Abstract
The firing activity of hypothalamic magnocellular neurosecretory neurons (MNNs) located in the PVN and SON is coordinated by the combined, fine-tuned action of intrinsic membrane properties, synaptic and extrasynaptic signaling. Among these, purinergic and glutamatergic signaling pathways have been shown to play a key role regulating the activity of MNNs. However, the precise cellular mechanisms by which ATP and glutamate act in concert to regulate osmotically-driven MNNs neuronal excitability remains unknown. Whole-cell patch clamp recordings obtained from MNNs showed that ATP (100 μM) induced an increase in firing rate, an effect that was blocked by either PPADS (10 μM) or kynurenic acid (1 mM). While ATP did not affect the frequency or magnitude of glutamatergic excitatory postsynaptic currents (EPSCs), it induced an inward shift in the holding current that was prevented by PPADS or kynurenic acid treatment, suggesting that ATP enhances a tonic extrasynaptic glutamatergic excitatory current. We observed that ATP potentiated glutamatergic receptors mediated-currents evoked by focal application of L-glu (1 mM) and NMDA (50 μM), but not AMPA (50 μM). ATP potentiation of NMDA-evoked currents was blocked by PPADS (10 μM) and by chelation of intracellular Ca2+ with BAPTA (10 mM). Finally, we report that a hyperosmotic stimulus (mannitol 1%, +55 mOsm/kgH2O) potentiated NMDA-evoked currents and increased MNNs firing activity, effects that were blocked by PPADS. Taken together, our data support a functional excitatory coupling between P2 and extrasynaptic NMDA receptors in MNNs, which is engaged in response to an acute hyperosmotic stimulus.
Keywords: ATP, NMDA, PVN, SON, magnocellular neurosecretory neurons, hyperosmolality, P2 receptors
INTRODUCTION
The neurohypophysial hormones vasopressin (VP) and oxytocin (OT) are synthesized in magnocellular neurosecretory neurons (MNNs) located within the hypothalamic paraventricular (PVN) and supraoptic nuclei (SON) (Bourque, 2008). While OT plays an important role in parturition and milk ejection, VP induces antidiuretic and pressor effects in response to osmotic and cardiovascular changes (Poulain et al., 1977; Antunes-Rodrigues et al., 2004).
VP and OT are stored in vesicles and transported to axonal nerve terminals within the neurohypophysis, from where they are released into the bloodstream in response to physiological challenges, such as an increase in plasma osmolarity (Bourque, 2008). Axonal release of VP and OT is regulated in an activity-dependent manner (Dutton & Dyball, 1979; Cazalis et al., 1985) which, in turn, is determined by the combined action of intrinsic membrane properties as well as synaptic and extrasynaptic signaling mechanisms (Bourque et al., 1993; Bourque, 2008; Brown et al., 2013). Several neurotransmitters within the PVN and SON have been shown to participate in the coordinated response during an osmotic challenge, such as glutamate (Inenaga et al., 1997; Kawasaki et al., 2005), angiotensin II (Chen & Toney, 2001; Sandgren et al., 2018), VP (Gillard et al., 2007), nitric oxide (Grassi et al., 2013) and adenosine triphosphate (ATP) (Ferreira-Neto et al., 2015; Ferreira-Neto et al., 2017).
ATP is well known as an essential molecule for cell energy and signaling. In addition, ATP has been shown in the last decades to be an important neurotransmitter involved in several physiological mechanisms as well as pathological conditions (Burnstock, 2007). The actions of ATP as an excitatory neurotransmitter are mediated by purinergic ionotropic P2X and metabotropic P2Y receptors expressed in different central nervous system sites and peripheral nerve terminals (Burnstock, 2007). In addition, several pieces of evidence demonstrated that ATP can be co-released with different neurotransmitters including GABA, acetylcholine, noradrenaline, and glutamate (Burnstock, 2007).
We have previously shown that within the PVN, ATP evokes a sympathoexcitatory response that was mediated by an increase excitability of presympathetic RVLM-projecting PVN neurons (Ferreira-Neto et al., 2013; Ferreira-Neto et al., 2015). Moreover, we showed that this action, as well as an osmotically-driven sympathoexcitation in the PVN involved a signaling coupling between P2 and non-NMDA (AMPA) receptors (Ferreira-Neto et al., 2013; Ferreira-Neto et al., 2015; Ferreira-Neto et al., 2017). Given that both purinergic and glutamatergic neurotransmission also regulate the activity of MNNs (Day et al., 1993; van den Pol & Trombley, 1993; Vavra et al., 2011), and that these neurosecretory neurons are also engaged in the homeostatic response to an osmotic challenge, we aimed to test the hypothesis in this study that a similar purinergic/glutamatergic coupling mechanism contributes to the osmotically-driven excitation of MNNs.
Here, we report that exogenous application of ATP increases the firing activity of MNNs, an effect that was significantly attenuated by blockade of both glutamate ionotropic and ATP P2 receptors. Moreover, we found that ATP potentiated the current evoked by exogenous application of NMDA, an effect that was significantly attenuated by PPADS or the Ca2+ chelator BAPTA. Finally, we report that a local hyperosmotic stimulus potentiated glutamatergic NMDA receptor-mediated currents and evoked firing of MNNs, effects that were significantly reduced by blockade of ATP P2 receptors. Together, these results support a functional coupling between ATP P2 and glutamate NMDA receptors that contributes to osmotically-driven activity of hypothalamic magnocellular neurons.
MATERIALS AND METHODS
Ethical approval
Male Wistar rats (180–220 g, 5–6 weeks old, n=35), purchased from Harlan Laboratories (Indianapolis, IN, USA), were used in this study. Rats were housed in rooms with constant temperature of 22–24 °C, relative humidity of 50–60% and under a controlled light/dark cycle (12/12 hours) with normal rat chow and drinking water ad libitum. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee (IACUC; approval number A18003) and carried out in agreement with IACUC guidelines. This study conforms to the Journal of Physiology policy on ethical use of animals.
Hypothalamic slice preparation
Hypothalamic brain slices containing either the SON or PVN were prepared according to methods previously described (Stern, 2001). Briefly, rats were deeply anesthetized with pentobarbital (80 mg/kg, i.p.). Then, rats were quickly decapitated by using a guillotine apparatus, brains dissected out and coronal slices cut (240 μm thick) using a vibroslicer (Leica VT1200 S, Leica Biosystems, Buffalo Grove, IL, USA). An oxygenated ice-cold artificial cerebrospinal fluid (aCSF) was used during slicing (containing in mM: 119 NaCl, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 2 CaCl2, and 2 pyruvic acid; pH 7.4; 295 mOsm/kgH2O). Slices were placed in a holding chamber containing aCSF and kept at room temperature until used.
Patch-clamp electrophysiology
Slices were bathed with aCSF (~2.0 mL/min) that were continuously bubbled with 95% O2–5% CO2 and maintained at ~32 °C. In order to facilitate measurements of NMDA currents (INMDA) in the focal application experiments with L-glu and NMDA, slices were bathed with a low Mg2+ aCSF (10 μM Mg2SO4) and in the presence of glycine (10 μM), as we previously reported (Fleming et al., 2011).Thin-walled (1.5 mm od, 1.17 mm id) borosilicate glass (G150TF-3, Warner Instruments, Sarasota, FL, USA) was used to pull patch pipettes (3–7 MΩ) on a horizontal Flaming/Brown micropipette puller (P-97, Sutter Instruments, Novato, CA). The internal solution contained in mM: 140 potassium gluconate, 5 EGTA, 10 HEPES, 10 KCl, 0.9 MgCl2, 0.5 CaCl2, 4 MgATP, 0.3 NaGTP, and 20 phosphocreatine (Na+); pH 7.2–7.3; 285 mOsm/kgH2O. Recordings were obtained with an Axopatch 700A amplifier (Axon Instruments, Foster City, CA, USA) from SON (n=93) and PVN (n=67) MNNs using infrared differential interference contrast (IR-DIC) video microscopy. PVN MNNs were identified by their anatomical location and their unique electrophysiological properties during depolarizing current injection, characterized by a robust outward rectification and the absence of the low threshold spike, which are hallmarks of MNNs (Luther et al., 2000; Luther & Tasker, 2000; Stern, 2001). Data collected from MNNs recorded from the SON and PVN were pooled for analysis. The voltage output was digitized at 16-bit resolution, 10 kHz and was filtered at 2 kHz (Digidata 1440A, Axon Instruments). Data were discarded if the series resistance was not stable throughout the entire recording (>20% change) (Stern, 2001). For current-clamp recordings, direct current injection was applied to bring the membrane potential near or at spike threshold (−50 to −45 mV). The mean firing rate was analyzed in segments of 1 min before, during and after ATP application, and the peak effect calculated and express as percent change from baseline levels (Li et al., 2003a).
Spontaneous glutamate-mediated excitatory postsynaptic currents (sEPSCs) were recorded and analyzed as previously described (Potapenko et al., 2011). Briefly, sEPSCs were recorded as inward currents in aCSF containing the GABAA receptor blocker picrotoxin (PIC, 100 μM), while holding the membrane at −70 mV. sEPSCs were analyzed using Mini Analysis software (Synaptosoft Inc, Leonia, NJ, USA), with a detection threshold of 12 pA. PSCs frequency and waveform parameters were analyzed using the same software. sEPSCs were analyzed in periods of one minute before, during and after ATP application.
Pharmacological activation of L-glu, NMDA and AMPA receptor-mediated current in MNNs was assessed by measuring the peak and the integrated area of the evoked change in holding current (Iholding) following a focal puff of L-glu (1 mM, 100 ms), NMDA (50 μM, 100 ms) or AMPA (50 μM, 50 ms) onto the recorded cell using a picospritzer device (Toohey, 5–10 psi) connected to a patch pipette positioned around ~10 μm from the recorded cell. Cell input resistance and cell capacitance were calculated in voltage clamp using a 5mV pulse while holding the cells at −70 mV. All data were analyzed using MiniAnalysis software version 6.03 (Fort Lee, NJ, USA). Adenosine 5’-triphosphate disodium salt (ATP), 4-[[4-Formyl-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-2-pyridinyl]azo]-1,3-benzenedisulfonic acid tetrasodium salt (PPADS) were purchased from Abcam (Cambridge, UK). Kynurenic acid sodium salt (KYN) was purchased from Ascent Scientific (Abcam, Cambridge, UK). NMDA and (RS)-AMPA (AMPA) was purchased from Tocris Bioscience (Bristol, UK). BAPTA Tetrapotassium Salt, cell impermeant was purchased from Invitrogen (Carlsbad, CA, USA). L-glutamate, Picrotoxin and Mannitol were purchased from Sigma Aldrich (St. Louis, MO, USA).
Statistical Analysis
All values are expressed as mean ± standard deviation (SD). One- or two-way analysis of variance (ANOVA) tests with Bonferroni post hoc tests were used as indicated. Differences were considered significant at p<0.05 and n refers to the number of cells. All statistical analyses were conducted using GraphPad Prism version 7.00 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Ionotropic glutamate receptors contribute to the ATP-evoked action potentials firing in MNNs
To evaluate if ATP affects the firing activity of MNNs, we obtained whole-cell current clamp recording from MNNs and bath applied ATP (100 μM, 1 min). In order to compare the time course effect of the ATP effect, we plotted the percent change of action potential (AP) frequency over time during ATP bath application. Results from a 2-way RM-ANOVA indicated that the change in MNNs firing varied significantly as a function of drug treatment (F=21.01, p<0.0001) and time (F=6.268, p<0.0001). As observed in figure 1A and D, ATP increased the firing rate of MNNs almost immediately, progressively enhancing firing activity and reaching a plateau at ~5 min after. (Fig. 1A and D, p<0.0001 vs baseline 2, Bonferroni post hoc test, n=6). In all cases, ATP effects were incompletely washed out 10 min after its application.
Figure 1. ATP increases firing rate of MNNs.

A, B and C: representative examples of a current-clamp whole-cell recording of the firing activity of MNN during ATP bath application (A, 100 ⎧M, 1 min) in control condition and in slices pretreated with PPADS (B, 10 ⎧M) or kynurenic acid (C, KYN, 1mM). D: Summary graph showing the mean percentage change in action potential (AP) frequency over time during ATP application in control condition (black bars with black circles, n=3 SON and 3 PVN; two rats, one cell per slice) and in slices pretreated with PPADS (red bars with red circles, n=5 SON and 4 PVN; two rats, one cell per slice) or kynurenic acid (green bars with green circles, n=3 SON and 2 PVN; one rat, one cell per slice). The numbers on the x axis represent minutes (min) of the recordings. Statistics in red: ATP vs ATP+PPADS at the same period analyzed; 2-way RM ANOVA, Bonferroni post hoc test. Statistics in green: ATP vs ATP+KYN at the same period analyzed; 2-way RM ANOVA, Bonferroni post hoc test.
To evaluate the mechanism by which ATP increased the firing activity of MNNs, a different set of slices were pretreated with the P2 receptor antagonist, PPADS (10 μM) for at least 10 min prior to ATP bath application. The ATP-induced excitation in MNNs was completely blocked by PPADS treatment (Fig. 1B and D, p<0.0001 vs ATP alone, Bonferroni post hoc test n=9), supporting a P2 receptor-mediated effect to the ATP-induced increase in firing frequency in MNNs.
We previously showed that ATP increased the firing activity of RVLM-projecting PVN neurons via an ionotropic glutamatergic receptors-dependent mechanism (Ferreira-Neto et al., 2015). To verify whether a similar mechanism operates in MNNs, a different set of slices were pretreated with the broad-spectrum ionotropic receptor blocker kynurenic acid (KYN, 1 mM) at least 10 min prior to ATP application. The blockade of ionotropic glutamatergic receptors blunted the ATP-induced increase in firing frequency of MNNs (Fig. 1C and D, p=0.0001 vs ATP alone, Bonferroni post hoc test, n=5). Together, these data indicate that ATP increases the firing activity of MNNs via a mechanism dependent on the activation of both purinergic and ionotropic glutamatergic receptors.
ATP does not alter the degree of glutamate synaptic activity
To determine whether the glutamatergic-dependent mechanism mediating ATP effects in MNNs involved changes in the degree of glutamatergic synaptic activity, we recorded spontaneous synaptic activity in MNNs voltage-clamped at −70 mV. Given that the increase in AP frequency evoked by ATP application in MNNS was blocked by previous treatment with KYN, we isolated glutamatergic receptors-mediated excitatory postsynaptic currents (EPSCs) from GABAergic inhibitory PSCs by treating slices with the GABAA receptor antagonist picrotoxin (100 μM) 6 min before bath applying a cocktail containing picrotoxin and ATP (both 100 μM, 1 min). Picrotoxin significantly decreased the amplitude, frequency, decay time and area of spontaneous PSCs in MNNs (Fig. 2A–E, amplitude: F=24.05; frequency: F=34.26; decay time: F=46.34, area: F=39.45, p<0.0001, 1-way RM-ANOVA, Bonferroni post hoc test, n=9), indicating an effective blockade of GABAergic currents. The properties of isolated glutamatergic EPSCs (amplitude, frequency, decay time and area) were not significantly affected by ATP application or during the washout periods evaluated (Fig. 2A–E, p>0.9999 for all parameters, 1-way RM-ANOVA, Bonferroni post hoc test, n=9).
Figure 2. ATP does not affect EPSCs properties.

A: representative example of a voltage-clamp whole-cell recording (holding potential: −70 mV) MNNs synaptic currents in baseline condition, during GABAergic synaptic currents blockade with picrotoxin (100 ⎧M), during ATP bath application (100 ⎧M, 1 min) and after ATP washout. B, C, D and E: Summary bar graphs (n=5 SON and 4 PVN; two rats, one cell per slice) showing the mean EPSCs amplitude (B), frequency (C), decay time (D) and area (F) in baseline condition (black bars), during picrotoxin application (100 ⎧M, gray bars), ATP bath application (1 min, red bar) and after ATP washout (blue bars). The numbers on the x axis represent minutes (min) of the recordings. Statistics represent differences vs baseline 5 and baseline 10, respectively; 2-way RM ANOVA, Bonferroni post hoc test.
ATP results in the activation of extrasynaptic NMDA receptors
We previously showed that in MNNs glutamate also evokes a sustained, tonic NMDA receptor-mediated inward current via activation of extrasynaptic NMDA receptors (eNMDARs) (Fleming et al., 2011; Potapenko et al., 2012, 2013; Naskar & Stern, 2014). In order to determine whether ATP would affect eNMDARs, we evaluated the effect of ATP (100 μM) on the holding current (Iholding) of MNNs voltaged-clamped at −70 mV in control conditions and in slices pretreated with PPADS (10 μM). Results from a 2-way RM-ANOVA indicated that the magnitude of Iholding varies significantly as a function of drug treatment (F=9.327, p=0.0007) and time (F=11.16, p<0.0001). As observed in figure 3A, while ATP induced a significant inward shift in Iholding (Fig. 3A, n=13), this effect was significantly blunted in the presence of PPADS (Fig. 3B and D, p=0.0016 vs ATP alone, Bonferroni post hoc test, n=10) and kynurenic acid (Fig. 3C and D, p=0.0058 vs ATP alone, Bonferroni post hoc test, n=10). These results indicate that exogenous ATP application induced a significant inward shift in MNNs which was dependent on P2 and ionotropic glutamate receptors. Taken together, these results support the notion that the ATP-glutamate crosstalk mediating the increased excitability in MNNs involves P2 and extrasynaptic (but not synaptic) ionotropic glutamatergic receptors.
Figure 3. ATP induces an inward holding current in P2-dependent manner.
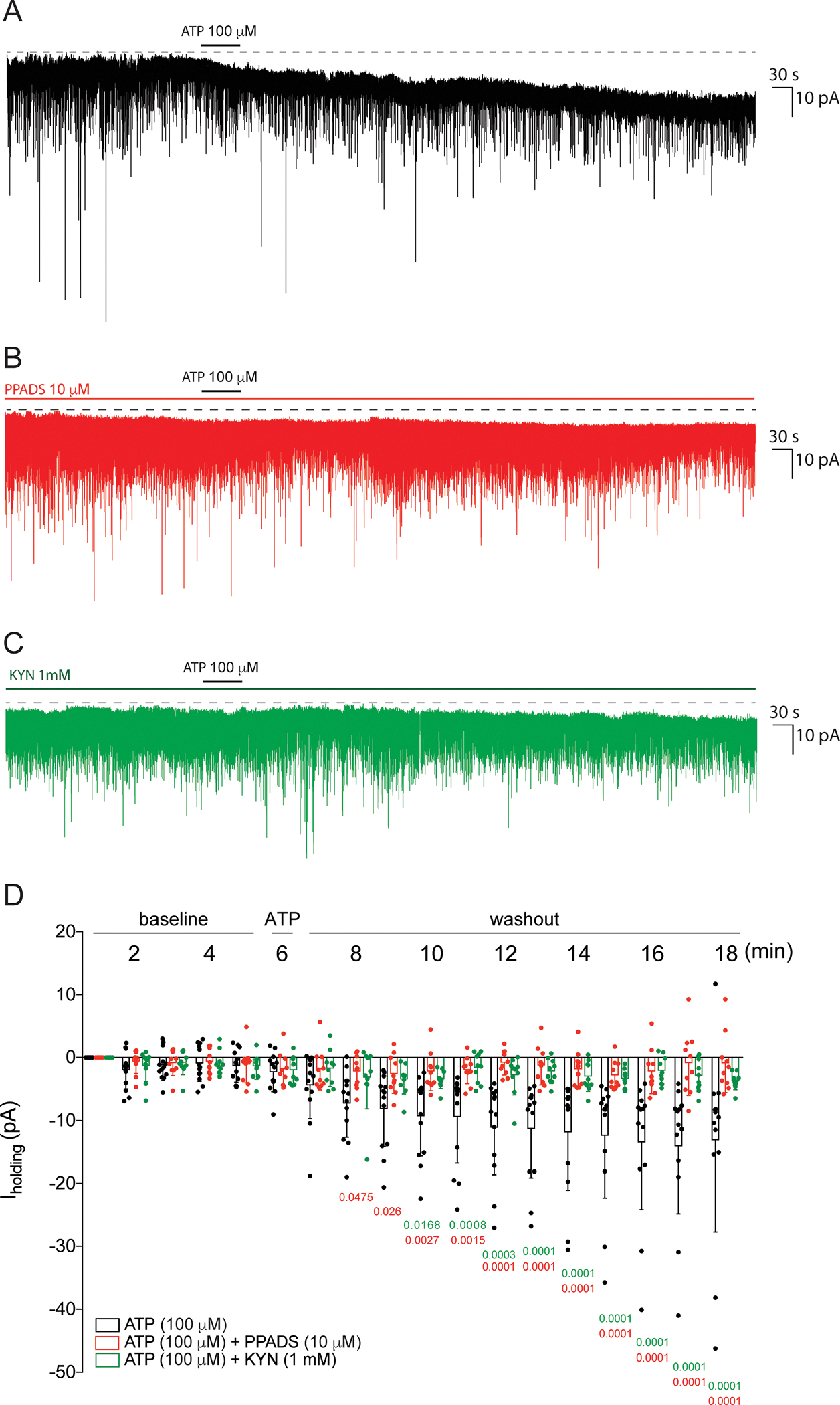
A and B: representative example of a voltage-clamp whole-cell recording (holding potential: −70 mV) of an PSCs before, during ATP bath application (100 ⎧M, 1 min) and after ATP washout in control conditions (A) and in slices pretreated with PPADS (B, 10 ⎧M) or KYN (C, 1mM) throughout the recording. D: Summary graph showing the mean Iholding before, during ATP application and the washout periods evaluated in the control condition (black bars with black circles, n=7 SON and 6 PVN; three rats, one cell per slice) and in slices pretreated with PPADS (red bars with red circles, n=6 SON and 4 PVN; two rats, one cell per slice) or kynurenic acid (green bars with green circles, n=6 SON and 4 PVN; two rats, one cell per slice). The numbers on the x axis represent minutes (min) of the recordings. Statistics in red: ATP vs ATP+PPADS at the same period analyzed; 2-way RM ANOVA, Bonferroni post hoc test. Statistics in green: ATP vs ATP+KYN at the same period analyzed; 2-way RM ANOVA, Bonferroni post hoc test.
ATP potentiates NMDA (but not AMPA) receptor-evoked currents in MNNs
To get further insights into the precise glutamatergic mechanisms mediating ATP effects in MNNs, we assessed the effects of ATP on L-glutamate (L-glu)-evoked currents (IL-glu) by focally applying L-glu onto those neurons, an approach that activates both synaptic and extrasynaptic receptors. To this end, 1 mM of L-glu was focally applied to the recorded cells (5–10 psi, 100 ms) before, during and after ATP bath application (100 μM, 1 min). As shown in Figure 4A, at a holding potential of −70 mV, L-glu evoked a large inward current (IL-glu amplitude: 2.09±1.05 nA, area: 1363.46±824.55 nA*ms, n=16). As shown in Fig. 4A–C, we found that ATP application increased IL-glu amplitude and area, an effect that became statistically significant 10 min after ATP washout (Fig. 4B–C, amplitude: F=7.777; area: F=9.049, p<0.0001, 1-way RM-ANOVA, Bonferroni post hoc test, n=16), supporting a slow-developing ATP effect.
Figure 4. ATP potentiated IL-glu and INMDA but not IAMPA in MNNs.

A, D, G and K: representative examples of MNNs in voltage-clamp whole-cell recording (holding potential: −70 mV) of inward currents evoked by focal application of L-glu (A, 1 mM, 100 ms, 5 psi), AMPA (D, 50 ⎧M, 50 ms, 5 psi), NMDA (G, 50 ⎧M, 50 ms, 5 psi) before, during ATP bath application (100 ⎧M, 1 min) and 5, 10 and 15 min after ATP washout and NMDA (K, 50 ⎧M, 50 ms, 5 psi) in control conditions. Summary bar graph showing the mean IL-glu (n=9 SON and 7 PVN; three rats, one cell per slice) amplitude (B) and area (C); mean IAMPA (n=11 SON and 8 PVN; four rats, one cell per slice) amplitude (E) and area (F), mean INMDA (n=8 SON and 6 PVN; three rats, one cell per slice) amplitude (H) and area (I) and before, during ATP application and the washout periods evaluated and mean INMDA (n=4 SON and 1 PVN; one rat, one cell per slice) amplitude (L) and area (M) in control conditions. Statistics in gray: Control vs Washout at different periods analyzed; 1-way RM ANOVA, Bonferroni post hoc test. Statistics in black: ATP vs Washout at different periods analyzed; 1-way RM ANOVA, Bonferroni post hoc test.
It is well established that L-glu activates both AMPA and NMDA receptors. Thus, we aimed to determine which type of glutamate receptor was affected by ATP. As observed in Fig. 4D, focal application of AMPA (50 μM, 50 ms) in control conditions induced an inward current (IAMPA amplitude: 0.73±0.39 nA, area: 1203.25±771.03 nA*ms, n=19) in MNNs, which was not significantly affected during ATP application or washout periods evaluated (Fig. 4D–F, amplitude: F=2.222, p=0.075; area: F=0.3681, p=0.8306, 1-way RM-ANOVA, Bonferroni post hoc test, n=19).
Next, we evaluated whether ATP potentiated NMDAR-evoked currents. As observed in figure 4G, NMDA puff (50 μM, 50 ms) induced an inward current (INMDA, amplitude: 0.26±0.13 nA, area: 200.39±93.43 nA*ms, n=14). Similar to IL-glu, ATP induced a slow-developing increase in INMDA that was statistically significant 5 min after ATP application, effects than continued to increase 10- and 15-min following ATP washout (Fig 4G–I, amplitude: F=23.06; area: F=16.34, p<0.0001, 1-way RM-ANOVA, Bonferroni post hoc test, n=14). Finally, to rule out that repetitive activation of NMDARs would induce a self-potentiating effect, we performed the same protocol without bath applying ATP. As shown in figure 4J, INMDA magnitude was not affected following repetitive applications of NMDA per se (Fig. 4J–L, amplitude: F=2.164, p=0.1198; area: F=1.341, p=0.2977, 1-way RM-ANOVA, Bonferroni post hoc test, n=5). Taken together, these data indicate that exogenous ATP potentiates NMDA receptors-evoked currents in MNNs.
ATP-mediated potentiation of NMDA receptors in MNNs is blocked by PPADS and intracellular Ca2+ chelation
To determine whether the ATP potentiation of INMDA in MNNs was P2 receptor-dependent, we repeated experiments in slices pretreated with PPADS 10 min prior to ATP application. Results from a 2-way RM-ANOVA indicated that INMDA amplitude and area varied significantly as a function of drug treatment (amplitude: F=7.033, p=0.0049, area, F=5.975, p=0.0092). and time (amplitude: F=8.635, p<0.0001, area, F=5.567, p=0.0005). As shown in Fig 5D and E, ATP bath application potentiated INMDA (amplitude: p<0.0001; area: p<0.0001, 2-way RM-ANOVA, Bonferroni post hoc test, n=14). The ATP-mediated potentiation of INMDA was significantly blunted by PPADS (Fig. 5B, D and E, amplitude: p=0.0453; area: p=0.0757 vs NMDA+ATP, 2-way RM-ANOVA, Bonferroni post hoc test, n=4).
Figure 5. ATP-induced potentiation of INMDA in MNNs are P2 receptor- and Ca2+-dependent.

A, B and C: representative examples of MNNs in voltage-clamp whole-cell recording (holding potential: −70 mV) of inward currents evoked by focal application of NMDA (50 ⎧M, 50 ms, 5 psi) before, during ATP bath application (100 ⎧M, 1 min) and 5, 10 and 15 min after ATP washout in control conditions (A), in slices pretreated with PPADS (B, 10 ⎧M) throughout the recording and in MNNs dialyzed with BAPTA (C, 10 mM). D and E: Summary bar graph showing INMDA mean percentage change in amplitude (D) and area (E) before, during ATP application and the washout periods evaluated in control conditions (black bars with black filled circles, n=8 SON and 6 PVN; three rats, one cell per slice), in the presence of PPADS (red bars with red filled squares, n=2 SON and 2 PVN; one rat, one cell per slice) and BAPTA (blue bars with blue filled triangles, n=3 SON and 2 PVN; one rat, one cell per slice). Statistics in red: NMDA+ATP vs NMDA+ATP+PPADS at the same period analyzed; 2-way RM ANOVA, Bonferroni post hoc test. Statistics in blue: NMDA+ATP vs NMDA+ATP+BAPTA at the same period analyzed; 2-way RM ANOVA, Bonferroni post hoc test.
It is well known that activation of P2 receptors by ATP increases intracellular Ca2+ (Khakh et al., 2003; Song et al., 2006, 2007; Bhattacharya et al., 2013). Thus, to determine if the ATP-induced INMDA potentiation in MNNs was dependent on changes in intracellular Ca2+, MNNs were dialyzed with the Ca2+ chelator BAPTA (10 mM, 10 min) via the recording pipette. As observed in figure 5C, BAPTA prevented the ATP-mediated increase in INMDA (Fig. 5C–E, amplitude: p=0.0132; area: p=0.0221 vs NMDA+ATP, 2-way RM-ANOVA, Bonferroni post hoc test, n=5). The basal INMDA amplitude and area in MNNs from slices pretreated with PPADS or dialyzed with BAPTA were not significantly affected. Taken together, these data suggest that ATP induces the potentiation of INMDA via intracellular Ca2+ increase through P2 receptor activation.
A hyperosmotic stimulation potentiates INMDA and increases the firing of MNNs in a P2 receptor-dependent manner
It has been previously shown that an osmotic challenge can evoke the endogenous release of ATP (Guzman-Aranguez et al., 2017; Hollborn et al., 2017; Hu et al., 2017; Okada et al., 2018; Trull et al., 2019). Thus, in order to identify a possible contribution of the P2-NMDA receptor coupling to the regulation of MNNs firing activity in the context of a physiological challenge, we subjected MNNs to a local and transient hyperosmotic milieu.
Firstly, we performed voltage-clamp recordings to investigate whether a transient hyperosmotic stimulus resulted in the potentiation of INMDA and if so, whether this occurred in an ATP-dependent manner. To this end, we performed whole-cell voltage clamp (−70 mV) recordings in MNNs and focally applied NMDA (50 μM, 50 ms) while neurons were challenged with a hyperosmotic stimulus (mannitol 1%, +55 mOsm/kgH2O, 1 min). As shown in the representative traces in figure 6A and B, a transient hyperosmotic stimulation (mannitol 1%, +55 mOsm/kgH2O, 1 min) increased the magnitude of INMDA, an effect that was attenuated by the pre-treatment of slices with PPADS (10 μM). Results from a 2-way RM-ANOVA indicated that INMDA amplitude and area varied significantly as a function of drug treatment (amplitude: F=16.37, p=0.0014; area: F=7.094, p=0.0195) and time (amplitude: F=13.78, p<0.0001; area: F=9.25, p<0.0001; n=7 for mannitol and n=8 for mannitol+PPADS). As shown in figure 6C and D the increase in INMDA amplitude and area evoked by mannitol was significantly blunted in the presence of PPADS.
Figure 6. Hyperosmotic stimulus potentiates NMDA-evoked currents by activation of P2 receptors.

A and B: representative examples MNNs in voltage-clamp whole-cell recording (holding potential: −70 mV) of inward currents evoked by focal application of NMDA (50 ⎧M, 50 ms, 5 psi) before, during mannitol bath application (1%, +55 mOsm/kgH2O, 1 min) and 5, 10 and 15 min after mannitol washout in control conditions (A) and in slices pretreated with PPADS (B, 10 ⎧M) throughout the recording. C and D: Summary bar graph showing INMDA mean percentage change in amplitude (C) and area (D) before, during mannitol application and the washout periods evaluated in the control conditions (black bars with black circles, n=4 SON and 3 PVN; two rats, one cell per slice) and in the presence of PPADS (red bars with red circles, n=5 SON and 3 PVN; two rats, one cell per slice). Statistics in red: NMDA+Mannitol vs Mannitol+PPADS at the same period analyzed; 2-way RM ANOVA, Bonferroni post hoc test.
These results suggest that a hyperosmotic stimulation engages the P2-NMDAR coupling in MNNs. To determine whether this coupling translated into an effect on MNN firing response to the osmotic stimulation, we performed additional experiments in current-clamp mode. As shown in the representative trace in figure 7A and B, a transient hyperosmotic stimulation (mannitol 1%, +55 mOsm/kgH2O, 1 min) increased the firing activity of MNNs, an effect that was attenuated by the pre-treatment of slices with PPADS (10 μM). Results from a 2-way RM-ANOVA indicated that SON firing activity varied significantly as a function of drug treatment and time (F=15.1, p=0.0011 and F=11.78, p<0.0001, respectively, n=9 for mannitol and n=11 for mannitol+PPADS). As shown in figure 7C, the increase in firing activity evoked by mannitol was significantly blunted in the presence of PPADS. Taken together, these data suggest that the P2-NMDA receptors coupling activation plays an important role in MNN firing response to a hyperosmotic challenge.
Figure 7. Acute hyperosmotic challenge increases the firing activity in MNNs partially via P2 receptors-dependent mechanism.

A and B: representative examples of a current-clamp whole-cell recording of the firing activity of MNN during mannitol bath application (1%, +55 mOsm/kgH2O, 1 min) in control condition (A) and in slices pretreated with PPADS (B, 10 ⎧M). C: Summary graph showing the mean percentage change in action potential (AP) frequency during mannitol application in the control condition (black bars with black circles, n=5 SON and 4 PVN; two rats, one cell per slice) and in slices pretreated with PPADS (red bars with red circles, n=7 SON and 4 PVN; two rats, one cell per slice). The numbers on the x axis represent minutes (min) of the recordings. Statistics in red: Mannitol vs Mannitol+PPADS at the same period analyzed; 2-way RM ANOVA, Bonferroni post hoc test.
DISCUSSION
Our study shows, as previously demonstrated, that ATP acts as an excitatory neurotransmitter in MNNs via activation of P2 receptors (Day et al., 1993; Hiruma & Bourque, 1995). Moreover, we demonstrate that the ATP-induced firing activity in these neurons involves intracellular Ca2+ changes (Song et al., 2006, 2007) and a Ca2+-dependent enhancement of a tonic eNMDAR-mediated current (INMDA), which as we previously showed (Fleming et al., 2011), was not dependent on a presynaptic increase of glutamate release from SON afferent inputs. Finally, we showed that the P2-eNMDAR coupling mechanism is engaged during an acute hyperosmotic challenge, contributing in turn the adaptive increase in the firing activity of MNNs in response to this physiological challenge.
Evidence of purinergic signaling/neurotransmission in MNNs
Despite its well-known role as an essential molecule that provides energy to countless metabolic and signaling processes in living cells, ATP has been recognized as an important neurotransmitter participating in several physiological and pathological processes in the central and peripheral nervous systems (Burnstock, 2020). ATP acts as a neurotransmitter by binding in P2 receptors divided in two main subtypes; P2X receptors that are ionotropic ligand-gated ion channels permeable to Na+, K+, and Ca2+, and P2Y metabotropic G protein-coupled receptors (Burnstock, 2020). The ionotropic P2X receptor family consists of seven different isoforms (P2X1-P2X7), while the metabotropic P2Y receptor family is composed of eight different isoforms described so far (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14) (Burnstock, 2020). Several studies based on different approaches, including PCR, Western Blot, immunohistochemistry and pharmacology (Yao et al., 2003; Song et al., 2011; Vavra et al., 2011; Ferreira-Neto et al., 2017) have shown that from all these subtypes, P2X1-P2X7 and P2Y1, P2Y2 and P2Y12 are found to be present in the SON. Additionally, it has been shown that ATP exogenously applied or endogenously released can induce VP and OT release from hypothalamo-neurohypophyseal system explants (Kapoor & Sladek, 2000; Song & Sladek, 2006; Song et al., 2006, 2007), revealing an important neuroendocrine physiological role for ATP. Our data demonstrate that the ATP-mediated increase in firing activity of MNNs is underlined by an excitatory inward shift in Iholding, an effect that was blocked by the broad-spectrum P2 receptor antagonist, PPADS. These results are in line with our previous studies showing that ATP has excitatory effects in RVLM-projecting PVN neurons and induce sympathoexcitation when microinjected into the PVN (Ferreira-Neto et al., 2013). Given that ATP can act synergistically, be co-released and/or be coupled to the actions of other neurotransmitters within the hypothalamus (Kapoor & Sladek, 2000; Song et al., 2006; Ferreira-Neto et al., 2013; Ferreira-Neto et al., 2015), we aimed to further investigate the precise mechanisms underlying ATP excitatory effects in MNNs.
ATP-glutamate coupling in MNNs
Glutamate is the main excitatory neurotransmitter in the control of MNNs excitability, acting via activation of both ionotropic NMDA and AMPA receptors (Hu & Bourque, 1992; Nissen et al., 1995; Stern et al., 1999; Hirasawa et al., 2003; Li et al., 2003b). It has been recently reported that ATP can be co-released from synaptic terminals with glutamate, either stored in the same vesicles, in separated vesicles in the same synaptic terminal or by ATP facilitating the release of glutamate-containing vesicles (Pankratov et al., 1998; Pankratov et al., 2002a; Pankratov et al., 2002b; Voigt et al., 2015). These different types of ATP-glutamate co-transmission have been shown to contribute to signaling and information processing in different brain nuclei. Here, we report a different type of interaction between ATP and glutamate than involves a postsynaptic signaling coupling between P2 and extrasynaptic NMDARs (eNMDARs). Namely, we found that the ATP-induced firing activity and the underlying excitatory inward shift in Iholding were dependent on the activation of ionotropic glutamatergic receptors, since both effects were blocked by KYN. Interestingly, we did not observe any significant change in glutamatergic sEPSCs, ruling out a presynaptic action of ATP on P2 receptors to facilitate glutamate release (no change in sEPSCs frequency) or a postsynaptic effect on glutamate synaptic receptors, given than neither the magnitude nor the kinetics of sEPSCs were affected. However, given that recordings were obtained at a relatively hyperpolarized Vm, we cannot completely rule out activation of synaptic NMDARs by ATP. These results are in agreement with our previous findings in PVN-RVLM neurons but it is different from reports by others in MNNs (Gordon et al., 2005; Vavra et al., 2011), who showed that exogenous or endogenous ATP can increase glutamatergic synaptic activity in MNNs. Possible explanations for the inconsistent results among these studies are the differences in age and/or strain of rats used, as well as differences in the composition of the recording pipette intracellular solution used. Clearly, additional studies are needed to further elucidate these inconsistencies.
Importantly, the P2-glutamate receptor coupling we report here in MNNs selectively involved NMDA receptors, as AMPARs were not affected. This is in quite contrast with our previous study in RVLM-projecting PVN neurons, in which ATP potentiated AMPA (but not NMDA) receptors (Ferreira-Neto et al., 2015), suggesting that the ATP-glutamate receptor coupling in the hypothalamus is regulated in a cell type-dependent manner.
Several lines of evidence suggest that the NMDARs coupled to ATP P2 receptors were of the extrasynaptic subtypes (Hardingham et al., 2002; Ivanov et al., 2006). We have previously shown that eNMDARs (but not synaptic NMDARs) contribute to a tonic, sustained excitatory inward current that potently drives firing activity in these neurons (Potapenko et al., 2012; Naskar & Stern, 2014; Stern et al., 2016; Zhang et al., 2017). Moreover, we have also shown that eNMDARs are amenable to positive modulation by relevant signaling molecules, such as AngII which is manifested as an inward shift in Iholding, resulting in an increased firing activity (Stern et al., 2016). Thus, the fact that ATP in our studies evoked a similar kynurenic-sensitive shift in Iholding, without affecting EPSCs properties, while at the same time potentiated exogenously evoked glutamate currents that predominantly affect extrasynaptic over synaptic currents support the notion than eNMDARs were involved in the ATP-glutamate coupling reported here. It is important to emphasize that INMDA did not induce a self-potentiation since repetitive NMDA focal applications did not increase INMDA in control conditions (Fig. 4D).
The precise mechanism by which P2 receptor activation increases INMDA in MNNs remains unknown. The fact that the P2-eNMDAR coupling was prevented in MNNs dialyzed with BAPTA supports an intracellular Ca2+-dependency. In this sense, a possible Ca2+-dependent mechanism than could lead to an enhancement of eNMDAR-mediated current could be the trafficking and insertion of additional NMDA receptors at extrasynaptic sites (Groc et al., 2006; Baxter et al., 2011; Papouin et al., 2012) Additional studies however are warranted to determine if this mechanism contributes to the P2-eNMDAR coupling reported here.
The P2-eNMDA receptor coupling contributes to osmotic-driven firing activity in MNNs
It is well known that MNNs play a key role in regulating fluid and electrolyte balance, in part by efficiently adjusting their firing activity based on extracellular fluid osmolarity levels (Bourque et al., 1994; Bourque, 2008). Osmosensitivity in MNNs involves a combination of both intrinsic osmosensitive mechanisms, mediated primarily by the nonselective cation channel transient receptor potential vanilloid type-1 (TRPV1) (Ciura & Bourque, 2006; Sharif Naeini et al., 2006; Prager-Khoutorsky et al., 2014), as well as a glutamatergic input arising mostly from the organum vasculosum laminae terminalis (OVLT) (Bourque et al., 1994; Richard & Bourque, 1995; Bourque & Richard, 2001; Trudel & Bourque, 2003). However, whether ATP and its receptors participate in the osmotic regulation of MNN firing activity is at present unknown. Here, we show for the first time that the P2X receptor blocker PPADs significantly blunted the firing activity response of MNNs evoked by a local, transient hyperosmotic stimulation. We also observed that an acute osmotic challenge potentiated INMDA in MNNs in similar manner to what we observed in response to the exogenous ATP application. Finally, the increase in INMDA induced by the hyperosmotic stimulus in MNNs was completely blocked by PPADS. Taken together, these results strongly support the notion that an acute hyperosmotic stimulus induces the endogenous release of ATP within the SON, which via potentiation of eNMDARs contribute to the osmosensitive increase in firing activity of MNNs. An intriguing question that remains to be determined is the cellular source of the endogenous ATP released during the osmotic challenge. One possible source is the release of ATP from osmosensitive axonal terminals, possibly from the OVLT that contact MNNs (Yang et al., 1994; Richard & Bourque, 1995; Bourque & Richard, 2001; Trudel & Bourque, 2003). Another cellular candidate as an ATP source during a hyperosmotic stimulus are astrocytes. It has been shown that astrocytes are important sources of ATP in several physiological and pathological processes (Marina et al., 2016). Importantly, a functional role for astrocyte-derived ATP has been previously shown within the PVN magnocellular system and other hypothalamic regions (Gordon et al., 2009; Crosby et al., 2018). Moreover, ATP released from astrocytes is involved in baroreflex sensitivity modulation in the nucleus of the solitary tract (Mastitskaya et al., 2020), hypoxia-induced ventilatory response by the pre-Bötzinger complex (Rajani et al., 2018) and in the increased sympathetic activity during heart failure driven by the RVLM (Marina et al., 2013). Together, these previous studies strongly support astrocytes as a likely source of ATP release in the SON during an osmotic challenge, acting thus in concert with other signaling mechanisms to adjust the firing activity of MNNs and, ultimately, regulate VP and OT release in the bloodstream to properly restore extracellular fluid osmolarity. One limitation of our study is that we did not identify and differentiate between oxytocin and vasopressin MNNs. However, our results were largely homogenous across all recordings, and no evidence for subgroups were observed for any of the parameters tested. Thus, it is reasonable to speculate that both MNN subtypes were similarly affected. Finally, while the magnitude and time course of the osmotic stimulation used in our study has been commonly used to study in vitro neuronal osmosensitive responses (Richard & Bourque, 1995; Zhang et al., 2007; Trudel & Bourque, 2010; Prager-Khoutorsky et al., 2014), we acknowledge, however that this osmotic challenge may not be representative of on that would occur in vivo under physiological conditions.
In conclusion, our data indicate that ATP, via activation of a P2-eNMDAR coupling signaling mechanism, efficiently increases the firing activity of SON/PVN MNNs. Moreover, we demonstrate that the P2-eNMDA receptor crosstalk is engaged during an acute hyperosmotic stimulus, contributing to the adaptive firing discharge of MNNs in order to cope with the osmotic challenge.
Supplementary Material
KEY POINTS.
Purinergic and glutamatergic signaling pathways play a key role regulating the activity of hypothalamic magnocellular neurosecretory neurons (MNNs).
However, the precise cellular mechanisms by which ATP and glutamate act in concert to regulate osmotically-driven MNNs neuronal excitability remains unknown.
Here, we report that ATP acts on purinergic P2 receptors in MNNs to potentiate in a Ca2+-dependent manner extrasynaptic NMDAR function.
The P2-NMDAR coupling is engaged in response to an acute hyperosmotic stimulation, contributing to osmotically-driven firing activity in MNNs.
These results help us understand better the precise mechanisms contributing to the osmotic regulation of firing activity and hormone release from MNNs.
FUNDING
This work was supported by a 1) National Institute of Neurological Disorders and Stroke Grant NIH R01NS094640 (Stern JE), 2) Sao Paulo State Research Foundation, FAPESP, #10/17997-0, #16/21991-3 (Antunes VR), #10/05037-1; #12/12444-8 (Ferreira-Neto HC); 3) Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 and; 4) National Council for Scientific and Technological Development (CNPq). Antunes VR is CNPq Research Fellow: # 304970/2017-4.
ABBREVIATIONS
- AP
action potentials
- ATP
adenosine triphosphate
- BAPTA
BAPTA tetrapotassium salt, cell impermeant
- IR-DIC
infrared differential interference contrast
- IAMPA
AMPA current
- IL-glu
L-glutamate current
- INMDA
NMDA current
- KYN
kynurenic acid
- MNNs
magnocellular neurosecretory neurons
- PIC
picrotoxin
- PPADS
4-[[4-Formyl-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-2-pyridinyl]azo]-1,3-benzenedisulfonic acid tetrasodium salt
- PVN
paraventricular nucleus
- RVLM
rostral ventrolateral medulla
- SON
supraoptic nucleus
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
Data Availability Statement
The authors state that all data supporting the results in the paper are in the manuscript itself and in the uploaded Statistical Summary Table as supporting information for online publication.
REFERENCES
- Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM & McCann SM. (2004). Neuroendocrine control of body fluid metabolism. Physiol Rev 84, 169–208. [DOI] [PubMed] [Google Scholar]
- Baxter AW, Choi SJ, Sim JA & North RA. (2011). Role of P2X4 receptors in synaptic strengthening in mouse CA1 hippocampal neurons. Eur J Neurosci 34, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Vavra V, Svobodova I, Bendova Z, Vereb G & Zemkova H. (2013). Potentiation of inhibitory synaptic transmission by extracellular ATP in rat suprachiasmatic nuclei. J Neurosci 33, 8035–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW. (2008). Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9, 519–531. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SH, Kirkpatrick K, Richard D & Fisher TE. (1993). Extrinsic and intrinsic modulatory mechanisms involved in regulating the electrical activity of supraoptic neurons. Ann N Y Acad Sci 689, 512–519. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SH & Richard D. (1994). Osmoreceptors, osmoreception, and osmoregulation. Front Neuroendocrinol 15, 231–274. [DOI] [PubMed] [Google Scholar]
- Bourque CW & Richard D. (2001). Axonal projections from the organum vasculosum lamina terminalis to the supraoptic nucleus: functional analysis and presynaptic modulation. Clin Exp Pharmacol Physiol 28, 570–574. [DOI] [PubMed] [Google Scholar]
- Brown CH, Bains JS, Ludwig M & Stern JE. (2013). Physiological regulation of magnocellular neurosecretory cell activity: integration of intrinsic, local and afferent mechanisms. J Neuroendocrinol 25, 678–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G (2007). Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87, 659–797. [DOI] [PubMed] [Google Scholar]
- Burnstock G (2020). Introduction to Purinergic Signalling in the Brain. Adv Exp Med Biol 1202, 1–12. [DOI] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G & Nordmann JJ. (1985). The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol 369, 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH & Toney GM. (2001). AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol 281, R1844–1853. [DOI] [PubMed] [Google Scholar]
- Ciura S & Bourque CW. (2006). Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci 26, 9069–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby KM, Murphy-Royal C, Wilson SA, Gordon GR, Bains JS & Pittman QJ. (2018). Cholecystokinin Switches the Plasticity of GABA Synapses in the Dorsomedial Hypothalamus via Astrocytic ATP Release. J Neurosci 38, 8515–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA, Sibbald JR & Khanna S. (1993). ATP mediates an excitatory noradrenergic neuron input to supraoptic vasopressin cells. Brain Res 607, 341–344. [DOI] [PubMed] [Google Scholar]
- Dutton A & Dyball RE. (1979). Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol 290, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Neto HC, Antunes VR & Stern JE. (2015). ATP stimulates rat hypothalamic sympathetic neurons by enhancing AMPA receptor-mediated currents. J Neurophysiol 114, 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Neto HC, Ribeiro IM, Moreira TS, Yao ST & Antunes VR. (2017). Purinergic P2 receptors in the paraventricular nucleus of the hypothalamus are involved in hyperosmotic-induced sympathoexcitation. Neuroscience 349, 253–263. [DOI] [PubMed] [Google Scholar]
- Ferreira-Neto HC, Yao ST & Antunes VR. (2013). Purinergic and glutamatergic interactions in the hypothalamic paraventricular nucleus modulate sympathetic outflow. Purinergic Signal 9, 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TM, Scott V, Naskar K, Joe N, Brown CH & Stern JE. (2011). State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol 589, 3929–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard ER, Coburn CG, de Leon A, Snissarenko EP, Bauce LG, Pittman QJ, Hou B & Curras-Collazo MC. (2007). Vasopressin autoreceptors and nitric oxide-dependent glutamate release are required for somatodendritic vasopressin release from rat magnocellular neuroendocrine cells responding to osmotic stimuli. Endocrinology 148, 479–489. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE & Bains JS. (2005). Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci 8, 1078–1086. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA & Bains JS. (2009). Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron 64, 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi D, Lagunas N, Amorin M, Pinos H, Panzica GC, Garcia-Segura LM & Collado P. (2013). Estrogenic regulation of NADPH-diaphorase in the supraoptic and paraventricular nuclei under acute osmotic stress. Neuroscience 248, 127–135. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L & Choquet D. (2006). NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A 103, 18769–18774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Aranguez A, Perez de Lara MJ & Pintor J. (2017). Hyperosmotic stress induces ATP release and changes in P2X7 receptor levels in human corneal and conjunctival epithelial cells. Purinergic Signal 13, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y & Bading H. (2002). Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5, 405–414. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Mouginot D, Kozoriz MG, Kombian SB & Pittman QJ. (2003). Vasopressin differentially modulates non-NMDA receptors in vasopressin and oxytocin neurons in the supraoptic nucleus. J Neurosci 23, 4270–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma H & Bourque CW. (1995). P2 purinoceptor-mediated depolarization of rat supraoptic neurosecretory cells in vitro. J Physiol 489 (Pt 3), 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollborn M, Fischer S, Kuhrt H, Wiedemann P, Bringmann A & Kohen L. (2017). Osmotic regulation of NFAT5 expression in RPE cells: The involvement of purinergic receptor signaling. Mol Vis 23, 116–130. [PMC free article] [PubMed] [Google Scholar]
- Hu B & Bourque CW. (1992). NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. J Physiol 458, 667–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Hui Z, Wei W, Yang J, Chen Z, Guo B, Xing F, Zhang X, Pan L & Xu J. (2017). Hypotonic stress promotes ATP release, reactive oxygen species production and cell proliferation via TRPV4 activation in rheumatoid arthritis rat synovial fibroblasts. Biochem Biophys Res Commun 486, 108–115. [DOI] [PubMed] [Google Scholar]
- Inenaga K, Cui LN, Nagatomo T, Honda E, Ueta Y & Yamashita H. (1997). Osmotic modulation in glutamatergic excitatory synaptic inputs to neurons in the supraoptic nucleus of rat hypothalamus in vitro. J Neuroendocrinol 9, 63–68. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y & Medina I. (2006). Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol 572, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor JR & Sladek CD. (2000). Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release. J Neurosci 20, 8868–8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki A, Hoshi K, Kawano M, Nogami H, Yoshikawa H & Hisano S. (2005). Up-regulation of VGLUT2 expression in hypothalamic-neurohypophysial neurons of the rat following osmotic challenge. Eur J Neurosci 22, 672–680. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Gittermann D, Cockayne DA & Jones A. (2003). ATP modulation of excitatory synapses onto interneurons. J Neurosci 23, 7426–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang W & Stern JE. (2003a). Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience 118, 585–601. [DOI] [PubMed] [Google Scholar]
- Li YF, Cornish KG & Patel KP. (2003b). Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93, 990–997. [DOI] [PubMed] [Google Scholar]
- Luther JA, Halmos KC & Tasker JG. (2000). A slow transient potassium current expressed in a subset of neurosecretory neurons of the hypothalamic paraventricular nucleus. J Neurophysiol 84, 1814–1825. [DOI] [PubMed] [Google Scholar]
- Luther JA & Tasker JG. (2000). Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol 523 Pt 1, 193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Kasymov V, Ackland GL, Kasparov S & Gourine AV. (2016). Astrocytes and Brain Hypoxia. Adv Exp Med Biol 903, 201–207. [DOI] [PubMed] [Google Scholar]
- Marina N, Tang F, Figueiredo M, Mastitskaya S, Kasimov V, Mohamed-Ali V, Roloff E, Teschemacher AG, Gourine AV & Kasparov S. (2013). Purinergic signalling in the rostral ventro-lateral medulla controls sympathetic drive and contributes to the progression of heart failure following myocardial infarction in rats. Basic Res Cardiol 108, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastitskaya S, Turovsky E, Marina N, Theparambil SM, Hadjihambi A, Kasparov S, Teschemacher AG, Ramage AG, Gourine AV & Hosford PS. (2020). Astrocytes Modulate Baroreflex Sensitivity at the Level of the Nucleus of the Solitary Tract. J Neurosci 40, 3052–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naskar K & Stern JE. (2014). A functional coupling between extrasynaptic NMDA receptors and A-type K+ channels under astrocyte control regulates hypothalamic neurosecretory neuronal activity. J Physiol 592, 2813–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen R, Hu B & Renaud LP. (1995). Regulation of spontaneous phasic firing of rat supraoptic vasopressin neurones in vivo by glutamate receptors. J Physiol 484 (Pt 2), 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Okada T, Islam MR & Sabirov RZ. (2018). Molecular Identities and ATP Release Activities of Two Types of Volume-Regulatory Anion Channels, VSOR and Maxi-Cl. Curr Top Membr 81, 125–176. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal MT & Krishtal O. (1998). A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur J Neurosci 10, 3898–3902. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal O & Verkhratsky A. (2002a). Ionotropic P2X purinoreceptors mediate synaptic transmission in rat pyramidal neurones of layer II/III of somato-sensory cortex. J Physiol 542, 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov YV, Lalo UV & Krishtal OA. (2002b). Role for P2X receptors in long-term potentiation. J Neurosci 22, 8363–8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP & Oliet SH. (2012). Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150, 633–646. [DOI] [PubMed] [Google Scholar]
- Potapenko ES, Biancardi VC, Florschutz RM, Ryu PD & Stern JE. (2011). Inhibitory-excitatory synaptic balance is shifted toward increased excitation in magnocellular neurosecretory cells of heart failure rats. J Neurophysiol 106, 1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapenko ES, Biancardi VC, Zhou Y & Stern JE. (2012). Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol 303, R291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapenko ES, Biancardi VC, Zhou Y & Stern JE. (2013). Astrocytes modulate a postsynaptic NMDA-GABAA-receptor crosstalk in hypothalamic neurosecretory neurons. J Neurosci 33, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB & Dyball RE. (1977). Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci 196, 367–384. [DOI] [PubMed] [Google Scholar]
- Prager-Khoutorsky M, Khoutorsky A & Bourque CW. (2014). Unique interweaved microtubule scaffold mediates osmosensory transduction via physical interaction with TRPV1. Neuron 83, 866–878. [DOI] [PubMed] [Google Scholar]
- Rajani V, Zhang Y, Jalubula V, Rancic V, SheikhBahaei S, Zwicker JD, Pagliardini S, Dickson CT, Ballanyi K, Kasparov S, Gourine AV & Funk GD. (2018). Release of ATP by pre-Botzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca(2+) -dependent P2Y1 receptor mechanism. J Physiol 596, 3245–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D & Bourque CW. (1995). Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. J Physiol 489 (Pt 2), 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandgren JA, Linggonegoro DW, Zhang SY, Sapouckey SA, Claflin KE, Pearson NA, Leidinger MR, Pierce GL, Santillan MK, Gibson-Corley KN, Sigmund CD & Grobe JL. (2018). Angiotensin AT1A receptors expressed in vasopressin-producing cells of the supraoptic nucleus contribute to osmotic control of vasopressin. Am J Physiol Regul Integr Comp Physiol 314, R770–R780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif Naeini R, Witty MF, Seguela P & Bourque CW. (2006). An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci 9, 93–98. [DOI] [PubMed] [Google Scholar]
- Song X, Guo W, Yu Q, Liu X, Xiang Z, He C & Burnstock G. (2011). Regional expression of P2Y(4) receptors in the rat central nervous system. Purinergic Signal 7, 469–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z & Sladek CD. (2006). Site of ATP and phenylephrine synergistic stimulation of vasopressin release from the hypothalamo-neurohypophyseal system. J Neuroendocrinol 18, 266–272. [DOI] [PubMed] [Google Scholar]
- Song Z, Vijayaraghavan S & Sladek CD. (2006). Simultaneous exposure to ATP and phenylephrine induces a sustained elevation in the intracellular calcium concentration in supraoptic neurons. Am J Physiol Regul Integr Comp Physiol 291, R37–45. [DOI] [PubMed] [Google Scholar]
- Song Z, Vijayaraghavan S & Sladek CD. (2007). ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. Am J Physiol Regul Integr Comp Physiol 292, R423–431. [DOI] [PubMed] [Google Scholar]
- Stern JE. (2001). Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537, 161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Galarreta M, Foehring RC, Hestrin S & Armstrong WE. (1999). Differences in the properties of ionotropic glutamate synaptic currents in oxytocin and vasopressin neuroendocrine neurons. J Neurosci 19, 3367–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Son S, Biancardi VC, Zheng H, Sharma N & Patel KP. (2016). Astrocytes Contribute to Angiotensin II Stimulation of Hypothalamic Neuronal Activity and Sympathetic Outflow. Hypertension 68, 1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel E & Bourque CW. (2003). A rat brain slice preserving synaptic connections between neurons of the suprachiasmatic nucleus, organum vasculosum lamina terminalis and supraoptic nucleus. J Neurosci Methods 128, 67–77. [DOI] [PubMed] [Google Scholar]
- Trudel E & Bourque CW. (2010). Central clock excites vasopressin neurons by waking osmosensory afferents during late sleep. Nat Neurosci 13, 467–474. [DOI] [PubMed] [Google Scholar]
- Trull KJ, Miller P, Tat K, Varney SA, Conley JM & Tantama M. (2019). Detection of Osmotic Shock-Induced Extracellular Nucleotide Release with a Genetically Encoded Fluorescent Sensor of ADP and ATP. Sensors (Basel) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN & Trombley PQ. (1993). Glutamate neurons in hypothalamus regulate excitatory transmission. J Neurosci 13, 2829–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavra V, Bhattacharya A & Zemkova H. (2011). Facilitation of glutamate and GABA release by P2X receptor activation in supraoptic neurons from freshly isolated rat brain slices. Neuroscience 188, 1–12. [DOI] [PubMed] [Google Scholar]
- Voigt J, Grosche A, Vogler S, Pannicke T, Hollborn M, Kohen L, Wiedemann P, Reichenbach A & Bringmann A. (2015). Nonvesicular release of ATP from rat retinal glial (Muller) cells is differentially mediated in response to osmotic stress and glutamate. Neurochem Res 40, 651–660. [DOI] [PubMed] [Google Scholar]
- Yang CR, Senatorov VV & Renaud LP. (1994). Organum vasculosum lamina terminalis-evoked postsynaptic responses in rat supraoptic neurones in vitro. J Physiol 477, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ST, Gourine AV, Spyer KM, Barden JA & Lawrence AJ. (2003). Localisation of P2X2 receptor subunit immunoreactivity on nitric oxide synthase expressing neurones in the brain stem and hypothalamus of the rat: a fluorescence immunohistochemical study. Neuroscience 121, 411–419. [DOI] [PubMed] [Google Scholar]
- Zhang M, Biancardi VC & Stern JE. (2017). An increased extrasynaptic NMDA tone inhibits A-type K(+) current and increases excitability of hypothalamic neurosecretory neurons in hypertensive rats. J Physiol 595, 4647–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kindrat AN, Sharif-Naeini R & Bourque CW. (2007). Actin filaments mediate mechanical gating during osmosensory transduction in rat supraoptic nucleus neurons. J Neurosci 27, 4008–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data supporting the results in the paper are in the manuscript itself and in the uploaded Statistical Summary Table as supporting information for online publication.


