Abstract
Introduction:
This study compared bacterial percolation and sealer penetration of a novel obturation technique with the ones of warm vertical condensation technique.
Methods and Materials:
A bacterial percolation test was carried out with 80 single rooted human teeth divided into 5 groups; A (n=20): warm vertical condensation and AH-Plus, B (n=20): CPoint with AH-Plus, C (n= 20): CPoint with EndoSequence BC, +ve Control (n=10): teeth with no canal obturation, -ve Control (n=10): teeth with no access cavity. The samples were inoculated with a multispecies bacterial incoulum. Bacterial percolation was evaluated by turbidity. Confocal laser scanning microscopy (CLSM) was used to observe the presence of gaps and voids. Further 48 extracted human mandibular single-canal premolars were used to determine the sealer penetration. Slices of the samples were observed by CLSM to evaluate tubules penetration of the sealer. Kaplan Meyer survival, ANOVA one way and Tuckey HSD test and a Wilcoxon signed-rank test were utilised.
Results:
A Kaplan-Meier test showed no significant difference overall (P>0.05) between groups A, B and C. At 43 days, the group B showed a significantly inferior ability to prevent bacterial passage (P<0.05). The group C showed a deeper sealer penetration than group A and B with statistically significant differences (P<0.05) for the total penetration (ANOVA one way and Tukey HSD). A Wilcoxon signed-rank test showed statistically significant differences for the penetration in the middle-and apical third of the 3 groups.
Conclusion:
Based on this in vitro study, the single polymer-cone obturation technique with a resin based- and bioceramic based-sealer behaved similarly to the warm vertical obturation technique in preventing bacterial passage. The bioceramic sealer showed the deepest penetration but did not fully prevent bacterial leakage.
Key Words: Bioceramic Endodontic Sealer, Epoxy Resin, Root Canal Sealer, Warm Vertical Condensation Technique
Introduction
The aim of root canal obturation is to prevent the leakage of microorganisms [1]. Once the hermetic seal is lost bacteria can re-colonise the root canal and cause a relapse of the treatment. The traditional material used to obturate root canals has been gutta percha and zinc-oxide eugenol (ZOE) and epoxy resin-based cements. Recently hydrophilic polymer master cone obturation systems were introduced. The development of simplified obturation techniques may be adopted in the clinical setting, with the proviso of a good material behaviour and results comparable to the standard warm vertical condensation and cold lateral techniques. A reduced number of technical passages may lead to less flare-ups and iatrogenic errors technique [2, 3]. According to the manufacturer, these materials improve the seal because of the ability of the cone to expand with moisture [4]. The CPoint is based on these principles (Endo Innovations Ltd, St. Austell, UK) with a hydrophilic cone. The CPoint has a two-part construction. A central core composed of two nylons (Trogamid T and Trogamid CX), a radiopacifier (ZrO2) and a hydrophilic polymer coating, bonded to the central core, which radially expands constituted of two monomers, acrylonitrile and N-vinyl-pyrrolidone (NVP), polymerized and cross-linked using allyl methacrylate and a thermal and UV active initiator and radiopacifier (ZrO2) [5]. To date little experimental data exists regarding the efficacy of this system, except for its good biocompatibility and proven expansion [6, 7].
The introduction of so called bioceramic sealer which are tricalcium silicate (TCS) are more aptly defined hydraulic cements as they can set in the presence of moisture. The utilisation of hydraulic cement-based sealer may represent an advantage in terms of bioactivity and increased seal compared with traditional obturation techniques even when combined with a single cone [8].
I n vitro studies have limitations assessing the behaviour of obturation system. Dye-leakage has been employed; nevertheless, the pure liquid filtration system has limited clinical significance [9]. The use of bacterial leakage test is considered more relevant to compare different materials [10].
Also, penetration of endodontic sealer into dentinal tubules is another important parameter to study. Efficient sealer penetration improves mechanical retention of the core material because of an increment of interface between the dentinal walls and the obturation core material [11]. Sealer penetration may also affect tubular infection via entombing phenomenon, where bacteria may be entrapped within the dentinal tubules with progressive loss of viability [12]. The penetration depth into dentinal tubules depends on several factors: irrigation regimen, smear layer removal [13] or physical-chemical characteristics of the sealer [14]. Regarding the last factor, studies have reported penetration depth of epoxy resin, ZOE, methacrylate resin and adhesive resin-based sealers [15-17]. To date only one paper analysed in depth the bioceramic tubular penetration [18].
The aim was to evaluate the ability of the CPoint obturation system to avoid bacterial leakage and to measure the penetration depth into tubules. Its behaviour was compared to single cone/resin-based sealer obturation and warm vertical condensation technique. The null hypothesis were: 1) No difference could be detected between the different systems bacterial percolation. 2) No difference in the sealer penetration could be measured.
Material and Methods
Eighty extracted single rooted teeth with similar length (20-22 mm) were obtained (Research Ethics Committee Number 10/H0804/056). The exclusion criteria were presence of root caries, blocked canals, incomplete apices were discarded. Access cavity were cut; patency filing carried out with 10 K-file (Dentsply Maillefer, Ballaigues, Switzerland) and the ProTaper sequence (Dentsply Maillefer, Ballaigues, Switzerland) was used up to size F3. Irrigation was performed with 5 mL of 1% NaOCl solution and a rinse with 2 mL of 17% ethylenediaminetetraacetic acid (EDTA) solution followed by a final rinse with NaOCl. A 27-gauge side-vented needle was used. The teeth were assigned to three experimental groups:
Group A: (n=20) Warm vertical condensation, using a Size F3 gutta-percha (GP; Dentsply Maillefer) point and AH-Plus (Dentsply Maillefer).
Group B: (n=20) CPoint (Endo Innovations Ltd, St. Austell, Cornwall, UK) (size F3) with AH-Plus.
Group C: (n=20) CPoint (Endo Innovations) (size F3) with EndoSequence BC (ESBC) sealer (Brasseler USA, Savannah, GA, USA).
Positive Control: Teeth with shaped canal and no canal obturation were used as positive control (n=10), and negative control: Virgin teeth with no access cavity represented the negative control (n=10).
A warm vertical compaction technique was used for group A (GP/AH-Plus). The cone was coated with AH-Plus and inserted in the canal. System B (Analytic, Sybron Endo, Orange, CA, USA) was used to down-pack to 3 mm from the WL followed by the Obtura backfill (Obtura II; Sparta, Fenton MO, USA) and vertical compaction with Matchou pluggers 1 and 2 (Dentsply Maillefer, Ballaigues, Switzerland).
In Group B and C master cones CPoint F3 were coated with AH-Plus prepared according to manufacturer’s instruction (Group B) and ESBC (Group C) sealers and inserted to WL. Once inserted the cones were trimmed with a round bur on a high-speed handpiece.
A bacterial percolation double chamber test was employed. This method allowed determining the the passage of bacteria from the top chamber to the bottom one through the root canal system in presence of different obturation materials. Three nail polish coatings (Boots Group, Nottingham, UK) were applied to the teeth, excluding the apical dome. Specimens were mounted under laminar flow, in the experimental apparatus (Figure 1). A 5 mL vial (LSL, Rochdale, UK) was used as lower chamber. The upper chamber was made with a sectioned 20 mL syringe. A small hole was pierced through the centre of the rubber lid with the system B tip and the specimen was forced to the cemento- enamel junction (CEJ) level. Cyanoacrylate was placed around the CEJ. The metal ring was used to secure the lid. The 26 G and 18 G needles were inserted through the rubber lid to allow ventilation and further medium sampling. The testing apparatuses were sterilized by γ-irradiation (25 kGy).
Figure1.
In vitro infection model A) 18G needle to replenish medium; B) 26G needle for ventilation; C) Sectioned 20 mL syringe; D) Rubber cap; E) Ring; F) 5 mL vial
Under laminar-flow cabinet Enterococcus faecalis, Actinomyces radicidentis, Propionibacterium acnes, Streptococcus epidermidis and Streptococcus mitis were inoculated. Starter cultures were set up in filter-sterilized modified fluid universal medium (mFUM) incubated anaerobically at 37ºC for 3 h. The turbidity was adjusted with mFUM to 0.5 OD at 540 nm by iEMS Reader MF (Labsystems, Helsinki, Finland). Bacterial species were mixed equally and specimens were infected with 30 µL of the inoculum in the access cavity. In the negative control group, the bacterial inoculum was placed around the CEJ margin to test the seal of the system. The inferior chamber of the system was filled with mFUM to reach a level flush to the apical dome. Medium turbidity was monitored daily and replenished weekly (up to 70 days) via 18 G needle. The system was covered with parafilm (Bemis, Neenah, WI, USA) to prevent evaporation of the medium. The turbidity was considered positive when OD was more than 0.5. Each positive event was plotted in a spreadsheet as bacterial percolation through the root canal obturation with GraphPad, Prism v5.0 (GraphPad Software, San Diego, CA, USA). After bacterial passage 10 random specimens were sectioned horizontally at 2 mm and 10 mm from the apex using a diamond-wafering blade (Extec, Enfield, CT, USA) in a low speed saw (Buehler Ltd, Coventry, UK) under water-cooling and observed qualitatively for the presence of gaps and voids by confocal laser scanning microscopy (CLSM).
The slices of the specimens were stained with live/dead Baclight bacterial viability kit (Molecular Probes, Invitrogen, Paisley, UK) and visualized under Leica SP2 CLSM to observe gaps between the obturation material and the dentinal walls and to detect bacterial penetration in the tubules.
The samples were placed in dry wells of 24 multi-well plates and stained with 400 μL of dye for 15 min. The root discs were washed twice with phosphate buffered saline (PBS) and placed inverted onto glass bottom 35 mm diameter Petri dishes (MatTek Corporation, Ashland, USA).
A Leica SP2 CLSM was used to observe the fluorescence emission of SYTO 9 and PI using 488 and 569 (Ar-Kr laser) as excitation source respectively. The images were acquired and analysed with Image J (NIH, Bethesda, MD, USA) to determine the depth of penetration of the bacteria in the tubules.
Further 48 extracted human mandibular single-canal premolars were used to determine the sealer penetration. The roots were divided into 3 groups (A, B and C) as previously described. Sealers were labeled with 0.1% rhodamine B isothiocyanate (Sigma-Aldrich, Química SA de CV, Toluca, Mexico) to promote its fluorescence [19]. Shaping and obturation were carried out as previously described. Horizontal sections (1 mm thick) of the roots were cut at 2 (apical) and 5 mm (middle) from the apex using a diamond disc at 200 rpm under continuous water-cooling. The sections were polished with a silicone carbide abrasive paper and prepared for observation with CLSM (Leica TCS-SPE confocal microscope; Leica, Mannheim, Germany) under 10× magnification. Absorption and emission wavelengths of rhodamine B isothiocyanate were 540 nm and 590 nm respectively. The penetration of the sealer was measured from the canal wall to a maximum depth of 1000 μm with the software LAS AF (Leica, Mannheim, Germany) as reported by others [20, 21].
Statistical analysis
Percolation level was analysed by Kaplan-Meier survival test to determine differences between the groups at a significance level set at P<0.05. A one-way ANOVA and Tukey HSD tests were applied to ascertain statistical significant differences among total penetration of the groups and a Wilcoxon Signed-Ranks Test was applied to determine statistical significance differences between penetration of the sealer in the middle and the apical third of roots of each group (significance P<0.05).
Results
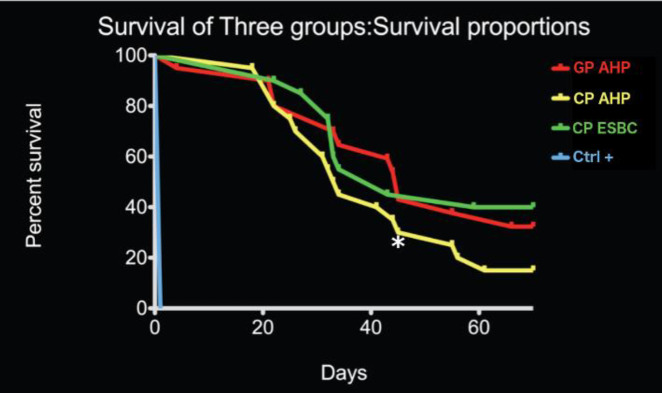
The Kaplan-Meier survival analysis revealed overall no significant difference (P-value=0.2046) between Group A, B and C (Figure 2). However, at 43 days, Group B showed a significantly inferior ability to prevent bacterial passage (P<0.05). The positive control showed immediate bacterial passage. The negative control did not show any bacterial passage for the whole duration of the experiment.
Figure 2.
Kaplan-Meier plot showing the survival to bacterial penetration following percolation test of the samples for each experimental group. No significant difference was observed between the three experimental groups. The rate of bacterial passage increased significantly 20 days after the bacterial infection. Group A: Size F3 Gutta-percha (GP) point and AH-Plus sealer; Group B: CPoint F3 with AH-Plus selaer; Group C: CPoint F3 with EndoSequence BC sealer, +ve Control: Teeth with no canal obturation
CLSM qualitative imaging revealed the presence of minimal gaps between the obturating material and the sealer and between the sealer and the root canal walls in teeth with bacterial percolation. Minimal voids allowed radial colonization of the tubules. Viable bacteria were detected as far as 400 μm in the depth of the tubules (Figure 3).
Figure 3.
CLSM images of a root disc from A group (warm condensation technique with gutta-percha in association with AH-Plus), positive for bacterial passage, stained with the live/dead stain showing the two channels [left for live bacteria (green), right for dead bacteria (red)]. Deep tubular infection could be observed
Table 1 shows the measurements regarding the penetration of the sealer. The Group C showed the deepest penetration and the group B showed the shortest one. For all groups, penetration in the middle third was deeper than penetration in the apical third (P<0.05). Figure 4 shows representative images of the samples observed with the CLSM.
Table 1.
Depth penetration of sealers (µm)
| Group (N) | Total (middle+apical third) | Middle third | Apical third |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| A (15) | 589.41 (296.18) | 715.75 (222.41) * | 435.88 (293.95) |
| B (15) | 324.16 (266.10)# | 463.31 (290.84) * | 176.06 (150.07) |
| C (15) | 709.84 (340.67) + | 913.25 (212.55) * | 506.44 (326.19) |
Group A: Warm vertical condensation, using a Gutta-percha point and AH-Plus Sealer; Group B: CPoint with AH-Plus; Group C: CPoint with EndoSequence BC sealer. Data with (+) showed statistically significant differences with the data of group B. Data with (#) showed statistically significant differences with the data of group A (One-way ANOVA, Tukey HSD). Data with (*) showed statistically significant differences with data of the apical third of its corresponding group (Wilcoxon Signed-Ranks Test)
Figure 4.
Representative images collected with a confocal scanning laser microscopy (10×). A1 and A2 show the penetration of the sealer in the middle third and in the apical third of Group A respectively. B1 and B2 show penetration in the middle third and in the apical third of Group B respectively. Images C1 and C2 show penetration in the middle third and in the apical third of Group C respectively
Discussion
The present study revealed that the use of single cone obturation material in conjunction with bioceramic sealers provides a level of seal to bacterial percolation comparable to traditional warm vertical condensation techniques. On the other hand, using a single cone technique in conjunction with epoxy-resin based sealer has a higher bacterial leakage further after 43 days. On a microscopic level the penetration of bioceramic sealer in the dentinal tubules was the highest.
The ideal obturation material would provide a hermetic seal. But several in vitro studies demonstrated that GP-obturated root canals tend to leak [22, 23].
The presence of discrepancy between the shaped root canal walls and the size and taper of the cone may affect to a greater extent single cone techniques [24, 25]. Warm vertical obturation techniques may lead to a better adaptation of softened gutta-percha to root canal walls irregularities [26]. Still recent studies addressed the concerns regarding the potential shrinkage while cooling of heated GP leading to voids and gaps [27]. The use of standardised cones matching the preparation of NiTi instruments may improve the adaptation. However, the thickness of the sealer may still be higher in the single cone obturation techniques [28, 29]. The use of expanding polymers may compensate the shrinkage of the cement during setting [24]. The use of bio-aggregates and calcium silicate (MTA) based sealers could represent a significant improvement compared with the traditional zinc oxide eugenol and epoxy resin based sealers [30-32].
In the present study, all groups showed a passage of bacteria in the course of the experiment (70 days). A significant difference could be detected between Group B, with a higher passage of bacteria. This could be ascribed to the type of sealer as Group C used the same type of cone (CPoint) but another type of calcium silicate-based sealer (ESBC), and interestingly had a similar behaviour to group A (GP+AH-Plus). Which is the same polymer single cone technique performed significantly better when associated with bioceramic sealer compared with epoxy-resin based ones.
Although not statistically significant, the least bacterial percolation was overall present with single cone used with bioceramic sealer. It may be hypothesized that either a sustained antibacterial effect of the calcium silicate sealer, or the progressive maturation of the portlandite phase with a better hermetic seal has been achieved [33].
The samples analysed with CLSM showed the development of live biofilms in the gap present between the root canal walls and the obturation materials. A tubular infection could be detected, although to a lesser extent (400 μm) compared with previous studies (1000 μm) [34].
The limitations of in vitro bacterial leakage models are represented by the fact that it is still unknown the number of bacteria necessary to cause pathology [35]. The quality of the obturation is technique dependant but it is also affected by the behaviour of the sealer. Therefore, the behaviour of the sealer is very important as it should not only fill the space between the core obturation material and the root canal walls but should also flow in the dentinal tubules to entomb viable bacteria.
The ESBC showed the deepest penetration level followed by the AH-Plus combined with GP and then the AH-Plus combined with CP. Differences in penetration could occur because the ESBC is a hydrophilic bioceramic sealer thus its interaction with residual water in the root canal could facilitate its entry in the tubules. Hence cone expansion represents an advantage for the penetration ability of the ESBC. The properties of sealers affect the efficacy of penetration. Balguerie et al. [15] reported that the AH-Plus sealer and the Acroseal -both epoxy resin based- showed deeper penetration than penetration of the Endobtur (ZOE), Ketac-Endo (glass ionomer) and RSA (silicon). The AH-Plus showed the deepest penetration with statistically significant differences when compared to the others [15]. Mamootil et al. [14] observed a deepest penetration of AH26 (statistically significant) when compared to EndoREZ (UDMA resin) and zinc oxide eugenol based sealer like Pulp Canal Selear EWT [14]. On the other hand, Ordinola-Zapata et al. [17] noticed that Sealapex, a calcium hydroxide-based sealer, showed the deepest mean penetration in comparison with Sealer 26 (calcium hydroxide) and GuttaFlow sealer (silicon). The studies concluded equally that the penetration varied because of the physical-chemical properties of the sealers [14, 15, 17]. A previous study, instead, found no significant difference between the penetration of AH-Plus and bioceramic sealers, however the latter had overall a better adaptation to dentine, differences in findings may be ascribed to differences in the irrigation regimen [36]. Indeed, the difference on the behaviour of the sealers origins from their physical-chemical properties expressed on solubility, flowing ability, viscosity, setting time and dimensional change among others [37-39]. Furthermore, the biological impact of bioceramic sealers on the periapical tissue may represent an important factor, considering the advantageous bioactivity of these sealers as demonstrated in a previous study [40].
Apical sealer penetration was the lower for all experimental groups. Other studies confirmed these findings as they have reported significant differences between the depth penetration between the middle and the apical third [16, 17, 20]. The finding is explained because of the distinct permeability of the dentin along the root canal. The density and the diameter of the tubules decreases from coronal to the apical third and sclerotic dentin occurs in the apical third [41, 42]. In addition to the effect of the properties of the dentine, the permeability is also impacted by the presence of smear layer [41]. Hence its removal favours penetration of sealers [13]. Accordingly, a final rinse with 2 mL of 17% EDTA solution was used to avoid presence of smear layer prior to obturation. Despite the chelating irrigant residual smear layer and debris might persist at the apical third [43]. Also, the penetration of bioceramic sealer could be greatly affected by the use of ultrasonic activation of endodontic irrigant [44].
A recent clinical study showed a promising outcome with single technique used with bioceramic sealer, hence supporting this simplified novel approach [8].
In our study, the ESBC sealer showed the deepest penetration and this result might suggest that the ESBC helps to achieve hermetic seal. However, if we observe the results obtained from the leakage test, we cannot assume that a deeper penetration will prevent bacterial re-invasion in the tubules. The CLSM imaging may be limited as the technique did not involve a 3D observation. The role of bacteria in the dentinal tubules has been extensively speculated and not fully understood. Once a tubular infection is established bacteria may harbour within the tubules and intermittently recolonize the root canal space.
Conclusion
Based on this in vitro study, single cone obturation techniques using novel materials performed similarly to warm vertical obturation technique in preventing bacterial passage. The ESBC showed the deepest penetration of the sealer, but it did not fully prevent bacterial percolation. Future developments of obturation materials should aim to further reduce the passage of bacteria from the coronal third to the apical third by reducing gaps present between the obturation material and the root canal walls.
Conflict of Interest:
‘None declared’.
References
- 1.Molander A, Warfvinge J, Reit C, Kvist T. Clinical and radiographic evaluation of one- and two-visit endodontic treatment of asymptomatic necrotic teeth with apical periodontitis: a randomized clinical trial. Journal of Endodontics. 2007;33(10):1145–8. doi: 10.1016/j.joen.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Onay EO, Ungor M, Yazici AC. The evaluation of endodontic flare-ups and their relationship to various risk factors. BMC Oral Health. 2015;15(1):142. doi: 10.1186/s12903-015-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haji-Hassani N, Bakhshi M, Shahabi S. Frequency of Iatrogenic Errors through Root Canal Treatment Procedure in 1335 Charts of Dental Patients. J Int Oral Health. 2015;7(Suppl 1):14–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Arora S, Hegde V. Comparative evaluation of a novel smart-seal obturating system and its homogeneity of using cone beam computed tomography: In vitro simulated lateral canal study. J Conserv Dent. 2014;17(4):364–8. doi: 10.4103/0972-0707.136512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hegde V, Murkey LS. Microgap Evaluation of Novel Hydrophilic and Hydrophobic Obturating System: A Scanning Electron Microscope Study. J Clin Diagn Res. 2017;11(5):ZC75–ZC8. doi: 10.7860/JCDR/2017/27458.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eid AA, Nikonov SY, Looney SW, Didato A, Niu LN, Levin MD, Rueggeberg FA, Pashley DH, Watanabe I, Tay FR. In Vitro biocompatibility evaluation of a root canal filling material that expands on water sorption. J Endod. 2013;39(7):883–8. doi: 10.1016/j.joen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Didato A, Eid AA, Levin MD, Khan S, Tay FR, Rueggeberg FA. Time-based lateral hygroscopic expansion of a water-expandable endodontic obturation point. J Dent. 2013;41(9):796–801. doi: 10.1016/j.jdent.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Zavattini A, Knight A, Foschi F, Mannocci F. Outcome of Root Canal Treatments Using a New Calcium Silicate Root Canal Sealer: A Non-Randomized Clinical Trial. J Clin Med. 2020;9:3. doi: 10.3390/jcm9030782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rechenberg DK, Thurnheer T, Zehnder M. Potential systematic error in laboratory experiments on microbial leakage through filled root canals: an experimental study. Int Endod J. 2011;44(9):827–35. doi: 10.1111/j.1365-2591.2011.01888.x. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira AC, Tanomaru JM, Faria-Junior N, Tanomaru-Filho M. Bacterial leakage in root canals filled with conventional and MTA-based sealers. Int Endod J. 2011;44(4):370–5. doi: 10.1111/j.1365-2591.2011.01852.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuci A, Alacam T, Yavas O, Ergul-Ulger Z, Kayaoglu G. Sealer penetration into dentinal tubules in the presence or absence of smear layer: a confocal laser scanning microscopic study. J Endod. 2014;40(10):1627–31. doi: 10.1016/j.joen.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Sedgley CM, Lennan SL, Appelbe OK. Survival of Enterococcus faecalis in root canals ex vivo. Int Endod J. 2005;38(10):735–42. doi: 10.1111/j.1365-2591.2005.01009.x. [DOI] [PubMed] [Google Scholar]
- 13.Bolles JA, He J, Svoboda KK, Schneiderman E, Glickman GN. Comparison of Vibringe, EndoActivator, and needle irrigation on sealer penetration in extracted human teeth. J Endod. 2013;39(5):708–11. doi: 10.1016/j.joen.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Mamootil K, Messer HH. Penetration of dentinal tubules by endodontic sealer cements in extracted teeth and in vivo. Int Endod J. 2007;40(11):873–81. doi: 10.1111/j.1365-2591.2007.01307.x. [DOI] [PubMed] [Google Scholar]
- 15.Balguerie E, van der Sluis L, Vallaeys K, Gurgel-Georgelin M, Diemer F. Sealer penetration and adaptation in the dentinal tubules: a scanning electron microscopic study. J Endod. 2011;37(11):1576–9. doi: 10.1016/j.joen.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Chandra SS, Shankar P, Indira R. Depth of penetration of four resin sealers into radicular dentinal tubules: a confocal microscopic study. J Endod. 2012;38(10):1412–6. doi: 10.1016/j.joen.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Ordinola-Zapata R, Bramante CM, Graeff MS, del Carpio Perochena A, Vivan RR, Camargo EJ, Garcia RB, Bernardineli N, Gutmann JL, de Moraes IG. Depth and percentage of penetration of endodontic sealers into dentinal tubules after root canal obturation using a lateral compaction technique: a confocal laser scanning microscopy study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):450–7. doi: 10.1016/j.tripleo.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Arikatla SK, Chalasani U, Mandava J, Yelisela RK. Interfacial adaptation and penetration depth of bioceramic endodontic sealers. J Conserv Dent. 2018;21(4):373–7. doi: 10.4103/JCD.JCD_64_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eymirli A, Sungur DD, Uyanik O, Purali N, Nagas E, Cehreli ZC. Dentinal Tubule Penetration and Retreatability of a Calcium Silicate-based Sealer Tested in Bulk or with Different Main Core Material. J Endod. 2019;45(8):1036–40. doi: 10.1016/j.joen.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Gharib SR, Tordik PA, Imamura GM, Baginski TA, Goodell GG. A confocal laser scanning microscope investigation of the epiphany obturation system. J Endod. 2007;33(8):957–61. doi: 10.1016/j.joen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Moon YM, Shon WJ, Baek SH, Bae KS, Kum KY, Lee W. Effect of final irrigation regimen on sealer penetration in curved root canals. J Endod. 2010;36(4):732–6. doi: 10.1016/j.joen.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Trope M, Chow E, Nissan R. In vitro endotoxin penetration of coronally unsealed endodontically treated teeth. Endod Dent Traumatol. 1995;11(2):90–4. doi: 10.1111/j.1600-9657.1995.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 23.Haikel Y, Wittenmeyer W, Bateman G, Bentaleb A, Allemann C. A new method for the quantitative analysis of endodontic microleakage. J Endod. 1999;25(3):172–7. doi: 10.1016/S0099-2399(99)80136-8. [DOI] [PubMed] [Google Scholar]
- 24.Monticelli F, Sadek FT, Schuster GS, Volkmann KR, Looney SW, Ferrari M, Toledano M, Pashley DH, Tay FR. Efficacy of two contemporary single-cone filling techniques in preventing bacterial leakage. J Endod. 2007;33(3):310–3. doi: 10.1016/j.joen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Nawal RR, Parande M, Sehgal R, Naik A, Rao NR. A comparative evaluation of antimicrobial efficacy and flow properties for Epiphany, Guttaflow and AH-Plus sealer. Int Endod J. 2011;44(4):307–13. doi: 10.1111/j.1365-2591.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- 26.Collins J, Walker MP, Kulild J, Lee C. A comparison of three gutta-percha obturation techniques to replicate canal irregularities. J Endod. 2006;32(8):762–5. doi: 10.1016/j.joen.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Lottanti S, Taubock TT, Zehnder M. Shrinkage of backfill gutta-percha upon cooling. J Endod. 2014;40(5):721–4. doi: 10.1016/j.joen.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 28.Diemer F, Sinan A, Calas P. Penetration depth of warm vertical Gutta-Percha pluggers: impact of apical preparation. J Endod. 2006;32(2):123–6. doi: 10.1016/j.joen.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Wu MK, Wesselink PR. A primary observation on the preparation and obturation of oval canals. Int Endod J. 2001;34(2):137–41. doi: 10.1046/j.1365-2591.2001.00361.x. [DOI] [PubMed] [Google Scholar]
- 30.De-Deus G, Canabarro A, Alves G, Linhares A, Senne MI, Granjeiro JM. Optimal cytocompatibility of a bioceramic nanoparticulate cement in primary human mesenchymal cells. J Endod. 2009;35(10):1387–90. doi: 10.1016/j.joen.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Leal F, De-Deus G, Brandao C, Luna AS, Fidel SR, Souza EM. Comparison of the root-end seal provided by bioceramic repair cements and White MTA. Int Endod J. 2011;44(7):662–8. doi: 10.1111/j.1365-2591.2011.01871.x. [DOI] [PubMed] [Google Scholar]
- 32.Camilleri J, Gandolfi MG, Siboni F, Prati C. Dynamic sealing ability of MTA root canal sealer. Int Endod J. 2011;44(1):9–20. doi: 10.1111/j.1365-2591.2010.01774.x. [DOI] [PubMed] [Google Scholar]
- 33.Morgental RD, Vier-Pelisser FV, Oliveira SD, Antunes FC, Cogo DM, Kopper PM. Antibacterial activity of two MTA-based root canal sealers. International Endodontic Journal. 2011 doi: 10.1111/j.1365-2591.2011.01931.x. [DOI] [PubMed] [Google Scholar]
- 34.Love RM, Jenkinson HF. Invasion of dentinal tubules by oral bacteria. Crit Rev Oral Biol Med. 2002;13(2):171–83. doi: 10.1177/154411130201300207. [DOI] [PubMed] [Google Scholar]
- 35.Stashenko P, Yu SM, Wang CY. Kinetics of immune cell and bone resorptive responses to endodontic infections. J Endod. 1992;18(9):422–6. doi: 10.1016/S0099-2399(06)80841-1. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadian F, Farahanimastary F, Dibaji F, Kharazifard MJ. Scanning Electron Microscopic Evaluation of the Sealer-Dentine Interface of Three Sealers. Iran Endod J. 2017;12(1):38–42. doi: 10.22037/iej.2017.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marin-Bauza GA, Silva-Sousa YT, da Cunha SA, Rached-Junior FJ, Bonetti-Filho I, Sousa-Neto MD, Miranda CE. Physicochemical properties of endodontic sealers of different bases. J Appl Oral Sci. 2012;20(4):455–61. doi: 10.1590/S1678-77572012000400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resende LM, Rached-Junior FJ, Versiani MA, Souza-Gabriel AE, Miranda CE, Silva-Sousa YT, Sousa Neto MD. A comparative study of physicochemical properties of AH Plus, Epiphany, and Epiphany SE root canal sealers. Int Endod J. 2009;42(9):785–93. doi: 10.1111/j.1365-2591.2009.01584.x. [DOI] [PubMed] [Google Scholar]
- 39.Flores DS, Rached FJ Jr, Versiani MA, Guedes DF, Sousa-Neto MD, Pecora JD. Evaluation of physicochemical properties of four root canal sealers. Int Endod J. 2011;44(2):126–35. doi: 10.1111/j.1365-2591.2010.01815.x. [DOI] [PubMed] [Google Scholar]
- 40.Jafari F, Jafari S, Etesamnia P. Genotoxicity, Bioactivity and Clinical Properties of Calcium Silicate Based Sealers: A Literature Review. Iran Endod J. 2017;12(4):407–13. doi: 10.22037/iej.v12i4.17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrari M, Mannocci F, Vichi A, Cagidiaco MC, Mjor IA. Bonding to root canal: structural characteristics of the substrate. Am J Dent. 2000;13(5):255–60. [PubMed] [Google Scholar]
- 42.Hargreaves KM, Cohen S. Cohen's Pathways of the Pulp Expert Consult. 10th Edition ed: Mosby 2010. [Google Scholar]
- 43.Mancini M, Armellin E, Casaglia A, Cerroni L, Cianconi L. A comparative study of smear layer removal and erosion in apical intraradicular dentine with three irrigating solutions: a scanning electron microscopy evaluation. J Endod. 2009;35(6):900–3. doi: 10.1016/j.joen.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 44.Zand V, Milani A, Yavari H, Majidi A. The Effects of Different Agitation Techniques of Canal Irrigant on Tubular Penetration of a Bioceramic Sealer. Iran Endod J. 2019;14(4):289–95. doi: 10.22037/iej.v14i4.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]