Abstract
BACKGROUND:
Avoidance of hypoxia and hyperoxia may reduce morbidity and mortality in critically ill civilian and military trauma patients. The objective of this study was to determine if a multimodal quality improvement intervention increases adherence to a consensus-based, targeted normoxia strategy. We hypothesized that this intervention would safely improve compliance with targeted normoxia.
METHODS:
This is a pre/postquasiexperimental pilot study to improve adherence to normoxia, defined as a pulse oximetry (SpO2) of 90% to 96% or an arterial partial pressure oxygen (PaO2) of 60 to 100 mm Hg. We used a multimodal informatics and educational intervention guiding clinicians to safely titrate supplemental oxygen to normoxia based on SpO2 monitoring in critically ill trauma patients admitted to the surgical-trauma or neurosurgical intensive care unit within 24 hours of emergency department arrival. The primary outcome was effectiveness in delivering targeted normoxia (i.e., an increase in the probability of being in the targeted normoxia range and/or a reduction in the probability of being on a higher fraction-inspired oxygen concentration [FiO2]).
RESULTS:
Analysis included 371 preintervention subjects and 201 postintervention subjects. Preintervention and postintervention subjects were of similar age, race/ethnicity, and sex and had similar comorbidities and Acute Physiologic and Chronic Health Evaluation II scores. Overall, the adjusted probability of being hyperoxic while on supplemental oxygen was reduced during the postintervention period (adjusted odds ratio, 0.74; 95% confidence interval, 0.57–0.97). There was a higher probability of being on room air (FiO2, 0.21) in the postintervention period (adjusted odds ratio, 1.38; 95% confidence interval, 0.83–2.30). In addition, there was a decreased amount of patient time spent on higher levels of FiO2 (FiO2, >40%) without a concomitant increase in hypoxia.
CONCLUSION:
A multimodal intervention targeting normoxia in critically ill trauma patients increased normoxia and lowered the use of supplemental oxygen. A large clinical trial is needed to validate the impact of this protocol on patient-centered clinical outcomes.
LEVEL OF EVIDENCE:
Therapeutic/care management, level II.
Keywords: Trauma, normoxia, supplemental oxygen, intensive care unit, combat
Providing supplemental oxygen to prevent hypoxia, a major contributor to mortality and morbidity, is widespread in critically ill patients and has been traditionally viewed as safe.1,2 However, it often results in supraphysiologic oxygen levels or hyperoxia. While hyperoxia as a potential therapy in traumatic brain injury and stroke has been explored,3 in the general population of intensive care unit (ICU) patients, both hypoxia and hyperoxia are associated with increased mortality.4,5
The importance of avoiding hypoxia is well understood; however, the effects of hyperoxia, especially in major trauma, are not as widely known. Hyperoxia has numerous deleterious effects that must be carefully balanced against the potential benefits of routine supplemental oxygen administration to prevent hypoxia. Higher levels of the fraction-inspired oxygen concentration (FiO2) can result in hyperoxic acute lung injury, impaired pulmonary gas exchange through a process of resorption atelectasis, and impaired mucocilliary transport function of the lungs leading to decreased bacterial clearance.6,7 Both duration of hyperoxia exposure and higher FiO2 correlate with the severity of hyperoxic acute lung injury.7 In addition to the many negative effects on the lungs, hyperoxia also impairs cardiac output, causes systemic vasoconstriction, and accentuates the ischemic-reperfusion injury through production of proinflammatory reactive oxygen species.6,8,9
Given the multiple deleterious effects of hyperoxia, several studies have explored targeted supplemental oxygen administration in ICU patients in an attempt to determine the safety and efficacy of various “conservative” oxygenation strategies. These trials have been promising, demonstrating the potential to reduce exposure to hyperoxia, reduce duration of mechanical ventilation, and reduce mortality.10–12 Nevertheless, even with growing awareness that hyperoxia is harmful, many clinicians do not routinely target normoxia or alter the dosage of supplemental oxygen provided in response to documented hyperoxia13—almost 75% of ICU patients experience prolonged hyperoxia.14,15 With growing evidence that avoidance of both hypoxia and hyperoxia plays an important role in clinical outcomes, prehospital, emergency, trauma, and critical care physicians endorse the concept of oxygen titration.6,16–18 Furthermore, in the context of military operations, the amount of supplemental oxygen has critical consequences related to logistics, size, weight, and power requirements, particularly in prolonged field care settings.19,20
The civilian and military implications of targeted normoxia warrant close examination of this approach from close to the time of injury through subsequent hospitalization. To date, few studies have looked at implementation strategies to increase compliance with targeted normoxia during these emergency department (ED) to ICU admissions,12,21,22 and the ability to target normoxia has not been specifically examined in critically ill trauma patients. The objective of this study was to determine if a multimodal quality improvement intervention increases adherence to a consensus-based, targeted normoxia strategy. We hypothesized that this intervention would (1) increase compliance with targeted normoxia (reduced probability of being on hyperoxic and on supplemental oxygen), (2) reduce supplemental oxygen use, and (3) not cause a significant increase in hypoxia.
PATIENTS AND METHODS
Study Design
This study was a quasiexperimental pre/postobservational pilot study to evaluate a multimodal quality improvement intervention aimed at improving adherence to guidelines targeting normoxia, a pulse oximetry (SpO2) of 90% to 96%, or an arterial partial pressure oxygen (PaO2) of 60 to 100 mm Hg, at a single level 1 trauma center at the University of Colorado Hospital (NCT03789396).
Study Sample
We included adult trauma patients, 18 years or older, who were admitted to the surgical-trauma or neurosurgical ICU from January 1, 2018, to December 31, 2018 (preintervention), and from January 1, 2019, to July 1, 2019 (postintervention), within 24 hours of ED arrival. The quasiexperimental protocol was approved by the Colorado Multiple Institutional Review Board (COMIRB 18-1528) with a waiver of consent, given that the intervention was designed as a quality improvement measure to increase compliance with an evidence-based guideline for oxygenation.
Intervention
We implemented a multimodal educational platform to guide clinicians to safely and feasibly titrate supplemental oxygen to normoxia based on the results of noninvasive SpO2 monitoring. We defined normoxia as a SpO2 90% to 96% or, when available, a PaO2 60 to 100 mm Hg; hypoxia as an SpO2 of <88%; borderline hypoxia as an SpO2 of 88% to 89%; and hyperoxia as an SpO2 of >96%. The optimal oxygenation target ranges were defined using a modified Delphi approach with 31 nationally and internationally recognized military and civilian experts in trauma surgery, emergency medicine, critical care, and military operational medicine. We asked the expert panel to rate how strongly they agreed or disagreed with specific SpO2 low thresholds, SpO2 high thresholds, PaO2 low thresholds, and PaO2 high thresholds. Based on our analysis of the data, we were able to identify what the majority of experts felt were appropriate oxygenation thresholds. The expert consensus panel agreed on the ranges used for this pilot implementation.
After physician and operational leadership support, the intervention was primarily administered by respiratory therapists and nurses, following a predefined protocolized clinical decision support tool and automated feedback. The study targeted providers in the ED and ICUs, but clinical decisions could override protocol recommendations. Implementation involved power point presentations for clinical staff, flyers, one-page protocols, trial staff attendance at ICU huddles, morning emails to respiratory therapists caring for mechanically ventilated patients, and an electronic health record (Epic eRecord) alert. The eRecord alert (Fig. 1) fired after identifying eligible patients with sustained oxygenation (at least 30 minutes) qualifying as hyperoxia. The preestablished protocol then made recommendations for titration of supplemental oxygen, that is, changes in FiO2 or positive end expiratory pressure (PEEP) in mechanically ventilated patients, and/or oxygen flow rate in nonmechanically ventilated patients. In some instances, no additional titration could be made.
Figure 1.
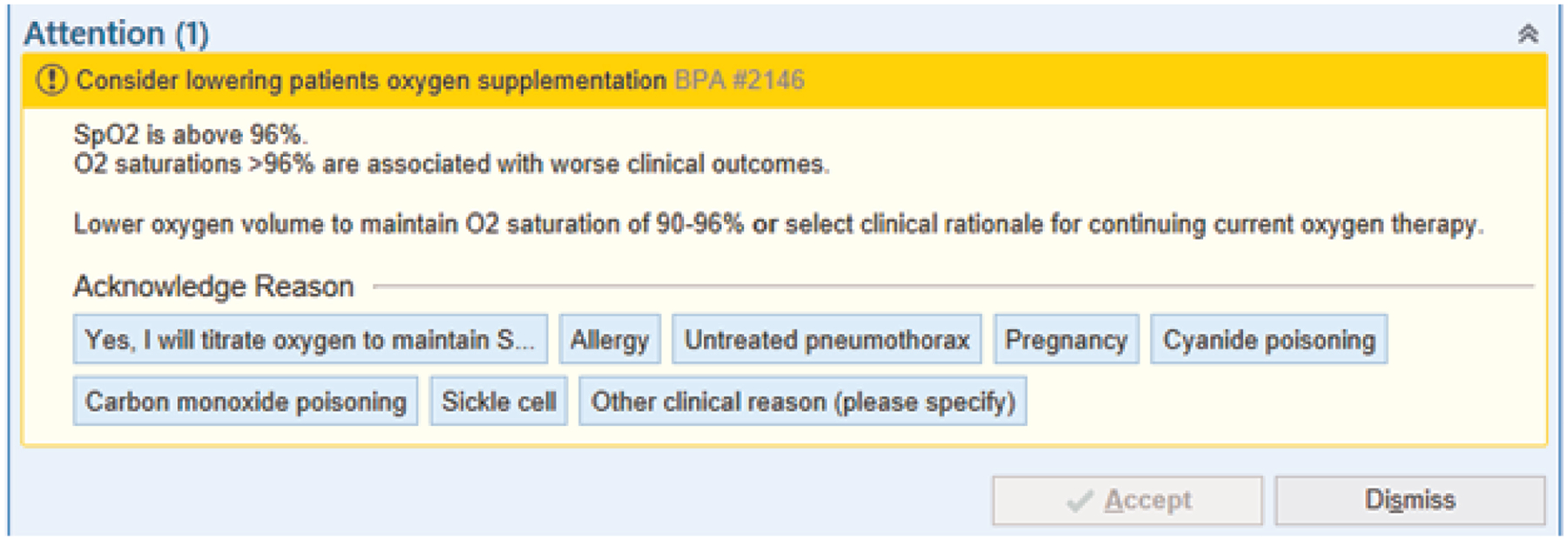
Electronic health record alert. Example of the electronic health record best practices alert triggered by patients found to have a sustained SpO2 of >96% for at least 30 minutes demonstrating common clinical exceptions to targeted normoxia.
Outcomes
The primary outcome was effectiveness in delivering targeted normoxia during the first 7 days of hospitalization, defined as an increase in the probability of being in the targeted normoxia range and a reduction in the probability of being on a higher FiO2 used. Secondary outcomes included in-hospital mortality (censored at discharge or 90 days), ventilator-free days (censored at 7 days), hospital-free days (censored at 90 days), and length of stay.
Sample Size and Power
In the design of this quasiexperimental pre/postintervention observational pilot study, a formal sample size and power calculation was not performed. This study was designed to gather preliminary data on the general effectiveness of the intervention that will inform the next larger, multicenter randomized control trial.
Statistical Analysis
Two 6-month intervals in the preintervention period (January to July and July to December) were included and analyzed separately and in combination to assess for potential seasonal variations. We compared baseline differences in patient and injury characteristics using χ2 test for categorical variables and Student’s t test for differences in age and length of stay. The results of the Acute Physiologic and Chronic Health Evaluation II scores and the number of other comorbidities were highly skewed.23 These and other ordinal and continuous variables that exhibited potential violations of normality assumptions were summarized as median and interquartile range with p values obtained via the Wilcoxon test. We summarized the primary outcome oxygenation variables using weighted averages and standard errors obtained by first aggregating outcome measures within person to best account for between-subject variability and total amount of time spent at each SpO2 or FiO2 level. We calculated descriptive statistics for patient time spent in predefined categories of SpO2 and FiO2. Separate mixed-effects logistic regression models for the probability of being hyperoxic and not on room air and the probability of being on room air alone were fit to the data, adjusting for demographic characteristics, and Injury Severity Score; the primary exposure variable was the patient’s pre/postintervention status. A random intercept for patient was included to account for correlation between measurements within a patient over time. Differences in secondary patient-centered clinical outcomes such as in-hospital mortality and ventilator-free days (Table 2) were evaluated using χ2 tests for comparison of proportions or Student’s t test for comparisons of continuous variables. Patients who died were assigned values of zero for ventilator-free days and hospital-free days. We defined a p value of <0.05 as statistically significant. Statistical analysis was performed using R statistical software (R Foundation for Statistical Computing, Vienna, Austria)24 with exception of the mixed effect models, which were analyzed using the PROC MIXED procedure in SAS software (SAS Institute, Cary, NC).25
TABLE 2.
Patient Time in a Given SpO2 Category
| SpO2 Category | Patient Time Preintervention, % | Patient Time Postintervention, % |
|---|---|---|
| Hypoxia (SpO2, <88%) | 1.1 | 1.0 |
| Mild hypoxia (SpO2, 88–89%) | <0.5 | <0.5 |
| Normoxia (90–96%) | 47.6 | 50.9 |
| Hyperoxia on FiO2 of 21% | 10.2 | 13.7 |
| Hyperoxia on FiO2 of >21% | 40.6 | 33.9 |
RESULTS
Overall Subject Characteristics
A total of 572 patients were included for analysis—371 in the preintervention phase and 201 in the postintervention phase. To assess for potential seasonal variation over the 12-month preintervention period, subjects were grouped and initially analyzed by two 6-month periods for differences in baseline characteristics and outcomes. However, no statistically significant differences were seen (results not shown), so these were combined into a single preintervention group for further analysis. Patients in the preintervention and postintervention period were similar with regards to baseline demographics, comorbidities, insurance type (a surrogate measure for possible socioeconomic status and access to care), mechanism of injury, mode of arrival, and Acute Physiologic and Chronic Health Evaluation II scores (Table 1). There was no difference between traumatic brain injury patients relative to those without a traumatic brain injury with respect to baseline characteristics or outcomes (data not shown).
TABLE 1.
Patient Demographic and Injury Characteristics
| Characteristics | Preintervention (n = 371) | Postintervention (n = 201) | χ2 or t Test p Value |
|---|---|---|---|
| Age, mean (SD), y | 55.3 (21.4) | 52.5 (21.4) | 0.14 |
| Sex, female, n (%) | 113 (30) | 62 (31) | 0.99 |
| Race/ethnicity, n (%) | |||
| Hispanic | 83 (22) | 51 (25) | 0.24 |
| Non-Hispanic Black | 49 (13) | 20 (10) | |
| Non-Hispanic White | 194 (52) | 114 (57) | |
| Other | 45 (12) | 16 (8) | |
| Insurance type, n (%) | |||
| Private | 101 (27) | 63 (31) | 0.08 |
| Medicare | 140 (38) | 56 (28) | |
| Medicaid | 102 (27) | 69 (34) | |
| Other | 5 (1) | 3 (1) | |
| Current/former smoker, n (%) | 121 (33) | 70 (35) | 0.72 |
| Alcohol use, n (%) | 128 (35) | 81 (40) | 0.30 |
| Substance abuse, n (%) | 50 (13) | 31 (15) | 0.86 |
| Comorbidities | |||
| Cardiopulmonary,* n (%) | 82 (22) | 56 (28) | 0.15 |
| No. other, median (IQR) | 3.0 (1.0–4.0) | 3.0 (1.0–4.0) | 0.68 |
| Mechanism of injury,** n (%) | |||
| Blunt trauma — fall | 156 (42) | 90 (45) | 0.18 |
| Blunt trauma — MVA | 132 (36) | 73 (36) | |
| Nonblunt — penetrating | 38 (10) | 10 (5) | |
| Nonblunt — other | 45 (12) | 28 (14) | |
| Mode of arrival, n (%) | |||
| EMS | 250 (67) | 148 (74) | 0.94 |
| Walk-in | 49 (13) | 30 (15) | |
| APACHE II score, median (IQR) | 8.0 (6.0–15.0) | 8.0 (5.0–15.4) | 0.87 |
Cardiopulmonary comorbidities were defined as congestive heart failure, valvular disease, pulmonary circulation disorders, and chronic pulmonary disease.
Injury classifications: blunt trauma — fall, that is, any falls; blunt trauma — MVA, that is, any motor vehicle accident; nonblunt — penetrating, that is, stabbing, cuts, gunshots wounds; nonblunt — other, that is, burns, drowning, struck by other object, assault, bicycle accident.
APACHE II, Acute Physiology and Chronic Health Evaluation II; EMS, emergency medical service; IQR, interquartile range; MVA, motor vehicle accident.
Effectiveness of Targeted Normoxia
The primary objective of this study was to determine if the multimodal intervention could effectively increase targeted normoxia. We measured the mean FiO2 and SpO2 levels over the first 7 days. The mean FiO2 and SpO2 levels over the first 7 days decreased overall during the postintervention period compared with the preintervention period with exception of the mean SpO2 on day 7, which was higher in the postintervention period (Fig. 2A and B). The multimodal intervention improved compliance with targeted normoxia, decreasing the odds of being hyperoxic (SpO2, >96%) and not on room air significantly in the postintervention period (aOR, 0.74 [95% confidence interval (CI), 0.57–0.97]; unadjusted OR, 0.77 [95% CI, 0.57–1.03]) (Fig. 2D) after adjusting for demographic characteristics, pre/postgrouping variable, and Injury Severity Score. In addition, the odds of being on room air (FiO2, 0.21) was higher in the postintervention period compared with the preintervention period (Fig. 2C). However, this effect did not reach statistical significance (aOR, 1.38 [95% CI, 0.83–2.30]; unadjusted OR, 1.38 [95% CI, 0.78–2.43]).
Figure 2.
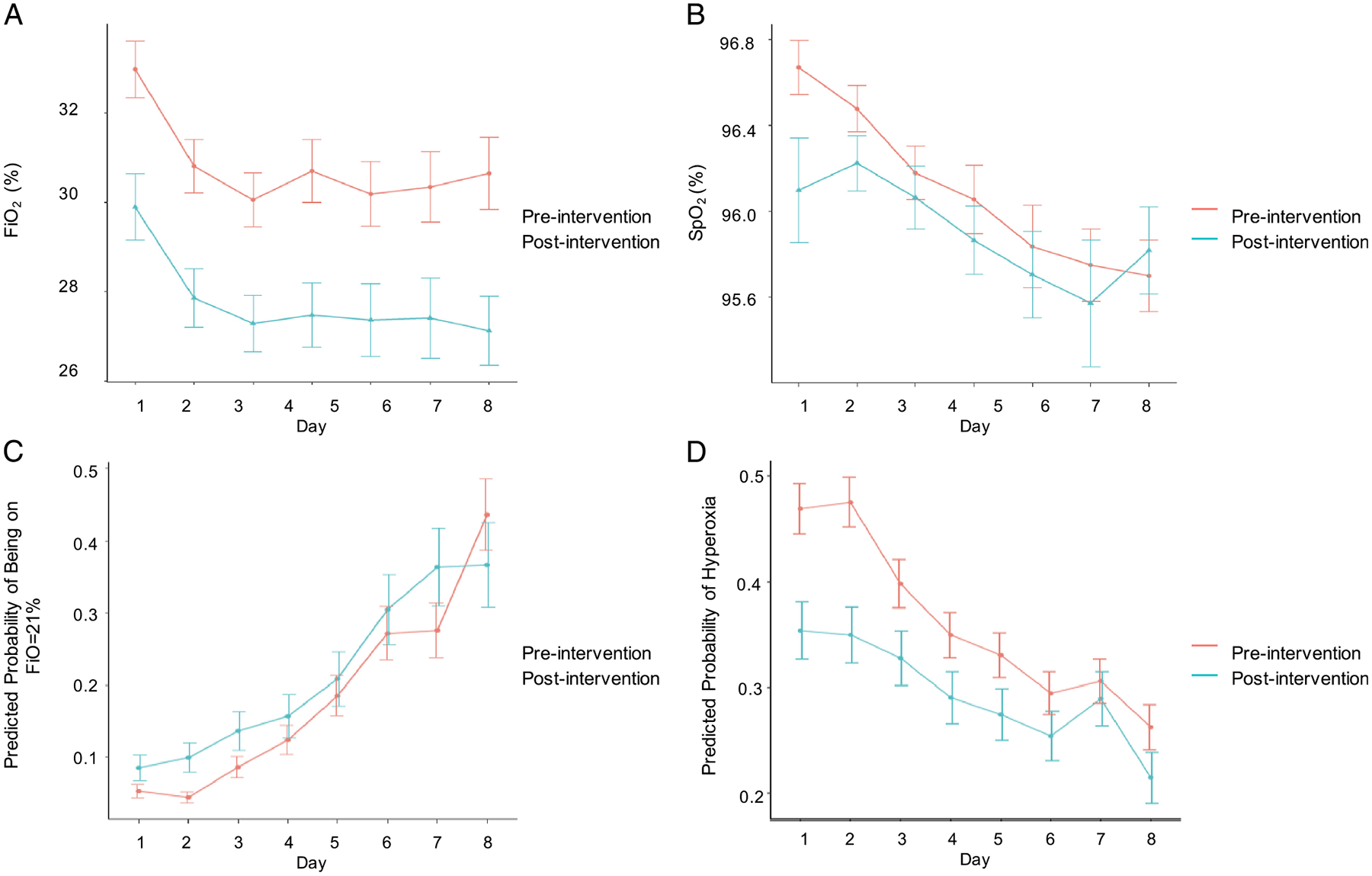
FiO2 and SpO2 over time. Comparison of the (A) mean FiO2 and (B) mean SpO2 by day in the preintervention and postintervention period. Data shown represent the mean value and SE of the mean. Comparison of the (C) predicted probability of being on room air (FiO2, 0.21) and (D) predicted probability of being hyperoxic (SpO2, >96%) and not on room air among the preintervention and postintervention period. Data shown represent the predicted probability with standard errors.
FiO2 Use Overall
There was an overall increase in the proportion of patient time spent on no supplemental oxygen (room air or FiO2, 21%) during the postintervention period (Fig. 3A). Specifically, in the group of patients found to be hyperoxic, 28.7% of patient time over the first 7 days in the postintervention period was spent on a FiO2 of 21% compared with only 20.1% of patient time on a FiO2 of 21% in the preintervention period (Fig. 3A). Similarly, in normoxic patients, 46.1% of patient time over the first 7 days in the postintervention period was spent on a FiO2 of 21% compared with only 42.6% in the preintervention period. Over the first 7 days, in both hyperoxic and normoxic patients, there was a decrease in the proportion of patient time spent on high levels of FiO2 (FiO2, >40% to >60%) (7.4% postintervention vs. 12.2% preintervention hyperoxic, and 4.7% postintervention vs. 9.3% preintervention normoxic). Similar trends were seen in the first 72 hours and on days 4 to 7 (Fig. 3B).
Figure 3.
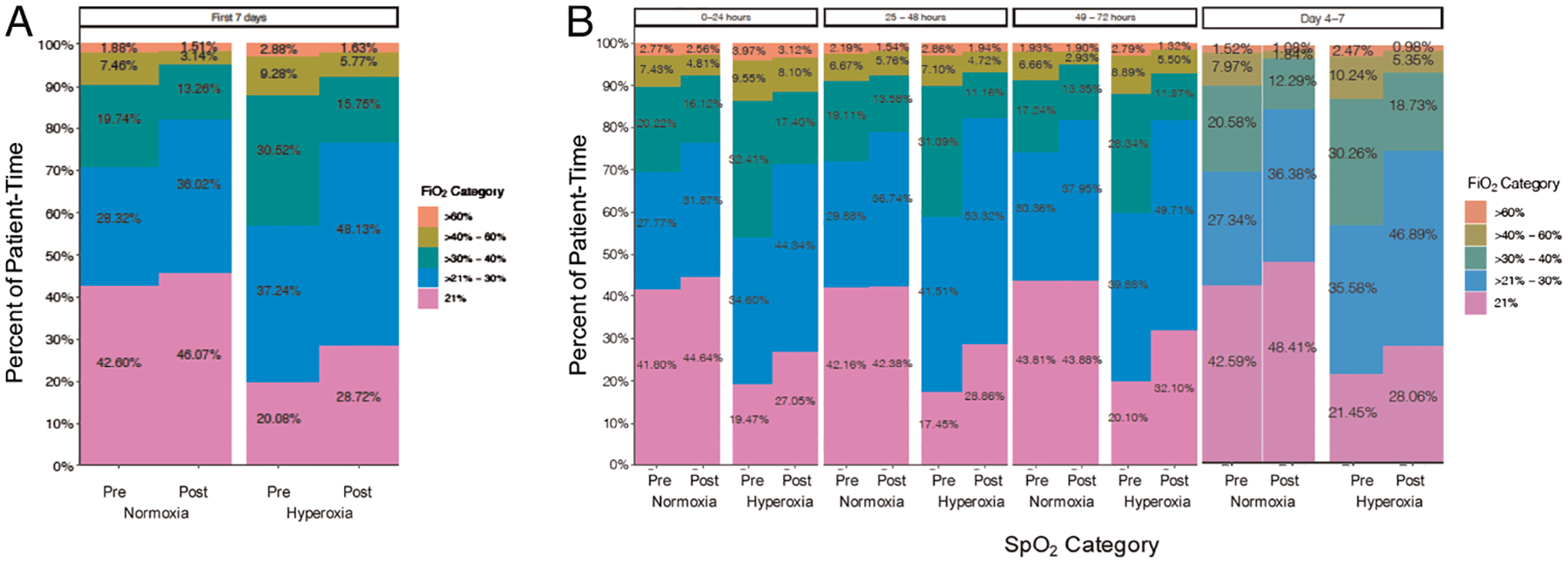
Percent total patient time at a given FiO2. Amount of patient time spent oxygenating at a given FiO2 for patients categorized as either normoxic (SpO2, 90–96%) or hyperoxic (SpO2, >96%) in the preintervention and postintervention periods. Including (A) all patients over the first 7 days and (B) all patients over the first 72 hours and days 4 to 7.
FiO2 Use in Mechanically Ventilated Patients
In a subgroup analysis, mechanically ventilated patients had a slight increase in the amount of patient time spent on minimal FiO2 settings (FiO2, >21% to 30%) over the first 7 days in both postintervention hyperoxic patients (52.9%) compared with the preintervention hyperoxia patients (29.1%) and normoxic patients (49.4% postintervention vs. 19.2% preintervention) (Supplemental Digital Content 1A, http://links.lww.com/TA/B962). In addition, there was a corresponding reduction in the percent of patient time spent in high FiO2 settings (FiO2, >40% to >60%), especially among hyperoxia patients from days 4 to 7 (19.2% preintervention to 7.8% postintervention) (Supplemental Digital Content 1B, http://links.lww.com/TA/B962).
FiO2 Use in Nonmechanically Ventilated Patients
In nonmechanically ventilated patients, among those found to be hyperoxic, there was again an increase in the proportion of patient time spent on room air (FiO2, 21%) (38.6% postintervention vs. 28.7% preintervention). There was also an increase in patient time spent on minimal FiO2 (FiO2, >21–30%) in the postintervention normoxic and hyperoxic patients during the first 7 days (34.7% postintervention vs. 29.2% preintervention normoxia; 46.2% postintervention vs. 40.9% preintervention hyperoxia) (Supplemental Digital Content 1C, http://links.lww.com/TA/B962) and the first 72 hours and days 4 to 7 (Supplemental Digital Content 1C and D, http://links.lww.com/TA/B962).
SpO2 Levels
Postintervention, there was a shift not only in the amount of FiO2 used but also in the amount of patient time spent within the targeted normoxia range (SpO2, 90–96%). Among all patients, there was found to be an overall increase in the amount of patient time with SpO2 of 90% to 96%, a decrease in the patient time spent above 98%, and no change in the patient time spent in the hypoxia or borderline hypoxia ranges (SpO2, <90%) (Supplementary Digital Content 2A, http://links.lww.com/TA/B963). The same shift was seen among mechanically ventilated (Supplementary Digital Content 2B, http://links.lww.com/TA/B963) and nonmechanically ventilated (Supplementary Digital Content 2C, http://links.lww.com/TA/B963) patients. Postintervention, in patients with an SpO2 of >96%, there was a decreased percentage of patient time spent with an FiO2 of >21% in patients overall and nonmechanically ventilated patients (Supplemental Digital Content 3A–C, http://links.lww.com/TA/B964).
Furthermore, there was no change in the percent of patient time over the first 7 days spent either hypoxic or mildly hypoxic in the postintervention group (1.0% and <0.5%, respectively) compared with the preintervention group (1.1% and <0.5%, respectively) (Table 2). In the postintervention period, there was a slight increase in the total patient time spent being normoxic (SpO2, 90–96%) (47.6% preintervention vs. 50.9% postintervention) and hyperoxic on room air (SpO2 of >96% with an FiO2 of 21%) (10.2% preintervention vs. 13.7% postintervention). Finally, there was a reduction in the patient time spent being hyperoxic on supplemental oxygen (SpO2 of >96% with an FiO2 of >21%) in the postintervention period (40.6% preintervention vs. 33.9% postintervention).
Secondary Patient-Centered Outcomes
The multimodal intervention to target normoxia resulted in no significant difference in in-hospital mortality (difference in proportions, 0.02; 95% CI, −0.03 to 0.07), ventilator-free days among all patients (mean difference, 0.04; 95% CI, −1.5 to 1.6), hospital-free days (mean difference, 2.1; 95% CI, −2.2 to 6.4), or length of stay (mean difference, 1.094; 95% CI, −2.373 to 4.561) (Table 3).
TABLE 3.
Overall Patient Outcomes by Pre/Postintervention Period
| Outcome | Preintervention (n = 371) | Postintervention (n = 201) | Difference (95% Cl) |
|---|---|---|---|
| In-hospital mortality, n (%) | |||
| Live | 250 (94) | 185 (92) | 0.02 (−0.03 to 0.07) |
| Died | 21 (6) | 16 (8) | |
| Ventilator-free days Overall | |||
| Mean (SD) | 23.3 (8.7) | 23.2 (9.2) | 0.04 (−1.5 to 1.6) |
| Median (IQR) | 28.0 (24.0–28.0) | 28.0 (24.0–28.0) | n/a |
| Ever mechanically ventilated only | |||
| Mean (SD) | 17.2 (10.3) | 16.4 (11.1) | 0.9 (−2.1 to 3.8) |
| Median (IQR) | 22.0 (6.0–26.0) | 22.0 (1.0–26.0) | n/a |
| Required mechanical ventilation, n (%) | 161 (43) | 78 (39) | 0.05 (−0.04 to 0.1) |
| Hospital-free days | |||
| Mean (SD) | 73.8 (23.2) | 71.7 (25.5) | 2.09 (−2.2 to 6.4) |
| Median (IQR) | 83.0 (74.0–87.0) | 83.0 (72.0–86.0) | n/a |
| Length of stay | |||
| Mean (SD) | 12.9 (27.5) | 11.8 (14.7) | 1.1 (−2.4 to 4.6) |
| Median (IQR) | 6.0 (3.0–13.0) | 7.0 (4.0–15) | n/a |
IQR, interquartile range; n/a, not applicable.
DISCUSSION
This multimodal intervention strategy to target normoxia successfully reduced the probability of being hyperoxic and on supplemental oxygen in critically ill trauma patients. Furthermore, the postimplementation phase had lower patient time spent on high FiO2 levels in both mechanically ventilated and nonmechanically ventilated patients identified as either normoxic or hyperoxic. The intervention resulted in a higher proportion of patient time spent on room air among those hyperoxic (SpO2, >96%) patients, meaning that no further adjustments to lower supplemental oxygen use were possible.
We used a simple multimodal approach to target normoxia through clinical decision support tools, provider education, and periodic reminders. This approach successfully reduced the probability of being hyperoxic, the probability of being on supplemental oxygen, and the time spent on supplemental oxygen. There appeared to be no difference in secondary clinical outcomes, although this pilot study was not powered for these effects. Still, safely reducing supplemental oxygen use in trauma patients has critical implementation for logistics and resource utilization, particularly in combat settings and other remote/austere environments. Our pilot study demonstrates the success of such an approach and lays the groundwork for a future larger-scale study to validate this practice and assess patient-centered outcomes in trauma patients (NCT04534959).
Previous studies demonstrate multiple methods for reducing oxygen supplementation with a variety of oxygenation targets and variable implementation outcomes. Mechanically ventilated ICU patients randomized to a conservative oxygenation (SpO2, 88–92%) spent less time being hyperoxic (4% patient time) but also spent more time being hypoxic (1% patient time) compared with those randomized to “liberal” oxygenation (SpO2, ≥96%) (22% patient time, hyperoxic; 0.3% patient time, hypoxic).12 Despite the increased rates of hypoxia in the conservative oxygenation group, there was no increase in ICU or 90-day mortality, allowing authors to argue that this increase was not clinically significant. By specifically targeting normoxia (SpO2, 90–96%), our intervention had minimal impact in the patient time spent hypoxic, while decreasing the overall probability of being hyperoxic and not on room air and increasing the probability of being on room air. This is also reflected by no change in patient time spent in hypoxia or borderline hypoxia ranges but an increase in patient time spent being normoxic or hyperoxic on room air (Table 2) among the postintervention group compared with the preintervention.
Similarly, when Intensive Care Unit Randomized Trial Comparing Two Approaches to Oxygen Therapy (ICU-Rox) investigators added a pulse-oximetry alarm for SpO2 of ≥97% compared with usual care without a protocol-defined upper limit SpO2, the time spent in the hyperoxic range was reduced, and there was no significant increase in hypoxia (SpO2, <88%).11 In both the conservative oxygenation and usual care groups, a lower limit pulse oximetry alarm was set for SpO2 of 90%, which likely helped to prevent an increase in hypoxia. In addition, this study, like the study of Panwar et al.,12 was focused on only mechanically ventilated patients, which may limit the applicability to other populations whose oxygenation may not be as closely followed and titrated. Taken together, our data and the Intensive Care Unit Randomized Trial Comparing Two Approaches to Oxygen Therapy (ICU-Rox) study suggest that targeting normoxia through alarms and limiting oxygen exposure as opposed to being focused on the target SpO2 without support alarms may be more optimal approach.
A common thread in these targeted normoxia studies is the use of a combination of educational objectives with technology component (i.e., best practices alert and/or pulse oximetry alarms). This approach relies on existing infrastructure and the ability of personnel to provide these educational opportunities. However, an alternative approach is being explored in combat settings where preservation of limited oxygenation supplies has broader implications for operational logistics and mission planning. One approach is to deploy a portable oxygen concentrator to a closed loop ventilation system. Such a system relies on a computer program to coordinate ventilator and concentrator functions and allows the program to automatically adjust the FiO2 delivered in response to measured SpO2 values. Currently, this system has been tested in proof-of-concept experiments and porcine models but is not widely used.26,27 Such a design may have important advantages over our approach to targeted normoxia, including the potential for tighter oxygenation control and less personnel requirements for titration. The main disadvantage compared with our approach is the requirement of specialized ventilator systems. However, further studies are needed to determine the best approach (closed loop systems vs. multimodal interventions) for specific populations and treatment settings.
Given our pilot study design, our results were meant to inform a larger-scale validation rather than provide conclusive analyses of all secondary outcomes proposed. While neither our approach nor the two previous approaches demonstrated a significant effect on mortality, targeting a conservative oxygenation practice (PaO2, 70–100 mm Hg; SpO2, 94–98%) instead of the “conventional” oxygenation practice (PaO2, up to 150 mm Hg; SpO2, 97–100%) was associated with reduced mortality (relative risk [RR], 0.57; 95% CI, 0.37–0.90) in a single-center randomized trial of primarily medical patients.10 In the previous studies, there was more direct oversight in terms of strict adherence to the protocol than would be expected from our quasiexperimental intervention, which more accurately reflects routine clinical implementation of targeted normoxia.
This study was limited by its design, as it was conducted at a single institution with a relatively small sample size over a limited time frame. In addition, the study is limited to validated SpO2 and FiO2 data collected in a patient’s medical record. It is possible that, outside of the times where data were pulled from monitors into eRecord, patients experienced transient episodes of hypoxia or hyperoxia that were not captured. However, by the nature of the patient monitoring in the ICU setting, these missed episodes would typically be brief and are less likely to be clinically significant. In addition, this study was conducted at an elevation of approximately 5,300 ft, which may limit the extrapolation of some results to sea-level populations. As a pilot study, it was not powered to detect a significant impact on patient-centered clinical outcomes. However, the data gathered were used to plan a larger, multicenter implementation clinical trial, which is underway (NCT04534959). In addition, this study is the first to broadly address normoxia implementation (targeting an SpO2 of 90–96%) specifically in critically ill trauma patients and takes a practical implementation strategy that could be deployed in a variety of clinical settings to improve patient outcomes.
In conclusion, this multimodal intervention strategy with educational activities and electronic health record alerts to target normoxia (SpO2, 90–06%) reduced the amount of supplemental oxygen used (lower patient time spent on high levels FiO2) and increased the amount of patient time spent normoxic. Future multicenter clinic trials are needed to evaluate the effect of such interventions on patient outcomes in a variety of critically ill trauma civilian and military populations who may respond differently to close oxygen titration.
ACKNOWLEDGMENT
We thank Caroline Ledbetter, MPH, for her work with preliminary data analysis.
Footnotes
DISCLOSURE
The authors declare no conflicts of interest. This project was funded by the Department of Defense/SOCOM (W81XWH-17-C-0241).
This abstract expresses the authors’ opinions and does not reflect the policy or opinions of the Department of the Army, Department of the Air Force, Department of Defense, or US Government.
This study was presented at the Special Operations Medical Association, September 2020, virtual conference, September 15–10, 2020.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
REFERENCES
- 1.Chi JH, Knudson MM, Vassar MJ, et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J Trauma 2006;61(5):1134–1141. [DOI] [PubMed] [Google Scholar]
- 2.Parke RL, Eastwood GM, McGuinness SP, George Institute for Global Health; Australian and New Zealand Intensive Care Society Clinical Trials Group. Oxygen therapy in non-intubated adult intensive care patients: a point prevalence study. Crit Care Resusc 2013;15(4):287–293. [PubMed] [Google Scholar]
- 3.Kumaria A, Tolias CM. Normobaric hyperoxia therapy for traumatic brain injury and stroke: a review. Br J Neurosurg 2009;23(6):576–584. [DOI] [PubMed] [Google Scholar]
- 4.de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, Bosman RJ, de Waal RA, Wesselink R, de Keizer NF. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008; 12(6):R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, Donati A. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18(6):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafner S, Beloncle F, Koch A, Radermacher P, Asfar P. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. 2015;5(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013; 58(1):123–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallet RH, Branson RD. Should oxygen therapy be tightly regulated to minimize hyperoxia in critically ill patients? Respir Care. 2016;61(6):801–817. [DOI] [PubMed] [Google Scholar]
- 9.Pannu SR. Too much oxygen: Hyperoxia and oxygen management in mechanically ventilated patients. Semin Respir Crit Care Med 2016;37(1):16–22. [DOI] [PubMed] [Google Scholar]
- 10.Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, Moreli A, Antonelli M, Singer M. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316(15):1583–1589. [DOI] [PubMed] [Google Scholar]
- 11.ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group, Mackel D, Bellomo R, Bailer M, et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med 2020;382(11):989–998. [DOI] [PubMed] [Google Scholar]
- 12.Panwar R, Hardie M, Bellomo R, Barrot L, Eastwood GM, Young PJ, Capellier G, Harrigan PW, Bailey M, CLOSE Study Investigators; ANZICS Clinical Trials Group. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med 2016;193(1):43–51. [DOI] [PubMed] [Google Scholar]
- 13.de Graaff AE, Dongelmans DA, Binnekade JM, de Jonge E. Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO2. Intensive Care Med 2011;37(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rachmale S, Li G, Wilson G, Malinchoc M, Gajic O. Practice of excessive F (IO(2)) and effect on pulmonary outcomes in mechanically ventilated patients with acute lung injury. Respir Care. 2012;57(11):1887–1893. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Eastwood GM, Peck L, Glassford NJ, Bellomo R. Current oxygen management in mechanically ventilated patients: a prospective observational cohort study. J Crit Care. 2013;28(5):647–654. [DOI] [PubMed] [Google Scholar]
- 16.Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342(18):1301–1308. [DOI] [PubMed] [Google Scholar]
- 17.Eastwood GM, Peck L, Young H, Suzuki S, Garcia M, Bellomo R. Intensive care clinicians’ opinion of conservative oxygen therapy (SpO2 90–92%) for mechanically ventilated patients. Aust Crit Care. 2014;27(3):120–125. [DOI] [PubMed] [Google Scholar]
- 18.Helmerhorst HJ, Schultz MJ, van der Voort PH, Bosman RJ, Jeffermans NP, de Jonge E, van Westerloo DJ. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naylor JF, Borgman MA, April MD, Hill GJ, Schauer SG. Normobaric hyperoxia in wartime pediatric trauma casualties. Am J Emerg Med 2020; 38(4):709–714. [DOI] [PubMed] [Google Scholar]
- 20.Schauer SG, April MD, Naylor JF, Mould-Millman NK, Bebart VS, Becker TE, Maddry JK, Ginde AA. Incidence of hyperoxia in combat wounded in Iraq and Afghanistan: a potential opportunity for oxygen conservation. Mil Med 2019;184(11–12):661–667. [DOI] [PubMed] [Google Scholar]
- 21.Helmerhorst HJ, Schultz MJ, van der Voort PH, et al. Effectiveness and clinical outcomes of a two-step implementation of conservative oxygenation targets in critically ill patients: a before and after trial. Crit Care Med 2016; 44(3):554–563. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S, Eastwood GM, Glassford NJ, Peck L, Young H, Garcia-Alvarez M, Schneider AG, Bellomo R. Conservative oxygen therapy in mechanically ventilated patients: a pilot before-and-after trial. Crit Care Med 2014;42(6): 1414–1422. [DOI] [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13(10):818–829. [PubMed] [Google Scholar]
- 24.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 25.Base SAS 9.4 Procedure Guide. Cary, NC: Base SAS 9.4 Procedure Guide. Version 9; 2015. [Google Scholar]
- 26.Gangidine MM, Blakeman TC, Branson RD, Johannigman JA. System design verification for closed loop control of oxygenation with concentrator integration. Mil Med 2016;181(Suppl 5):177–183. [DOI] [PubMed] [Google Scholar]
- 27.Blakeman T, Rodriquez D, Johannigman J, Branson R. Pulsed dose oxygen delivery during mechanical ventilation: impact on oxygenation. Mil Med 2019;184(5–6):e312–e318. [DOI] [PubMed] [Google Scholar]


