Abstract

Chalcogenide perovskites (CPs), with the general composition ABX3, where A and B are metals and X = S and Se, have recently emerged as promising materials for application in photovoltaics. However, the development of CPs and their applications has been hindered by the limitations of available preparation methods. Here we present a new approach for the synthesis of CPs, based on the sulfurization of ternary and binary oxides or carbonates with in situ formed boron sulfides. In contrast to the previously described approaches, the method presented here uses chemically stable starting materials and yields pure-phase crystalline CPs within several hours, under low hazard conditions. CP yields over 95% are obtained at temperatures as low as 600 °C. The generality of the approach is demonstrated by the preparation of CPs with compositions BaZrS3, β-SrZrS3, BaHfS3, SrHfS3, and EuHfS3. Mechanistic insights about the formation of CPs are discussed.
Short abstract
Chalcogenide perovskites with compositions BaZrS3, β-SrZrS3, BaHfS3, SrHfS3, and EuHfS3 were synthesized from ternary and binary oxides or carbonates in a vacuum by reaction with in situ formed boron sulfides. This new synthetic approach offers several advantages over previously reported methods, including the use of chemically stable starting materials, shorter reaction times, and less hazardous preparation conditions. The effects of the reaction time, temperature, and starting mixture composition and mechanistic insights about the reactions are discussed.
A great level of interest in solid materials with perovskite crystalline structure in recent years has been stimulated primarily by the discovery that a subset of these materials, halide perovskites (HPs), possess a combination of properties excellent for exploitation in photovoltaics and, more broadly, optoelectronics.1,2 Integration of HPs into thin-film solar cells led to unprecedented performance enhancements.3 HPs were also shown to be very promising materials for the development of efficient light-emitting diodes, lasers, detectors, and other technologies.4−7 Despite the promise of HPs, their limited thermal and chemical stability and inclusion of toxic ions, such as lead, pose challenges for their commercial exploitation.
Chalcogenide perovskites (CPs), crystalline solids with the composition ABX3, where A and B are metal cations and X = S and Se, have recently been suggested as a possible alternative to the structurally related HPs.8−11 Although more research is needed to fully assess their potential, theoretical12,13 and early experimental13−15 studies indicate that CPs have electronic properties similar to those of HPs, are comparable or better light absorbers,13,16 have better chemical and thermal stability,17,18 and can be prepared without the use of toxic elements. Experimental studies of CPs have so far been limited, however, mainly because of challenges with their preparation.
To date, all known CPs (all sulfides)11 were prepared by one of two solid-state, high-temperature (>600 °C) syntheses methods first described more than 60 years ago.19,20 In one method, the elements and/or binary sulfides are reacted in sealed, evacuated reaction tubes, according to eq 1:
| 1a |
| 1b |
| 1c |
The second method is based on sulfurization of oxide or carbonate precursors with H2S or CS2 gas at temperatures of >1000 °C, according to eq 2:
| 2a |
| 2b |
In eqs 1 and 2, the typical examples of ion A are Ca2+, Sr2+, Ba2+, and Ln2+/3+ and those of ion B are Zr4+, Hf4+, Sc3+, and Ln3+/4+ (Ln = lanthanide). One drawback of both methods is long reaction time, with days to weeks often required for complete conversion. Another drawback of approach (1) is a high propensity of the starting materials A, B, AS, and BS2 to oxidize even at ambient conditions (Figure S6). This often leads to product contamination with hard to separate oxides. In method (2), complete sulfurization is often difficult because of the limited thermal stability of H2S and CS2.21,22 Additional drawback is that both H2S and CS2 are toxic and form explosive mixtures with air.23,24
In studies of binary oxides, Wu and Seo described a sulfurization approach based on a solid-state metathesis reaction with boron sulfides.25,26 The O → S metathesis reaction was shown to be very effective in the sulfurization of binary oxides of transition metals and lanthanides25,26 as well as actinides.27 The synthesis of α-EuZrS3 from Eu2O3 and ZrO2, in the presence of elemental boron and sulfur, was also recently reported.28 The approach has not yet been explored in the preparation of CPs.
In this work, we investigated a preparation of sulfide CPs by solid-state reactions of ternary and binary oxides and carbonates in the presence of boron and sulfur. The synthetic approach is schematically shown in the top part of Scheme 1. In a typical reaction, ternary oxide (a), binary oxides (b), element and binary oxide (c) or carbonate (d) were thoroughly mixed with a small (∼10%) molar excess of elemental boron and a stoichiometric amount of sulfur and sealed under vacuum in a quartz reaction ampule. The ampule was heated in a high-temperature furnace at 500–1100 °C for several hours to several days. The full details of the syntheses are provided in the Supporting Information, SI.
Scheme 1. (Top) Diagram of the Synthetic Approach for the Preparation of CPs by Sulfurization in the Presence of Boron and (Bottom) Schematic Energy Diagram Showing the Relative Enthalpies of Sulfurization in the Absence and Presence of Boron.
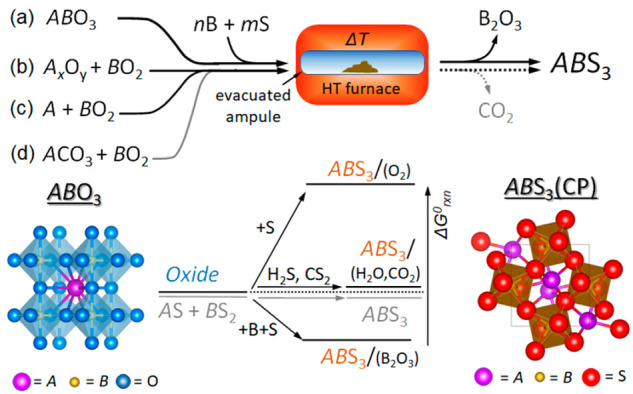
The italicized symbols A and B represent metal cations, and the nonitalic letter B represents boron. Also shown are the crystal structures of a starting material ABO3 (oxide) and the product ABS3 (CP).
High-Temperature Chemistry of Boron Sulfides
As described for the first time nearly 200 years ago,29 heating of a boron–sulfur mixture past the sulfur boiling point (444.6 °C) produces boron sulfides. More recent studies revealed that, in a vacuum, where sulfur sublimes below 120 °C, the reaction yields B2S3 and/or BS2, depending on the B/S ratio in the starting mixture.30 B2S3 and BS2 sublime at temperatures above 300 °C26 and form various oligomers and polymers.31 The high effectivity of gaseous boron sulfides as sulfurizing agents for oxides is, in part, due to a large difference in the thermodynamic stability of the byproduct B2O3 (ΔGf°298 = −1192.3 kJ/mol) and B2S3 (ΔGf°298 = −247.6 kJ/mol) and BS2 (ΔGf°298 = −120 kJ/mol).32,33,26 Thus, boron sulfides serve not only as sources of sulfur but also as an effective “oxygen trap”.
As shown in the bottom part of Scheme 1, the high stability of B2O3 makes the sulfurization of metal oxides with boron sulfides thermodynamically more favorable than the sulfurization with H2S [ΔGf°298(H2O) = −228.6 kJ/mol and ΔGf°298(H2S) = −33.3 kJ/mol]32 or CS2 [ΔGf°298(CO2) = −394.4 kJ/mol and ΔGf°298(CS2) = 66.8 kJ/mol]32 or the direct reaction of binary sulfides. While the specific reaction mechanisms and kinetic barriers for reactions (a)–(d) are not known, a comparison of the reaction enthalpies calculated from the available experimental and theoretical data (Table TS7) suggests that they are highly thermodynamically favorable. This was one of the motivating factors for us to explore these processes experimentally.
Sulfurization of Ternary Oxides
The metathesis of ternary oxides was investigated for A = Ba2+ and Sr2+ and B = Zr4+ and Hf4+, according to reaction (3)((a) in Scheme 1):
| 3 |
In the reaction, the italicized symbols A and B represent metal cations, and the nonitalic letter B represents boron. In the experiments the reaction mixtures were prepared with n = 2.2 and m = 3 and carried out at T = 1000 °C, with holding time t = 36 h. The X-ray diffraction (XRD) patterns of the reaction products are summarized in Figure 1. The peaks match the reference patterns of the corresponding CPs from the ICDD database. No Bragg reflections of any other phases were detected. The product lattice parameters are in good agreement with the literature values (see TS1 in the SI), indicating the formation of pure phases. The sharpness of the diffraction peaks and flat background indicate a high degree of crystallinity of the products.
Figure 1.

Powder XRD patterns (blue traces) of the CPs prepared from the corresponding ABO3 oxides and the reference literature patterns (orange traces) of the CPs obtained from the ICDD database. In all cases, n = 2.2 and m = 3, and the heating program (schematically shown by green traces) is as follows: ramp at 5 °C/min to 300 °C, hold 5 h, ramp at 5 °C/min to 600 °C, hold 5 h, ramp at 5 °C/min to 1000 °C, hold 36 h, and cool at 5 °C/min.
Observation of a high efficiency of the metathesis reaction at T = 1000 °C prompted us to investigate it at more moderate temperatures, T = 500–600 °C. This temperature range is an important threshold from the technological standpoint because it represents an upper limit of thermal stability of the substrates typically used in the preparation of optoelectronic devices. The O → S metathesis of BaZrO3 was performed according to eq 3, with n = 2.2 and m = 3 and the temperatures and holding times systematically varied. The results are summarized in the bottom two panels of Figure 2 and in Table TS2.
Figure 2.

Sulfurization of BaZrO3 at moderate temperatures. Powder XRD patterns (blue traces) of the reaction products as a function of the reaction temperature T and holding time t. In all cases, n = 2.2 and m = 3. The heating programs are schematically shown by green traces. In the bottom three panels, the heating program is as follows: ramp at 5 °C/min to 300 °C, hold 5 h, ramp at 5 °C/min to T, hold t h, and cool to RT at 5 °C/min. In the top panel, the heating program is as follows: ramp at 5 °C/min to 300 °C and cool to RT at 5 °C/min. Also shown are reference literature patterns (orange traces) of BaZrO3, BaZrS3, ZrS3, and ZrO2 obtained from the ICDD database.
A Rietveld analysis of the XRD patterns of the reaction products for T = 500 °C shows that for t = 48 h conversion to BaZrS3 was about ∼48.5% complete and increased to ∼63.8% at t = 168 h (bottom XRD pattern in Figure 2). The other components of the product mixture were BaZrO3 and ZrS3. Analysis of the results for T = 600 °C shows that, at t = 5 h, the product comprises ∼97% BaZrS3. At t = 10 h, the yield decreases to ∼92% (XRD trace shown in Figure 2). In both cases, traces of ZrO2 were detected in the product mixture (Figure S2). Other possible side products were present in the amounts below our detection limit. To find the temperature required for full conversion, the samples heated at T = 600 °C for t = 5 h were subsequently exposed to temperatures of 650, 700, 800, 900, and 1000 °C, without any holding time at maximum temperature. The experiments revealed that complete conversion was achieved at 900 °C (Figure 2) or above. Interestingly, the full conversion to BaZrS3 was also achieved by a simple temperature ramp to 900 °C (at 5 °C/min) without any preheating at lower temperatures (top trace in Figure 2). Notably, this reaction, including the cool down, was complete in less than 6 h (Table TS2).
The above analysis leads to several observations. Although partial conversion of BaZrO3 to CP at T = 500 °C is encouraging, conditions for higher conversion rates and shorter reaction times have to be identified for the reaction at this temperature to be useful for applications. Increasing T to 600 °C leads to near-complete conversion in as little as 5 h. Surprisingly, extension of the reaction time does not lead to more complete conversion. This implies the presence of a kinetic barrier, which at this temperature is difficult to overcome. Our results show that a brief heating to 900 °C is sufficient to overcome the barrier and achieve full conversion.
To further investigate the possibility of complete BaZrO3 → BaZrS3 conversion at T = 500–600 °C, reaction (3) was carried out in the presence of excess sulfur (m = 5). This was motivated by observations described previously in a report by Wang et al.,34 who showed that, in the reaction of BaS with ZrS2 with excess sulfur and a small amount of BaCl2, up to 90% conversion to BaZrS3 can be achieved at T = 500 °C with t = 12 h and 96% conversion at T = 550 °C with t = 1 h. In our experiments with BaZrO3, performed in the temperature range T = 500–600 °C, with and without BaCl2 added to the reaction mixture, we found the conversion to be more efficient without BaCl2, with a maximum conversion rate of ∼88% at T = 600 °C and t = 1 h. At longer reaction times and lower temperatures, we observed lower conversion rates. Compared to the stoichiometric reactions described above, the reactions with excess sulfur yielded higher fractions of ZrO2, ZrS3, and unreacted sulfur in the product mixtures.
Our results offer some mechanistic insights. Specifically, the presence of ZrO2 and ZrS3 in the product mixture indicates that at least a fraction of BaZrO3 decomposes during the reaction. In addition, in several reactions carried out at 500–560 °C, we detected small amounts of barium sulfides BaSn (n = 1–3). Because no binary compounds were identified in the product mixture upon a brief heating to ≥900 °C, we hypothesize that the detected binary compounds are reaction intermediates. Thus, under the studied conditions, one of the possible pathways for BaZrO3 → BaZrS3 conversion involves the initial decomposition of the former, with ZrO2 as one of the products. Upon ZrO2 sulfurization, the CP is likely formed by the reaction of zirconium sulfide(s) with barium sulfides. The reaction is fast at ≥900 °C but inefficient below 600 °C. Other reaction pathways may be contributing as well.
Sulfurization of Binary Oxides and Carbonates
The high efficiency of the sulfurization of ternary oxides prompted us to also explore similar reactions with the starting materials binary oxides and carbonates, according to reactions (4)–(6) ((b)-(d) in Scheme 1):
| 4 |
| 5 |
| 6 |
The reactions were investigated for A = Ba2+, Sr2+, and Eu3+ and B = Zr4+ and Hf4+. The details of the reaction conditions and the product compositions are summarized in Tables TS3–TS5. The highest conversion rates to CPs were in all three types of reactions observed for A = Sr2+ and B = Zr4+. The XRD patterns of the products of the three reactions, carried out at T = 1000 °C and t = 36 h, are shown in Figure 3. Rietveld analysis revealed that for reaction (4) the conversion rate to β-SrZrS3 CP is ∼99%, with about 1% ZrO2. For reaction (5), the CP yield was 74% with ∼8% ZrO2 and ∼18% ZrS2. Because reaction (5) is thermodynamically more favorable than reaction (4) (Table TS6), the lower conversion rate suggests a larger kinetic barrier for the former. In the case of reaction (6), the conversion rate was 82% with ∼18% ZrO2 in the product mixture. The lower conversion rate compared to reactions (3) and (4) may be due to a lower thermodynamic favorability (Table TS6) and/or a negative effect of CO2 formed as a side product during the reaction.
Figure 3.

Effect of the starting material on the sulfurization efficiency. Powder XRD patterns (blue traces) of β-SrZrS3 CP prepared from the starting materials indicated in the legends by reaction with boron sulfides. In all cases, the heating program is as follows: ramp at 5 °C/min to 300 °C, hold 5 h, ramp at 5 °C/min to 600 °C, hold 5 h, ramp at 5 °C/min to 1000 °C, hold 36 h, and cool at 5 °C/min. Also shown are the reference literature patterns (orange traces) of β-SrZrS3, ZrS2, and ZrO2 obtained from the ICDD database.
Finally, we found that heating of partially oxidized binary sulfides in the presence of boron sulfides can also be used to effectively remove the oxide impurities. The details are provided in section 10 in the SI.
In summary, we presented a new method for the preparation of CPs, based on the sulfurization of ternary oxides with boron sulfides in closed ampules. The method offers high conversion rates using chemically stable starting materials, significantly shorter reaction times, and improved safety over the previously reported methods. Optimization of the method for synthesis at more moderate temperatures and the preparation of new compositions are currently in progress.
Acknowledgments
This work was supported by the European Union’s Horizon 2020 research and innovation programme under Grant Agreement 810701 and by the Slovak Research and Development Agency under Grant Agreement APVV-19-410. S.K.T. acknowledges partial support from Project USCCCORD (ŽoNFP: NFP313020BUZ3), cofinanced by the European Regional Development Fund within the Operational Programme Integrated Infrastructure.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.2c03200.
Synthesis details, characterization methods, reaction conditions and product composition for reactions with binary oxides and carbonates, SEM images, AES data, and selected thermodynamic data (PDF)
Author Contributions
R.B.: conceptualization, materials synthesis, characterization, data analysis, visualization, manuscript editing. S.K.T.: materials synthesis, visualization, manuscript editing. P.H.: materials characterization, data analysis, visualization, manuscript editing. L.V.: materials characterization, data analysis, manuscript editing. M.S.: conceptualization, funding acquisition, project administration, original manuscript writing, data analysis, visualization, editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Green M. A.; Ho-Baillie A.; Snaith H. J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8 (7), 506–514. 10.1038/nphoton.2014.134. [DOI] [Google Scholar]
- Stranks S. D.; Snaith H. J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10 (5), 391–402. 10.1038/nnano.2015.90. [DOI] [PubMed] [Google Scholar]
- Best Research-Cell Efficiency Chart. https://www.nrel.gov/pv/cell-efficiency.html.
- Liu X. K.; Xu W. D.; Bai S.; Jin Y. Z.; Wang J. P.; Friend R. H.; Gao F. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2021, 20 (1), 10–21. 10.1038/s41563-020-0784-7. [DOI] [PubMed] [Google Scholar]
- Lei L.; Dong Q.; Gundogdu K.; So F. Metal Halide Perovskites for Laser Applications. Adv. Funct. Mater. 2021, 31, 2010144. 10.1002/adfm.202010144. [DOI] [Google Scholar]
- Kim H.; Han J. S.; Choi J.; Kim S. Y.; Jang H. W. Halide Perovskites for Applications beyond Photovoltaics. Small Methods 2018, 2, 1700310. 10.1002/smtd.201700310. [DOI] [Google Scholar]
- Chouhan L.; Ghimire S.; Subrahmanyam C.; Miyasaka T.; Biju V. Synthesis, optoelectronic properties and applications of halide perovskites. Chem. Soc. Rev. 2020, 49 (10), 2869–2885. 10.1039/C9CS00848A. [DOI] [PubMed] [Google Scholar]
- Swarnkar A.; Mir W. J.; Chakraborty R.; Jagadeeswararao M.; Sheikh T.; Nag A. Are Chalcogenide Perovskites an Emerging Class of Semiconductors for Optoelectronic Properties and Solar Cell?. Chem. Mater. 2019, 31 (3), 565–575. 10.1021/acs.chemmater.8b04178. [DOI] [Google Scholar]
- Buffiere M.; Dhawale D. S.; El-Mellouhi F. Chalcogenide Materials and Derivatives for Photovoltaic Applications. Energy Technol. 2019, 7, 1900819. 10.1002/ente.201900819. [DOI] [Google Scholar]
- Tiwari D.; Hutter O. S.; Longo G. Chalcogenide perovskites for photovoltaics: current status and prospects. Journal of Physics-Energy 2021, 3, 034010. 10.1088/2515-7655/abf41c. [DOI] [Google Scholar]
- Sopiha K. V.; Comparotto C.; Marquez J. A.; Scragg J. J. S. Chalcogenide Perovskites: Tantalizing Prospects, Challenging Materials. Adv. Opt. Mater. 2022, 10, 2101704. 10.1002/adom.202101704. [DOI] [Google Scholar]
- Sun Y. Y.; Agiorgousis M. L.; Zhang P. H.; Zhang S. B. Chalcogenide Perovskites for Photovoltaics. Nano Lett. 2015, 15 (1), 581–585. 10.1021/nl504046x. [DOI] [PubMed] [Google Scholar]
- Meng W. W.; Saparov B.; Hong F.; Wang J. B.; Mitzi D. B.; Yan Y. F. Alloying and Defect Control within Chalcogenide Perovskites for Optimized Photovoltaic Application. Chem. Mater. 2016, 28 (3), 821–829. 10.1021/acs.chemmater.5b04213. [DOI] [Google Scholar]
- Perera S.; Hui H. L.; Zhao C.; Xue H. T.; Sun F.; Deng C. H.; Gross N.; Milleville C.; Xu X. H.; Watson D. F.; Weinstein B.; Sun Y. Y.; Zhang S. B.; Zeng H. Chalcogenide perovskites - an emerging class of ionic semiconductors. Nano Energy 2016, 22, 129–135. 10.1016/j.nanoen.2016.02.020. [DOI] [Google Scholar]
- Niu S. Y.; Huyan H. X.; Liu Y.; Yeung M.; Ye K.; Blankemeier L.; Orvis T.; Sarkar D.; Singh D. J.; Kapadia R.; Ravichandran J. Bandgap Control via Structural and Chemical Tuning of Transition Metal Perovskite Chalcogenides. Adv. Mater. 2017, 29, 1604733. 10.1002/adma.201604733. [DOI] [PubMed] [Google Scholar]
- Nishigaki Y.; Nagai T.; Nishiwaki M.; Aizawa T.; Kozawa M.; Hanzawa K.; Kato Y.; Sai H.; Hiramatsu H.; Hosono H.; Fujiwara H. Extraordinary Strong Band-Edge Absorption in Distorted Chalcogenide Perovskites. Solar Rrl 2020, 4, 1900555. 10.1002/solr.201900555. [DOI] [Google Scholar]
- Niu S. Y.; Milam-Guerrero J.; Zhou Y. C.; Ye K.; Zhao B. Y.; Melot B. C.; Ravichandran J. Thermal stability study of transition metal perovskite sulfides. J. Mater. Res. 2018, 33 (24), 4135–4143. 10.1557/jmr.2018.419. [DOI] [Google Scholar]
- Gupta T.; Ghoshal D.; Yoshimura A.; Basu S.; Chow P. K.; Lakhnot A. S.; Pandey J.; Warrender J. M.; Efstathiadis H.; Soni A.; Osei-Agyemang E.; Balasubramanian G.; Zhang S. B.; Shi S. F.; Lu T. M.; Meunier V.; Koratkar N. An Environmentally Stable and Lead-Free Chalcogenide Perovskite. Adv. Funct. Mater. 2020, 30, 2001387. 10.1002/adfm.202001387. [DOI] [Google Scholar]
- Hahn H.; Mutschke U. Untersuchungen uber ternare chalkogenide 11. Versuche zur darstellung von thioperowskiten. Z. Anorg. Allg. Chem. 1957, 288 (5–6), 269–278. 10.1002/zaac.19572880505. [DOI] [Google Scholar]
- Clearfield A. Synthesis and crystal structures of some alkaline earth titanium and zirconium sulfides. Acta Crystallogr. 1963, 16 (2), 135. 10.1107/S0365110X6300030X. [DOI] [Google Scholar]
- Kaloidas V. E.; Papayannakos N. G. Hydrogen-Production from the Decomposition of Hydron-Sulfide - Equilibrium Studies on the System H2S/H2/SI, (I = 1,···8) in the Gas-Phase. Int. J. Hydrogen Energy 1987, 12 (6), 403–409. 10.1016/0360-3199(87)90159-5. [DOI] [Google Scholar]
- Stull D. R. Thermodynamics of Carbon Disulfide Production. Ind. Eng. Chem. 1949, 41 (9), 1968–1973. 10.1021/ie50477a031. [DOI] [Google Scholar]
- CS2 safety information. https://www.osha.gov/chemicaldata/574.
- H2S safety information. https://www.osha.gov/chemicaldata/652.
- Wu L. M.; Sharma R.; Seo D. K. Metathetical conversion of Nd2O3 nanoparticles into NdS2 polysulfide nanoparticles at low temperatures using boron sulfides. Inorg. Chem. 2003, 42 (19), 5798. 10.1021/ic034575j. [DOI] [PubMed] [Google Scholar]
- Wu L. M.; Seo D. K. New solid-gas metathetical synthesis of binary metal polysulfides and sulfides at intermediate temperatures: Utilization of boron sulfides. J. Am. Chem. Soc. 2004, 126 (14), 4676–4681. 10.1021/ja0392521. [DOI] [PubMed] [Google Scholar]
- Breton L. S.; Klepov V. V.; Zur Loye H. C. Facile Oxide to Chalcogenide Conversion for Actinides Using the Boron-Chalcogen Mixture Method. J. Am. Chem. Soc. 2020, 142 (33), 14365–14373. 10.1021/jacs.0c06483. [DOI] [PubMed] [Google Scholar]
- Guo S. P.; Chi Y.; Zou J. P.; Xue H. G. Crystal and electronic structures, and photoluminescence and photocatalytic properties of alpha-EuZrS3. New J. Chem. 2016, 40 (12), 10219–10226. 10.1039/C6NJ02106A. [DOI] [Google Scholar]
- Berzelius J. J. Untersuchungen uber flufspathsaure und deren merwurdigsten verbindungen. Ann. Phys. 1824, 78, 113–150. 10.1002/andp.18240781002. [DOI] [Google Scholar]
- Hurter H. U.; Krebs B.; Eckert H.; Mullerwarmuth W. Solid-State B-11 NMR-Studies on Boron Chalcogenide Systems. Inorg. Chem. 1985, 24 (9), 1288–1292. 10.1021/ic00203a006. [DOI] [Google Scholar]
- Chen H. Y.; Gilles P. W. High Molecular Weight Boron Sulfides 5. Vaporization Behavior of Boron-Sulfur System. J. Am. Chem. Soc. 1970, 92 (8), 2309. 10.1021/ja00711a018. [DOI] [Google Scholar]
- Barin I.Thermochemical Data of Pure Substances, 3rd ed.; VCH Verlagsgesellshaft mbH: Weinheim, Germany, 1995. [Google Scholar]
- ΔGf°800(B2S3) = −232.9 kJ/mol, ΔGf°1100(B2S3) = −199.2 kJ/mol, ΔGf°800(B2O3) = −1062.9 kJ/mol, and ΔGf°1100(B2O3) = −996.9 kJ/mol.
- Wang Y. R.; Sato N.; Yamada K.; Fujino T. Synthesis of BaZrS3 in the presence of excess sulfur. J. Alloys Compd. 2000, 311 (2), 214–223. 10.1016/S0925-8388(00)01134-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


