Sometimes what starts out as a side project can develop into an important aspect of the whole. For example, plant architecture is variable and hard to predict precisely—indeed, slight changes in environmental conditions or endogenous factors can transform a languishing stem into an exuberant leafy monster. This plasticity, which is key to adaptation and agronomic traits such as yield, is conferred by the meristems. In these specialized regions, stem cells produce new cells that make plant organs. The shoot apical meristem (SAM) produces almost all the above-ground tissue and this potential is amplified by the development of “side projects” in the form of axillary meristems (AMs) (Bennett and Leyser, 2006). AMs are located at the angle joining the cauline or rosette leaf petioles and the stem (the axils), where they form new branches upon activation. In the new work, Antoine Nicolas and colleagues (Nicolas et al., 2022) bring a detailed morphological description of the establishment of cauline AMs (CaAMs) in Arabidopsis thaliana and show the relevance of regulating CUP-SHAPED COTYLEDON (CUC) genes to this process.
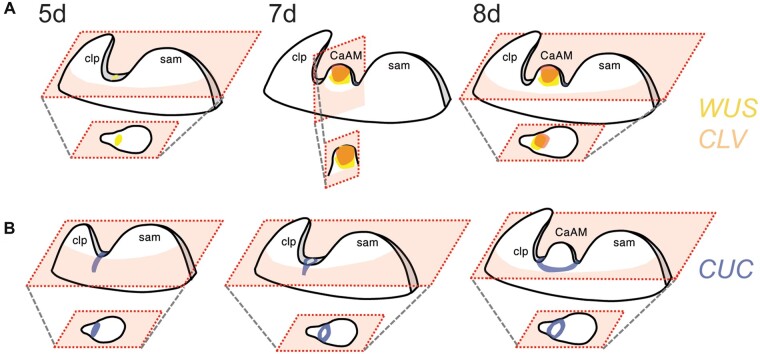
To induce AM formation, the authors transferred the plants to long-day conditions and were able to identify the boundary region that will form the AM 6 days later. It is composed of small narrow cells that will transform into a dome from which new leaf and flower primordia will emerge. These transitions are organized by a genetic choreography that Nicolas and colleagues explored using reporter lines. To establish the organizing center and stem cells in the bulge, WUSCHEL (WUS) is expressed first and CLAVATA3 (CLV3) follows. Both partially overlap at the beginning and finally settle in a distribution like the one observed in the SAM (Figure A). In contrast, CUC genes are expressed at the boundary region but progressively form an eye-shaped pattern that ultimately circles the meristematic dome and is excluded from the central region (Figure B).
Figure.
A, WUS and CLV3 expression in the CaAM, days after transition to long-day conditions. B, CUC expression in the CaAM. Adapted from Nicolas et al. (2022), Figure 1.
Wondering which factors could be relevant to guide CUC expression, the authors performed a yeast one-hybrid screening. This identified SUPPRESSOR OF DA1-1 7 (SOD7)/NGAL2, from the NGATHA-LIKE (NGAL) family of transcription factors, as capable of binding the promoter of CUC3. Single- and higher-order mutants of genes in the same family (such as DEVELOPMENT-RELATED PcG TARGET IN THE APEX4 (DPA4)/NGAL3) were grown to assess AM development. Instead of the eye-shaped pattern, CUC reporters spread to the meristem at the dome stage in dpa4-2 sod7-2 double mutants and return to the boundaries when leaf primordia emerge. In this mutant background, CaAM formation is delayed and asynchronous, with cell abnormalities at the dome stage that revert when CUC expression goes back to the standard at leaf stage. To further test whether CUC clearing in the central domain at the dome stage is relevant for CaAM development, cuc mutant alleles were introduced into the double mutant dpa4-2 sod7-2, rescuing the developmental defects. Furthermore, mutating binding motifs for DPA4 and SOD7 in the promoter of CUC3 induces ectopic CUC3 expression and reproduces the defects observed in dpa4-2 sod7-2.
In contrast to animals, where stem cells can migrate (Laird et al., 2008), in plants a new stem cell niche must be established from scratch post-embryonically. Regarding the stem cell niche, when there is ectopic expression of CUC genes in the double mutant background, CLV3 expression is delayed and WUS expression is distorted, reverting only when CUC expression returns to normal. Altogether, the data indicate that DPA4 and SOD7 redundantly repress CUC boundary genes in the central region to allow the establishment of the stem cell niche in the new meristem. Similar results were observed in other AM, hinting that these transcription factors may regulate the formation of new stem cell niches in other aerial meristems.
AMs are important in shaping plants and influence plant success as well as agricultural yield and other practical applications. CUC repression is therefore a very relevant side project.
References
- Bennett T, Leyser O. (2006) Something on the side: axillary meristems and plant development. Plant Mol Biol 60: 843–854 [DOI] [PubMed] [Google Scholar]
- Laird DJ, von Andrian UH, Wagers AJ (2008) Stem cell trafficking in tissue development, growth, and disease. Cell 132: 612–630 [DOI] [PubMed] [Google Scholar]
- Nicolas A, Maugarny-Calès A, Adroher B, Chelysheva L, Li Y, Burguet J, Bågman A-M, Smit ME, Brady SM, Li Y, et al. (2022) De novo stem cell establishment in meristems requires repression of organ boundary cell fate. Plant Cell https://doi.org/10.1093/plcell/koac269 [DOI] [PMC free article] [PubMed] [Google Scholar]