Abstract
This systematic review provides an overview of the effects of menopausal symptom treatment options on palpitations, defined as feelings of missed or exaggerated heart beats, reported by peri- and postmenopausal women. Guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, searches were conducted in PubMed, CINAHL, and PsycINFO to identify articles meeting pre-specified inclusion criteria. Of 670 unique articles identified, 37 were included in the review. Treatments included drug therapies and non-drug therapies. Palpitations were studied as an outcome in 89% of articles and as an adverse effect in 11%. Articles provided mostly level II/III evidence due to their design and/or small sample sizes. Based on available evidence, no therapies can be fully recommended for clinical practice. Only some hormonal agents (e.g., estradiol) can be recommended with caution based on some positive evidence for reducing palpitations prevalence or severity. However, other drug therapies (e.g., moxonidine, atenolol), dietary supplementary treatments (e.g., isoflavones, Rheum rhaponticum, sage), cognitive-behavioral intervention, and auricular acupressure cannot be recommended giving the existing evidence. Additional well-designed randomized controlled treatment trials focusing on palpitations during the menopause transition as an inclusion criteria and outcome are needed to advance the field.
Keywords: Menopausal therapy, palpitations, menopause, postmenopause, perimenopause, menopausal symptoms, systematic review
Introduction
About 21 million women living in the US today and 1.2 billion women worldwide will experience menopausal symptoms by 2030 [1–5], and most (75%) will seek help from a health care provider [6]. Menopausal symptoms are more likely to occur during perimenopause and postmenopause as a result of estrogen decline caused by ovarian insufficiency [7,8]. The average age of menopause in women is about 51 years [8,9]. Women experience menopausal symptoms for 10 years or more [9]. Menopause symptoms affect women’s life and work [7,8]. Research to date has focused mainly on vasomotor symptoms (e.g., hot flashes, night sweats) [10] and strong efficacy evidence is available for drug and non-drug treatment options [11,12].
When compared to the vast literature available on vasomotor symptoms, palpitations during the menopause transition appear to be seldomly studied. Palpitations are reported by 20% to 42% of perimenopausal women and 16% to 54% of postmenopausal women as sensations of skipped, missed, or exaggerated heartbeats [13]. Distress from palpitations during the menopause transition has been associated with more severe insomnia, worse depressive symptoms, and poorer menopausal quality of life [14]. Whether palpitations are associated with electrocardiogram (ECG) abnormalities is not fully known. In the Tromsø population-based study (n=22,815), the sensation of palpitations (e.g., sudden changes in heart rate or rhythm) in the past year was associated with greater risk of developing incident, ECG-verified, atrial fibrillation (ORwomen=1.62 [1.29–2.02]) when controlling for other known risk factors [15]. Atrial fibrillation is one of several arrhythmias that increases morbidity and mortality [16]. Similar to untreated vasomotor symptoms, untreated palpitations may increase direct care costs and lead to poorer health, lower work productivity, and greater healthcare utilization and costs [17–20].
Although evidence-informed reviews of therapies for vasomotor symptoms regularly appear in the literature [11,12], the same is not true for palpitations. To date, the effect of menopausal symptom treatment options on palpitations have not been reviewed and summarized to create recommendations for practice. Palpitations are often measured with vasomotor and other menopausal symptoms [21]. Menopausal symptom treatments may have potential benefits on palpitations or may cause palpitations as an adverse effect. A systematic review could help assist (1) clinicians in engaging women in shared decision making about treatment options and (2) researchers in understanding gaps in treatment efficacy/effectiveness research to help design future articles. Therefore, given this large gap in the literature, the purpose of this review was to evaluate the effects of menopausal symptom treatment options on palpitations.
Methods
The systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [22].
Review strategy
The review was not registered and did not require institutional review board approval. Articles were included if they were full-length, peer-reviewed, English-language, randomized, non-randomized, controlled, and uncontrolled trials of menopausal symptom therapies that included data on palpitations as an outcome or adverse effect. The latter criteria permitted summarization of evidence on treatments that could alleviate as well as exacerbate palpitations. Articles were excluded if they (1) included transgender or gender transitioning populations, men, or animals due to different menopausal experience from biologically female women, or (2) did not specify menopausal status for the study sample.
Literature search strategy
Literature searches were conducted in May 2020 by a health science librarian using three online search engines with no restriction on publication date: PubMed, CINAHL, and PsycINFO. All searches used (MeSH terms) “Menopause” OR “Menopaus*”) AND (keywords) (“Palpitation*” OR “Heart racing” OR “Hear Pounding” OR “Irregular heart”). Additional articles were identified by searching for articles that used standard menopausal symptom assessment tools, including the Menopause Rating Scale (Heinemann) [23], Greene Climacteric Symptom Rating Scale [24], Midlife Women’s Symptom Index [25], Holte/Mikkelsen Menopause Checklist [26], Hunter’s Women’s Health Questionnaire [27], Neugarten and Kraines’ Symptom Checklist [28], Study of Women Across the Nation menopausal symptom checklist, Menopause Symptoms List [29], and Kupperman/Blatt Index [30–32].
Study selection
All citations from the database searches were screened independently by two authors (JSC, CDE, JA). Disagreements were resolved through discussion and erring on the side of inclusion to full-text screening, which was performed independently by two reviewers from the same group of three. All disagreements were resolved through discussion.
Data collection
Data extraction was performed by one author (JSC) and then verified by two reviewers (YS, CDE, MY, CXC, JET). Discrepancies were resolved through discussion and referral back to the article contents. The data extraction form included fields about treatment categories, the article (author, year, country), design (number of groups, masking, randomization, control, and crossover), sample characteristics (number, age, symptoms when recruited, and menopausal status), data collection and timepoints, intervention and control or comparison condition details, palpitations as outcome or adverse effect, study findings, and quality and bias ratings. Cochrane levels of evidence ratings were noted, and the ROBINS-I and ROBINS-II tools were used to assess for presence of bias and study quality [33,34]. Because this appeared to be the first review of its kind and we aimed to conduct an inclusive/comprehensive review, papers were not excluded solely on the basis of quality ratings.
Data synthesis
To aid the data synthesis, the tables were organized by author last name from the included articles within larger categories of drug and non-drug therapies. Within each category, extracted data were synthesized. Effects of the interventions on palpitations prevalence and/or severity were noted as significant or non-significant and details about the significant findings were further described. Heterogeneity across the reviewed articles prohibited meta-analysis. We included an evidence summary statement in the results pertaining to each treatment, was done in a prior vasomotor treatment position paper [35]. We included a statement about whether each treatment could be recommended (positive level 1 evidence from multiple articles), could be recommended with caution (positive level II evidence from multiple articles), or could not be recommended at this time (insufficient, negative, or equivocal evidence) [35].
Results
This search initially identified 1574 citations. After 904 duplicates were removed, there were 670 unique articles. Of these, 62 were excluded, most because of no palpitations data or not being data-based. Of the remaining 608 articles reviewed in full text, 571 were excluded, most for not containing data on palpitations or data not separately reported. This resulted in 37 eligible articles for the review. The selection process is illustrated in the PRISMA [22] diagram (Figure 1).
Figure 1.
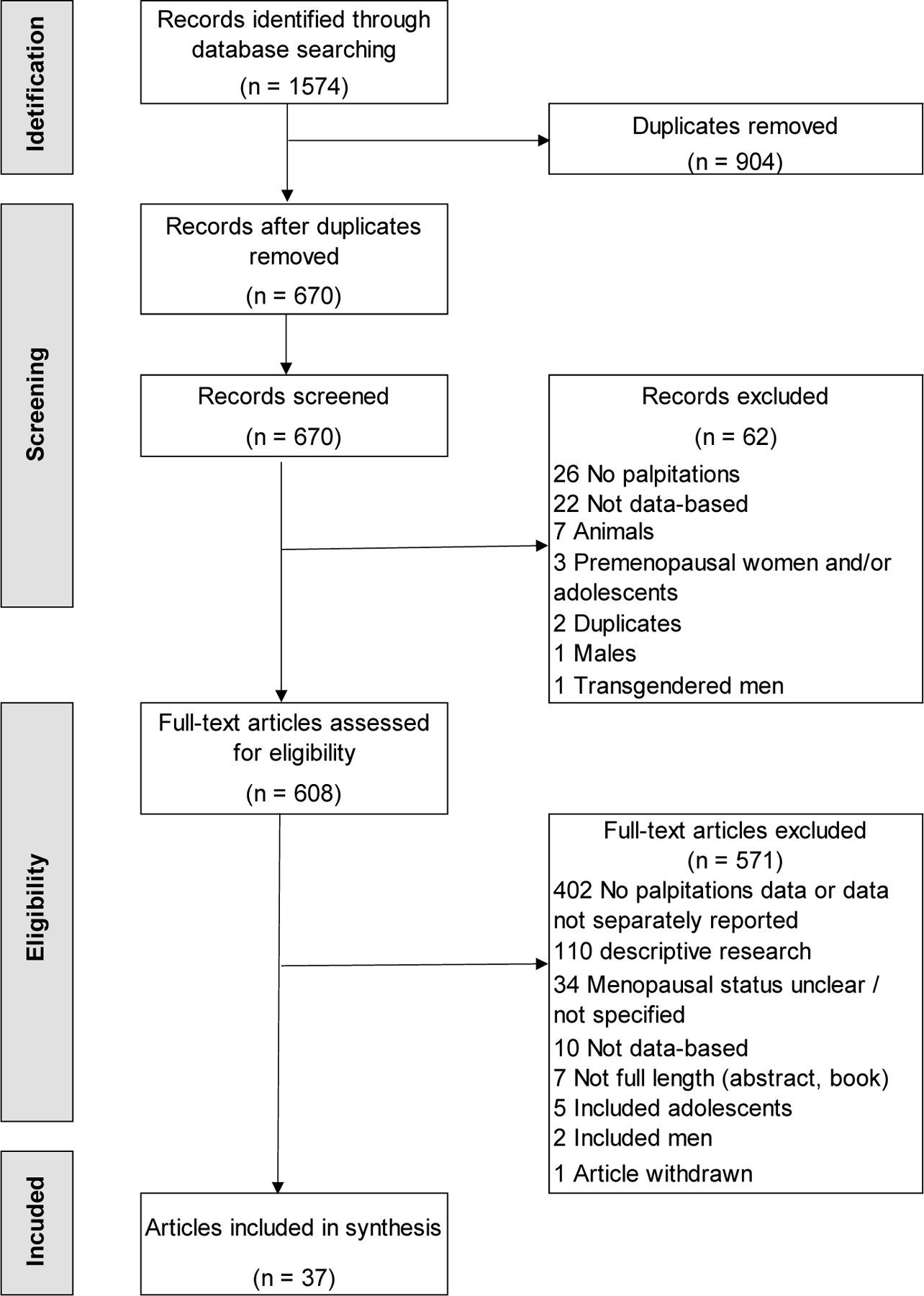
Flow diagram depicting disposition of the articles.
Articles are summarized in tables and figures. Table 1 contains descriptive information about all articles, organized by treatment option category of drug therapies followed by non-drug therapies. Table 2 pertains to 2- and 3-group trials and shows comparative effects on palpitations. Additional details on results for treatments on palpitations prevalence and/or severity are in Supplement A. Bias assessment for articles is shown in Supplement B.
Table 1.
Descriptive information from interventions articles (N=37).
| Author, year, country | Design | Sample size and characteristics | Measure, timepoints | Intervention | O or AE | QR |
|---|---|---|---|---|---|---|
| DRUG THERAPIES (n=19) | ||||||
| Hormonal agents (n=15) | ||||||
| Anarte et al., 1998 [36], Spain | 2-group RT Unclear masking |
|
|
|
O | II |
| Bhattacharya & Jha, 2010 [37], India | 2-group single blind RT |
|
|
|
AE | NA |
| Carmignani et al., 2010 [38], Brazil | 3-group double blind RCT |
|
|
|
O | II |
| Checa et al., 2005 [39], Spain | 3-group evaluator blind RT |
|
|
|
O | II |
| Chittacharoen et al., 2004 [40], Thailand | 1-group open-label pre-post trial |
|
|
|
O | III |
| Elfituri et al., 2005 [41], Libya | 2-group RT, unclear masking |
|
|
|
O | II |
| Fluck et al., 2002 [49], United Kingdom | Follow-up from 2-group open-label trial |
|
|
|
O | III |
| Kim et al., 2019 [42], Korea | 2-group open-label trial |
|
|
|
O | III |
| Moyer et al., 2018 [43], USA | 3-group RCT, unclear masking |
|
|
|
O | II |
| Nevinny-Stickel, 1983 [50], Germany | 2-group single blind crossover RT |
|
|
|
O | II |
| Polo-Kantola et al., 1998 [44], Finland | 2-group double blind crossover RCT |
|
|
|
O | II |
| Pornel 1996 [45], United Kingdom, Australia, New Zealand, Italy, Belgium, France, Netherlands | 1st trial: 2-group double blind RT 2nd trial: 2 group open label RT |
1st trial:
|
|
1st trial:
|
O | II |
| Takamatsu et al., 2001 [46], Japan | 2-group open-label trial |
|
|
|
O | III |
| Tit et al., 2017 [47], Romania | 3-group open label trial |
|
|
|
O | III |
| Glaser et al., 2011 [48], USA & Greece | 1-group open label trial |
|
|
|
O | III |
| Non-hormonal drug therapies (n=4) | ||||||
| SSRI/SNRI (n=3) | ||||||
| Callegari et al., 2019 [51], Italy | 2-group open label trial |
|
|
|
AE | NA |
| Freeman et al., 2017 [52], USA | 1-group open label trial |
|
|
|
AE | NA |
| Kornstein et al., 2015 [53], USA | 2-group double blind RCT |
|
|
|
AE | NA |
| Antihypertensives (n=1) | ||||||
| Kujala et al., 2014 [54], Finland | 2-group RT Unclear masking |
|
|
|
O | III |
| NON-DRUG THERAPIES (n=18) | ||||||
| Supplementary treatments – isoflavones and other phytoestrogens (n=5) | ||||||
| Agosta et al., 2011 [55], Italy | 2-group RCT (unclear if open-label or masked) |
|
|
|
O | III |
| Ahsan & Mallick, 2017 [56], India | 1-group open-label trial |
|
|
|
O | III |
| Auerbach et al., 2012 [57], Austria | 2-group double blind RCT |
|
|
|
O | II |
| Costa et al., 2017 [58], Brazil | 2-group double blind RT |
|
|
|
O | II |
| Davinelli et al., 2017 [59], Italy | 2-group double blind RCT |
|
|
|
O | II |
| Supplementary treatments – Rheum rhaponticum or false rhubarb (n=3) | ||||||
| Hasper et al., 2009 [60], Ukraine | 2-group double blind RCT |
|
|
|
O | II, IV |
| Heger et al., 2006 [61], Ukraine | 2-group double blind RCT |
|
|
|
O | II |
| Kaszkin-Bettag et al., 2009 [62], Ukraine | 2-group double blind RCT |
|
|
|
O | II |
| Supplementary treatments – Salvia officinalis or sage (n=3) | ||||||
| Bommer et al., 2011 [63], Switzerland | 1-group open-label trial |
|
|
|
O | III |
| Dadfar & Bamdad, 2019 [64], Iran | 1-group open-label trial |
|
|
|
O | III |
| Zeidabadi et al., 2020 [65], Iran | 2-group double blind RCT |
|
|
|
O | II |
| Supplementary treatments – other (n=4) | ||||||
| Fatima et al., 2017 [66], India | 2-group patient blind RCT |
|
|
|
O | II |
| Modi et al., 2012 [67], India | 1-group open-label trial |
|
|
|
O | III |
| Nayak et al., 2011 [68], India | 1-group open-label trial |
|
|
|
O | III |
| Park & Kim, 2016 [69], South Korea | 2-group double blind RCT |
|
|
|
O | II |
| Psychological intervention (n=2) | ||||||
| Alder et al., 2006 [70], Switzerland | 1-group open-label trial |
|
|
|
O | III |
| Qian et al., 2010 [71], China | 3-group RT, partial factorial trial, unclear if masked |
|
|
|
O | II |
| Auricular acupressure (n=1) | ||||||
| Kung et al., 2011 [72], Taiwan | 1-group open-label trial |
|
|
|
O | III |
O = outcome, AE = adverse event, QR = level of evidence quality rating, R = randomized, C = controlled, T = trial, HT = hormonal therapy, IG = intervention group, CG = control group, M = mean, sx = symptoms, meno = menopausal, premeno = premenopausal, peri = perimenopausal, postmeno = postmenopausal, NA = not applicable, MRS = menopausal rating scale, SSRI = selective serotonin reuptake inhibitor, SNRI = serotonin-norepinephrine reuptake inhibitor, MDD = major depressive disorder, oCEE = oral conjugated equine estrogen, ERr 731 = a special extract of Rheum rhaponticum, DDCYSS = Distress During Climacteric Years Symptom Scale.
Table 2.
Palpitations findings from two or three groups articles of drug therapies and non-drug therapies (n=24).
| 1st author year | Placebo benefit | Treatment benefit | Comparison of treatment and placebo benefit | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No | Yes | No | Yes | Same | Difference | ||
| DRUG THERAPIES (n=13) | |||||||
| Hormonal agents (n=12) | |||||||
| Anarte 1998 [36] | √ | ♦ | HT + psychological treatment > HT | ||||
| √ | |||||||
|
| |||||||
| Carmignani 2010 [38] | ● | √ | ♦ | HT = Isoflavone = placebo | |||
| √ | |||||||
|
| |||||||
| Checa 2005 [39] | √ | ♦ | HT > Calcium or raloxifene at 6 not 12 months | ||||
| √ | |||||||
| √ | |||||||
|
| |||||||
| Elfituri 2005 [41] | √ | ♦ | Estradiol+dydrogesterone = Tibolone | ||||
| √ | |||||||
|
| |||||||
| Fluck 2002 [49] | √ | ♦ | Tibolone > untreated group | ||||
|
| |||||||
| Kim 2019 [42] | √ | ♦ | Estrogen = Tibolone | ||||
| √ | |||||||
|
| |||||||
| Moyer 2018 [43] | ● | √ | ♦ | oCEE = Transdermal E = placebo | |||
| √ | |||||||
|
| |||||||
| Nevinny-Stickel 1983 [50] | ● | √ | ♦ | Org OD 14 = placebo | |||
|
| |||||||
| Polo-Kantola 1998 [44] | ● | √ | ♦ | HT = placebo | |||
|
| |||||||
| Pornel 1996 [45] | √ | ♦ | (HT) Menorest = Premarin = Estraderm | ||||
| √ | |||||||
| √ | |||||||
|
| |||||||
| Takamatsu 2001 [46] | √ | ♦ | HT < Counseling | ||||
| √ | |||||||
|
| |||||||
| Tit 2017 [47] | √ | HT, isoflavones not reported | |||||
| √ | |||||||
|
Non-hormonal drug therapies (n=1) | |||||||
| Kujala 2014 [54] | √ | ♦ | Moxonidine = Atenolol | ||||
| √ | |||||||
|
NON-DRUG THERAPIES (n=11) | |||||||
| Supplementary treatments - isoflavones&other phytoestrogens (n=4) | |||||||
| Agosta 2011[55] | √ | ♦ | Estromineral = Estromineral Serena | ||||
| √ | |||||||
|
| |||||||
| Auerbach 2012 [57] | ● | √ | ♦ |
Pomegranate seed oil = placebo | |||
|
| |||||||
| Costa 2017 [58] | √ | ♦ | Isoflavone + exercise = Placebo + exercise | ||||
| √ | |||||||
|
| |||||||
| Davinelli 2017 [59] | ● | √ | ♦ | Equopausa (resveratrol) > placebo | |||
|
Supplementary treatments - Rheum rhaponticum (n=3) | |||||||
| Hasper 2009 [60] | ● | √ | ♦ | ERr 731 = placebo | |||
|
| |||||||
| Heger 2006 [61] | ● | √ | ♦ | ERr 731 > placebo | |||
|
| |||||||
| Kaszkin-Bettag 2009 [62] | ● | √ | ♦ | ERr 731 > placebo | |||
|
Supplementary treatments - Salvia officinalis or sage (n=1) | |||||||
| Zeidabadi 2020 [65] | ● | √ | ♦ | Salvia officinalis extract > placebo | |||
|
Supplementary treatments - other (n=2) | |||||||
| Fatima 2017 [66] | ● | √ | ♦ | Tribulus terrestris L. > placebo | |||
|
| |||||||
| Park & Kim, 2016 [69] | ● | √ | ♦ | Schisandra chinensis > placebo | |||
|
Psychological intervention (n=1) | |||||||
| Qian 2010 [71] | √ | √ | ♦ | Psychological + herbals > single treatment | |||
| √ | |||||||
> indicates that one intervention is superior to another one or placebos; = indicates no effect difference between interventions and/or placebos.
HT = hormonal therapy, oCEE = oral conjugated equine estrogen, ERr 731 = a special extract of Rheum rhaponticum.
Drug therapy options (n=19)
Drug therapies (n=19) for menopausal symptoms included: (1) hormonal agents (n=15); (2) non-hormonal drug therapies (n=4) including selective serotonin reuptake inhibitor/serotonin-norepinephrine reuptake inhibitor (SSRI/SNRI) (n=3), and antihypertensives (n=1). Each category is discussed below.
Hormonal agents (n=15)
As shown in Table 1, 15 articles focused on various hormonal agents (e.g., estrogen, progesterone, tibolone, raloxifene) with differing mechanisms of action [36–50]. Articles were from 19 different countries. Most articles included a control or comparison group (86.7%) [36–39,41–47,49,50]. Most articles focused on peri- and postmenopausal women reporting one or more menopausal symptoms. Sample sizes varied with 60% below 100 (n=9) [36–38,40,42,44,46,49,50], 20% between 100 and 200 (n=3) [39,41,43], and 20% over 200 (n=3) [45,47,48]. Palpitations were measured using a single self-reported item in 10 different measures, such as “palpitations” in the Kupperman index [36] and “heart discomfort” in the Menopause Rating Scale (MRS) [38,42]. Intervention doses and treatment periods varied across all the articles. For example, one article [36] used transdermal 17 β-estradiol plus medroxyprogesterone on days 14 and 25 for 6 months, while another [43] applied transdermal 17 β-estradiol 50ug daily with micronized progesterone 200mg daily for first 12 days of the month for 48 months. Most data collection periods were within one year and slightly more than half of the articles collected data at three or more timepoints [36,39–41,43,44,47,50]. Palpitations were studied as an adverse effect in one article, where one subject withdrew from transdermal estradiol due to palpitations [37]. In the remaining 14 articles, palpitations were an outcome usually assessed as severity (Supplement A). Of these 14, none (0%) were level I, 57.1% were level II [36,38,39,41,43–45,50] and 42.9% were level III evidence [40,42,46–49].
As shown in Table 2 and Supplement A, in most articles, hormonal agents showed treatment benefit in relation to baseline but not necessarily in relation to placebo or other comparators [38,39,41,42,45–47,49]. When hormonal agents were compared to other interventions, various forms of hormonal agents were equally beneficial, but hormonal agents were not superior to isoflavone [38]. When hormonal agents were combined with psychological treatment, benefits were superior to hormonal agents alone in one study [36], yet in another study, hormonal agents alone was less efficacious than counseling [46]. In two studies, neither hormonal agents or placebo showed benefit [44,50]. In two uncontrolled trials [40,48] shown in Supplement A, severity decreased from pre- to post-treatment with 3 or 6 months of hormonal agents. However, in one report, most women (73.7%) reported unchanged or worsened palpitations [40]. Based on the evidence, hormonal agents should be recommended with caution for menopausal palpitations.
Non-hormonal drug therapies (n=4)
SSRI/SNRI.
As shown in Table 1, palpitations were studied as an adverse effect in three articles [51–53]. One [51] focused on postmenopausal women and two [52,53] on peri- and postmenopausal women reporting depressive symptoms. Of the three articles, two [51,52] had less than 40 women, while one [53] had about 800 women. Palpitations were assessed as a self-reported adverse effect by a checklist or a scale for adverse effects. Palpitations were reported following treatment with vortioxetine (3.7% [51], 25.0% [52]), and desvenlafaxine (3.5%) [53] but not paroxetine (0%) [51]. Although all articles used an outcome measure that included palpitations, none reported the number of participants with palpitations at baseline. Thus, it is unclear whether palpitations were present at baseline or truly an adverse effect of treatment. Given that there is no efficacy data for these medications on palpitations, they cannot be recommended at this time.
Antihypertensives.
As shown in Table 1, one article compared moxonidine and atenolol in 112 postmenopausal women in Finland [54]. Palpitations were an outcome measured by a single self-reported item in a questionnaire. Both medications showed benefit over baseline on decreasing palpitations severity, but there was no placebo group [54] (Table 2, Supplement A). Based on this single article of level III evidence, moxonidine and atenolol are not recommended for menopausal palpitations.
Assessment of bias in articles of drug therapies
All articles of drug therapies (100%) had a critical risk of bias, defined as bias in three or more categories. Article bias was from: no confounders included in the analysis (93.3% articles, 14/15), participant selection bias present or unclear (94.7%, 18/19), no palpitations-specific inclusion criteria (100%), no treatment adherence assessment or analysis (94.7%, 18/19), no dropout analysis among five trials with dropout (100%), and/or no specified measurement recall period (100%) (Supplement B).
Non-drug therapy options (n=18)
Non-drug therapies (n=18) for menopausal symptoms included: supplementary treatments (n=15), psychological intervention (n=2) and auricular acupressure (n=1). Each category is discussed below.
Supplementary treatments – isoflavones and other phytoestrogens (n=5)
As shown in Table 1, five articles evaluated isoflavones and other phytoestrogens and were conducted in four countries, including Italy, India, Austria, and Brazil [55–59]. Four articles compared an intervention to another intervention or placebo [55,57–59], while one was a single-group design [56]. Four articles (80%) included 100 or fewer peri- or postmenopausal women. In all studies, the sample mean age ranged from 42.3 to 55 years. Palpitations were measured as an outcome using a single self-reported item in a survey, MRS, and Blatt-Kupperman index. Of the five articles, three were level II [57–59], and two were level III evidence [55,56].
As shown in Table 2 and Supplement A, the effects of isoflavones/other phytoestrogens were equivocal. One uncontrolled trial of isoflavones showed positive effects on palpitations [56], and another controlled trial showed an isoflavone together with resveratrol was beneficial over placebo [59]. However, in controlled trials, phytoestrogens from pomegranate seed oil showed no benefit over placebo [57], and isoflavone plus exercise showed no benefit over exercise plus placebo [58]. One comparative study showed no differences between two isoflavone supplementary treatments, one of which also contained magnolia bark extract [55]. Based on this evidence, isoflavones and other phytoestrogens are not recommended for menopausal palpitations.
Supplementary treatments– Rheum rhaponticum (n=3)
Three randomized controlled trials compared Rheum rhaponticum extract (ERr 731) to placebo for menopausal symptoms [60–62] (Table 1). All had about 100 perimenopausal Ukrainian women with a mean age of about 49 years old. Palpitations as an outcome were measured using a single self-reported item, “heart complaints” in the MRS. All women in Hasper et al. [60] were from a sample who previously participated in the trial by Heger et al. [61]. Hasper et al. [60] measured palpitations for every 12 weeks from baseline to 48 weeks and the other two measured every 28 days from baseline to 84 days [61,62]. All the articles were level II evidence, except a sub-study from Hasper et al., which was level IV [60]. As shown in Table 2 and Supplement A, results were positive in two trials [61,62] but not another trial [60]. Based on the evidence, Rheum rhaponticum is not recommended for menopausal palpitations.
Supplementary treatments – Salvia officinalis or sage (n=3)
Three articles used Salvia officinalis or sage for menopausal symptoms in postmenopausal women from Switzerland or Iran [63–65] (Table 1). Palpitations were an outcome measured by a single item in the MRS from baseline to post-treatment with a range of 2 to 12 weeks. One article was level II [65] and two were level III evidence [63,64]. As shown in Table 2 and Supplement A, one controlled trial [65] found Salvia officinalis to be more effective than placebo for reducing palpitations severity. In two uncontrolled trials [63,64], severity was decreased from pre- to post-treatment after 4-week and 8-week treatments with Salvia officinalis. Because of the level of evidence, Salvia officinalis cannot be recommended for menopausal palpitations.
Supplementary treatments – other (n=4)
As shown in Table 1, four articles evaluated other miscellaneous supplementary treatments, including Tribulus Terrestris L. (fruits) powder, ayurvedic agents, homeopathic agents, and Schisandra chinensis [66–69]. Three articles were from India [66–68] and one from South Korea [69]. The majority (75%) had small sample sizes of 36 to 60 peri- or postmenopausal women. Palpitations were assessed using a single self-reported item differently across articles, such as “heart discomfort” [66,67] and “heart palpitations” [69]. Half were level II [66,69] and half were level III evidence [67,68].
Compared to placebo, Tribulus terrestris [66] significantly reduced palpitations prevalence and severity [66], and Schisandra chinesis reduced severity [69] (Table 2, Supplement A). In uncontrolled trials, ayurvedic agents decreased palpitations severity over a 3-month period, and [67] and homeopathic agents decreased palpitations prevalence and severity over one year [68]. Based on only one level II/III evidence article of each supplementary treatment, none of these agents can be recommended for menopausal palpitations.
Psychological intervention (n=2)
Two articles evaluated psychological interventions for menopausal symptoms [70,71] (Table 1, Table 2, Supplement A). In an uncontrolled trial (level III evidence) in 30 women, palpitation severity significantly decreased from pre- to post-treatment after 7 weekly 1.5-hour group cognitive-behavioral sessions [70]. In a partial factorial trial (level II evidence) in 164 women, a psychological intervention plus Chinese herbals were significantly more beneficial in reducing palpitations severity compared to either intervention alone [71]. Based on limited level II evidence, a psychological intervention cannot be recommended for palpitations at this time.
Auricular acupressure (n=1)
One article evaluated 4-week auricular acupressure in 45 postmenopausal Taiwanese women reporting insomnia [72] (Table 1). Palpitations were measured at baseline and 4 weeks post-treatment using a single self-report item in the MRS. Although severity decreased over time (Supplement A), based on this single article of level III evidence, auricular acupressure cannot be recommended for palpitations at this time.
Assessment of bias in articles of non-drug therapies
All non-drug therapy articles (100%) had a critical risk of bias, defined as bias in three or more categories. Article bias was from: no confounders in the analysis (94.4%, 17/18), participant selection bias present or unclear (88.9%, 16/18), no palpitations-specific inclusion criteria (100%), no treatment adherence assessment or analysis (94.4%, 17/18), no dropout analysis among nine trials with dropout (100%), and/or no specified measurement recall period (100%) (Supplement B).
Discussion
This is the first review of treatments for palpitations occurring during the menopause transition. The major findings from this review were the relative scarcity of articles, the heterogeneity in treatments, design, and outcome measures, and the poor quality of articles. Only 37 articles were identified, which pales in comparison to the number of intervention articles related to other menopausal symptoms such as hot flashes or sleep disturbances. In addition, articles varied in terms of intervention (category, agent, dose, treatment length), design, and outcome measures, thus preventing comparisons and meta-analysis. All articles showed a critical risk of bias when being evaluated for efficacy on palpitations. Finally, there was no level I evidence for any intervention, and level II evidence was not always positive. Thus, based on this review, no treatments can be fully recommended. Only hormonal agents can be recommended with caution, and all other treatments in the reviewed articles cannot be recommended given the existing evidence [35].
The second finding in this review was the lack of focus on palpitations as an outcome. In articles where palpitations were assessed as an outcome, measurement tools and statistical power were problematic. For example, palpitations were assessed mainly via a single item on a checklist with response options related to the severity, and the recall period for the measure was commonly unspecified. In addition, because articles focused on menopausal symptoms in general, and only a subset of women reported palpitations at baseline, negative findings in many of the smaller trials could be because studies were not powered on palpitations as the outcome. In addition, in four articles [37,51–53] it was unclear whether palpitations resulted from treatments, including hormonal agents (transdermal estrogen [37]) and non-hormonal SSRI/SNRI (vortioxetine [51,52] and desvenlafaxine [53]), or whether they were present at baseline.
In regard to safety, some of the treatment options studied have been associated with adverse effects. For example, in a Cochrane review, tibolone was associated with vaginal bleeding and recurrent breast cancer in those with a history of breast cancer and may be associated with stroke in women over 60 years old [73]. SSRI/SNRI were associated with risks of bleeding, hyponatremia [74], nausea, headache, insomnia, and sexual dysfunction [75]. Long-term phytoestrogens (e.g., 5-year treatment) were associated with an increased risk of endometrial hyperplasia [76]. Adverse effects of Rheum rhaponticum include hypersensitivity or rash, gastrointestinal symptoms, and nervous system effects [77]. Adverse effects of Salvia officianalis include mild abdominal pain, mild diarrhea, acneiform skin eruption, and mild gastrointestinal complaints [78]. Herbal dietary supplementary treatments are associated with adverse effects in different physiological systems, such as the central nervous system, hematologic, and cardiovascular [79]. Assessment and documentation of adverse effects may be missing, particularly due to misconceptions that natural products are safe because they come from plants. In future research testing interventions for palpitations, it will be critical to examine intervention efficacy and safety to generate the evidence women and providers need to make informed choices about treatment options.
Results from this review should be considered in light of some limitations. Only articles in English were included, which may have led to the inadvertent omission of some articles or some types of interventions (e.g., Traditional Chinese medicine reported in Chinese language publications). Palpitations do not have a standardized subject search term in any of the databases we searched; therefore, some articles may have been missed in our search. Finally, we did not conduct meta-analysis to quantitatively calculate the overall effect size due to the high heterogeneity across studies, and thus avoided the likelihood of producing a questionable summary [80].
This review contributes to future research and clinical practice in the field of palpitations. None of the reviewed articles used ECG devices to evaluate palpitations and exclude arrhythmias, such as atrial fibrillation. Several wearable ECG devices are approved as clinically diagnostic for accurately capturing heart rate and rhythm disturbances [81]. Future studies should use clinically diagnostic ECG devices to evaluate rhythm and rate disturbances occurring with the felt sensation of palpitations to better understand the symptom and develop treatments. Clinicians should be aware that women reporting palpitations may need referral to a cardiologist to further evaluate the symptoms, any underlying arrhythmia, and determine a best course of treatment. We note that while beta-blockers effectively control heart rhythm when used appropriately [82], there is scant evidence regarding their use for menopausal symptoms [54,83].
Conclusions
This review serves as a call to action for rigorous clinical research evaluating the efficacy and safety of commonly researched menopausal symptom treatment options on palpitations. This review also informs clinicians about the current state of evidence related to menopausal symptom treatment options and palpitations. Based on available evidence, no treatment options can be fully recommended, only hormonal agents can be recommended with caution, and many other treatments cannot be recommended based on currently available evidence.
Supplementary Material
References
- 1.North American Menopause Society. Menopause Practice: A Clinician's Guide 6th ed. Mayfield Heights, OH: North American: Menopause Society; 2019. [Google Scholar]
- 2.Islam RM, Bell RJ, Billah B, et al. Prevalence and severity of vasomotor symptoms and joint pain in women at midlife in Bangladesh: a population-based survey. Menopause 2016;23(7):731–739. [DOI] [PubMed] [Google Scholar]
- 3.Islam RM, Bell RJ, Rizvi F, et al. Vasomotor symptoms in women in Asia appear comparable with women in Western countries: a systematic review. Menopause 2017;24(11):1313–1322. [DOI] [PubMed] [Google Scholar]
- 4.Minkin MJ, Reiter S, Maamari R. Prevalence of postmenopausal symptoms in North America and Europe. Menopause 2015;22(11):1231–1238. [DOI] [PubMed] [Google Scholar]
- 5.Sriprasert I, Pantasri T, Piyamongkol W, et al. An International Menopause Society study of vasomotor symptoms in Bangkok and Chiang Mai, Thailand. Climacteric 2017;20(2):171–177. [DOI] [PubMed] [Google Scholar]
- 6.Obermeyer CM, Reynolds RF, Price K, et al. Therapeutic decisions for menopause: results of the DAMES project in central Massachusetts. Menopause 2004;11(4):456–465. [DOI] [PubMed] [Google Scholar]
- 7.Nelson HD. Menopause. Lancet (London, England) 2008;371(9614):760–770. [DOI] [PubMed] [Google Scholar]
- 8.Bruce D, Rymer J. Symptoms of the menopause. Best Pract Res Clin Obstet Gynaecol 2009;23(1):25–32. [DOI] [PubMed] [Google Scholar]
- 9.Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol 2015;126(4):859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costanian C, Zangiabadi S, Bahous SA, et al. Reviewing the evidence on vasomotor symptoms: the role of traditional and non-traditional factors. Climacteric 2020;23(3):213–223. [DOI] [PubMed] [Google Scholar]
- 11.Drewe J, Bucher KA, Zahner C. A systematic review of non-hormonal treatments of vasomotor symptoms in climacteric and cancer patients. SpringerPlus 2015;4(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iliodromiti S, Wang W, Lumsden MA, et al. Variation in menopausal vasomotor symptoms outcomes in clinical trials: a systematic review. BJOG 2020;127(3):320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter JS, Sheng Y, Elomba CD, et al. A systematic review of palpitations prevalence by menopausal status. Curr Obstet Gynecol Rep 2021;10:7–13. [Google Scholar]
- 14.Carpenter JS, Tisdale JE, Chen CX, et al. A menopause strategies–finding lasting answers for symptoms and health (MsFLASH) investigation of self-reported menopausal palpitation distress. J Womens Health (Larchmt) 2021;30(4):533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyrnes A, Mathiesen EB, Njølstad I, et al. Palpitations are predictive of future atrial fibrillation. An 11-year follow-up of 22,815 men and women: the Tromsø Study. Eur J Prev Cardiol 2013;20(5):729–736. [DOI] [PubMed] [Google Scholar]
- 16.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129(8):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinkerton JV. Money talks: untreated hot flashes cost women, the workplace, and society. Menopause 2015;22(3):254–255. [DOI] [PubMed] [Google Scholar]
- 18.Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause 2015;22(3):260–266. [DOI] [PubMed] [Google Scholar]
- 19.Whiteley J, Wagner JS, Bushmakin A, et al. Impact of the severity of vasomotor symptoms on health status, resource use, and productivity. Menopause 2013;20(5):518–524. [DOI] [PubMed] [Google Scholar]
- 20.Assaf AR, Bushmakin AG, Joyce N, et al. The relative burden of menopausal and postmenopausal symptoms versus other major conditions: a retrospective analysis of the medical expenditure panel survey data. Am Health Drug Benefits 2017;10(6):311–321. [PMC free article] [PubMed] [Google Scholar]
- 21.Sheng Y, Carpenter JS, Elomba CD, et al. Review of menopausal palpitations measures. Womens Midlife Health 2021. Advance online publication. doi: 10.1186/s40695-021-00063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinemann LA, DoMinh T, Strelow F, et al. The Menopause Rating Scale (MRS) as outcome measure for hormone treatment? A validation study. Health Qual Life Outcomes 2004;2(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene JG. Constructing a standard climacteric scale. Maturitas 1998;29(1):25–31. [DOI] [PubMed] [Google Scholar]
- 25.Im EO. The Midlife Women's Symptom Index (MSI). Health Care Women Int 2006;27(3):268–287. [DOI] [PubMed] [Google Scholar]
- 26.Holte A, Mikkelsen A. The menopausal syndrome: a factor analytic replication. Maturitas 1991;13(3):193–203. [DOI] [PubMed] [Google Scholar]
- 27.Hunter M The south-east England longitudinal study of the climacteric and postmenopause. Maturitas 1992;14(2):117–126. [DOI] [PubMed] [Google Scholar]
- 28.Neugarten BL, Kraines RJ. "Menopausal symptoms" in women of various ages. Psychosom Med 1965;27:266–273. [DOI] [PubMed] [Google Scholar]
- 29.Perz JM. Development of the menopause symptom list: a factor analytic study of menopause associated symptoms. Women Health 1997;25(1):53–69. [DOI] [PubMed] [Google Scholar]
- 30.Delaplaine RW, Bottomy JR, Blatt M, et al. Effective control of the surgical menopause by estradiol pellet implantation at the time of surgery. Surg Gynecol Obstet 1952;94(3):323–333. [PubMed] [Google Scholar]
- 31.Blatt MH, Wiesbader H, Kupperman HS. Vitamin E and climacteric syndrome; failure of effective control as measured by menopausal index. AMA Arch Intern Med 1953;91(6):792–799. [DOI] [PubMed] [Google Scholar]
- 32.Kupperman HS, Wetchler BB, Blatt MH. Contemporary therapy of the menopausal syndrome. J Am Med Assoc 1959;171:1627–1637. [DOI] [PubMed] [Google Scholar]
- 33.Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366(14898). [DOI] [PubMed] [Google Scholar]
- 35.Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause 2015;22(11):1155–1172. [DOI] [PubMed] [Google Scholar]
- 36.Anarte MT, Cuadros JL, Herrera J. Hormonal and psychological treatment: therapeutic alternative for menopausal women? Maturitas 1998;29(3):203–213. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharya SM, Jha A. Effects of transdermal estradiol gel and oral tibolone on health-related quality of life after surgical menopause. Int J Gynaecol Obstet 2010;110(3):213–216. [DOI] [PubMed] [Google Scholar]
- 38.Carmignani LO, Pedro AO, Costa-Paiva LH, et al. The effect of dietary soy supplementation compared to estrogen and placebo on menopausal symptoms: a randomized controlled trial. Maturitas 2010;67(3):262–269. [DOI] [PubMed] [Google Scholar]
- 39.Checa MA, Garrido A, Prat M, et al. A comparison of raloxifene and calcium plus vitamin D on vaginal atrophy after discontinuation of long-standing postmenopausal hormone therapy in osteoporotic women. A randomized, masked-evaluator, one-year, prospective study. Maturitas 2005;52(1):70–77. [DOI] [PubMed] [Google Scholar]
- 40.Chittacharoen A, Domhardt R, Manonai J, et al. Alleviation of the climacteric symptoms with oral sequential hormone replacement therapy. J Med Assoc Thai 2004;87(1):1–7. [PubMed] [Google Scholar]
- 41.Elfituri A, Sherif F, Elmahaishi M, et al. Two hormone replacement therapy (HRT) regimens for middle-eastern postmenopausal women. Maturitas 2005;52(1):52–59. [DOI] [PubMed] [Google Scholar]
- 42.Kim HK, Jeon SH, Ryu KJ, et al. Comparison of the efficacy of tibolone and transdermal estrogen in treating menopausal symptoms in postmenopausal women. J Menopausal Med 2019;25(3):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moyer AM, de Andrade M, Faubion SS, et al. SLCO1B1 genetic variation and hormone therapy in menopausal women. Menopause 2018;25(8):877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polo-Kantola P, Erkkola R, Helenius H, et al. When does estrogen replacement therapy improve sleep quality? Am J Obstet Gynecol 1998;178(5):1002–1009. [DOI] [PubMed] [Google Scholar]
- 45.Efficacy Pornel B. and safety of Menorest in two positive-controlled studies. Eur J Obstet Gynecol Reprod Biol 1996;64(Suppl):S35–37. [DOI] [PubMed] [Google Scholar]
- 46.Takamatsu K, Ohta H, Makita K, et al. Effects of counseling on climacteric symptoms in Japanese postmenopausal women. J Obstet Gynaecol Res 2001;27(3):133–140. [DOI] [PubMed] [Google Scholar]
- 47.Ţiţ DM, Pallag A, Iovan C, et al. Somatic-vegetative symptoms evolution in postmenopausal women treated with phytoestrogens and hormone replacement therapy. Iran J Public Health 2017;46(11):1528–1534. [PMC free article] [PubMed] [Google Scholar]
- 48.Glaser R, York AE, Dimitrakakis C. Beneficial effects of testosterone therapy in women measured by the validated Menopause Rating Scale (MRS). Maturitas 2011;68(4):355–361. [DOI] [PubMed] [Google Scholar]
- 49.Fluck E, File SE, Rymer J. Cognitive effects of 10 years of hormone-replacement therapy with tibolone. J Clin Psychopharmacol 2002;22(1):62–67. [DOI] [PubMed] [Google Scholar]
- 50.Nevinny-Stickel J Double-blind cross-over study with Org OD 14 and placebo in postmenopausal patients. Arch Gynecol 1983;234(1):27–31. [DOI] [PubMed] [Google Scholar]
- 51.Callegari C, Ielmini M, Caselli I, et al. Paroxetine versus vortioxetine for depressive symptoms in postmenopausal transition: a preliminary study. Psychopharmacol Bull 2019;49(1):28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman MP, Cheng LJ, Moustafa D, et al. Vortioxetine for major depressive disorder, vasomotor, and cognitive symptoms associated with the menopausal transition. Ann Clin Psychiatry 2017;29(4):249–257. [PubMed] [Google Scholar]
- 53.Kornstein SG, Clayton AH, Bao W, et al. A pooled analysis of the efficacy of desvenlafaxine for the treatment of major depressive disorder in perimenopausal and postmenopausal women. J Womens Health (Larchmt) 2015;24(4):281–290. [DOI] [PubMed] [Google Scholar]
- 54.Kujala SM, Pöyhönen-Alho M, Kaaja RJ. Effects of sympatholytic therapy on postmenopausal symptoms in hypertensive postmenopausal women. Climacteric 2014;17(4):356–362. [DOI] [PubMed] [Google Scholar]
- 55.Agosta C, Atlante M, Benvenuti C. Randomized controlled study on clinical efficacy of isoflavones plus Lactobacillus sporogenes, associated or not with a natural anxiolytic agent in menopause. Minerva Ginecol 2011;63(1):11–17. [PubMed] [Google Scholar]
- 56.Ahsan M, Mallick AK. The effect of soy isoflavones on the menopause rating scale scoring in perimenopausal and postmenopausal women: a pilot study. J Clin Diagn Res 2017;11(9):Fc13–fc16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Auerbach L, Rakus J, Bauer C, et al. Pomegranate seed oil in women with menopausal symptoms: a prospective randomized, placebo-controlled, double-blinded trial. Menopause 2012;19(4):426–432. [DOI] [PubMed] [Google Scholar]
- 58.Costa JG, Giolo JS, Mariano IM, et al. Combined exercise training reduces climacteric symptoms without the additive effects of isoflavone supplementation: a clinical, controlled, randomised, double-blind study. Nutr Health 2017;23(4):271–279. [DOI] [PubMed] [Google Scholar]
- 59.Davinelli S, Scapagnini G, Marzatico F, et al. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: a randomized, placebo-controlled study. Maturitas 2017;96:77–83. [DOI] [PubMed] [Google Scholar]
- 60.Hasper I, Ventskovskiy BM, Rettenberger R, et al. Long-term efficacy and safety of the special extract ERr 731 of Rheum rhaponticum in perimenopausal women with menopausal symptoms. Menopause 2009;16(1):117–131. [DOI] [PubMed] [Google Scholar]
- 61.Heger M, Ventskovskey BM, Borzenko I, et al. Efficacy and safety of a special extract of Rheum rhaponticum (ERr 731) in perimenopausal women with climacteric complaints: a 12-week randomized, double-blind, placebo-controlled trial. Menopause 2006;13(5):744–759. [DOI] [PubMed] [Google Scholar]
- 62.Kaszkin-Bettag M, Ventskovskiy BM, Solskyy S, et al. Confirmation of the efficacy of ERr 731 in perimenopausal women with menopausal symptoms. Altern Ther Health Med 2009;15(1):24–34. [PubMed] [Google Scholar]
- 63.Bommer S, Klein P, Suter A. First time proof of sage's tolerability and efficacy in menopausal women with hot flushes. Adv Ther 2011;28(6):490–500. [DOI] [PubMed] [Google Scholar]
- 64.Dadfar F, Bamdad K. The effect of Saliva officinalis extract on the menopausal symptoms in postmenopausal women: an RCT. Int J Reprod Biomed (Yazd) 2019;17(4):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeidabadi A, Yazdanpanahi Z, Dabbaghmanesh MH, et al. The effect of Salvia officinalis extract on symptoms of flushing, night sweat, sleep disorders, and score of forgetfulness in postmenopausal women. J Family Med Prim Care 2020;9(2):1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fatima L, Sultana A. Efficacy of Tribulus terrestris L. (fruits) in menopausal transition symptoms: a randomized placebo controlled study. Adv Integr Med 2017;4(2):56–65. [Google Scholar]
- 67.Modi MB, Donga SB, Dei L. Clinical evaluation of Ashokarishta, Ashwagandha Churna and Praval Pishti in the management of menopausal syndrome. Ayu 2012;33(4):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nayak C, Singh V, Singh K, et al. Management of distress during climacteric years by homeopathic therapy. J Altern Complement Med 2011;17(11):1037–1042. [DOI] [PubMed] [Google Scholar]
- 69.Park JY, Kim KH. A randomized, double-blind, placebo-controlled trial of Schisandra chinensis for menopausal symptoms. Climacteric 2016;19(6):574–580. [DOI] [PubMed] [Google Scholar]
- 70.Alder J, Besken KE, Armbruster U, et al. Cognitive-Behavioural Group Intervention for Climacteric Syndrome. Psychother Psychosom 2006;75(5):298–303. [DOI] [PubMed] [Google Scholar]
- 71.Qian L, Wang B, Niu J, et al. Assessment of the clinical effect of Chinese medicine therapy combined with psychological intervention for treatment of patients of peri-menopausal syndrome complicated with hyperlipidemia. Chin J Integr Med 2010;16(2):124–130. [DOI] [PubMed] [Google Scholar]
- 72.Kung YY, Yang CC, Chiu JH, et al. The relationship of subjective sleep quality and cardiac autonomic nervous system in postmenopausal women with insomnia under auricular acupressure. Menopause 2011;18(6):638–645. [DOI] [PubMed] [Google Scholar]
- 73.Formoso G, Perrone E, Maltoni S, et al. Short‐term and long‐term effects of tibolone in postmenopausal women. Cochrane Database Syst Rev 2016(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orleans RJ, Li L, Kim M-J, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med 2014;370(19):1777–1779. [DOI] [PubMed] [Google Scholar]
- 75.Al-Safi ZA, Santoro N. Menopausal hormone therapy and menopausal symptoms. Fertil Steril 2014;101(4):905–915. [DOI] [PubMed] [Google Scholar]
- 76.Unfer V, Casini ML, Costabile L, et al. Endometrial effects of long-term treatment with phytoestrogens: a randomized, double-blind, placebo-controlled study. Fertil Steril 2004;82(1):145–148. [DOI] [PubMed] [Google Scholar]
- 77.Chang J-L, Montalto MB, Heger PW, et al. Rheum rhaponticum extract (ERr 731): postmarketing data on safety surveillance and consumer complaints. Integr Med (Encinitas) 2016;15(3):34–39. [PMC free article] [PubMed] [Google Scholar]
- 78.Lopresti AL. Salvia (sage): a review of its potential cognitive-enhancing and protective effects. Drugs in R&D 2017;17(1):53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bellanger RA, Seeger CM, Smith HE. Safety of complementary and alternative medicine treatments and practices. In: Ray SD, editor. Side effects of drugs annual: Elsevier; 2019. p. 559–571. [Google Scholar]
- 80.Haidich AB. Meta-analysis in medical research. Hippokratia 2010;14(Suppl 1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 81.Marston HR, Hadley R, Banks D, et al. Mobile self-monitoring ecg devices to diagnose arrhythmia that coincide with palpitations: a scoping review. Healthcare (Basel) 2019;7(3):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary: a Report of the American College of Cardiology/American HeartAssociation Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias) Developed in collaboration with NASPE–Heart Rhythm Society. Eur Heart J 2003;24(20):1857–1897. [DOI] [PubMed] [Google Scholar]
- 83.Carranza-Lira S, Cortés-Fuentes E. Modification of vasomotor symptoms after various treatment modalities in the postmenopause. Int J Gynecol Obstet 2001;73(2):169–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


