayers treated with 20 mM NH4Cl or 100 nM bafilomycin for 30 min prior to infection and during the whole experiment. Cell monolayers were infected with logarithmically grown or stationary E. coli HB101(pRI203). NH4Cl or bafilomycin pretreatment had no effect on either the entry or the intracellular survival of E. coli strains (Table 6). These results rule out the possibility that intracellular killing was due to a susceptibility to low pH mediated by the altered rpoS gene in E. coli HB101(pRI203). Similar experiments were carried out with E. coli HB101(pRIEcSOD), and similar levels of bacterial survival within treated and untreated cells were observed (data not shown).
TABLE 6.
Effect of endosome pH-neutralizing reagents on E. coli HB101(pRI203) intracellular survivala
| E. coli strain and additive | Growth phase | No. of intracellular bacteria at 2 h | Intracellular survival at:
|
|
|---|---|---|---|---|
| 24 h | 48 h | |||
| HB101(pRI203) | Logarithmic | (9.3 ± 0.9) × 105 | (4.6 ± 0.5) × 103 | 0 |
| HB101(pRI203) plus NH4Cl | (3.3 ± 0.7) × 105 | (5.9 ± 3.3) × 103 | 0 | |
| HB101(pRI203) plus bafilomycin | (8.4 ± 0.7) × 105 | (2.8 ± 0.8) × 103 | 0 | |
| HB101(pRI203) | Stationary | (3.2 ± 0.5) × 105 | 0 | 0 |
| HB101(pRI203) plus NH4Cl | (3.8 ± 0.7) × 105 | 0 | 0 | |
| HB101(pRI203) plus bafilomycin | (2.6 ± 0.5) × 105 | 0 | 0 | |
Effect of 20 mM NH4Cl or 100 nM bafilomycin A1 on invasion efficiency (number of intracellular bacteria at 2 h) and intracellular survival of E. coli HB101(pRI203) infecting HeLa cell monolayers at an MOI of 100. Values are the means ± standard deviations of four independent experiments.
Electron microscopy analysis.
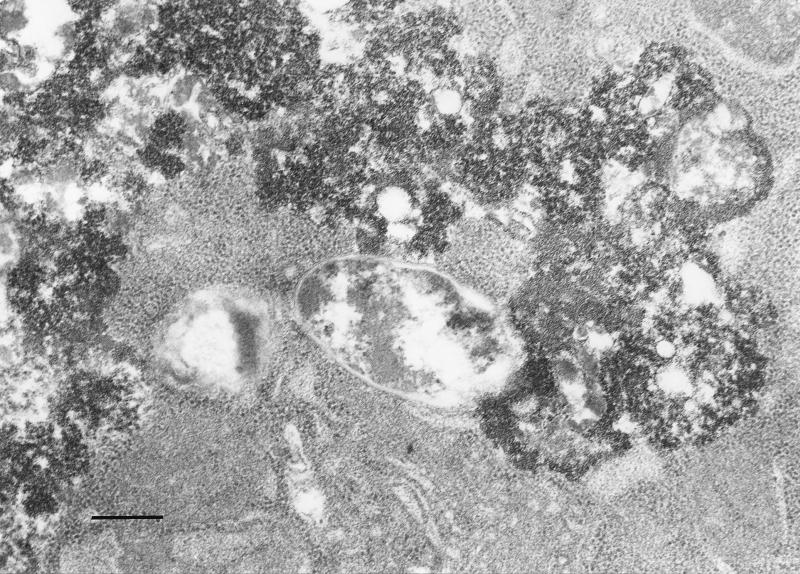
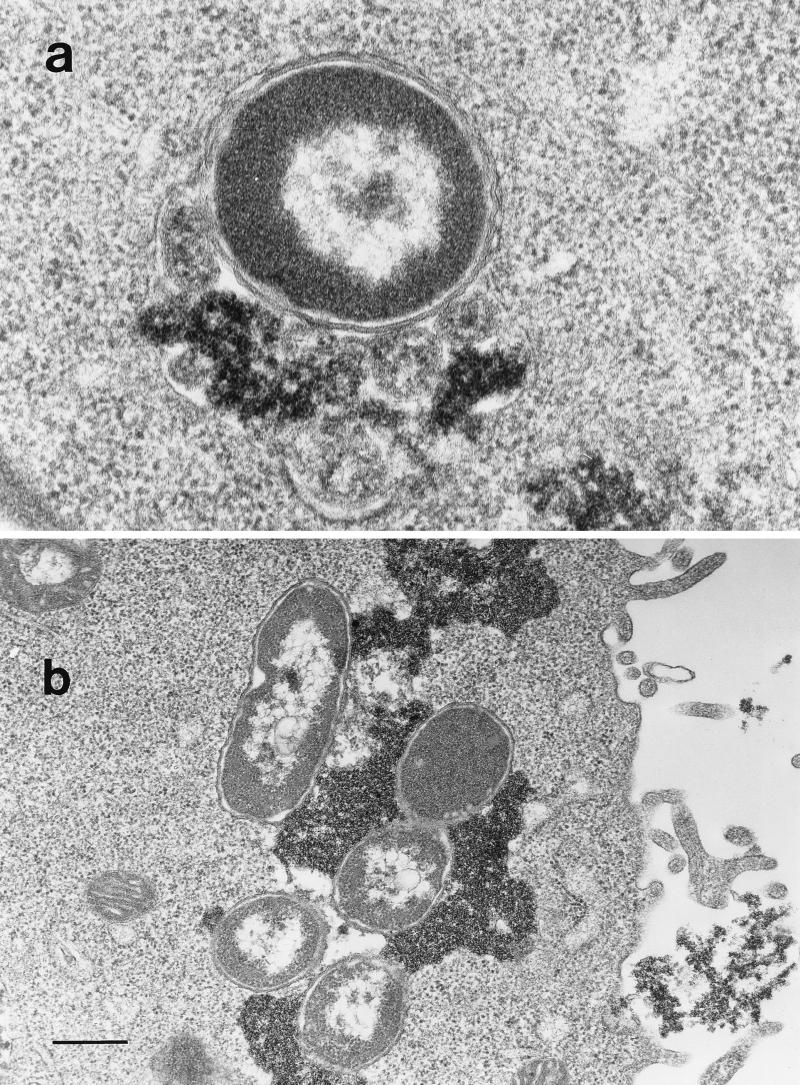
The fate of E. coli HB101(pRI203) and E. coli HB101(pRIEcSOD) during cell infection was visualized by transmission electron microscopy, and ferritin was used as a lysosomal marker to demonstrate phagosome-lysosome fusion. After 2 h of infection, the strain appeared intact, while after 24 or 48 h E. coli HB101(pRI203) appeared damaged within the endosome-lysosome (Fig. 1). In contrast, cells infected with E. coli HB101(pRIEcSOD) showed that the endosome-lysosome still contained intact bacteria (Fig. 2a); in some observations, it was not possible to visualize a membrane surrounding E. coli pRIEcSOD cells that appeared undamaged, even though they were in close contact with ferritin-labeled material (Fig. 2b).
FIG. 1.
Electron micrograph of HeLa cells infected with E. coli HB101(pRI203) at 24 h postinfection. Bar, 250 nm.
FIG. 2.
Electron micrographs of HeLa cells infected with E. coli HB101(pRIEcSOD) 24 h postinfection. Bars, 450 (a) and 160 (b) nm.
DISCUSSION
Several authors have investigated the possible involvement of periplasmic Cu,Zn superoxide dismutase in the mechanisms of bacterial protection against the respiratory burst elicited by phagocytes. It has been shown that Cu,ZnSOD-deficient mutants of several bacterial genera are less virulent in in vivo models and that, at least in some cases, the enzyme plays a protective role against superoxide generated by macrophages. Some bacteria producing Cu,ZnSOD are facultative intracellular microorganisms. This lifestyle, in a protected cellular niche, is thought to allow bacteria to escape the host defense mechanisms, thus contributing to the establishment of chronic and recurrent diseases. In order to evaluate whether oxidative species participate in bacterial killing within epithelial cells and whether periplasmic Cu,Zn superoxide dismutase could afford protection, we have chosen an experimental model consisting of nonpathogenic E. coli strains able to invade eukaryotic cells by the expression of the inv gene from Y. pseudotuberculosis but unable to escape from the endosome.
Our data clearly show that overproduction of Cu,ZnSOD protects E. coli strains from killing upon infection by HeLa cell monolayers. In fact, E. coli HB101, GC4468, and QC2725 bearing pRIEcSOD were noticeably more resistant to intracellular killing than E. coli strains bearing pRI203 (Table 5). As also demonstrated by electron microscopy observations, 24 h after the infection E. coli HB101 cells harboring pRIEcSOD were shown to be intact within the endosome-lysosome (Fig. 2a), whereas E. coli HB101(pRI203) cells were damaged (Fig. 1). It is intriguing that in some observations, E. coli HB101(pRIEcSOD) appeared undamaged in close contact with ferritin-labeled material, without a visible endosome-lysosome membrane (Fig. 2b).
The increase of SOD activity in strains bearing pRIEcSOD was only two- to threefold higher than the basal level of strains containing only a single copy of the chromosomal sodC gene (Table 3). This finding is in apparent contradiction with the high expression of β-Gal from a sodC-lacZ fusion carried on plasmid pMC1403 (Table 4). Whether this reflects a difference in plasmid copy number or an in vivo-reduced stability of Cu,ZnSOD compared to β-Gal or whether the measurement of Cu,ZnSOD led to an underestimation of the amount of enzyme expressed from the plasmid is unclear. However, several factors could be responsible for an underestimation: the SOD assay used (which relies on the use of an inhibitor to discriminate between the activity of Cu,ZnSOD and that of contaminating cytoplasmic SODs), protease sensitivity, the low stability of E. coli Cu,ZnSOD (2, 3, 5), and the requirement of adequate amounts of copper to ensure full catalytic activity to the enzyme (2, 3).
Considering the effect of Cu,ZnSOD overproduction on E. coli survival within epithelial cells, we expected that a sodC-null mutant could be more sensitive to intracellular killing than its isogenic wild-type strain. However, this expectation was only partially supported by our results, which showed that a differential survival between the two strains could be detected only at 48 h postinfection. As the strains overexpressing sodC are much more resistant to intracellular killing, we suggest that the small difference in the intracellular survival of the wild type and the sodC mutant in HeLa cells could be due to the low level of expression of chromosomal sodC in E. coli.
Moreover, we have also shown that the intracellular survival of E. coli HB101(pRI203) infecting HeLa cells in the logarithmic or stationary phase is independent of the vacuole pH. In fact, intracellular survival of E. coli HB101(pRI203) did not change following treatment of monolayers with NH4Cl or bafilomycin, either of which prevents endosome acidification (Table 6). These results indicate that the acid-sensitive phenotype of E. coli HB101 (39) is not responsible for the low viability of this strain within epithelial cells. The characterization of the peculiar features responsible for the different survival in epithelial cells of invasive E. coli HB101 and GC4468 is outside the aims of this work. However, it is interesting that overexpression of sodC enhances survival of both of these strains and appears to be sufficient to increase the survival of invasive E. coli HB101 (which is known to be highly sensitive to oxidative stress due to mutations in the genes encoding RecA and RpoS) to a level close to that of invasive E. coli GC4468.
Taken together, our findings suggest that oxyradical damage is involved in the mechanisms of bacterial killing by epithelial cells. Such a proposal is in agreement with a previous study which showed that Caco-2 and IEC-18 intestinal epithelial cells are able to kill bacteria by an oxidative pathway (16). While it is well established that superoxide production by an NADPH oxidase located on the cell membrane plays a pivotal role in the oxygen-dependent antimicrobial systems of phagocytic cells, no information is available about the presence of specific antimicrobial mechanisms based on free radicals generating enzymes in epithelial cells. The observation that Cu,ZnSOD protects E. coli from killing within epithelial cells, however, suggests that free radicals can also be produced on the endosomal membrane of nonphagocytic cells.
Although obtained with a model which uses nonpathogenic recombinant E. coli K-12 strains rendered invasive by the expression of a Y. pseudotuberculosis gene, our data encourage investigations of whether sodC plays a role in the intracellular survival of those pathogens that are naturally able to invade epithelial cells.
ACKNOWLEDGMENTS
This work was partially supported by the MURST PRIN grant “Role of free or chelated metal ions in intracellular infections” and by the CNR target project on “Biotechnology.”
REFERENCES
- 1.Bannister J V, Bannister W H, Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. Crit Rev Biochem. 1987;22:111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- 2.Battistoni A, Rotilio G. Isolation of an active and heat-stable monomeric form of Cu,Zn superoxide dismutase from the periplasmic space of Escherichia coli. FEBS Lett. 1995;374:199–202. doi: 10.1016/0014-5793(95)01106-o. [DOI] [PubMed] [Google Scholar]
- 3.Battistoni A, Folcarelli S, Gabbianelli R, Capo C, Rotilio G. The Cu,Zn superoxide dismutase from Escherichia coli retains monomeric structure at high protein concentration. Evidence for altered subunit interaction in all the bacteriocupreins. Biochem J. 1996;320:713–716. doi: 10.1042/bj3200713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battistoni A, Donnarumma G, Greco R, Valenti P, Rotilio G. Overexpression of a hydrogen peroxide-resistant periplasmic Cu,Zn superoxide dismutase protects Escherichia coli from macrophage killing. Biochem Biophys Res Commun. 1998;243:804–807. doi: 10.1006/bbrc.1998.8182. [DOI] [PubMed] [Google Scholar]
- 5.Battistoni A, Folcarelli S, Cervone L, Polizio F, Desideri A, Giartosio A, Rotilio G. Role of the dimeric structure in Cu,Zn superoxide dismutase. pH-dependent, reversible denaturation of the monomeric enzyme from Escherichia coli. J Biol Chem. 1998;273:5655–5661. doi: 10.1074/jbc.273.10.5655. [DOI] [PubMed] [Google Scholar]
- 6.Beck B L, Tabatabai L B, Mayfield J E. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry. 1990;16:372–376. doi: 10.1021/bi00454a010. [DOI] [PubMed] [Google Scholar]
- 7.Benov L T, Fridovich I. Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J Biol Chem. 1994;269:25310–25314. [PubMed] [Google Scholar]
- 8.Boyer H, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 9.Canvin J, Langford P R, Wilks K E, Kroll J S. Identification of sodC encoding periplasmic [Cu,Zn]-superoxide dismutase in Salmonella. FEMS Microbiol Lett. 1996;136:215–220. doi: 10.1111/j.1574-6968.1996.tb08052.x. [DOI] [PubMed] [Google Scholar]
- 10.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadaban M J, Chou J, Cohen S N. In vitro gene fusions that join an enzymatically active β-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980;143:971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C Y, Eckmann L, Libby S J, Fang F C, Okamoto S, Kagnoff M F, Fierer J, Guiney D G. Expression of Salmonella typhimurium rpoS and rpoS-dependent genes in the intracellular environment of eukaryotic cells. Infect Immun. 1996;64:4739–4743. doi: 10.1128/iai.64.11.4739-4743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conte M P, Mastromarino P, Nicoletti M, Visca P, Valenti P, Seganti L. Effect of polyelectrolytes on entry of Escherichia coli HB101 (pRI203) into HeLa cells. Microb Pathog. 1990;9:191–198. doi: 10.1016/0882-4010(90)90021-h. [DOI] [PubMed] [Google Scholar]
- 14.D'Arcy P, Hart J, Young M R. Interference with normal phagosome-lysosome fusion in macrophage, using ingested yeast cells and suramin. Nature. 1975;256:47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- 15.De Groote M A, Ochsner U A, Shiloh M U, Nathan C, McCord J M, Dinauer M C, Libby S J, Vazquez-Torres A, Xu Y, Fang F C. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci USA. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deitch E A, Haskel Y, Cruz N, Xu D, Kvietys P R. Caco-2 and IEC-18 intestinal epithelial cells exert bactericidal activity through an oxidant-dependent pathway. Shock. 1995;4:345–350. doi: 10.1097/00024382-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Eisenstark A, Calcutt M J, Becker-Hapak M, Ivanova A. Role of Escherichia coli rpoS and associated genes in defense against oxidative damage. Free Radic Biol Med. 1996;21:975–993. doi: 10.1016/s0891-5849(96)00154-2. [DOI] [PubMed] [Google Scholar]
- 18.Fang F C, DeGroote M A, Foster J W, Baumler A J, Ochsner U, Testerman T, Bearson S, Giard J C, Xu Y, Campbell G, Laessig T. Virulent Salmonella typhimurium has two periplasmic Cu,Zn-superoxide dismutases. Proc Natl Acad Sci USA. 1999;96:7502–7507. doi: 10.1073/pnas.96.13.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrant J L, Sansone A, Canvin J R, Pallen M J, Langford P R, Wallis T S, Dougan G, Kroll J S. Bacterial copper- and zinc-cofactored superoxide dismutase contributes to the pathogenesis of systemic salmonellosis. Mol Microbiol. 1997;25:785–796. doi: 10.1046/j.1365-2958.1997.5151877.x. [DOI] [PubMed] [Google Scholar]
- 20.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Gort A S, Ferber D M, Imlay J A. The regulation and role of the periplasmic copper, zinc superoxide dismutase of Escherichia coli. Mol Microbiol. 1999;32:179–191. doi: 10.1046/j.1365-2958.1999.01343.x. [DOI] [PubMed] [Google Scholar]
- 21.Hassan H M, Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979;254:10846–10852. [PubMed] [Google Scholar]
- 22.Imlay K R, Imlay J. Cloning and analysis of sodC, encoding the copper-zinc superoxide dismutase of Escherichia coli. J Bacteriol. 1996;178:2564–2571. doi: 10.1128/jb.178.9.2564-2571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isberg R R, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 24.Kroll J S, Langford P R, Loynds B M. Copper-zinc superoxide dismutase of Haemophilus influenzae and H. parainfluenzae. J Bacteriol. 1991;173:7449–7457. doi: 10.1128/jb.173.23.7449-7457.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroll J S, Langford P R, Wilks K E, Keil A D. Bacterial [Cu,Zn]superoxide dismutase: phylogenetically distinct from the eukaryotic enzyme, and not so rare after all! Microbiology. 1995;141:2271–2279. doi: 10.1099/13500872-141-9-2271. [DOI] [PubMed] [Google Scholar]
- 26.Langford P R, Loynds B M, Kroll J S. Cloning and molecular characterization of Cu,Zn superoxide dismutase from Actinobacillus pleuropneumoniae. Infect Immun. 1996;64:5035–5041. doi: 10.1128/iai.64.12.5035-5041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loewen P C, Hengge-Aronis R. The role of the sigma factor sigma S (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 28.Loewen P C, Triggs B L. Genetic mapping of KatF, a locus that affect the synthesis of a second catalase species in Escherichia coli. J Bacteriol. 1985;160:668–675. doi: 10.1128/jb.160.2.668-675.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 31.McCord J M, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 32.Murphy K C. Use of bacteriophage lambda recombination functions to promote gene replacement in Escherichia coli. J Bacteriol. 1998;180:2063–2071. doi: 10.1128/jb.180.8.2063-2071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickerson C A, Curtiss R., III Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect Immun. 1997;65:1814–1823. doi: 10.1128/iai.65.5.1814-1823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rathman M, Sjaastad M D, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.San Mateo L R, Toffer K L, Orndorff P E, Kawula T H. Neutropenia restores virulence to an attenuated Cu,Zn superoxide dismutase-deficient Haemophilus ducreyi strain in the swine model of chancroid. Infect Immun. 1999;67:5345–5351. doi: 10.1128/iai.67.10.5345-5351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnell S, Steinman H M. Function of stationary-phase induction of periplasmic copper-zinc superoxide dismutase and catalase/peroxidase in Caulobacter crescentus. J Bacteriol. 1995;177:5924–5929. doi: 10.1128/jb.177.20.5924-5929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Small P L, Isberg R R, Falkow S. Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yersinia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within HEp-2 cells. Infect Immun. 1987;55:1674–1679. doi: 10.1128/iai.55.7.1674-1679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Small P, Blankenhorn D, Welty D, Zinser E, Slonczewski J L. Acid and base resistance in Escherichia coli and Shigella flexneri: role of rpoS and growth pH. J Bacteriol. 1994;176:1729–1737. doi: 10.1128/jb.176.6.1729-1737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St. John G, Steinman H M. Periplasmic copper-zinc superoxide dismutase of Legionella pneumophila: role in stationary-phase survival. J Bacteriol. 1996;178:1578–1584. doi: 10.1128/jb.178.6.1578-1584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swords W E, Cannon B M, Benjamin W H., Jr Avirulence of LT2 strains of Salmonella typhimurium results from a defective rpoS gene. Infect Immun. 1997;65:2451–2453. doi: 10.1128/iai.65.6.2451-2453.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatum F M, Detilleux P G, Sacks J M, Hallings S M. Construction of Cu-Zn superoxide dismutase deletion mutants of Brucella abortus: analysis of survival in vitro in epithelial and phagocytic cells and in vivo in mice. Infect Immun. 1992;60:2863–2869. doi: 10.1128/iai.60.7.2863-2869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilks K E, Dunn K L, Farrant J L, Reddin K M, Gorringe A R, Langford P R, Kroll J S. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect Immun. 1998;66:213–217. doi: 10.1128/iai.66.1.213-217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilmes-Riesenberg M R, Foster J W, Curtiss R., III An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect Immun. 1997;65:203–210. doi: 10.1128/iai.65.1.203-210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu C H, Tsai-Wu J J, Huang Y T, Lin C Y, Lioua G G, Lee F J. Identification and subcellular localization of a novel Cu,Zn superoxide dismutase of Mycobacterium tuberculosis. FEBS Lett. 1998;13:192–196. doi: 10.1016/s0014-5793(98)01373-8. [DOI] [PubMed] [Google Scholar]