Abstract
Objective
To estimate the prevalence of post-vaccination seropositivity against SARS-CoV-2 and identify its predictors in Peruvian Social Health Insurance (EsSalud) personnel in 2021.
Methods
We conducted a cross-sectional study in a representative simple stratified sample of EsSalud workers. We evaluated IgG anti-SARS-CoV-2 antibodies response (seropositivity) by passive (previous infection) and active immunization (vaccination), and epidemiological and occupational variables obtained by direct interview and a data collection form. Descriptive and inferential statistics were used with correction of sample weights adjusted for non-response rate, and crude and adjusted odds ratio (OR) and geometric mean ratio (GMR) with their respective 95% confidence intervals (95%CI) were estimated.
Results
We enrolled 1077 subjects. Seropositivity was 67.4% (95%CI: 63.4–71.1). Predictors of seropositivity were age (negative relation; p < 0.001), previous infection (aOR = 11.7; 95%CI: 7.81–17.5), working in COVID-19 area (aOR = 1.47; 95%CI: 1.02–2.11) and time since the second dose. In relation to antibody levels measured by geometric means, there was an association between male sex (aGMR = 0.77; 95%CI: 0.74–0.80), age (negative relation; p < 0.001), previous infection (aGMR = 13.1; 95%CI:4.99–34.40), non-face-to-face/licensed work modality (aGMR = 0.78; 95%CI: 0.73–0.84), being a nursing technician (aGMR = 1.30; 95%CI: 1.20–1.41), working in administrative areas (aGMR = 1.17; 95%CI: 1.10–1.25), diagnostic support (aGMR = 1.07; 95%CI: 1.01–1.15), critical care (aGMR = 0.85; 95%CI: 0.79–0.93), and in a COVID-19 area (aGMR = 1.30; 95%CI: 1.24–1.36) and time since receiving the second dose (negative relation; p < 0.001).
Conclusions
Seropositivity and antibody levels decrease as the time since receiving the second dose increases. Older age and no history of previous infection were associated with lower seropositivity and antibody values. These findings may be useful for sentinel antibody surveillance and the design of booster dose strategies.
Keywords: COVID-19, Vaccine, Serology, Antibodies, Doses (source: MeSH)
1. Introduction
The pandemic caused by the SARS-CoV-2 virus has reported figures of up to 458 million cases and 6 million deaths worldwide up to March 13, 2022 [1]. To mitigate the contagion and spread of the disease, many countries have responded with the development of vaccines.
One of the vaccines designed was BBIBP-CorV, developed by the Beijing Bio-Institute of Biological Products of the Chinese national BIOTEC group and known internationally as Sinopharm [2]. This vaccine was produced from the HB02 strain and consists of viral particles cultured in the laboratory and inactivated to lose their ability to produce disease while stimulating the host immune response [3].
Despite being one of the first countries to initiate mandatory social immobilization to reduce the spread of COVID-19, Peru has registered more than 2 million cases and has one of the highest mortality rates in the world at 9.3% [4]. These figures can be explained considering labor informality, agglomeration, precariousness of the health system and intradomiciliary overcrowding which prevail in Peruvian society [5,6].
Nonetheless, in order to address the further spread of COVID-19, one of the fundamental pillars implemented by the Peruvian government was the acquisition and administration of vaccines to immunize the population, starting with high-risk target groups such as health personnel [7]. This first group was inoculated with the BBIBP-CorV vaccine, requiring the application of 2 doses with a 21-day interval between doses [8].
Although efforts have been focused on maximizing vaccine uptake and coverage, the question of passive immunity conferred arose taking into consideration studies showing that active immunization did not necessarily lead to the generation of antibodies [9] and/or in which a drop in these antibodies was described months after completing the vaccination schedule [10]. In addition, we can introduce terms related to active immunity, such as infection-induced immunity (defined as immune protection in an unvaccinated individual after an episode of SARS-CoV-2), vaccine-induced immunity (immune protection in someone who has not previously been infected with SARS -CoV-2 but have received at least one dose of vaccine) and hydrid immunity (occurs in people who suffered at least one episode of COVID-19 and have received at least one dose of vaccine) [11]. This aspect is currently the subject of studies worldwide, focusing their attention on neutralizing antibodies as a strategy for monitoring the individual's immune response to infection and vaccination [[12], [13], [14], [15]].
Studies with the BNT162b2 vaccine reported an exponential increase in neutralizing antibodies on days 11 and 21 after vaccination [16]. However, studies on vaccines with inactivated virus technologies, such as BBIBP-CorV and CoronaVac, are still scarce. A study in Chile, in health care workers who completed the 2 doses in 0–14 day schedules, reported activation of interferon gamma secreting T cells and favorable antibody levels at 14, 28 and 42 days after immunization [17].
In Peru, it was possible to vaccinate health personnel at the beginning of the second wave of COVID-19 (February to April 2021), despite various controversies regarding the efficacy and effectiveness of the BBIBP-CorV vaccine, the social and scientific scandal of “vacunagate” [18] and the deep-rooted infodemics and misinformation surrounding COVID-19 [19]. A study in health personnel vaccinated with 2 doses of BBIBP-CorV reported an effectiveness of 50.4% in preventing infection and 94% in preventing mortality due to SARS-CoV-2 [20], while a study on the CoronaVac vaccine reported an effectiveness in preventing infection of 65.9%, hospitalization of 87.5% and mortality of 86.3% [21]. These effectiveness were lower compared to efficacy reported by the World Health Organization (WHO) Phase III report for the same vaccine in relation to preventing symptomatic infection [22].
It has been shown that the BBIBP-CorV vaccine provides protection against severe forms of COVID-19 that can lead to hospitalization and death. However, to date evidence regarding the prevention of symptomatic infection is questionable. Even within the current context of the circulation of different SARS-CoV-2 variants (Omicron, Delta, and Lambda) and heterologous vaccination schemes with booster doses (BBIBP-CorV + BNT162b2, BBIBP-CorV + ChAdOx1, BNT162b2 + ChAdOx1), there is no evidence of the generation of immune response by the BBIBP-CorV vaccine and even less by the heterologous vaccination schemes. This situation highlights the importance of immunological monitoring of antibody seropositivity in vaccinees to identify specific groups of low seropositivity, as well as temporal trends of the antibodies generated. Therefore, the objective of this study was to estimate post-vaccination seropositivity against COVID-19 in Peruvian Social Health Insurance (EsSalud) personnel vaccinated with two doses of BBIBP-CorV in Lima, Peru, 2021.
2. Methods
2.1. Study design and population
We conducted a cross-sectional study in a representative sample of health workers from five secondary and tertiary level hospitals of the Peruvian Social Health Insurance (EsSalud). The hospitals were Hospital Nacional Edgardo Rebagliati Martins, Hospital Nacional Guillermo Almenara Irigoyen, Hospital Nacional Alberto Sabogal Sologuren and Villas Panamericana and Mongrut. EsSalud health workers in whom there was an interval of at least 14 days since the first vaccination with the BBIBP-CorV vaccine, and who provided consent to participate in the study were enrolled. Those with contraindications for venous blood collection, active symptoms suggestive of COVID-19 and any condition related to hospitalization or quarantine hospitalization were excluded.
2.2. Sample
Based on the sampling frame defined by the list of vaccinated workers of the Health Care Centers (N = 2539), a probability, uni-stage, stratified sampling was performed with independent and representative strata corresponding to the domains represented by the occupational groups (physicians, nurses, nursing technicians, others and administrative personnel). A sample size per domain was calculated considering a nonresponse rate of 20% and a precision of 9% for each of the 24 area-occupancy strata (6 areas for each occupational group), and an estimated prevalence of seropositivity based on a previous study of post-vaccination IgG antiprotein S antibody production of 79.5% [23]. The sample size calculated was a total of 1436 participants. The sample weights were adjusted for nonresponse by the propensity score-matched class method [24]. The present analysis was restricted to health personnel who received two doses of the BBIBP-CorV vaccine in Peru as part of the vaccination campaigns promoted by the Peruvian government.
2.3. Participant recruitment, data and blood sample collection
All the participants were invited to participate by telephone and agreed on a specific date to come to the enrollment site to sign the informed consent form and to provide a blood sample. At recruitment, the participants were given a data collection form prepared by the research team to collect information on the variables of interest. All doubts or questions the participants had, were answered by a team of professionals assigned for this purpose. In addition, 5 cc of venous blood were drawn from each participant, to which EDTA was added and the sample was transported and stored in the laboratory for processing. A cold chain was maintained at all times to ensure sample stability.
In personnel who confirmed participation but were unable to do so for reasons of distance and workload, an additional period of sample collection from May to July 2021 was developed in 3 hospital sites to facilitate sample collection in these participants.
2.4. Laboratory methods
Blood samples were processed at the Clinical Pathology Service Laboratory of the Hospital Nivel II Suárez Angamos following standardized protocols and the manufacturer's recommendations.
IgG anti-SARS-CoV-2 antibodies were measured using the LIAISON® SARS-CoV-2 TrimericS IgG test (DiaSorin Inc., Stillwater, USA), Stillwater, USA). This chemiluminescence immunoassay has a positive and negative concordance greater than 96% with the microneutralization plate test and has proven to be an excellent substitute for the Plate Reduction Neutralization Test - PRNT (gold standard) [[25], [26]]. Likewise, the equipment complied with the verification method recommended by the National Institute of Quality - INACAL [27] and the Clinical & Laboratory Standards Institute - CLSI, under the EP06-A, EP12-A2 and EP15A3 evaluation protocols [[28], [29], [30]].
3. Variables
3.1. Outcomes and covariates
A participant was defined as seropositive with antibody levels greater than or equal to 33.8 BAU/ml, which is the cut-off value recommended by the WHO harmonization process [31]. Antibody levels were also analyzed as a quantitative variable after transformation as a logarithm. Variables related to sex, age, occupation, work area, work modality (referring to the participants main work and classified as non-attendance, face-to-face or mixed), work in COVID-19 area, comorbidities, full dose of BBIBP-CorV vaccine, and time from the first/second dose to sampling were measured. In addition, we collected history of previous SARS-CoV-2 infection through self-report.
3.2. Statistical analysis
All analyses were performed with the R statistical program [32]. The data were entered into the REDCap® capture platform [33] and were subjected to a quality control process that checked for missing, extreme and/or inconsistent values. Missing data were completed by simple multivariate imputation processing by random Forest [34].
Numerical variables were described as means (standard deviation [SD]) or medians (25th and 75th percentiles), as appropriate. Categorical variables were described as absolute and relative frequencies. Bivariate analyses were performed using the Wald or Mann-Whitney U test (both adjusted for the sample design) to compare numerical variables between groups; and the Chi-2 test with Rao-Scott second-order correction for association of categorical variables. Prevalences of seropositivity were reported together with 95% confidence intervals (95%CI) obtained by the logit method.
The association between the probability of seropositivity with predictors of interest was assessed using a logistic generalized additive model (GAM). Odds ratios (OR) were estimated with their respective 95%CI. On the other hand, the relationship between antibody level and predictors of interest was evaluated by tobit GAM, with identity link function and censoring on both sides corresponding to the lower (3.81 BAU/mL) and upper (2080 BAU/mL) limit of the test. Considering evidence that the response to vaccination would vary differentially according to age, time since vaccination, and the existence of previous infection [35], we constructed models that evaluated the interaction between these three variables. The interactions were evaluated by specifying a tensor product of B-splines that allowed modeling the nonlinearity among these variables. To reduce the risk of overfitting we performed a smoothing penalty by restricted maximum likelihood. We assessed collinearity using generalized variance inflation factor and concurvity, a generalization of collinearity that can make estimates unstable, as previously described [36]. We selected variables a priori based on an epidemiological approach, considering previous studies [13,20].
3.3. Ethical issues
The study was approved by the Institutional Research Ethics Committee of the National Heart Institute (INCOR) (12/2021-CEI). Participants provided informed consent prior to enrollment in the study. The information was anonymized and coded to avoid any subsequent identification of the participant.
4. Results
4.1. General characteristics according to seropositivity
A total of 1077 subjects were enrolled. Seventeen participants were excluded for only having received one dose and 18 because they did not have the variables of interest. The prevalence of seropositivity was 67.4% (95%CI: 63.4–71.1) with a coefficient of variation of 2.9%. Among the main characteristics of the sample, we found that 66.7% (n = 786) were female, the median age was 44.9 years (IQR: 35.0–55.0), 69.6% (n = 720) had no comorbidities, 58.5% (n = 651) had no previous SARS-CoV-2 infection, 82.1% (n = 866) worked in an office and 50.9% (n = 527) worked in an area with COVID-19 patients. In addition, the median time since receipt of the second dose was 130 days (interquartile range: 124 to 134). There were no statistically significant differences between groups according to seropositivity and sex, reported comorbidities, area of work, work setting, number of BBIBP-CorV doses received, additional vaccination abroad, and time in days since receiving the first and second doses (Table 1 ).
Table 1.
Characteristics of the study sample including ineligible individuals.
| Characteristics | Total |
Seropositivity |
|||
|---|---|---|---|---|---|
| Missing data | n = 1077 |
Negative |
Positive |
P valuea | |
|
(n = 378) |
(n = 687) |
||||
| n (%) | n (%) | ||||
| Sex | 0 | 0.200 | |||
| Female | 786 (66.7%) | 268 (30.2%) | 514 (69.8%) | ||
| Male | 291 (33.3%) | 110 (35.5%) | 173 (64.5%) | ||
| Age (years) | 0 | <0.001 | |||
| Mean (SD) | 45.1 (11.8) | 47.6 (11.7) | 43.9 (11.7) | ||
| Median (p25-p75) | 0 | 44.9 (35.0–55.0) | 49.0 (36.0–57.7) | 42.0 (35.0–52.0) | |
| Range (minimum-maximum) | 24.0–70.0 | 25.0–69.0 | 24.0–70.0 | ||
| Age | 0 | 0.004 | |||
| 18 to 44 | 500 (49.8%) | 150 (25.7%) | 345 (74.3%) | ||
| 45 to 59 | 395 (34.1%) | 152 (37.0%) | 237 (63.0%) | ||
| 60 or more | 182 (16.1%) | 76 (40.5%) | 105 (59.5%) | ||
| Nationality | 0 | 0.018 | |||
| Peruvian | 1069 (99.6%) | 377 (32.0%) | 680 (68.0%) | ||
| Foreign | 8 (0.4%) | 1 (4.7%) | 7 (95.3%) | ||
| Comorbidities | 10 | 0.300 | |||
| None | 720 (69.6%) | 250 (30.5%) | 465 (69.5%) | ||
| One | 266 (24.2%) | 92 (35.0%) | 169 (65.0%) | ||
| Two or more | 81 (6.2%) | 34 (40.8%) | 45 (59.2%) | ||
| Previous SARS-CoV-2 infection | 4 | <0.001 | |||
| No | 651 (58.5%) | 344 (49.7%) | 297 (50.3%) | ||
| Yes | 422 (41.5%) | 34 (7.5%) | 386 (92.5%) | ||
| Profession | 0 | <0.001 | |||
| Physician | 289 (16.0%) | 130 (45.4%) | 151 (54.6%) | ||
| Administrative or other | 283 (45.3%) | 103 (32.3%) | 177 (67.7%) | ||
| Nurse | 284 (25.9%) | 100 (31.7%) | 183 (68.3%) | ||
| Nursing technician | 221 (12.9%) | 45 (14.7%) | 176 (85.3%) | ||
| Work modality | 1 | 0.002 | |||
| Non-attendance | 137 (11.9%) | 65 (46.1%) | 71 (53.9%) | ||
| Face-to-face | 866 (82.1%) | 278 (29.0%) | 577 (71.0%) | ||
| Mixed | 38 (3.2%) | 22 (55.8%) | 16 (44.2%) | ||
| Licensed | 35 (2.8%) | 12 (29.0%) | 23 (71.0%) | ||
| Work area | 0 | 0.076 | |||
| Hospitalization/Surgery | 226 (46.9%) | 67 (29.2%) | 155 (70.8%) | ||
| Administrative or other related | 151 (11.3%) | 56 (32.8%) | 95 (67.2%) | ||
| Diagnostic support and other related | 145 (12.3%) | 54 (37.9%) | 88 (62.1%) | ||
| Outpatient, extramural and other related | 191 (8.3%) | 81 (45.2%) | 107 (54.8%) | ||
| Critical care | 185 (6.2%) | 69 (35.7%) | 116 (64.3%) | ||
| Emergency or urgent care | 179 (15.0%) | 51 (26.1%) | 126 (73.9%) | ||
| Main work area | 66 | 0.034 | |||
| ICU | 167 (8.8%) | 54 (26.4%) | 112 (73.6%) | ||
| Emergency | 174 (16.9%) | 43 (22.5%) | 127 (77.5%) | ||
| Hospitalization | 212 (28.7%) | 59 (27.6%) | 153 (72.4%) | ||
| Non-COVID-19 Clinic | 89 (5.1%) | 32 (41.5%) | 55 (58.5%) | ||
| Home care | 10 (2.6%) | 5 (36.3%) | 5 (63.7%) | ||
| Administrative care | 61 (7.6%) | 26 (37.3%) | 34 (62.7%) | ||
| Research | 1 (0.0%) | 1 (100.0%) | 0 (0.0%) | ||
| Remote work | 86 (7.7%) | 45 (52.3%) | 40 (47.7%) | ||
| Other | 211 (22.6%) | 85 (34.6%) | 124 (65.4%) | ||
| Works in COVID-19 area | 40 | 0.002 | |||
| No | 527 (50.9%) | 205 (36.8%) | 317 (63.2%) | ||
| Yes | 510 (49.1%) | 154 (24.5%) | 349 (75.5%) | ||
| EsSalud hospital of work | 0 | 0.600 | |||
| I Octavio Mongrut Muñoz Hospital | 52 (4.5%) | 18 (40.7%) | 34 (59.3%) | ||
| Alberto Sabogal Sologuren National Hospital | 341 (23.3%) | 128 (33.4%) | 212 (66.6%) | ||
| Edgardo Rebagliati Martins National Hospital | 465 (42.3%) | 156 (31.3%) | 308 (68.7%) | ||
| Guillermo Almenara Irigoyen National Hospital | 198 (24.3%) | 70 (32.5%) | 118 (67.5%) | ||
| Villa Panamericana | 21 (5.5%) | 6 (20.5%) | 15 (79.5%) | ||
| BBIBP-CorV doses | 0 | 0.070 | |||
| One dose | 17 (2.4%) | 1 (7.9%) | 16 (92.1%) | ||
| Two doses | 1060 (97.6%) | 377 (32.5%) | 671 (67.5%) | ||
| Additional vaccination abroad | 0 | 0.150 | |||
| No | 1071 (99.6%) | 378 (32.0%) | 681 (68.0%) | ||
| Yes | 6 (0.4%) | 0 (0.0%) | 6 (100.0%) | ||
| Time since first dose (days) | 3 | 0.130 | |||
| Median (p25-p75) | 152.0 (145.0–155.0) | 153.0 (145.0–155.0) | 152.0 (145.0–155.0) | ||
| Range (minimum-maximum) | 13.0–163.0 | 95.0–163.0 | 13.0–163.0 | ||
| Time since second dose (days) | 19 | 0.033 | |||
| Median (p25-p75) | 130.0 (124.0–134.0) | 131.0 (124.0–134.0) | 130.0 (123.0–134.0) | ||
| Range (minimum-maximum) | 14.0–142.0 | 14.0–142.0 | 14.0–142.0 | ||
n: unweighted absolute frequency; %: weighted percentage; ICU: intensive care unit; SD: standard deviation.
*Ineligible individuals were those who had only one dose of vaccine, were vaccinated with a vaccine other than BBIBP-CorV and/or were vaccinated abroad. Likewise. we also excluded those who did not have complete data on the response variable.
Squared chi-square test with Rao and Scott second-order correction; Wilcoxon rank sum test for complex samples.
4.2. Seropositivity predictors
In the adjusted analysis, we found a negative association between seropositivity and age (p < 0.001). In addition, belonging to the occupational group of nursing technicians (aOR = 2.24; 95%CI: 1.14–4.37), belonging to the Villa Mongrut or Panamericana group (aOR = 0.27; 95%CI: 0.14–0.53) and Sabogal Hospital (aOR = 0.60; 95%CI: 0.40–0.94), time since second vaccination (p < 0.001), previous SARS-CoV-2 infection (aOR = 11.7; 95%CI: 7.81–17.5) and working in a COVID-19 area (aOR = 1.47; 95%CI: 1.02–2.11) were associated with presenting seropositivity (see Table 2).
Table 2.
Predictors of seropositivity 14 to 142 days after receiving the second dose in health personnel vaccinated with BBIBP-CorV.
| Characteristics | 14–142 days post second dose |
Crude analysis |
Adjusted analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Negative |
Positive |
P valuea | cORb | 95%CIc | P value | aORd | 95%CIc | P value | ||
|
(n = 377) |
(n = 665) |
|||||||||
| n (%) | n (%) | |||||||||
| Sex | 0.200 | |||||||||
| Female | 267 (31.0%) | 495 (69.0%) | – | – | – | – | ||||
| Male | 110 (36.0%) | 170 (64.0%) | 0.80 | 0.61–1.04 | 0.091 | 0.82 | 0.58–1.17 | 0.300 | ||
| Age (years) | 0.001 | <0.001 | <0.001 | |||||||
| Mean (SD) | 47.5 (11.6) | 44.0 (11.6) | ||||||||
| Median (p25-p75) | 49.0 (36.0–57.3) | 43.0 (35.0–52.0) | ||||||||
| Range (minimum-maximum) | 25.0–69.0 | 24.0–70.0 | ||||||||
| Comorbidities | 0.200 | |||||||||
| None | 252 (31.1%) | 460 (68.9%) | – | – | – | – | ||||
| One or more | 125 (36.4%) | 205 (63.6%) | 0.79 | 0.60–1.03 | 0.086 | 0.96 | 0.68–1.35 | 0.800 | ||
| Previous SARS-CoV-2 infection | <0.001 | |||||||||
| No | 343 (49.9%) | 292 (50.1%) | – | – | – | – | ||||
| Yes | 34 (7.8%) | 373 (92.2%) | 11.7 | 8.00–17.2 | <0.001 | 11.7 | 7.81–17.5 | <0.001 | ||
| Profession | <0.001 | |||||||||
| Physician | 130 (46.3%) | 147 (53.7%) | – | – | – | – | ||||
| Administrative or other | 102 (32.9%) | 172 (67.1%) | 1.76 | 1.25–2.49 | 0.001 | 1.24 | 0.77–2.02 | 0.400 | ||
| Nurse | 100 (32.2%) | 178 (67.8%) | 1.82 | 1.24–2.66 | 0.002 | 1.53 | 0.91–2.58 | 0.110 | ||
| Nursing technician | 45 (15.6%) | 168 (84.4%) | 4.67 | 2.73–8.00 | <0.001 | 2.24 | 1.14–4.37 | 0.019 | ||
| Work modality | 0.017 | |||||||||
| Face-to-face/Mixed | 300 (30.7%) | 576 (69.3%) | – | – | – | – | ||||
| Non-attendance/Licensed | 77 (43.9%) | 89 (56.1%) | 0.57 | 0.40–0.80 | 0.001 | 0.94 | 0.58–1.51 | 0.800 | ||
| Work area | 0.089 | |||||||||
| Hospitalization/Surgery | 66 (30.3%) | 146 (69.7%) | – | – | – | – | ||||
| Administrative or other related | 56 (32.8%) | 94 (67.2%) | 0.89 | 0.58–1.35 | 0.600 | 0.61 | 0.36–1.03 | 0.065 | ||
| Diagnostic support or other related | 54 (38.4%) | 86 (61.6%) | 0.70 | 0.47–1.04 | 0.078 | 0.77 | 0.46–1.28 | 0.300 | ||
| Outpatient. extramural and other related | 81 (45.9%) | 102 (54.1%) | 0.51 | 0.32–0.81 | 0.005 | 0.83 | 0.48–1.42 | 0.500 | ||
| Critical care | 69 (36.1%) | 113 (63.9%) | 0.77 | 0.45–1.31 | 0.300 | 0.53 | 0.28–1.02 | 0.058 | ||
| Emergency or urgent care | 51 (26.2%) | 124 (73.8%) | 1.22 | 0.82–1.82 | 0.300 | 0.77 | 0.47–1.27 | 0.300 | ||
| Works in COVID-19 area | <0.001 | |||||||||
| No | 220 (39.0%) | 318 (61.0%) | – | – | – | – | ||||
| Yes | 157 (25.6%) | 347 (74.4%) | 1.86 | 1.44–2.40 | <0.001 | 1.47 | 1.02–2.11 | 0.040 | ||
| EsSalud hospital of work | >0.900 | |||||||||
| Edgardo Rebagliati Martins National Hospital | 155 (31.6%) | 298 (68.4%) | – | – | – | – | ||||
| Guillermo Almenara Irigoyen National Hospital | 70 (33.2%) | 114 (66.8%) | 0.93 | 0.67–1.29 | 0.700 | 0.87 | 0.57–1.32 | 0.500 | ||
| I Octavio Mongrut Muñoz Hospital/Villa Panamericana | 24 (33.9%) | 45 (66.1%) | 0.90 | 0.57–1.42 | 0.600 | 0.27 | 0.14–0.53 | <0.001 | ||
| Alberto Sabogal Sologuren National Hospital | 128 (33.5%) | 208 (66.5%) | 0.92 | 0.66–1.26 | 0.600 | 0.61 | 0.40–0.94 | 0.024 | ||
| Time since second dose (days) | 0.030 | <0.001 | <0.001 | |||||||
| Median (p25-p75) | 131.0 (124.0–134.0) | 130.0 (123.0–134.0) | ||||||||
| Range (minimum-maximum) | 14.0–142.0 | 14.0–142.0 | ||||||||
n: unweighted absolute frequency; %: weighted percentage.
The associations of seropositivity with age and time since second vaccination are shown in Fig. 1, Fig. 2.
Squared chi-square test with Rao and Scott second-order correction; Wilcoxon rank sum test for complex samples.
cOR: crude odds ratio.
CI: confidence interval.
aOR: adjusted odds ratio; SD: standard deviation.
The associations of seropositivity and time since the second vaccination are shown in the graphs in Fig. 1 . Fig. 1A shows the trend to a decrease in the predicted probability of seropositivity to Ac IgG SARS-CoV-2 with a longer time since the second vaccination dose, showing a notable reduction after day 110. In addition, in Fig. 1B we describe a slightly negative association between SARS-CoV-2 IgG antibody positivity and age. This is also shown in Fig. 1C in individuals aged 25, 45 and 60 years, with a sustained reduction in individuals aged 60 years after day 110. The predicted probability of seropositivity remained high over time in individuals who reported having had a previous infection compared to those who did not. Fig. 1E, F and 1G show the predicted probability of seropositivity according to occupational groups, working in a COVID-19 area and the hospital work site.
Fig. 1.
Association between SARS-CoV-2 IgG antibody positivity and time since receiving the second vaccination dose (days) (A), age (B), according to age group (C), history of previous SARS-CoV-2 infection (D), profession (E), work in COVID-19 area (F) and EsSalud hospital (G).
4.3. Predictors of anti-SARS-CoV-2 antibody levels
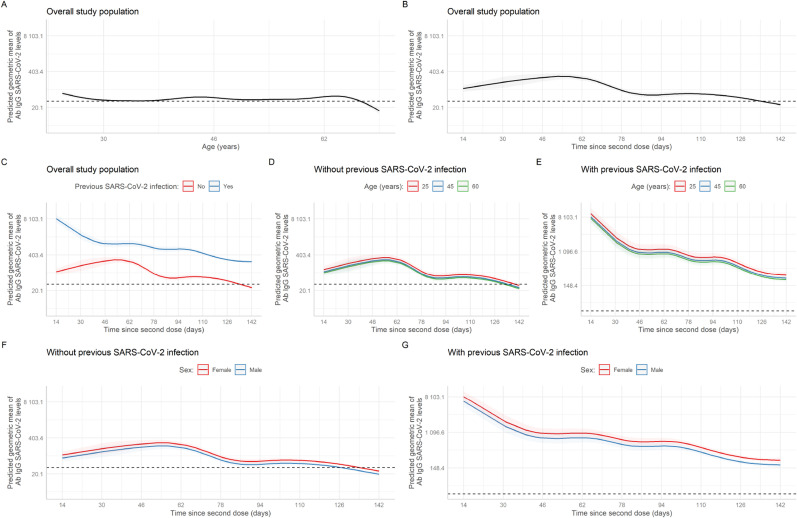
In the adjusted analysis, a statistically significant association was found for male sex (aGMR = 0.77; 95%CI: 0.74–0.80), non-face-to-face/licensed work modality (aGMR = 0.78; 95%CI: 0.73–0.84), type of administrative or other related service (aGMR = 1.17; 95%CI: 1.10–1.25), work in critical care (aGMR = 0.85; 95%CI: 0.79–0.93), the nursing technician occupational group (aGMR = 1.30; 95%CI: 1.20–1.41), having worked at Villa Mongrut/Panamericana (aGMR = 0.62; 95%CI: 0.58–0.68) and Sabogal Hospital (aGMR = 0.84; 95%CI: 0.80–0.89), having reported a previous SARS-CoV-2 infection (aGMR = 13.1; 95%CI: 4.99–34.3), having worked in a COVID-19 area (aGMR = 1.30; 95%CI: 1.24–1.36), age (p < 0.001) and time since second vaccination (p < 0.001) (Table 3 ). The association between the geometric mean of SARS-CoV-2 IgG antibody levels and time since second vaccination is shown in Fig. 2 . Fig. 2A shows the trend to a reduction in the geometric mean of antibody levels in individuals 62 years of age or older. In addition, Fig. 2B presents a negative relation between antibody levels and time since second dose. Likewise, Fig. 2D and E presents the geometric mean of antibody levels and time since second vaccination comparing previous SARS-CoV-2 infection and disease presentation in individuals 25, 45 and 60 years old. In all these cases there was a reduction in the time of the geometric mean which was especially notable in those without previous infection and 60 years of age. Graphs 2F and 2G show the behavior of the curve according to previous infection and by sex. In Fig. 3 , it is important to highlight that the occupational group of nursing technicians and those who worked in the on-site modality had a higher predicted geometric mean of IgG SARS-CoV-2 antibodies. In addition, we showed in supplementary material the behavior of the curve according to groups by EsSalud hospital of work and working areas (by previous SARS-CoV-2 infection).
Table 3.
Predictors of anti-SARS-CoV-2 antibody levels 14 to 142 days after receiving the second dose in health personnel vaccinated with BBIBP-CorV.
| Characteristics | Crude analysis |
Adjusted analysis |
||||
|---|---|---|---|---|---|---|
| cGMRa | 95%CI2 | P value | aGMR3 | 95%CI2 | P value | |
| Sex | ||||||
| Female | – | – | – | – | ||
| Male | 0.88 | 0.83–0.93 | <0.001 | 0.77 | 0.74–0.80 | <0.001 |
| Age (years) | <0.001 | <0.001 | ||||
| Comorbidities | ||||||
| None | – | – | – | – | ||
| More than one | 0.87 | 0.83–0.92 | <0.001 | 1.00 | 0.96–1.05 | 0.900 |
| Previous SARS-CoV-2 infection | ||||||
| No | – | – | – | – | ||
| Yes | 7.7 | 7.40–8.02 | <0.001 | 13.1 | 4.99–34.40 | <0.001 |
| Profession | ||||||
| Physician | – | – | – | – | ||
| Administrative or other | 1.57 | 1.46–1.69 | <0.001 | 0.98 | 0.92–1.05 | 0.600 |
| Nurse | 1.41 | 1.30–1.52 | <0.001 | 1.01 | 0.94–1.08 | 0.800 |
| Nursing technician | 3.18 | 2.90–3.50 | <0.001 | 1.30 | 1.20–1.41 | <0.001 |
| Work modality | ||||||
| Face-to-face/Mixed | – | – | – | – | ||
| Non-attendance/Licensed | 0.48 | 0.45–0.51 | <0.001 | 0.78 | 0.73–0.84 | <0.001 |
| Work area | ||||||
| Hospitalization/Surgery | – | – | – | – | ||
| Administrative or other related | 1.39 | 1.28–1.51 | <0.001 | 1.17 | 1.10–1.25 | <0.001 |
| Diagnostic support or other related | 0.92 | 0.85–1.00 | 0.053 | 1.07 | 1.01–1.15 | 0.045 |
| Outpatient. extramural and other related | 0.65 | 0.59–0.72 | <0.001 | 1.04 | 0.97–1.12 | 0.300 |
| Critical care | 0.98 | 0.88–1.08 | 0.600 | 0.85 | 0.79–0.93 | <0.001 |
| Emergency or urgent care | 1.52 | 1.41–1.63 | <0.001 | 1.06 | 1.00–1.13 | 0.057 |
| Works in COVID-19 area | ||||||
| No | – | – | – | – | ||
| Yes | 1.71 | 1.62–1.80 | <0.001 | 1.30 | 1.24–1.36 | <0.001 |
| EsSalud hospital of work | ||||||
| Edgardo Rebagliati Martins National Hospital | – | – | – | – | ||
| Guillermo Almenara Irigoyen National Hospital | 1.01 | 0.95–1.08 | 0.700 | 1.02 | 0.97–1.07 | 0.500 |
| I Octavio Mongrut Muñoz Hospital/Villa Panamericana | 1.09 | 0.99–1.20 | 0.067 | 0.62 | 0.58–0.68 | <0.001 |
| Alberto Sabogal Sologuren National Hospital | 0.99 | 0.93–1.05 | 0.700 | 0.84 | 0.80–0.89 | <0.001 |
| Time since second dose (days) | <0.001 | <0.001 | ||||
| Interaction (Time since second dose (days) * Previous SARS-CoV-2 infection) | <0.001 | |||||
cGMR: crude geometric mean ratio; 2CI: confidence interval; 3aGMR: adjusted geometric mean ratio.
Fig. 2.
Association between the SARS-CoV-2 IgG antibodies (BAU/mL) levels and age (A), time (days) since receipt of the second vaccination dose (B) according to: age groups with no history of previous SARS-CoV-2 infection (C), age groups with this history (D), previous SARS-CoV-2 infection (E), groups according to sex and no history of previous SARS-CoV-2 infection (F), groups by sex with this history (G).
Fig. 3.
Association between the SARS-CoV-2 IgG antibodies (BAU/mL) levels and time since receipt of the second vaccination dose (days) according to: groups by work modality according to previous SARS-CoV-2 infection, groups by profession according to previous SARS-CoV-2 infection, groups according to work in COVID-19 area and -CoV-2 infection and groups by working area according to previous SARS-CoV-2 infection.
5. Discussion
Our study estimated the prevalence of post-vaccination seropositivity against SARS-CoV-2 and identified the predictors in Peruvian health personnel during 2021. Approximately 70% of the participants were seropositive, with younger age, having a history of COVID-19, and working in a COVID-19 area being associated with higher seropositivity. In addition, being male, younger age, having previous COVID-19 infection, working in a non-face-to-face modality, as well as in a COVID-19 area were predictors of higher antibody levels. We also found that antibody levels progressively decreased from day 110 after receiving the second vaccine dose.
About seven out of ten participants presented seropositivity for anti-SARS-CoV-2 IgG anti-SARS-CoV-2 protein S antibodies generated from vaccination. There was a negative significant association with age and in subcategories of age group, highlighting a marked difference in seropositivity between the groups of 18–44, 45 to 59, and greater than or equal to 60 years of age. Some studies have reported similar findings on the relationship of age and antibody quantification. One study, by Ferenci et al. evaluated the levels of neutralizing antibodies after receiving a second dose of the BBIBP-CorV vaccine, and reported that 90% of participants younger than 50 had detectable antibodies, while 50% of those older than 80 had no detectable antibodies [37]. Likewise, a cohort study evaluating antibody response in participants after receiving the ChAdOx1 and BNT162b2 vaccines identified a group of non-responders, mainly composed of those over 75 years of age, males and individuals with chronic health problems [35]. The decrease in antibody levels with increasing age could be explained by immunosenescence [38], which would produce a reduced adaptive immune response and a decline in humoral and cellular immune response [39,40], indicating a greater need for booster doses in this age group.
We found that being female was predictive of higher antibody levels compared to males. However, we observed a similar sustained reduction in antibody levels from day 110 onwards regardless of having had previous infection or not. Our finding is consistent with that described by Wei et al. who reported that being male was a predictor of lower antibody positivity [35]. Likewise, sex differences have been described after natural infection with COVID-19 [41]. This finding could be explained by the fact that biological sex affects the response of the innate and adaptive immune system, inducing different responses to a pathogen or vaccines [42,43]. In addition, males are at higher risk for diseases caused by X-linked alleles [44,45] and epigenetic expression in this group would determine exposure to sex steroids that would have a direct effect on immune function [[46], [47], [48]].
Our results showed that working in an area with patients with COVID-19 infection was a predictor of seropositivity and higher antibody levels and working in the non-face-to-face modality was a predictor of lower antibody levels. These findings could be explained in that the participants who worked in a face-to-face setting as well as in COVID-19 areas had an additional risk of becoming infected. These two characteristics are important because they could be associated with having a history of COVID-19. Thus, passive re-exposure would cause the immune system to produce a greater proportion of antibodies [49] compared to individuals without these occupational characteristics.
Self-reported previous COVID-19 infection was a predictor of seropositivity and higher antibody levels. In addition, this group of participants recorded a lower reduction in antibody levels during the time after receiving the second dose compared to those who had no history of COVID-19. Previous studies have reported similar findings, describing higher antibody levels in vaccinated persons with a history of COVID-19 [50,51] independently of the age group [35]. Thus, it was proposed that, in a scenario of vaccine shortage, a dose of mRNA-type BNT162b2 vaccine could generate a robust immune response in persons who have had a COVID-19 infection three to six months prior to vaccination [35,52]. Immune response following inoculation with a COVID-19 vaccine involves two processes; the first is related to cellular response involving the production of different T-cell lineages, interleukins and interferons, while the second process is triggered by the first and involves the production of IgG immunoglobulins against viral antigens, such as protein S [53]. This process must be understood as a whole for the correct study of the immune response of a particular individual. These antibodies usually persist for up to six months [[54], [55], [56]] and then decline by 5 to 10-fold [57,58]. However, B and T cells can be detected even longer and are essential for protection against possible reinfections [[56], [57], [58], [59]], highlighting the role of cellular immunity [56,60]. Thus, our finding on the reduction of antibody levels 110 days after receiving the second vaccination dose indicate the need to administer a booster dose in the event of the circulation of new variants.
Previous studies have highlighted the reduction in antibody levels generated after passive immunization over time. One study showed a decrease in antibody levels three months after receiving the second dose of the BNT162b2 vaccine (mRNA type vaccine produced by the Pfizer laboratory) [61]. On the other hand, another report indicated that between days 21–70 after receiving the second dose of ChAdOx1 (viral vector vaccine produced by the University of Oxford and the AstraZeneca laboratory) and BNT162b2 there was a 5-fold and 2-fold reduction in antibody levels, respectively [62]. Similarly, a study evaluating antibody levels in two groups after receiving a type of inactivated virus vaccine produced by the Sinopharm laboratory described a significant reduction in antibody levels after the third- or fourth week following receipt of the second dose. However, after receiving a third dose, the humoral immune response was very high [63]. The evidence is consistent in showing a decrease in the level of antibodies three months after receiving the second dose, a finding that is consistent with our results. This evidence supports the need for a booster dose after this period to increase antibody levels.
Although low antibody values would not imply greater vulnerability and decreased protection against the virus, the need for a booster dose should be considered due to the adaptive mutability of the virus. This characteristic has generated different variants that can compromise the protection of vaccines against severe forms of the disease, hospitalization, admission to the intensive care unit and death [64,65]. By compromising vaccine protection, new waves, collapse of health systems and greater impact on the population could be generated [6,66], all of which are preventable scenarios.
Our results elucidate the need for follow-up and immunologic surveillance (at humoral and cellular levels) in the risk group of health personnel. This surveillance would generate information for evidence-based decision making to identify the times at which a booster dose is necessary to maintain elevated antibody levels and provide better protection against SARS-CoV-2 and its variants [67].
Our study has limitations. Since antibody levels tend to decline over time, some of the seronegative measurements could previously have been seropositive. Despite having considered non-response and loss rates, we were unable to reach the estimated sample size for the study due to the high workload of the participants, limiting their availability for sample collection. To deal with this drawback, we went to the hospitals in which the health personnel worked to try to extract samples and increase the recruitment of participants. Another limitation is that memory bias could affect the validity of the information obtained in some questions such as the date of vaccination, self-report of previous SARS-CoV-2 infection, and a history of a positive diagnostic test for COVID-19. However, to reduce this bias, we provided support for filling out the survey and obtaining the vaccination date from the official portal of the Peruvian Ministry of Health. In addition, although previous studies have evaluated seropositivity and antibody production after receiving different vaccines worldwide, information related to the BBIBP-CorV vaccine is still scarce, thereby limiting comparison with other vaccines. Finally, this study only evaluated the humoral response of the immune system but not the cellular response. Studies on cellular immunity are needed for better evidence-based decision making. To our knowledge, this study is one of the first reports of active surveillance of SARS-CoV-2 anti-SARS-CoV-2 IgG antiprotein S antibody levels and post-vaccination seropositivity in a high-risk group, such as health care workers.
6. Conclusions
Two out of three participants achieved seropositivity after receiving both doses of the inactivated BBIBP-CorV vaccine produced by the Sinopharm laboratory. We found predictors of seropositivity to be male sex, younger age, self-reported COVID-19 and working in an area with COVID-19 patients. In addition, a longer time after receiving the second dose was a predictor of lower antibody levels. We described a sustained drop in antibody levels after day 110, elucidating the need for a booster dose three months after the second dose.
Funding
This research was carried out with funding from the Peruvian Social Health Insurance - EsSalud.
CRediT authorship contribution statement
Aleksandar Cvetkovic-Vega: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. Diego Urrunaga-Pastor: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. Percy Soto-Becerra: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Luis E. Figueroa-Montes: Conceptualization, Investigation, Writing – review & editing. Lizette Fernandez-Bolivar: Conceptualization, Investigation, Writing – review & editing. Sergio Alvizuri-Pastor: Conceptualization, Investigation, Writing – review & editing. Martin Oyanguren-Miranda: Conceptualization, Investigation, Writing – review & editing. Ibeth Neyra-Vera: Conceptualization, Investigation, Writing – review & editing. Elizabeth Carrillo-Ramos: Conceptualization, Investigation, Writing – review & editing. Arturo Sagástegui: Conceptualization, Investigation, Writing – review & editing. Roxana Contreras-Macazana: Conceptualization, Investigation, Writing – review & editing. Diana Lecca-Rengifo: Conceptualization, Investigation, Writing – review & editing. Nikolai Grande-Castro: Conceptualization, Investigation, Writing – review & editing. Moises Apolaya-Segura: Conceptualization, Investigation, Methodology, Writing – review & editing. Jorge L. Maguina: Conceptualization, Investigation, Methodology, Writing – review & editing.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
The research team would like to thank all the health and non-health personnel who participated in the study. We would also like to thank the EsSalud 107 call team, as well as the teams of the IETSI units and the Flexible Supply Management that provided on-site support in the execution of the research and to the health personnel of the clinical pathology service of the EsSalud Hospital Nivel III Suárez Angamos.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmaid.2022.102514.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.https://coronavirus.jhu.edu/map.html COVID-19 Map [Internet]. Johns Hopkins Coronavirus Resource Center [Available from:
- 2.https://covid-19pharmacovigilance.paho.org/ Farmacovigilancia de vacunas para COVID-19 - Sinopharm/BIBP [Internet]. Farmacovigilancia de vacunas para COVID-19. [Available from:
- 3.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713–721. doi: 10.1016/j.cell.2020.06.008. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministerio de Salud Sala situacional de COVID-19 Perú. 2020. https://covid19.minsa.gob.pe/sala_situacional.asp
- 5.Alcalde-Rabanal J.E., Lazo-González O., Nigenda G. Sistema de salud de Perú. Salud Publica Mex. 2011;53:s243–s254. [PubMed] [Google Scholar]
- 6.Gianella C., Gideon J., Romero M.J. What does COVID-19 tell us about the Peruvian health system? Canadian Journal of Development Studies/Revue canadienne d'études du développement. 2021;42(1–2):55–67. [Google Scholar]
- 7.Ministerio de Salud . [Internet]; 2021. Resolución ministerial N° 345-2021-MINSA. [Google Scholar]
- 8.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Z.R.L., Yang J., Guo L., Feng L., Ma C., et al. The Lancet; 2021. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng H., Mao J., Ye Q. Booster vaccination strategy: necessity, immunization objectives, immunization strategy and safety. J Med Virol. 2022;94:2369–2375. doi: 10.1002/jmv.27590. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . 2022. Interim statement on hybrid immunity and increasing population seroprevalence rates. [Google Scholar]
- 12.Food and Drug Administration La FDA autoriza la primera prueba que detecta anticuerpos neutralizantes de una infección reciente o anterior de SARS-CoV-2. 2020. https://www.fda.gov/news-events/press-announcements/actualizacion-sobre-el-coronavirus-la-fda-autoriza-la-primera-prueba-que-detecta-anticuerpos [Internet] [Available from:
- 13.Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D., Miller T.E., Feldman J., et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2):476–488. doi: 10.1016/j.cell.2020.12.015. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. What level of neutralising antibody protects from COVID-19? medRxiv. 2021 doi: 10.1101/2021.03.09.21252641. [DOI] [Google Scholar]
- 15.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 16.Vickers M.A., Sariol A., Leon J., Ehlers A., Locher A.V., Dubay K.A., et al. Exponential increase in neutralizing and spike specific antibodies following vaccination of COVID-19 convalescent plasma donors. Transfusion. 2021;61(7):2099–2106. doi: 10.1111/trf.16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bueno S.M., Abarca K., González P.A., Gálvez N.M., Soto J.A., Duarte L.F., et al. Interim report: safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 in healthy chilean adults in a phase 3 clinical trial. medRxiv. 2021 doi: 10.1101/2021.03.31.21254494. [DOI] [Google Scholar]
- 18.Kenyon G. Vacuna-gate escalates in Peru. Lancet Infect Dis. 2021;21(4):463. doi: 10.1016/S1473-3099(21)00157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez-Risco A., Mejia C.R., Delgado-Zegarra J., Del-Aguila-Arcentales S., Arce-Esquivel A.A., Valladares-Garrido M.J., et al. The Peru approach against the COVID-19 infodemic: insights and strategies. Am J Trop Med Hyg. 2020;103(2):583. doi: 10.4269/ajtmh.20-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silvia Valencia J., Soto Becerra P., Escobar Agreda S., Fernández Navarro M., Moscoso Porras M., Solari L., et al. 2021. Efectividad de la vacuna BBIBP-CorV para prevenir infección y muerte en personal de salud; p. 2021. Perú. [Google Scholar]
- 21.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO . World Health Organization; 2021. Evidence assessment: Sinopharm/BBIBP COVID-19 vaccine. [Google Scholar]
- 23.Stefanelli P., Bella A., Fedele G., Fiore S., Pancheri S., Benedetti E., et al. medRxiv; 2020. Longevity of seropositivity and neutralizing titers among SARS-CoV-2 infected individuals after 4 months from baseline: a population-based study in the province of Trento. [Google Scholar]
- 24.Valliant R., Dever J.A. Stata Press College Station, TX; 2018. Survey weights: a step-by-step guide to calculation. [Google Scholar]
- 25.Favresse J., Gillot C., Di Chiaro L., Eucher C., Elsen M., Van Eeckhoudt S., et al. Neutralizing antibodies in COVID-19 patients and vaccine recipients after two doses of BNT162b2. Viruses. 2021;13(7):1364. doi: 10.3390/v13071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkmann T., Perkmann-Nagele N., Koller T., Mucher P., Radakovics A., Marculescu R., et al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr. 2021;9(1) doi: 10.1128/spectrum.00247-21. e00247-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Instituto Nacional de la Calidad . INACAL; Lima: 2022. https://www.gob.pe/inacal Available at: [Google Scholar]
- 28.Clinical and Laboratory Standards Institute . In: User verification of precision and estimation of bias; approved guideline. 3rd. Wayne P., editor. CLSI; USA: 2014. CLSI document EP15-A3. [Google Scholar]
- 29.Wayne P., editor. For CaLSIUp, guideline-2nd eoqtpa. CLSI; USA: 2008. CLSI document EP12-A2. [Google Scholar]
- 30.Institute CaLS . CLSIe; Wayne (PA): 2003. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. CLSI Document EP06-a. [Google Scholar]
- 31.Infantino M., Pieri M., Nuccetelli M., Grossi V., Lari B., Tomassetti F., et al. The WHO International Standard for COVID-19 serological tests: towards harmonization of anti-spike assays. Int Immunopharm. 2021;100 doi: 10.1016/j.intimp.2021.108095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Team R.C.R. R Core Team; Vienna, Austria: 2013. A language and environment for statistical computing; p. 2021. [Google Scholar]
- 33.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 35.Wei J., Stoesser N., Matthews P.C., Ayoubkhani D., Studley R., Bell I., et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nature Microbiology. 2021;6(9):1140–1149. doi: 10.1038/s41564-021-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood S.N. chapman and hall/CRC; 2006. Generalized additive models: an introduction with R. [Google Scholar]
- 37.Ferenci T., Sarkadi B. medRxiv; 2021. Virus neutralizing antibody responses after two doses of BBIBP-CorV (Sinopharm, Beijing CNBG) vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blomberg B.B., Frasca D. Quantity, not quality, of antibody response decreased in the elderly. J Clin Invest. 2011;121(8) doi: 10.1172/JCI58406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafson C.E., Kim C., Weyand C.M., Goronzy J.J. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 2020;145(5):1309–1321. doi: 10.1016/j.jaci.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frasca D., Blomberg B.B. Aging induces B cell defects and decreased antibody responses to influenza infection and vaccination. Immun Ageing. 2020;17(1):1–10. doi: 10.1186/s12979-020-00210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grzelak L., Velay A., Madec Y., Gallais F., Staropoli I., Schmidt-Mutter C., et al. Sex differences in the evolution of neutralizing antibodies to severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2021;224(6):983–988. doi: 10.1093/infdis/jiab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 43.Markle J., Fish E.N. SeXX matters in immunity. Trends Immunol. 2014;35(3):97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Carrel L., Brown C.J. When the Lyon (ized chromosome) roars: ongoing expression from an inactive X chromosome. Phil Trans Biol Sci. 2017;372(1733) doi: 10.1098/rstb.2016.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tukiainen T., Villani A.-C., Yen A., Rivas M.A., Marshall J.L., Satija R., et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550(7675):244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furman D., Hejblum B.P., Simon N., Jojic V., Dekker C.L., Thiébaut R., et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci USA. 2014;111(2):869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vom Steeg L.G., Klein S.L., editors. Seminars in immunopathology. Springer; 2019. Sex and sex steroids impact influenza pathogenesis across the life course. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vom Steeg L.G., Klein S.L. Sex steroids mediate bidirectional interactions between hosts and microbes. Horm Behav. 2017;88:45–51. doi: 10.1016/j.yhbeh.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gobbi F., Buonfrate D., Moro L., Rodari P., Piubelli C., Caldrer S., et al. Antibody response to the BNT162b2 mRNA COVID-19 vaccine in subjects with prior SARS-CoV-2 infection. Viruses. 2021;13(3):422. doi: 10.3390/v13030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demonbreun A.R., Sancilio A., Velez M.P., Ryan D.T., Saber R., Vaught L.A., et al. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buonfrate D., Piubelli C., Gobbi F., Martini D., Bertoli G., Ursini T., et al. Antibody response induced by the BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers, with or without prior SARS-CoV-2 infection: a prospective study. Clin Microbiol Infect. 2021;27(12):1845–1850. doi: 10.1016/j.cmi.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burckhardt R.M., Dennehy J.J., Poon L.L., Saif L.J., Enquist L.W. Are COVID-19 vaccine boosters needed? The science behind boosters. J Virol. 2021;96(3) doi: 10.1128/jvi.01973-21. e01973-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Müller L., Andrée M., Moskorz W., Drexler I., Walotka L., Grothmann R., et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73(11):2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doria-Rose N., Suthar M.S., Makowski M., O'Connell S., McDermott A.B., Flach B., et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384(23):2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pegu A., O'Connell S.E., Schmidt S.D., O'Dell S., Talana C.A., Lai L., et al. Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. Science. 2021;373(6561):1372–1377. doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho A., Muecksch F., Schaefer-Babajew D., Wang Z., Finkin S., Gaebler C., et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature. 2021;600(7889):517–522. doi: 10.1038/s41586-021-04060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goel R.R., Painter M.M., Apostolidis S.A., Mathew D., Meng W., Rosenfeld A.M., et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374(6572):abm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Favresse J., Bayart J.-L., Mullier F., Elsen M., Eucher C., Van Eeckhoudt S., et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg Microb Infect. 2021;10(1):1495–1498. doi: 10.1080/22221751.2021.1953403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shrotri M., Navaratnam A.M., Nguyen V., Byrne T., Geismar C., Fragaszy E., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saeed U., Uppal S.R., Piracha Z.Z., Uppal R. SARS-CoV-2 spike antibody levels trend among Sinopharm vaccinated people. Iran J Public Health. 2021;50(7):1486. doi: 10.18502/ijph.v50i7.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bian L., Gao Q., Gao F., Wang Q., He Q., Wu X., et al. Impact of the Delta variant on vaccine efficacy and response strategies. Expet Rev Vaccine. 2021;20(10):1201–1209. doi: 10.1080/14760584.2021.1976153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vasireddy D., Vanaparthy R., Mohan G., Malayala S.V., Atluri P. Review of COVID-19 variants and COVID-19 vaccine efficacy: what the clinician should know? J Clin Med Res. 2021;13(6):317. doi: 10.14740/jocmr4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.da Silva S.J.R., Pena L. Collapse of the public health system and the emergence of new variants during the second wave of the COVID-19 pandemic in Brazil. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubin R. COVID-19 vaccines vs variants—determining how much immunity is enough. JAMA. 2021;325(13):1241–1243. doi: 10.1001/jama.2021.3370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.