Abstract
Background
Extraosseous Ewing's sarcoma/peripheral primitive neuroectodermal tumours(EWS/pPNETs) of the kidney are rare. Signs and symptoms are atypical in EWS patients. Presenting symptoms include haematuria, abdominal pain, or a palpable mass. A comprehensive review of the literature shows that it is difficult to make an accurate diagnosis based on physical examination alone. The imaging findings of EWS/pPNETs are nonspecific. We used contrast-enhanced ultrasound (CEUS) to diagnose an EWS/pPNET in our patient, which had never been reported previously to our knowledge.
Case presentation
This article reports the case of a 20-year-old female with an abdominal mass and gross haematuria for 1 month. The ultrasound revealed a hypoechoic mass with a clear margin at the lower pole in the left kidney. CEUS demonstrated signs of annular enhancement and heterogeneous enhancement of the tumour, and simultaneous wash-in was predominant. Computed tomography images showed an elliptical low-density tumour. The patient underwent a left kidney resection, and the pathological diagnosis was an EWS/pPNET. Twenty-one days after the kidney operation, the patient underwent 8 cycles of a CAV (vinorelbine, ifosfamide, epirubicin) + IE (isocyclophosphamide, etoposide) chemotherapy regimen. Subsequently, radiotherapy (dose: 45 Gy, radiation field:the tumour bed following surgical resection) was administered for nearly 30 days. The patient had no signs of local recurrence or metastasis within a follow-up of 4 years.
Conclusions
As a radiation-free, inexpensive, convenient, and repeatable examination method, ultrasound was the primary choice for kidney examination. Early CEUS was helpful to make an accurate diagnosis. Surgery and adjuvant radiation or chemotherapy administered in a timely manner can prevent further deterioration.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12894-022-01146-w.
Keywords: Extraosseous Ewing's sarcoma/peripheral primitive neuroectodermal tumour, Nephrectomy, Contrast-enhanced ultrasound, Case report
Background
Ewing's family of tumours (ESFTs), the rarest of oncologic disorders [1], are highly malignant tumours of soft tissue origin. ESFTs are common in adolescents and young adults and occur primarily in the bones or soft tissues of the limbs and rarely in the internal organs [2–4]. ESFTs include Ewing's sarcoma (ES), extraosseous sarcoma, and primitive neuroectodermal tumours (PNETs) [1]. The origin of these tumours is unclear, but they appear to be derived from cells migrating from the neural tube, with different ectodermal or neuronal differentiation abilities [5]. Histologically, ES/PNETs consist of a single circle of small cells, forming Homer Wright rosettes [6]. Primary Ewing's sarcomas of the kidney (ESKs) account for less than 1% of all renal tumours [7]. The clinical symptoms of ESKs are atypical and resemble renal colic symptoms, including an abdominal mass, abdominal pain, and haematuria [8]. More than 65% of ESK patients have distant metastases, and the common sites of metastases include the regional lymph nodes, lungs, and liver, of which the lungs are the most common site. The overall survival rate of patients is meagre, and most patients die from metastatic lung carcinoma [9, 10]. Since the first case was reported in 1975 by Seemayer et al. [11], there has been increasing interest in ESKs. However, there is still no clear understanding of this disease at present. To our knowledge, most researchers have reported their clinical symptoms, computed tomography (CT) or magnetic resonance imaging (MRI) manifestations, and treatment methods; however, few researchers have analysed the ultrasound appearance of ESKs. In this article, we summarize a patient's symptoms, imaging findings, and treatment method, as well as the findings of previously published studies. The investigators would like to discuss and share ultrasound findings that are consistently found in ESKs as well as criteria for developing imaging and treatment guidelines for ESKs.
Case presentation
A 20-year-old female presented to the hospital with an abdominal mass and gross haematuria for 1 month. She recently complained of left abdominal pain, nausea and vomiting, and noticeable weight loss. The patient did not have a family history of malignant tumours. The physical examination revealed abdominal distention with a large mass in the left upper quadrant. The mass was irregular, hard, immovable, nontender and without overlying skin changes. The laboratory test results for tumour markers were as follows: carbohydrate antigen 125 (CA-125), 136.89 U/mL (reference range: 0.00–35.00 U/ml); the levels of other tumour markers, such as carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA-199), and alpha-fetoprotein (AFP), were normal. Urine analysis showed that the red blood cell (RBC) count was 56/µl (reference range: 0.0–25.0), and the white blood cell (WBC) count was 59/µl (reference range: 0.0–25.0). Liver and kidney function test results and the complete blood count were within normal levels. Conventional ultrasound (US) examination showed a 4.5 × 3.2 cm irregular lesion at the lower pole of the left kidney. To further clarify the diagnosis of the tumour, contrast-enhanced ultrasound (CEUS) was performed, which presented signs of annular enhancement and heterogeneous enhancement of the tumour, and simultaneous wash-in was predominant. The possibility of a tumour lesion was considered (Fig. 1). A CT scan showed a 3.7 × 3.8 × 4.0 cm heterogeneous mass in the left kidney, which had blurry edges and a high-density dissepiment in the interior. Three-dimensional reconstruction of the kidney showed that the lower one-third of the left kidney was occupied (Fig. 2). Gross total removal of the tumour was achieved. The tumour was a 3.5 × 3 × 2.6 cm well-defined grey‒white nodular mass with necrosis (Fig. 3). Hematoxylin–eosin staining (HE) and immunohistochemistry (Leica DM4 B, DFC7000 T camera and LAS X software) were performed. Under the microscope, the tumour cells were small, round, short spindle-shaped, and densely arranged; Homer Wright rosettes were found; the cytoplasm of the tumour cells was sparse; and the nuclei were slightly enlarged and hyperchromatic. The tumour tissue was accompanied by extensive haemorrhage and necrosis. Immunohistochemistry showed the following: CD99 ( +), Vimentin ( +), EMA (−), CD10 (−), CD56 (−), syn (−), and NSE (−) (Fig. 4). Other immunohistochemistry staining see supplementary information (Additional file 1). The pathological diagnosis was an EWS/pPNET that did not invade the ureters. Subsequently, 21 days after the operation, the patient received 8 cycles of a CAV (vinorelbine, ifosfamide, epirubicin) + IE (isocyclophosphamide, etoposide) regimen from December 1, 2017, to May 11, 2018. Adjuvant radiotherapy (dose: 45 Gy, radiation field: the tumour bed following surgical resection) was also administered from June 11, 2018, to July 13, 2018. At the same time, the serum CA-125 level of the patient showed a gradual downwards trend after surgery and chemotherapy. The serum CA-125 levels returned to normal at the end of chemotherapy and radiotherapy. The patient had no signs of local recurrence or metastasis on CT scan within a follow-up of 4 years.
Fig. 1.
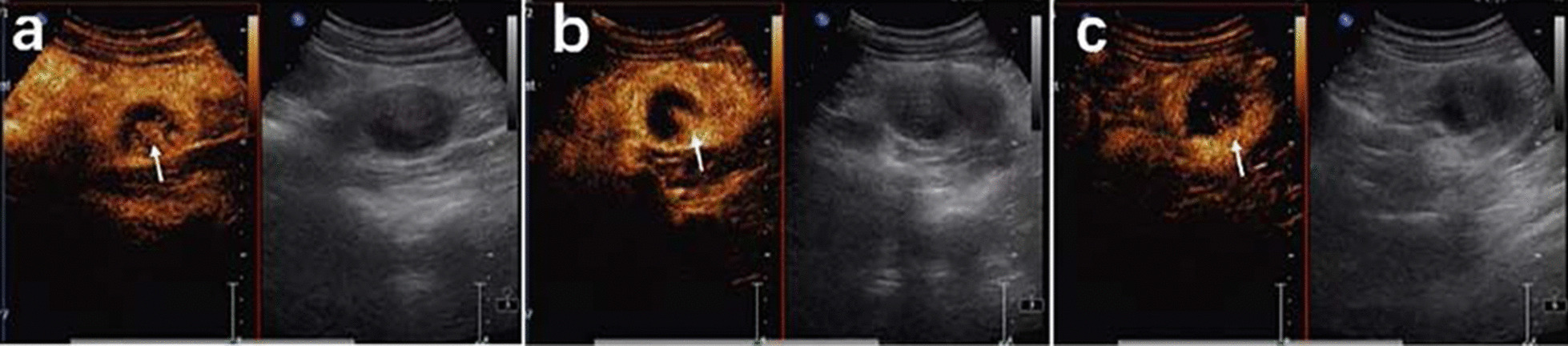
CEUS patterns of EWS/pPNET of the kidney in a 20-year-old female patient. a The lesion demonstrates annular enhancement in the cortical phase (arrows). b The lesion showed heterogeneous enhancement in the parenchymal phase (arrow). c The lesion demonstrates simultaneous wash-in with surrounding renal parenchyma in the late enhancement phase (arrow)
Fig. 2.
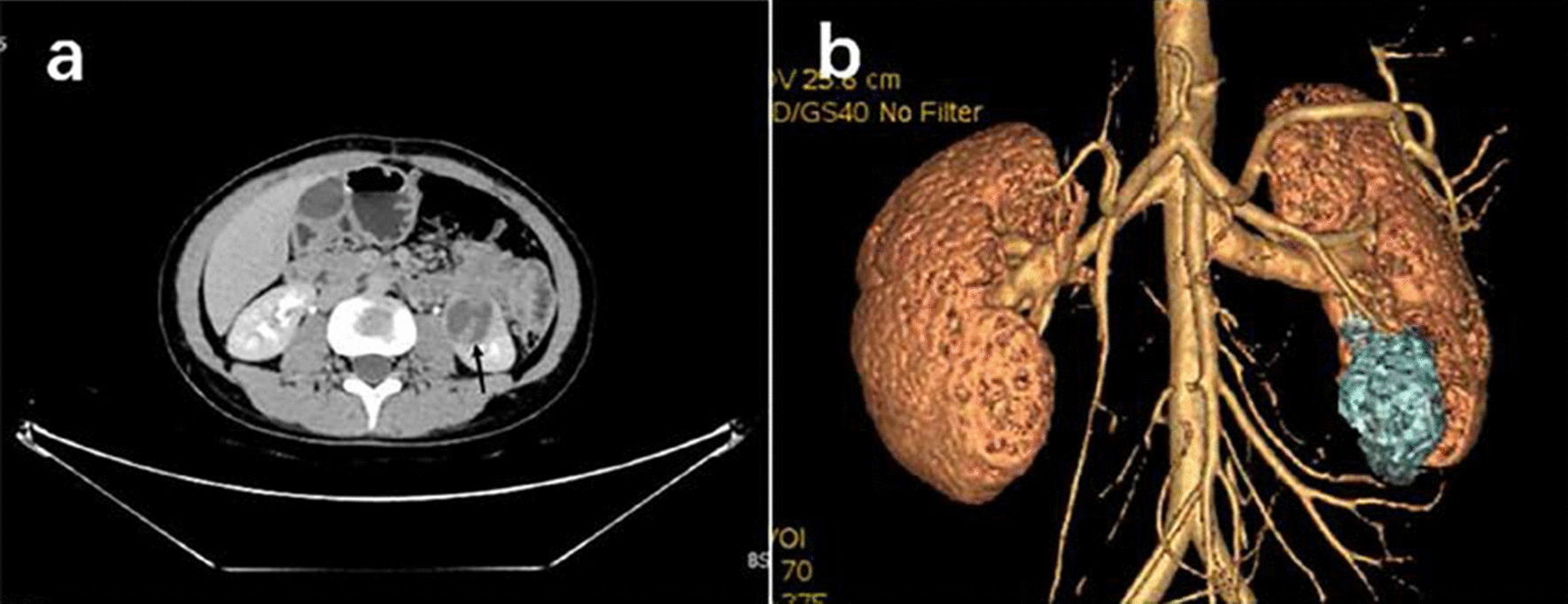
a Computerized tomography (CT)of the urinary system showed a heterogeneous mass in the left kidney(arrow). b Three-dimensional reconstruction of the kidney showed the lower 1/3 of the left kidney was occupied
Fig. 3.
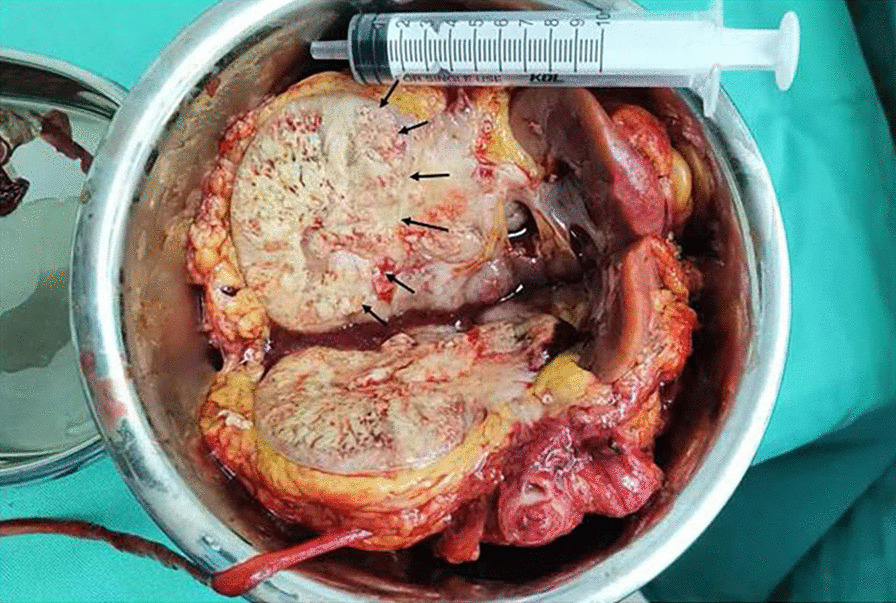
Postoperative gross pathology (left kidney): Gross total removal of the kidney was achieved. The tumor was a well-defined gray-white nodular mass with necrosis(arrow)
Fig. 4.

HE view suggests that the tumor cells were small, round, short spindle shape, densely arranged. The structure of the Homer right rosettes (arrow)can be seen. b Tumor cells showed positive immunoreactivity for CD99 (Fiqure 4 was acquired by Leica LAS X. The measured resolution was 1920*1440. We enhanced the resolution of the image to 300dpi (3862*1497) by PS(Adobe Photoshop 2020) in order to conform to standards of journal.)
Discussion and conclusions
EWS/pPNETS of the kidney are sporadically seen in clinical practice. The main pathogenesis is not clear thus far. Eighty to ninety-five percent of EWS/pPNET patients exhibit the chromosomal translocation T (11; 22) (q24; Q12), and 5–20% of patients often present with mutations in the EWS-ETS gene [9–13]. A comprehensive review of the literature shows that it is difficult to make an accurate diagnosis based on physical examination alone. The imaging findings of EWS/pPNETs are nonspecific. We used contrast-enhanced ultrasound to make a diagnosis of EWS/pPNETs in our patient, which had never been reported previously to our knowledge.EWS/pPNETs of the kidney are more common in male adolescents, with an average age of 29 years, and the male:female sex incidence ratio ranges from 2:1 to 3:1 [14]. The clinical symptoms and imaging findings are nonspecific. The clinical symptoms of EWS/pPNETs are atypical and resemble renal colic symptoms, including an abdominal mass, abdominal pain, and haematuria [8]. More than 65% of ESK patients have distant metastases, and the common sites of metastases include the regional lymph nodes, lungs, and liver, of which the lungs are the most common site. The overall survival rate of patients is meagre, and most patients die from metastatic carcinoma of lung [9, 10].
Imaging methods, such as CT and MRI, have their own specific indications [14] to can help make a correct diagnosis [15]. The EWS/pPNET of the kidney was an inhomogeneous mass with unobvious renal vessels, no signs of invasion, and no calcifications on CT [16, 17]. In a 60-year-old patient with an EWS/pPNET of the kidney, ultrasound revealed an exophytic cortical cyst of the left kidney with irregular echogenic septa. Abdominal MRI and CT scans revealed a large lesion with necrosis of the mass. MRI showed homogeneous hypointensity on T1-weighted images and hyperintensity on T2-weighted images [18]. According to previous literature reports [19–23], we concluded that the CT characteristics of an EWS/pPNET of the kidney are as follows: (1) a large soft tissue mass, (2) the mass can be well defined, (3) necrosis can be found, (4) the renal vein or inferior vena cava may be involved, and (5) calcification is rare. Areas of high density correspond to areas of internal haemorrhage, and areas of low density correspond to areas of necrosis. The CT findings of the patient whose case is presented here are consistent with those previously reported [16–23]. As a radiation-free, inexpensive, and convenient examination method, US can be the primary choice in the diagnosis of EWS/pPNETs. Conventional US may fail to differentiate cystic and necrotic areas due to factors such as resolution. However, CEUS can solve this problem. The EWS/pPNET of the kidney mainly manifested as annular enhancement and heterogeneous enhancement on CEUS, and simultaneous wash-in was predominant in the EWS/pPNET of the kidney. Other common renal malignancies, such as clear cell renal cell carcinomas (ccRCCs), papillary renal cell carcinomas (pRCCs), and chromophobe renal cell carcinomas (chRCCs), can be differentiated from EWS/pPNETs of the kidney on CEUS. ccRCCs are rich in blood vessels, and the vessels of ccRCCs are large, irregular and distorted, with arteriovenous fistulas, which lead to the characteristics of early wash-in and hyperenhancement on CEUS [24]. Furthermore, the rapid tumour growth and proneness to ischaemic necrosis of ccRCCs lead to heterogeneous enhancement. In contrast, pRCCs and chRCCs, owing to the relative lack of vessels or the thick walls of vessels, often show hypoenhancement on CEUS. Previous studies [25] have reported that chRCCs mainly demonstrated simultaneous wash-in, while pRCCs mainly demonstrated slow wash-in. For the wash-out pattern, rapid wash-out mostly appeared in pRCCs and chRCCs. Additionally, EWS/pPNETs need to be distinguished from other uncommon renal tumours, such as adult Wilms, tumours, rhabdoid tumours, and renal clear cell sarcomas. Preoperative diagnosis of these tumours is difficult because there are no specific radiographic findings, and diagnosis relies primarily on histopathology.
Immunohistochemical and molecular diagnostic methods are critical to diagnose EWS/pPNETs. Pathological examination showed that the tumour cells were small, round, short spindle-shaped, and densely arranged. Homer Wright rosettes were found. Immunohistochemical results showed that the glycoprotein CD99 was expressed on the cell surface. The chromosomal translocation T (11;22) (q24; Q12) can be observed in 95% of EWS/pPNETs. However, despite the availability of immunohistochemical and molecular diagnostic methods, these tumours were also misdiagnosed on biopsy [2, 26, 27].
Currently, there is no universally accepted treatment regimens for EWS/pPNETs of the kidney based on treatment guidelines [28, 29], and the best treatment and natural history are unknown. The National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology [28, 29] recommend that the current treatment of EWS/pPNETs of the kidney be based on the treatment experience of other ESFTs, which include radical nephrectomy combined with chemotherapy and adjuvant radiotherapy [10, 28, 30, 31]. The most commonly used chemotherapy regimen is CAV/IE or VACD/IE [5]. In the Grier study [32], alternating VACD or VACD/IE was used for nonmetastatic EWS/pPNET patients, which significantly improved the overall survival (72% vs. 61%) of those patients. The VDC/IE alternating cycle did not improve the outcome of patients with metastatic disease. Approximately 30–40% of Ewing’s sarcoma patients will relapse [5], and the following regimen has been proposed: alternating ifosfamide with etoposide and carboplatin, ifosfamide with etoposide, docetaxel with gemcitabine, and temozolomide with irinotecan [33–36]. The determination of the chemotherapy regimen for the patient whose case is presented here was based on the situation of the patient, who was without metastasis or recurrence. The prognosis for EWS/pPNET patients with active treatment remains objective. The 4-year overall survival was 85% for patients without metastasis and 47% for patients in whom the tumour had invaded the renal vein or inferior vena cava or who had distant metastasis [37]. The overall survival rate of EWS/pPNET patients is higher than that of Ewing's sarcoma of the bone (ESB) patients [38, 39]. If no metastasis is found after the end of chemotherapy for an EWS/pPNET of the kidney, the 5-year and 10-year survival rates are significantly increased to 70% and 60%, respectively [21, 40–42].
In conclusion, EWS/pPNETs of the kidney are very rare tumours that can be diagnosed by CEUS. However, multimodal imaging combined with pathological examination is necessary. Total surgical resection and auxiliary treatment can improve the prognosis of patients with EWS/pPNETs.
Supplementary Information
Additional file 1. Supplement of immunohistochemical staining.
Acknowledgements
Thanks for the patients and everyone involved in this study. We wish to thank Wei Shi for participating in the surgery and treatment.
Abbreviations
- EWS/pPNET
Ewing's sarcoma/peripheral primitive neuroectodermal tumour
- CEUS
Contrast-enhanced ultrasound
- CT
Computed tomography
- ESFT
Ewing's family of tumours
- ES
Ewing's sarcoma
- PNET
Primitive neuroectodermal tumour
- ESK
Ewing's sarcoma of the kidney
- MRI
Magnetic resonance imaging
- CA-125
Carbohydrate antigen 125
- CEA
Carcinoembryonic antigen
- CA-199
Carbohydrate antigen 199
- AFP
Alpha-fetoprotein
- RBC
Red blood cells
- WBC
White blood cells
- US
Ultrasound
- HE
Hematoxylin–eosin staining
- ccRCC
Clear cell renal cell carcinoma
- pRCC
Papillary renal cell carcinoma,
- chRCC
Chromophore renal cell carcinoma
- ESB
Ewing's sarcoma of the bone
Author contributions
JL participated in CEUS diagnosis, follow-up, literature review and manuscript writing. FN helped in consulting the relevant literature and revised manuscript. YL edited the manuscript and performed literature review. All authors read and approved the final manuscript. All authors have agreed both to be personally accountable for the author's own contributions.
Funding
There is no funding for study.
Availability of data and materials
All data and figure generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study had obtained the approval of ethics Committee of Lanzhou University Second Hospital. The patient agreed to participate in our study and signed an informed consent prior to CEUS.
Consent for publication
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Babapour S, Mohseni I, Piri R, Basi A. Left renal Ewing's sarcoma: a case study and a review of imaging literature. Radiol Case Rep. 2020;15(4):391–395. doi: 10.1016/j.radcr.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11(2):184–192. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 3.Wedde TB, Lobmaier IV, Brennhovd B, Lohne F, Hall KS. Primary Ewing's sarcoma of the kidney in a 73-year-old man. Sarcoma. 2011;2011:978319. doi: 10.1155/2011/978319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lalwani N, Prasad SR, Vikram R, Katabathina V, Shanbhogue A, Restrepo C. Pediatric and adult primary sarcomas of the kidney: a cross-sectional imaging review. Acta Radiol. 2011;52(4):448–457. doi: 10.1258/ar.2011.100376. [DOI] [PubMed] [Google Scholar]
- 5.Kairouani M, Mokrim M, Mellas N, Khennoussi B, M'rabti HE, Boutayeb S, Errihani H. Metastatic Ewing's sarcoma/PNET of kidney in 40 year old patient. Int J Surg Case Rep. 2012;3(6):215–217. doi: 10.1016/j.ijscr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, Juergens H. Ewing's sarcoma family of tumors: current management. Oncologist. 2006;11(5):503–519. doi: 10.1634/theoncologist.11-5-503. [DOI] [PubMed] [Google Scholar]
- 7.Lai TC, Lin YJ, Yang MH. Primary Ewing sarcoma of the kidney with inferior vena cava and right atrial tumor thrombi successfully treated with two-stage surgery. Asian J Surg. 2021;44(5):757–758. doi: 10.1016/j.asjsur.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Hakky TS, Gonzalvo AA, Lockhart JL, Rodriguez AR. Primary Ewing sarcoma of the kidney: a symptomatic presentation and review of the literature. Ther Adv Urol. 2013;5(3):153–159. doi: 10.1177/1756287212471095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Zhang L, Wei Y, Wang H, Xiong W, Chen Z, Hes O, Zheng J. Detection of EWSR1 translocation with nuclear extraction-based fluorescence in situ hybridization for diagnosis of Ewing's sarcoma/primitive neuroectodermal tumor. Anal Quant Cytol Histol. 2007;29(4):221–230. [PubMed] [Google Scholar]
- 10.Risi E, Iacovelli R, Altavilla A, Alesini D, Palazzo A, Mosillo C, Trenta P, Cortesi E. Clinical and pathological features of primary neuroectodermal tumor/Ewing sarcoma of the kidney. Urology. 2013;82(2):382–386. doi: 10.1016/j.urology.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Seemayer TA, Thelmo WL, Bolande RP, Wiglesworth FW. Peripheral neuroectodermal tumors. Perspect Pediatr Pathol. 1975;2:151–172. [PubMed] [Google Scholar]
- 12.Zucman J, Delattre O, Desmaze C, Plougastel B, Joubert I, Melot T, Peter M, De Jong P, Rouleau G, Aurias A, et al. Cloning and characterization of the Ewing's sarcoma and peripheral neuroepithelioma t(11;22) translocation breakpoints. Genes Chromosom Cancer. 1992;5(4):271–277. doi: 10.1002/gcc.2870050402. [DOI] [PubMed] [Google Scholar]
- 13.de Alava E, Gerald WL. Molecular biology of the Ewing's sarcoma/primitive neuroectodermal tumor family. J Clin Oncol. 2000;18(1):204–213. doi: 10.1200/JCO.2000.18.1.204. [DOI] [PubMed] [Google Scholar]
- 14.Liang L, Song H, Ma B, Zhang Z, Zhu K, Li Q, Zhou C, Li A, Liu J, Zhang Q, Zhu S, Zhang Q. Renal Ewing's sarcoma/primitive neuroectodermal tumor (PNET): a case series of 7 patients and literature review. Transl Androl Urol. 2021;10(2):548–554. doi: 10.21037/tau-20-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javery O, Krajewski K, O'Regan K, Kis B, Giardino A, Jagannathan J, Ramaiya NH. A to Z of extraskeletal Ewing sarcoma family of tumors in adults: imaging features of primary disease, metastatic patterns, and treatment responses. AJR Am J Roentgenol. 2011;197(6):W1015–W1022. doi: 10.2214/AJR.11.6667. [DOI] [PubMed] [Google Scholar]
- 16.Alghamdi MHA, Alawad SA, Alharbi MG, Alabdulsalam AK, Almodhen F, Alasker A. A rare case of Ewing's sarcoma of the kidney. Urol Case Rep. 2019;16(29):101094. doi: 10.1016/j.eucr.2019.101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manescu MR, Sahyoun A, Froment N, Crisan N, Girot V. Ewing's sarcoma of the kidney complicated by a wunderlich syndrome. Case Rep Urol. 2015;2015:601038. doi: 10.1155/2015/601038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilgetekin I, Karaca M, Gönül II, Üner A, Şahinli H, Demir H, Aytekin A, Çiltaû A, Benekli M. Ewing's sarcoma of kidney in a 60-year-old patient with local recurrence: a rare occurrence. J Cancer Res Ther. 2018;14(6):1422–1424. doi: 10.4103/0973-1482.191062. [DOI] [PubMed] [Google Scholar]
- 19.Angel JR, Alfred A, Sakhuja A, Sells RE, Zechlinski JJ. Ewing's sarcoma of the kidney. Int J Clin Oncol. 2010;15(3):314–318. doi: 10.1007/s10147-010-0042-0. [DOI] [PubMed] [Google Scholar]
- 20.Ellinger J, Bastian PJ, Hauser S, Biermann K, Müller SC. Primitive neuroectodermal tumor: rare, highly aggressive differential diagnosis in urologic malignancies. Urology. 2006;68(2):257–262. doi: 10.1016/j.urology.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 21.Ekram T, Elsayes KM, Cohan RH, Francis IR. Computed tomography and magnetic resonance features of renal Ewing sarcoma. Acta Radiol. 2008;49(9):1085–1090. doi: 10.1080/02841850802345618. [DOI] [PubMed] [Google Scholar]
- 22.Parada D, Godoy A, Liuzzi F, Pelia KB, Romero A, Parada AM. Primary Ewing's sarcoma/primitive neuroectodermal tumor of the kidney. An infrequent finding. Arch Esp Urol. 2007;60(3):321–325. doi: 10.4321/s0004-06142007000300020. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Li Y, Wang R, Song B. Ewing's sarcoma/primitive neuroectodermal tumor of the kidney: a case report and literature review. Transl Androl Urol. 2019;8(5):562–566. doi: 10.21037/tau.2019.09.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun D, Wei C, Li Y, Lu Q, Zhang W, Hu B. Contrast-enhanced ultrasonography with quantitative analysis allows differentiation of renal tumor histotypes. Sci Rep. 2016;11(6):35081. doi: 10.1038/srep35081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue LY, Lu Q, Huang BJ, Li CX, Yan LX, Wang WP. Differentiation of subtypes of renal cell carcinoma with contrast-enhanced ultrasonography. Clin Hemorheol Microcirc. 2016;63(4):361–371. doi: 10.3233/CH-152024. [DOI] [PubMed] [Google Scholar]
- 26.Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. MIC2 is a specific marker for Ewing's sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991;67(7):1886–1893. doi: 10.1002/1097-0142(19910401)67:7<1886::aid-cncr2820670712>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 27.Rowe RG, Thomas DG, Schuetze SM, Hafez KS, Lawlor ER, Chugh R. Ewing sarcoma of the kidney: case series and literature review of an often overlooked entity in the diagnosis of primary renal tumors. Urology. 2013;81(2):347–353. doi: 10.1016/j.urology.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Das S, Aggarwal G, Gupta S, Midha D. Primary renal Ewing's sarcoma in an adult: an enigma. Innov Surg Sci. 2021;6(1):20200022. doi: 10.1515/iss-2020-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito T, Mitomi H, Kurisaki A, Torigoe T, Takagi T, Suehara Y, Okubo T, Kaneko K, Yao T. Low-grade myofibroblastic sarcoma of the distal femur. Int J Surg Case Rep. 2013;4(2):195–199. doi: 10.1016/j.ijscr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukkunda R, Venkitaraman R, Thway K, Min T, Fisher C, Horwich A, Judson I. Primary Adult Renal Ewing's Sarcoma: A Rare Entity. Sarcoma. 2009;2009:504654. doi: 10.1155/2009/504654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thyavihally YB, Tongaonkar HB, Gupta S, Kurkure PA, Amare P, Muckaden MA, Desai SB. Primitive neuroectodermal tumor of the kidney: a single institute series of 16 patients. Urology. 2008;71(2):292–296. doi: 10.1016/j.urology.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 32.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore S, Rausen AR, Vietti TJ, Miser JS. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 33.Miser JS, Kinsella TJ, Triche TJ, Tsokos M, Jarosinski P, Forquer R, Wesley R, Magrath I. Ifosfamide with mesna uroprotection and etoposide: an effective regimen in the treatment of recurrent sarcomas and other tumors of children and young adults. J Clin Oncol. 1987;5(8):1191–1198. doi: 10.1200/JCO.1987.5.8.1191. [DOI] [PubMed] [Google Scholar]
- 34.Van Winkle P, Angiolillo A, Krailo M, Cheung YK, Anderson B, Davenport V, Reaman G, Cairo MS. Ifosfamide, carboplatin, and etoposide (ICE) reinduction chemotherapy in a large cohort of children and adolescents with recurrent/refractory sarcoma: the Children's Cancer Group (CCG) experience. Pediatr Blood Cancer. 2005;44(4):338–347. doi: 10.1002/pbc.20227. [DOI] [PubMed] [Google Scholar]
- 35.Navid F, Willert JR, McCarville MB, Furman W, Watkins A, Roberts W, Daw NC. Combination of gemcitabine and docetaxel in the treatment of children and young adults with refractory bone sarcoma. Cancer. 2008;113(2):419–425. doi: 10.1002/cncr.23586. [DOI] [PubMed] [Google Scholar]
- 36.Hunold A, Weddeling N, Paulussen M, Ranft A, Liebscher C, Jürgens H. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer. 2006;47(6):795–800. doi: 10.1002/pbc.20719. [DOI] [PubMed] [Google Scholar]
- 37.Tarek N, Said R, Andersen CR, Suki TS, Foglesong J, Herzog CE, Tannir NM, Patel S, Ratan R, Ludwig JA, Daw NC. Primary Ewing sarcoma/primitive neuroectodermal tumor of the kidney: the MD anderson cancer center experience. Cancers (Basel) 2020;12(10):2927. doi: 10.3390/cancers12102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Covelli HD, Beekman JF, Kingry RL. Extraskeletal Ewing's sarcoma: prolonged survival with recurrence after operation. South Med J. 1980;73(9):1294–1295. doi: 10.1097/00007611-198009000-00053. [DOI] [PubMed] [Google Scholar]
- 39.Rud NP, Reiman HM, Pritchard DJ, Frassica FJ, Smithson WA. Extraosseous Ewing's sarcoma. A study of 42 cases. Cancer. 1989;64(7):1548–1553. doi: 10.1002/1097-0142(19891001)64:7<1548::aid-cncr2820640733>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 40.Paulussen M, Ahrens S, Dunst J, Winkelmann W, Exner GU, Kotz R, Amann G, Dockhorn-Dworniczak B, Harms D, Müller-Weihrich S, Welte K, Kornhuber B, Janka-Schaub G, Göbel U, Treuner J, Voûte PA, Zoubek A, Gadner H, Jürgens H. Localized Ewing tumor of bone: final results of the cooperative Ewing's Sarcoma Study CESS 86. J Clin Oncol. 2001;19(6):1818–1829. doi: 10.1200/JCO.2001.19.6.1818. [DOI] [PubMed] [Google Scholar]
- 41.Obata H, Ueda T, Kawai A, Ishii T, Ozaki T, Abe S, Tanaka K, Tsuchiya H, Matsumine A, Yabe H; Japanese Musculoskeletal Oncology Group. Clinical outcome of patients with Ewing sarcoma family of tumors of bone in Japan: the Japanese Musculoskeletal Oncology Group cooperative study. Cancer. 2007;109(4):767–75. 10.1002/cncr.22481. [DOI] [PubMed]
- 42.Sadiq M, Ahmad I, Shuja J, Ahmad K. Primary Ewing sarcoma of the kidney: a case report and treatment review. CEN Case Rep. 2017;6(2):132–135. doi: 10.1007/s13730-017-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplement of immunohistochemical staining.
Data Availability Statement
All data and figure generated or analyzed during this study are included in this published article.


