Abstract
Cyanation of benzylic C–N bonds is useful in the preparation of important α-aryl nitriles. The first general catalytic cyanation of α-(hetero)aryl amines, analogous to the Sandmeyer reaction of anilines, was developed using reductive cyanation with CO2/NH3. A broad array of α-aryl nitriles was obtained in high yields and regioselectivity by C–N cleavage of intermediates as ammonium salts. Good tolerance of functional groups such as ethers, CF3, F, Cl, esters, indoles, and benzothiophenes was achieved. Using 13CO2, a 13C-labeled tryptamine homologue (five steps, 31% yield) and Cysmethynil (six steps, 37% yield) were synthesized. Both electronic and steric effects of ligands influence the reactivity of alkyl nickel species with electrophilic silyl isocyanates and thus determine the reactivity and selectivity of the cyanation reaction. This work contributes to the understanding of the controllable activation of CO2/NH3 and provides the promising potential of the amine cyanation reaction in the synthesis of bio-relevant molecules.
Keywords: reductive cyanation, utilization of CO2/NH3, nitrile synthesis, α-(hetero)aryl amines, C−N activation, nickel, isotope labeling
Introduction
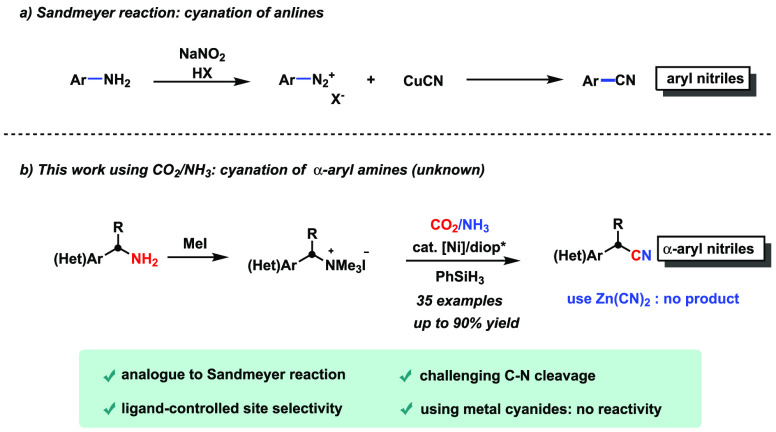
Selective transformation of C–N bonds is attractive but challenging.1−3 Although the transformation of amines to nitriles is a straightforward process, examples have been published only infrequently. The Sandmeyer reaction is the traditional method for the cyanation of anilines and involves in situ preparation of aryldiazonium salts (Figure 1a).4,5 In addition, the cyanation of C–N bonds is difficult to achieve via traditional SN2- or SN1-type reactions. Recently, Watson et al. developed the nickel-catalyzed cyanation of Katritzky pyridinium salts with Zn(CN)2 and one example of benzylic pyridinium salt was reported.6 C–N bond cleavage of enaminones promoted by I2 for synthesis of β-cyano enones was also realized.7 Expanding the diversity of C–C coupling reactions is a main topic in modern chemistry,8−28 and utilization of CO2 as the most abundant, nontoxic C1 synthon provides a promising approach to economically generate desirable products.29,30 Recently, Martin et al. and Yu et al. have reported carboxylation of C–N bonds in benzyl ammonium salts with CO2.31,32 The utilization of CO2 in catalytic synthesis of nitriles however is yet to be explored.
Figure 1.
(a) Sandmeyer reaction and (b) cyanation of α-aryl amines using CO2/NH3.
Inspired by the biological 2e-reduction process for the formation of cyanide ligands in [NiFe]-hydrogenase from CO2 and NH3,33 we have prepared aryl nitriles by cyanation of aryl halides using CO2.34,35 The wide application of the Sandmeyer reaction of anilines led us to envision a Sandmeyer reaction-like system for cyanation of α-aryl amines, and to the best of our knowledge, a general procedure of C–N bond cyanation to afford α-aryl nitriles has not yet been reported. Moreover, the selective incorporation of cyano group has attracted tremendous attention.36−43 Even by far, the most prominent production process for adiponitrile (>106 tons per year) is suffering from toxic cyanide and costly purification.44 Herein, we report the first example of general cyanation of α-aryl amines via the challenging C–N bond cleavage for the synthesis of α-aryl nitriles using CO2/NH3 (Figure 1b). This nickel-catalyzed reaction allows the synthesis of one carbon longer nitrile via a cyanation–reduction–cyanation sequence from a simple substrate, and it has been successfully applied to the convenient synthesis of isotopically labeled tryptamine precursors.45,46 Notably, no desired product was detected when metal cyanides were used. Thus, the reductive electrophilic cyanation process using cheap and abundant CO2/NH3 provides an alternative chemical platform for C–C coupling reactions and offers a hitherto unrecognized opportunity for cyanide-free synthesis of bio-relevant α-(hetero)aryl nitriles.
Results and Discussion
Initially, our investigation began by carrying out reactions on benzyl ammonium salt (1a) formed in situ by the reaction of the amine (1a′) with MeI (Table 1). During optimization, careful selection of reductants is crucial to evade the undesired reduction pathways, such as hydrolysis of ammonium salt, reduction of CO2, and/or reduction of nitrile products. Under CO2 and NH3, both at atmospheric pressure, the use of phenyl silane efficiently provided the cyanated products, albeit in low to moderate regioselectivities (Table 1, entries 1 and 2, 49–87% yields; 18–68% selectivity for 2a). DIOP turned to be the best ligand among the various ligands tested (Table S2).47−50 Since ligands containing a DIOP backbone proved to be suitable for the reaction, various DIOP derivatives (L1–L7) with different steric and electronic properties were synthesized and tested. It was found that the more sterically hindered ligand L3 ((R,R)-3,5-Me-DIOP) provided a better reactivity (entries 3–8 and Table S2).51−53 Also, when using L3 as the ligand, the switchable regioselectivity between the α-aryl nitrile (2a) and linear nitrile (4a) could be well tuned, and the desired product (2a) was obtained in good yield with high regioselectivity (entry 4, 80% yield; 84:5:11 rr). These results highlight the adjustment of the ability of ligands for selective cyanation on the benzylic carbon over other positions.
Table 1. Ligand Screening for Nickel-Catalyzed C–N Bond Cyanationa.
| entry | ligand (mol %) | yield (%) | rr (2a:3a:4a) |
|---|---|---|---|
| 1 | dppb | 49 | 18:29:53 |
| 2 | L1 | 87 | 68:10:22 |
| 3 | L2 | trace | trace |
| 4 | L3 | 80 | 84:5:11 |
| 5 | L4 | 66 | 60:16:24 |
| 6 | L5 | 22 | 76:16:8 |
| 7 | L6 | 26 | 72:15:13 |
| 8 | L7 | 35 | 86:6:8 |
Reaction conditions: benzyl ammonium salts 1a (0.15 mmol, 1.0 equiv), NiBr2·(diglyme) (12 mol %), ligand (15 mol %), CO2/NH3 (15/15 mL), Zn (1.2 equiv), PhSiH3 (5.0 equiv), and ZnF2 (50 mol %) were stirred in NMP:diglyme (0.5:0.1 mL) at 120 °C for 28 h. Yields and regioselectivities were determined by GC. dppb = 1,4-bis(diphenylphosphino)butane.
With the optimal conditions in hand, we next examined the substrate scope in the synthesis of different α-(hetero)aryl nitriles (Table 2). Considering the electronic properties, we found that benzyl ammonium salts (1) bearing electron-rich or electron-deficient groups could deliver the α-aryl nitriles in moderate to good yields with high regioselectivity (2a–2f). However, the reaction gave racemic products. Substrates containing various functional groups such as fluorine (1c), phenyl (1d), and naphthalene (1f) were examined. All were smoothly converted to the corresponding α-aryl nitriles in yields of 74–81%. Notably, selective cyanation between C–N and C–O bonds to form the α-aryl nitrile product (2e) could be achieved, albeit in diminished yield under the established conditions.
Table 2. Applicability Study of α-(Hetero)Aryl Amines.
Reaction conditions: ammonium salt (1) (0.15 mmol, 1.0 equiv), NiBr2·(diglyme) (12 mol %), L3 (15 mol %), CO2/NH3 (15/15 mL), Zn (1.2 equiv), ZnF2 (50 mol %), NMP:diglyme (0.5:0.1 mL), 120 °C, 28 h. Isolated yields.
NiBr2 (12 mol %), dppe (12 mol %), NMP:toluene (0.5:0.1 mL), 120 °C, 24 h. Isolated yields.
GC yields reported using n-tetradecane as the internal standard.
30 h. rr represents the ratio of the major product to the sum of all other isomers as determined by GC analysis.
Aryl acetonitrile compounds are valuable intermediates and are generally used for diverse modification at their α-carbon to synthesize chiral α-amino acid precursors.54−57 Remarkably, the devised protocol is applicable to synthesize aryl acetonitriles (2) from the corresponding primary benzyl ammonium salts (1), showcasing the versatile utility of this methodology. Initially, we evaluated the catalytic cyanation of the benzyl ammonium salt (1g) with CO2 and NH3 (Tables S3–S5). Examination of the reaction parameters showed that the cyanation of 1g with CO2/NH3 and organophosphorus ligands catalyzed by NiBr2 proceeds efficiently (Table S4). Evaluation of various ligands showed that phosphine-containing ligands outperformed ligands containing nitrogen in both reactivity and chemoselectivity. Systematic investigation of phosphine ligands by changing the chain length between two phosphorus atoms was found to improve the yield of 2g. The ligand 1,2-bis(diphenylphosphino)ethane (dppe) showed a remarkable performance, producing 2g in 81% yield.
Generally, studies of the substrate scope revealed that ammonium salts bearing various substituents are tolerated and afford the desired aryl acetonitriles in moderate to high yields (Table 2). Use of 13CO2 led us to explore a catalytic protocol to prepare isotopically labeled nitriles. First, using 13CO2, we obtained 13C-2g in 83% yield, showing that the carbon source of the CN group in the product was derived from CO2. This reaction exhibited excellent chemoselectivity, and it was found that benzyl ammonium salts substituted with either electron-donating groups (Me, OMe, Et, t-Bu, and OCF3) or electron-withdrawing groups (CF3, F, COOMe, and COOt-Bu) are well tolerated, giving 2h–2n, 2p–2s, and 2v–2w. The reaction is applicable to aromatic substrates with fused rings (2o, 2x, and 2y), indicating that molecules with expanded π-conjugated systems are tolerated. A substrate with chlorine on the aromatic ring provided the chlorophenyl nitrile (2t) in 50% yield, together with phenyl nitrile as a byproduct formed by dehalogenative hydrogenolysis. It is noteworthy that the benzylic C–N bond of the ammonium salt (1z) was selectively converted to the corresponding nitrile (2z) in 89% yield with the benzylic C–O bond remaining intact. In contrast, only a trace amount of product was formed in the presence of the cyanating reagent, Zn(CN)2.6 We studied the reaction of heterocyclic substrates such as indole and furan and found the method to be applicable, obtaining 2aa–2ae in yields of 77–90%. Specifically, cyanation of the 3-substituted indole ammonium salt provided the tryptamine precursor (2ad) in a good yield. Subsequent reduction of 2ad with LiAlH4 provided the tryptamine derivative in two steps, a method that is far superior to the classical method that requires more steps and a tedious workup. At the same time, different aliphatic amines were tested and almost no desired nitrile products could be obtained.45
Tryptamine precursors produced from indole are a privileged motif in biological and pharmaceutical research, but only limited methods are known for their preparation.45,46,58,59 We prepared the intermediate indole-3-carboni-trile (3c) in 72% yield with a nickel-catalyzed reaction with CO2 and NH3. Treatment of 13CO2 with ammonium salt (3e) and NH3 under standard conditions gave the 13C-labeled tryptamine precursor (13C-2ad) in 66% yield (Scheme 1). This protocol provides an alternative valuable route to construct homologous tryptamines or 13C-labeled indole derivatives efficiently. This methodology has also been applied to the production of 13C-labeled Cysmethynil (Scheme 2). The reaction of the indole ammonium salt (4e) with 13CO2 and NH3 provided the 13C-labeled intermediate indole derivative (4f) in 62% yield, and this was finally converted by treatment with potassium hydroxide to 13C-labeled Cysmethynil (4g) in 89% yield.60,61
Scheme 1. Synthetic Application for Aryl Acetonitrile. I: Cyanation–Reduction Sequence for 13C-Labeled Tryptamine Precursors.
Scheme 2. Synthetic Application for Aryl Acetonitrile. II: Synthesis of Isotopically Labeled Cysmethynil.
Studies of the Mechanism
Additional experiments were conducted in an effort to understand the mechanism of this transformation. Radical clock experiments were conducted with benzyl ammonium salts (1n) and α-cyclopropylstyrene (5). The ring-expanded product (6) was obtained in 20% yield (Scheme 3a-I).62,63 The benzyl ammonium salt (1n) was converted to o-methoxyphenyl acetonitrile (2n) in 66% yield along with the ring-expanded product (6) (Scheme 3a-II) in 13% yield. These results indicate that although the well-known oxidative addition of ammonium salts to Ni(0) is regarded as a major pathway, the alternative radical pathway cannot be excluded.64 In the presence of a Hantzsch ester, the benzyl ammonium salt (1n), a hydrogen donor capable of trapping carbon radicals (Scheme 3b), was transformed into the nitrile (2n), which was formed as a major product in 63% yield, with the formation of a minor product, 2-methylanisole, under standard conditions.63,65 This result is in accord with the hypothesis that a benzylic radical was formed in the mixture and subsequently reacted with a hydrogen of the Hantzsch ester to give 2-methylanisole. Control experiments were conducted to gain more information on the active benzyl-nickel species. As shown in Scheme 3c, in the absence of zinc powder, only trace amounts of branched and linear nitriles were detected to be formed, with 95:0:5 regioselectivity. Since nickel(II) complexes can be reduced to nickel(I) complexes by zinc powder through a single-electron transfer process and nickel(I) species can participate in Ni-catalyzed coupling or chain walking processes, a radical mechanism with nickel(I) active intermediates in which cyanation is taking place. As shown in Scheme 3d, when the ammonium salt of 2-phenyl ethylamine (7) was the substrate, only trace amounts of nitriles were obtained. This result implies that an efficient C–N bond activation and the formation of benzyl-nickel intermediate are crucial for the efficient cyanation of terminal sp3C-H bonds. We attempted to isolate the active intermediate in the reaction of the ortho-COOMe benzyl ammonium salt (1w) (see III-8 in the SI).66,67 Ni(dppe)2 (8) was obtained, and its structure was confirmed by X-ray crystallography (Scheme 3e). Using compound 8 as the catalyst in the cyanation of the benzyl ammonium salt (1n), the desired product was obtained in 15% yield. This implies that the nickel(0) active species is involved and the use of more ligand (in a ratio to Ni of 2:1) decreases the reactivity. When DIPAMP* (L9: (1,2-Bis((R)-(2-methoxyphenyl)(phenyl)phosphino)ethane) was used as the ligand, only 13% selectivity for 2a was observed (Table S2). In order to understand the regioselectivity differences between the bisphosphine ligand, DIOP* (L3), and DIPAMP* (L9), density functional theory (DFT) calculations based on the hybrid of the Becke’s three-parameter exchange functional and the Lee, Yang, and Parr correlation functional (B3LYP) were performed for the benzylic nickel species.68−70 The basis set used for C, H, O, P, and Br atoms was 6-31G, and the LANL2DZ pseudopotential basis set was employed for the Ni atom. The vibrational frequency was computed at the same level of theory to determine whether each optimized structure represented an energy minimum or a transition state. The natural population analysis charge was also calculated using the same method as in the optimization. As shown in Figure S3, different electronic densities on the Ni center (0.365 vs 0.345) and bite angles (P-Ni-P: 94.59 vs 78.22°) were obtained for these two ligands. This suggests that both the electronic and steric effects of ligands influence the reactivity with silyl isocyanates of alkyl nickel species and the tendency of chain walking via an iterative β-H elimination and reinsertion process.
Scheme 3. (a–e) Mechanistic Studies.
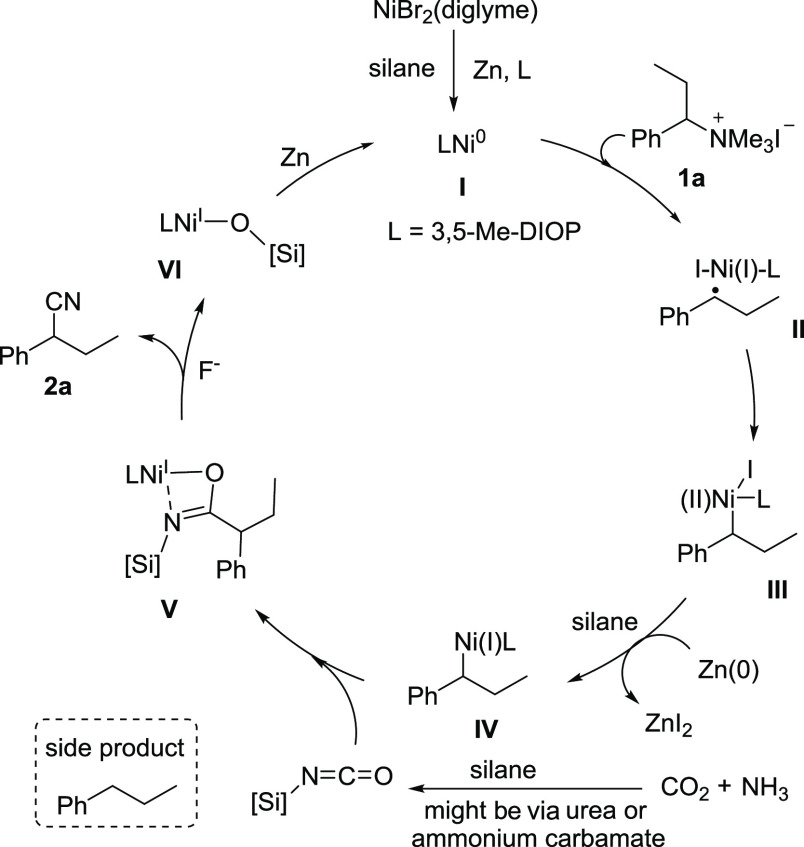
Based on the experimental results and previous reports, we propose a plausible reaction mechanism for the benzyl nitriles (Figure 2).34,35,63,64 First, the nickel(II)-precursor formed in situ is reduced and the nickel(0)-species (I) is generated in the presence of silanes and Zn. Subsequently, the benzyl ammonium salt (1a) can be reduced by nickel(0) to give a benzyl radical and a nickel(I) species (II) followed by a radical addition reaction, which delivers the nickel(II) intermediate (III). This intermediate is then reduced by Zn and silanes to generate the thermodynamically favored benzyl-nickel(I) intermediate (IV), which is inserted by silyl isocyanate intermediates to give a transient imidate species (V) in the presence of DIOP* (L3) as the ligand. As a minor process, the β-hydrogen elimination of the intermediate benzyl-nickel(I) (IV) would form the nickel(I) hydride species, subsequently delivering the chain walking nitrile products.71−74 The key to success is the careful choice of the ligand, which favors the reactivity of the benzyl-nickel(I) intermediate and/or the silyl isocyanate insertion step. The transient imidate species (V) are then transformed into benzyl nitriles via a plausible 1,3-silyl N-to-O migration,27,75,76 accompanied by the formation of a nickel siliconate intermediate (VI), which upon reduction by hydrosilane and zinc, regenerates the species (I), closing the catalytic cycle.
Figure 2.
Plausible reaction pathway for nitriles using CO2 and NH3.
Conclusions
In summary, we have developed the catalytic cyanation of α-aryl amines via ammonium salt intermediates with CO2/NH3 as a source of the cyano group. This versatile protocol provides a straightforward and cyanide-free route to an array of valuable benzylic nitriles in moderate to good yields. The success of this cyanation reaction is attributed to the careful selection of a bisphosphine ligand that can control the formation and/or reactivity of the benzyl-nickel(I) intermediate. This reaction exhibits broad functional group tolerance and operational simplicity. 13C-containing nitriles can be obtained conveniently using 13CO2. The cyanation of C–N bonds with CO2/NH3 allows electrophilic cyanation of the C–N bond to form cyano products and supports downstream applications in synthesis and their prospective use in the synthesis of bio-relevant molecules.
Methods
General Procedure for the Reductive Cyanation of Ammonium Salts Forming Nitriles
Under a nitrogen atmosphere, the nickel-catalyst (12 mol %, 0.018 mmol), ligand (15 mol %, 0.0225 mmol), ZnF2 (50 mol %, 0.075 mmol), Zn (1.2 equiv, 0.18 mmol), ammonium salts (1.0 equiv, 0.15 mmol), and a stirring bar were placed in a 10 mL oven-dried sealed tube (Figure S4). Then, the respective solvents and PhSiH3 (5.0 equiv, 0.75 mmol) were injected by a syringe. The tube was sealed, and CO2 (15 mL) and NH3 (15 mL) were injected by a syringe after N2 was removed under vacuum. Then, the mixture was stirred for the indicated time in a preheated alloy block. After the reaction was finished, the tube was cooled to room temperature and the pressure was released. The yield was measured by GC analysis or isolated by preparative thin-layer chromatography on silica gel plates to give nitriles (for the detailed procedure, see Figures S5 and S6).
Acknowledgments
The authors would like to dedicate this work to Professor Matthias Beller (LIKAT) on the occasion of his 60th birthday and acknowledge financial support by the NSFC (22022204, 22072167, and 22001257). F.Y. thanks Dr. Qiming Sun (Soochow University) for valuable discussions and help.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.2c00392.
General notes, reaction details, characterization data, and 1H, 13C, and heteroatom NMR spectral data (PDF)
Author Contributions
Y.L. supervised this study. F.Y. performed the catalytic experiments and mechanistic studies. J.-F.B., Y.D., C.L., and C.-X.D. discussed the result. S.L. performed the theoretical calculations. Y.D. performed part of synthetic experiments. Y.L. and F.Y. wrote the paper. All authors have given approval to the final version of the manuscript. CRediT: Fachao Yan data curation, investigation, methodology, writing-original draft; Jian-Fei Bai conceptualization, investigation; Yanan Dong data curation, formal analysis, investigation, methodology, validation; Shaoli Liu data curation, investigation, software; Chen Li formal analysis, methodology, validation; Chen-xia Du methodology, resources; Yuehui Li conceptualization, funding acquisition, methodology, project administration, supervision, writing-original draft, writing-review & editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Ouyang K.; Hao W.; Zhang W.-X.; Xi Z. Transition-metal-catalyzed cleavage of C-N single bonds. Chem. Rev. 2015, 115, 12045–12090. 10.1021/acs.chemrev.5b00386. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Su Y.; Li L.; Huang H. Transition-metal catalysed C-N bond activation. Chem. Soc. Rev. 2016, 45, 1257–1272. 10.1039/C5CS00534E. [DOI] [PubMed] [Google Scholar]
- García-Cárceles J.; Bahou K. A.; Bower J. F. Recent methodologies that exploit oxidative addition of C-N bonds to transition metals. ACS Catal. 2020, 10, 12738–12759. 10.1021/acscatal.0c03341. [DOI] [Google Scholar]
- Sandmeyer T. Ueber die ersetzung der amid-gruppe durch Chlor, brom und cyan in den aromatis substanzen. Ber. Dtsch. Chem. Ges. 1884, 17, 1633–1635. 10.1002/cber.18840170219. [DOI] [Google Scholar]
- Xu W.; Xu Q.; Li J. Sandmeyer cyanation of arenediazonium tetrafluoroborate using acetonitrile as a cyanide source. Org. Chem. Front. 2015, 2, 231–235. 10.1039/C4QO00301B. [DOI] [Google Scholar]
- Xu J.; Twitty J. C.; Watson M. P. Nickel catalyzed deaminative cyanation: nitriles and one-carbon homologation from alkyl amines. Org. Lett. 2021, 23, 6242–6245. 10.1021/acs.orglett.1c01959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Wan J.; Liu Y. Metal-free enaminone C-N bond cyanation for stereoselective synthesis of (E)- and (Z)-β-cyano enones. Chem. Commun. 2021, 57, 9112–9115. 10.1039/D1CC03292E. [DOI] [PubMed] [Google Scholar]
- Sundermeier M.; Zapf A.; Beller M. Palladium-catalyzed cyanation of aryl halides: recent developments and perspectives. Eur. J. Inorg. Chem. 2003, 2003, 3513–3526. 10.1002/ejic.200300162. [DOI] [Google Scholar]
- Anbarasan P.; Schareina T.; Beller M. Recent developments and perspectives in Palladium-catalyzed cyanation of aryl halides: synthesis of benzonitriles. Chem. Soc. Rev. 2011, 40, 5049–5067. 10.1039/c1cs15004a. [DOI] [PubMed] [Google Scholar]
- Wang C.-S.; Dixneuf P. H.; Soulé J.-F. Photoredox catalysis for building C–C bonds from C(sp2)–H bonds. Chem. Rev. 2018, 118, 7532–7585. 10.1021/acs.chemrev.8b00077. [DOI] [PubMed] [Google Scholar]
- Kurono N.; Ohkuma T. Catalytic asymmetric cyanation reactions. ACS Catal. 2016, 6, 989–1023. 10.1021/acscatal.5b02184. [DOI] [Google Scholar]
- Pimparkar S.; Koodan A.; Maiti S.; Ahmed N. S.; Mostafa M. M.; Maiti D. C-CN bond formation: an overview of diverse strategies. Chem. Commun. 2021, 57, 2210–2232. 10.1039/D0CC07783F. [DOI] [PubMed] [Google Scholar]
- Ding S.; Jiao N. N,N-Dimethylformamide: a multipurpose building block. Angew. Chem., Int. Ed. 2012, 51, 9226–9237. 10.1002/anie.201200859. [DOI] [PubMed] [Google Scholar]
- Wang T.; Jiao N. Direct approaches to nitriles via highly efficient nitrogenation strategy through C–H or C–C bond cleavage. Acc. Chem. Res. 2014, 47, 1137–1145. 10.1021/ar400259e. [DOI] [PubMed] [Google Scholar]
- Ahmad M. S.; Pulidindi I. N.; Li C. Recent advances in C–CN and C–H bond activation of green nitrile (MeCN) for organo-complexation, cyanation and cyanomethylation. New J. Chem. 2020, 44, 17177–17197. 10.1039/D0NJ01996H. [DOI] [Google Scholar]
- Wang F.; Chen P.; Liu G. Copper catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 2018, 51, 2036–2046. 10.1021/acs.accounts.8b00265. [DOI] [PubMed] [Google Scholar]
- Schareina T.; Zapf A.; Cotté A.; Gotta M.; Beller M. A versatile protocol for copper-catalyzed cyanation of aryl and heteroaryl bromides with acetone cyanohydrin. Adv. Synth. Catal. 2011, 353, 777–780. 10.1002/adsc.201000200. [DOI] [Google Scholar]
- Mills L. R.; Graham J. M.; Patel P.; Rousseaux S. A. L. Ni-catalyzed reductive cyanation of aryl halides and phenol derivatives via transnitrilation. J. Am. Chem. Soc. 2019, 141, 19257–19262. 10.1021/jacs.9b11208. [DOI] [PubMed] [Google Scholar]
- Jagadeesh R. V.; Junge H.; Beller M. Green synthesis of nitriles using non-noble metal oxides-based nanocatalysts. Nat. Commun. 2014, 5, 4123. 10.1038/ncomms5123. [DOI] [PubMed] [Google Scholar]
- Malapit C. A.; Reeves J. T.; Busacca C. A.; Howell A. R.; Senanayake C. H. Rhodium-catalyzed transnitrilation of aryl boronic acids with dimethylmalononitrile. Angew. Chem., Int. Ed. 2016, 55, 326–330. 10.1002/anie.201508122. [DOI] [PubMed] [Google Scholar]
- Sundermeier M.; Zapf A.; Beller M. A convenient procedure for the palladium-catalyzed cyanation of aryl halides. Angew. Chem., Int. Ed. 2003, 42, 1661–1664. 10.1002/anie.200250778. [DOI] [PubMed] [Google Scholar]
- Sundermeier M.; Mutyala S.; Zapf A.; Spannenberg A.; Beller M. A convenient and efficient procedure for the palladium-catalyzed cyanation of aryl halides using trimethylsilylcyanide. J. Organomet. Chem. 2003, 684, 50–55. 10.1016/S0022-328X(03)00503-5. [DOI] [Google Scholar]
- Ding S.; Jiao N. Direct transformation of N,N-dimethylformamide to -CN: Pd-catalyzed cyanation of heteroarenes via C-H functionalization. J. Am. Chem. Soc. 2011, 133, 12374–12377. 10.1021/ja204063z. [DOI] [PubMed] [Google Scholar]
- Li J.; Zhang Z.; Wu L.; Zhang W.; Chen P.; Lin Z.; Liu G. Site-specific allylic C-H bond functionalization with a copper-bound N-centred radical. Nature 2019, 574, 516–521. 10.1038/s41586-019-1655-8. [DOI] [PubMed] [Google Scholar]
- Yu X.; Tang J.; Jin X.; Yamamoto Y.; Bao M. Manganese-catalyzed C–H cyanation of arenes with N-cyano-N-(4-methoxy)phenyl-p-toluenesulfonamide. Asian J. Org. Chem. 2018, 7, 550–553. 10.1002/ajoc.201700628. [DOI] [Google Scholar]
- Kim J.; Choi J.; Shin K.; Chang S. Copper-mediated sequential cyanation of aryl C-B and arene C-H bonds using ammonium iodide and DMF. J. Am. Chem. Soc. 2012, 134, 2528–2531. 10.1021/ja211389g. [DOI] [PubMed] [Google Scholar]
- Liu R. Y. M.; Bae M.; Buchwald S. L. Mechanistic Insight Facilitates discovery of a mild and efficient copper-catalyzed dehydration of primary amides to nitriles Using hydrosilanes. J. Am. Chem. Soc. 2018, 140, 1627–1631. 10.1021/jacs.8b00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X.; Yu P.; Morandi B. Catalytic reversible alkene-nitrile interconversion through controllable transfer hydrocyanation. Science 2016, 351, 832–836. 10.1126/science.aae0427. [DOI] [PubMed] [Google Scholar]
- Aresta M.Carbon dioxide as chemical feedstock. Wiley-VCH, Weinheim, 2010. [Google Scholar]
- Liu Q.; Wu L.; Jackstell R.; Beller M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. 10.1038/ncomms6933. [DOI] [PubMed] [Google Scholar]
- Moragas T.; Gaydou M.; Martin R. Nickel-catalyzed carboxylation of benzylic C-N bonds with CO2. Angew. Chem., Int. Ed. 2016, 55, 5053–5057. 10.1002/anie.201600697. [DOI] [PubMed] [Google Scholar]
- Liao L.-L.; Cao G.-M.; Ye J.-H.; Sun G.-Q.; Zhou W.-J.; Gui Y.-Y.; Yan S.-S.; Shen G.; Yu D.-G. Visible-light-driven external-reductant-free cross-electrophile couplings of tetraalkyl ammonium salts. J. Am. Chem. Soc. 2018, 140, 17338–17342. 10.1021/jacs.8b08792. [DOI] [PubMed] [Google Scholar]
- Reissmann S.; Hochleitner E.; Wang H.; Paschos A.; Lottspeich F.; Glass R. S.; Böck A. Taming of a poison: biosynthesis of the NiFe-hydrogenase cyanide ligands. Science 2003, 229, 1067–1070. 10.1126/science.1080972. [DOI] [PubMed] [Google Scholar]
- Wang H.; Dong Y.; Zheng C.; Sandoval C. A.; Wang X.; Makha M.; Li Y. Catalytic cyanation using CO2 and NH3. Chem 2018, 4, 2883–2893. 10.1016/j.chempr.2018.09.009. [DOI] [Google Scholar]
- Dong Y.; Yang P.; Zhao S.; Li Y. Reductive cyanation of organic chlorides using CO2 and NH3 via triphos-Ni(I) species. Nat. Commun. 2020, 11, 4096. 10.1038/s41467-020-17939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Kim H. J.; Chang S. Synthesis of aromatic nitriles using nonmetallic cyano-group sources. Angew. Chem., Int. Ed. 2012, 51, 11948–11959. 10.1002/anie.201206168. [DOI] [PubMed] [Google Scholar]
- Rodrigues R. M.; Thadathil D. A.; Ponmudi K.; George A.; Varghese A. Recent advances in electrochemical synthesisof nitriles: a sustainable approach. ChemistrySelect 2022, 7, e202200081 10.1002/slct.202200081. [DOI] [Google Scholar]
- Hu H.; Wu S.; Yan F.; Makha M.; Sun Y.; Du C.-X.; Li Y. Recent developments in electrosynthesis of nitriles and electrocatalytic cyanations. J. Energy Chem. 2022, 70, 542–575. 10.1016/j.jechem.2022.02.054. [DOI] [Google Scholar]
- Cheng H.-C.; Guo P.-H.; Ma J.-L.; Hu X.-Q. Directing group strategies in catalytic sp2 C-H cyanations: scope, mechanism and limitations. Catal. Sci. Technol. 2021, 11, 3308–3325. 10.1039/D1CY00241D. [DOI] [Google Scholar]
- Wu W.-B.; Yu J.-S.; Zhou J. Catalytic enantioselective cyanation: recent advances and perspectives. ACS Catal. 2020, 10, 7668–7690. 10.1021/acscatal.0c01918. [DOI] [Google Scholar]
- Nakao Y. Metal-mediated C-CN bond activation in organic synthesis. Chem. Rev. 2021, 121, 327–344. 10.1021/acs.chemrev.0c00301. [DOI] [PubMed] [Google Scholar]
- Patil R. D.; Gupta M. K. Methods of nitriles synthesis from amines through oxidative dehydrogenation. Adv. Synth. Catal. 2020, 362, 3987–4009. 10.1002/adsc.202000635. [DOI] [Google Scholar]
- Zeng X.-P.; Sun J.-C.; Liu C.; Ji C.-B.; Peng Y.-Y. Catalytic asymmetric cyanation reactions of aldehydes and ketones in total synthesis. Adv. Synth. Catal. 2019, 361, 3281–3305. 10.1002/adsc.201900015. [DOI] [Google Scholar]
- Tolman C. A. Steric and electronic effects in olefin hydrocyanation at Du Pont: A scientific and industrial success story. J. Chem. Educ. 1986, 63, 199–201. 10.1021/ed063p199. [DOI] [Google Scholar]
- Hock K. J.; Knorrscheidt A.; Hommelsheim R.; Ho J.; Weissenborn M. J.; Koenigs R. M. Tryptamine synthesis by iron porphyrin catalyzed C-H functionalization of indoles with diazoacetonitrile. Angew. Chem., Int. Ed. 2019, 58, 3630–3634. 10.1002/anie.201813631. [DOI] [PubMed] [Google Scholar]
- Takano S.; Nishimura T.; Ogasawawa K. Efficient synthesis of tryptamine. Heterocycles 1977, 6, 1167–1171. 10.3987/R-1977-08-1167. [DOI] [Google Scholar]
- Gao J.; Jiao M.; Ni J.; Yu R.; Cheng G.-J.; Fang X. Nickel-catalyzed migratory hydrocyanation of internal alkenes: unexpected diastereomeric-ligand-controlled regiodivergence. Angew. Chem., Int. Ed. 2021, 60, 1883–1890. 10.1002/anie.202011231. [DOI] [PubMed] [Google Scholar]
- Yu R.; Rajasekar S.; Fang X. Enantioselective nickel-catalyzed migratory hydrocyanation of nonconjugated dienes. Angew. Chem., Int. Ed. 2020, 59, 21436–21441. 10.1002/anie.202008854. [DOI] [PubMed] [Google Scholar]
- Gao J.; Ni J.; Yu R.; Cheng G.-J.; Fang X. Ni-catalyzed isomerization-hydrocyanation tandem reactions: access to linear nitriles from aliphatic internal olefins. Org. Lett. 2021, 23, 486–490. 10.1021/acs.orglett.0c04007. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Neumann H.; Beller M. Pd-catalyzed cyanation of (hetero)aryl halides by using biphosphine ligands. Chem. – Eur. J. 2018, 24, 67–70. 10.1002/chem.201704178. [DOI] [PubMed] [Google Scholar]
- Koschker P.; Kähny M.; Breit B. Enantioselective redox-neutral Rh-catalyzed coupling of terminal alkynes with carboxylic acids toward branched allylic esters. J. Am. Chem. Soc. 2015, 137, 3131–3137. 10.1021/jacs.5b01131. [DOI] [PubMed] [Google Scholar]
- Steib P.; Breit B. Enantioselective rhodium-catalyzed dimerization of omega-allenyl carboxylic acids: straightforward synthesis of C2-symmetric macrodiolides. Angew. Chem., Int. Ed. 2018, 57, 6572–6576. 10.1002/anie.201803369. [DOI] [PubMed] [Google Scholar]
- Ganss S.; Breit B. Enantioselective rhodium-catalyzed atom-economical macrolactonization. Angew. Chem., Int. Ed. 2016, 55, 9738–9742. 10.1002/anie.201604301. [DOI] [PubMed] [Google Scholar]
- Wu L.; Hartwig J. F. Mild palladium-catalyzed selective monoarylation of nitriles. J. Am. Chem. Soc. 2005, 127, 15824–15832. 10.1021/ja053027x. [DOI] [PubMed] [Google Scholar]
- Shang R.; Ji D.-S.; Chu L.; Fu Y.; Liu L. Synthesis of α-aryl nitriles through palladium-catalyzed decarboxylative coupling of cyanoacetate salts with aryl halides and triflates. Angew. Chem., Int. Ed. 2011, 50, 4470–4474. 10.1002/anie.201006763. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Wang F.; McCann S. D.; Wang D.; Chen P.; Stahl S. S.; Liu G. Enantioselective cyanation of benzylic C-H bonds via copper-catalyzed radical relay. Science 2016, 353, 1014–1018. 10.1126/science.aaf7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Xu L.; Jiang Y.; Ma D. Assembly of α-(hetero)aryl nitriles via copper-catalyzed coupling reactions with (hetero)aryl chlorides and bromides. Angew. Chem., Int. Ed. 2021, 60, 7082–7086. 10.1002/anie.202014638. [DOI] [PubMed] [Google Scholar]
- Wenkert E.; Alonso M. E.; Gottlieb H. E.; Sanchez E. L.; Pellicciari R.; Cogolli P. Reactions of ethyl diazoacetate with thianaphthene, indoles, and benzofuran. J. Org. Chem. 1977, 42, 3945–3949. 10.1021/jo00444a034. [DOI] [Google Scholar]
- Tsotinis A.; Vlachou M.; Papahatjis D. P.; Calogeropoulou T.; Nikas S. P.; Garratt P. J.; Piccio V.; Vonhoff S.; Davidson K.; Teh M.-T.; Sugden D. Mapping the melatonin receptor. 7. Subtype selective ligands based on β-substituted N-acyl-5-methoxytryptamines and β-substituted N-acyl-5-methoxy-1-methyltryptamines. J. Med. Chem. 2006, 49, 3509–3519. 10.1021/jm0512544. [DOI] [PubMed] [Google Scholar]
- Leow J. L.; Casey M.-W.; Casey P. J.; Go M. L.; Suresh K. G.. Small molecule inhibitors of isoprenylcysteine carboxyl methyltransferase with potential anticancer activity. 2014, U.S. Patent 8,742,100.
- Winter-Vann A. M.; Baron R. A.; Wong W.; dela Cruz J.; York J. D.; Gooden D. M.; Bergo M. O.; Young S. G.; Toone E. J.; Casey P. J. A small-molecule inhibitor of isoprenylcysteine carboxyl methyltransferase with antitumor activity in cancer cells. Proc. Natl. Acad. Sci. 2005, 102, 4336–4341. 10.1073/pnas.0408107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatalova-Sazepin C.; Wang Q.; Sammis G. M.; Zhu J. Copper-catalyzed intermolecular carboetherification of unactivated alkenes by alkyl nitriles and alcohols. Angew. Chem., Int. Ed. 2015, 54, 5443–5446. 10.1002/anie.201412357. [DOI] [PubMed] [Google Scholar]
- He R.-D.; Li C.-L.; Pan Q.-Q.; Guo P.; Liu X.-Y.; Shu X.-Z. Reductive coupling between C-N and C-O electrophiles. J. Am. Chem. Soc. 2019, 141, 12481–12486. 10.1021/jacs.9b05224. [DOI] [PubMed] [Google Scholar]
- Wang Z.-X.; Yang B. Chemical transformations of quaternary ammonium salts via C-N bond cleavage. Org. Biomol. Chem. 2020, 18, 1057–1072. 10.1039/C9OB02667C. [DOI] [PubMed] [Google Scholar]
- Huang W.; Cheng X. Hantzsch esters as multifunctional reagents in visible-light photoredox catalysis. Synlett 2017, 28, 148–158. 10.1055/s-0036-1588129. [DOI] [Google Scholar]
- Maity P.; Shacklady-McAtee D. M.; Yap G. P. A.; Sirianni E. R.; Watson M. P. Nickel-catalyzed cross couplings of benzylic ammonium salts and boronic acids: stereospecific formation of diarylethanes via C-N bond activation. J. Am. Chem. Soc. 2013, 135, 280–285. 10.1021/ja3089422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K.; Fukahori Y.; Inatomi T.; Tazaki S.; Yamada Y.; Koga Y.; Kanegawa S.; Nakamura T. Monomeric three-coordinate N-heterocyclic carbene nickel(I) complexes: synthesis, structures, and catalytic applications in cross-coupling reactions. Organometallics 2016, 35, 3281–3287. 10.1021/acs.organomet.6b00419. [DOI] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Becke A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; He J.; Song P.; Wang Y.; Zhu S. Ligand-enabled NiH-catalyzed migratory hydroamination: chain walking as a strategy for regiodivergent/regioconvergent remote sp3C-H amination. CCS Chem. 2020, 2, 2259–2268. 10.31635/ccschem.020.202000490. [DOI] [Google Scholar]
- Chen J.; Zhu S. Nickel-catalyzed multicomponent coupling: synthesis of α-chiral ketones by reductive hydrocarbonylation of alkenes. J. Am. Chem. Soc. 2021, 143, 14089–14096. 10.1021/jacs.1c07851. [DOI] [PubMed] [Google Scholar]
- Kochi T.; Kanno S.; Kakiuchi F. Nondissociative chain walking as a strategy in catalytic organic synthesis. Tetrahedron Lett. 2019, 60, 150938. 10.1016/j.tetlet.2019.07.029. [DOI] [Google Scholar]
- Zhou F.; Zhu J.; Zhang Y.; Zhu S. NiH-Catalyzed reductive relay hydroalkylation: a strategy for the remote C(sp3)-H alkylation of alkenes. Angew. Chem., Int. Ed. 2018, 57, 4058–4062. 10.1002/anie.201712731. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Addis D.; Das S.; Junge K.; Beller M. New catalytic properties of iron complexes: dehydration of amides to nitriles. Chem. Commun. 2009, 4883–4885. 10.1039/b910145d. [DOI] [PubMed] [Google Scholar]
- Enthaler S. Straightforward Uranium-Catalyzed Dehydration of Primary Amides to Nitriles. Chem. – Eur. J. 2011, 17, 9316–9319. 10.1002/chem.201101478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.