Abstract
With the rapid approval of immune checkpoint inhibitors for lung, melanoma, breast, genitourinary, and hematological malignancies, the hematopoietic cells in the tumor microenvironment (TME) are now considered an important, if not essential, consideration for cancer scientists. In many instances, syngeneic murine models have not been highly predictive for responsiveness in clinical trials. Our limited understanding of the human TME have, therefore, precluded a rational translation of immunotherapeutic combinations. This has led to the adoption of hematopoietic humanized murine models for the study of human tumor immunology in vivo. However, concerns about chimerism rates, HLA mismatching, and incomplete reconstitution of the innate immune system have driven a quest for improvements in these allogeneic humanized murine systems. Presented in this unit is a completely autologous xenotransplantation method for reconstituting the human tumor immune microenvironment in vivo without the use of a patient’s peripheral blood which is known to be associated with low engraftment rates. With this new approach, the current limitations of allogeneic humanized models are avoided by using matched bone marrow cells (BMC) and derived tumor xenoplants (PDX) from solid cancer patients. This autologous system provides a platform for studying endogenous lymphocytic and myeloid cell infiltration into the human tumor in vivo.
Keywords: tumor microenvironment, autologous reconstitution, humanized mice, head neck carcinoma
Introduction
Lasting responses to immune checkpoint inhibitors have stimulated clinical trials with various immunotherapy-based combination platforms for the treatment of cancer (Brahmer, et.al., 2012; Larkins, et.al., 2015). Unfortunately, retrospective, correlative biomarker analyses of biospecimens obtained from these trials provide only limited information on the mechanistic complexities of the dynamic human tumor microenvironment that is needed to inform the next generation of clinical studies (Anagnostou, et.al. 2017). Preclinical testing of immunotherapeutic agents and strategies relies upon data obtained from syngeneic immunocompetent murine models or genetically engineered mouse models (GEMM). Both of these approaches are limited by inadequate genetic and biological representation of corresponding human cancer cells, as well as intrinsic differences between human and murine immune systems (Singh, et.al., 2012; Alizadeh, et.al., 2015; Gould, et.al., 2015). Because of these problems, investigators have turned to using humanized murine models for studying human TME.
Patient derived xenotransplant (PDX) models that introduce human tumor xenografts into immunodeficient (γc deficient NSG, NOG, or NRG) mice engrafted with human hematopoietic cells (“humanized mice”)(Schultz, et.al., 2007; Ito, et.al., 2008) are limited by the fact that the adaptive human immune system developing in these mice is allogeneic to the tumor, even when the human thymus is concurrently implanted or when human HLA transgenic mice are used as recipients (Lan, et.al., 2006; Schultz, et.al. 2010; Schultz, et.al. 2014). While efforts are being made to generate transgenic mice with the different combinations of human HLA genes to overcome this allogeneic response, the extensive polymorphism of HLA genes makes it virtually impossible to match all HLA alleles with a given tumor and the engrafted immune system (Serreze, et.al., 2010; Legrand, et.al., 2009; Kalscheuer, et.al., 2012). Attempts to address this allogeneic problem have included the use of matched peripheral blood (PB) and tumor for the dual engraftment (Morton, et.al., 2016). However, chimerism rates for this strategy are low and variable.
The protocol described in this unit details a method that involves the harvesting matched bone marrow and tumor cells to autologously reconstitute the human TME in vivo (Protocol 1) (Fu, et.al., 2016). This procedure requires the collaboration of a team of clinicians (surgeons and hematologists) for the collection of tumor and BMC used to separately engraft a parallel set of immunodeficient mice (Fig. 1). The second phase of the protocol requires passaging the PDX tumor into the matched BMC humanized mice. The protocol allows for banking the matched cells for later reconstitution (Support Protocol 1). Some of the limitations of other humanized mice protocols are applicable to this approach as well. These include the presence of murine cytokine microenvironments and the need for long incubation times. Nonetheless, the protocol detailed herein allows for testing hypotheses requiring the use of an autologous immune context (i.e. neoantigen immunogenicity). Studies in our laboratory have shown that this autologous system is amenable for studying tumor infiltrating human myeloid suppressor cells as well as for screening novel agents for their ability to alter endogenous leukocytic infiltration into the human TME (Protocol 2).
Figure 1.
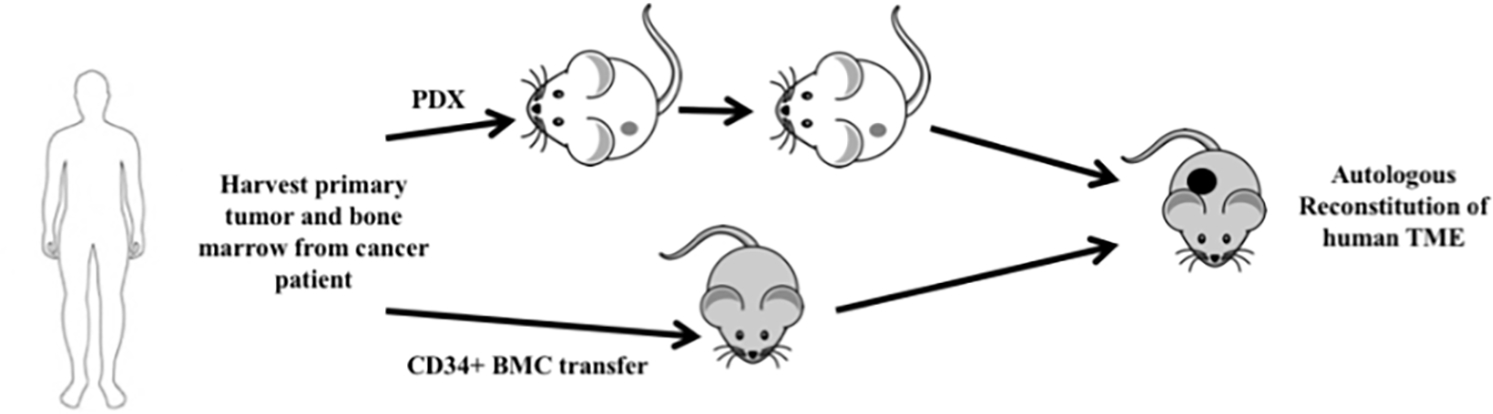
Schematics of autologous reconstitution of human solid tumors. PDX is patient derived xenoplants, BMC bone marrow cells, and TME tumor microenvironment. The recipient mice (e.g. NOG, NSG, NSG-HLA-A2, NSG-IL-6, etc.) can vary depending on the aims of the experiment. Both engraftment of the PDX tumor and BMC should be performed with NOG/NSG mice.
Basic Protocol 1: Autologous reconstitution of human tumors
Execution of this protocol initially requires a parallel set of mice recipients, one for BMC and one for tumor xenotransplantation (Fig 1). The engrafted humanized mice will be the recipients of the serially passaged matched tumor xenoplants. While the BMC can be successfully engrafted after a single freeze/thaw, the tumor xenoplants should not be frozen before implantation. After the initial tumor transplantation, the tumor xenoplants can be frozen and subsequently passaged in mice. We stored BMC aliquots in liquid nitrogen. The BMC and the tumor are typically engrafted at the same time into a parallel set of mice.
MATERIALS:
Mice:
NOD scid gamma, HLA-A2.1 mice: pairs keep breeding, 6–8 weeks, strain designation, NOD. Cg-Mcph1 Tg (HLA-A2.1)1Enge Prkdcscid Il2rgtm1wjl/SzJ, (The Jackson Laboratory, Stock no. 009617)
NOG mice: male and female, 4–8 weeks old, keep breeding, strain designation, NOD.Cg-Prkdcscid Il2rg tmlsug/JicTac, Model no: NOG-F and NOG-M, HSCFTL-NOG homozygous, (Taconic Biosciences)
Antibodies: (conjugated fluorophores)
Anti-human CD3(clone sp3, Cat no.556611, BD), CD4(clone SK3, Cat. no.565999, BD), CD8(clone RPA-T8, Cat. no.565165, BD), CD11c (clone39, Cat. no.748269, BD), CD11b (clone 44, Cat. no. 747357, BD), CD14 (clone M5E2, Cat. no. 746304, BD), CD19 (clone S125C1, no. 566103, BD), CD33 (clone P67.6, Cat. no. 745556, BD), CD34 (clone 9E2, Cat, no. 745556, Biolegend), CD45 (clone 2D1, Cat. no. 368505, Biolegend), CD56 (clone NCAM16.2, Cat. no. 564057, BD), NKP46 (clone 9E2, Cat. no. 743710, BD), HLA-DR (clone G46–6, Cat. no. 745782, BD).
Kits:
Human CD34 microbead kit and pan T-cell isolation kit (Miltenyi Biotec, catalogue no. 130-046-702)
AmpFISTR Identifier PCR Amplification kit (Applied Biosystems)
Human bone marrow cells (hBMC):
hBMC are harvested from the fibular bone marrow cells from cancer patient donors undergoing head and neck cancer resection and reconstruction surgery. BMC can be aspirated from iliac crest from cancer patients during diagnostic surgeries as well.
RPMI medium 1640 (Gibco, 11875119)
150 mm tissue culture dish (TPP, 93150)
Human donor fibular bone marrow cells (see above)
70% (v/v) EtOH (pharmco-AAPER, DSP-CT-18)
Phosphate-buffered saline (PBS), (Quality Biological, 114-058-101)
Ice bucket and ice (Hoshizaki ice maker)
Kim Wipe tissue (KimTech Science Brand)
5 ml syringes (BD, catalogue no. 309646-1)
25G5/8-in needles (BD, catalogue no.305122)
70-micron cell strainer (Corning, 352350)
50 ml falcon tubes (Falcon, catalogue no.352098)
Ficoll/Paque ™ premium 1.084 (GE Healthcare)
Centrifuge (Beckman Coulter, Allegra X-14R)
DNAse I (Roche) and Liberase Blendzyme 2 (20,000 Mandl U/ml) (Roche)
MACS Buffer (Miltenyi Biotec 130-091-376)
LS Column (Miltenyi Biotec 130-042-401)
QuadroMACS Separator (Miltenyi Biotec 130-091-051)
Trimethoprim/Sulfamethoxazole (TMS, Aurobindo Pharma USA, Inc. catalogue no NDC 65862-496-47)
ACK lysing buffer (Quality Biological, catalogue no. 118-156-721)
BD Matrigel ™ basement membrane matrix (BD Bioscience 356234)
Tissue-Tek cryomold (Sakura, 4557)
Tissue-Tek O.C.T. compound (Sakura, 4583)
Sterile surgical instruments: forceps, scissors
Isolation of human bone marrow cells
-
1
Human donor fibular bone marrow cells or BMC aspirates from iliac crest from cancer patients undergoing head and neck cancer resection and reconstruction are harvested during surgery. Remnant bone after reconstructive surgery that is typically discarded is an excellent source for these cells. Alternatively, BMC aspirates can be obtained from needle aspirates from iliac crest during diagnostic surgery. Approaches generally yield cellularity in the range of 108 BMC, depending on the patients (see below). Because autologous reconstitutions are needed, BMC cannot be pooled from multiple patients. BMC can be harvested, prepared, and transferred into mice on the same day. However, these BMC can be frozen, and the thawed BMC will have comparable engraftment rates as fresh BMC.
-
2
Use 50 ml 70% EtOH and 50 ml cold PBS to clean the human sample if large bony pieces (~10 cm long) are harvested. Remove muscle and joint tissue from fibular surface using scissors and sterile Kim-wipe tissue. If BMC from aspirates are collected, skip to step 5 below.
-
3
Place the long bone into a clean dish with 50 ml cold RPMI 1640 medium on ice.
-
4
Using 5–10 ml syringes with 25G 5/8 needles aspirate 5–10 ml RPMI 1640 medium, flushing bone marrow cavities from one end of the bone. Repeat flushing 3–5 times.
-
5
Collect 25–50 ml of the bone marrow cells and smash pass through 70 μm cell strainers to prepare single cells.
-
6
Centrifuge the cell suspension for 5 min at 500 × g at 4 ° C. Discard the supernatant.
-
7
Suspend the cell pellet and dilute in 30–35 ml sterile PBS. The more diluted the sample, the better the purity of the mononuclear cells.
-
8
Carefully layer 30–35 ml of the cell suspension over 15 ml of Ficoll-Paque in a 50 ml conical tube at room temperature.
-
9
Centrifuge the sample at 400×g for 30–40 min at 20°C in a swinging-buck rotor without brake and acceleration.
-
10
Carefully aspirate the mononuclear cell layer to a fresh 50 ml conical tube, fill with RPMI 1640 medium and centrifuge at 300×g for 10 min at 20°C. Carefully remove supernatant using pipette.
-
11
Resuspend the cell pellet and count the monocytes. The yields should be 1–5×108 mononuclear cells. Centrifuge resuspended cells at 300×g for 10 min at 20°C then carefully remove supernatant for CD34 bead enrichment.
-
12
Resuspend the cell pellet with 300 μl MACS buffer for up to 108 total cells.
-
13
Add 100 μl of FcR Blocking Reagent for up to 108 total cells.
-
14
Add 100 μl of CD34 MicroBeads for up to 108 total cells.
-
15
Mix the sample and incubate for 30 min at 4°C.
-
16
Wash cells with 5–10ml MACS buffer, centrifuge at 300×g for 10 min, and carefully remove all the supernatant.
-
17
Resuspend the cell pellet in 500 μl MACS buffer.
-
18
Proceed to magnetic separation.
-
19
Place LS column in the magnetic field of the MACS Separator.
-
20
Rinse the LS column following the manufacturer’s instructions and add pour the cell suspension onto the column.
-
21
Remove the column from the separator and place it on a collection tube.
-
22
Pipette 5 ml MACS buffer onto the column. Immediately flush out the magnetically separated CD34+ BMC.
-
23
Wash the cells with PBS and resuspend the cell pellet in PBS. Yields should total 1–2 × 106 CD34+ cells.
-
24
Prepare 1.0 × 105 CD34+ BMC in 500 μl PBS per mouse for tail i.v. injection into lethally irradiated mice. Typically, there are 10–20 recipient mice from 1–2 × 106 CD34+ cells. Transferring less than 50,000 BMC results in poor engraftments.
The recipient mice age can be 8–10 weeks, and they can be irradiated by 4–5Gy at least 4–6 hr prior to injection with human bone marrow CD34+ cells.
-
25
House the mice in a sterile environment where they are fed Trimethoprim/Sulfamethoxazole (TMS) containing water.
One week before irradiation, begin feeding the mice with antibiotic containing water. Prepare 3.4 ml Trimethoprim /Sulfamethoxazole (TMS, 40mg/ml/200mg/ml) into 378.5ml bottle. Mice should be fed with antibiotic water for 6–8 weeks.
-
26
Analyze peripheral blood (or spleen) by FACS 6–8 weeks after transfer to determine chimerism rates (Fig. 2).
Six to eight weeks after human CD34+ cell injection the humanized mice peripheral blood can be collected from retro-orbital or tail vein access. Isolate the white blood cells using Ficoll-paque TM plus. Flow cytometry (BD Celesta) can be performed with anti-human CD3, CD4, CD8, CD45, CD11b, CD11c, CD56, CD16, NKp46, CD123, CD33, HLA-DR staining, and analyzed with FlowJo software.
Figure 2.
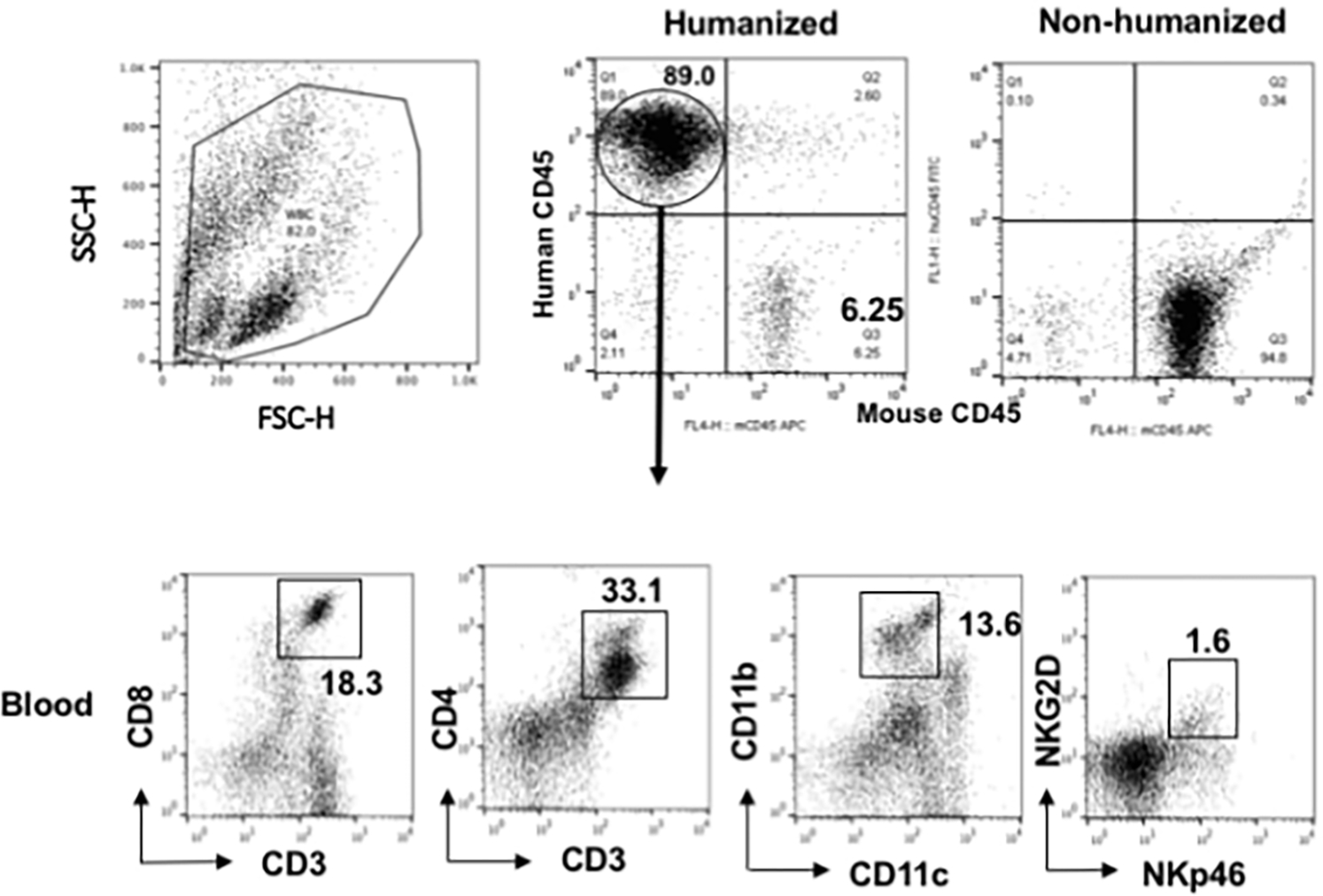
After engraftment with CD34+ BMC from an HNSCC patient undergoing surgery, humanized mice showed the presence of human lymphocytic and myeloid cells (CD11b+), including quantifiable human NK cells (NKG2D+NKp46+)_in blood. The percentages are normalized to total mononuclear cells from the spleen or blood (blood as shown). Comparable figures were noted in the spleen. Gated mononuclear cells were plotted for human CD45 and murine CD45 levels (upper panels). Gated human CD45 were plotted for human CD markers as noted on the axis (lower panels) (adopted from Fu, et.al., 2016).
Xenotransplantation of matched human tumor into non-humanized mice
-
27
Wash human tumor tissue (at least 0.125cm3) from a single patient with PBS.
-
28
Cut the tumor into small pieces (1–2mm in size) in sterile 150 mm tissue culture dishes and digest it for 5 min at room temperature in 5 ml of RPMI 1640 medium containing 1 ml 1% DNAse I (Roche).
-
29
Add 1 ml of Liberase Blendzyme 2 (400 Mandl U/ml) (Roche) to above the tissue culture dish and incubate for 20 min at room temperature.
-
30
Pass 6 ml of the cell mixture via the cell strainer and smash the remaining pieces with the plunger of a 1 ml syringe.
-
31
Centrifuge the tumor cells about 1–2×107 at 300×g for 10 min. Wash the cells with PBS 10 ml twice.
-
32
Resuspend the cell pellet with PBS 1 ml and count the tumor cell number.
-
33
Mix 1–2×106 tumor cells per mouse with 100 uL of BD Matrigel ™ basement membrane matrix (BD, 35426). Inject subcutaneously in the flank 50–100 μl of the tumor cell mixture into selected immunodeficient mice.
Before using BD Matrigel ™ basement membrane matrix, remove from −20°C storage and thaw by submerging the vial into ice in a 4°C refrigerator. Maintain on ice at all times. Before injection shave the mice fur on the flank to facilitate subsequent tumor measurements.
Autologous reconstitution of matched human tumor into humanized mice
-
34
The matched tumor xenoplant is implanted subcutaneously once the mice engrafted with BMC are confirmed to have human CD4+ and CD8+ T-cells in the peripheral blood 6–8 weeks after CD34+ injection. See above 26 and 33. Engraftment typically range 80–90% human CD45 cells in mice peripheral blood.
In some cases, the tumor cells and/or BMC are transduced with lentivirus prior to engraftments. See the below support protocol (Support Protocol 1) for lentiviral transduction.
-
35
Transgenic NOG that overexpress HLA-A2 (Jackson Laboratory, stock no. 014570) are used to ensure the T-cells are restricted to HLA-A2.
-
36
After the PDX becomes palpable (>1–2 mm), they can be harvested for immunophenotyping – flow cytometry (see above step 26), multiplexed immunofluorescent histological analysis (Fu, et.al, 2016), or immunotherapeutic assays (see below)(Fig. 3.)
One week after human tumor injection with BD Matrigel ™ basement membrane matrix, examine for palpable tumors. The time to palpable growth varies among tumor samples. Once palpable, (~ 1mm×1mm×1mm), tumor measurements should be taken every other day or as needed. Tumors can be collected for immunotyping once they exceed 20mm in size.
-
37
Harvest the thymus to assess whether engrafted BMC are “educated” into single positive cells (Fu, et.al., 2016) to ensure tumor-infiltrating lymphocytes (TILs) noted in step 36 come from do novo development of engrafted human stem cells rather than homeostatic expansion of mature T-cells from the bone marrow or from the engrafted tumor tissue. Thymocytes should be predominantly double positive (CD4+CD8+ human T-cells).
-
38
For TIL analysis, cut the harvested the tumors into small pieces (<1–2mm), resuspend the pellet with PBS 5 ml, and smash pass through cell strainers. Centrifuge at 1500 rpm 10 for min, resuspend the cells with 4 ml 80% Percoll, transfer the cell mixture into 14 ml tubes and gently lay on 4 ml 40% Percoll. Centrifuge the sample at 3000 rpm×30 min at 25 °C with no brakes. Collect the TILs at the interface between 80% Percoll and 40% Percoll, and wash the cells with 5–10 ml PBS twice. Stain the cells for flow cytometry (see step 26 above).
Figure 3.
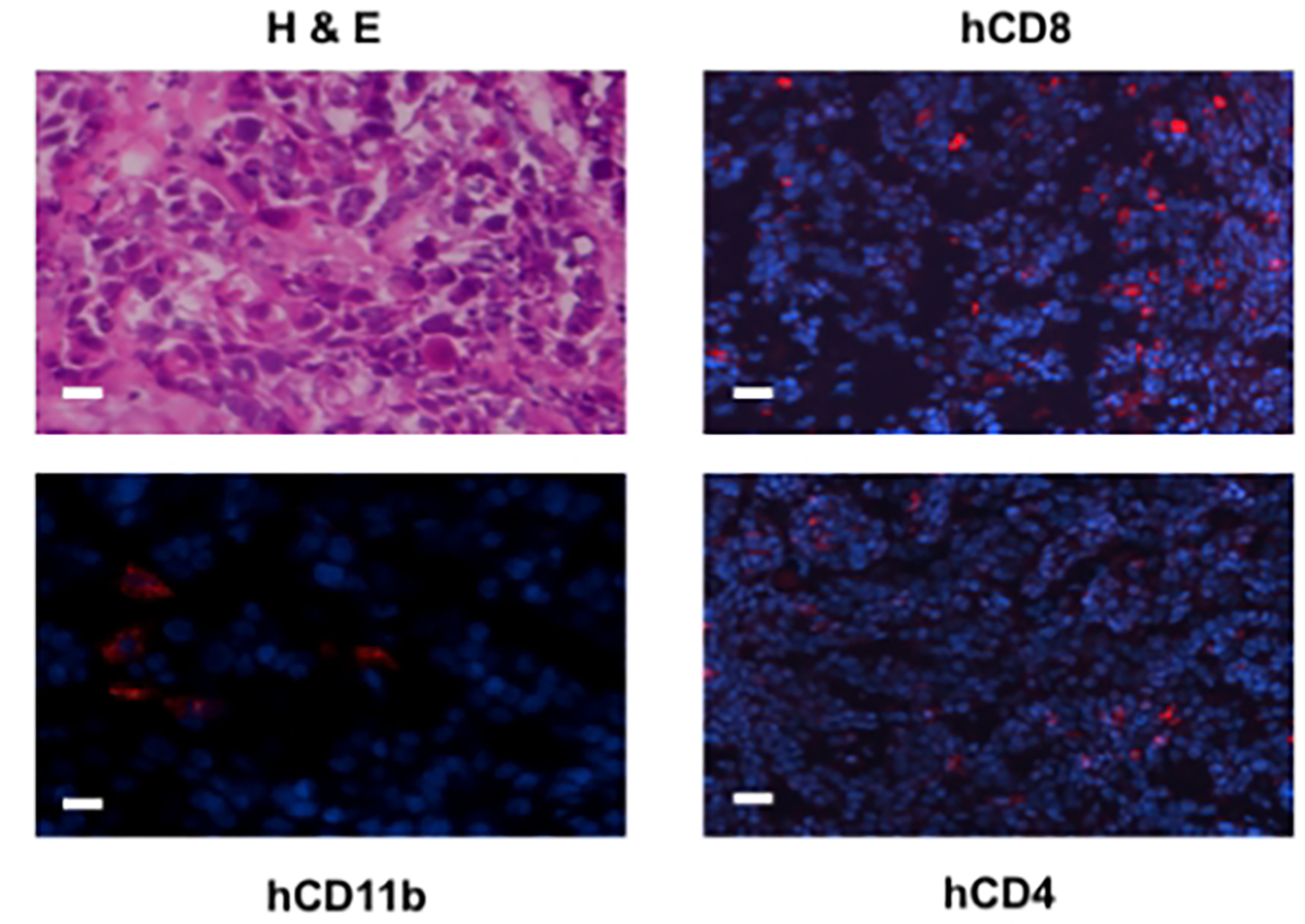
TME assessment of autologously reconstituted tumor. Surgical tumor specimens from the same patient whose CD34+ BMC were used to engraft NOG-A2 mice were implanted into the same humanized mice. The engrafted tumor was harvested and stained for tumor infiltrating human CD8, CD4, and CD11b cells. The primary antibodies for these stainings were rabbit anti-human CD8, rat anti-human CD4, and rabbit anti-human CD11b, all conjugated to Alexa Fluor® 568. The bar scale is 100 μ. The upper left panel is an H&E stain of the same tumor (adopted from Fu, et.al., 2016).
Support Protocol 1: Transduction of BMC and/or tumor cells prior to autologous reconstitution.
Lentiviral siRNA or transgenic constructs are now available from many companies. These commercial reagents should be used only after confirming their reported titers. Alternatively, investigators can prepare their own lentiviral vectors from DNA constructs as detailed below. GFP lentivirus (or other fluorophore lentivirus) can also be used to initially label either the BMC or the cancer cells used for engraftment.
MATERIALS LIST:
GFP plasmid (Santa Cruz) and pPACKF1 ™ lentivector packaging kit (SBI, Cat#LV100A-1) can be used. Most lentiviral kits have been shown to be effective.
Titers should be measured and compared to the titers predicted by the manufacturer.
Global UltraRapid Titer Kit (SBI, cat#LV961A-1)
TransDux (SBI, Cat#LV850A-1).
293 cells (ATCC)
DMEM (Thermofisher Scientific, 11965118)
150 mm tissue culture plates (TPP)
pPACK Lentivector Packaging kits (SBI, Cat# LV500A-1)
Lipofectamine ®2000 (Thermofisher Scientific, 11668019)
100×15 mm Bacteriological Petri Dish (Falco, 351029)
24 well cell culture plate (Falco)
Polybrene Infection/Transfection Reagent (Sigma, TR-1003)
Vortex
Protocol Steps
-
1
Seed 7×106 293 cells per 150 cm2 cell culture plates in 20 ml of DMEM (without antibiotics) 18–24 hours prior to transfection. Culture the cells at 37°C in 5% CO2 until the cell density is at 60–80% confluency at the time of transfection.
-
2
Add 1ml DMEM (serum free) to an autoclaved Eppendorf tube.
-
3
Add 45 μl pPACK packaging mix and 4.5 μg of lentivector construct into the EMDM, mix with pipetting. This is tube 1.
-
4
Add 55 μl Lipofectamine 2000 into another tube and vortex for 10 sec. This is tube 2.
-
5
Mix the contents of tube 1 and tube 2 at room temperature and let it sit for 15 min.
-
6
Add the above mixture drop-wise to the 293 cells tissue culture dish, and swirl to disperse evenly throughout the plate.
-
7
Return the infected cells tissue culture dish to the cell culture incubator at 37°C in 5% CO2 for 48–72 hours.
Monitor the cells daily and observe the percentage surviving using microscopy and fluorescent microscopy to detect GFP green color. GFP expression on fluorescent microscopy or flow cytometry can be performed. If less than 90% of the cell population is green, puromycin selection recommended to reduce injection of non-transduced cells into the mice.
-
8
Collect the medium and centrifuge at 3000 × g for 15 min at room temperature to pellet cell debris. Transfer the viral supernatant into a fresh tube.
-
9
Check titer using the Global UltraRapid Titer Kit.
Transduction of tumor cells
-
10
Day 1: Plate 5–10×104 tumor cells (Basic Protocol 1.27) in a 24 well plate in 1ml RPMI 1640. These tumor cells are the passaged PDX from Protocol 1.
-
11
Day 2: Grow the cells to 50–70% confluency in the 37°C incubator at 5% CO2 and aspirate the medium from the cells.
-
12
Thaw the lentivirus particle-containing supernatant on ice. Add 2.5 μl of TransDux to 500 μl of culture medium and then transfer to each well.
-
13
Remove the culture medium from the tumor cells and add the lentiviral particle-containing supernatant (8 μg/ml) mixture and swirl the mix.
-
14
If working with cells that are difficult to transduce, perform a spinoculation step, centrifuging for 90 min at 800×g.
-
15
Day 5: At 72 hr post transduction use fluorescence microscopy or flow cytometry (GFP reporter gene) to determine whether the lentiviral genome has been integrated into the host cell genome.
-
16
GFP+ cells can be sorted prior to engraftment to ensure only GFP+ BMC are engrafted. Alternatively, add 1–10 μg/ml puromycin for selection of stable cells.
-
17
Aspirate medium wash the cells with PBS and then count the cells.
-
18
Inject subcutaneously into the humanized NOG-A2 mouse flank 1–2×106 GFP positive tumor cells with 100μl BD Matrigel ™ basement membrane matrix (BD Biosciences, catalog no. 354262).
-
19
After tumor engraftment, usually 2–4 weeks after injection, the palpable tumor can be measured, typically when the tumor measures > 2×2×1mm3. The TME of the harvested tumor can be imaged. (Fig. 4).
Place fresh tumor tissue onto a pre-labeled tissue mold and cover it entirely with cry-embedding media O.C.T. compound. Slowly place the base mold containing the tissue block into liquid nitrogen until it is completely frozen. Store the frozen tissue at −80 °C until sectioning. Cut the frozen tissue into 5μm thick sections. Place the tissue sections onto glass slides for immunofluorescence staining (Unit 21.4).
Figure 4.
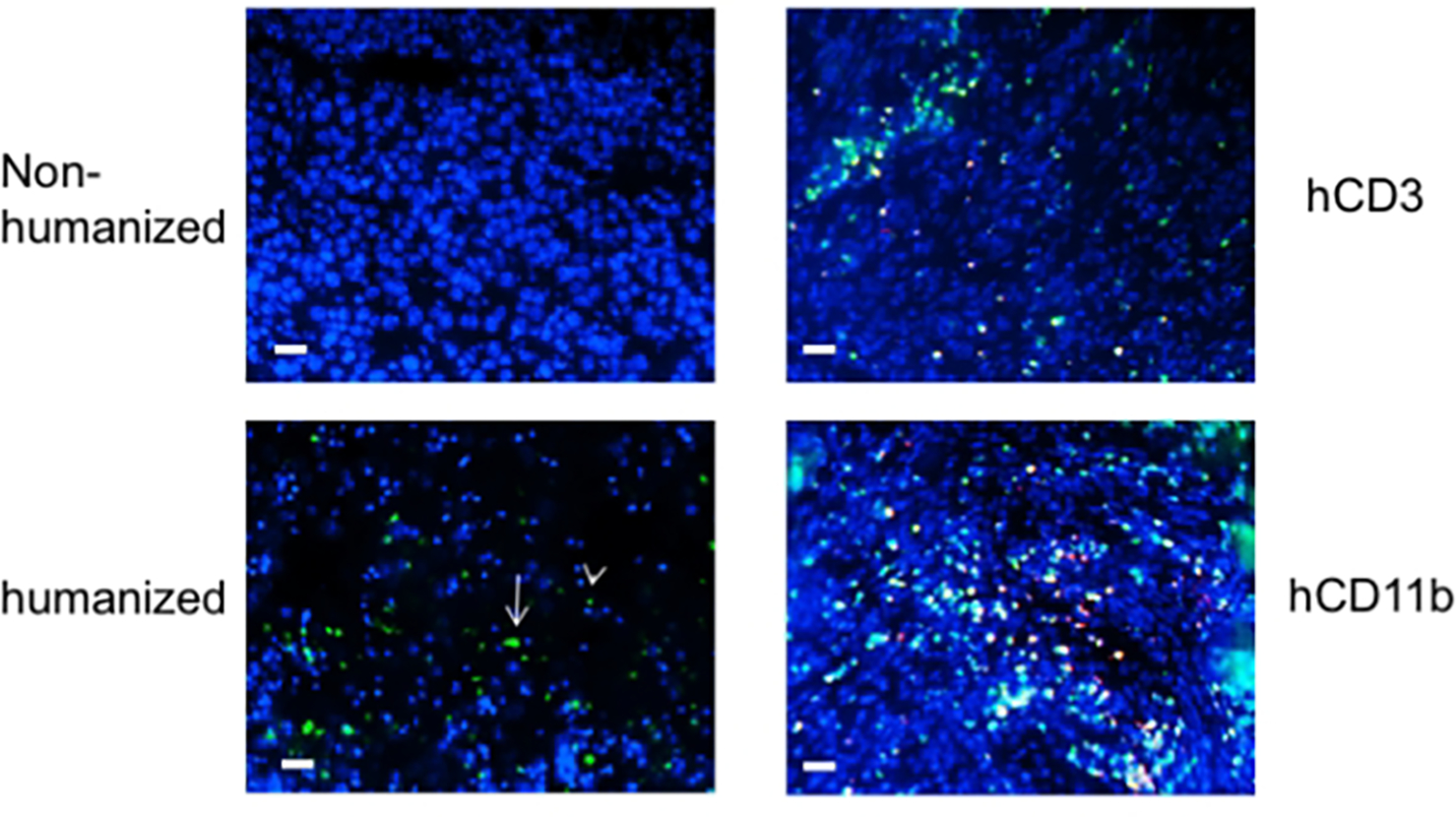
Autologously reconstituted human carcinoma is infiltrated with endogenous human hematopoietic lymphocytes that mature in the thymus. CD34+ BMC were transduced with lentivirus vectors containing GFP protein prior to BMC engraftment. Autologously reconstituted tumor was harvested, stained with DAPI, and immunofluorescent microscopy performed. The left panels show tumor with only DAPI counterstain. The left upper panel is tumor xenografted into non-humanized mice, while the left lower panel is the tumor in autologously humanized mice (white arrowhead – lymphocyte; white arrow – myeloid cells). Slides were co-stained with human CD3 and CD11b conjugates (right panels). The bar scale is 100 μ (from Fu, et.al., 2016).
Transduction of BMC
-
20
Harvest human fibular bone marrow cells or BMC aspirates from iliac crest from HLA-A2+ patients undergoing head and neck cancer surgery.
-
21
Culture the bone marrow cells in a 37° culture incubator with 5% CO2..
-
22
Prepare human pSIH1-Hi-siLuc-copGFP positive plasmid and pPAKH1 packaging plasmid mixture (SBI, Cat# LV500A-1).
-
23
Day 1 - plate 5–10×104 bone marrow cells in a 24 well plate in RPMI 1640.
-
24
Day 2 - add lentivirus (8 μg) to each well and swirl to mix with 2.5 μl TransDux and 500 μl culture medium.
-
25
Day 5 - after 72 hours transduction, check GFP using fluorescence microscopy or flow cytometry to ensure the viral genome is integrated into the host cell genome.
-
26
Remove the medium and wash the cells with PBS and determine the percentage of GFP positive cells.
-
27
Inject i.v. into irradiated mice a total of 0.5–2 × 105 GFP+CD34+ cells in 500 μl PBS per mouse.
Support Protocol 2: Modeling immunotherapeutic agents in autologously humanized model
These autologous humanized mice can be used to model immuno-oncological (IO) agents and treatment strategies in anticipation of clinical trials, much like PDX for targeted therapy development. Allogeneic reactions against the tumor are minimized because the human tumor infiltrating cells are autologous. Hence, immune cell targeting agents used in this autologous setting minimize off-target effects in vivo. Described below is an intratumoral immuno-oncological agent, STING agonist, that can repolarize the myeloid cells towards an immunogenic mediator of anti-tumor response. The IO agent to be used will vary depending the hypothesis being tested and the aim of the study.
MATERIALS:
Mice:
NOD scid gamma, HLA-A2.1 mice: pairs keep breeding, 6–8 weeks, strain designation, NOD. Cg-Mcph1 Tg (HLA-A2.1)1Enge Prkdcscid Il2rgtm1wjl/SzJ, (The Jackson Laboratory, Stock no. 009617)
NOG mice: male and female, 4–8 weeks old, keep breeding, stain designation, NOD.Cg-Prkdcscid Il2rg tmlsug/JicTac, Model no: NOG-F and NOG-M, HSCFTL-NOG homozygous, (Taconic Biosciences)
Antibodies: (conjugated fluorophores)
Anti-human CD3 (clone sp3, Cat no.556611, BD), CD4 (clone SK3, Cat. no.565999, BD), CD8 (clone RPA-T8, Cat. no.565165, BD), CD11c (clone39, Cat. no.748269, BD), CD11b (clone 44, Cat. no. 747357, BD), CD14 (clone M5E2, Cat. no. 746304, BD), CD19 (clone S125C1, no. 566103, BD), CD33 (clone P67.6, Cat. no. 745556, BD), CD34 (clone 9E2, Cat, no. 745556, Biolegend), CD45 (clone 2D1, Cat. no. 368505, Biolegend), CD56 (clone NCAM16.2, Cat. no. 564057, BD), NKP46 (clone 9E2, Cat. no. 743710, BD), HLA-DR (clone G46–6, Cat. no. 745782, BD).
Kits:
Human CD34 microbead and pan T-cell isolation kits (Miltenyi Biotec, catalogue no. 130–046-702)
AmpFISTR Identifier PCR Amplification kit (Applied Biosystems)
Human bone marrow cells (hBMC):
hBMC are harvested from fibular bone marrow cells obtained from cancer patient donors undergoing head and neck cancer resection and reconstruction surgery. BMC can be aspirated from iliac crest from cancer patients during diagnostic surgeries as well.
RPMI medium 1640 (Gibco, 11875119)
150 mm tissue culture dish (TPP, 93150)
Human donor fibular bone marrow cells (see above)
70% (v/v) EtOH (pharmco-AAPER, DSP-CT-18)
Phosphate-buffered saline (PBS, Quality Biological, 114–058-101)
Ice bucket and ice (Hoshizaki ice maker).
Kim Wipe tissue (KimTech)
5 ml syringes (BD)
25G5/8-in needles (BD)
70-micron cell strainer (Corning, 352350)
50 ml falcon tubes (Falcon)
Ficoll/Paque ™ premium 1.084 (GE Healthcare)
Centrifuge (Beckman Coulter, Allegra X-14R)
DNAse I (Roche) and Liberase Blendzyme 2 (20,000 Mandl U/ml) (Roche)
MACS Buffer (Miltenyi Biotec 130-091-376)
LS Column (Miltenyi Biotec 130-042-401)
QuadroMACS Separator (Miltenyi Biotec 130-091-051)
Trimethoprim/Sulfamethoxazole (TMS, Aurobindo USA, Inc, catalogue no. NDC65862-496-47)
BD Matrigel ™ basement membrane matrix (BD Bioscience 356234)
Tissue-Tek cryomold (Sakura, 4557)
Tissue-Tek O.C.T. compound (Sakura, 4583)
Sterile surgical instruments: forceps, scissors
Protocol Steps:
Ensure BMC engraftment using FACS analysis on the peripheral blood from the chimeric 6–8 weeks after engraftment (Fig. 2) (Basic Protocol 1, step 26).
Once it is confirmed the mice engrafted with their matched BMC to have CD4+ and CD8+ T-cells in the peripheral blood, the matched tumor xenoplant can be implanted subcutaneously into the matched humanized NOG-A2 mice.
Shave the fur on the flank 1 day prior to injecting tumor cells.
Inject subcutaneously into the flank of humanized NOG-A2 mice 5–10×104 tumor cells with 100 μl BD Matrigel ™ basement membrane matrix.
Begin treatment on day 7–10 after cell injection when the tumors are palpable at ~50 mm3 in volume. Refer to Wang, et.al. who used pembroluzimab for immunotherapeutic modeling. The autologous model described in the present unit can be used for intratumoral interventions. As an example, 20 μg STING (stimulator of interferon genes) agonist (Fu, et al. 2015) can be injected intratumorally in 50 μl PBS per mouse, twice per week (Corrales, et.al., 2015; Fu, et.al., 2015).
Measure the tumor volume daily until the control tumor is 2 cm in diameter (Fig 5).
Figure 5.
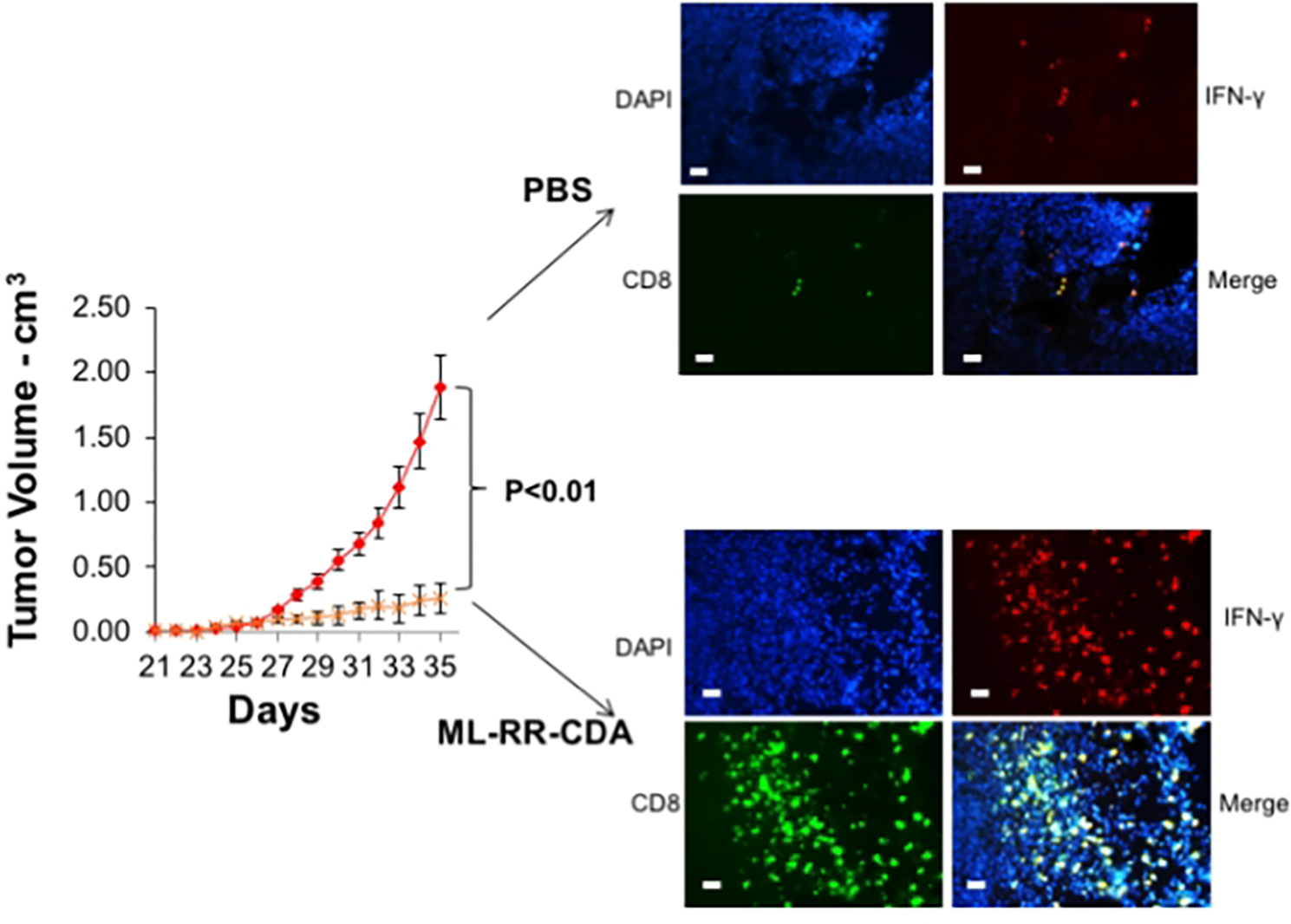
Intratumoral STING agonist modeling in autologous humanized mice. Palpable tumors of equivalent size in both groups were treated intratumorally with either PBS control or ML-RR-CDA (STING agonist) at 20 μg per injection and the tumor growth monitored. The experiment was repeated twice with 4–5 mice per group. At the completion of experiment the tumor was harvested and stained with human CD8, IFNγ, and DAPI counterstain (right panels). The bar scale is 100 μ (adopted from Fu, et.al., 2016).
Commentary
Background Information
The translational limitations of GEMM have been reviewed elsewhere (Gould, et.al., 2015). While patient derived xenograft (PDX) models in immunodeficient mice may recapitulate some of the genetic heterogeneity of human tumor cells (Stewart, et.al., 2015; Cassidy, et.al., 2015), they cannot be used to study their immunological context, which is important for in vivo preclinical characterization of potential immunotherapeutic agents (Cohen, et.al., 2015). Syngeneic GEMM also have limitations for preclinical testing of immunomodulating agents because some of the phenotypes derived from genetic manipulations may minimize the stromal and immunological context of the autochthonous tumor (Gould, et.al., 2015). Although xenografts into humanized models are used to study the potential responses to immune checkpoint inhibitors (Wang, et.al., 2018), an important limitation is the unavoidable allogeneic responses that may confound the anti-tumor responses in these mice. While autologous adoptive transfer of T cells has been reported, this method does not allow for endogenous immune infiltration into the developing tumor (Yokota, et.al., 2013). The autologous system developed in our laboratory and described in this unit alleviates many of these problems, thereby providing in vivo method for studying endogenous lymphocytic and myeloid cell trafficking into the human tumor.
Like all the humanized mouse models, there are limitations to the model described in this report. One is that the mature naïve T-cells are educated either on the murine MHC or the human MHC transgene in the IL2rγnull recipient mice. Common human HLA transgenes in NSG or NOG can be purchased commercially. Other investigators have used adenoviral vector to transduce human HLA genes prior to the BMC engraftment (Huang, et.al., 2014). Secondly, while many of the minor subsets of endogenous human immune cells, for example, CD123+ plasmacytoid dendritic cells, can be detected, these humanized mice do not have the human cytokine profile to promote, maintain, and/or homeostatically differentiate some of these minor immune cell subsets. For example, uneven development of NK cells is observed in these humanized mice. This limitation has been offset with IL-15 injections. Likewise, investigators focused on tumor associated macrophages or Th17-mediated responses obtained encouraging results when the recipient humanized mice overexpressed human IL-6 (Hanazawa, et.al. 2018)(Yu, et.al. 2017). Lastly, secondary lymphoid structure development in all humanized models is limited (Rongvaux, 2013). Work is ongoing to increase relevant human chemokines and cytokines to (e.g. CXCL13, TSLP) offset these deficiencies in humanized murine systems (Li, 2018).
Using the autologous model described in this unit, we were able to genetically modify both the tumor cells and donor BMC to study how different signaling pathways in both compartments regulate each other (Fu, et.al., 2016). Rather than relying on correlative relationships from specimens, it is possible to directly test specific immune-oncologic signaling pathways in the human TME in vivo to complement mechanistic studies on genetically engineered murine tumor systems (Shuvalov, et.al., 2015). Because this system is completely autologous, any in vivo effect can be attributed to the experimental interventions rather than the allogeneic response inherent in non-matched humanized models. The results, however, must be interpreted with the caveats and limitations noted above. This protocol can also be used to develop rare human tumor models that do not have corresponding syngeneic murine models to enable the preclinical in vivo evaluation of immune-oncologic agents.
Critical Parameters
Several considerations are important when using these humanized murine models. One is the source of BMC. After poor engraftments of HSC from the fibula of those who were older than 70 years of age, we now use primarily BMC obtained from those under 65 years old. While most of the surgical BMC employed for these studies are from fibula, some are harvested from scapula or radial bones used for reconstruction of the primary defect in the head and neck anatomic region. To accommodate tumor types that do not frequently require donor bones for cancer treatment, a procedure has been designed to use BMC harvested from the iliac crest aspiration during the time of diagnostic surgical procedures. The protocols for these aspirations are commonly performed by board certified hematologists at medical institutions. Typical BMC cellularity range from 1–10 ×108 from for the long bones, to 0.1–2×108 per patient for the ileac crest. The BMC value dictates the number of recipient mice that can be humanized. As noted in the protocol, transferring in less than 5×104 CD34+ BMC will result in poor engraftments, and the lethally irradiated mice will undergo blast crisis within 1–2 weeks of BMC injection. Because obtaining BMC (surgical and aspirate) are typically not considered standard of care for the management of solid tumors, this protocol must receive IRB approved prior to creating the reconstitution models. Coordination between acquisition of BMC and tumor tissue from the patients and the benchwork of engraftment must be planned carefully because the reconstitution time requires over 6 months.
Another critical feature of the humanized platform is the recipient mice. This topic is important as it underlies the limitations of the humanize mouse model. That is, the murine immune system of the NSG and NOG background (e.g., cytokines) cannot fully reconstitute and maintain the full human lymphocytic and myeloid repertoire. Some of the minor effector cell subsets such as NK cells or Th17 subsets may require transgenic expression of relevant human cytokines (IL-15, IL-6) in the recipient mice to demonstrate their functional activity (Rongvaux, 2013). Improved engraftment of the human hematopoietic cells, particularly to develop mature myeloid cells, was reported with MISTRG mice that “knocked-in” human M-CSF, IL-3, GM-CSF, SIRPα, and TPO in the Rag2−/−IL2rγnull recipient mice (Rongvaux, 2014). Unfortunately, this strain has been discontinued at Jackson Lab. While we were successful in obtaining greater than 70% chimerism without these modified NSG/NOG mice strains, the adoption of some of the specialized recipient mice may be important for any given experiment.
An additional critical parameter is the engraftment of the tumor tissue in the humanized host. Unlike frozen BMC that can maintain their ability to engraft into Rag2−/− IL2rγnull or SCID IL2rγnull recipient mice, successful PDX engraftment requires freshly processed tumor cells be transplanted into mice for the first generation of PDX. Viable xenoplants may then be frozen for further passaging into recipient mice. However, if the selected tumor type has low engraftment rate as a PDX this approach will yield variable results. Tumor “take” rates can be increased consistently when the recipient mice are humanized with allogeneic CD34+ BMC or cord blood.
Understanding Results and Troubleshooting
Typically, the rate of chimerism of the hematopoietic system ranges from 70–90% as defined by human CD45 levels with respect to total mononuclear cell from the spleen (Fig 2). Depending on the needs of the investigator, these global chimerism rates may or may not be sufficient. As noted above, some of the minor subsets of immune cells, such as Th17, may require that the recipient mouse express a human transgene. While BMC engraftments with cord blood is limited by the available abundance of CD34+ cells (104–5 per mouse), the greater abundance of fibular BMC allows the transfer of 2×105 CD34+ cells (per mouse) to yield engraftment rates comparable to those with cord blood (Fu, et.al. 2016; Scholbach J, et.al., 2012). Unlike the implantation of PDX into non-humanized mice, most, if not all, of the mice display human HNSCC tissue growing in subcutaneous tissue for the analysis. Therefore, the “rate-limiting step” of this autologous reconstitution is the engraftment of the BMC.
To ensure that the tumor infiltrating human T-cells is derived from the engrafted BMC hematopoietic cells and not from the tumor infiltrating lymphocytes (TIL) from the PDX tumor tissue, we labeled the human CD34+ BMC with lentiviral vectors that expressed GFP proteins. Once the engrafted lymphocyte progenitors were found to produce GFP positive lymphocytes in the peripheral blood (data not shown), autologous tumor was implanted, with the immunofluorescent microscopy clearly showing GFP+ immune cells in the TME with both lymphocytic and myeloid histology (Fig 4a – left lower panel). Immunohistochemical staining with human CD3 and CD11b conjugates co-localized to these GFP positive cells confirmed the presence of both human lymphoid and myeloid cells in the TME (Fig 4a – right panels). While these experiments demonstrate that a significant proportion of T cells in the TME are derived from the engrafted BMC, they do not formally distinguish which portion is from homeostatic expansion of mature T cells in the bone marrow vs. differentiation of CD34+ progenitor cells. When the thymus from these mice was examined, thymocytes from these engrafted animals were predominantly double positive GFP+ human T-cells, suggesting that de novo development of human T cells in the murine thymus with human HLA-A2 expression was operative (Fu, et.al., 2016). Lastly, the transplanted PDX tumor and the human leukocytes from peripheral blood of these autologously humanized mice can be HLA typed to ensure a complete match.
Time considerations
These experiments can take over 4–6 months to complete. Each of the engraftment steps, for both BMC and for PDX tumor tissue, can require up to 3 months to complete. Preliminary experiments should be performed on the engraftment times for the tumor types and the BMC prior to undertaking reconstitution.
Statistics
A paired t-test is used to calculate two-tailed p values to estimate the statistical significance of differences between two treatment groups using Excel software. Error bars are standard error of means, with P values shown on the figures. Kaplan–Meier curves are generated using GraphPad Prism software and analyzed with a log-rank test.
Acknowledgments
YJK is supported by Barry and Amy Baker Endowment, NIH R01 CA178613, R01 DE027749, Dept. of Defense CDMRP Breakthrough Award, Rowen fund.
References
- Alizadeh AA, Aranda V, Bardelli A, Blanpain C, Bock C, Borowski C, Caldas C, Califano A, Doherty M, Elsner M, Esteller M, Fitzgerald R, Korbel JO, et al. Toward understanding and exploiting tumor heterogeneity. Nat Med. 2015; 21:846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou V, Yarchoan M, Hansen A, Wang H, Verde F, Sharon E, Collyar D, Chow L, Forde P. Immuno-oncology Trial Endpoints: Capturing Clinically Meaningful Activity. Clin Can Res. 2017; 23: 4959–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012; 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy JW, Caldas C, Bruna A. Maintaining Tumor Heterogeneity in Patient-Derived Tumor Xenografts. Cancer Res. 2015; 75: 2963–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Sznol M. Therapeutic combinations of immune-modulating antibodies in melanoma and beyond. Semin Oncol. 2015; 42: 488–494. [DOI] [PubMed] [Google Scholar]
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW Jr, Gajewski TF. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015; 11: 1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, Sivick KE, Zeng Q, Soares KC, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015; 7: 283ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Sen R, Masica D, Karchin R, Pardoll D, Walter V, Hayes D, Chung C, Kim YJ. Autologous reconstitution of human cancer and immune system in vivo. Oncotarget. 8: 2053–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SE, Junttila MR, de Sauvage FJ. Translational value of mouse models in oncology drug development. Nat Med. 2015; 21: 431–439. [DOI] [PubMed] [Google Scholar]
- Hanazawa A, Ito R, Katano I, Kawai K, Goto M, Suemizu H, Kawakami Y, Ito M, Takahasi T. Generation of human immunosuppressive myeloid cell population in human IL-6 transgenic NOG mice. Front Immunol. 9:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li X, Coelho-dos-Reis JG, Wilson JM, Tsuji M. An AAV vector mediated gene delivery approach facilitates reconstitution of functional human CD8+ Tcells in mice. PLoS One. 9:e88205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Kobayashi K, Nakahata T. NOD/Shi-scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models. Curr Top Microbiol Immunol. 2008; 324: 53–76. [DOI] [PubMed] [Google Scholar]
- Kalscheuer H, Danzl N, Onoe T, Faust T, Winchester R, Goland R, Greenberg E, Spitzer TR, Savage DG, Tahara H, Choi G, Yang YG, Sykes M. A model for personalized in vivo analysis of human immune responsiveness. Sci Transl Med. 2012; 4, 125ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006; 108: 487–492. [DOI] [PubMed] [Google Scholar]
- Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015; 373: 23–34. [DOI] [PubMed] [Google Scholar]
- Legrand N, Ploss A, Balling R, Becker PD, Borsotti C, Brezillon N, Debarry J, de Jong Y, Deng H, Di Santo JP, Eisenbarth S, Eynon E, Flavell RA, et al. Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host Microbe. 2009; 6: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Masse-Ranson G, Garcia Z, Bruel T, Kok A, Strick-Marchand H, Jouvion G, Serafini N, Lim AI, Dusseaux M, Hieu T, Bourgade F, Toubert A, Finke D, Schwartz O, Bousso P, Mouquet H, Di Santo JP. A human immune system model with robust lymph node development. Nat Methods. 15: 623–630. [DOI] [PubMed] [Google Scholar]
- Morton J, Bird G, Keysar SB, Astling D, Lyons T, et al. XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient derived xenograft model of head and neck cancer. Oncogene. 35: 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A, Takizawa H, Strowig T, Willinger T, Eynon EE, Flavell RA, Manz MG. Human hemato-lymphoid system mice: current use and future potential for medicine. Ann Rev Immunol. 31: 635–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, Manz GA, Flavell RA. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 32: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholbach J, Schulz A, Westphal F, Egger D, Wege AK, Patties I, Köberle M, Sack U, Lange F. Comparison of hematopoietic stem cells derived from fresh and cryopreserved whole cord blood in the generation of humanized mice. PLoS One. 2012; 7(10), e46772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serreze DV, Niens M, Kulik J, Dilorenzo TP. Bridging mice to men: using HLA transgenic mice to enhance the future prediction and prevention of autoimmune type 1 diabetes in humans. Methods Mol Biol. 2010; 602: 119–134. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007; 7: 118–130. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Goodwin N, Ishikawa F, Hosur V, Lyons BL, Greiner DL. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc. 2014; 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, Doi T, Sone A, Suzuki N, Fujiwara H, Yasukawa M, Ishikawa F. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci USA. 2010; 107:13022–13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvalov O, Petukhov A, Daks A, Fedorova O, Ermakov A, Melino G, Barlev NA. Current Genome Editing Tools in Gene Therapy: New Approaches to Treat Cancer. Curr Gene Ther. 2015; 15: 511–529. [DOI] [PubMed] [Google Scholar]
- Singh M, Murriel CL, Johnson L. Genetically engineered mouse models: closing the gap between preclinical data and trial outcomes. Cancer Res. 2012; 72: 2695–2700. [DOI] [PubMed] [Google Scholar]
- Stewart EL, Mascaux C, Pham NA, Sakashita S, Sykes J, Kim L, Yanagawa N, Allo G, Ishizawa K, Wang D, Zhu CQ, Li M, Ng C, et al. Clinical Utility of Patient-Derived Xenografts to Determine Biomarkers of Prognosis and Map Resistance Pathways in EGFR-Mutant Lung Adenocarcinoma. J Clin Oncol. 2015; 33: 2472–2480. [DOI] [PubMed] [Google Scholar]
- Wang M, Yao LC, Cheng M, Cai D, Martinek J, Pan CX, Shi W, Ma AH, De Vere White RW, Airhart S, Liu ET, Banchereau J, Brehm MA, Greiner DL, Shultz LD, Palucka K, Keck JG. Humanizing mice in studying efficacy and mechanisms of PD-1 targeted cancer immunotherapy. FASEB J. 32: 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota SJ, Facciponte JG, Kelleher RJ Jr, Shultz LD, Loyall JL, Parsons RR, Odunsi K, Frelinger JG, Lord EM, Gerber SA, Balu-Iyer SV, Bankert RB. Changes in ovarian tumor cell number, tumor vasculature, and T cell function monitored in vivo using a novel xenograft model. Cancer Immun. 2013; 13: 11. [PMC free article] [PubMed] [Google Scholar]
- Yu H, Borsotti C, Schickel JN, Zhu S, Strowig T, Eynon EE, Frleta D, Gurer C, Murphy AJ, Yancopoulos GD, Meffre E, Manz MG, Flavell RA. A novel humanized mouse model with significant improvement of class switched antigen specific antibody production. Blood. 129: 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, David BK, Meredith L, et al. STING agoinst formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Science Translation Medicine. 15 Apr 2015, vol 7, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]


