Abstract
Objective
To examine whether baseline model for end-stage liver disease (MELD) score in patients with cirrhosis and ascites predicts the future development of first spontaneous bacterial peritonitis (SBP) episode.
Methods
A retrospective case-control study was performed at three academic centers to select patients admitted with first SBP episode (cases) and those with ascites admitted for decompensation without SBP (controls). Medical records from these centers were reviewed between January 1, 2008, and December 31, 2013. Cases and controls were matched (1:2) for age, sex, and race. Conditional logistic recession models were built to determine whether baseline MELD score (within a month before hospitalization) predicts first SBP episode.
Results
Of 697 patients (308, 230, and 159 from centers A, B, and C, respectively), cases and controls were matched in 94%, 89%, and 100% at three respective centers. In the pooled sample, probability of SBP was 11%, 31%, 71%, and 93% at baseline MELD scores less than or equal to 10, from 11 to 20, from 21 to 30, and greater than 30, respectively. Compared with MELD score less than or equal to 10, patients with MELD scores from 11 to 20, 21 to 30, and greater than 30 had six- (3- to 11-), 29- (12- to 69-), and 115- (22- to 598-) folds (95% CI) risk of SBP, respectively. Based on different MELD score cutoff points, MELD score greater than 17 was most accurate in predicting SBP occurrence. Analyzing 315 patients (152 cases) with available data on ascitic fluid protein level controlling for age, sex, and center, MELD score but not ascitic fluid protein associated with first SBP episode with respective odds ratios of 1.20 (1.14 to 1.26) and 0.88 (0.70 to 1.11).
Conclusion
Baseline MELD score predicts first SBP episode in patients with cirrhosis and ascites.
Video Abstract
Cirrhosis has significant impact on morbidity, mortality, and quality of life.1, 2 Patients with cirrhosis are immune compromised with propensity to develop infections. Infected patients with cirrhosis are four times more likely to die compared with uninfected patients with cirrhosis.3, 4 Spontaneous bacterial peritonitis (SBP) occurs in 10% to 30% of patients with cirrhosis and carries an in-hospital mortality rate of 30% to 50 %.5, 6, 7 Current guidelines suggest antibiotic prophylaxis for prevention of SBP among patients with an established SBP episode (secondary prophylaxis) and those with cirrhosis who are admitted with gastrointestinal (GI) bleeding (primary prophylaxis).8, 9 However, data on the benefit of antibiotics for primary prophylaxis of SBP among patients with cirrhosis without GI bleeding are conflicting.10, 11, 12, 13
A meta-analysis of four randomized studies showed benefit of antibiotics in primary prophylaxis of SBP.14 However, due to inconsistent results and heterogeneity among studies, this practice is not a mainstream approach despite the American Association for the Study of Liver Diseases recommending antibiotic prophylaxis in high-risk groups.8 Among patients without GI bleeding and who have not had a previous episode of SBP, antibiotic prophylaxis is suggested for patients with low-protein ascites. However, antibiotic prophylaxis is associated with development of resistance, and a shift to gram-positive infections is associated with higher mortality.
The model for end-stage liver disease (MELD) score is a simple and accurate score in predicting 3-month mortality in patients with cirrhosis, and is used worldwide for listing patients for liver transplantation.15 MELD score is also shown to be higher among SBP patients and to predict mortality in these patients.16, 17 However, MELD score in these studies was calculated after the onset of SBP. To our knowledge, there are no studies examining the association of baseline MELD score as with the first episode of SBP.
METHODS
Study Design
This is a multicenter retrospective case-controlled study, conducted at three tertiary referral academic institutions. A common protocol was developed for institutional review board approval.
Study Population
Medical records of patients with discharge diagnosis of cirrhosis (International Classification of Diseases volume 9 [ICD-09] codes 571.2, 571.5, 571.6, 571.8, and 571.9) and SBP (ICD-09 code 567.23) during January 1, 2008, and December 31, 2013, were reviewed at University of Texas Medical Branch Galveston (center A), University of Alabama at Birmingham, Birmingham (center B), and Mayo Clinic, Rochester (center C). Cases with confirmed diagnosis of first episode of SBP (ascitic fluid cell count >250 neutrophils/m3 per minute and/or positive ascitic fluid culture) were selected. Patients with GI bleed (>2 g decrease in hemoglobin within 48 hours, or associated with orthostatic changes), peritoneal instrumentation or procedure within previous 1 week of the diagnosis, and previous episode of SBP were excluded. Controls were selected from patients discharged with diagnosis of cirrhosis (ICD-09 codes 571.2, 571.5, 571.6, 571.8, and 571.9), and admitted for reasons other than SBP. Similar to cases, none of the controls had any previous episode of SBP.
Exposure
Baseline MELD score was calculated within 1 month before SBP diagnosis for cases and within 1 month before hospitalization for controls.
Outcomes
The primary outcome of the study was SBP, and the secondary outcome was in-hospital mortality.
Data Collection Variables
Apart from patient demographics used for matching cases and controls, medical charts of cases and controls were reviewed to collect data within 90 days before hospitalization for (1) MELD score labs (serum bilirubin, serum creatinine, institutional normalized ratio, and use of dialysis), (2) ascitic fluid protein level, (3) previous hospitalization, and (4) use of proton pump inhibitor (PPI) or antibiotic. Data on inpatient mortality was confirmed using the social security death index. Data were pooled from each center into a common sheet for analysis.
Statistical Analysis
For every case an effort was made to match two controls for calendar year of admission, age, sex, and race within each institution. Cases and controls were compared using the χ2 test for categorical variables and the t test for the continuous variables. MELD score was stratified to less than or equal to 10, 11 to 20, 21 to 30, and greater than 30 for both cases and controls. Frequency of SBP occurrence at various MELD stratification scores was calculated. A conditional logistic regression model was built to determine risk of development of SBP associated with various MELD stratifications with MELD score less than or equal to 10 as reference. A similar conditional model was built to examine independent effect of MELD score being associated with SBP development, with MELD score used as a continuous variable. For validation of the data at each center, similar models were built separately for the data at each respective center. These separate models were built using MELD score only as a continuous variable as such models could not be built using MELD score as categorical variable due to small cell sizes in the conditional model. Patient age, sex, race, use of PPI, antibiotic use, and recent hospitalization were entered in each model for analysis. Subgroup analysis limited to patients with available ascitic fluid protein level was also performed. As this subgroup may not have been matched for cases and controls, this regression model was unconditional and controlled for age, sex, and center. A logistic regression model, which could be predictive of inpatient mortality, was also built to examine independent effect of SBP for inpatient mortality using all the variables. Data from logistic regression models are reported as odds ratio (OR) with 95% CIs. Analyses were performed using statistical analysis SAS software (SAS Institute Inc., Cary, NC), and P less than .05 is considered significant.
RESULTS
Study Population
A total of 697 patients (308 from center A with 106 cases, 230 from center B with 81 cases, and 159 from center C, 53 cases) were analyzed. Cases and controls were matched in 1:2 ratio in 94% at center A, 89% at center B and 100% at center C (Figure 1).
Figure 1.
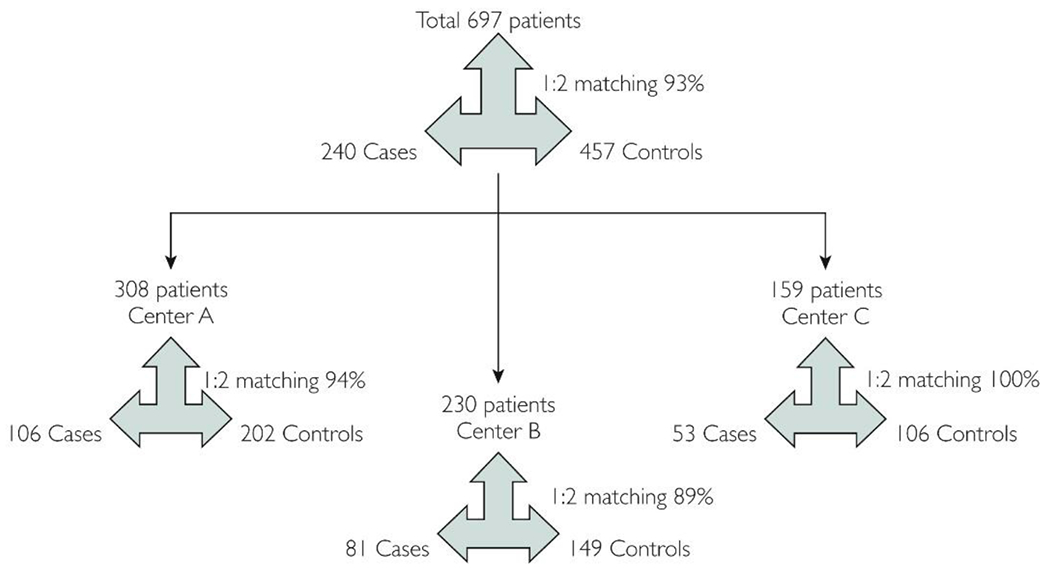
Study population and matching of cases to controls.
Baseline Characteristics Comparing Cases and Controls
Of 697 patients (240 cases with SBP), 75% were white males with mean age of 55 years. Antibiotic use within previous 3 months of index admission was reported in 38% of cases, and 21% of controls. Similar proportions for the use of PPI within previous 3 months were 46% and 38%, respectively. History of previous hospitalization within previous 3 months was reported in 55% of cases and 21% of controls (Table 1). The study cohort was stratified based on MELD score to less than or equal to 10, 11 to 20, 21 to 30, and greater than 31. Most patients were in the MELD score 11 to 20 strata (n=394, 57%) followed by less than or equal to 10 (n=175, 25%), 21 to 30 (n=98, 14%), and greater than 30 (n=30, 4%), respectively. Among cases, only 8% had MELD scores less than or equal to 10 and 41% had MELD scores greater than 20. Similar respective figures for controls were 34% and 7%, respectively (Table 1). However, there were center-based differences on patient demographics and other baseline characters (Supplemental Tables 1 and 2, available online at http://www.mayoclinicproceedings.org).
Table 1.
Baseline Characteristics Comparing Cumulated Cases and Controls at all Three Centersa
| Variable | Cases (n=240) | Controls (n=457) | P |
|---|---|---|---|
| Age, years | 55.1±10.5 | 55.3±10.0 | .78 |
| Males | 181 (75.4) | 339 (74.3) | .72 |
| Whites | 182 (75.8) | 350 (76.6) | .97 |
| Blacks | 19 (7.9) | 34 (7.4) | |
| Hispanics | 39 (16.3) | 73 (16) | |
| AB useb | 90 (37.5) | 97 (21.2) | <0.001 |
| PPI useb | 110 (45.8) | 172 (37.6) | .07 |
| Hospitalizationb | 131 (54.6) | 94 (20.6) | <0.001 |
| MELD ≤0 | 19 (7.9) | 156 (34.1) | <0.001 |
| MELD 11-20 | 123 (51.3) | 271 (59.3) | |
| MELD 21-30 | 70 (29.2) | 28 (6.1) | |
| MELD >30 | 28 (11.7) | 2 (0.4) |
Values shown are n (%) unless otherwise stated.
AB = antibiotic; MELD = model for end-stage liver disease score; PPI = proton pump inhibitor.
Within previous 90 days before index admission.
Probability and Predictors of Occurrence of SBP
Of 697 patients with cirrhosis in this study 240 (34.4%) were admitted with first episode of SBP (cases). Probability of occurrence of SBP in this pooled data was 11%, 31%, 71%, and 93% at baseline MELD score of less than or equal to 10, 11 to 20, 21 to 30, and greater than or equal to 31, respectively (Figure 2). Center-specific analysis also showed linear association of baseline MELD score with the probability of first SBP episode (Supplemental Figure 1, available online at http://mayoclinicproceedings.org).
Figure 2.
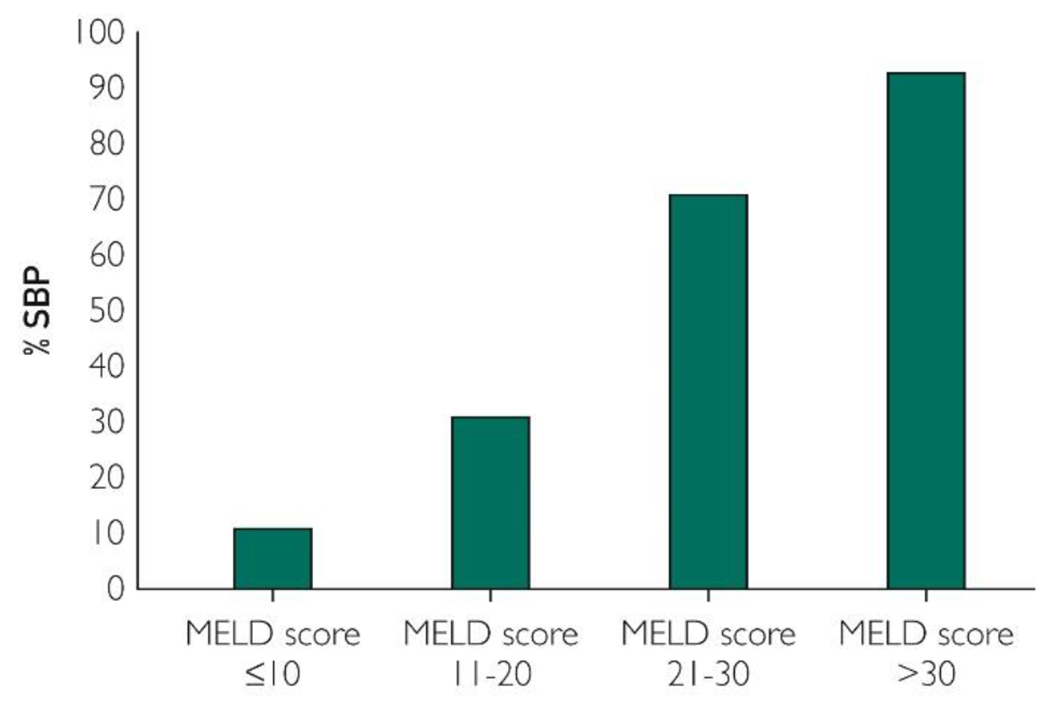
Probability of spontaneous bacterial peritonitis (SBP) at various model for end-stage liver disease (MELD) scores strata. The probability of SBP occurrence increases linearly with baseline MELD score with 11%, 31%, 71%, and 93% at baseline MELD score less than or equal to 10, 11 to 20, 21 to 30, and greater than 30, respectively.
On conditional logistic regression analysis, compared with patients with baseline MELD score less than or equal to 10, OR (95% CI) for first episode of SBP at baseline MELD score 11 to 20, 21 to 30, and greater than 30 were 6 (95% CI, 3-11), 29 (95% CI, 12-69), and 115 (95% CI, 22-598), respectively. Hospitalization within the previous 90 days was associated with greater than five- (3- to 10- ) fold (95% CI) risk of SBP occurrence (Table 2). Use of antibiotics or PPI was not associated with the risk of developing first episode of SBP. Comparing Akaike information criterion based on different MELD score cutoff values, baseline MELD score greater than 17 was most accurate for predicting the first episode of SBP with sensitivity, specificity, and positive predictive value of 60%, 86%, and 69%, respectively (Supplemental Table 3, available online at http://www.mayoclinicproceedings.org). We performed this analysis after randomly splitting the data from each center to training set (two-thirds of the data) and the validation set (one-third of data). The results on the validation set remained similar with a MELD score of 17 as on the whole original dataset (Supplemental Table 3, available online at http://www.mayoclinicproceedings.org). In a smaller validation dataset of 231 subjects of cases and controls, best agreement on probability of SBP was noted at a MELD score cutoff of 16, 17, and 19 (Supplemental Table 3).
Table 2.
Logistic Regression Analysis Model Examining Predictors of Occurrence of SBPa
| Variable | Model with MELD score as categorical variable OR (95% CI) | Model with MELD score as continuous variable OR (95% CI) |
|---|---|---|
| MELD score | ||
| 11-20 vs ≤10 | 5.86 (3.05-11.28) | |
| 21-30 vs ≤10 | 29.35 (12.42-69.34) | ↑4 units baseline MELD 2.41 (1.98-2.94) |
| >30 vs ≤10 | 115.64 (22.36-598.0) | |
| Age, years | 1.01 (0.96-1.06) | 1.01 (0.96-1.06) |
| Females vs males | 0.88 (0.27-2.83) | 0.98 (0.27-0.36) |
| Blacks vs Whites | 0.38 (0.1-1.42) | 0.34 (0.09-1.37) |
| Hispanic/other vs Whites | 0.93 (0.45-1.90) | 0.91 (0.43-1.91) |
| AB useb | 1.18 (0.68-2.03) | 1.12 (0.62-2.0) |
| PPI useb | 0.94 (0.58-1.50) | 0.87 (0.52-1.43) |
| Hospitalizationb | 5.67 (3.25-9.90) | 5.9 (3.25-10.7) |
AB = antibiotic; MELD = model for end-stage liver disease score; OR = odds ratio; PPI = proton pump inhibitors; SBP = spontaneous bacterial peritonitis.
Within previous 90 days before index admission.
A similar conditional logistic regression analysis model was built using the MELD score as continuous variable. The occurrence of SBP increased by 2.41 (95% CI, 1.98-2.94) for each 4-unit increase in baseline MELD score (Table 2). Results on other variables were similar with recent hospitalization being associated with a six- (3- to 11- ) fold (95% CI) increased risk of SBP.
Subgroup Analysis
One hundred fifty-two cases and 163 controls (45.2%) of 697 patients had available data on ascitic fluid protein level. On subgroup analysis limited to these patients, baseline MELD score remained a significant predictor of SBP (OR, 1.20; 95% CI, 1.14-1.26) after adjusting for age, sex, and center. Ascitic fluid protein level, however, did not predict the occurrence of SBP (OR, 0.88; 95% CI, 0.70-1.11) (Table 3).
Table 3.
Subgroup Analysis Limiting to Patients With Available Ascitic Protein Fluid Levelsa
| Variable | Odds ratio for whole group (95% CI) | Odds ratio for subgroup (95% CI) |
|---|---|---|
| MELD score | 1.20 (1.14-1.26) | NA |
| Ascitic fluid protein | NA | 0.88 (0.70-1.11) |
| Age, years | 0.99 (0.97-1.03) | 1.01 (0.98-1.03) |
| Females vs males | 1.65 (0.87-3.15) | 1.41 (0.80-2.49) |
| Center A vs center C | 0.68 (0.34-1.36) | 0.75 (0.40-1.39) |
| Center B vs center C | 0.72 (0.33-1.60) | 1.26 (0.63-2.53) |
Center A = University of Texas Medical Branch, Galveston; Center B = University of Alabama at Birmingham; Center C = Mayo Clinic, Rochester, MN; MELD = model for end-stage liver disease; NA = not available.
Inpatient Mortality
Forty-eight of 697 (6.9%) patients died during the index hospitalization, with higher mortality among cases compared with controls (15% vs. 2.6%, P=0.0001) (Figure 3). This pattern of higher mortality among cases compared with controls was noted at each academic center in this study (Supplemental Figure 2, available online at http://www.mayoclinicproceedings.org). On logistic regression analysis, patients admitted with SBP had more than two- (1.03- to 5.5-) fold (95% CI) risk of inpatient mortality compared with patients admitted for reasons other than SBP. Of the other variables examined in the model, baseline MELD score predicted inpatient mortality with 13% (95% CI, 8%-18%) increased risk with each unit increase in MELD score (Table 4).
Figure 3.
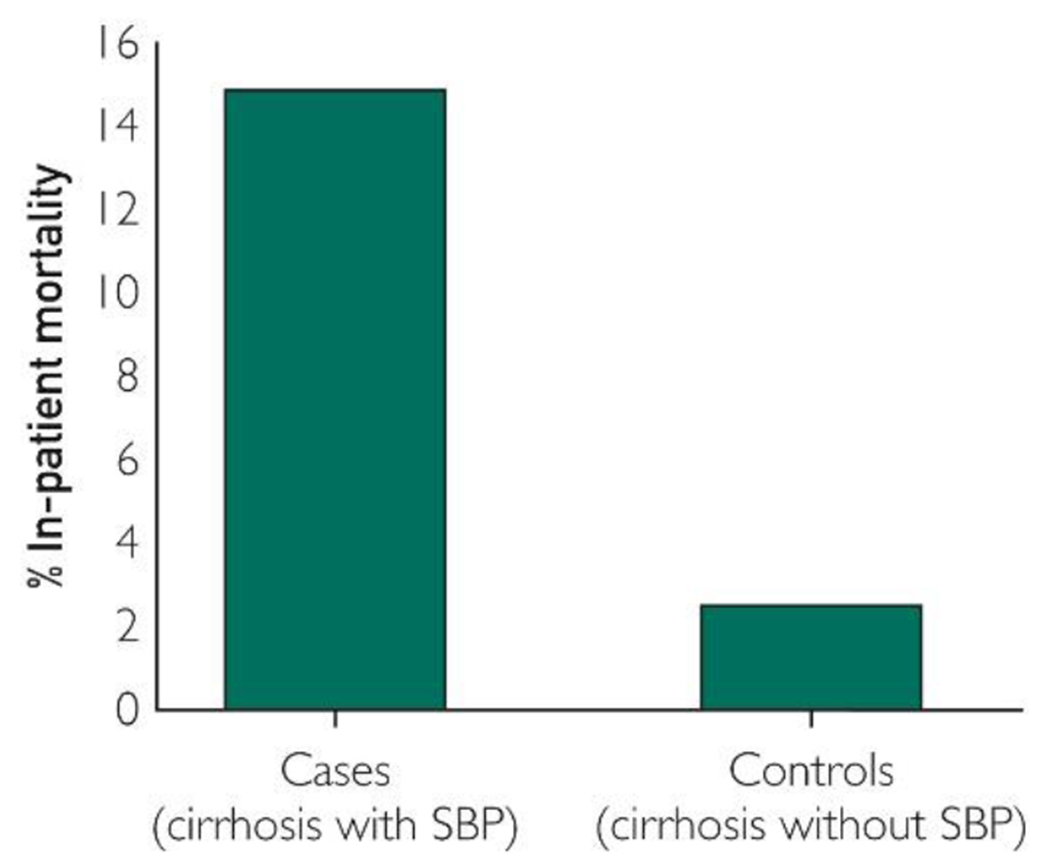
Inpatient mortality comparing cases (hospitalized patients with cirrhosis and spontaneous bacterial peritonitis [SBP]) and controls (hospitalized patients with cirrhosis decompensated for reasons other than SBP). The figure shows an overall inpatient mortality of 7%, and higher among cases compared with controls (15% vs. 2.5%, P<0.001).
Table 4.
Multivariate Logistic Regression Analysis Model Examining for Predictors of Inpatient Mortalitya
| Variable | Odds ratio (95% CI) |
|---|---|
| SBP | 2.38 (1.03-5.50) |
| MELD score | 1.13 (1.08-1.18) |
| Age, years | 1.03 (1.00-1.06) |
| Females vs males | 0.59 (0.28-1.23) |
| Blacks vs whites | 1.49 (0.47-4.68) |
| Hispanic/other vs whites | 1.19 (0.47-3.02) |
| Antibiotic use within previous 90 days | 1.42 (0.66-3.05) |
| PPI use within previous 90 days | 0.71 (0.35-1.47) |
| Hospitalization within previous 90 days | 0.84 (0.38-1.85) |
MELD = Model for end-stage liver disease; PPI = proton pump inhibitor; SBP = spontaneous bacterial peritonitis.
Discussion
The novel finding of our study is a linear association of baseline MELD score with occurrence of SBP, with the best cutoff at a MELD score greater than 17 in independently predicting future development of first SBP episode. We also found that recent hospitalization within the prior 90 days of index admission is associated with SBP. Further, SBP occurrence predicted inpatient mortality among hospitalized patients for decompensation of cirrhosis.
Factors such as low ascitic fluid total protein, serum sodium, serum bilirubin, and serum creatinine have been shown to predict the development of the first episode of SBP.10, 11, 12, 13 These studies have conflicting data regarding antibiotic prophylaxis with benefit in two,10, 12 and no benefit in another two studies.11, 13 In a subgroup analysis in this study, we were not able to show ascitic fluid protein level predictive of first SBP episode after controlling for baseline MELD score. In another study of 296 patients with cirrhosis and ascites, 75 (25.3%) had SBP. In this study, thrombocytopenia, age greater than 60 years, and C-reactive protein were associated with SBP occurrence, and a scoring system combining these three parameters had accuracy of 82% in predicting SBP. MELD score in this study did not predict the SBP.18 However, the MELD score in this study was calculated at or after the development of SBP and not the baseline MELD score within 90 days before admission as we did in this study.
Another major predictor for occurrence of SBP was history of hospitalization within 3 months before index admission. Patients with cirrhosis and recent hospitalization are known to develop health care–associated infections with multidrug-resistant organisms.3 In one prospective study on 333 patients with cirrhosis, of 669 infections including SBP, more than 30% of these infections were health care–associated infections.19
Data on the association of SBP occurrence with antibiotics and PPI use are conflicting. For example, increased risk of SBP was observed with use of PPI in one study.16 In another study, development of infections was associated with PPI and antibiotic use, although occurrence of SBP was not specifically examined.20 A recent large multicenter prospective study from Argentina showed that use of PPI is not associated with the development of SBP,21 similar to what we observed in this study.
Many studies have shown that SBP is associated with increased use of hospital resources and inpatient mortality as observed in our study.5, 6, 7, 17, 22 Septic shock, delay in antibiotic administration, serum lactate levels, MELD score, and acute kidney injury predict inpatient mortality among patients with cirrhosis admitted with SBP. In another study on 184 SBP patients and externally validated on 109 patients showed white blood cell count greater than or equal to 11,000/μl and MELD score greater than or equal to 22 to be independent predictors of 30-day mortality.18 However, the MELD score in all these studies was calculated after the onset of SBP and not before development of SBP.
Large sample size with data from three tertiary academic centers, strict criteria to define SBP, validation of findings at each center, and study design of matching cases to controls are strengths of our study. However, our study has similar limitations found in any retrospective study design.
Findings from this study provide implications on the use of prophylactic antibiotics for primary prophylaxis of SBP among cirrhosis patients with a MELD score greater than 17, especially those with recent hospitalization. However, the positive predictive value of 69% at a MELD greater than 17 in predicting first SBP episode is relatively low for recommending its application in clinic and changing the current practices on SBP prophylaxis. To our knowledge, this is the first such study showing our novel finding of baseline MELD score being predictive of future development of a first SBP episode. Further, use of prophylactic antibiotics has a potential for emergence of infections due to gram-positive and multidrug-resistant organisms, which are more difficult to control and are associated with a higher mortality.6, 20, 23, 24 Clearly, large well-designed randomized controlled trials are needed to examine use of antibiotic prophylaxis in preventing the first episode of SBP as basis for identify at risk population and recommendations for prophylactic antibiotic use in patients with cirrhosis and ascites for primary prophylaxis of SBP.
Conclusion
Baseline MELD score predicts the first episode of SBP occurrence among patients with cirrhosis and ascites, with best accuracy with a MELD score greater than 17. Randomized placebo-controlled studies are suggested among patients with cirrhosis and ascites to identify those who will benefit from prophylactic antibiotics for prevention of a first episode of SBP.
Supplementary Material
Abbreviations and Acronyms
- MELD
model for end-stage liver disease
- OR
odds ratio
- PPI
proton pump inhibitor
- SBP
spontaneous bacterial peritonitis
References
- 1.Kim WR, Brown RS Jr, Terrault NA, El-Serag H Burden of liver disease in the United States: summary of a workshop Hepatology, 36 (1) (2002), pp. 227–242 [DOI] [PubMed] [Google Scholar]
- 2.Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR Underestimation of liver-related mortality in the United States Gastroenterology, 145 (2) (2013), pp. 375–382 e371-e372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnel AR, Bunchorntavakul C, Reddy KR Immune dysfunction and infections in patients with cirrhosis Clin Gastroenterol Hepatol, 9 (9) (2011), pp. 727–738 [DOI] [PubMed] [Google Scholar]
- 4.Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis Gastroenterology, 139 (4) (2010), pp. 1246–1256 1256.e1241-e1245 [DOI] [PubMed] [Google Scholar]
- 5.Singal AK, Salameh H, Kamath PS Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: a nationwide study of hospitalised patients in the United States Aliment Pharmacol Ther, 40 (1) (2014), pp. 105–112 [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS, O’Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience Hepatology, 56 (6) (2012), pp. 2328–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thuluvath PJ, Morss S, Thompson R Spontaneous bacterial peritonitis—in-hospital mortality, predictors of survival, and health care costs from 1988 to 1998 Am J Gastroenterol, 96 (4) (2001), pp. 1232–1236 [DOI] [PubMed] [Google Scholar]
- 8.Runyon BA, Practice Guidelines Committee AASLD Management of adult patients with ascites due to cirrhosis Hepatology, 39 (3) (2004), pp. 841–856 [DOI] [PubMed] [Google Scholar]
- 9.Saab S, Hernandez JC, Chi AC, Tong MJ Oral antibiotic prophylaxis reduces spontaneous bacterial peritonitis occurrence and improves short-term survival in cirrhosis: a meta-analysis Am J Gastroenterol, 104 (4) (2009), pp. 993–1001 [DOI] [PubMed] [Google Scholar]
- 10.Fernandez J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis Gastroenterology, 133 (3) (2007), pp. 818–824 [DOI] [PubMed] [Google Scholar]
- 11.Terg R, Fassio E, Guevara M, et al. Ciprofloxacin in primary prophylaxis of spontaneous bacterial peritonitis: a randomized, placebo-controlled study J Hepatol, 48 (5) (2008), pp. 774–779 [DOI] [PubMed] [Google Scholar]
- 12.Grange JD, Roulot D, Pelletier G, et al. Norfloxacin primary prophylaxis of bacterial infections in cirrhotic patients with ascites: a double-blind randomized trial J Hepatol, 29 (3) (1998), pp. 430–436 [DOI] [PubMed] [Google Scholar]
- 13.Novella M, Sola R, Soriano G, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin Hepatology, 25 (3) (1997), pp. 532–536 [DOI] [PubMed] [Google Scholar]
- 14.Loomba R, Wesley R, Bain A, Csako G, Pucino F Role of fluoroquinolones in the primary prophylaxis of spontaneous bacterial peritonitis: meta-analysis Clin Gastroenterol Hepatol, 7 (4) (2009), pp. 487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease Hepatology, 33 (2) (2001), pp. 464–470 [DOI] [PubMed] [Google Scholar]
- 16.Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites Am J Gastroenterol, 104 (5) (2009), pp. 1130–1134 [DOI] [PubMed] [Google Scholar]
- 17.Bal CK, Daman R, Bhatia V Predictors of fifty days in-hospital mortality in decompensated cirrhosis patients with spontaneous bacterial peritonitis World J Hepatol, 8 (12) (2016), pp. 566–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehmeyer MH, Krohm S, Kastein F, Lohse AW, Luth S Prediction of spontaneous bacterial peritonitis in cirrhotic ascites by a simple scoring system Scand J Gastroenterol, 49 (5) (2014), pp. 595–603 [DOI] [PubMed] [Google Scholar]
- 19.Fernandez J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study Hepatology, 55 (5) (2012), pp. 1551–1561 [DOI] [PubMed] [Google Scholar]
- 20.O’Leary JG, Reddy KR, Wong F, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis Clin Gastroenterol Hepatol, 13 (4) (2015), pp. 753–759 e751-e752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terg R, Casciato P, Garbe C, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study J Hepatol, 62 (5) (2015), pp. 1056–1060 [DOI] [PubMed] [Google Scholar]
- 22.Karvellas CJ, Abraldes JG, Arabi YM, Kumar A, Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: a retrospective cohort study Aliment Pharmacol Ther, 41 (8) (2015), pp. 747–757 [DOI] [PubMed] [Google Scholar]
- 23.Wade JJ, Rolando N, Hayllar K, Philpott-Howard J, Casewell MW, Williams R Bacterial and fungal infections after liver transplantation: an analysis of 284 patients Hepatology, 21 (5) (1995), pp. 1328–1336 [DOI] [PubMed] [Google Scholar]
- 24.Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis N Engl J Med, 365 (19) (2011), pp. 1790–1800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


