Abstract
Introduction:
This study aimed to evaluate the in vitro effectiveness of N-acetylcysteine (NAC), photodynamic therapy (PDT) and NAC with supplemental PDT in optimizing the removal of bacteria from infected dentinal tubules of root canals infected with Enterococcus (E.) faecalis biofilm.
Methods and Materials:
Eighty human teeth were randomly divided into 5 groups (n=16) according to the intracanal medication used: saline solution (control); calcium hydroxide (CH); NAC; PDT; NAC+PDT. Ten samples from each group were prepared for microbiological culture analysis (CFU/mL) and were inoculated with E. faecalis suspension for 21 days for biofilm development; the other six samples from each group were prepared for scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) and submitted to a 5-days contamination protocol including eight centrifugation cycles on every other day for dentinal tubules infection. For antimicrobial activity analysis by microbiological culture (CFU/mL), the root canals were contaminated with E. faecalis biofilm, instrumented and then medicated according to the experimental groups. Three samples were collected from the root canals: after 21-days of contamination, immediately after the instrumentation and 14-days after the medication according to the experimental groups. The morphology of E. faecalis biofilm on the root canal walls and bacterial cells viability were assessed by means of SEM and CLSM, respectively. One-way ANOVA and Repeated Measures ANOVA tests were used to analyze the obtained data statistically.
Results:
CFU/mL analysis showed that CH, NAC and NAC+PDT promoted greater antibacterial activity with statistically significant difference compared to saline solution and PDT (P<0.0001). However, saline solution and PDT were statistically similar (P>0.07). Illustrative images by SEM confirmed partially the CFU/mL results. CLSM showed that all groups were effective eliminating E. faecalis except for the saline solution group.
Conclusions:
Based on this in vitro study NAC was bactericidal against E. faecalis biofilms regardless PDT stimulation, presenting similar antimicrobial activity to CH.
Key Words: Enterococus faecalis, Confocal Laser Scanning Microscopy, N-Acetylcysteine, Photodynamic Therapy, Scanning Electron Microscopy
Introduction
The success of endodontic therapy relies on the elimination of microorganisms and their byproducts and prevention of reinfection [1]. The majority of bacteria from infected and necrotic tissue is generally removed by chemical irrigation and mechanical instrumentation [2]. However, the effectivity of these combined procedures is limited due to the complexity of root canal system [3]. Some inaccessible areas such as ramifications, isthmii and dentinal tubules remain untouched and thus may favor bacterial retention and regrowth [4].
Despite the significant progress made in “cleaning and shaping” techniques, the resistance of microorganisms is frequently associated with the ecological organization of bacteria in a three-dimensional structure known as biofilm [5]. Notably, biofilm can be defined as a sessile bacterial community characterized by cells embedded in a self-produced polymeric matrix [6, 7], which is firmly attached to surfaces or interfaces. Biofilms formed on the root canal surfaces can be remarkably difficult to eradicate since it is 2–1000-fold more resistant thanthe corresponding floating planktonic forms [8]. Therefore, the use of intracanal medication to disrupt biofilms and thereby eradicate residual microbiological infections within root canals has been recommended to achieve a successful endodontic treatment [9, 10].
Enterococcus faecalis (E. faecalis) is a gram-positive facultative anaerobic bacterium frequently isolated from root canals of teeth with pulpal necrosis and apical periodontitis and have been reported as a potential cause of endodontic failure in root-filled teeth [11,12]. The high prevalence of E. faecalis in persistent infection is partially explained by its capacity to form biofilms, dentinal penetration ability [13], and high tolerance to alkaline pH [14].
N-acetylcysteine (NAC) is derivative of amino acid L-cysteine, with antioxidant properties and widely used as mucolytic agent in medical treatments [15,16]. In addition, NAC has been actively studied by clinical microbiologists due to its high antibacterial activity, including against biofilm phenotypes, such as E. faecalis [17]. It has been reported that NAC reduces a self-produced polymeric matrix production, preventing bacterial adherence to surfaces, inhibiting biofilm formation and disrupting mature biofilms [18–20].
Photodynamic therapy (PDT) has been proposed as a supplemental method to optimize the root canal infection and overcome the challenge of disinfecting inaccessible areas. PDT consists on the use of a light source generated by a low-power laser and a nontoxic photosensitizer, which causes bacterial cell damage and death of microorganisms [21, 22]. This technique is minimally invasive, and causes no damage for periodontal tissue, and thus has been recommended to be used as an adjuvant to conventional endodontic treatment to eliminate the bacterial load. In vitro studies have revealed a notable bactericidal potential of PDT against E. faecalis [23–25].
Up to now, there is no study evaluating the effectiveness of NAC associated to PDT in reducing and/or eliminating E. faecalis biofilms. Therefore, this study evaluated, in vitro, the effectiveness of NAC, PDT and NAC with supplemental PDT in optimizing the removal of bacteria from root canals infected with E. faecalis.
Materials and Methods
The present study was performed under approval of the human Ethics Committee of São Paulo State University, São José dos Campos, Brazil. Eighty freshly extracted single rooted human teeth (with closed apex ,without caries and calcification of the canal, which was confirmed by radiography) were selected and stored in saline (Eurofarma, São Paulo, SP, Brazil) until the moment of use [26]. Fifty teeth were used for microbiological culture analysis and thirty were prepared for scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) analysis.
The microbiological procedures were carried out in a laminar flow chamber to ensure aseptic condition (Veco Bioseg 09 Ltda., Campinas, Brazil). E. faecalis bacterial strain (ATCC 29212) was plated onto sterile brain-heart infusion (BHI) agar (BHI, Difco, Kansas City, MO, USA) and incubated at 37 °C under aerobic conditions for 24 h.
Microbiological culture analysis
Preparation of specimens : Fifty extracted single rooted human teeth were selected based on dimensional and morphological similarities. The crowns were cross-cut with a carborundum disc and the specimens length was standardized to 16±0.5 mm. Specimens were instrumented in the root canal total length to K-file #30 (Dentsply, Petrópolis, RJ, Brazil) in order to standardize the specimens’ diameter and irrigated with 3 mL of 1% NaOCl through each instrument change. Then, the canals were filled with 17% trisodium ethylenediaminetetraacetic acid (EDTA) (Porto Alegre, RS, Brazil) for 3 min followed by a 10 mL final irrigation with saline solution [27]. The external apical portion of specimens were sealed with light-cured composite resin (Z-100; 3M do Brazil Ltda, Sumaré, SP, Brazil) and the outer surfaces of the roots were sealed with an epoxy adhesive layer, except for the cervical opening region. The specimens were randomly distributed into 5 different 24-wells cell culture plates with ten specimens in each one and were fixed with chemically activated acrylic resin. All materials used in the present study were sterilized by gamma radiation with cobalt-60 (20 kGy for 6 h) [28].
Contamination of specimens: Initially, E. faecalis (ATCC 29212) suspension from the culture previously seeded was prepared containing 106 cells/mL in exponential phase confirmed by a spectrophotometer reading (B582, Micronal, São Paulo, SP, Brazil) [29]. The optical density and wavelength parameters used were 0.298 and 760 nm, respectively. Then, 5 𝜇L of E. faecalis suspension was inoculated into each root canal followed by 10 𝜇L of brain heart infusion (BHI) broth (HiMedia Laboratories, Mumbai, India). Then, a sterile cotton pellet was soaked in the culture medium and placed in the cervical opening of each specimen. All specimens were stored in an incubator at 37±1ºC with relative humidity. BHI broth was added to root canals every alternate day for 21 days [29].
Sample collection: For microbiological culture, sterile paper points (size #25; Dentsply Maillefer, Ballaigues, Switzerland) were introduced into the full length of the root canal and retained in position for 60 sec. In order to confirm the root canal infection, after the contamination period an initial sample (S1) was collected to serve as the baseline. Immediately after, the paper point was transferred to sterile microtubes (Axygen, Union city, CA, USA) containing 1000 𝜇L of sterile saline solution [30]. The root canals were instrumented with Reciproc system file R40 (VDW, Munich, Germany), adapted to an electric motor (VDW, Munich, Germany) in reciprocation movement. Instrumentation was performed along the total length of the canals associated to irrigation of 5 mL of sterile saline solution for each radicular third, totalizing 15 mL. After instrumentation, another sample was collected (S2) in the same standard way as previously described. Then, the specimens were submitted to the intracanal medication protocol described below according to the group assigned:
Saline solution: To serve as a control group, the root canals were filled with 30 μL of saline.
CH: The powder of CH (Biodinâmica Química e Farmacêutica LTDA, Ibiporã, PR, Brazil) was mixed with sterile saline solution (1 g : 1 mL proportion) over a sterile glass plate until smooth and no lumps remain, resembling the consistency of commercial toothpaste. The paste was inserted into the root canal using a K-file #30 [30].
PDT: PDT was performed using 0.005% methylene blue as a photosensitizer and a diode LASER (DL) (660 nm wavelength) with a power of 40 mW (MMOptics Ltda, São Carlos, SP, Brazil). The photosensitizer was placed into the canals for 5 min. The optical tip of the DL (0.40 mm diameter and 16±0.5 mm active surface length) was placed into the canal and the DL was activated for 2 min without interval, using a helical movement from apical to cervical. A density of approximately 120.0 J/cm2 was applied into each root canal. Subsequently, the photosensitizer rinsed out using 5 mL of saline and the canals were dried with sterile #40 paper points [31].
NAC: NAC powder (Sigma Aldrich, Merck KGaA, Darmstadt, Germany) was mixed with sterile saline solution (1 gr: 1 mL proportion), over a sterile glass plate until smooth and no lumps remain, resembling the consistency of commercial toothpaste. The paste was inserted into the root canal using a K-file #30 [30]. This proportion was determined based on the results of a pilot study that tested the antibacterial efficacy of different formulations of NAC in aqueous paste by the agar diffusion method. This is a validated method to test different concentrations of a compound against a large number of individual bacterial strains, providing a preliminary suggestion as to varying degrees of antibacterial efficacy. The inhibitory activity of three concentrations of NAC powder (Sigma Aldrich, Merck KGaA, Darmstadt, Germany) mixed with sterile saline were compared. These mixed NAC concentrations were chosen because they all had a paste-like consistency enough to be inserted with a K-file #30 but also to remain on the agar disks when tested during incubation. The different concentrations of NAC mixture in aqueous solution were placed in 24-well plates and each well was filled with 2.5 mL sterile PBS. After incubation for 24 h at conditions of 5% CO2 at 37°C, paper discs of 5 mm in diameter were soaked in the original extracts and left to dry in an incubator at 37°C. Then, the discs were placed in petri dishes in direct contact with E. faecalis in Brain Heart Infusion broth (BHI) agar (Himedia, Mumbai, India) for 24 h at 37°C. The mixture of NAC in aqueous solution in the proportion of 50% (1: 1) was more effective at eliminating and inhibiting the bacterial growth, showing wider zones of inhibition compared to the other tested concentrations.
NAC+PDT: First, PDT protocol was applied in the same standard way as previously described and then NAC paste was prepared and inserted into the root canal also in the same standard way as previously described.
All specimens were stored at 37°C for 14 days. The medications were then removed with 10 mL of saline, and another sample was collected with sterile paper point size #45 (S3) and transferred to microtubes containing 1 mL of sterile saline.
Culture procedure: To determine the antimicrobial activity, all samples collected at S1 (baseline samples), S2 (after instrumentation) and S3 (after final intracanal medication protocol) were serial diluted, and 100 μL aliquots of each dilution were seeded onto duplicate petri dishes containing Enterococcosel agar (HiMedia Laboratories, Mumbai, India). Then, microorganisms were incubated at 37°C for 24 h and the number of colony-forming units/mL (CFU/mL) was counted.
Imaging analysis
Specimens preparation: Thirty extracted single rooted human teeth were selected and initially stored for 48 h in 1% NaOCl solution for decontamination [32]. The tooth crowns were removed and the roots sectioned at a distance of 4±0.5 mm from the apex, using a diamond disc attached to a low-speed saw (Isomet 1000, Buehler Ltd, Lake Bluff, IL, USA), under irrigation. The roots segments were standardized at lengths of 12±0.5 mm and prepared with K-files up to size #30 (Dentsply Ind. Com. Ltda, Petrópolis, RJ, Brazil) and irrigated with 3 mL of 1% NaOCl through each instrument change. The smear layer was removed using 17% EDTA (Inodon, Porto Alegre, RS, Brazil) for 3 min followed by final irrigation with 10 mL of saline solution. In order to prevent external microbial contamination, two layers of red polish nail (L’Oreal Colorama, Rio de Janeiro, RJ, Brazil) were applied to the external surface of the roots. After 24 h, the specimens were allocated into microtubes containing distilled water and submitted to a regular sterilization cycle in autoclave at 121°C.
Contamination of specimens: After sterilization, the water was removed and 1 mL of sterilized BHI broth was added individually into all microtubes, which were submitted to an ultrasonic bath (Cristofoli Equipamento de Biossegurança LTDA, Campos Mourao, PR, Brazil) for 15 min to allow the maximum penetration of the culture medium into the dentinal tubules [32]. The E. faecalis inoculum was adjusted to 3×108 CFU/mL according to the McFarland standard No.1 using a spectrophotometer, and exponential bacterial grown was achieved in 7h [32]. After this period, 1mL of the inoculum was added into the microtubes containing the specimens, which were taken for centrifugation (Eppendorf 542R, Eppendorf, Hamburg, Germany). The tubes were submitted to a sequence of eight centrifugation cycles at 1400, 2000, 3600 and 5600 g, at 25°C, in two cycles of 5 min for each speed. Between every centrifugation cycle, the solution that had penetrated through the dentine specimen was discarded, and a fresh solution of inoculum was added to the microtube. After the centrifugation procedures, sterilized BHI broth was inserted into the microtubes, which were agitated in a vortex and incubated at 37°C under aerobic conditions for 24 h. Dentinal tubules were submitted to the contamination protocol for 5 days, with centrifugation on alternative days. On the fifth day, the specimens were removed from the microtubes and prepared for treatment with the different intracanal medication protocol: Saline solution, CH, NAC, PDT and NAC+PDT paste as previously described. Of the 6 specimens from each group, 2 specimens were submitted to SEM analysis and 4 specimens were submitted to CLSM analysis.
SEM analysis: Two specimens from each group were longitudinally sectioned by implementing two lengthwise grooves along the external surface of the root in the mesiodistal direction. For this purpose, a diamond disc was used at low rotation with caution to avoid penetrating the inner wall of the root canal until the samples could be gently cleaved with a chisel into two semi-cylindrical halves [26]. Then, the halves were fixed with 4% paraformaldehyde for 30 min followed by dehydration by increasing concentrations of ethanol (60, 70, 80, and 100) and stored at 37ºC for 24 h. Next, they were metalized by 12 nm gold layer by means of a metallizer (Emitech SC7620; Quorum Technologies Ltd, Lewes, East Sussex, UK). The presence of bacteria in the dentinal tubules was observed by SEM (Inspect S50 FEI, Brno, Moravia, Czech Republic) at a magnification of 5000–10000× [33] operating at 7-8 kV.
CLSM analysis: The 4 specimens of each group were longitudinally sectioned using a diamond disc attached to an Isomet saw, under constant irrigation with a sterilized saline solution. The smear layer produced by these cutting procedures was removed with 17% EDTA for 10 min, followed by a wash ofsterilized saline solution [32]. To evaluate bacterial penetration and viability in dentinal tubules, the specimens were firstly washed with phosphate-buffered saline and then stained with SYTO 9/propidium iodide (Live/Dead BacLight Viability Kit; Molecular Probes, Eugene, OR, USA) in a dark environment with 15 mL of the dyes for 15 min. Then, they were newly washed and directly observed by inverted confocal laser scanning microscopy (Leica Microsystems GmbH, Mannheim, Baden-Württemberg, Germany) using a 40× magnification oil lens. The Live/Dead reagents stained live bacteria with a green stain and dead bacteria with a red stain, thus making it possible to identify viable bacteria. The 488 and 532 nm wave-lengths were used to excite the Live/Dead stain, and emission was detected between 490 and 575 nm for green fluorescence and between 600 and 720 nm for red fluorescence [32]. Two confocal “stacks” of cervical and apical third were obtained for each sample. There were 4 samples per groups, thus 8 stacks for each medication protocol. The images were collected by Leica application Suite-Advanced fluorescence program (LAS AF; Leica, Mannheim, Germany), and then analyzed with Leica LAS AF Lite software. For quantification of bacterial viability, bioImage_L (www.bioImage.com) was used to calculate the percentage of green and red (live and dead bacteria, respectively) found after the intracanal medication treatment [34].
Statistical analysis
The Shapiro-Wilk test was used to verify the normal distribution of data. In the absence of normality, the data were transformed into log10 to obtain a normal distribution. After log10 transformation parametric methods were used: One-way ANOVA test was used for intergroup comparison, while the Repeated Measure ANOVA test was used for intra-group comparison (between sample collections). The significance level was established at 5% for all tests.
Results
Microbiological culture analysis
All specimens were contaminated, which was confirmed by S1 serving as a baseline. Table 1 shows the mean number of CFU/mL of all groups initially (S1), after instrumentation procedure (S2) and after 14 days of intracanal medication (S3).
Table 1.
The mean (SD) of E. faecalis CFU/mL counts for all groups at baseline samples (S1), after instrumentation (S2), after treatment protocol (S3). Data were presented in log10 due to the absence of normality in Shapiro-Wilk test
| Groups | Baseline (S1) | After instrumentation (S2) | After treatment (S3) |
|---|---|---|---|
| Saline solution | 3.475 (0.3290) Aa | 3.182 (0.9020) ABa | 2.761 (0.9769) Ba |
| CH | 3.556 (0.2872) Aa | 3.557 (0.3944) Aab | 0.0 (0.0) Bb |
| PDT | 4.060 (0.6145) Aab | 3.251 (0.5644) Aa | 1.807 (0.7093) Bc |
| NAC | 3.722 (0.4656) Aa | 3.942 (0.2765) Ab | 0.0 (0.0) Bb |
| NAC+PDT | 4.775 (1.092) Ab | 4.077 (0.1185) Ab | 0.0 (0.0) Bb |
*Different uppercase letters mean statistically significant differences intragroup (P<0.05) (Repeated Measure ANOVA); Different lowercase letters indicate statistically significant difference in intergroup (Ordinary one-way ANOVA)
In S2, after instrumentation procedure, no significant reduction of E. faecalis was observed in any of the groups.
In S3, after using intracanal medication, there was a statistically significant reduction of E. faecalis in all experimental groups except for the control group.
However, the CH, NAC and NAC+PDT groups were the most effective in eliminating E. faecalis, with a statistically significant difference compared to the PDT group alone (P<0.0001) and the Saline control group (P<0.0001).
SEM analysis
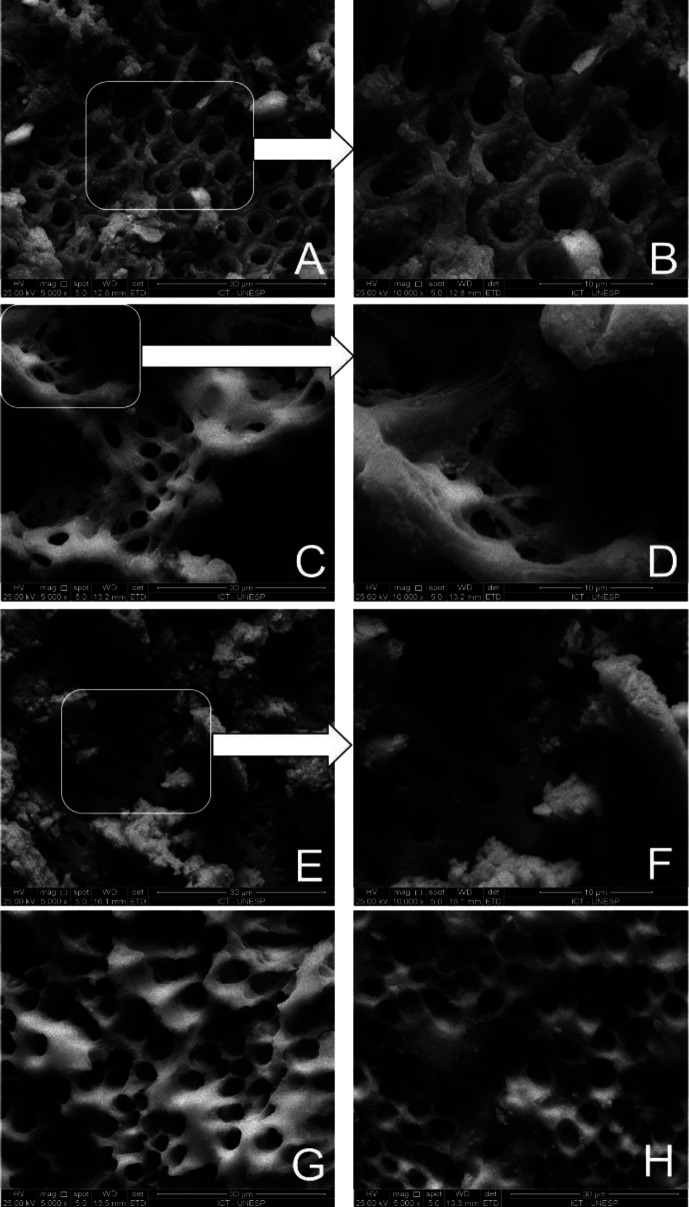
Figure 1 presents representative SEM images of cleaning obtained by the intracanal medications used after biomechanical preparation in the different experimental groups. As shown, in the Saline control group, dentinal surface presented a thick biofilm of E. faecalis with bacterial cells extending into dentinal tubules (Figures 1A and 1B). Similarly, the PDT group also showed bacterial clusters attached to the dentin surface, however with a lower number of bacterial cells in the opening of the dentin tubules (Figures 1E and 1F). In the CHCH group, the dentin surface was mostly clean with only a few remaining bacterial colonies (Figures 1C and 1D). Surprisingly, in the groups treated with NAC and NAC+PDT, despite the surface showing some remaining bacteria, a noticeable reduction was observed in relation to bacteria in dentinal tubules among the treated groups (Figures 1G and 1H).
Figure 1.
Representative photomicrography of canal walls after treatment: A and B) Saline solution group (Positive control) showed the presence of E. faecalis in dentinal tubules entrance (original magnification, 5000× and 10000×, respectively); C and D) CH group showed presence of E. faecalis (original magnification, 5000× and 10000×, respectively); E and F) PDT group showed presence of E. faecalis (original magnification, 5000× and 10000×, respectively); G) NAC group showed absence of E. faecalis in canal walls and dentinal tubules (original magnification, 5000×); H) NAC+PDT group showed absence of E. faecalis in canal walls and dentinal tubules (5000×)
CLSM analysis
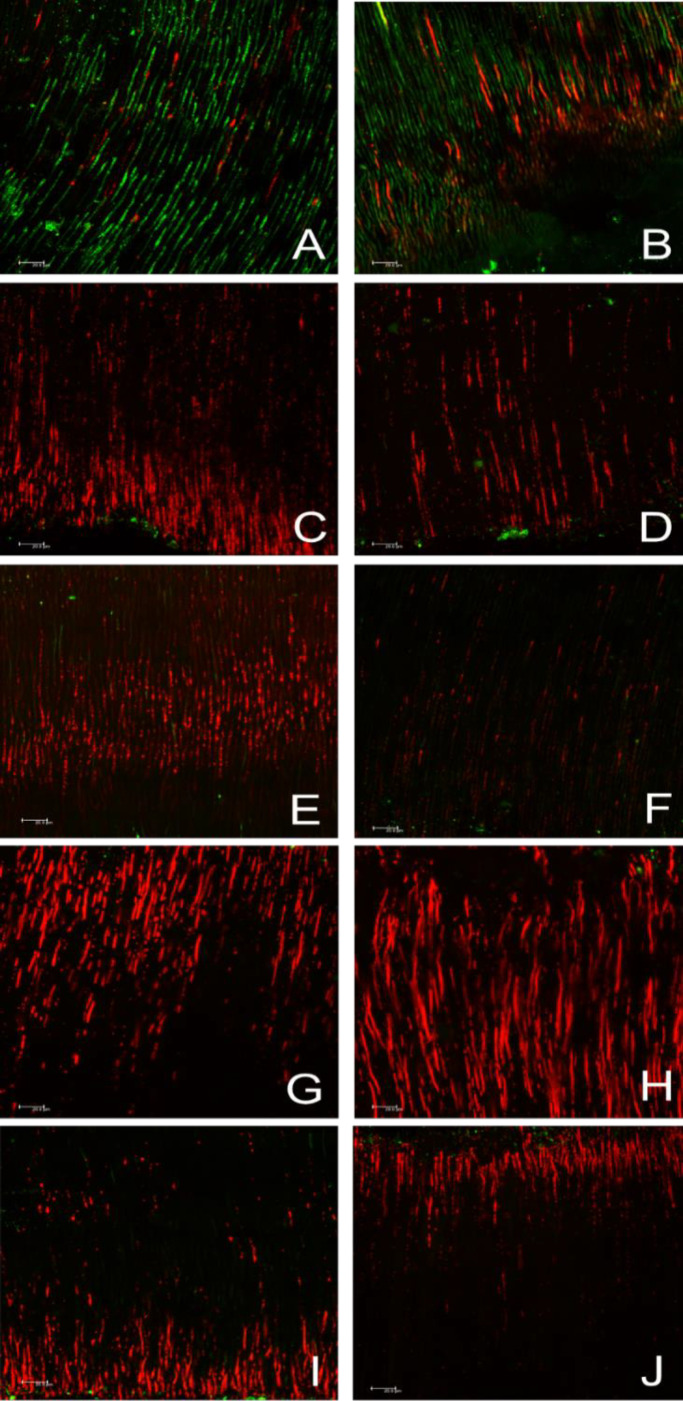
The results of viable bacteria and total biovolume of E. faecalis biofilm after intracanal medication are shown in Table 2. The highest biovolume of viable bacteria was found in the Saline Solution control group (76.90%), with a statistical significantly difference compared to all experimental groups (P<0.0001). NAC group was less effective presenting (32.63%) of viable bacteria followed by NAC+PDT (28.85%), PDT (26.95%), and CH (20.09%), however, no statistical difference was observed between these groups (P>0.05).
Table 2.
The mean (SD) values of the percentage of viable cells and of E. faecalis biofilm after intracanal medication treatment in all groups
| Groups | Saline solution | CH | PDT | NAC | NAC+PDT |
|---|---|---|---|---|---|
| Percentage of viable cells (green) | Total biovolume | ||||
| 76.90 (17.82) A | 20.09 (24.13) B | 26.95 (23.41) B | 32.63 (31.27) B | 28.85 (27.45) B | |
| Percentage of viable cells (green) | Cervical third | ||||
| 69.17 (22.64) A | 17.76 (19.11) B | 27.23 (26.79) B | 30.25 (30.96) B | 36.97 (30.13) B | |
| Percentage of viable cells (green) | Apical third | ||||
| 73.80 (20.79) A | 28.84 (30.64) B | 46.58 (36.16) A | 38.17 (31.38) B | 41.82 (40.28) A | |
*Different uppercase letters in each row indicate significant differences between the groups regarding of percentage of viable cells (One-way ANOVA) (P<0.05)
Table 2 also shows the percentage of viable bacteria within the dentinal tubules in the cervical and apical third after treatment with the intracanal medications tested. After analysis of CLSM images, the saline control group showed the highest percentage of viable bacteria in both the cervical and apical thirds, being statistically different from the other experimental groups (P<0.005). Comparing the experimental groups, in the cervical third, the NAC+PDT group had the greatest percentage of viable bacteria (36.97%) followed by the NAC (30.25%), PDT (27.23%) and CH (17.76%) groups, however without statistical difference between the groups (P>0.05). Meanwhile, in the apical third, the PDT and PDT+NAC groups had the highest percentages of viable bacteria (46.58%) and (41.82%) respectively, with a statistically significant difference compared to the NAC (38.17%) and CH (28.84%) groups (P<0.005) (Figure 2).
Figure 2.
Confocal laser scanning microscopy analysis of E. faecalis infected dentinal tubules after treatment: Illustrative images of dentinal tubules in the cervical (left column) and apical (right column) third, respectively, by CLSM after treatment with A and B) Saline solution; C and D) Ca(OH)2; E and F) PDT; G and H) NAC; I and J) NAC+PDT. Red stain indicates the presence of dead bacteria, while green stain represents live bacteria
Discussion
In the present study, it was proposed the use of NAC as intracanal medication, associated or not with PDT on intracanal biofilm of E. faecalis. After 21 days of E. faecalis inoculation it was possible to observe the bacterial growth of E. faecalis in all groups through S1 (baseline). Data obtained by microbiological culture analysis after instrumentation, showed a great reduction of CFU/mL compared to S1 (baseline), but none of the groups were completely free of E. faecalis. It should be noted that during the biomechanical preparation a saline solution was used as an irrigating solution in order to avoid that the action of other antimicrobial agent could mask the results of the disinfection obtained by the intracanal medication. Probably for this reason, the results of the collection after the instrumentation procedure was not as satisfactory as generally expected, which highlights the importance of the irrigation solution during biomechanical preparation in endodontic therapy.
Calcium hydroxide has been widely used in Endodontics as antimicrobial dressing since its introduction. Its antimicrobial mechanisms are related to the high alkaline pH and the dissociation of calcium and hydroxyl ions in an aqueous environment. The hydroxyl free radical leads to harmful effects on bacterial cells, due to protein denaturation causing destruction of bacterial DNA and cytoplasmic membrane [35].
However, in some infections calcium hydroxide may not be the most advantageous intracanal medicament as enterococci have been shown to adapt to extreme conditions such as nutritional deprivation or an alkaline environment [9,36,37]. Indeed, E. faecalis has been demonstrated to be one of the most prevalent species associated with endodontic failure [38]. Besides its capacity to penetrate in root canal ramifications, E. faecalis binds to dentinal tubules by adhering to collagen, which is the main organic component of dentine [39]. In view of the resistance factors of E. faecalis, neither biomechanical preparation nor calcium hydroxide as an intracanal medication has been shown to be sufficient to eliminate this microorganism completely from the root canal systems [4]. In this context, several adjuvant techniques and combinations of drug therapies have been investigated in the search for new strategies to improve the disinfection procedure [40–42], like the effect of ultrasonic agitation of the final irrigating solution in apical leakage reduction reported by Ramazani et al. [42]. In another study, Neves et al. [41] found that PDT associated with different irrigation protocols was more effective against E. faecalis growth than PDT alone.
In the present study, the use of NAC was proposed as an intracanal medication. NAC is an important antioxidant which contains thiol and has antimicrobial action over a wide microorganisms spectrum [18,19,43]. Within the limitations of in vitro studies, several authors have demonstrated excellent antibacterial and antibiofilm effectiveness against different endodontic pathogens, such as Actinomyces naeslundii, Lactobacillus salivarius, Streptococcus mutans, and Staphylococcus epidermidis [43]. In addition, a study by Marchese et al. [19] demonstrated that NAC associated with Fosfomycin, another antimicrobial agent, exhibits a synergistic effect by breaking down E. coli biofilms and reduces the viability of sessile cells and Khosravi et al. [40] evaluated the effect of different antibiotic combinations and reported that levofloxacin associated with NAC showed a great antibacterial potential against E. faecalis biofilm compared to other tested drugs. Its antimicrobial activity is possibly due to tiol reactions (-SH) with the bacteria proteins which induces irreversible protein damage, and also due to inhibition of cysteine use in bacteria [19]. Besides that, in some biofilms, NAC is capable to reduce extracellular matrix production, reducing the bacteria adhesion on surfaces, thus inhibiting the biofilms formation [44]. Pinar Karapinar et al. [45] recommended NAC as an alternative therapeutic agent of CH, whereas the NAC was highly effective against several microorganisms including E. faecalis [45] in both the planktonic and biofilm forms [17].
In order to circumvent the adverse effects of some medicaments on the physical properties of dentin and microbial resistance [46,47], new approaches such as PDT have been introduced to Endodontics in order to improve the disinfection of the root canal system [41]. The benefits of PDT include instantaneous action, feasibility, low toxicity and causes no microbial resistance [48,49].
After intracanal medication period, the microbiological analysis showed that CH, NAC and NAC+PDT presented similar results regarding the antibacterial activity over CFU/mL of E. faecalis, differing statistically from Saline solution and PDT groups. Although some studies have shown the potential antimicrobial effects using PDT as an adjunctive therapy, it is important to note that several factors related to laser-assisted therapies can interfere with their effectiveness in microbial elimination such as: photosensitizers type and its concentration, irradiation time, light source wavelength, photosensitizers absorption peak, and the exposure time [50]. As there is still no consensus on a standardized protocol which can be clinically recommended [50], in the present study we used 0.005% methylene blue with 660 nm wavelength [51,52].
In addition, it is worth mentioning that the microbiological culture technique has some limitations. Although the literature supports the sensitivity of culture-based methods to detect E. faecalis, bacteriological sampling with paper points are often unable to detect bacteria present within grooves, or dentinal tubules of the root canal system [53]. Therefore, in addition to the microbiological evaluation by the culture technique, the present study also performed the CLSM analysis, an effective immunofluorescence technique for detecting bacterial viability in dentinal tubules [32]. It has used the total biovolume and percentage of viable cells as parameters for evaluating the antimicrobial activity of a material. The measurement of the entire area in which the live and dead cells had been detected is required to define the percentage of viable cells in the dentinal tubules. Hence, the percentage was measured in this study as previously described [32].
When evaluating the different thirds, the CLSM analysis demonstrated that in the cervical third all groups had a better performance when compared to saline solution group and in the apical third, PDT, CH and NAC+PDT differed statistically from the control Saline Solution group.
CH was evaluated in the present study because despite its widespread use as intracanal medication, there are some conflicting studies regarding its effectiveness against E. faecalis [13,54]. However , the present results are in accordance with previous studies which also confirmed the antimicrobial activity of CH as intracanal medication [55].
Although there are few studies evaluating NAC as an intracanal medication, to our knowledge there is no study assessing the association between NAC and PDT. Quah et al. [17] evaluated the antibacterial and biofilm eradication effectiveness of NAC on E. faecalis and found superior results for NAC compared to CH. However, in this study E. faecalis biofilms were grown on a dentine disk model, which although simulating a chemically in vivo situation, this fails to mimic the anatomical obstacles inherent in a root canal. In addition, in the study by Quah et al. [17] the medications were used for only 7 days, which may be a reason why the authors found significant amounts of E. faecalis after calcium hydroxide treatment. According to a previous study by Hosoya et al. [56], peak pH change using calcium hydroxide with aqueous vehicle is found after 14 days, after which pH declines over time. Therefore, it is suggested that the time required for optimum intracanal activity when using CH with aqueous vehicle is at least 14 days. In another study, Ulusoy et al. [57] showed that CH was more effective than NAC when both NAC and CH were evaluated for 21 days of exposure. In the present study, microbiological analyzes were performed on root canals of extracted teeth in order to achieve a greater similarity with the clinical situation in vivo. The CH and NAC powders were handled with saline and intracanal dressing from all groups and maintained for 14 days. In this context, although the results of the present study show an effectiveness of NAC similar to calcium hydroxide, more research is needed in order to evaluate the physico-chemical properties of this substance over time, in different concentrations and its penetrability when associated with other types of vehicles.
The images obtained by SEM analysis showed presence of E. faecalis after treatment in NAC+PDT and NAC groups, while CH group showed the dentin surface was mostly clean with only a few remaining bacterial colonies. In PDT and saline solution group’s E. faecalis was more evident. However, the analysis by SEM are illustrative and complement the results of the other analysis carried out. These differences may be related to the subjective character of analysis by SEM.
Several methodologies have been used to evaluate the antimicrobial activity of different intracanal medication and thus the findings of different studies should be carefully interpreted. Possibly, different methodologies may justify the different results between the analyses performed in the present study.
In addition, it must be considered the polymicrobial nature of endodontic infections [58]. Therefore, some structural and physico-chemical characteristics of the root canal wall may be different in a clinical setting [59].
Conclusion
Within the limitation of the present in vitro study, it was concluded that the use of NAC as an intracanal medication was as effective as calcium hydroxide against E. faecalis biofilm, regardless of its association with PDT. However; further studies are needed to investigate the effectiveness of NAC on other endodontic pathogens and to assess their physicochemical and biological properties over time.
Acknowledgments
Supported by the Brazilian agencies Fapesp (grant n: 2014/25789-9).
Conflict of Interest:
‘None declared’.
References
- 1.Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am. 2010 Apr;54(2):291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Cavalli D, Toia CC, Flores Orozco EI, Khoury RD, Cardoso FG da R, Alves MC, et al. Effectiveness in the removal of endotoxins and microbiological profile in primary endodontic infections using 3 different instrumentation systems: A randomized clinical study. J Endod. 2017 Aug;43(8):1237–1245. doi: 10.1016/j.joen.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 3.Markvart M, Darvann TA, Larsen P, Dalstra M, Kreiborg S, Bjørndal L. Micro-CT analyses of apical enlargement and molar root canal complexity. Int Endod J. 2012 Mar;45(3):273–281. doi: 10.1111/j.1365-2591.2011.01972.x. [DOI] [PubMed] [Google Scholar]
- 4.Gomes BPFA, Pinheiro ET, Gadê-Neto CR, Sousa ELR, Ferraz CCR, Zaia AA, et al. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004 Apr;19(2):71–76. doi: 10.1046/j.0902-0055.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Singh SK, Chowdhury I, Singh R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol J. 2017 Apr;:11:53–62. doi: 10.2174/1874285801711010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricucci D, Siqueira JF. Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod. 2010 Aug;36(8):1277–1288. doi: 10.1016/j.joen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Jiang L-M, Hoogenkamp MA, van der Sluis LWM, Wesselink PR, Crielaard W, Deng DM. Resazurin metabolism assay for root canal disinfectant evaluation on dual-species biofilms. J Endod. 2011 Jan;37(1):31–35. doi: 10.1016/j.joen.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Svensater G, Bergenholtz G. Biofilms in endodontic infections. Endod Topics. 2004 Nov;9(1):27–36. [Google Scholar]
- 9.Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Dent Traumatol. 1985 Oct;1(5):170–175. doi: 10.1111/j.1600-9657.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee J-K, Chang SW, Perinpanayagam H, Lim S-M, Park Y-J, Han SH, et al. Antibacterial efficacy of a human β-defensin-3 peptide on multispecies biofilms. J Endod. 2013 Dec;39(12):1625–1629. doi: 10.1016/j.joen.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Rôças IN, Siqueira JF, Santos KRN. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004 May;30(5):315–320. doi: 10.1097/00004770-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Endo MS, Ferraz CCR, Zaia AA, Almeida JFA, Gomes BPFA. Quantitative and qualitative analysis of microorganisms in root-filled teeth with persistent infection: Monitoring of the endodontic retreatment. Eur J Dent. 2013 Jul;7(3):302–309. doi: 10.4103/1305-7456.115414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siqueira JF, de Uzeda M. Disinfection by calcium hydroxide pastes of dentinal tubules infected with two obligate and one facultative anaerobic bacteria. J Endod. 1996 Dec;22(12):674–676. doi: 10.1016/S0099-2399(96)80062-8. [DOI] [PubMed] [Google Scholar]
- 14.Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002 Mar;35(3):221–228. doi: 10.1046/j.1365-2591.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 15.El-Feky MA, El-Rehewy MS, Hassan MA, Abolella HA, Abd El-Baky RM, Gad GF. Effect of ciprofloxacin and N-acetylcysteine on bacterial adherence and biofilm formation on ureteral stent surfaces. Pol J Microbiol. 2009;58(3):261–267. [PubMed] [Google Scholar]
- 16.Zhao T, Liu Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010 May;10:140. doi: 10.1186/1471-2180-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quah SY, Wu S, Lui JN, Sum CP, Tan KS. N-acetylcysteine inhibits growth and eradicates biofilm of Enterococcus faecalis. J Endod. 2012 Jan;38(1):81–85. doi: 10.1016/j.joen.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Giraldo C, Rodríguez-Benito A, Morán FJ, Hurtado C, Blanco MT, Gómez-García AC. Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J Antimicrob Chemother. 1997 May;39(5):643–646. doi: 10.1093/jac/39.5.643. [DOI] [PubMed] [Google Scholar]
- 19.Marchese A, Bozzolasco M, Gualco L, Debbia EA, Schito GC, Schito AM. Effect of fosfomycin alone and in combination with N-acetylcysteine on E. coli biofilms. Int J Antimicrob Agents. 2003 Oct;22 Suppl 2:95–100. doi: 10.1016/s0924-8579(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 20.Silveira LFM, Baca P, Arias-Moliz MT, Rodríguez-Archilla A, Ferrer-Luque CM. Antimicrobial activity of alexidine alone and associated with N-acetylcysteine against Enterococcus faecalis biofilm. Int J Oral Sci. 2013 Sep;5(3):146–149. doi: 10.1038/ijos.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rios A, He J, Glickman GN, Spears R, Schneiderman ED, Honeyman AL. Evaluation of photodynamic therapy using a light-emitting diode lamp against Enterococcus faecalis in extracted human teeth. J Endod. 2011 Jun;37(6):856–859. doi: 10.1016/j.joen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Chiniforush N, Pourhajibagher M, Shahabi S, Kosarieh E, Bahador A. Can antimicrobial photodynamic therapy (apdt) enhance the endodontic treatment? J Lasers Med Sci. 2016 Mar;7(2):76–85. doi: 10.15171/jlms.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiwar A, Usha HL, Meena N, Ashwini P, Murthy CS. The efficiency of root canal disinfection using a diode laser: in vitro study. Indian J Dent Res. 2013 Feb;24(1):14–18. doi: 10.4103/0970-9290.114916. [DOI] [PubMed] [Google Scholar]
- 24.Asnaashari M, Mojahedi SM, Asadi Z, Azari-Marhabi S, Maleki A. A comparison of the antibacterial activity of the two methods of photodynamic therapy (using diode laser 810 nm and LED lamp 630 nm) against Enterococcus faecalis in extracted human anterior teeth. Photodiagnosis Photodyn Ther. 2016 Mar;13:233–237. doi: 10.1016/j.pdpdt.2015.07.171. [DOI] [PubMed] [Google Scholar]
- 25.Hoedke D, Enseleit C, Gruner D, Dommisch H, Schlafer S, Dige I, et al. Effect of photodynamic therapy in combination with various irrigation protocols on an endodontic multispecies biofilm ex vivo. Int Endod J. 2018 Jan;51 Suppl 1:e23–e34. doi: 10.1111/iej.12763. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho AS, Camargo CHR, Valera MC, Camargo SEA, Mancini MNG. Smear layer removal by auxiliary chemical substances in biomechanical preparation: a scanning electron microscope study. J Endod. 2008 Nov;34(11):1396–1400. doi: 10.1016/j.joen.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Matos F de S, da Silva FR, Paranhos LR, Moura CCG, Bresciani E, Valera MC. The effect of 17% EDTA and QMiX ultrasonic activation on smear layer removal and sealer penetration: ex vivo study. Sci Rep. 2020 Jun;10(1):10311. doi: 10.1038/s41598-020-67303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Csako G, Elin RJ, Hochstein HD, Tsai CM. Physical and biological properties of U S standard endotoxin EC after exposure to ionizing radiation. Infect Immun. 1983 Jul;41(1):190–196. doi: 10.1128/iai.41.1.190-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valera MC, Silva KCG da, Maekawa LE, Carvalho CAT, Koga-Ito CY, Camargo CHR, et al. Antimicrobial activity of sodium hypochlorite associated with intracanal medication for Candida albicans and Enterococcus faecalis inoculated in root canals. J Appl Oral Sci. 2009 Dec;17(6):555–559. doi: 10.1590/S1678-77572009000600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maekawa LE, Valera MC, Oliveira LD de, Carvalho CAT, Camargo CHR, Jorge AOC. Effect of Zingiber officinale and propolis on microorganisms and endotoxins in root canals. J Appl Oral Sci. 2013 Feb;21(1):25–31. doi: 10.1590/1678-7757201302129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider M, Kirfel G, Berthold M, Frentzen M, Krause F, Braun A. The impact of antimicrobial photodynamic therapy in an artificial biofilm model. Lasers Med Sci. 2012 May;27(3):615–620. doi: 10.1007/s10103-011-0998-7. [DOI] [PubMed] [Google Scholar]
- 32.Andrade FB de, Arias MPC, Maliza AGA, Duarte MAH, Graeff MSZ, Amoroso-Silva PA, et al. A new improved protocol for in vitro intratubular dentinal bacterial contamination for antimicrobial endodontic tests: standardization and validation by confocal laser scanning microscopy. J Appl Oral Sci. 2015 Dec;23(6):591–598. doi: 10.1590/1678-775720140261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwang S, Abbott PV. Bacterial contamination of the fitting surfaces of restorations in teeth with pulp and periapical disease: a scanning electron microscopy study. Aust Dent J. 2012 Dec;57(4):421–428. doi: 10.1111/adj.12007. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues CT, de Andrade FB, de Vasconcelos LRSM, Midena RZ, Pereira TC, Kuga MC, et al. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int Endod J. 2018 Aug;51(8):901–911. doi: 10.1111/iej.12904. [DOI] [PubMed] [Google Scholar]
- 35.Siqueira JF, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999 Sep;32(5):361–369. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 36.Waltimo TM, Sirén EK, Orstavik D, Haapasalo MP. Susceptibility of oral Candida species to calcium hydroxide in vitro. Int Endod J. 1999 Mar;32(2):94–98. doi: 10.1046/j.1365-2591.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 37.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006 Feb;32(2):93–98. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 38.Rôças IN, Jung I-Y, Lee C-Y, Siqueira JF. Polymerase chain reaction identification of microorganisms in previously root-filled teeth in a South Korean population. J Endod. 2004 Jul;30(7):504–508. [PubMed] [Google Scholar]
- 39.Love RM. Enterococcus faecalis--a mechanism for its role in endodontic failure. Int Endod J. 2001 Jul;34(5):399–405. doi: 10.1046/j.1365-2591.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 40.Rastegar Khosravi M, Khonsha M, Ramazanzadeh R. Combined effect of levofloxacin and N-acetylcysteine against enterococcus faecalis biofilm for regenerative endodontics: An in vitro study. Iran Endod J. 2019;14:40–46. doi: 10.22037/iej.v14i1.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Vasconcelos Neves G, Kátia Simone Alves dos Santos, de Souza Sales Rocha EAL, de Moura RQ, Danyllo Guimarães Morais Barros, Gominho LF, et al. Antibacterial Effect of Photodynamic Therapy on Root Canal Disinfection Combined with Different Irrigation Protocols. Iran Endod J. 2020 May 17; doi: 10.22037/iej.v15i2.27801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramazani M, Asnaashari M, Ahmadi R, Zarenejad N, Rafie A, Yazadani Charati J. The Effect of Final Rinse Agitation with Ultrasonic or 808 nm Diode Laser on Coronal Microleakage of Root-canal Treated Teeth. Iran Endod J. 2018;13(1):108–113. doi: 10.22037/iej.v13i1.17248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon J-H, Choi Y-S, Lee H-W, Heo JS, Chang SW, Lee J-Y. Antibacterial effects of N-acetylcysteine against endodontic pathogens. J Microbiol. 2016 Apr;54(4):322–329. doi: 10.1007/s12275-016-5534-9. [DOI] [PubMed] [Google Scholar]
- 44.Olofsson A-C, Hermansson M, Elwing H. N-acetyl-L-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl Environ Microbiol. 2003 Aug;69(8):4814–4822. doi: 10.1128/AEM.69.8.4814-4822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinar Karapinar S, Ulum YZA, Ozcelik B, Dogan Buzoglu H, Ceyhan D, Balci Peynircioglu B, et al. The effect of N-acetylcysteine and calcium hydroxide on TNF-α and TGF-β1 in lipopolysaccharide-activated macrophages. Arch Oral Biol. 2016 Aug;68:48–54. doi: 10.1016/j.archoralbio.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Estrela C, Pimenta FC, Ito IY, Bammann LL. In vitro determination of direct antimicrobial effect of calcium hydroxide. J Endod. 1998 Jan;24(1):15–17. doi: 10.1016/S0099-2399(98)80205-7. [DOI] [PubMed] [Google Scholar]
- 47.Baik JE, Jang K-S, Kang S-S, Yun C-H, Lee K, Kim B-G, et al. Calcium hydroxide inactivates lipoteichoic acid from Enterococcus faecalis through deacylation of the lipid moiety. J Endod. 2011 Feb;37(2):191–196. doi: 10.1016/j.joen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007 Aug;86(8):694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 49.Soukos NS, Goodson JM. Photodynamic therapy in the control of oral biofilms. Periodontol. 2011 Feb;55(1):143–166. doi: 10.1111/j.1600-0757.2010.00346.x. [DOI] [PubMed] [Google Scholar]
- 50.Bordea IR, Hanna R, Chiniforush N, Grădinaru E, Câmpian RS, Sîrbu A, et al. Evaluation of the outcome of various laser therapy applications in root canal disinfection: A systematic review. Photodiagnosis Photodyn Ther. 2020 Mar;29:101611. doi: 10.1016/j.pdpdt.2019.101611. [DOI] [PubMed] [Google Scholar]
- 51.Rabello DGD, Corazza BJM, Ferreira LL, Santamaria MP, Gomes APM, Martinho FC. Does supplemental photodynamic therapy optimize the disinfection of bacteria and endotoxins in one-visit and two-visit root canal therapy? A randomized clinical trial. Photodiagnosis Photodyn Ther. 2017 Sep;19:205–211. doi: 10.1016/j.pdpdt.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Susila AV, Sugumar R, Chandana CS, Subbarao CV. Combined effects of photodynamic therapy and irrigants in disinfection of root canals. J Biophotonics. 2016 Jun;9(6):603–609. doi: 10.1002/jbio.201500112. [DOI] [PubMed] [Google Scholar]
- 53.Sathorn C, Parashos P, Messer HH. How useful is root canal culturing in predicting treatment outcome? J Endod. 2007 Mar;33(3):220–225. doi: 10.1016/j.joen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Valera MC, de Moraes Rego J, Jorge AO. Effect of sodium hypochlorite and five intracanal medications on Candida albicans in root canals. J Endod. 2001 Jun;27(6):401–403. doi: 10.1097/00004770-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Maekawa LE, Valera MC, Oliveira LD de, Carvalho CAT, Koga-Ito CY, Jorge AOC, et al. In vitro evaluation of the action of irrigating solutions associated with intracanal medications on Escherichia coli and its endotoxin in root canals. J Appl Oral Sci. 2011 Apr;19(2):106–112. doi: 10.1590/S1678-77572011000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hosoya N, Takahashi G, Arai T, Nakamura J. Calcium concentration and pH of the periapical environment after applying calcium hydroxide into root canals in vitro. J Endod. 2001 May;27(5):343–346. doi: 10.1097/00004770-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Ulusoy AT, Kalyoncuoğlu E, Reis A, Cehreli ZC. Antibacterial effect of N-acetylcysteine and taurolidine on planktonic and biofilm forms of Enterococcus faecalis. Dent Traumatol. 2016 Jun;32(3):212–218. doi: 10.1111/edt.12237. [DOI] [PubMed] [Google Scholar]
- 58.Machado FP, Khoury RD, Toia CC, Flores Orozco EI, de Oliveira FE, de Oliveira LD, et al. Primary versus post-treatment apical periodontitis: microbial composition, lipopolysaccharides and lipoteichoic acid levels, signs and symptoms. Clin Oral Investig. 2020 Jan 13; doi: 10.1007/s00784-019-03191-6. [DOI] [PubMed] [Google Scholar]
- 59.George S, Kishen A, Song KP. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J Endod. 2005 Dec;31(12):867–872. doi: 10.1097/01.don.0000164855.98346.fc. [DOI] [PubMed] [Google Scholar]