Abstract
In the present study we evaluated the role of B cells in acquired immunity to Salmonella infection by using gene-targeted B-cell-deficient innately susceptible mice on a C57BL/6 background (Igh-6−/−). Igh-6−/− mice immunized with a live, attenuated aroA Salmonella enterica serovar Typhimurium vaccine strain showed impaired long-term acquired resistance against the virulent serovar Typhimurium strain C5. Igh-6−/− mice were able to control a primary infection and to clear the inoculum from the reticuloendothelial system. However, Igh-6−/− mice, unlike Igh-6+/+ C57BL/6 controls, did not survive an oral challenge with strain C5 at 4 months after vaccination. Transfer of immune serum did not restore resistance in Igh-6−/− mice. Total splenocytes and purified CD4+ T cells obtained from Igh-6−/− mice 4 months after vaccination showed reduced ability to release Th1-type cytokines (interleukin 2 and gamma interferon) upon in vitro restimulation with serovar Typhimurium soluble cell extracts compared to cells obtained from Igh-6+/+ C57BL/6 control mice. Therefore, the impaired resistance to oral challenge with virulent serovar Typhimurium observed in B-cell-deficient mice, which cannot be restored by passive transfer of Salmonella-immune serum, may be in part due to a reduced serovar Typhimurium-specific T-cell response following primary immunization.
Salmonella infections are still a serious health problem worldwide. The recent emergence of multidrug-resistant Salmonella strains calls for a more rational approach to the treatment of the disease and increases the need for the rapid development of safer and more effective vaccines. A better understanding of the protective mechanisms of acquired immunity to Salmonella would undoubtedly be beneficial for improving vaccination strategies.
The mouse model is commonly used to study the mechanisms of natural resistance and acquired immunity to Salmonella. Early bacterial growth in mice is controlled by the Nramp (Ity) gene, expressed in macrophages (43), and is suppressed by a T-cell-independent host response which requires production of tumor necrosis factor alpha (TNF-α), interleukin 12 (IL-12), IL18, and gamma interferon (IFN-γ) as well as granuloma formation and the production of nitric oxide (10, 15, 18, 19, 25, 27, 28, 34, 35, 41, 42). Clearance of bacteria from the tissues requires functional CD4+ T cells (10), is under the control of genes within and outside the H-2 complex, and can leave long-lasting specific immunity to rechallenge (reviewed in references 14 and 24).
Mice immunized with protective live attenuated Salmonella vaccines mount antibody responses against lipopolysaccharide and protein antigens in addition to acquiring Th1-type T-cell memory (8). The relative importance of T- and B-cell-associated immunity in long-term vaccine-induced acquired protection against virulent salmonellae has been intensively investigated and much debated (reviewed in references 6, 14, and 24). Members of our group and others have demonstrated that both CD4+ and CD8+ T cells, with the contribution of TNF-α and IFN-γ, are needed for the expression of full acquired resistance to Salmonella (7, 23, 41). A role for B-cell-dependent humoral immunity in vaccine-induced protection has also been demonstrated (4–6, 11).
It is now becoming increasingly clear that antibodies or T cells alone can confer only a moderate level of protection against salmonellosis, especially when innately susceptible animals are challenged with fully virulent salmonellae. Passive administration of immune serum or B cells alone can protect innately resistant mice against virulent salmonellae or susceptible mice against moderately virulent organisms (5, 6, 11). In such host-parasite combinations, passively administered antibody can reduce the number of organisms in a moderate, but lethal, challenge to sublethal levels such that humoral immunity alone can confer protection (5). Adoptive transfer of immune T cells can protect mice against infection with very low doses of virulent salmonellae or against moderately virulent microorganisms (7, 37). However, adoptive transfer of both immune serum and immune T cells is absolutely required to protect innately susceptible mice against highly virulent salmonellae (26), indicating that neither humoral nor cell-mediated immunity is dispensable for resistance to virulent salmonellae in Nramps/s animals.
In addition to antibody production, B cells display a wide range of functions within the immune system, including antigen presentation and production of cytokines (9, 33). Epstein-Barr virus-transformed B cells have been successfully used to present Salmonella antigens to T cells (40). Recent reports indicate that gene-targeted B-cell-deficient mice display increased susceptibility to infections by some agents but not by others (1, 3, 13, 17, 21, 22, 44, 45). In murine models of infection with Chlamydia trachomatis, lymphocytic choriomeningitis virus, and Bordetella pertussis, a role for B cells in the initiation and persistence of T-cell responses has been demonstrated (13, 22, 45).
In the present study, we evaluated the role of B cells in long-term acquired immunity to virulent Salmonella enterica serovar Typhimurium in mice vaccinated with live, attenuated serovar Typhimurium strain SL3261 (aroA).
MATERIALS AND METHODS
Animals.
C57BL/6 mice were purchased from Harlan Olac Ltd., Blackthorn, Bicester, United Kingdom. B-cell-deficient mice on a C57BL/6 background homozygous for a targeted mutation in the gene for immunoglobulin heavy chain 6 (Igh-6) (20) were bred either at BαK Universal Ltd. (Hull, United Kingdom) or at the Imperial College animal unit from breeder mice originally obtained from The Jackson Laboratory, Bar Harbor, Maine. Age- and sex-matched groups were used when over 8 weeks old.
Bacteria.
S. enterica serovar Typhimurium strain SL3261 is an aroA attenuated live vaccine strain (12) with an intravenous (i.v.) 50% lethal dose (LD50) for C57BL/6 mice of ca. 107 CFU. S. enterica serovar Typhimurium strain C5 is a virulent strain with an i.v. LD50 for C57BL/6 mice of <10 CFU (26) and an oral LD50 for C57BL/6 mice of ca. 107 CFU (unpublished data). Strain C5 rfa is an O-rough derivative of strain C5 kindly provided by B. A. D. Stocker, Stanford University, Stanford, Calif. For i.v. inoculation, bacteria were grown at 37°C as stationary-phase overnight cultures in Luria-Bertani (LB) broth (Difco). Aliquots were snap frozen and stored in liquid nitrogen. The inoculum was diluted in phosphate-buffered saline (PBS) and injected into a lateral tail vein. For oral inoculations, bacteria were grown as described above, harvested by centrifugation, resuspended in sterile PBS, and administered intragastrically to mice lightly anesthetized with fluoroalothane by using a gavage tube (26). The numbers of viable bacteria in the inocula were checked by pour plating on LB agar plates.
Bacterial enumeration in organ homogenates.
Mice were killed by cervical dislocation. Spleens and livers were aseptically removed and homogenized in a Colworth stomacher in 10 ml of cold distilled water (15). Viable counts were performed by using pour plates of LB agar.
Collection of sera and passive transfer.
For serum transfer, the donor mice were sample bled from a lateral tail vein or exsanguinated under anesthesia by cardiac puncture. The sera were collected and used fresh or were stored at −20°C. For passive transfer, immune serum from vaccinated (i.v. with ca. 5 × 105 CFU of strain SL3261) and subsequently reinfected (orally with strain C5) C57BL/6 donors was injected i.v. or intraperitoneally (i.p.) into Igh-6−/− recipient mice, 2 h before challenge and at various times thereafter (days 1, 3, and 6 in experiment A and days 1, 3, 4, 6, and 12 in experiment B). Each mouse received 0.5 ml of immune serum at each time point. Sera injected 2 h before challenge were collected from donor mice (and stored at −20°C) 2 days before the designated challenge date for the recipients; sera to be transferred at any other time points were collected on the same day of the serum transfer (days 1, 3, and 6 in experiment A and days 1, 3, 4, 6, and 12 in experiment B).
Preparation of T-cell antigens.
Antigens for T-cell assays were prepared as described previously (8). Briefly, an overnight, stationary-phase culture of strain C5 rfa in LB broth was pelleted, washed once in PBS containing 5 mM EDTA, and washed once more in PBS. The suspension was sonicated on ice, and cellular debris were removed by centrifugation at 13,000 × g. The supernatant (C5SE) was sterile filtered through a 0.22-μm-pore-size filter (Sartorius, Epsom, Surrey, United Kingdom) and stored at −70°C. The protein concentration was determined by using the bicinchoninic acid kit (Pierce Biochemicals, Rockford, Ill.) according to the instructions of the manufacturer. Alkali-treated antigen (C5SENaOH) was prepared from C5SE by addition of NaOH up to 0.25 M; the mixture was incubated at 37°C for 3 h before it was neutralized with HCl and sterile filtered through a 0.22-μm-pore-size filter.
T-cell assays.
Unless otherwise stated all tissue culture reagents were purchased from Sigma Chemical Co. (Poole, Dorset, United Kingdom). Splenocytes for T-cell assays were prepared as described previously (8). Briefly, mice were sacrificed by cervical dislocation and single cell suspensions were prepared. Cells were washed once in RPMI 1640 medium and incubated in Gey's solution to lyse the erythrocytes. Leukocytes were washed twice more and resuspended in RPMI 1640 medium supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM glutamine, 2 × 10−5 M β-mercaptoethanol, 1 mM HEPES, and 10% heat-inactivated fetal calf serum (FCS). For in vitro stimulation, cells were dispensed at a concentration of 4 × 105/well in round-bottom 96-well plates (Corning Glass Works, Corning, N.Y.) in 200 μl and triplicates were incubated with the relevant concentration of antigen (1.25 μg of concanavalin A [ConA] per ml, 20 μg of C5SENaOH per ml, and 1.2 μg of C5SE per ml). Preliminary titration experiments using cells from naive C57BL/6 mice determined the highest concentration of T-cell antigens which would not exert an inhibitory effect on cell proliferation or IL-2 release from mouse splenocytes in response to an optimal dose of ConA. Within the noninhibitory range, the optimal stimulatory concentrations of C5NaOH (20 μg/ml) and C5SE (1.2 μg/ml) were determined by titration experiments by using spleen cells of C57BL/6 mice immunized 4 months earlier with 5 × 105 CFU of strain SL3261.
CD4+ T cells were positively enriched by using magnetic beads coated with anti-mouse CD4 antibodies (Miltenyi Biotec, Camberley, Surrey, United Kingdom). The CD4+ cells were >94% pure as assessed by flow cytometry. Gamma-irradiated (3,000 rads) total spleen cells from age- and sex-matched naive C57BL/6 mice were used as feeder cells in the in vitro assays.
For IL-2 and IFN-γ measurements, the supernatants were harvested at 24 and 48 h, respectively, aliquoted, and stored at −70°C.
IL-2 and IFN-γ ELISA.
IL-2 and IFN-γ were measured by capture enzyme-linked immunosorbent assay (ELISA) with antibody pairs and cytokine standards purchased from Pharmingen (Becton Dickinson Ltd., Cowly, Oxford, United Kingdom).
For IFN-γ concentration determinations, 96-well ELISA plates (Maxisorp Nunc Immuno plate, Nunc, Roskilde, Denmark) were coated overnight at 4°C with 50 μl of a capture rat anti-mouse IFN-γ immunoglobulin G1 (IgG1) monoclonal antibody (clone R4-6A2) per well in 0.1 M NaHCO3 buffer (pH 9.5) at 2 μg/ml. After blocking with PBS supplemented with 10% FCS at 37°C for 1 h, supernatants from T-cell assays were loaded in 50 μl in triplicate and the plates were incubated at 37°C for 2 h. Serial twofold dilutions of recombinant IFN-γ ranging from 20 ng/ml to 40 pg/ml were included as standards. Biotinylated rat anti-mouse IFN-γ IgG1 monoclonal antibody (clone XMG1.2; 100 μl/well) at 1 μg/ml in PBS–10% FCS was then added for 1 h at 37°C, after which 100 μl of peroxidase-labelled streptavidin per well at 2.5 μg/ml (Sigma) in PBS–10% FCS was added for 45 min at room temperature. ortho-Phenylenediamine (1 mg/ml in 0.2 M Na2HPO4–0.1 M citrate buffer) in the presence of H2O2 was used to develop the plates. The reaction was stopped by adding 15 μl of 3 M H2SO4 per well. The optical density was read at 490 nm. IFN-γ values were determined by comparison with the standard curve. We considered 80 pg/ml the lower limit of sensitivity of our IFN-γ ELISA.
For IL-2 measurement, the OptEIA set (Pharmingen) was used according to the instructions provided by the manufacturer, the only modification being in the use of ortho-phenylenediamine as the substrate. The sensitivity of the IL-2 ELISA was ≥7 pg/ml.
Statistical analysis.
Student's t test was used to determine the significance of differences between controls and experimental groups. Differences between experimental groups were considered significant for P values of <0.05.
RESULTS
Infection of Igh-6−/− mice with S. enterica serovar Typhimurium strain SL3261.
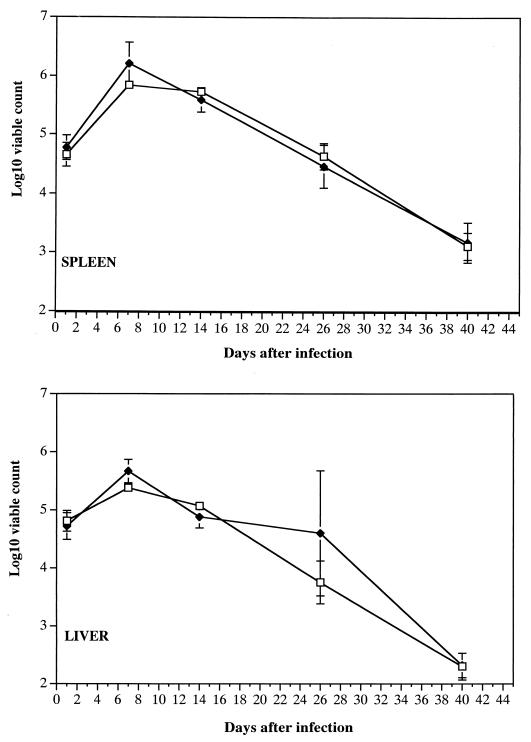
Igh-6−/− mice and C57BL/6 control mice were infected i.v. with 5 × 105 CFU of strain SL3261. Spleen and liver counts of viable bacteria obtained thereafter showed that the courses of the infection in B-cell-deficient and control animals were very similar (Fig. 1). The initial bacterial growth was efficiently controlled and suppressed by both groups of mice, followed by a steady decline in the numbers of bacteria in the tissues, with low bacterial numbers being detectable on day 40 of the infection. The microorganisms were cleared from the spleen and liver by day 60 as assessed by pour plating of the whole-organ homogenate.
FIG. 1.
Igh-6−/− mice (closed diamonds) and C57BL/6 mice (open squares) were infected i.v. with 5 × 105 CFU of serovar Typhimurium strain SL3261. Spleen and liver counts of viable bacteria were obtained thereafter. Results are means ± standard deviations from groups of four mice.
Thus, B cells are not required for control of the growth of an aromatic-dependent S. enterica serovar Typhimurium aroA strain. B cells are also dispensable for the clearance of Salmonella from the reticuloendothelial system (RES), which is known to require the contribution of T cells (10, 39).
Protection against oral challenge with virulent strain C5 in Igh-6−/− mice immunized with strain SL3261.
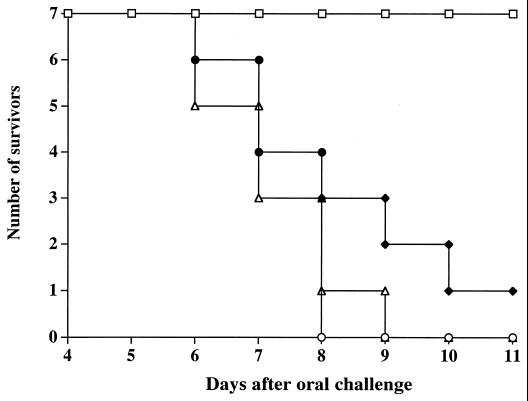
Groups of seven Igh-6−/− mice and seven C57BL/6 control mice were immunized with strain SL3261 as for Fig. 1. A similar number of age- and sex-matched naive mice for each strain were used as unimmunized controls. Four months after vaccination, all mice were challenged orally with ca. 2.5 × 109 CFU of strain C5. As expected, all naive mice of either strain succumbed to the challenge with the virulent strain C5 within 8 or 9 days after infection. No deaths were observed among the immunized C57BL/6 mice; conversely, six of seven immunized Igh-6−/− mice died within 11 days of challenge (Fig. 2). Two repeat experiments gave similar results.
FIG. 2.
Igh-6−/− mice (closed diamonds) and C57BL/6 mice (open squares) were immunized with serovar Typhimurium strain SL3261 as for Fig. 1. Age-matched naive Igh-6−/− mice (open triangles) and naive C57BL/6 mice (open circles) were used as unimmunized controls. Four months after vaccination, all mice were challenged orally with ca. 2.5 × 109 CFU of virulent serovar Typhimurium strain C5.
Thus, B-cell-deficient mice have an impaired ability to develop long-term acquired resistance against oral challenge with S. enterica serovar Typhimurium strain C5.
Effect of transfer of immune serum on resistance to virulent oral challenge in Igh-6−/− mice immunized with the attenuated strain SL3261.
We have previously shown that both T cells and antibodies are required for optimal vaccine-induced protection against oral challenge with virulent S. enterica serovar Typhimurium in innately susceptible mice (26). Although the lack of Salmonella-specific antibody could be the only cause of the impaired resistance to reinfection seen in B-cell-deficient mice, other B-cell-dependent functions could also play a role in the development of long-term vaccine-induced acquired immunity to rechallenge with virulent salmonellae. To investigate this, we attempted to restore resistance to oral challenge in B-cell-deficient mice by passive transfer of immune serum. Previous work established that immune serum in the presence of immune T cells can protect innately susceptible mice against oral challenge with virulent serovar Typhimurium (26).
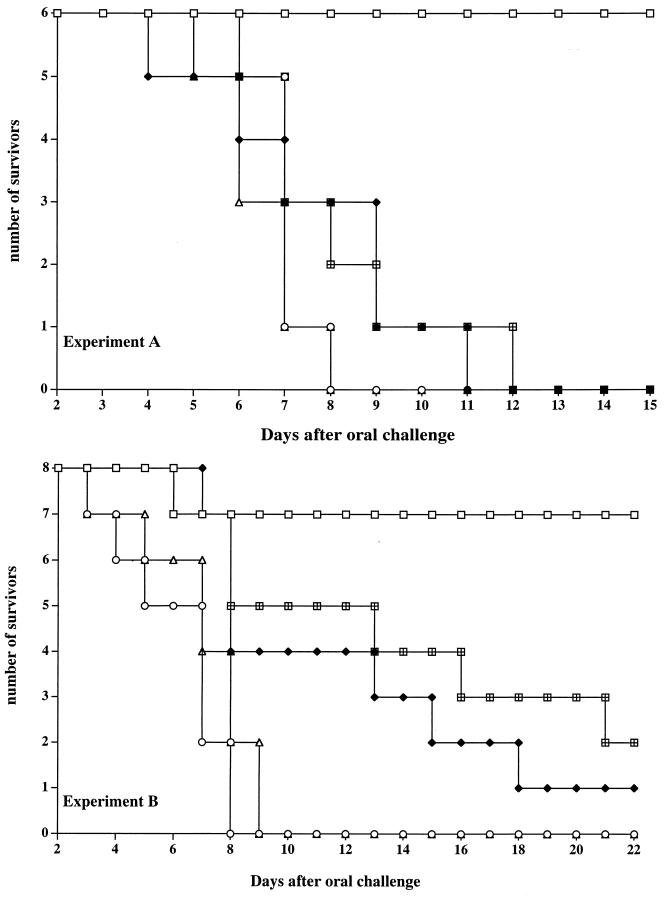
Groups of Igh-6−/− and C57BL/6 mice were immunized as for Fig. 1, and parallel groups of naive mice of both strains were used as unimmunized controls. Six mice per group were used in experiment A, and eight mice per group were used in experiment B. All mice were challenged orally with ca. 5 × 109 CFU (experiment A) or 1 × 109 CFU (experiment B) of virulent strain C5 4 months after the strain SL3261 vaccination. In each experiment, a parallel group of similarly immunized and challenged C57BL/6 mice were used as donors for adoptive transfer of serum. Figure 3 shows that all naive mice from either strain died within 9 days postchallenge in both experiments. Six of six and seven of eight immunized C57BL/6 mice survived the oral challenge in experiments A and B, respectively. Six of six immunized B-cell-deficient animals died within 11 days postinfection in experiment A, and seven of eight B-cell-deficient mice died within 18 days in experiment B. One additional group of Igh-6−/− mice in each experiment received immune serum from immunized (with strain SL3261) and reinfected (with strain C5) C57BL/6 donors 2 h before challenge and at various times thereafter (days 1, 3, and 6 in experiment A and days 1, 3, 4, 6, and 12 in experiment B). Despite the transfer of immune serum, six of six B-cell-deficient mice died within 12 days after challenge in experiment A and six of eight B-cell-deficient mice died within 21 days in experiment B.
FIG. 3.
Igh-6−/− mice (closed diamonds) and C57BL/6 mice (open squares) were immunized with serovar Typhimurium strain SL3261 as for Fig. 1. Age-matched naive Igh-6−/− mice (open triangles) and naive C57BL/6 mice (open circles) were used as unimmunized controls. One additional group of Igh-6−/− mice in each experiment (squares with bars) received immune serum from similarly immunized and orally reinfected C57BL/6 donors 2 h before challenge and at various times thereafter (days 1, 3, and 6 in experiment A and days 1, 3, 4, 6, and 12 in experiment B). All mice were challenged orally with serovar Typhimurium strain C5 (5 × 109 CFU [experiment A] or 1 × 109 CFU [experiment B]).
Thus, adoptive transfer of immune serum does not restore acquired immunity in vaccinated B-cell-deficient mice, indicating that antibody production alone does not appear to be the only function of B cells in salmonellosis.
T-cell responses in Igh-6−/− mice.
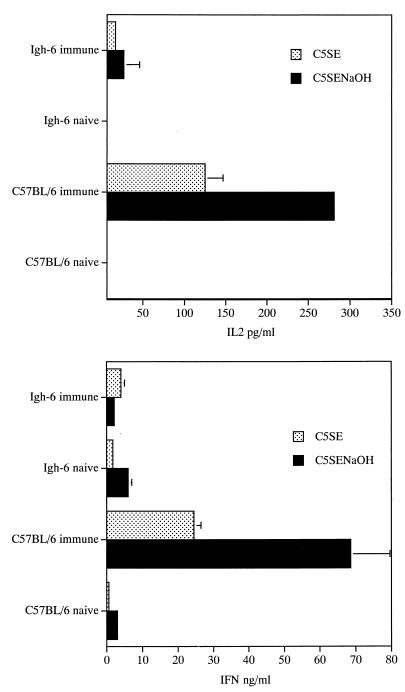
Splenocytes from immunized and naive C57BL/6 mice and Igh-6−/− mice taken 4 months after vaccination with strain SL3261 were restimulated in vitro with optimal concentrations of crude C5SE or detoxified C5SENaOH soluble extract prepared from the O-rough derivative C5 rfa. Figure 4 shows that IL-2 and IFN-γ production from splenocytes of immunized C57BL/6 mice was significantly higher than cytokine release by spleen cells of Igh-6−/− mice upon restimulation with antigen. Cells from naive mice of either strain produced undetectable levels of IL-2 and very low levels of IFN-γ. Spleen cells from Igh-6−/− mice and C57BL/6 mice produced similar amounts of IL-2 and IFN-γ in response to in vitro stimulation with the T-cell mitogen ConA (not shown). Three repeat experiments gave similar results.
FIG. 4.
Splenocytes were prepared from naive C57BL/6 mice and Igh-6−/− mice as well as from mice of either strain which had been vaccinated 4 months earlier with 5 × 105 CFU of serovar Typhimurium strain SL3261. The cells were restimulated in vitro with a crude (C5SE at 1.2 μg/ml) or detoxified (C5SENaOH at 20 μg/ml) Salmonella soluble extract. IL-2 and IFN-γ production was measured by ELISA. Values are means ± standard deviations of triplicate cultures.
Results similar to the ones obtained with whole splenocyte populations were obtained when CD4+ T cells from C57BL/6 and Igh-6−/− mice were restimulated in vitro with the detoxified Salmonella soluble cell extract in the presence of irradiated feeder cells (splenocytes) prepared from naive C57BL/6 mice (results not shown).
Thus, total spleen cells and purified CD4+ T cells from Igh-6−/− mice showed impaired ability to produce Th1-type cytokines in response to in vitro restimulation with Salmonella soluble cell extracts.
DISCUSSION
In the present paper we report that Igh-6−/− mice immunized with a live, attenuated, aromatic-dependent S. enterica serovar Typhimurium aroA vaccine strain exhibited an impaired long-term acquired immunity against virulent Salmonella microorganisms. Igh-6−/− mice were able to control a primary infection and to clear the inoculum from their RESs. However, Igh-6−/− mice, unlike C57BL/6 controls, did not survive an oral challenge with virulent strain C5 4 months after vaccination. Transfer of immune serum did not restore resistance in Igh-6−/− mice. Total splenocytes and purified CD4+ T cells from Igh-6−/− mice showed a reduced ability to release the Th1-type cytokines IL-2 and IFN-γ upon in vitro restimulation with serovar Typhimurium soluble extracts.
The control and eradication of Salmonella infection in the mouse model occur in a number of phases that involve distinct cellular and humoral mechanisms (reviewed in reference 24). The early bacterial growth in the RES is controlled by the Nramp gene. The suppression of bacterial growth in the tissues requires bone marrow-derived cells and a wide array of cytokines but does not need conventional T cells (15, 25, 28–30, 34, 35). CD4+ T lymphocytes are essential for the clearance of the organisms from the RES and for the development of T-cell-dependent long-term immunity (24, 39). The role of B cells and antibody in host resistance to primary Salmonella infection has been studied with xid mice, which lack B1 cells and have a partial defect in a proportion of B2 cells (36). It has been shown that xid mice have increased susceptibility to i.p. infection with S. enterica serovar Typhimurium. xid mice were able to mount an IgM response to Salmonella although they displayed impaired, but not abolished, IgG production. Resistance in xid mice was restored by passive transfer of Salmonella-immune serum generated in immunocompetent mice, leading the authors to conclude that the diminished or delayed antibody response in xid mice was responsible for the increased susceptibility to infection. Nevertheless, the passive transfer of serum from immunocompetent to xid mice was performed before the mice were infected with salmonellae. This protocol is known to confer a degree of protection to mice infected by the i.p. route due to a reduction in bacterial numbers very early in infection (before colonization of the RES) and is not representative of the physiological production of protective antibodies, which certainly starts after infection (5). Furthermore, the xid mutation might have other effects on host resistance to infection independent of the lack of B1 cells (2). More work is needed to ascertain whether deficient antibody production is the only cause of the impaired resistance to primary serovar Typhimurium infection seen in xid mice.
In the present study we used Igh-6−/− mice, which are B cell deficient and incapable of antibody production. We did not find differences in bacterial viable counts between wild-type and Igh-6−/− mice during the course of a primary infection with an aromatic-dependent strain of serovar Typhimurium. Members of our group previously reported that the growth of an aroA serovar Typhimurium strain is not exacerbated in xid mice compared to that in wild-type control animals (16). The results obtained in the present study confirm that B cells and antibody are not needed for the control and clearance of attenuated aro mutant salmonellae. It must be noted that aromatic-dependent serovar Typhimurium strains can grow in immunocompromised mouse strains and can cause lethal infections. nu/nu mice, T-cell receptor α/β−/− mice, IFN-γ−/− mice, mice treated with aminoguanidine (an inhibitor of inducible nitric oxide synthase), NOS2−/− mice, and anti-IL-12-treated mice all succumb to infections with aro mutant Salmonella strains (10, 28, 31, 39, and our unpublished observations). The results shown in the present paper demonstrate that unlike other forms of immunodeficiency, the lack of B cells does not impair host resistance during a primary infection. We obtained similar results by infecting B-cell-deficient mice with wild-type Salmonella strains of intermediate virulence (results not shown), thus proving that the unimpaired resistance to primary infection seen in Igh-6−/− mice is not limited to the attenuated serovar Typhimurium aroA vaccine strain described in this study.
In the mouse model, immunization with live attenuated aromatic-dependent Salmonella vaccines elicits good antibody responses against protein and polysaccharide determinants, long-lasting Th1-type immunological memory, and CD8+ cytotoxic T cells (7, 23). Vaccination confers solid and long-lasting protection against oral challenge with virulent microorganisms which persists long after the initial serovar Typhimurium vaccine strain has been cleared from the RES (12, 14, 24). It has been shown that both humoral and cellular factors play a role in acquired immunity to salmonellae; the relative importance of either type of factor depends on the susceptibility of the mouse strain used and on the relative virulence of the bacterial challenge strain (5, 6, 26). When innately susceptible mice are challenged with virulent salmonellae, survival of the host requires the presence of antilipopolysaccharide antibodies, immune CD4+ T cells and CD8+ T cells, IFN-γ, TNF-α, IL-12, and granuloma formation (7, 23, 28, 41).
In the present paper we show that B cells are essential for the expression of full acquired immunity to virulent oral challenge. Immunized B-cell-deficient mice succumb to an oral challenge with virulent serovar Typhimurium strain C5. At the lowest challenge dose used (Fig. 3, experiment B) a proportion of immunized Igh-6−/− mice showed a delay in time to death compared to that of unimmunized controls, indicating that in the absence of B cells a low degree of protection is still present, although it is clearly insufficient to control the infection.
A role for B cells in acquired immunity to Salmonella could be due to the absolute requirement for antibodies in secondary resistance to salmonellae in innately susceptible mice (26). However, our present results indicate a more complex contribution of B cells to specific immunity to Salmonella infections. Antibody production alone does not appear to be the only function of B cells in salmonellosis. In fact, acquired resistance in Igh-6−/− mice could not be restored, even at the lowest challenge dose used, by passive transfer of large amounts of immune serum, despite the documented (6, 26) efficacy of adoptive transfer of antibodies in the presence of functional immune T cells. Noticeably, a marked reduction in the ability of mouse splenocytes from Igh-6−/− mice to produce Th1-type cytokines in response to antigen-specific (but not ConA-mediated) restimulation was observed. This indicates a significant impairment of Th1-type memory, which is known to be essential for vaccine-induced acquired resistance to virulent salmonellae (8, 23). The inadequate Th1-type memory in immunized Igh-6−/− mice would explain, at least in part, the increased susceptibility to rechallenge despite the transfer of immune serum. Evidence from other infection models also indicates that B cells are required for the establishment and/or persistence of long-lasting T-cell-mediated immunity. In fact, in some infections B cells are required for the initial establishment of cell-mediated immunity during a primary infection (e.g., malaria [21], tuberculosis [44], or lymphocytic choriomeningitis virus [13] or C. trachomatis infection [45]) or for long-term persistence of solid T-cell immunity (e.g., in the B. pertussis mouse model [22]).
At this stage we cannot rule out a role for B cells in the modulation of CD8+-T-cell functions. In fact, this T-cell subset is needed for the full expression of immunity to oral challenge with virulent serovar Typhimurium in vaccinated mice (23). We cannot rule out the possibility that in the absence of B cells (especially B1 cells) an impairment of gut humoral immunity might contribute to the reduced vaccine-induced resistance to oral challenge with virulent organisms seen in Igh-6−/− immunized mice. In fact, B1 cells have been shown to be important for IgA responses in the gut mucosa following oral immunization with serovar Typhimurium (38). Evidence for a protective role of IgA against very low oral doses of virulent Salmonella has been provided (32).
In primary Salmonella infections in the mouse model, clearance of the inoculum from the tissues requires functional CD4+ T cells and the development of Th1-type T-cell immunity (10, 39). The results shown in this report indicate that Igh-6−/− mice can control and clear a primary infection (within 60 days after primary vaccination) despite their apparent inability to display antigen-specific long-lasting Th1-type T-cell immunity (at 4 months after immunization). It is possible that either (i) T-cell immunity to Salmonella is normally initiated but is short lasting in the absence of B cells, (ii) the lower degree of T-cell memory seen in Igh-6−/− mice is sufficient to control an infection with salmonellae of low virulence yet is insufficient for resistance against virulent organisms, or (iii) clearance of the inoculum from the tissues and resistance to rechallenge are mediated by different T-cell-dependent mechanisms. This is currently under investigation.
ACKNOWLEDGMENTS
This work was supported by grants from The Wellcome Trust and BBSRC.
REFERENCES
- 1.Aguirre K M, Johnson L L. A role for B cells in resistance to Cryptococcus neoformans in mice. Infect Immun. 1997;65:525–530. doi: 10.1128/iai.65.2.525-530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babai B, Louriz H, Cazenave P A, Dellagi K. Depletion of peritoneal CD5+ B-cells has no effect on the course of Leishmania major infection in susceptible mice. Clin Exp Immunol. 1999;117:123–129. doi: 10.1046/j.1365-2249.1999.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berglof A, Sandstedt K, Feinstein R, Bolske G, Smith C I E. B cell deficient mice as an experimental model for Mycoplasma infections in X-linked agammaglobulinemia. Eur J Immunol. 1997;27:2118–2121. doi: 10.1002/eji.1830270841. [DOI] [PubMed] [Google Scholar]
- 4.Casadevall A. Antibody mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–107. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 5.Collins F M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974;38:371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenstein T K, Killar L M, Sultzer B M. Immunity to infection with Salmonella typhimurium: mouse strain differences in vaccine and serum mediated protection. J Infect Dis. 1984;150:425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- 7.Guilloteau L, Buzoni-Gatel D, Bernard F, Lantier I, Lantier F. Salmonella abortus ovis infection in susceptible BALB/cby mice: importance of Lyt-2+ and L3T4+ T cells in acquired immunity and granuloma formation. Microb Pathog. 1993;14:45–55. doi: 10.1006/mpat.1993.1005. [DOI] [PubMed] [Google Scholar]
- 8.Harrison J, Villarreal-Ramos B, Mastroeni P, Demarco de Hormaeche R, Hormaeche C E. Correlates of protection induced by live Aro− Salmonella typhimurium vaccines in the murine typhoid model. Immunology. 1997;90:618–625. doi: 10.1046/j.1365-2567.1997.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinzel F P, Sadick M D, Mutha S S, Locksley R M. Production of interferon-γ, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess J, Ladel C, Miko D, Kaufmann S H E. Salmonella typhimurium aroA− infection in gene targeted immunodeficient mice. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 11.Hochadel J F, Keller K F. Protective effects of passively transferred immune T- or B-lymphocytes in mice infected with Salmonella typhimurium. J Infect Dis. 1977;135:813–823. doi: 10.1093/infdis/135.5.813. [DOI] [PubMed] [Google Scholar]
- 12.Hoiseth S K, Stocker B A D. Aromatic dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 13.Homann D, Tishon A, Berger D P, Weigle W O, von Herrath M G, Oldstone M B A. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from μMT/μMT mice. J Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hormaeche C E, Khan C M A, Mastroeni P, Villarreal B, Dougan G, Chatfield S N. Salmonella vaccines: mechanisms of immunity and their use as carriers of recombinant antigens. In: Ala'Aldeen D, Hormaeche C E, editors. Molecular and clinical aspects of bacterial vaccine development. Chichester, United Kingdom: John Wiley; 1994. pp. 119–153. [Google Scholar]
- 15.Hormaeche C E, Mastroeni P, Arena A, Uddin J, Joysey H S. T cells do not mediate the initial suppression of a salmonella infection in the RES. Immunology. 1990;70:247–250. [PMC free article] [PubMed] [Google Scholar]
- 16.Izhar M, DeSilva L, Joysey H S, Hormaeche C E. Moderate immunodeficiency does not increase susceptibility to Salmonella typhimurium aroA live vaccines in mice. Infect Immun. 1990;58:2258–2261. doi: 10.1128/iai.58.7.2258-2261.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson M, Ward M, Lycke N. B-cell deficient mice develop complete immune protection against genital tract infection with Chlamydia trachomatis. Immunology. 1997;92:422–428. doi: 10.1046/j.1365-2567.1997.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagaya K, Watanabe K, Fukazawa Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect Immun. 1989;57:609–615. doi: 10.1128/iai.57.2.609-615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kincy-Cain T, Clements J D, Bost K L. Endogenous and exogenous interleukin-12 augment the protective immune response in mice orally challenged with Salmonella dublin. Infect Immun. 1996;64:1437–1440. doi: 10.1128/iai.64.4.1437-1440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B-cell deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 21.Langhorne J, Cross C, Seixas E, Li C, von der Weid T. A role for B-cells in the development of T-cell helper function in a malaria infection in mice. Proc Natl Acad Sci USA. 1998;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahon B P, Sheahan B J, Griffin F, Murphy G, Mills K H G. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J Exp Med. 1997;186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastroeni P, Villarreal-Ramos B, Hormaeche C E. Role of T-cells, TNFα and IFNγ in recall of immunity to oral challenge with virulent salmonellae in mice immunised with live attenuated aroA Salmonella vaccines. Microb Pathog. 1992;13:477–491. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 24.Mastroeni P, Harrison J A, Hormaeche C E. Natural resistance and acquired immunity to Salmonella. Fundam Clin Immunol. 1994;2:83–95. [Google Scholar]
- 25.Mastroeni P, Arena A, Costa G B, Liberto M C, Bonina L, Hormaeche C E. Serum TNFα in mouse typhoid and enhancement of a Salmonella infection by anti-TNFα antibodies. Microb Pathog. 1991;11:33–38. doi: 10.1016/0882-4010(91)90091-n. [DOI] [PubMed] [Google Scholar]
- 26.Mastroeni P, Villarreal-Ramos B, Hormaeche C E. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastroeni P, Villarreal-Ramos B, Hormaeche C E. Effect of late administration of anti-TNFα antibodies on a Salmonella infection in the mouse model. Microb Pathog. 1993;14:473–480. doi: 10.1006/mpat.1993.1046. [DOI] [PubMed] [Google Scholar]
- 28.Mastroeni P, Harrison J A, Robinson J H, Clare S, Khan S, Maskell D J, Dougan G, Hormaeche C E. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun. 1998;66:4767–4776. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastroeni P, Skepper J N, Hormaeche C E. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect Immun. 1995;63:3674–3682. doi: 10.1128/iai.63.9.3674-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastroeni P, Clare S, Khan S, Harrison J A, Hormaeche C E, Okamura H, Kurimoto M, Dougan G. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–483. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFarlane A S, Schwacha M G, Eisenstein T K. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect Immun. 1999;67:891–898. doi: 10.1128/iai.67.2.891-898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michetti P, Mahan M J, Slauch J M, Mekalanos J J, Neutra M R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milich D R, Chen M, Schoedel F, Peterson D L, Jones J E, Hughes J L. Role of B-cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci USA. 1997;94:14648–14653. doi: 10.1073/pnas.94.26.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muotiala A, Makela P H. The role of IFNγ in murine Salmonella typhimurium infection. Microb Pathog. 1990;8:135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- 35.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien A D, Scher I, Metcalf E. Genetically conferred defect in anti-Salmonella antibody formation renders CBA/N mice innately susceptible to Salmonella typhimurium infection. J Immunol. 1981;126:1368–1372. [PubMed] [Google Scholar]
- 37.Paul C, Shalala K, Warren R, Smith R. Adoptive transfer of murine host protection to salmonellosis with T-cell growth factor-dependent, Salmonella-specific T-cell lines. Infect Immun. 1985;48:40–43. doi: 10.1128/iai.48.1.40-43.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pecquet S S, Ehrat C, Ernst P B. Enhancement of mucosal antibody responses to Salmonella typhimurium and the microbial hapten phosphorylcholine in mice with X-linked immunodeficiency by B-cell precursors from the peritoneal cavity. Infect Immun. 1992;60:503–509. doi: 10.1128/iai.60.2.503-509.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha K A, Mastroeni P, Harrison J, Demarco de Hormaeche R, Hormaeche C E. Salmonella typhimurium aroA, htrA, and aroD htrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infect Immun. 1997;65:1566–1569. doi: 10.1128/iai.65.4.1566-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sztein M B, Tanner M K, Polotsky Y, Orenstein J M, Levine M M. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J Immunol. 1995;155:3987–3993. [PubMed] [Google Scholar]
- 41.Tite J P, Dougan G, Chatfield S N. The involvement of tumor necrosis factor in immunity to Salmonella infection. J Immunol. 1991;147:3161–3164. [PubMed] [Google Scholar]
- 42.Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidal S M, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 44.Vordermeier H M, Venkataprasad N, Harris D P, Ivanyi J. Increase of tuberculous infection in the organs of B-cell deficient mice. Clin Exp Immunol. 1996;106:312–316. doi: 10.1046/j.1365-2249.1996.d01-845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, Brunham R C. Gene knockout B cell deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]