Abstract
Background
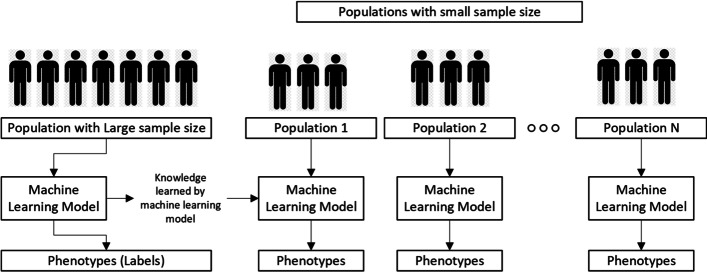
For some understudied populations, genotype data is minimal for genotype-phenotype prediction. However, we can use the data of some other large populations to learn about the disease-causing SNPs and use that knowledge for the genotype-phenotype prediction of small populations. This manuscript illustrated that transfer learning is applicable for genotype data and genotype-phenotype prediction.
Results
Using HAPGEN2 and PhenotypeSimulator, we generated eight phenotypes for 500 cases/500 controls (CEU, large population) and 100 cases/100 controls (YRI, small populations). We considered 5 (4 phenotypes) and 10 (4 phenotypes) different risk SNPs for each phenotype to evaluate the proposed method. The improved accuracy with transfer learning for eight different phenotypes was between 2 and 14.2 percent. The two-tailed p-value between the classification accuracies for all phenotypes without transfer learning and with transfer learning was 0.0306 for five risk SNPs phenotypes and 0.0478 for ten risk SNPs phenotypes.
Conclusion
The proposed pipeline is used to transfer knowledge for the case/control classification of the small population. In addition, we argue that this method can also be used in the realm of endangered species and personalized medicine. If the large population data is extensive compared to small population data, expect transfer learning results to improve significantly. We show that Transfer learning is capable to create powerful models for genotype-phenotype predictions in large, well-studied populations and fine-tune these models to populations were data is sparse.
Keywords: Bioinformatics, Genotype-phenotype, Transfer learning, Deep learning, Genetics
Background
Human characteristics are different for various reasons; classified into nature (genetics and heritability) and nurture (Environment and food consumption). The physical characteristic or the presence of a particular disease is known as a phenotype. For example, eye color is a phenotype, and blue, green, brown, and black eye colors are the four possibilities of this particular phenotype. Genetic data (genotype, DNA) plays a vital role in determining some characteristics or diseases. Researchers inferred that the variations or mutations in the DNA result in the variation in the phenotype. Mutations at a particular location in the DNA that can be used to find this phenotype-genotype relationship are SNPs, known as Single-nucleotide polymorphism. SNPs associated with an increased risk of developing a particular disease or trait are called risk SNPs.
Following is a list of acronyms used in this manuscript:
PhenotypeSimulator = PS
Utah Residents with Northern and Western European ancestry = CEU
Yoruba in Ibadan, Nigera = YRI
Single-nucleotide polymorphism = SNPs
Genome-wide association studies = GWAS
Artificial neural network = ANN
Recurrent neural network= RNN
Gated recurrent unit = GRU
Long short-term memory = LSTM
Bidirectional LSTM = BILSTM
In genetics, case/control studies for humans, animals, and different species [1–7] are of great importance for the diagnosis of a particular disease in organisms. Genotype data available for genetic analysis can significantly improve the final results [8]. Although next-generation sequencing [9, 10] and imputation algorithms [11, 12] have increased the genetic data available for analysis, genomics is failing on diversity [13]. There are still some small understudied populations [14, 15] for which data is not enough for genetic analysis or case/control classification. We refer to a population as small, if the available genotype data for analysis is insufficient for reliable conclusions [16], i.e., a statistical power analysis would deem the dataset to contain too few samples to achieve statistical significance. To mitigate this issue, we can use knowledge extracted from a large population (for which data is in abundance), to make predictions about the small population with a fine-tuned model [17]. Indeed, transfer learning is currently an increasing research sub-field of machine learning. It is used in computer vision [18], natural language processing tasks like sentiment analysis [19], and to improve the clustering of single-cell RNA-Seq data [20]. Furthermore, it is often used in conjunction with deep neural networks [21], which require a lot of data and computing power.
Pio et al. [22] used transfer learning to improve the human gene regulatory network reconstruction accuracy using gene-related metabolic features (generated through gene expression data) from human and mouse. The task at hand is to identify regulatory links between genes in a network. This approach is similar to what we present in this work, but instead of using it for gene network reconstruction, we use it for classification of genotype-phenotype prediction tasks.
Mignone et al. [23] also uses a specific variant of transfer learning in which the number of negative examples or the labels for particular class instances are missing (negative samples), but positive samples are known. In that case, positive interactions between genes are known, but negative interaction are not. They derived the information from the common genes between humans and mouse, and used those features to train the machine learning model, and used that model to identify the negative interactions. Similarly, this article [24] used the sample approach to the prediction of virus-human protein-protein interactions.
Transfer learning is also used for genetic data [25–27] and offers several advantages described below.
Transfer learning helps to solve complex real-world problems having little or almost no labeled data availability [17].
It allows transferring knowledge from one model to another based on domains and tasks.
With transfer learning, the final model achieved has better generalization ability than directly training the data on a small population.
In the following, we elaborate on the applications and advantages of using transfer learning, particularly for genotype data.
The motivation for using transfer learning is personalized medicine [28], which is a medical model that separates people into different groups with medical decisions and products being tailored to the individual patient based on their risk of disease. Genotype data for one person belonging to a particular population cannot train the deep learning model. However, with transfer learning, using genotype data of another population, we can make some valuable insights about that person, leading to personalized medicine for a specific person. In the case of humans, cancer is the perfect example. Suppose we have 1000 cancer patients from the CEU (Northern Europeans from Utah) population and 10 cancer patients from the YRI (Yoruba in Ibadan) population. Direct statistical analysis on 10 (YRI) cancer patients would not lend itself to a Genome Wide Association Study, as it would be underpowered for this purpose. However, using genotype data from 1000 (CEU) cancer patients can improve case/control classification. Most deep learning algorithms work best when given a lot of data [29], and we can leverage samples from large populations to make case/control predictions on small populations, after adjusting for the characteristics of the small population. Many species have biological function resemblances [30], so transfer learning using genotype information would be an invaluable resource for the effective management of breeding programs and cases/controls studies in small and endangered populations. Consider Bornean elephants (smallest Asian elephant subspecies) [31] which is among endangered species. Suppose that the Bornean elephants, which are small in quantity, suffer from some disease. In that case, we would not have enough genetic information for any analysis or prediction. However, we can use case/control studies of other elephant species that are large in quantity to get insights into the Bornean elephants.
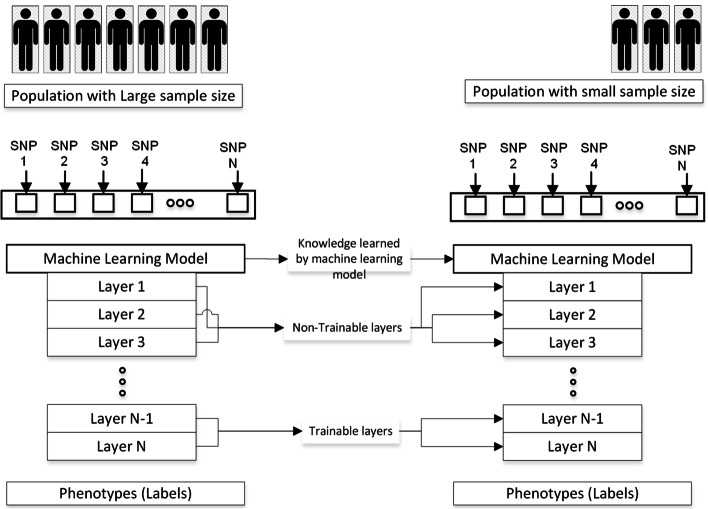
Deep learning models perform well when the amount of available datasets is sufficiently high.The deep learning models trained on the large population store the knowledge that can be used for classification; however, these models do not contain the exact representation required for classifying small population samples, so we used fine-tunning (transfer learning) to improve the representation learned by the models using the training data from the small population to improve the classification score. Deep learning models store the information in various layers, which can be made trainable/non-trainable. It helps to extract the particular knowledge or update the stored knowledge allowing for fine-tuning the model to improve the classification score.
The second reason is that existing tools like Plink and LDPred-2 consider the linear interaction of SNPs weighted by the corresponding Odds ratio to find the polygenic risk scores. These models cannot be directly used for transferring knowledge as it will result in only SNP transfer learning methodology with limited information from the large population.
In general, deep learning algorithms perform well when the dataset is large, whereas the performance is poor when the dataset is in low quantity. With that in mind, we used classic machine learning algorithms, which are good when the dataset is low. Second, the genotype-phenotype prediction depends on the phenotype under consideration. We first tested classic machine learning and then employed a deep learning algorithm for prediction. So, if classical machine learning algorithms can work for transfer learning, there is no need to use deep learning algorithms.
We considered only simulated data for testing the applicability of transfer learning for genotype data and the following are the reasons for this:
Phenotype: For transfer learning, data for a specific phenotype for both populations is required, and we did not have the data for a specific phenotype for both populations. If we directly use the 1000 Genome data, we can have a phenotype for all people, but for the large population, data will be low, which is one of the assumptions for successful transfer learning.
Heritability: Second, the performance of the deep learning models depends on the dataset’s quality and heritability [32]. The real dataset mostly has missing values, which may reduce the number of common SNPs between both populations, whereas simulated data has fewer missing values for SNPs.
Different populations: If the dataset is a mix, one has to classify people into different populations. Moreover, there are many possibilities for transfer learning. We transferred knowledge from CEU to YRI, whereas transfer from YRI to CEU is also possible. Due to multiple possibilities, we restricted the scope to a particular transfer to test the applicability.
The proposed method is distinct from the existing studies for the following two reasons. We searched for existing transfer learning methodologies for genotype data, but we did not find any article specifically designed for genotype data. Second, the method presented in the article can assist in studying the transfer learning approach for various populations. For example, we considered CEU as a large population and YRI as a small population, but they can be interchanged too. Phenotypes with various heritability and number of risk SNPs can be generated, and the effectiveness of transfer learning can be studied. All the above reasons make this article distinct, and there is no such article tackling the above issue.
Section (Material, Module 1 to 3) contains the data generation part. Section (Methods, Module 4 to 5) provides technical context and describes the entire pipeline for transfer learning. Section (Results) demonstrates the results. Section (Discussion) contains a closing discussion.
Materials
This section contains the datasets used in this manuscript. We explained the data generation part in detail for the following reasons.
We worked on simulated data, and using the tools used to generate data, one can generate data with multiple possibilities. For instance, genotype data with varying heritability, genetic variation, and the number of risk SNPs can be generated, and the effectiveness of transfer learning for various phenotypes can be explored. It may work for some simulated phenotypes and may give negative results for others. A dataset with the same parameters must be generated for both populations to support such studies, making the data generation process crucial in the pipeline. The overall methodology is explained in Fig. 1.
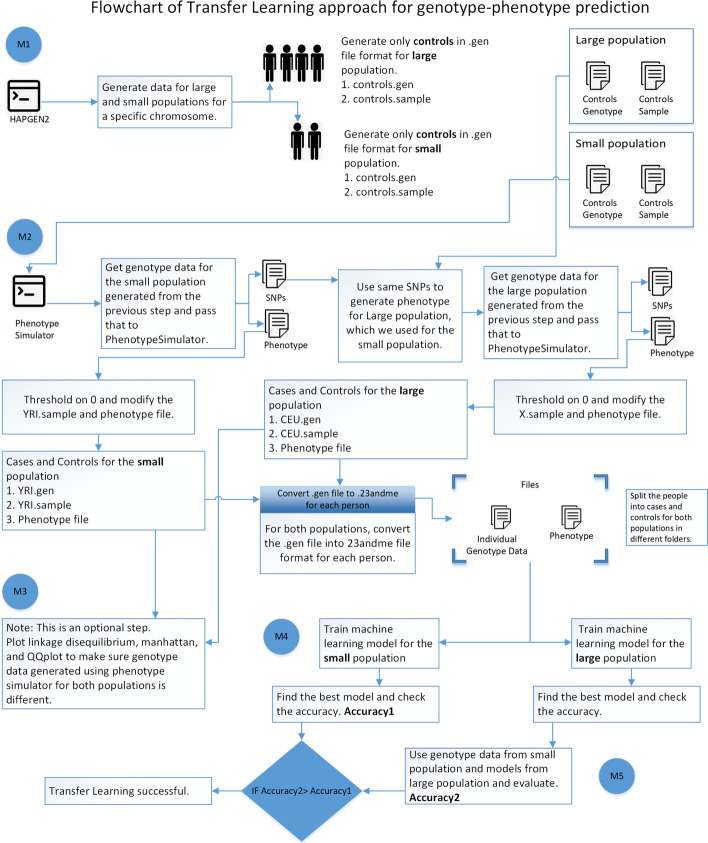
Fig. 1.
This diagram shows the flowchart of the deep learning-based transfer learning approach for the genotype-phenotype prediction that consists of 5 modules
The transfer learning approach for the actual genotype data is straightforward, but when working on simulated genotype data, there are various parameters, which can change the outcome of the whole process, and it requires an in-depth explanation of all the processes involved in the data generation part. These processes include handling directory structure and file formats, coordinating genotype data between populations, and applying transfer learning to a small population. As we presented the pipeline along with the use case, the data generation part also includes the information for the use case to comprehend the methodology section. We feel not explaining this section in detail would miss the overall gist of the manuscript, making it possibly difficult to understand.
Module 1: Generate data using HAPGEN2
We generated genotype data for YRI (Small population) and CEU (Large population) using HAPGEN2, which is a tool that takes 1000 Genomes Pilot + HapMap 3 data [33], and simulates cases/controls genotype data by specifying the disease model through a set of disease-causing SNPs together with their relative risks [34].
1000 controls for CEU and 100 controls for YRI
Generated genotype data for Chromosome 21
This step generates four files: YRI.gen and YRI.sample (genotype data and sample file for the small population), CEU.gen, and CEU.sample (genotype data and sample file for the large population)
Module 2: Generate phenotype using phenotype simulator
PhenotypeSimulator takes genotypes to simulate the basic genetic, non-genetic covariates, observational noise, and non-genetic correlation structures. The effect structure of the four abovementioned components is divided into a shared effect across traits or an independent effect for a number of traits, allowing for complex phenotype structures. Finally, the simulated phenotype and its components are saved into genetic output formats. Using PS, generate phenotype for each sample in the YRI.samples and CEU.samples files, and perform further analysis on common SNPs between the two populations. Transfer learning requires training a machine learning model on the common SNPs between both populations because each SNP is a feature when training the model. If SNP is missing from the small population, we cannot transfer knowledge.
Number of SNPs in CEU: 101053
Number of SNPs in YRI: 136021
Number of common SNPs between CEU and YRI: 62283
Generate phenotype for the small population
YRI.sample and YRI.gen files, generated in the previous step, are passed to PS to simulate a phenotype for each person. We considered the default values for each parameter as shown on page 3,example 2, https://cran.r-project.org/web/packages/PhenotypeSimulator/vignettes/PhenotypeSimulator.pdf in the PhenotypeSimulator documentation. A detailed explanation of each variable is mentioned in PhenotypeSimulator documentation https://cran.r-project.org/web/packages/PhenotypeSimulator/PhenotypeSimulator.pdf. We generate 4 phenotypes for 5 risk SNPs and 10 risk SNPs, labeled with a specific code as shown below.
4 phenotypes with 5 risk SNPs: YRI_5_1, YRI_5_2, YRI_5_3, and YRI_5_4
4 phenotypes with 10 risk snps: YRI_10_1, YRI_10_2, YRI_10_3, and YRI_10_4
The actual risk SNPs are different and randomly decided by PS for each phenotype.
Generate phenotype for the large population
CEU.sample and CEU.gen file, generated in the previous step, are passed to PS to simulate the phenotype, using the same parameters and the same risk SNPs to simulate the data for the small population.
4 phenotypes with risk SNPs 5: CEU_5_1, CEU_5_2, CEU_5_3, and CEU_5_4
4 phenotypes with risk SNPs 10: CEU_10_1, CEU_10_2, CEU_10_3, and CEU_10_4
One crucial point is that the actual risk SNPs for YRI_5_1 are the same for CEU_5_1, and the machine learning model trained on CEU_5_1 is used for the prediction of YRI_5_1.
PS also outputs the risk SNPs for the small population, which can be extracted and passed to PS as causal SNPs when generating data for the large population pass.
Each phenotype is represented in this format, X_Y_Z, X is the population code (CEU or YRI), Y is the number of risk SNPs (5 or 10), and Z is the iteration number (1 to 4). The number of risk SNPs (Y) can be mutated in the PS. Z is a specific iteration. We generated a new dataset for each iteration, and the risk SNPs selected by PS are different for the same Y.
At this stage, we have genotype data for eight phenotypes. Considering these two datasets, CEU_5_1 and YRI_5_1, we explained the next steps in the pipeline, and these steps are the same for each phenotype.
Convert continuous phenotype to cases/controls
PS generates continuous phenotype, which we converted to binary phenotype after thresholding on 0 (below 0 a control and above 0 is a case), for CEU_5_1 and YRI_5_1. Represent controls with 0 and cases with 1, and update the CEU_5_1/CEU.sample and YRI_5_1/YRI.sample files accordingly with new phenotypes values after thresholding. Generate a separate phenotype file for both populations, which contains the sample id and the phenotype, and convert CEU_5_1/CEU.gen and YRI_5_1/YRI.gen files in 23andme file format, so the machine learning techniques specified in this article [35] are applicable to genotype-phenotype prediction.
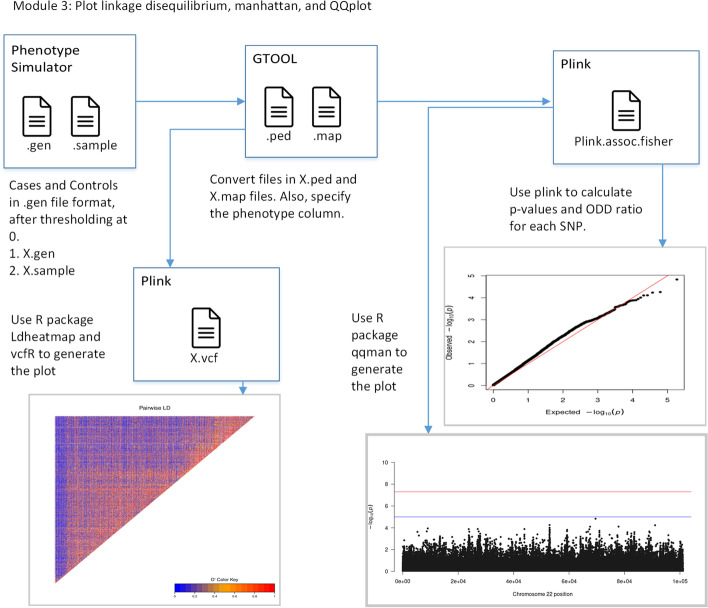
Module 3: Plot linkage disequilibrium, manhattan, and QQplot
Module 3 is optional used to generate the manhattan, QQ, and Linkage disequilibrium plot for a particular phenotype of both populations. Figure 2 elaborates the visualization step. For visualization, use X_5_1/X.gen and X_5_1/X.sample files.
Fig. 2.
Module 3: Plot linkage disequilibrium, manhattan, and QQplot. Genotype data generated from PS is passed to Gtool -> Plink to convert genotype data into VCF file format, and that file is used to generate linkage disequilibrium. Gtool output is also used to find p-values for each SNP and generate the manhattan plot
Methods
This section provides in-depth detail of the proposed pipeline. In each section, some subsections describe the sub-steps in each module.
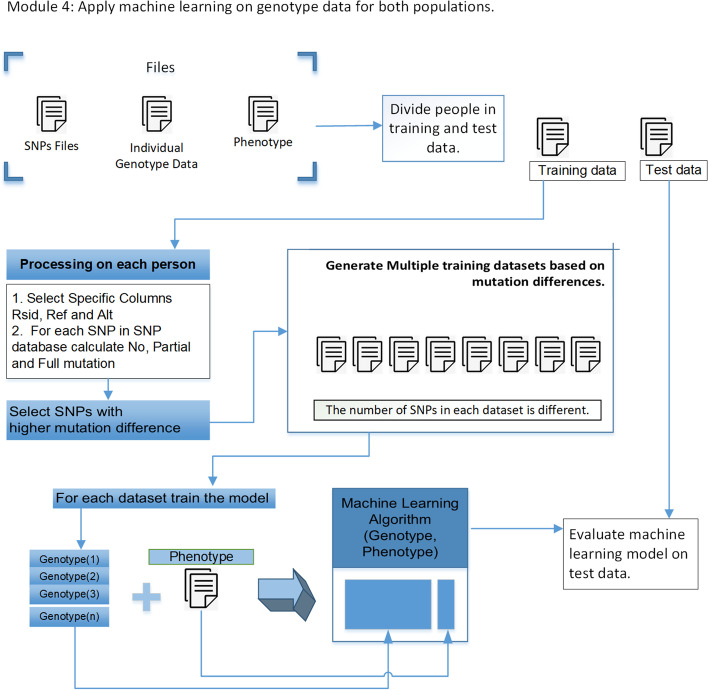
Module 4: Machine learning for both small and large populations
At this stage, we have these files for both populations.
Genotype files in 23andme file format.
SNPs file
Phenotype
Quality control
Quality control steps are performed on the genotype data to ensure the quality of the data is of a high standard for any interpretation [36–38], but for the simulated data, some quality control steps, like removing SNPs with missing alleles and duplicate SNPs, can be skipped. Perform these steps independently for both large and small populations.
SNPs pre-selection
Using 62,283 SNPs for training may overfit the model, so SNPs pre-selection process (p-value threshold or mutation difference between cases/controls at each SNP [35]) will reduce the dimensionality of input data leading to a generalized model. Generate multiple datasets using the SNPs pre-selection process on the training data for both populations. Tables 1 and 2 show the number of SNPs in each sub-dataset of the small population and the large population for each phenotype.
Table 1.
13 sub-datasets for each phenotype containing different number of SNPs
| SNPs in sub-dataset | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YRI_5_1 | 3 | 6 | 14 | 32 | 51 | 105 | 154 | 236 | 287 | 435 | 822 | 1112 | 1803 |
| YRI_5_2 | 1 | 4 | 7 | 26 | 28 | 60 | 65 | 192 | 233 | 314 | 662 | 935 | 1868 |
| YRI_5_3 | 0 | 1 | 10 | 21 | 38 | 67 | 116 | 202 | 239 | 436 | 728 | 995 | 1616 |
| YRI_5_4 | 4 | 5 | 24 | 83 | 127 | 153 | 193 | 255 | 295 | 487 | 732 | 1048 | 1729 |
| YRI_10_1 | 2 | 7 | 18 | 47 | 91 | 137 | 180 | 345 | 422 | 551 | 1086 | 1899 | |
| YRI_10_2 | 7 | 11 | 12 | 67 | 136 | 171 | 248 | 279 | 494 | 563 | 656 | 1158 | 2032 |
| YRI_10_3 | 3 | 5 | 13 | 43 | 65 | 103 | 130 | 146 | 341 | 431 | 590 | 1110 | 1974 |
| YRI_10_4 | 1 | 3 | 20 | 36 | 75 | 109 | 179 | 264 | 312 | 561 | 913 | 1282 | 2130 |
0 means sub-dataset does not contain any phenotype and such sub-datasets are ignored
Table 2.
10 sub-datasets for each phenotype containing different number of SNPs
| SNPs in sub-dataset | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| CEU_5_1 | 17 | 18 | 48 | 154 | 193 | 1135 | 1196 | 3421 | 4901 | 6629 |
| CEU_5_2 | 0 | 4 | 18 | 74 | 89 | 949 | 987 | 3551 | 4867 | 6583 |
| CEU_5_3 | 0 | 4 | 10 | 73 | 80 | 921 | 1016 | 3411 | 4780 | 6641 |
| CEU_5_4 | 0 | 1 | 28 | 101 | 126 | 932 | 969 | 3438 | 4718 | 6751 |
| CEU_10_1 | 2 | 20 | 96 | 101 | 809 | 894 | 3485 | 4816 | 6601 | |
| CEU_10_2 | 1 | 4 | 22 | 150 | 162 | 1005 | 1076 | 3545 | 4999 | 6673 |
| CEU_10_3 | 1 | 5 | 134 | 243 | 296 | 1119 | 1162 | 3732 | 5015 | 6731 |
| CEU_10_4 | 0 | 1 | 44 | 151 | 164 | 924 | 980 | 3522 | 4962 | 6699 |
0 means sub-dataset does not contain any phenotype and such sub-datasets are ignored
Machine learning for the small population
Before applying machine learning for genotype-phenotype prediction, we must generate multiple sub-datasets for both populations. Each sub-dataset contains a different number of SNPs. It is essential to try different SNPs to find the model that generalizes well. We generated about 13 sub-datasets containing a different number of SNPs for each phenotype. As the SNPs pre-selection process is performed on the training data, we must extract the same SNPs from the test data. Make sub-datasets like this YRI_5_1/snps_X, where X represents the number of SNPs in the particular sub-dataset. The number of SNPs selected for a specific dataset depends on the linear threshold value. Dataset split for YRI population is like this.
Training data 70 samples (YRI_TD)
Validation data 10 samples (YRI_VD)
Test data 20 samples (YRI_ED)
Final test data 50 samples (YRI_FD)
For genotype-phenotype prediction of the small population we used five machine learning algorithms: SVM = Support vector machine [39], Cart = Decision tree classifier [40], Rus = Random under sampler, Forest = Random forest classifier [41], Ub = Bagging classifier. We trained each sub dataset on these 5 models and selected the sub-dataset which performed well on the test data. For example, if YRI_5_1/snps_1000/SVM generated the best accuracy for the test data YRI_ED, save that model and test newly generated data YRI_FD.
To test the generalization of the best model, generate a new dataset YRI_FD.
A new dataset shows how well genotype-phenotype prediction for the small population works without transfer learning.
Use this newly generated data for the evaluation of the transfer learning methodology.
Table 3 shows the results of best machine learning without transfer learning for both YRI_ED and YRI_FD datasets.
Table 3.
The first, second, third, fourth, and fifth columns show the phenotype, the optimal number of SNPs, the best model for the YRI_ED dataset, accuracy on the YRI_ED dataset, and the final accuracy on the YRI_FD dataset
| Phenotype | SNPs | Best model | YRI_ED accuracy | YRI_FD accuracy |
|---|---|---|---|---|
| YRI_5_1 | 236 | UB | 66 | 56 |
| YRI_5_2 | 26 | CART | 85 | 52 |
| YRI_5_3 | 10 | SVM | 61 | 44 |
| YRI_5_4 | 1048 | UB | 71 | 42 |
| YRI_10_1 | 1086 | SVM | 76 | 48 |
| YRI_10_2 | 11 | Random forest | 66 | 52 |
| YRI_10_3 | 1110 | CART | 71 | 52 |
| YRI_10_4 | 36 | SVM | 61 | 46 |
Machine learning for the large population
For each phenotype of the large population, we generated about ten sub-datasets containing a different number of SNPs. For each sub-dataset, we used nine different architectures of deep learning algorithms ANN (3, Table 4 shows the architecture), LSTM(2), GRU(2), and BILSTM(2) (Table 5 shows the architecture) for transfer learning; after training, save all models.
Table 4.
Model 1, 2, 3 architecture
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Layers | Parameters | Parameters | Parameters |
| Layer 1—FullyConnected | Input layer | Input layer | Input layer |
| Layer 2—FullyConnected | 30 | 80 | 80 |
| Layer 3—FullyConnected | 10 | 70 | 70 |
| Layer 4—FullyConnected | 2 | 40 | 50 |
| Layer 5—FullyConnected | – | 10 | 20 |
| Layer 6—FullyConnected | – | 2 | 10 |
| Layer 7- FullyConnected | – | 2 |
The number of layers and the number of neurons in each layer can vary. Moreover, the hyper-parameters can be tuned to improve the final performance. The number of trainable and non-trainable layers can vary, but transfer learning does not perform well if all layers are trainable and the performance is improved
Table 5.
Model (4, 5, 6), (7, 8, 9) architecture
| Model 4/5/6 | Model 7/8/9 | |
|---|---|---|
| Layers | Parameters | Parameters |
| Layer 1—FullyConnected | Input layer | Input layer |
| Layer 2—GRU/LSTM/BILSTM | 50 | 50 |
| Layer 3—GRU/LSTM/BILSTM | 20 | 20 |
| Layer 4—FullyConnected | 2 | 10 |
| Layer 5—FullyConnected | – | 5 |
| Layer 6—FullyConnected | – | 2 |
The number of layers and the number of neurons in each layer can vary. Moreover, the hyper-parameters can be tuned to improve the final performance. The number of trainable and non-trainable layers can vary, but transfer learning does not perform well if all layers are trainable and the performance is improved
Following is the dataset split for training the machine learning model on the CEU population.
Training data 700 samples (CEU_TD)
Validation data 100 samples (CEU_VD)
Test data 200 samples (CEU_ED)
Figure 3 shows the machine learning methodology for genotype-phenotype prediction.
Fig. 3.
Module 4: Machine learning approach for training the large population genotype data. Train the machine learning model for a large population, and save all models. We can also choose those models for which the test accuracy is high, but we would have a limited number of models to test when evaluating the transfer learning performance
Module 5: Transfer learning for the small population
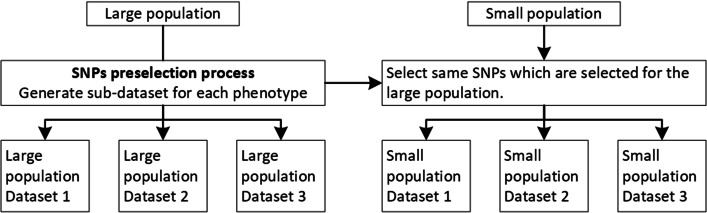
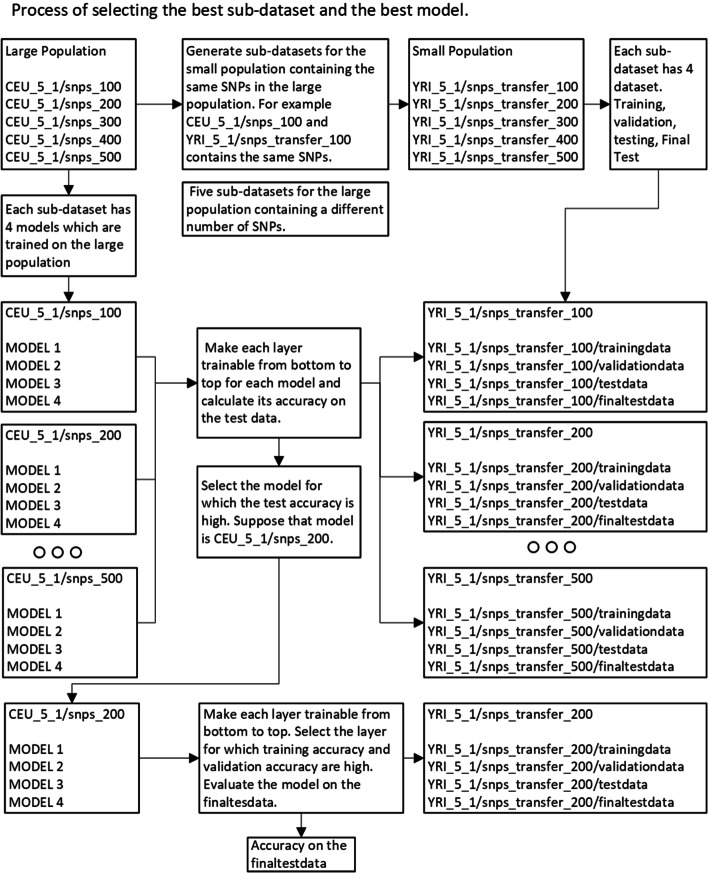
In the case of transfer learning, we have to select SNPs from two populations. If the machine learning model trains on N features (encoded SNPs) from the large population (CEU_5_1/snps_1000/), then we have to choose the same SNPs from a small population (YRI_5_1/snps_transfer_1000/). For each sub-dataset of the large population, make a corresponding sub-dataset of the small population, which contains the same SNPs in the large population as shown in Fig. 4.
Fig. 4.
The SNPs selection process for the small population to use transfer learning for a particular phenotype. Before the SNPs preselection process, it is necessary to select the common SNPs between both populations using Rsid
In two ways, transfer learning methods can be used for genotype data.
Transfer learning with SNPs transfer learning
SNPs selected to classify large population datasets into cases/controls based on mutation differences are good features for machine learning. Same SNPs can also be used for the classification of a small population. For each sub-dataset for a particular phenotype train the 5 classific machine learning algorithm on (CEU_5_1/snps_transfer_1000/training data), to find the optimal sub-dataset test the model on (YRI_5_1/snps_transfer_1000/test data) and for generalization test the model on (YRI_5_1/snps_transfer_1000/YRI_FD).
Transfer learning with deep transfer learning
In deep transfer learning, use the model trained on the large population for the small population genotype-phenotype prediction. Models trained on the large population can substantially increase the classification accuracy of the small population. The procedure to use deep transfer learning is explained in the following bullets and graphically shown in Fig. 5.
For each large population sub-dataset (CEU_5_1/snps_X, X is the number of SNPs in sub-dataset), train 9 deep learning models.
For each large population sub-dataset (CEU_5_1/snps_X), generate a corresponding sub-dataset for the small population containing the same SNPs of the large population (YRI_5_1/snps_transfer_X).
For each model (CEU_5_1/snps_1000_Model1), make layers trainable and nontrainable from bottom to top. When the model is trainable, pass (YRI_5_1/snps_transfer_1000_TD) for training, (YRI_5_1/snps_transfer_1000_VD) for validation, and evaluate the model on (YRI_5_1/snps_transfer_1000_ED). Select models which generate the best accuracy for (YRI_5_1/snps_transfer_1000_ED).
Repeat this process for each sub-dataset of a particular phenotype.
There are about 10 sub-datasets for the large population and 9 models for each sub-dataset. Based on the (YRI_5_1/snps_transfer_1000_ED) select the model for the final evaluation on (YRI_5_1/snps_transfer_1000/YRI_FD).
Fig. 5.
The process of selecting the best sub-dataset and model for transfer learning. Choose the model for which the training, validation, and test accuracy is high and test the final model on the final dataset
Models and implementation
This section contains information about the deep neural networks and transfer learning types used in the methodology.
Models
For any deep learning model, the number of layers, the number of neurons, and hyper-parameter selection can affect transfer learning performance [42]. We used nine different architectures of deep learning algorithms ANN(3), LSTM(2), GRU(2), and BILSTM(2) for transfer learning. All the models should be trained again if we change the base dataset.
Artificial neural network
An ANN goes through a training process where it learns to identify patterns in the input data [43, 44]. It contrasts its actual output generated with what it was intended to achieve during the back-propagation process. The activation function produces non-linearity, which is the most significant benefit of using ANN. Every sub-dataset has a different number of SNPs, so we used an ANN with different processing units in the first layer for each sub-dataset.
Recurrent neural network
The recurrent neural networks, known for their memory, which allow them to use past inputs to influence the current input and output, are used for time-series or sequential data such as language translation [45], natural language processing, voice recognition [46] and image captioning and classification [47].
We considered three types of recurrent neural networks: GRU, LSTM, and Bi-directional LSTM.
GRU: This deep learning algorithm consists of two gates (update and reset gate) to predict the next output in the sequence. The update gate determines the amount of prior knowledge to be transferred to the subsequent state, enables the model to copy all prior knowledge if necessary, and eliminates the danger of vanishing gradient. How much of the prior data will be ignored is determined by the reset gate.
LSTM: This deep learning algorithm consists of three gates (input, forget, and output gate) to predict the next output in the sequence. The input gate chooses what data from the input signal and short-term memory from the previous phase should be kept in long-term memory. The forget determines which long-term memory details should be retained or ignored. The output gate creates new short-term memory and transfers it to the cell in the following step using the current input, the old short-term memory, and the freshly computed long-term memory.
BILSTM: In a bidirectional LSTM, we consider two separate sequences (One from right to left and the other in the reverse order) and two LSTM networks.
These algorithms have already been used for genotype-phenotype [48–50] prediction making them applicate for genotype-phenotype transfer learning.
The use of the algorithm depends on the application. For example, CNN and its variants (ImageNet, ResNet, VGG) are good when transfer learning is used for image data. Similarly, models like word2vec and GloVe are used for natural language-based transfer learning. For genotype data, there is no existing model which can be used, so we must design such models from scratch. Among deep learning models, the best algorithms which can be used for transfer learning are ANN and RNN.
Types of transfer learning
There are three different types of transfer learning we consider here, simple transfer learning, pre-trained models, and fine-tuning.
Figure 6 shows the primary mechanism of transfer learning to transfer knowledge for genotype-phenotype prediction.
Fig. 6.
Basic transfer learning mechanism for genotype data. The model trained on the large population can be used to predict multiple small populations, and one has to ensure that before starting the analysis, common SNPs should be selected among all populations
Simple transfer learning
Transfer learning means when the model trained on some task A is used to make predictions for some other but related task B. Similarly, a deep learning model trained on a large population can predict a small population for a particular phenotype. For simple transfer learning, train the deep learning model on the large population, evaluate model performance on test data of the large population, and use that model to predict the small population.
The word “transfer learning” is described using domains and tasks. A domain D consists of: a feature space X and a marginal probability distribution P(X), where . Given a specific domain, , a task consists of two components: a label space Y and an objective predictive function . The function f is used to predict the corresponding label f(x) of a new instance x. This task, denoted by , is learned from the training data consisting of pairs , where and
Given a source domain and learning task , a target domain and learning task , where , or , transfer learning aims to help improve the learning of the target predictive function in using the knowledge in and .
Pre-trained model
Pre-trained type means when a model that has already been learned is used for transfer learning, and the nature of the problem specifies the number of layers to reuse and retrain. For transfer learning, estimation, function extraction, and fine-tuning, Keras contains nine pre-trained models. Many research institutions make trained models accessible regularly. Unfortunately, there is no existing pre-trained model for genotype-phenotype prediction. But it is important to discuss this strategy here. Figure 7 shows the transfer learning mechanism using the pre-trained model for genotype-phenotype prediction.
Fig. 7.
Module 5.2: Transfer learning using pre-trained model. All layers are non-trainable, and the same SNPs (on which the large population model is trained) are passed to the machine learning model for small population classification
One important point to understand here is that when we transfer knowledge which features should be used for a small population. From small population data, we will choose the same SNPs for which the initial model was trained using the large population data. In a real dataset, there is a possibility that in a small population, SNPs are missing. In that case, the missing SNPs should be imputed [12] or ignored.
Fine-tuning
In fine-tuning-based transfer learning, train a deep learning model on the large population, retrain the model on the training data of the small population by making some layers trainable and non-trainable, and finally, evaluate the model on test data of the small population. This technique is common in computer vision because it decreases the size of the dataset required to train the model, which saves time and makes it more suitable for traditional algorithms. Figure 8 shows the transfer learning mechanism using fine-tuning for genotype-phenotype prediction.
Fig. 8.
Module 5.3: Transfer learning with fine-tuning. Some of the layers are trainable, and some are not. A different number of trainable and non-trainable layers can be tried to improve the performance of the model
Results
It is important to notice that we should also look for bad results. As we increase the number of SNPs, the deep learning models will be more biased towards the large population, and that is why sub-datasets containing a different number of SNPs and models with different hyper-parameters were generated to generalize the final results [51]. Before using deep learning, it is also important to understand what we will transfer as knowledge. There are two important parameters that we can learn from the large population.
Selected SNPs from a large population
Selected SNPs and weights learned by deep learning model from a large population.
Model 1
When we choose SNPs based on mutation differences to classify large population datasets (B1) into cases/controls, those SNPs can act as good features for machine learning. Same SNPs can also be used to classify a small population (T1). Table 6 shows the results of SNPs transfer learning using a classical machine learning model. Results show that this technique did not work on YRI_FD of each phenotype.
Table 6.
Results of SNPs transfer learning
| Phenotype | SNPs | Best model | YRI_ED accuracy | YRI_FD accuracy |
|---|---|---|---|---|
| YRI_5_1 | 236 | RUS | 66 | 48 |
| YRI_5_2 | 26 | Random forest | 85 | 54 |
| YRI_5_3 | 10 | Random forest | 61 | 44 |
| YRI_5_4 | 1048 | SVM | 71 | 38 |
| YRI_10_1 | 1086 | SVM | 76 | 46 |
| YRI_10_2 | 11 | Random forest | 66 | 48 |
| YRI_10_3 | 1110 | Random forest | 71 | 48 |
| YRI_10_4 | 36 | RUS | 61 | 32 |
The first, second, third, fourth, and fifth columns show the phenotype, the optimal number of SNPs, the best model for the YRI_ED dataset, accuracy on the YRI_ED dataset, and the final accuracy on the YRI_FD dataset
Model 2
We used about 9 different architectures of 4 models ANN, LSTM, GRU, and BILSTM. Results show there is a bit of improvement in the results. Tables 7, 8, and 9 show the results when selected SNPs and weights learned by the deep learning model from a large population are used for transfer learning.
Table 7.
Results of deep transfer learning
| Phenotype | SNPs | Best model | Best model accuracy on YRI_ED |
|---|---|---|---|
| CEU_5_1 | 1196, 1196, 4901 | Model 1,2,5 | 66 |
| CEU_5_2 | 3551, 6583 | Model 3,5 | 75 |
| CEU_5_3 | 921, 80 | Model 2,4 | 71 |
| CEU_5_4 | 126,932,932,969 | Model 2,3,4,5 | 71 |
| CEU_10_1 | 3485, 6601 | Model 4,6 | 71 |
| CEU_10_2 | 150,150,162,3545 | Model 6,9,4,2 | 76 |
| CEU_10_3 | 134, 134, 5015 | Model 5,7,7 | 76 |
| CEU_10_4 | 164 | Model 4 | 76 |
The first, second, third, and fourth columns show the phenotype, the optimal number of SNPs, the best model for the YRI_ED dataset, and the accuracy of the YRI_ED dataset. These models are further used on YRI_FD
Table 8.
Results of deep transfer learning for 5 risk SNPs
| 5_1 | 5_2 | 5_3 | 5_4 | |
|---|---|---|---|---|
| Results without transfer learning | 56 | 52 | 44 | 42 |
| With snps transfer learning | 48 | 54 | 44 | 38 |
| Improvement with snps transfer learning | − 8 | 2 | 0 | − 4 |
| With deep transfer learning | 58 | 58 | 64 | 56 |
| Improvement with deep transfer learning | 2 | 6 | 20 | 14 |
| Max achieved | 58 | 58 | 64 | 56 |
| Final improvement | 2 | 6 | 20 | 14 |
The two-tailed P value equals 0.0306, t = 2.8146, df = 6, standard error of difference = 3.731
Table 9.
Results of deep transfer learning for 10 risk SNPs
| 10_1 | 10_2 | 10_3 | 10_4 | |
|---|---|---|---|---|
| Results without transfer learning | 48 | 52 | 52 | 46 |
| With snps transfer learning | 46 | 48 | 48 | 32 |
| Improvement with snps transfer learning | − 2 | − 4 | − 4 | − 14 |
| With deep transfer learning | 62 | 66 | 54 | 52 |
| Improvement with deep transfer learning | 14 | 14 | 2 | 6 |
| Max achieved | 62 | 66 | 54 | 52 |
| Final improvement | 14 | 14 | 2 | 6 |
The two-tailed P value equals 0.0478, t = 2.4803, df = 6, standard error of difference = 3.629
It is worth mentioning here what each layer is learning. In the deep learning model, the top layers correspond to minor features, whereas lower layers are population-specific. The reason that transfer learning may work in the real application is the low genetic diversity in the small populations [52]. So features learned from the large population are more robust than features from a small population. We do not know in advance which layers should be trainable and non-trainable. So, for in-depth analysis, we should try to make each layer non-trainable for each model and then test the model performance. There is also a possibility that the model performs negatively, which is called negative transfer [17].
Computational analysis
Computation time depends on several factors like the number of neurons in each layer , the number of layers in a model N, model type (ANN or LSTM), the number of epochs E, the number of trainable layers for transfer learning t, and the number of neurons in each trainable layer for transfer learning , where .
Consider the time to train the neurons in layer L for one iteration is , where l ranges from .
Among the three types of transfer learning, we used only two, and each transfer learning type has a different computation time. Consider the computation time for the following two cases: Transfer learning with SNPs transfer learning and Transfer learning with deep transfer learning.
Transfer learning with SNPs transfer learning. This process involves extracting the SNPs from the large population using the p-value threshold on the large population’s GWAS summary statistic file O(1), extracting the values for the corresponding SNPs from the small population’s genotype data O(1), and training/testing the machine learning model on the small population genotype data . The total computation time is , and it was approximately 10 minutes for one dataset and one machine learning model.
Transfer learning with deep transfer learning. The time to train the model on a large population’s genotype is . When transferring knowledge from a large population, one must decide the number of trainable and non-trainable layers. If the number of trainable layers is = 0, the final computation time would be . If some layers are trainable t, the actual computation time would be , where is t is the number of trainable layers from bottom to top. It was approximately 20 minutes for L = 5, t = 2, and E = 50.
Conclusion
Machine learning and transfer learning already exist in the literature, but we applied these methodologies for genotype data to show that transfer learning is applicable for genotype data. The whole pipeline requires an in-depth explanation of all the processes involved, like handling directory structure and file formats [53], coordinating genotype data between populations, and applying transfer learning to a small population. As highlighted in the manuscript, we explained these processes and developed sub-modules for processing data. For instance, when generating data for both populations, how to produce symmetric between both datasets.
Any algorithm, TCA, CORAL, 1DCNN, and SVC can also be used for transfer learning, and there is a possibility that these algorithms yield more accuracy when transferring knowledge. So, in the model section, any number of algorithms can be employed without affecting the methodology. We worked on simulated data, and when transferring knowledge to the real dataset for a specific phenotype (cancer or type-2 diabetes), results would be clearer and more interpretable.
Other parameters can also be used, for example, sensitivity, specificity, F1 score, and AUC. The dataset we considered is almost balanced, so accuracy and AUC are almost the same in this case. The other point is that when the best model among multiple models is to be selected for the final testing, we have to decide based on validation accuracy or AUC, so there is a possibility that the model which gives the best AUC is different from the one which gave the best accuracy. It is recommended to select one evaluation metric when evaluating the final performance of transfer learning.
We can use this pipeline to analyze endangered species. Although we tested this approach on a generated dataset, this approach will be more transparent with the real data. However, if the base data is extensive compared to the target data, we can expect transfer learning results to be better.
Acknowledgements
The author(s) wish to acknowledge the contribution of Khalifa University’s high-performance computing and research computing facilities to the results of this research.
Author contributions
MM and AH wrote the main manuscript text and did methodology development. MM prepared figures and generated results. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work is supported by Khalifa University’s Competitive Internal Research Award (CIRA) 2019-076.
Availability of data and materials
Dataset for both populations is available on this link https://mathgen.stats.ox.ac.uk/impute/data_download_1000G_pilot_plus_hapmap3.html.
Declarations
Ethics approval and consent to participate
All the data considered for this study is from a publically available source, so no approval is required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Muhammad Muneeb and Samuel Feng contributed equally to this work
Contributor Information
Muhammad Muneeb, Email: muneebsiddique007@gmail.com.
Samuel Feng, Email: samuel.feng@sorbonne.ae.
Andreas Henschel, Email: andreas.henschel@ku.ac.ae.
References
- 1.Qiu F-Z, Shen X-X, Li G-X, Zhao L, Chen C, Duan S-X, Guo J-Y, Zhao M-C, Yan T-F, Qi J-J, Wang L, Feng Z-S, Ma X-J. Adenovirus associated with acute diarrhea: a case–control study. BMC Infect Dis. 2018 doi: 10.1186/s12879-018-3340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zondervan KT, Cardon LR. Designing candidate gene and genome-wide case–control association studies. Nat Protoc. 2007;2(10):2492–2501. doi: 10.1038/nprot.2007.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saad MN, Mabrouk MS, Eldeib AM, Shaker OG. Genetic case–control study for eight polymorphisms associated with rheumatoid arthritis. PLoS ONE. 2015;10(7):0131960. doi: 10.1371/journal.pone.0131960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas J, Fernandez I, Pastor JC, MacLaren RE, Ramkissoon Y, Harsum S, Charteris DG, Meurs JCV, Amarakoon S, Ruiz-Moreno JM, Rocha-Sousa A, Brion M, Carracedo A. A genetic case–control study confirms the implication of SMAD7 and tnf locusin the development of proliferative vitreoretinopathy. Investig Opthalmol Vis Sci. 2013;54(3):1665. doi: 10.1167/iovs.12-10931. [DOI] [PubMed] [Google Scholar]
- 5.Clarke GM, Anderson CA, Pettersson FH, Cardon LR, Morris AP, Zondervan KT. Basic statistical analysis in genetic case–control studies. Nat Protoc. 2011;6(2):121–133. doi: 10.1038/nprot.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen JN, Sargeant JM, Makielski KM, O’Connor AM. The case–control design in veterinary sciences: a survey. Prevent Vet Med. 2016;134:179–87. doi: 10.1016/j.prevetmed.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Griffin JM, Martin SW, Thorburn MA, Eves JA, Hammond RF. A case–control study on the association of selected risk factors with the occurrence of bovine tuberculosis in the republic of Ireland. Prevent Vet Med. 1996;27(3–4):217–29. doi: 10.1016/0167-5877(95)00571-4. [DOI] [Google Scholar]
- 8.Subramanian S. The effects of sample size on population genomic analyses—implications for the tests of neutrality. BMC Genom. 2016 doi: 10.1186/s12864-016-2441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vecchio FD, Mastroiaco V, Marco AD, Compagnoni C, Capece D, Zazzeroni F, Capalbo C, Alesse E, Tessitore A. Next-generation sequencing: recent applications to the analysis of colorectal cancer. J Transl Med. 2017 doi: 10.1186/s12967-017-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besser J, Carleton HA, Gerner-Smidt P, Lindsey RL, Trees E. Next-generation sequencing technologies and their application to the study and control of bacterial infections. Clin Microbiol Infect. 2018;24(4):335–341. doi: 10.1016/j.cmi.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchini J, Howie B. Genotype imputation for genome-wide association studies. Nat Rev Genet. 2010;11(7):499–511. doi: 10.1038/nrg2796. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genom Hum Genet. 2009;10(1):387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538(7624):161–164. doi: 10.1038/538161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirugo G, Williams SM, Tishkoff SA. The missing diversity in human genetic studies. Cell. 2019;177(1):26–31. doi: 10.1016/j.cell.2019.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehghani N, Bras J, Guerreiro R. How understudied populations have contributed to our understanding of Alzheimer’s disease genetics. Brain. 2021;144(4):1067–1081. doi: 10.1093/brain/awab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onlinelink: uregina.ca/ morrisev/Sociology/Sampling from small populations.htm. http://uregina.ca/~morrisev/Sociology/Sampling%20from%20small%20populations.htm. Accessed 30 Mar 2021.
- 17.Weiss K, Khoshgoftaar TM, Wang D. A survey of transfer learning. J Big Data. 2016 doi: 10.1186/s40537-016-0043-6. [DOI] [Google Scholar]
- 18.Hussain M, Bird JJ, Faria DR. A study on CNN transfer learning for image classification. In: Advances in intelligent systems and computing. Springer; 2018. p. 191–202. 10.1007/978-3-319-97982-3_16.
- 19.Ruder S, Peters ME, Swayamdipta S, Wolf T. Transfer learning in natural language processing. In: Proceedings of the 2019 conference of the north. Association for Computational Linguistics; 2019. 10.18653/v1/n19-5004.
- 20.Mieth B, Hockley JRF, Görnitz N, Vidovic MM-C, Müller K-R, Gutteridge A, Ziemek D. Using transfer learning from prior reference knowledge to improve the clustering of single-cell RNA-seq data. Sci Rep. 2018 doi: 10.1038/s41598-019-56911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López-Sánchez D, Arrieta AG, Corchado JM. Deep neural networks and transfer learning applied to multimedia web mining. In: 14th International conference distributed computing and artificial intelligence. Springer; 2017. p. 124–131. 10.1007/978-3-319-62410-5_15.
- 22.Pio G, Mignone P, Magazzù G, Zampieri G, Ceci M, Angione C. Integrating genome-scale metabolic modelling and transfer learning for human gene regulatory network reconstruction. Bioinformatics. 2021;38(2):487–493. doi: 10.1093/bioinformatics/btab647. [DOI] [PubMed] [Google Scholar]
- 23.Mignone P, Pio G, Džeroski S, Ceci M. Multi-task learning for the simultaneous reconstruction of the human and mouse gene regulatory networks. Sci. Rep. 2020 doi: 10.1038/s41598-020-78033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong TN, Brogden G, Gerold G, Khosla M. A multitask transfer learning framework for the prediction of virus-human protein–protein interactions. BMC Bioinform. 2021 doi: 10.1186/s12859-021-04484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koumakis L. Deep learning models in genomics; are we there yet? Comput Struct Biotechnol J. 2020;18:1466–1473. doi: 10.1016/j.csbj.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taroni JN, Grayson PC, Hu Q, Eddy S, Kretzler M, Merkel PA, Greene CS. MultiPLIER: a transfer learning framework for transcriptomics reveals systemic features of rare disease. Cell Syst. 2019;8(5):380–3944. doi: 10.1016/j.cels.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhruba SR, Rahman R, Matlock K, Ghosh S, Pal R. Application of transfer learning for cancer drug sensitivity prediction. BMC Bioinform. 2018 doi: 10.1186/s12859-018-2465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutter GR, Liu Y. Personalized medicine: the return of the house call? Neurol Clin Pract. 2012;2(4):343–351. doi: 10.1212/cpj.0b013e318278c328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soekhoe D, van der Putten P, Plaat A. On the impact of data set size in transfer learning using deep neural networks. In: Lecture notes in computer science. Springer; 2016. p. 50–60. 10.1007/978-3-319-46349-0_5.
- 30.Dowell RD. The similarity of gene expression between human and mouse tissues. Genome Biol. 2011;12(1):101. doi: 10.1186/gb-2011-12-1-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onlinelink: Bornean Elephant | Species | WWF. https://www.worldwildlife.org/species/bornean-elephant. Accessed 24 Mar 2021.
- 32.Muneeb M, Feng SF, Henschel A. Heritability, genetic variation, and the number of risk SNPs effect on deep learning and polygenic risk scores AUC. In: 2022 14th International conference on bioinformatics and biomedical technology. ACM; 2022. 10.1145/3543377.3543387.
- 33.Onlinelink: 1000 Genome and HapMap3 data. https://mathgen.stats.ox.ac.uk/impute/data_download_1000G_pilot_plus_hapmap3.html. Accessed 12 Oct 2021.
- 34.Su Z, Marchini J, Donnelly P. HAPGEN2: simulation of multiple disease SNPs. Bioinformatics. 2011;27(16):2304–2305. doi: 10.1093/bioinformatics/btr341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muneeb M, Henschel A. Eye-color and type-2 diabetes phenotype prediction from genotype data using deep learning methods. 2020. 10.21203/rs.3.rs-125397/v1. [DOI] [PMC free article] [PubMed]
- 36.Zeng P. Statistical analysis for genome-wide association study. J Biomed Res. 2015 doi: 10.7555/jbr.29.20140007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JPA, Hirschhorn JN. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9(5):356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- 38.Clayton DG, Walker NM, Smyth DJ, Pask R, Cooper JD, Maier LM, Smink LJ, Lam AC, Ovington NR, Stevens HE, Nutland S, Howson JMM, Faham M, Moorhead M, Jones HB, Falkowski M, Hardenbol P, Willis TD, Todd JA. Population structure, differential bias and genomic control in a large-scale, case–control association study. Nat Genet. 2005;37(11):1243–1246. doi: 10.1038/ng1653. [DOI] [PubMed] [Google Scholar]
- 39.Evgeniou T, Pontil M. Support vector machines: theory and applications. In: Machine learning and its applications. Springer; 2001. p. 249–257. 10.1007/3-540-44673-7_12.
- 40.Rokach L, Maimon O. Decision trees. In: Data mining and knowledge discovery handbook. Springer. p. 165–192. 10.1007/0-387-25465-x_9.
- 41.Cutler A, Cutler DR, Stevens JR. Random forests. In: Zhang C, Ma Y, editors. Ensemble machine learning. Boston: Springer; 2012. pp. 157–175. [Google Scholar]
- 42.Jabbar HK, Khan RZ. Methods to avoid over-fitting and under-fitting in supervised machine learning (comparative study). In: Computer science, communication and instrumentation devices. Research Publishing Services; 2014, p. 163–172. 10.3850/978-981-09-5247-1_017.
- 43.Grossi E, Buscema M. Introduction to artificial neural networks. Eur J Gastroenterol Hepatol. 2007;19(12):1046–1054. doi: 10.1097/meg.0b013e3282f198a0. [DOI] [PubMed] [Google Scholar]
- 44.Ma W, Qiu Z, Song J, Cheng Q, Ma C. DeepGS: predicting phenotypes from genotypes using deep learning. BioRxiv. 2017 doi: 10.1101/241414. [DOI] [Google Scholar]
- 45.Liu Y, Zhang J. Deep learning in machine translation. In: Deep learning in natural language processing. Springer; 2018, p. 147–183. 10.1007/978-981-10-5209-5_6.
- 46.Amberkar A, Awasarmol P, Deshmukh G, Dave P. Speech recognition using recurrent neural networks. In: 2018 International conference on current trends towards converging technologies (ICCTCT). IEEE; 2018. 10.1109/icctct.2018.8551185.
- 47.Muneeb M, Feng SF, Henschel A. Deep learning pipeline for image classification on mobile phones. 2022. 10.48550/ARXIV.2206.00105.
- 48.Muneeb M, Feng S, Henschel A. An empirical comparison between polygenic risk scores and machine learning for case/control classification. 2022. 10.21203/rs.3.rs-1298372/v1.
- 49.Muneeb M, Henschel A. Eye-color and type-2 diabetes phenotype prediction from genotype data using deep learning methods. BMC Bioinform. 2021 doi: 10.1186/s12859-021-04077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muneeb M, Feng SF, Henschel A. Can we convert genotype sequences into images for cases/controls classification? Front Bioinform. 2022 doi: 10.3389/fbinf.2022.914435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Najafabadi MM, Villanustre F, Khoshgoftaar TM, Seliya N, Wald R, Muharemagic E. Deep learning applications and challenges in big data analytics. J Big Data. 2015 doi: 10.1186/s40537-014-0007-7. [DOI] [Google Scholar]
- 52.Furlan E, Stoklosa J, Griffiths J, Gust N, Ellis R, Huggins RM, Weeks AR. Small population size and extremely low levels of genetic diversity in island populations of the platypus, ornithorhynchus anatinus. Ecol Evol. 2012;2(4):844–857. doi: 10.1002/ece3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muneeb M, Feng SF, Henschel A. Tutorial on 8 genotype files conversion. In: 2022 10th International conference on bioinformatics and computational biology (ICBCB). IEEE; 2022. 10.1109/icbcb55259.2022.9802470.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Dataset for both populations is available on this link https://mathgen.stats.ox.ac.uk/impute/data_download_1000G_pilot_plus_hapmap3.html.