Abstract
Histone acetyltransferase (HAT)- and histone deacetylase (HDAC)-mediated histone acetylation and deacetylation regulate nucleosome dynamics and gene expression. HDACs are classified into different families, with HD-tuins or HDTs being specific to plants. HDTs show some sequence similarity to nucleoplasmins, the histone chaperones that aid in binding, storing, and loading H2A/H2B dimers to assemble nucleosomes. Here, we solved the crystal structure of the N-terminal domain (NTD) of all four HDTs (HDT1, HDT2, HDT3, and HDT4) from Arabidopsis (Arabidopsis thaliana). The NTDs form a nucleoplasmin fold, exist as pentamers in solution, and are resistant to protease treatment, high temperature, salt, and urea conditions. Structurally, HDTs do not form a decamer, unlike certain classical nucleoplasmins. The HDT-NTD requires an additional A2 acidic tract C-terminal to the nucleoplasmin domain for interaction with histone H3/H4 and H2A/H2B oligomers. We also report the in-solution structures of HDT2 pentamers in complex with histone oligomers. Our study provides a detailed structural and in vitro functional characterization of HDTs, revealing them to be nucleoplasmin family histone chaperones. The experimental confirmation that HDTs are nucleoplasmins may spark new interest in this enigmatic family of proteins.
Arabidopsis HDT family histone deacetylases are nucleoplasmins that can interact with histone H2A/H2B dimers and H3/H4 tetramers.
IN A NUTSHELL.
Background: The eukaryotic genome gets condensed by a group of basic proteins called histones; DNA is wrapped around histones to form chromatin. Chromatin dynamics is crucial for regulating all DNA-templated processes. Histone acetylation and histone deacetylation are histone modifications that control chromatin dynamics and gene expression. A plant-specific family of histone deacetylases (HDACs) called HDTs has been reported. Arabidopsis thaliana possesses four HDT paralogs, namely HDT1, HDT2, HDT3, and HDT4. Although the HDTs are classified as HDACs, they share no sequence similarity to other known HDACs, and there has been no direct evidence for HDTs performing deacetylation.
Question: The secondary structural analysis of HTDs showed a domain organization similar to the histone chaperone family of nucleoplasmins. We asked whether HDTs are indeed nucleoplasmins and, if so, what kind of histone interaction features they possess.
Findings: Crystal structures of HDT N-terminal domains revealed a nucleoplasmin fold with the classical pentameric organization. The pentameric nature of the proteins in solution was also confirmed. The nucleoplasmin core domain showed resistance to high temperature and salt and urea concentrations, similar to known nucleoplasmins. The core domain of HDTs could not interact with histone oligomers. However, upon including the acidic tract A2 C-terminal to the nucleoplasmin core domain, HDT2 pentamers interacted with H2A/H2B dimers and H3/H4 tetramers in a 1:1 stoichiometry. Although the putative histone deacetylation catalytic residues come in close proximity within the core of the pentamer, the recombinant HDTs showed no deacetylation activity.
Next steps: Through this work, we understand the nucleoplasmin function of HDTs. However, this study could not answer the question of whether the HDTs act as deacetylases. Additional co-factors could be needed for their deacetylation function. Understanding the exact mechanism of action of these multifunctional proteins will help us understand chromatin dynamics in plants.
Introduction
Nucleosomes, the fundamental units of the chromosome, require positioning and unwrapping for chromatin stability and dynamics, respectively (Lans et al., 2012). Nucleosome dynamics are regulated through replacement of canonical histones with variant histones and through covalent modifications in histones. These histone modifications lead to changes in the energy landscape of histone–DNA interactions and thus in DNA accessibility (Bannister and Kouzarides, 2011). The structure and function of chromatin are affected by covalent post-translational modifications (PTMs) of histones such as phosphorylation, acetylation, ubiquitylation, and sumoylation, especially during key processes like chromatin remodeling, DNA repair, DNA replication, and transcription (Rothbart and Strahl, 2014; Bowman and Poirier, 2015). Histone acetylation and deacetylation are catalyzed by histone acetyltransferases (HATs) and histone deacetylases (HDACs), respectively (Rothbart and Strahl, 2014). HDACs are enzymes whose primary function is to remove an acetyl group from an ɛ-N-acetyl-lysine residue on histones, causing an increase in their positive charge as well as DNA-binding ability (Gray and Ekström, 2001; Khochbin et al., 2001). Three families of HDACs are known in yeast and animals; these are called the Class I or Reduced Potassium Dependency 3 (RPD3) family, Class II or Histone deacetylase 1 (Hda1) family, and Class III or Silent information regulator 2 (Sirtuin/Sir2) family (Gray and Ekström, 2001; Khochbin et al., 2001). Separately, an unrelated family of HDACs has been reported from plants, known as the HD2 or HDAC tuin (HD-tuin/HDT) family (Brosch et al., 1996; Lusser et al., 1997; Aravind and Koonin, 1998; Hollender and Liu, 2008; Bourque et al., 2016).
HDT was first identified in maize (Zea mays) as a 400-kDa complex with three polypeptides, p39, p42, and p45, which were later found to be different phosphorylated forms of the same protein (Lusser et al., 1997; Aravind and Koonin, 1998; Hollender and Liu, 2008), but with no clear information regarding their actual function. They have been reported from Arabidopsis (Arabidopsis thaliana), maize, tobacco (Nicotiana tabacum), rice (Oryza sativa), barley (Hordeum vulgare), and longan (Dinocarpus longan; Zhou et al., 2004; Grandperret et al., 2014). Arabidopsis possesses four different HDTs, namely, HDT1, HDT2, HDT3, and HDT4 (Wu et al., 2000; Dangl et al., 2001; Hollender and Liu, 2008; Luo et al., 2012b). They all have an N-terminal domain (NTD) with a conserved “MEFWG” motif and show sequence similarity to certain insect FK506-binding proteins (FKBPs), a trypanosomal RNA-binding protein, and yeast Fpr3 and Fpr4 (Aravind and Koonin, 1998; Edlich-Muth et al., 2015; Bourque et al., 2016). The NTD of HDTs contains two conserved residues, His and Asp, important for their function as HDACs (Grandperret et al., 2014; Bourque et al., 2016). In addition, Arabidopsis HDTs possess a nuclear localization signal aiding localization into the nucleus (Supplemental Figure S1A). HDTs from a few other plants contain a nuclear export signal as well (Zhou et al., 2004; Grandperret et al., 2014; Bourque et al., 2016). All HDTs possess acidic tracts rich in Asp and Glu residues in the C-terminal half (Supplemental Figure S2). HDTs also contain multiple Ser and Thr residues, which are predicted to be phosphorylated by Casein Kinase 2α (Bourque et al., 2016). Furthermore, HDT2 and HDT4 contain a C2H2-type zinc-finger motif at their C-termini, speculated to interact with other protein factors (Ciftci-Yilmaz and Mittler, 2008; Kuang et al., 2012; Luo et al., 2012b). HDTs are expressed in the whole plant but with decreased expression in older tissues (Zhou et al., 2004). They are also involved in seed development and plant stress responses (Sridha and Wu, 2006; Colville et al., 2011). HDTs are of particular interest because they are present only in plants and have no sequence similarity with other known HDACs. Also, they control and participate in fundamental biological processes of plants like seed germination, flowering, plant development, and stress responses (Wu et al., 2000; Yano et al., 2013). The structure and function of the HDT family of HDACs are still poorly understood.
Nucleoplasmins are involved in sperm and somatic chromatin condensation (Prado et al., 2004; Bañuelos et al., 2007; Frehlick et al., 2007; Taneva et al., 2009) as histone chaperones aiding binding, storing, and loading H2A/H2B dimers to assemble nucleosomes (Philpott and Leno, 1992; Akey and Luger, 2003). Recent structural and biophysical characterization has revealed AtFKBP53 to be an FKBP-nucleoplasmin, the first experimental report of a plant nucleoplasmin (Singh et al., 2020). Sequence similarity and phylogenetic analysis of nucleoplasmins from different vertebrates, arthropods, plants, and yeast species have shown that HDTs form a separate and distinct clade of nucleoplasmins, along with FKBP-nucleoplasmins (Hollender and Liu, 2008; Edlich-Muth et al., 2015; Bourque et al., 2016; Kumar and Vasudevan, 2020). Also, the NTD of HDT2 has been reported to have a fold similar to the N-terminal nucleoplasmin domain of FKBP39 from Drosophila melanogaster (Edlich-Muth et al., 2015). Sequence alignment of HDTs with other structurally characterized nucleoplasmins revealed significant similarity, although identity appeared low (∼20%) between the sequences (Supplemental Figure S3). Phylogenetic analysis revealed that HDTs and nucleoplasmins have descended from different ancestors. Moreover, HDTs cluster together with FKBP nucleoplasmins, whereas the NPM and NLP families form separate clusters (Figure 1). HDTs are paralogs that have evolved from a gene duplication event (Bourque et al., 2016). Herein, we structurally characterized the NTD of Arabidopsis HDTs, namely HDT1, HDT2, HDT3, and HDT4, showing that they adopt a nucleoplasmin fold. HDTs form a stable pentamer in solution and show attributes characteristic of classical nucleoplasmins, such as protease resistance, temperature resistance, and stability toward high salt and urea conditions. Also, they interact with histone oligomers, wherein the acidic tract A2 plays a crucial role in binding. The detailed structural and in vitro functional characterization of HDTs reveals them to be nucleoplasmin family histone chaperones.
Figure 1.
Molecular phylogenetic analysis of nucleoplasmins. The phylogenetic tree was constructed for all structurally characterized nucleoplasmins and AtHDTs, illustrating the evolutionary relationship between these proteins. The bootstrap values from 1,000 replicates are given for the nodes. The species identities of the source organisms are represented as prefixes on the protein names—Hs (Homo sapiens), Mm (Mus musculus), Xl (Xenopus laevis), Dm (Drosophila melanogaster), and At (Arabidopsis thaliana).
Results
HDT-NTDs possess a nucleoplasmin fold
The crystal structures of the NTD of Arabidopsis HDT1, HDT2, HDT3, and HDT4 packed five polypeptide chains in the asymmetric unit, indicative of a nucleoplasmin fold that assembles as a pentamer. The crystal data collection and structure refinement statistics are provided (Supplemental Table S1). All four HDT-NTDs form a doughnut-shaped homo-pentameric structure with the five-fold rotational symmetry axis running roughly parallel to the β-sheets of the monomers (Figure 2, A–D; Supplemental Figure S1B). The face where the N- and C-termini of each monomer go in and come out in a pentamer is termed the proximal face, and the opposite face is termed the distal face (Supplemental Figure S1, B–E). A structural similarity search using the PDBeFold server (Krissinel and Henrick, 2004) revealed that the NTD of all four HDTs displays a nucleoplasmin-fold, similar to known animal nucleoplasmin and FKBP nucleoplasmin structures (Dutta et al., 2001; Namboodiri et al., 2003; Platonova et al., 2011; Edlich-Muth et al., 2015; Singh et al., 2020). The monomer–monomer interfaces of the HDT pentamers are stabilized by many hydrogen bonds, along with van der Waals contacts and electrostatic interactions. The interface area between the two monomers ranges from 759 to 930 Å2 (Supplemental Table S2). In each monomer of the HDTs, several aromatic residues are conserved and buried inside to give a compact hydrophobic core.
Figure 2.
Structural features of HDT-NTDs. A–D, Cartoon representation of the four pentamers revealing typical nucleoplasmin-like organization; HDT1, HDT2, HDT3, and HDT4. E–H, Cartoon representation of the monomeric subunits. The monomers of all four HDTs reveal the characteristic nucleoplasmin-fold with a triangular cross-section. The β-hairpin formed by the strands β4 and β5 is highlighted for all the structures. The acidic residues in the region corresponding to the A1 acidic tract of other nucleoplasmins are shown in the stick model and labelled. Unlike the other HDTs, HDT4 has an extra acidic stretch in the loop region of its β-hairpin, also shown in the stick model, and a 310 helix on the distal face shown in red. I–L, Rigid body SAXS in-solution models generated in P5 symmetry with GASBOR. The crystal structure and SAXS envelope images were prepared using PyMOL and UCSF Chimera, respectively.
The HDT-NTD monomers have a triangular cross-section, composed of eight antiparallel β-strands, forming a β-sandwich fold with four-stranded β-sheets in the opposing halves arranged in an antiparallel fashion (Figure 2, E–H; Supplemental Figure S1B); wherein β1, β3, β8, and β6 form an antiparallel β-sheet in one half, and β2, β7, β4, and β5 forms the antiparallel β-sheet in the other half in such a way that a hydrophobic core lies within the opposing faces of each monomer (Supplemental Figure S4). The β-strands of one half get connected to β-strands of the other half, except for β4 and β5, giving it a jelly roll topology. The β4–β5 region forms a β-hairpin structure that is considered important for histone interaction and contributes significantly to the stability of a nucleoplasmin pentamer (Dutta et al., 2001; Namboodiri et al., 2003; Platonova et al., 2011). The hydrogen-bonding pattern between the adjacent antiparallel β-strands that stabilizes the β-sheets of the two halves gets disturbed by β-bulges in β3 and β5 strands and a break in the strand β6 (Supplemental Figure S5). Additionally, a wedge-shaped structure is formed by the monomers of all four structures due to the asymmetric distribution of the bulky aromatic residues at β1 and β8, forming an aromatic corner (Supplemental Figure S6).
The solved crystal structures and analytical gel-filtration profiles (Supplemental Figure S7A) revealed the HDT-NTDs to be predominantly pentameric. The same was further confirmed by sedimentation velocity analytical ultra-centrifugation (SV-AUC) experiments. A single sedimentation boundary and continuous c(s) distribution in the sedimentation velocity experiments revealed that the NTDs of HDT1, HDT2, HDT3, and HDT4 exist as pentamers in solution with sedimentation coefficient values of 3.9S, 4.0S, 3.9S, and 4.1S, and with estimated molecular masses of 57.6 kDa, 56.8 kDa, 62.5 kDa, and 61.7 kDa, respectively (Supplemental Figure S7, B–F and Supplemental Table S3).
Small-angle X-ray scattering (SAXS) data also suggested a pentameric organization for all four HDTs (Supplemental Figure S8). The dimensionless Kratky plots and Guinier analysis showed the proteins to be properly folded and monodispersed (Supplemental Figure S8, B and C). The pairwise distance distribution function P(r) indicated a compact globular shape for all four NTD pentamers (Supplemental Figure S8D). SAXS parameters are provided in Supplemental Table S4. Structural envelopes of pentamers based on P(r) data were generated in P1 and P5 symmetry (Figure 2, I–L; Supplemental Figure S9, A–D). An acceptable χ2 value was obtained from Crysol for all four pentamers (2.89, 0.92, 0.65, and 0.41 for HDT1, HDT2, HDT3, and HDT4, respectively), indicating the crystal structure to be in agreement with the in-solution structure (Supplemental Figure S9, E–H).
HDT-NTDs show stability features similar to known nucleoplasmins
Nucleoplasmin family proteins form a stable pentamer, wherein the inside of the core is rich in non-polar and hydrophobic residues. This feature has been reported for the AtFKBP53 nucleoplasmin domain, which retains its pentameric organization at high temperatures, in high salt, and in the presence of high concentrations of a denaturing agent like urea (Singh et al., 2020). Furthermore, the sequence of the NTDs of AtHDT1, AtHDT2, AtHDT3, and AtHDT4, when compared to AtFKBP53 and other nucleoplasmins (Supplemental Figure S3), revealed a similar distribution of non-polar and hydrophobic residues.
The thermal stability of HDTs was explored by subjecting the heat-treated protein samples to analytical gel-filtration chromatography. The elution profiles revealed the existence of HDT1, HDT2, and HDT3-NTDs predominantly as pentamers with minimal reduction in peak heights, even after subjecting to high temperatures (Figure 3, A–C). However, HDT4 appeared less thermostable, as indicated by a considerable reduction in the peak height with increasing temperature (Figure 3D).
Figure 3.
Stability attributes of HDT pentamers. A–D, Analytical gel-filtration chromatogram of the NTDs of HDT1, HDT2, HDT3, and HDT4, respectively, after subjecting to different temperatures: 20°C, 40°C, 60°C, 80°C. E, CD-based thermal denaturation analysis of HDT-NTDs. The plots represent the fraction unfolded for each of the NTDs against the increase in temperature. Solid lines represent the fit, whereas the dotted lines show the thermal spectra for the NTDs of HDT1, HDT2, HDT3, and HDT4. F, 18% SDS–PAGE image of the HDT-NTDs before and after limited proteolysis with α-chymotrypsin. The HDT-NTDs showed no proteolytic digestion, whereas BSA, used as a control, got digested by α-chymotrypsin.
Secondary structure analysis using circular dichroism (CD) spectroscopy revealed the HDTs to be β-sheet-rich proteins (Supplemental Figure S10). Thermal ramping using CD revealed HDT2, HDT3, and HDT4-NTDs to exhibit a single transition melt curve with melting temperatures (Tm) in the range of 56.9°C to 61.8°C (Figure 3E; Supplemental Table S5). HDT4-NTD had the lowest Tm of 56.9°C. However, a Tm could not be calculated for the HDT1-NTD pentamer due to resistance to temperature-induced changes in its pentameric organization, possibly due to strong intra- and inter-subunit hydrophobic interactions and a closed central pore.
Nucleoplasmin pentamers stabilized by hydrophobic interactions are known to withstand high concentrations of salt and denaturing agents (Taneva et al., 2008). Therefore, to probe their chemical stability, HDT-NTDs were subjected to analytical gel-filtration chromatography in the presence of increasing concentrations of sodium chloride and urea, separately. From the elution profiles, it was evident that all four HDT-NTDs are stable up to 2 M sodium chloride (Supplemental Figure S11, A–D) and 2 M urea (Supplemental Figure S12, A–D).
Brief digestion with α-chymotrypsin showed no major differences between digested and undigested HDT samples in sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis (Figure 3F), suggesting that the pentameric core domain of all four HDTs is highly resistant to protease digestion, a characteristic feature of nucleoplasmins. Though the primary sequence analysis of the HDT-NTDs revealed multiple potential cleavage sites for α-chymotrypsin, they form part of the hydrophobic core and are hence protected from protease digestion (Supplemental Table S6 and Supplemental Figure S13).
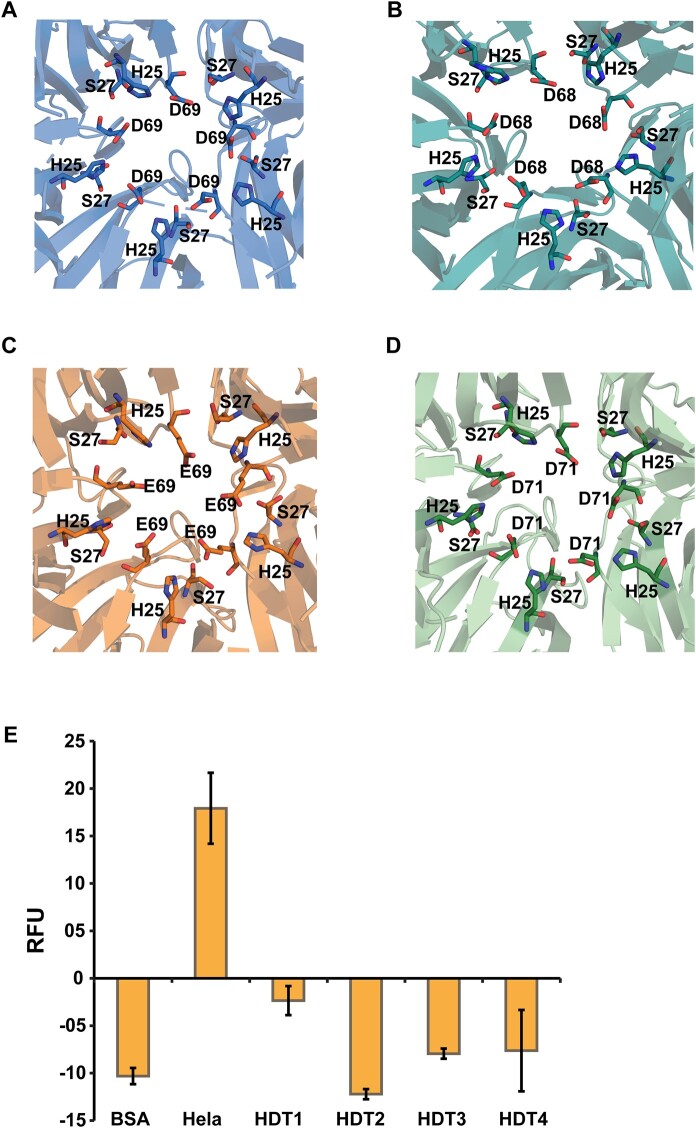
HDTs possess inactive deacetylase catalytic triads
A conserved His residue at position 25 (His25) and an Asp/Glu residue at position 68, 69, or 71 are common to all four HDTs (Supplemental Figure S2) and have been implicated in their HDAC activity. The structures reveal that the His and Asp residues are arranged alternatively in the inner core of the pentamer. Further, in addition to the conserved His and Asp residues, a Ser residue (Ser27) is also conserved among the HDTs (Supplemental Figure S2). Subsequent analysis revealed that these three residues form a triad, wherein the Ser residue is placed between the His and Asp residues on each of the subunits of the pentamer (Figure 4, A–D). Thus, five such triads are arranged in a ring-like pattern at the core of the pentamer. The central core of HDT1 is closed behind the triad by Phe67. A similar triad organization of His28–Ser30–Asp69 residues could also be observed in the structure of the DmFKBP39 nucleoplasmin, but not in other nucleoplasmins (Supplemental Figure S14, A–D). However, in vitro deacetylation assays with recombinant HDT-NTDs used for the structural work showed them to be inactive as HDACs (Figure 4E). The orientation and position of the three residues play a crucial role in deciding whether they form a catalytically active triad. A comprehensive study on catalytic triads has reported that certain criteria must be fulfilled for them to be functional (Gupta et al., 2010). The hydrogen bond angles between histidine and serine did not fall in the allowed range (Supplemental Table S7), suggesting it to be the possible reason for HDTs becoming inactive as HDACs.
Figure 4.
Putative catalytic triads and in vitro deacetylation activity assay of HDTs. A–D, Residues forming the putative catalytic triads are represented in the stick model for HDT1, HDT2, HDT3, and HDT4, respectively. E, In vitro deacetylation assay plot for recombinant HDT-NTDs. The relative fluorescence unit (RFU) values show that the HDT-NTDs do not possess deacetylation activity. The bars represent means ± sd values for three independent experiments.
HDT-NTDs show subtle structural differences
The superposition of the four HDT monomer structures revealed that HDT1 aligns with HDT2, HDT3, and HDT4, with root mean sqaure deviation (RMSD) values of 0.42 Å (for 73 Cα atoms), 0.56 Å (for 75 Cα atoms), and 0.75 Å (for 65 Cα atoms), respectively (Figure 5A); whereas HDT4 gave an RMSD value of 0.58 Å when aligned with HDT2 and HDT3 (for 70 and 68 Cα atoms, respectively). Unlike other HDTs, HDT4 possesses a 310 helix in its distal face on loop L3 connecting β3 and β4. Among the HDTs, L3 is the longest in HDT4 and the shortest in HDT2 (Figure 5A). The β-hairpin loop, L4 of HDT4, is offset out of the plane from that of other HDTs by ∼4.5 Å (Figure 5A). Moreover, L4 is the longest in HDT4 and possesses three acidic residues, a feature not seen in HDT1, HDT2, and HDT3. The HDT4 β-hairpin, with its acidic loop L4, gets packed onto the lateral face and imparts an acidic charge to the surface, similar to Xenopus laevis nucleoplasmin (Dutta et al., 2001). The relatively poor electron density in the L4 loop region of HDT4 suggests its flexibility, which could be of significance for the histone interaction properties of its pentamer. Though HDTs are concatenated, HDT4 appears to be divergent from the other three HDTs, as observed from the phylogenetic analysis as well (Supplemental Figure S1).
Figure 5.
Structure comparison between HDT-NTDs and other nucleoplasmins. A, Structural alignment of HDT-NTDs; HDT1, HDT2, HDT3, and HDT4. B–E, Structural alignment of DmFKBP39 and AtFKBP53 with HDT1, HDT2, HDT3, and HDT4.
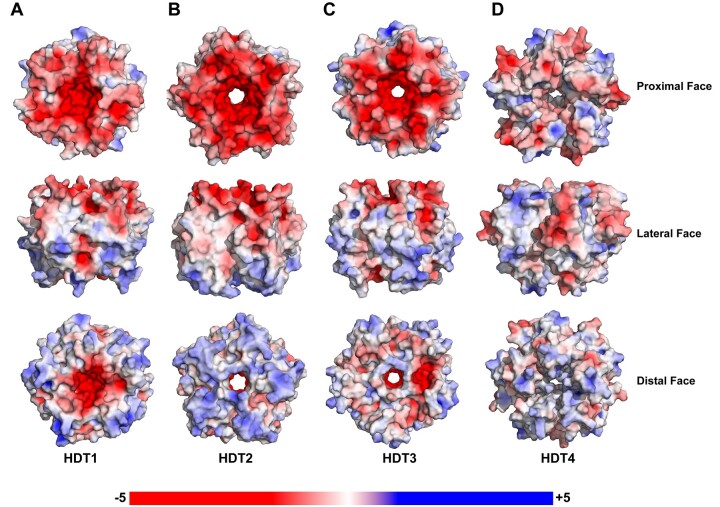
The NTD of nucleoplasmins usually has an acidic tract (A1) in its loop L2. However, in the case of HDTs, loop L2 contains fewer acidic residues. HDT1 has two acidic residues (Glu19 and Glu20), HDT2 has three acidic residues (Glu19, Glu20, and Asp21), whereas HDT3 and HDT4 have a single acidic residue Asp21 and Asp20, respectively, in loop L2 (Figure 2, E–H). The presence of three consecutive acidic residues in loop L2 provides a stronger negative charge to the proximal face of the HDT2-NTD pentamer, followed by the proximal face of HDT1 (Figure 6, A and B), which has two acidic residues in each of its monomers. The proximal face of HDT3 shows a greater negative charge distribution than the proximal face of HDT4 (Figure 6, C and D) due to the presence of acidic residues closer to the center of the pentamer. A strong negative charge on the proximal faces of HDT1 and HDT2 might suggest stronger histone interactions. Moreover, the entrance of the central core of the pentamers on the proximal face has conserved Asp/Glu residues giving the region an overall negative charge (Figure 6, A–D). The asymmetric charge distribution between the proximal and distal faces is the most prominent for HDT2, followed by HDT4. The central core of HDT1 is closed because the side chains of Phe67 in the monomers are flipped into the core (Figure 6A). The other HDTs do not have a Phe residue at this position and have their central core open.
Figure 6.
Surface electrostatic features of HDT-NTD pentamers. A–D, The top, middle, and lower panels show the surface charge features on the proximal, lateral, and distal faces, respectively, for the HDT-NTD pentamers–HDT1, HDT2, HDT3, and HDT4. The electrostatic surface charge distributions of HDT-NTD pentamers are represented with a linear ramp of –5 kT/e to +5 kT/e.
HDT-NTD structures compare well with other nucleoplasmins
HDT-NTD structures were compared with the nucleoplasmin domain of XlNP, DmNLP, AtFKBP53, and DmFKBP39, the closest structural homologs identified by PDBeFold (Krissinel and Henrick, 2004; Supplemental Table S8). The structural alignment of the monomers of these nucleoplasmins with the HDT-NTDs revealed that the overall topology is well conserved (Figure 5, B–E; Supplemental Figure S15, A–D). However, although the β-strands in the monomer of all nucleoplasmins follow the same topology, AtFKBP53 and DmFKBP39 nucleoplasmins appear wider than HDT-NTDs. Moreover, differences were observed in the length of loop L3, the β-hairpin, and the overall surface charge distribution for the pentamers.
The acidic tract A1 in XlNP loop L2 is longer, more flexible, and more disordered when compared to the corresponding short stretches in the HDTs. Loop L2 of AtFKBP53 contains a 310-helix, due to which it shows an offset of ∼6.5 Å above the plane compared to the HDTs (Figure 5, B–E). Interestingly, the 310-helix in loop L2 of AtFKBP53 is present at the same position where an acidic tract is present in other nucleoplasmins. The five-residue long A1-tract in AtFKBP53 is shifted to loop L6 on the proximal face making it extremely acidic compared to other nucleoplasmins. Similar to the AtFKBP53 nucleoplasmin domain, the proximal face of HDT2-NTD is also highly acidic in charge (Figure 6B), and asymmetric distribution of charges could be observed on the proximal and distal faces.
Unlike that of XlNp (Dutta et al., 2001) and the human nucleoplasmin NPM2 (Platonova et al., 2011), the crystal packing of HDT-NTDs did not show a decamer formation (Supplemental Figure S16, A–D). Analytical gel-filtration, SAXS, and SV-AUC experiments also identified only pentamers.
HDT with extended acidic tract interacts with histone oligomers and forms stable complexes
As the HDT-NTDs revealed a nucleoplasmin fold, their ability to interact with histone oligomers was explored. However, the HDT-NTDs did not bind to histone H2A/H2B or H3/H4 oligomers in our interaction studies. Therefore, to explore the possibility that the acidic tract A2 C-terminal to the nucleoplasmin core could be important for histone interaction, a longer stretch of HDT2-NTD, covering the A2 tract and spanning residues 1–120 (hereafter referred to as HDT2-A2), was used. The pentameric organization of HDT2-A2 was confirmed by analytical gel-filtration chromatography (Figure 7, A and B), SV-AUC (Figure 7C; Supplemental Figure S17 and Supplemental Table S3), and SAXS (Figure 8D; Supplemental Figure S18, D and E, and Supplemental Table S4).
Figure 7.
Interaction of HDTs with histone oligomers. A, Analytical gel-filtration chromatography profile for HDT2-A2, H2A/H2B, H3/H4, HDT2-A2–H2A/H2B, and HDT2-A2–H3/H4. The early eluting peaks with the mixtures of HDT2-A2 and the histone oligomers indicate complex formation. B, The peak fractions from analytical gel-filtration experiments analyzed on 18% SDS–PAGE, showing the presence of the respective histones along with HDT2-A2, confirm complex formation. C, SV-AUC analysis for H2A/H2B, H3/H4, HDT2-A2, HDT2-A2–H2A/H2B, and HDT2-A2–H3/H4. D and E, 18% SDS–PAGE images for pull-down experiments of HDT2-A2 with H2A/H2B and HDT2-A2 with H3/H4, respectively, under increasing salt concentrations. H2A/H2B interaction is more sensitive to increasing salt concentrations, suggesting a predominantly electrostatic interaction.
Figure 8.
Interaction of HDTs with histone oligomers analyzed by SV-AUC, ITC, and SAXS. A and B, ITC profiles for HDT2-A2 titrated against H2A/H2B and H3/H4, respectively. The left parts show the heat change during titration, and the right panels show the corresponding isotherms fitted with the “one set of binding site” model. C, Pairwise distance distribution of P(r) function for HDT2-A2, HDT2-A2 in complex with H2A/H2B, and HDT2-A2 in complex with H3/H4. D, SAXS envelope obtained for HDT2-A2 in P5 symmetry. E, SAXS envelope obtained for the HDT2-A2–H2A/H2B complex reveals an extended conformation with the H2A/H2B dimer–HDT2-A2 complex. F, SAXS envelope obtained for the HDT2-A2–H3/H4 complex reveals a compact structure. SAXS envelope images were prepared using UCSF Chimera.
Analytical gel-filtration chromatography was carried out for HDT2-A2 individually with H3/H4 and H2A/H2B. The shift in the position of the elution peak and subsequent analysis of the peak fractions by SDS–PAGE revealed that HDT2-A2 forms a complex with H3/H4 and H2A/H2B (Figure 7, A and B). SV-AUC experiments with the oligomers of H3/H4, H2A/H2B, and HDT2-A2 gave sedimentation coefficient values of 2.8S, 1.9S, and 3.7S, corresponding to molecular masses of 51.6 kDa (H3/H4 tetramer), 27.7 kDa (H2A/H2B dimer), and 73.9 kDa (HDT-A2 pentamer), respectively (Figure 7C). A sedimentation coefficient value of 4.8S and an estimated molecular mass of 93.8 kDa for HDT2-A2 in complex with the H2A/H2B dimer revealed a 1:1 ratio complex formation between the HDT pentamer and a histone dimer. The SV-AUC experiment for the complex of HDT2-A2 with H3/H4 yielded two peaks, with the major one corresponding to a sedimentation coefficient value of 8.9S and a molecular mass of 139 kDa, which is slightly more than the expected values for a pentamer in complex with a H3/H4 tetramer. Overall, the H3/H4 interaction with the HDT2-A2 pentamer appears weak.
The binding stoichiometry and thermodynamic parameters of the interaction were determined using isothermal titration calorimetry (ITC). As expected, HDT2-A2 interacted with both H3/H4 and H2A/H2B (Figure 8, A and B). The titrations showed that the binding of HDT2-A2 with the H3/H4 tetramer and the H2A/H2B dimer are both endothermic, with a 1:1 stoichiometry for the pentamer to the histone oligomers. The Kd values suggested that HDT2-A2 interacts with comparable, but slightly higher affinity for H2A/H2B (Kd = 0.173 µM) than for H3/H4 (Kd = 0.334 µM), further corroborating the SV-AUC results. The interaction between HDT and histone oligomers is predominantly electrostatic in nature, although hydrogen bonds and hydrophobic interactions also play a role in the interaction with H3/H4, as evident from the salt gradient pull-down assays (Figure 7, D and E).
Further, SAXS experiments of HDT2-A2 and its complex with histone oligomers were conducted to endorse our results (Supplemental Figure S18, A–C). SAXS data showed that a complex of HDT2-A2 with H2A/H2B and H3/H4 has higher Dmax values than that of HDT2-A2 alone; 22.6 nm and 14.0 nm versus 8.9 nm, respectively (Figure 8C). Moreover, the molecular mass estimation from the Porod volume corresponds to the 1:1 stoichiometry for the H2A/H2B dimer and H3/H4 tetramer in complex with HDT2-A2, individually (Supplemental Table S3). Finally, the ab initio model generated using DAMMIF for HDT2-A2 with H2A/H2B adopted an extended conformation with a radius of gyration (Rg) value of 5.1 nm, possibly suggesting an extended shape of the complex (Figure 8E; Supplemental Figure S18F), whereas the H3/H4 complex appeared more globular, as evident from an Rg value of 4.4 nm (Figure 8F; Supplemental Figure S18G).
The nucleoplasmin domain of AtFKBP53 has been reported to interact with preformed nucleosomes (Singh et al., 2020). However, such an interaction was not observed for HDT2-A2 (Supplemental Figure S19) or any of the HDT-NTDs.
Discussion
The HDT family is a special and dissimilar family of HDACs unique to plants (Dangl et al., 2001). Albeit, HDTs show moderate sequence similarity with the N-terminal nucleoplasmin domains of the nuclear peptidyl-prolyl cis–trans isomerases (PPIases) reported from insects, yeasts, and plants (Edlich-Muth et al., 2015). The similarity suggests that HDTs could perform functions similar to nucleoplasmins apart from being HDACs. We characterized these HDTs to gain insight into their structure and possible functions. The crystal structures revealed all four HDTs to be pentamers, with their NTD taking up the classical nucleoplasmin fold despite considerable sequence differences. In addition, biophysical studies revealed that HDT-NTDs do not form decamers like some other well-characterized nucleoplasmins. The AKDE and GSGP (or their analogous) motifs in the loop region on the distal face of the nucleoplasmins XlNP (Dutta et al., 2001), HsNPM2 (Platonova et al., 2011), and XlNO38 (Namboodiri et al., 2004) have been reported to aid their decamer formation. However, these motifs are absent in HDTs. To check whether the incorporation of these motifs can allow decamer formation of an HDT, we carried out loop exchange experiments wherein we replaced the corresponding residues in HTD2 NTD with AKDE and GSGP. Surprisingly, including these motifs in HDT2 did not allow decamerization. This result suggests that other factors such as the orientation of the loops and the charge distribution pattern on the distal face might also play a crucial role in decamer formation.
HDT1, HDT2, and HDT3 show thermal stability features similar to other nucleoplasmins. An in vivo analysis identified HDT1, HDT2, and HDT3, but not HDT4, as thermostable (Volkening et al., 2019), supporting our in vitro thermal stability data, which suggests that HDT1, HDT2, and HDT3 might be functional under stress conditions (Luo et al., 2012a; Buszewicz et al., 2016), whereas HDT4 may be functional otherwise. Although HDTs reveal differences in thermal stability, their core NTDs are protease resistant.
The NTD of HDT1, HDT2, and HDT3 stay as a stable pentamer irrespective of the salt concentrations. However, HDT4-NTD seems to require higher salt concentrations to stabilize the pentamer, as evident from the analytical gel-filtration peak getting narrower with increased salt concentration. The physiological relevance of this phenomenon is unclear but suggests that HDT4 may stay as lower oligomers or a monomer in plants. Furthermore, the crystal structure of the HDT4 pentamer shows the proximal face to be relatively broad compared to other HDTs. This feature is due to the acidic β-hairpin packed against the lateral face, possibly destabilizing the pentamer, unlike the other nucleoplasmins where the β-hairpin aids the formation of a stable pentamer (Dutta et al., 2001). HDT1 and HDT4 have a Lys-rich loop L3 on their distal face. L3 is disordered in HDT1; however, the presence of a 310 helix stabilizes this loop in HDT4. The AtFKBP53 NTD also possesses a 310 helix, but on the proximal face instead (Singh et al., 2020).
Two alternative models have been suggested for the interaction of nucleoplasmins with histones. The first is based on crystal structures and Förster resonance energy transfer studies, which propose that a nucleoplasmin decamer interacts with a histone octamer through its lateral face (Dutta et al., 2001; Namboodiri et al., 2003; Platonova et al., 2011). However, decamer formation has never been observed for isolated egg nucleoplasmin. The second model suggests a nucleoplasmin pentamer binding to H2A/H2B dimer and H3/H4 tetramer (Arnan et al., 2003). A previous report showed that HDT interacts with histone peptides while exploring the HDAC function (Lee and Cho, 2016). The histone interaction property of the various histone chaperones, including nucleoplasmins, is mediated primarily through long acidic tracts (Fernández-Rivero et al., 2016). The NTD of HDT1 and HDT2 contains a two-residue-long acidic tract A1 in its core for each monomer. However, HDT3 and HDT4 lack such an acidic tract.
We report for the first time that an HDT pentamer can interact individually with the H2A/H2B dimer and H3/H4 tetramer under physiological ionic strength conditions. In fact, it is a longer version of the pentameric HDT2-NTD (HDT2-A2), including the acidic tract A2, that interacted with the H2A/H2B dimer and H3/H4 tetramer. Thus, the addition of 20 residues covering the A2 tract helped increase the affinity of HDT2-NTD for histone oligomers. The SV-AUC and SAXS experiments ruled out the possibility of decamerization of HDT upon histone interaction. The HDT2-A2 pentamer interacted with histone oligomers with µM affinity in a 1:1 ratio for the H2A/H2B dimer and H3/H4 tetramer. The hydrodynamic properties showed that HDT2-A2 adopted an extended (nonglobular) conformation upon forming a complex with the H2A/H2B dimer. In contrast, the H3/H4 tetramer complex indicated a globular conformation. Though we could not build the in-solution model of the complexes from SAXS data, we could predict the mode and site of interaction of histone oligomers based on the hydrodynamic and thermodynamic parameters obtained from the AUC and ITC experiments, respectively. The peak fraction of the complex of HDT2-A2 with the H3/H4 tetramer from gel-filtration chromatography revealed a mixture of two different molecular masses in SV-AUC that was not obvious from the gel-filtration data. Thus, the complex of HDT2-A2 with H3/H4 could be weak and dissociate easily. However, the major fraction in SV-AUC for the H3/H4 tetramer complex gave a molecular mass of 139 kDa, close to the expected size of the HDT2-A2 complex with an H3/H4 tetramer in a 1:1 ratio, which is consistent with the SAXS results. In contrast, a frictional ratio of 4.49 for the HDT2-A2 and H2A/H2B dimer complex suggests an elongated yet compact conformation, again indicating a 1:1 stoichiometry. Our data suggest that HDT2-A2 interacts with the H2A/H2B dimer through charge-based interactions, with major contributions from the A2 tract. Though stabilized by the acidic tracts, the H3/H4 interaction seems to have a significant contribution coming from hydrophobic interactions as well. Structural characterization of the complexes by cryo-electron microscopy could be a way forward to understanding the exact mode of complex formation.
Our work confirms a major role for the HDT acidic tract A2 in the histone interaction. As reported earlier for other nucleoplasmins, both the core domain and the acidic tract are involved in the histone interaction (Warren et al., 2017). Fluorimetry revealed that the recombinant X. laevis nucleoplasmin binds with an affinity of 0.011 µM for the H2A/H2B dimer and 16 µM for the H3/H4 tetramer (Fernández-Rivero et al., 2016). ITC revealed that HDT2-A2 has a higher binding affinity for the H3/H4 tetramer than that of the recombinant X. laevis nucleoplasmin core, probably because of the acidic tract A2. Additionally, compared to the previously characterized plant nucleoplasmin AtFKBP53 (Singh et al., 2020), HDT2-A2 shows a higher affinity for histone oligomers.
We show that the NTD of HDTs is important for their oligomerization and histone interaction, which gets enhanced with the addition of the acidic tract, and we also confirmed that the C-terminal His-tag of the recombinant proteins does not interfere with oligomerization or histone binding (Supplemental Figure S20, A–D). The presence of multiple nucleoplasmins in Arabidopsis may suggest a redundant function. It is also possible that the different nucleoplasmins function during different stages of development or in different tissues. However, HDT2-A2 did not interact with preformed nucleosomes, unlike AtFKBP53 (Singh et al., 2020), signifying a difference in the function between the FKBP and HDT family of plant nucleoplasmins.
Because the HDTs were classified as HDACs, we analyzed the deacetylation activity of the recombinant HDTs, which confirmed that they are not functional as HDACs. To overrule the possibility of the C-terminal His-tag of the recombinant protein to be interfering with HDAC activity, we carried out an HDAC assay for HDT2-NTD and HDT2-A2 without His-tag, and the proteins without His-tag also did not show any HDAC activity (Supplemental Figure S20E). No in vitro studies have shown HDTs to be active as HDACs. In vivo reports showed HDT knockouts to have increased histone acetylation levels, and HDTs in plant extracts showed deacetylation activity (Bourque et al., 2011; Ding et al., 2012). The conserved N-terminal “MEFWG” motif and the His residue at position 25 are reported to be important for the HDAC activity of HDTs (Zhou et al., 2004). Immunoblotting studies have claimed the specificity of HDTs for different lysine residues on murine and chicken histone H4, even though the histones share 100% sequence identity (Kölle et al., 1999). Our analysis revealed that the possible HDAC active site, including His, Ser, and Asp/Glu, could be a catalytic triad on each monomer. However, it appears difficult for five closely placed catalytic triads to function simultaneously, as observed from our in vitro HDAC assay. The N-terminal “MEFWG” motif reported to be important for deacetylation activity could actually be important for histone interaction and not for the HDAC function per se. The PTMs like phosphorylation lacking in bacterial expressed recombinant HDTs may regulate the HDAC activity. Alternatively, RPD3 family HDACs, which associate with HDTs (Luo et al., 2012a, 2012b), may be responsible for the HDAC activity where HDTs help to increase the overall rate of histone deacetylation by bringing the histones and functional HDACs together. The absence of HDAC activity for HDTs could also be due to the lack of a specific cofactor yet to be characterized. If HDTs are indeed functional HDACs, how exactly they function and what the actual acetylated histone substrates for each of them need to be determined.
In summary, our study forms the first detailed structural and functional characterization report for the plant-specific HDT nucleoplasmins. Furthermore, we propose a histone chaperone function for HDTs by assisting histone binding and storage.
Materials and methods
Multiple sequence and phylogenetic analyses
Multiple sequence alignment for HDTs and other nucleoplasmins were carried out using T-Coffee (Di Tommaso et al., 2011) and formatted using ESPript3 (Robert and Gouet, 2014) for visualization. For phylogenetic analyses, the primary sequences of all structurally characterized nucleoplasmins were aligned using the MUSCLE 3.8 program (Edgar, 2004) from the MEGA 11 suite (Kumar et al., 2016b). The phylogenetic tree was then constructed using the maximum likelihood method with a bootstrap test of 1,000 replicates. Alignments are provided in Supplemental Files 1–3.
Cloning, expression, and purification of recombinant HDT-NTDs
The full-length cDNA constructs of Arabidopsis (Arabidopsis thaliana) HDTs, namely HDT1 (NP_566872.1; RAFL06-70-K17), HDT2 (NP_851056; RAFL09-88-N19), HDT3 (NP_195994.3; RAFL03-05-D06), and HDT4 (NP_565661.2; RAFL19-56-M20), were obtained from the RIKEN BioResource Center, Japan. The N-terminal regions of each of the HDTs (HDT1, 1–96; HDT2, 1–95; HDT3, 1–95; and HDT4, 1–97) were polymerase chain reaction amplified using primers as described in Supplemental Table S9 and sub-cloned with a noncleavable C-terminal His-tag into the NdeI–XhoI sites of the expression vector pET22b(+) (Novagen). Similarly, a construct for HDT2-A2 (spanning residues 1–120) was also prepared. The His-tagged proteins were expressed in Escherichia coli strain BL21 (DE3) cells in a 2xYT medium. The expression of NTD of HDTs was induced at an optical density at 600 nm of 0.6 with 0.6-mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C for 5 h. The cells were harvested by centrifugation, homogenized, and lysed by sonication in a lysis buffer [50-mM Tris–HCl (pH 8.0), 10% glycerol, 750-mM KCl, 1-mM MgCl2, 10-mM imidazole, 1-mM β-mercaptoethanol (β-ME), and 1-mM phenylmethylsulfonyl fluoride (PMSF)]. The cell debris was removed by centrifugation of cell lysate at 42,000g for 60 min at 4°C, and the supernatant was loaded onto a pre-equilibrated 5-mL HisTrap column (Cytiva) at 4°C. The column was washed with 12 column volumes of wash buffer [20-mM Tris–HCl (pH 8.0), 300-mM KCl, 1-mM MgCl2, 50-mM imidazole, 1-mM β-ME, and 1-mM PMSF]. The bound proteins were eluted by gradient elution using an elution buffer [20-mM Tris–HCl (pH 8.0), 300-mM KCl, 1-mM MgCl2, 300-mM imidazole, 1-mM β-ME, and 1-mM PMSF]. The pure fractions were pooled together, concentrated by ultrafiltration, and loaded onto a HiLoad 16/600 Superdex 200 pg gel-filtration chromatography column (Cytiva) pre-equilibrated with an SEC buffer [20-mM Tris–HCl (pH 8.0), 200-mM NaCl, and 1-mM β-ME]. The peak fractions from gel-filtration were analyzed using 18% SDS–PAGE after staining with Coomassie Brilliant Blue R250. Pure fractions were pooled together and concentrated as required for crystallization and other biochemical/biophysical studies. HDT2-NTD and HDT2-A2 constructs were prepared with a cleavable N-terminal His-tag in the pET-28a(+) vector as well and expressed in BL21 (DE3) cells. His-tag of the proteins after HisTrap column purification was cleaved and further purified by gel-filtration chromatography as described above.
Crystallization, data collection, and structure solution
The purified NTDs of HDT1 (1–96), HDT2 (1–95), HDT3 (1–95), and HDT4 (1–97) were used for crystallization. The best diffracting crystals were grown using the sitting-drop method in 96-well plates at a concentration range of 7–15 mg·mL−1 in the following conditions for the four proteins: HDT1—0.05-M MgCl2.6H2O, 0.1-M HEPES (pH 7.5), and 30% v/v PEG MME 550; HDT2—0.2-M ammonium phosphate dibasic (pH 8.0), and 20% w/v PEG 3350; HDT3—0.1-M succinic acid (pH 7.0), 0.1-M bicine (pH 8.5), and 30% v/v PEG MME 550; and HDT4—0.2-M L-proline, 0.1-M HEPES (pH 7.5), and 24% w/v PEG 1500. The crystal of HDT2-NTD was cryo-protected in a 50% mixture of paratone and mineral oil. Mother liquor supplemented with 20% PEG was used to cryo-protect the HDT4-NTD crystal. However, no cryoprotectants were used for HDT1 and HDT3-NTD crystals. The crystals were flash cooled in liquid nitrogen and shipped for data collection. The diffraction data sets were collected at two different synchrotron sources. HDT2-NTD crystal data collection was carried out at the beamline ID29 of the European Synchrotron Radiation Source (ESRF) Grenoble, France, with a Pilatus 6M detector. Datasets for HDT1, HDT3, and HDT4-NTD crystals were collected at the PX BL21 beamline (Kumar et al., 2016a) of the Indian synchrotron Indus-2 of the Raja Ramanna Centre for Advanced Technology (RRCAT), Indore, India, equipped with a MarCCD detector.
All the data sets were processed using XDS (Kabsch, 2010) and further by AIMLESS (Evans and Murshudov, 2013) from the CCP4 software suite (Winn et al., 2011). The structure of HDT2-NTD was solved using the molecular replacement method by MolRep (Vagin and Teplyakov, 2010) from the CCP4 suite using the coordinates of the native FKBP53 NTD monomer structure (PDB id: 6J2Z) as the search model. The two domains have only 23% sequence identity. However, the similarity in secondary structural organization aided the structure solution. Further, the structures of the remaining three HDT-NTDs were solved by MolRep using the coordinates of a monomer of HDT2-NTD as the search model. All four HDT-NTD structures had five monomers in the asymmetric units and revealed a pentameric organization. The crystal data collection parameters and the final refinement statistics are given in Supplemental Table S1. The initial rounds of the rigid body and restrained refinement steps for the structures were carried out by Refmac5 (Murshudov et al., 1997). The model building was done in Coot (Emsley and Cowtan, 2004), and the final models were validated using MolProbity (Chen et al., 2010). The coordinates and structure factors for the crystal structures have been deposited in the protein data bank (PDB) with the accession codes 7VRR (HDT1), 7VMF (HDT2), 7VMI (HDT3), and 7VMH (HDT4). All the structure figures were prepared using PyMOL (Schrodinger LLC). The surface electrostatics figures were prepared with the help of the electrostatics plugin Adaptive Poisson-Boltzmann Solver (Baker et al., 2001) in PyMOL.
Expression and purification of core histones
The pET21a(+) constructs for the core histones H2A, H2B, H3, and H4, codon-optimized for bacterial expression, were a generous gift from Dr Curtis A. Davey (Nanyang Technological University, Singapore). The tag-less recombinant histones were purified as described previously (Luger et al., 1999a, 1999b), but with slight modifications. In brief, plasmids for individual histones were transformed into E. coli strain Lemo21 (DE3) and were grown on LB agar plates containing ampicillin (100 µg·mL−1) and chloramphenicol (30 µg·mL−1). Cells were further inoculated into a 2xYT medium containing the antibiotics and grown at 37°C until OD600 reached 0.6. The histone overexpression was induced using 0.5 mM of IPTG for 4 h at 37°C. The cells were harvested and purified under denaturing conditions using gel-filtration chromatography from the inclusion bodies. The purified histones were dialyzed against deionized water, freeze-dried, and stored at −80°C for further use.
Preparation of histone oligomers
For preparing the histone oligomers such as the H2A/H2B dimer, H3/H4 tetramer, and histone octamer, the individually lyophilized core histones were dissolved in unfolding buffer [7-M guanidinium HCl, 20-mM Tris–HCl (pH 7.5), and 10-mM β-ME]. The concentrations of the histones were determined by measuring the Abs276. For reconstitution of the dimer, tetramer, and octamer, the appropriate histones were mixed in equimolar ratios and diluted to obtain a final concentration of 1 mg·mL−1. The mixture was dialyzed against a refolding buffer [2-M NaCl, 20-mM Tris–HCl (pH 7.5), 1-mM EDTA, and 5-mM β-ME]. After three exchanges of the dialysis buffer, the precipitates in the samples were removed by centrifugation. The supernatants were further concentrated to 5 mL each before loading into a HiLoad 16/600 Superdex 200 pg gel-filtration column pre-equilibrated in the refolding buffer. The peak fractions of the histone oligomers were pooled together, concentrated, and stored at −20°C in the same buffer with 50% (v/v) glycerol.
Analytical gel-filtration chromatography
Analytical gel-filtration chromatography was carried out for the initial characterization and stability analysis of the HDT-NTDs and their complexes using a Superdex 200 Increase 10/300 GL analytical gel-filtration chromatography column (Cytiva). For salt stability analysis, the protein samples were subjected to analytical gel-filtration chromatography in buffers containing 20-mM Tris–HCl (pH 8.0) with increasing NaCl concentrations from 300 mM to 2 M. A thermal stability assay was performed using analytical gel-filtration chromatography as described previously (Bobde et al., 2021). Briefly, the individual protein samples were heated at 25°C, 40°C, 60°C, and 80°C for 10 min each, centrifuged at 16,200g for 10 min to remove any precipitate, and the supernatant was passed through the same column. For the urea stability assay, the individual protein samples were incubated in a buffer containing increasing urea concentrations (300 mM to 2 M). The samples were then analyzed using the respective urea concentration buffers in the analytical gel-filtration column.
HDT2-A2, histone oligomers, and their complexes were incubated in a buffer containing 20-mM HEPES (pH 7.5), 300-mM NaCl, and 1-mM DTT and analyzed by analytical gel-filtration chromatography in the same buffer. The peak fractions from the analytical gel-filtration chromatographic steps were analyzed using 18% SDS–PAGE.
Protease digestion assay
To check how resistant the HDT-NTDs are to protease activity, their sensitivity to α-chymotrypsin was evaluated. Towards this end, 0.02 g of the lyophilized α-chymotrypsin enzyme was dissolved in a 2-mL mixture containing 1-mM HCl and 2-mM CaCl2 to make a stock of 10 mg·mL−1. The stock was diluted to 5 mg·mL−1 for setting up the protease digestion reactions. The enzyme and the substrate were taken in a 1:50 ratio, kept for digestion at 37°C for 30 min, and checked on 18% SDS gel. BSA was taken as a positive control to confirm the efficiency of the enzyme.
SV-AUC
SV-AUC experiments were carried out using an Optima AUC (Beckman-Coulter) with an absorbance detector. The two sectors of an Epon charcoal-filled double-sector centerpiece were filled with 400 µL of the sample buffer and 380 µL of the protein sample, respectively. HDT-NTDs, HDT2-A2, H2A/H2B dimer, H3/H4 tetramer, HDT-A2–H2A/H2B complex, and HDT-A2–H3/H4 complex purified by analytical gel-filtration chromatography in a Superdex 200 Increase 10/300 GL column, in a sample buffer containing 20-mM HEPES (pH 8.0), 300-mM NaCl, and 5-mM β-ME, were loaded into one of the sectors of the Epon centerpieces, with the sample buffer in the second (reference) sector. The sedimentation velocity runs were performed in an An-60 Ti rotor (Beckman Coulter) at 545,287,680g and 20°C. Data were collected at 280 nm with a spacing of 120 s between two scans. Parameters such as buffer viscosity, density, and protein partial specific volume were calculated using SEDNTREP (Goldberg, 1953). Data analyses were performed with SEDFIT (Schuck, 2000) using a c(s) distribution analysis, and c(s) distribution plots were prepared using the program GUSSI (Schuck, 2000).
CD spectroscopy
CD spectroscopy experiments were performed using a J-1500 Circular dichroism spectrophotometer (Jasco), equipped with a water bath for temperature control, in a buffer containing 5-mM Tris–HCl (pH 8.0) and 50-mM NaCl. The concentration of the proteins was 5 µM for each experiment. The CD spectra were collected using a quartz cuvette with a 1-mm path length at 25°C (except for thermal ramping experiments), and the step size was 0.5 nm with a bandwidth of 1.0 nm and a scan speed of 100 nm·min−1. An average of three scans was used for quantifying the secondary structures using the BeStSel (Micsonai et al., 2018) algorithm for all the protein samples. Thermal ramping experiments were carried out at a temperature range of 25°C–90°C at a ramp rate of 1°C·min−1, and the unfolding signal was followed at 218 nm. Fraction unfolded versus temperature plots were fitted into the Boltzmann equation. The values were normalized in CDPal software (Niklasson et al., 2015), and the melting temperatures (Tm) were calculated.
Salt-gradient pull-down assay
Twenty micrograms of 6× His-tagged HDT2-A2 was mixed with 50 µg of histone dimer or 50 µg of histone tetramer individually in a binding buffer [20-mM HEPES (pH 8.0), 300-mM NaCl, 10-mM imidazole, 10 µg·mL−1 BSA, 1-mM β-ME] along with nickel–nitrilotriacetic acid (Ni–NTA) agarose beads (Invitrogen). The mixture was incubated on ice for 4 h. The beads were then washed with wash buffer [20-mM HEPES (pH 8.0), 50-mM imidazole, 0.5% Triton-X, 1-mM β-ME] containing increasing concentrations of NaCl such as 0.3 M, 0.4 M, 0.5 M, 0.6 M, 0.8 M, and 1.0 M. The bound proteins after the wash step were eluted from the beads using an elution buffer [20-mM HEPES (pH 8.0), 300-mM NaCl, 300-mM imidazole, 1-mM β-ME]. The eluted fractions were analyzed using 18% SDS–PAGE after staining with Coomassie brilliant blue R250.
SAXS
SAXS was employed to characterize the oligomeric conformations of HDT-NTDs in solution. The SAXS data for HDT2, HDT3, and HDT4 NTDs were collected at an in-house SAXS facility on a SAXSpace machine (Anton Paar) with a Mythen 2R1K detector (Dectris). The protein samples were dialyzed in a SAXS buffer [20-mM Tris–HCl (pH 8.0), 200-mM NaCl], concentrated, and subjected to gel-filtration chromatography using a Superdex 200 Increase 10/300 GL column pre-equilibrated with the same buffer just before data collection. The peak fractions were directly taken for SAXS data collection. The data were acquired at concentrations of 1.8 mg·mL−1 for HDT2-NTD, 2 mg·mL−1 for HDT3-NTD, and 2.5 mg·mL−1 for HDT4-NTD. The SAXS data for HDT1-NTD (2 mg·mL−1), HDT2-A2 (2.5 mg mL−1), HDT2-A2–H2A/H2B (2.8 mg·mL−1), and HDT2-A2–H3/H4 (0.6 mg·mL−1) were collected at the beamline BM29 of ESRF, Grenoble, France. The data analysis and modeling were carried out with ATSAS 2.8 (Franke et al., 2017), and Guinier analysis was carried out with Primusqt software (Konarev et al., 2003) from the ATSAS suite. The scattering intensity I(0) and Rg were estimated using AUTOGNOM (Petoukhov et al., 2007). The molecular size and shape of the protein samples were evaluated using pair-distance distribution functions using AUTOGNOM. The results from AUTOGNOM were used to generate ab initio models using GASBOR (Svergun et al., 2001; Petoukhov et al., 2012) and DAMMIF (Franke and Svergun, 2009) in p1 and p5 symmetries. The molecular weight was estimated using Porod volume. The theoretical scattering profiles of the crystal structures of HDT-NTDs were fitted into the experimental scattering curves using CRYSOL (Svergun et al., 1995). The crystal structures of HDT-NTDs were fitted in the SAXS envelope using UCSF Chimera (Pettersen et al., 2004). The model for HDT2-A2 obtained from GalaxyHomomer (Baek et al., 2017) and HDT2-A2 histone dimer and tetramer complexes were manually placed in the SAXS envelope using UCSF Chimera.
ITC
All the ITC experiments were performed using a MicroCal PEAQ-ITC machine (Malvern Panalytical) at 25°C. The HDT2-A2 pentamer, H2A/H2B dimer, and H3/H4 tetramer were dialyzed overnight against an ITC buffer [25-mM HEPES (pH 8.0), 300-mM NaCl, 1-mM β-ME] at 4°C. The heat of the reaction was measured by 20 sequential 2.0-µL injections of 60–80 µM HDT2-A2 pentamer from the syringe into 6–8 µM H3/H4 tetramer or 6–8 µM H2A/H2B dimer solution, taken in the cell, spaced at intervals of 120 s. The heat of dilution obtained by titrating HDT2-A2 and histone oligomers into the ITC buffer was subtracted from the heat of reaction before fitting the data. The binding isotherm was fitted using a one-site binding model with the MicroCal PEAQ-ITC analysis software (Malvern Panalytical).
HDAC assay
HDAC activity was measured with a fluorometric histone deacetylase kit (Sigma-Aldrich) as described previously (Du et al., 2014). Briefly, stock solutions of HDT-NTDs were diluted into the provided assay buffer containing the HDAC substrate to a final concentration of 100 nM in 100-μL volume. Then, the assay reaction was incubated at 30°C for 30 min, followed by adding 10 μL of the developer solution. The developer allows the release of the fluorescent reporter upon deacetylation of the substrate. A Varioskan Flash plate reader (Thermo Scientific) with an excitation at 355 nm and emission at 460 nm was used for measuring fluorescence. Triplicate measurements were taken, and the readings were normalized.
Preparation of “601” DNA
“601” strong positioning 145-bp DNA cloned as eight repeats into the pUC57 vector was gifted by Dr Curtis A. Davey (Nanyang Technological University, Singapore). The plasmid DNA was transformed into E. coli HB101 cells and the plasmid was isolated by the alkaline lysis method from large volumes of the transformed cells. Phenol–chloroform extractions were used to remove RNA and protein contaminations. Finally, EcoRV restriction digestion was performed on the plasmid to release the eight copies of 145-bp “601” DNA, further separated from the plasmid backbone by PEG fractionation (Vasudevan et al., 2010).
Nucleosome core particle reconstitution
Nucleosome core particle (NCP) was reconstituted by serially dialyzing the 145-bp “601” DNA and histone octamer mixture in an equimolar ratio from a high salt buffer to a buffer without salt, as has been described before (Luger et al., 1999b; Vasudevan et al., 2010). The quality of NCP formed was analyzed on a 6% native–PAGE gel and stained with ethidium bromide (EtBr) for visualization.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay was performed to check the ability of HDT2-A2 to interact with preformed NCP. NCP was mixed with increasing stoichiometries of HDT2-A2 in a buffer containing 20-mM Tris (pH 8.0), 50-mM NaCl, 1-mM EDTA, and 1-mM DTT. The samples were incubated for 1 h at 4°C and then subjected to electrophoresis on 6% native–PAGE in 0.5× TBE buffer for 200 min at 60 V and stained with EtBr. AtFKBP53 NTD was taken as a positive control.
Accession numbers
The structure factors and coordinates have been deposited to the protein data bank (PDB; https://www.rcsb.org) with the accession codes 7VRR (HDT1), 7VMF (HDT2), 7VMI (HDT3), and 7VMH (HDT4).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Comparison of domain organization of HDT with other nucleoplasmins and structural features of the HDT1 NTD.
Supplemental Figure S2. Sequence alignment of Arabidopsis HDTs.
Supplemental Figure S3. Sequence alignment of Arabidopsis HDT-NTDs with other nucleoplasmins.
Supplemental Figure S4. Distribution of apolar and hydrophobic residues in HDT-NTDs.
Supplemental Figure S5. 2D-topology diagrams of HDT-NTDs.
Supplemental Figure S6. Distribution of aromatic residues towards the proximal face.
Supplemental Figure S7. Analytical gel-filtration chromatography and SV-AUC analysis for HDT-NTDs.
Supplemental Figure S8. Oligomeric status of HDT-NTDs as studied by SAXS.
Supplemental Figure S9. SAXS envelopes and CRYSOL fits for HDT-NTDs.
Supplemental Figure S10. Secondary structure analysis of HDT-NTDs.
Supplemental Figure S11. Salt stability of HDT-NTDs.
Supplemental Figure S12. Urea stability of HDT-NTDs.
Supplemental Figure S13. α-chymotrypsin cleavage sites on HDT-NTD structures.
Supplemental Figure S14. The residues in other structurally characterized nucleoplasmins corresponding to the catalytic triad in HDTs.
Supplemental Figure S15. Comparison of HDT-NTD structures with other classical nucleoplasmin structures.
Supplemental Figure S16. Packing of HDT-NTD molecules in the crystal lattice.
Supplemental Figure S17. SV-AUC experiments to characterize HDT2-A2 histone oligomer complexes.
Supplemental Figure S18. SAXS analysis of HDT2-A2 and its histone oligomer complexes.
Supplemental Figure S19. EMSA of the interaction between NCP and HDT2-A2.
Supplemental Figure S20. Characterization of untagged HDT2-NTD and HDT2-A2.
Supplemental Table S1. Crystal data collection, processing, and refinement statistics.
Supplemental Table S2. Hydrogen bonds and interface area analysis for HDT-NTDs calculated using PDBsum.
Supplemental Table S3. SV-AUC data for HDT-NTDs, HDT2-A2, and histone oligomers alone and in complex.
Supplemental Table S4. SAXS parameters for HDT-NTDs, HDT2-A2, and its complexes with histone oligomers.
Supplemental Table S5. Melting temperature of HDT-NTDs calculated using CD spectroscopy.
Supplemental Table S6. α-chymotrypsin cleavage site as predicted by ExPASy PeptideCutter.
Supplemental Table S7. Hydrogen bond angle, hydrogen bond distance between the catalytic triad in HDTs.
Supplemental Table S8. List of all the nucleoplasmin and nucleoplasmin-like structures and their differences with HDT-NTDs structure.
Supplemental Table S9. List of oligonucleotides used for cloning.
Supplemental Files 1–3. Alignments used for phylogenetic analysis
Supplementary Material
Acknowledgments
The authors would like to thank Dr Curt A. Davey (NTU, Singapore) for providing the plasmid constructs of histones and “601” DNA; Dr R Ravishankar (CDRI, Lucknow) and the beamline scientists at BM29 (ESRF, Grenoble) for SAXS experiments; Dr Ravindra D. Makde, Dr Biplab Ghosh, and Dr Ashwani Kumar (RRCAT, Indore) for their help with synchrotron XRD data collection at the PX beamline BL21; Dr Deepak T. Nair (RCB, Faridabad) for his help with synchrotron XRD data collection at the ESRF ID29 beamline and for help in arranging and coordinating the ESRF trips. Furthermore, the authors thank their colleague Asima Nayak for her help with histone expression work, Dr Rajivgandhi Sundaram for his critical comments, and Dr Aritreyee Datta for editing the manuscript to its current form. Finally, the authors wish to dedicate this manuscript to the late Prof. M Vijayan (MBU, IISc, Bangalore) for his unwavering support for the crystallography community in the country.
Funding
The extramural grant to D.V. from the Science and Engineering Research Board, Government of India [CRG/2018/000695/PS]; The intramural support to D.V. from the Institute of Life Sciences, Bhubaneswar; The extramural grant to D.V. from the Department of Biotechnology, Ministry of Science and Technology, Government of India [BT/INF/22/SP33046/2019]; The ESRF Access Program of the Department of Biotechnology, Ministry of Science and Technology, Government of India [BT/INF/22/SP22660/2017]; The institutional JRF to R.C.B. from the Institute of Life Sciences, Bhubaneswar; The DBT-JRF to A.K. from the Department of Biotechnology, Ministry of Science and Technology, Government of India.
Conflict of interest statement. None declared.
Contributor Information
Ruchir C Bobde, Institute of Life Sciences, Bhubaneswar, Odisha 751023, India; Regional Centre for Biotechnology, Faridabad 121001, Haryana, India.
Ashish Kumar, Institute of Life Sciences, Bhubaneswar, Odisha 751023, India.
Dileep Vasudevan, Institute of Life Sciences, Bhubaneswar, Odisha 751023, India.
R.C.B. carried out the experiments on HDT3, HDT4, and HDT2-A2. A.K. carried out the experiments on HDT1, HDT2, and HDT2-A2. R.C.B. and A.K. analyzed data and wrote the manuscript. D.V. envisaged the project, planned, obtained funding support, guided the experiments, and wrote the manuscript.
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Dileep Vasudevan (dileep@ils.res.in).
References
- Akey CW, Luger K (2003) Histone chaperones and nucleosome assembly. Curr Opin Struct Biol 13: 6–14 [DOI] [PubMed] [Google Scholar]
- Aravind L, Koonin EV (1998) The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci 23: 469–472 [DOI] [PubMed] [Google Scholar]
- Arnan C, Saperas N, Prieto C, Chiva M, Ausió J (2003) Interaction of nucleoplasmin with core histones. J Biol Chem 278: 31319–31324 [DOI] [PubMed] [Google Scholar]
- Baek M, Park T, Heo L, Park C, Seok C (2017) GalaxyHomomer: a web server for protein homo-oligomer structure prediction from a monomer sequence or structure. Nucleic Acids Res 45: W320–W324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA 98: 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21: 381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañuelos S, Omaetxebarria MJ, Ramos I, Larsen MR, Arregi I, Jensen ON, Arizmendi JM, Prado A, Muga A (2007) Phosphorylation of both nucleoplasmin domains is required for activation of its chromatin decondensation activity. J Biol Chem 282: 21213–21221 [DOI] [PubMed] [Google Scholar]
- Bobde RC, Saharan K, Baral S, Gandhi S, Samal A, Sundaram R, Kumar A, Singh AK, Datta A, Vasudevan D (2021) In vitro characterization of histone chaperones using analytical, pull-down and chaperoning assays. J Vis Exp 178: e63218 [DOI] [PubMed] [Google Scholar]
- Bourque S, Jeandroz S, Grandperret V, Lehotai N, Aimé S, Soltis DE, Miles NW, Melkonian M, Deyholos MK, Leebens-Mack JH, et al. (2016) The evolution of HD2 proteins in green plants. Trends Plant Sci 21: 1008–1016 [DOI] [PubMed] [Google Scholar]
- Bourque S, Dutartre A, Hammoudi V, Blanc S, Dahan J, Jeandroz S, Pichereaux C, Rossignol M, Wendehenne D (2011) Type-2 histone deacetylases as new regulators of elicitor-induced cell death in plants. New Phytol 192: 127–139 [DOI] [PubMed] [Google Scholar]
- Bowman GD, Poirier MG (2015) Post-translational modifications of histones that influence nucleosome dynamics. Chem Rev 115: 2274–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch G, Lusser A, Goralik-Schramel M, Loidl P (1996) Purification and characterization of a high molecular weight histone deacetylase complex (HD2) of maize embryos. Biochemistry 35: 15907–15914 [DOI] [PubMed] [Google Scholar]
- Buszewicz D, Archacki R, Palusiński A, Kotliński M, Fogtman A, Iwanicka-Nowicka R, Sosnowska K, Kuciński J, Pupel P, Olędzki J, et al. (2016) HD2C histone deacetylase and a SWI/SNF chromatin remodelling complex interact and both are involved in mediating the heat stress response in Arabidopsis. Plant. Cell Environ 39: 2108–2122. [DOI] [PubMed] [Google Scholar]
- Chen VB, Arendall WB 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66: 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciftci-Yilmaz S, Mittler R (2008) The zinc finger network of plants. Cell Mol Life Sci 65: 1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville A, Alhattab R, Hu M, Labbé H, Xing T, Miki B (2011) Role of HD2 genes in seed germination and early seedling growth in Arabidopsis. Plant Cell Rep 30: 1969–1979 [DOI] [PubMed] [Google Scholar]
- Dangl M, Brosch G, Haas H, Loidl P, Lusser A (2001) Comparative analysis of HD2 type histone deacetylases in higher plants. Planta 213: 280–285 [DOI] [PubMed] [Google Scholar]
- Ding B, del Rosario Bellizzi M, Ning Y, Meyers BC, Wang G-L (2012) HDT701, a histone H4 deacetylase, negatively regulates plant innate immunity by modulating histone H4 acetylation of defense-related genes in rice. Plant Cell 24: 3783–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso P, Moretti S, Xenarios I, Orobitg M, Montanyola A, Chang J-M, Taly J-F, Notredame C (2011) T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res 39: W13–W17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Risinger AL, King JB, Powell DR, Cichewicz RH (2014) A potent HDAC inhibitor, 1-alaninechlamydocin, from a Tolypocladium sp. induces G2/M cell cycle arrest and apoptosis in MIA PaCa-2 cells. J Nat Prod 77: 1753–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Akey IV, Dingwall C, Hartman KL, Laue T, Nolte RT, Head JF, Akey CW (2001) The crystal structure of nucleoplasmin-core: implications for histone binding and nucleosome assembly. Mol Cell 8: 841–853 [DOI] [PubMed] [Google Scholar]
- Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich-Muth C, Artero J-B, Callow P, Przewloka MR, Watson AA, Zhang W, Glover DM, Debski J, Dadlez M, Round AR, et al. (2015). The pentameric nucleoplasmin fold is present in Drosophila FKBP39 and a large number of chromatin-related proteins. J Mol Biol 427: 1949–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60: 2126–2132 [DOI] [PubMed] [Google Scholar]
- Evans PR, Murshudov GN (2013) How good are my data and what is the resolution? Acta Crystallogr D Biol Crystallogr 69: 1204–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Rivero N, Franco A, Velázquez-Campoy A, Alonso E, Muga A, Prado A (2016) A quantitative characterization of nucleoplasmin/histone complexes reveals chaperone versatility. Sci Rep 6: 32114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke D, Petoukhov MV, Konarev PV, Panjkovich A, Tuukkanen A, Mertens HDT, Kikhney AG, Hajizadeh NR, Franklin JM, Jeffries CM, et al. (2017) ATSAS 2.8: a comprehensive data analysis suite for small-angle scattering from macromolecular solutions. J Appl Crystallogr 50: 1212–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke D, Svergun DI (2009) DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J Appl Crystallogr 42: 342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehlick LJ, Eirín-López JM, Ausió J (2007) New insights into the nucleophosmin/nucleoplasmin family of nuclear chaperones. Bioessays 29: 49–59 [DOI] [PubMed] [Google Scholar]
- Goldberg RJ (1953) Sedimentation in the ultracentrifuge. J Phys Chem 57: 194–202 [Google Scholar]
- Grandperret V, Nicolas-Francès V, Wendehenne D, Bourque S (2014) Type-II histone deacetylases: elusive plant nuclear signal transducers. Plant Cell Environ 37: 1259–1269 [DOI] [PubMed] [Google Scholar]
- Gray SG, Ekström TJ (2001) The human histone deacetylase family. Exp Cell Res 262: 75–83 [DOI] [PubMed] [Google Scholar]
- Gupta V, Prakash NAU, Lakshmi V, Boopathy R, Jeyakanthan J, Velmurugan D, Sekar K (2010) Recognition of active and inactive catalytic triads: a template based approach. Int J Biol Macromol 46: 317–323 [DOI] [PubMed] [Google Scholar]
- Hollender C, Liu Z (2008) Histone deacetylase genes in Arabidopsis development. J Integr Plant Biol 50: 875–885 [DOI] [PubMed] [Google Scholar]
- Kabsch W (2010) XDS. Acta Crystallogr D Biol Crystallogr 66: 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khochbin S, Verdel A, Lemercier C, Seigneurin-Berny D (2001) Functional significance of histone deacetylase diversity. Curr Opin Genet Dev 11: 162–166 [DOI] [PubMed] [Google Scholar]
- Kölle D, Brosch G, Lechner T, Pipal A, Helliger W, Taplick J, Loidl P (1999) Different types of maize histone deacetylases are distinguished by a highly complex substrate and site specificity. Biochemistry 38: 6769–6773 [DOI] [PubMed] [Google Scholar]
- Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI (2003) PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J Appl Crystallogr 36: 1277–1282 [Google Scholar]
- Krissinel E, Henrick K (2004) Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr 60: 2256–2268 [DOI] [PubMed] [Google Scholar]
- Kuang J, Chen J, Luo M, Wu K, Sun W, Jiang Y, Lu W (2012) Histone deacetylase HD2 interacts with ERF1 and is involved in longan fruit senescence. J Exp Bot 63: 441–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Ghosh B, Poswal HK, Pandey KK, Jagannath, Hosur MV, Dwivedi A, Makde RD, Sharma SM (2016a) Protein crystallography beamline (PX-BL21) at Indus-2 synchrotron. J Synchrotron Radiat 23: 629–634 [DOI] [PubMed] [Google Scholar]
- Kumar A, Vasudevan D (2020) Structure-function relationship of H2A-H2B specific plant histone chaperones. Cell Stress Chaperones 25: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K (2016b) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lans H, Marteijn JA, Vermeulen W (2012) ATP-dependent chromatin remodeling in the DNA-damage response. Epigenetics Chromatin 5: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WK, Cho MH (2016) Telomere-binding protein regulates the chromosome ends through the interaction with histone deacetylases in Arabidopsis thaliana. Nucleic Acids Res 44: 4610–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Rechsteiner TJ, Richmond TJ (1999a) Expression and purification of recombinant histones and nucleosome reconstitution. Methods Mol Biol 119: 1–16 [DOI] [PubMed] [Google Scholar]
- Luger K, Rechsteiner TJ, Richmond TJ (1999b) Preparation of nucleosome core particle from recombinant histones. Methods Enzymol 304: 3–19 [DOI] [PubMed] [Google Scholar]
- Luo M, Wang Y-Y, Liu X, Yang S, Lu Q, Cui Y, Wu K (2012a) HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J Exp Bot 63: 3297–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Wang Y-Y, Liu X, Yang S, Wu K (2012b) HD2 proteins interact with RPD3-type histone deacetylases. Plant Signal Behav 7: 608–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusser A, Brosch G, Loidl A, Haas H, Loidl P (1997) Identification of maize histone deacetylase HD2 as an acidic nucleolar phosphoprotein. Science 277: 88–91 [DOI] [PubMed] [Google Scholar]
- Micsonai A, Wien F, Bulyáki É, Kun J, Moussong É, Lee Y-H, Goto Y, Réfrégiers M, Kardos J (2018) BeStSel: a web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra. Nucleic Acids Res 46: W315–W322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53: 240–255 [DOI] [PubMed] [Google Scholar]
- Namboodiri VMH, Akey IV, Schmidt-Zachmann MS, Head JF, Akey CW (2004) The structure and function of Xenopus NO38-core, a histone chaperone in the nucleolus. Structure 12: 2149–2160 [DOI] [PubMed] [Google Scholar]
- Namboodiri VMH, Dutta S, Akey IV, Head JF, Akey CW (2003) The crystal structure of Drosophila NLP-core provides insight into pentamer formation and histone binding. Structure 11: 175–186 [DOI] [PubMed] [Google Scholar]
- Niklasson M, Andresen C, Helander S, Roth MGL, Zimdahl Kahlin A, Lindqvist Appell M, Mårtensson L-G, Lundström P (2015) Robust and convenient analysis of protein thermal and chemical stability. Protein Sci 24: 2055–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoukhov MV, Franke D, Shkumatov AV, Tria G, Kikhney AG, Gajda M, Gorba C, Mertens HDT, Konarev PV, Svergun DI (2012) New developments in the ATSAS program package for small-angle scattering data analysis. J Appl Crystallogr 45: 342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoukhov MV, Konarev PV, Kikhney AG, Svergun DI (2007) ATSAS 2.1 - towards automated and web-supported small-angle scattering data analysis. J Appl Crystallogr 40: s223–s228 [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Philpott A, Leno GH (1992) Nucleoplasmin remodels sperm chromatin in Xenopus egg extracts. Cell 69: 759–767 [DOI] [PubMed] [Google Scholar]
- Platonova O, Akey IV, Head JF, Akey CW (2011) Crystal structure and function of human nucleoplasmin (npm2): a histone chaperone in oocytes and embryos. Biochemistry 50: 8078–8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado A, Ramos I, Frehlick LJ, Muga A, Ausió J (2004) Nucleoplasmin: a nuclear chaperone. Biochem Cell Biol 82: 437–445 [DOI] [PubMed] [Google Scholar]
- Robert X, Gouet P (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42: W320–W324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart SB, Strahl BD (2014) Interpreting the language of histone and DNA modifications. Biochim Biophys Acta 1839: 627–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck P (2000) Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys J. 78: 1606–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Datta A, Jobichen C, Luan S, Vasudevan D (2020) AtFKBP53: a chimeric histone chaperone with functional nucleoplasmin and PPIase domains. Nucleic Acids Res 48: 1531–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridha S, Wu K (2006) Identification of AtHD2C as a novel regulator of abscisic acid responses in Arabidopsis. Plant J 46: 124–133 [DOI] [PubMed] [Google Scholar]
- Svergun D, Barberato C, Koch MHJ (1995) CRYSOL - a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J Appl Crystallogr 28: 768–773 [Google Scholar]
- Svergun DI, Petoukhov MV, Koch MH (2001) Determination of domain structure of proteins from X-ray solution scattering. Biophys J 80: 2946–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneva SG, Bañuelos S, Falces J, Arregi I, Muga A, Konarev PV, Svergun DI, Velázquez-Campoy A, Urbaneja MA (2009) A mechanism for histone chaperoning activity of nucleoplasmin: thermodynamic and structural models. J Mol Biol 393: 448–463 [DOI] [PubMed] [Google Scholar]
- Taneva SG, Muñoz IG, Franco G, Falces J, Arregi I, Muga A, Montoya G, Urbaneja MA, Bañuelos S (2008) Activation of nucleoplasmin, an oligomeric histone chaperone, challenges its stability. Biochemistry 47: 13897–13906 [DOI] [PubMed] [Google Scholar]
- Vagin A, Teplyakov A (2010) Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr 66: 22–25 [DOI] [PubMed] [Google Scholar]
- Vasudevan D, Chua EYD, Davey CA (2010) Crystal structures of nucleosome core particles containing the “601” strong positioning sequence. J Mol Biol 403: 1–10 [DOI] [PubMed] [Google Scholar]
- Volkening JD, Stecker KE, Sussman MR (2019) Proteome-wide analysis of protein thermal stability in the model higher plant Arabidopsis thaliana. Mol Cell Proteomics 18: 308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren C, Matsui T, Karp JM, Onikubo T, Cahill S, Brenowitz M, Cowburn D, Girvin M, Shechter D (2017) Dynamic intramolecular regulation of the histone chaperone nucleoplasmin controls histone binding and release. Nat Commun 8: 2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, et al. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 67: 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Tian L, Malik K, Brown D, Miki B (2000) Functional analysis of HD2 histone deacetylase homologues in Arabidopsis thaliana. Plant J 22: 19–27 [DOI] [PubMed] [Google Scholar]
- Yano R, Takebayashi Y, Nambara E, Kamiya Y, Seo M (2013) Combining association mapping and transcriptomics identify HD2B histone deacetylase as a genetic factor associated with seed dormancy in Arabidopsis thaliana. Plant J 74: 815–828 [DOI] [PubMed] [Google Scholar]
- Zhou C, Labbe H, Sridha S, Wang L, Tian L, Latoszek-Green M, Yang Z, Brown D, Miki B, Wu K (2004) Expression and function of HD2-type histone deacetylases in Arabidopsis development. Plant J 38: 715–724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.