Abstract
Sleep disruption (SD) promotes stress which may mediate the effect of SD induced by noise on bodyweight gain and food intake. We determined if the change in bodyweight during SD caused by noise was driven by stress (assessed by corticosterone) and whether the effects of noise on SD, stress and bodyweight were specific to the method of SD or a consequence of SD per se. We isolated stress from SD due to noise by exposing rats to noise during the darkphase to test whether darkphase noise stimulated weight gain, stress and food intake. Male Sprague-Dawley rats slept undisturbed, were exposed to noise during both circadian phases (lightphase vs. darkphase) and lightphase gentle handling. Bodyweight, food intake, physical activity, vigilance states, and plasma corticosterone were determined. Darkphase noise did not affect vigilance states. Unlike lightphase noise, darkphase noise and lightphase gentle handling did not stimulate weight gain or food intake. Only gentle handling significantly increased corticosterone levels. Noise during the lightphase increasesed weight gain and food intake by causing SD and these effects were not driven by stress assessed by corticosterone. These results may have significant implications for developing translational models of insomnia-induced obesity in humans.
Keywords: arousal, brain, obesity, insomnia, sleep disruption
INTRODUCTION
Noise pollution, a consequence of urban environments, causes sleep disruption (SD) [5] and increases the risk for obesity [9, 13]. However, mechanisms linking SD and obesity remain uncertain. SD is a stressor that modulates the hypothalamic-pituitary-adrenal axis, since SD increases corticosterone and alters the sympatho-adrenal system [1, 14, 24, 29, 30]. While the relationship between stress and bodyweight is well-documented [23, 28], the contribution of stress to the change in bodyweight during SD remains unresolved as there are conflicting reports about the effect of SD on bodyweight [2, 3, 8, 12, 17, 18, 22, 26, 31].
Separating the relationship between stress, SD and bodyweight is not straightforward. First, animal models of SD require external stimuli to disrupt sleep (water, locomotion, gentle handling, noise, etc.). Thus, it’s difficult to know if SD itself or the stimuli causing SD drives changes in stress and bodyweight. Second, although stress contributes to physiological outcomes during forced wakefulness [19, 29], SD has bi-directional effects on stress biomarkers [7, 30]. Therefore, it remains unknown whether the stress responses observed in rodents subjected to experimental SD are a direct consequence of SD alone, the stress induced by the stimuli used to cause SD or are driven by the effects of SD [19, 20, 24, 27]. Overall, stress is inherent to SD regardless of whether stress is driven by SD or vice versa [27]. Furthermore, the role of stress in some methods that cause SD, including noise exposure, remains untested
Here we sought to separate the effects of SD on stress and bodyweight. We used a validated and translational method of SD that stimulates weight gain and food intake (pre-recorded noise exposure during the lightphase when rats sleep [8, 18, 22]). Because SD and noise are stressors [15] it’s unclear whether SD or stress is the physiological mechanism underlying the change in bodyweight in SD studies. The primary goal was to determine if the change in bodyweight was driven by stress during SD caused by lightphase noise. First, we exposed rats to noise during the darkphase, a time-period when rats spend more time awake. Previous studies have used this strategy (i.e., SD administered during the darkphase) to separate sleep and stress by minimizing changes in sleep caused by administration of the same SD method during the lightphase [20, 21]. Thus, we tested whether darkphase noise stimulated weight gain, food intake and stress. Our secondary goal was to determine if these effects were specific to the method of SD. We compared the effect of two SD methods (noise versus gentle handling) and tested whether lightphase gentle handling, a common SD method in rodents [2], affected weight gain and stress in male rats as reported after noise [8, 18, 22]. Plasma corticosterone was measured as an indicator of stress. Based on the reported effects of SD administered during the darkphase [20, 21], we anticipated that in contrast to less sleep and increased weight gain and hyperphagia during lightphase noise [8, 18]: 1) darkphase noise would not reduce sleep or stimulate weight gain and food intake and 2) SD due to lightphase gentle handling would not stimulate weight gain or food intake but would increase corticosterone and physical activity (PA).
MATERIALS AND METHODS
Animals
Three-month old male Sprague-Dawley rats (N=56, Charles River Laboratories, Kingston, NY) were housed individually on a perforated plexiglass floor (21–22 °C, 12h lightphase (inactive)/12h darkphase (active), lights-on/lights-off at 0600h/1800h). Rodent chow (Harlan Teklad 8604) and water (Hydropac) were available ad libitum except when food was withheld for 4h (0700–1100h) prior to euthanasia (1100h) to avoid the potential confound of recent food intake on endpoints measured at euthanasia (studies 2–3). All procedures were approved by the Institutional Animal Care and Use Committees (Minneapolis VA Health Care System and University of Arizona), were performed in accordance with the ARRIVE guidelines and the National Institutes of Health guidelines on the use of animals in research.
Surgery
Anesthetized rats (ketamine and xylazine, 75 and 7mg/kg i.p., respectively) were surgically implanted with a radiotelemetric transmitter connected to EEG and EMG leads (HDS-02, DSI, Saint Paul, MN) to determine vigilance states [22]. All efforts were made to minimize trauma during the surgical procedure. Experiments began 10d post-surgery.
Vigilance state determination
Cumulative time spent in each vigilance state [waketime, non-rapid eye movement sleep (NREMStime) and REMStime], episodes (wakeepisodes, NREMSepisodes, REMSepisodes), duration of episodes (wakeduration, NREMSduration, REMSduration) and transitions between vigilance states were determined from manually scored 15-sec. epochs of EEG and EMG [22].
Concurrent determination of PA and vigilance states.
EEG and EMG were recorded by radiotelemetry concurrently with PA registered by infrared sensors (Sable Systems International Inc. Las Vegas, NV) [8, 22].
Sleep disruption by noise exposure
After completing measurements for bodyweight and food intake, rats were exposed to noise (8h/d) that began at 0600h (lightphase) or 1800h (darkphase) by placing two speakers in front of the animals’ housing enclosures. Lightphase noise has been previously validated to reduce sleep [8, 18, 22].
Sleep disruption by gentle handling
After completing measurements for bodyweight and food intake, rats were subjected to gentle handling (8h/d) that began at 0600h (lightphase). Briefly, rats were gently touched with a flexible clear tube when they exhibited any indication of sleep or sleepiness (e.g., eyes closed or a sleep-like posture). Gentle handling has been validated to cause SD [2, 25]. We confirmed that the clear tube did not interrupt the infrared beams used to measure PA.
Corticosterone ELISA
Plasma was obtained from blood collected during euthanasia (1100–1200h) by decapitation from rats (studies 2–3). Corticosterone was determined in duplicate by ELISA following the manufacturer’s instructions (Invitrogen).
Experimental Design
Study 1. Effect of darkphase noise on vigilance states:
Male rats (N=9) were instrumented with EEG/EMG electrodes as stated above. Bodyweight and food weight were measured 30 min. before the darkphase began. Then EEG, EMG, and PA were determined for 8h in the absence (control) or presence of darkphase noise. Bodyweight, food intake (corrected for uneaten food including food in the rodent’s feeder and uneaten food beneath the perforated floor) were determined 24h after each test began to determine change in bodyweight and food intake. 96h after the first treatment, these procedures were repeated in a repeated measures design (Fig. 1A).
Figure 1. In male rats, darkphase noise did not affect time in vigilance states and failed to increase weight gain or food intake. This contrasts with the effects of lightphase noise.
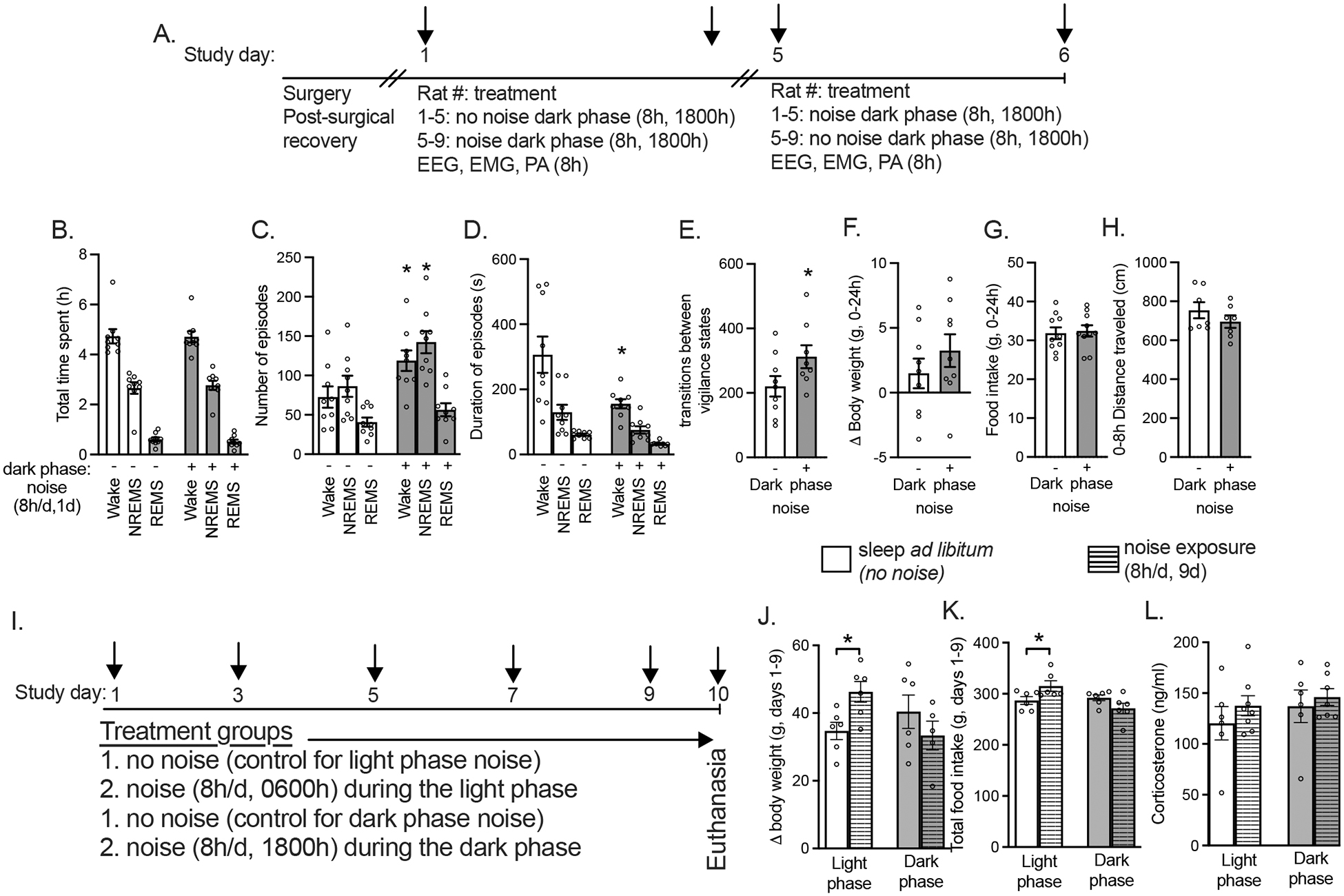
(A and I) Experimental designs. Time spent in (B) wake, non-rapid eye movement sleep (NREMS), and REMS, (C) episodes and (D) duration of each episode, (E) transitions between vigilance states, (F-G) cumulative weight gain and food intake during the 24h period after the test began and (H) physical activity in the presence (+) or absence (-) of darkphase noise (8h/d, 1d, 1800–2600h) in male rats. Cumulative (J-K) weight gain and food intake during the 9d treatment, and (l) plasma corticosterone levels in male rats that slept undisturbed (no noise) or were exposed to noise (8h/d) during either the lightphase or darkphase for 9-days. Panel A and I: arrows indicate bodyweight and food intake measurements prior to beginning treatments (no noise or noise) at either 0600 or 1800h. *P<0.05 versus absence of darkphase noise (panels D) or control (Panels J-K). Data expressed as mean±SEM. N=9 (A-H) and n=6/group (I-L).
Study 2. Effect of light versus darkphase noise on bodyweight:
Male rats were randomized (n=6/group) to sleep undisturbed (control for lightphase noise) or were exposed to lightphase noise (8h/d) for 9-days (Fig.1I). A separate cohort of male rats was randomized (n=6/group) to sleep undisturbed (control for darkphase noise and to account for variation in weight gain between set of rats) or darkphase noise (8h/d) for 9-days.
Study 3. Effect of two methods of SD on vigilance and bodyweight:
Another cohort of male rats (n=7–8/group) was randomized to sleep undisturbed (control), gentle handling (8h/d, lightphase) or noise (8h/d, lightphase) for 9-days (Fig. 2A). PA, EEG and EMG were recorded for 24h before and on the final day of the treatment.
Figure 2. In male rats, sleep disruption caused by lightphase gentle handling elicits greater stress than SD induced by lightphase noise and gentle handling does not increase weight gain.
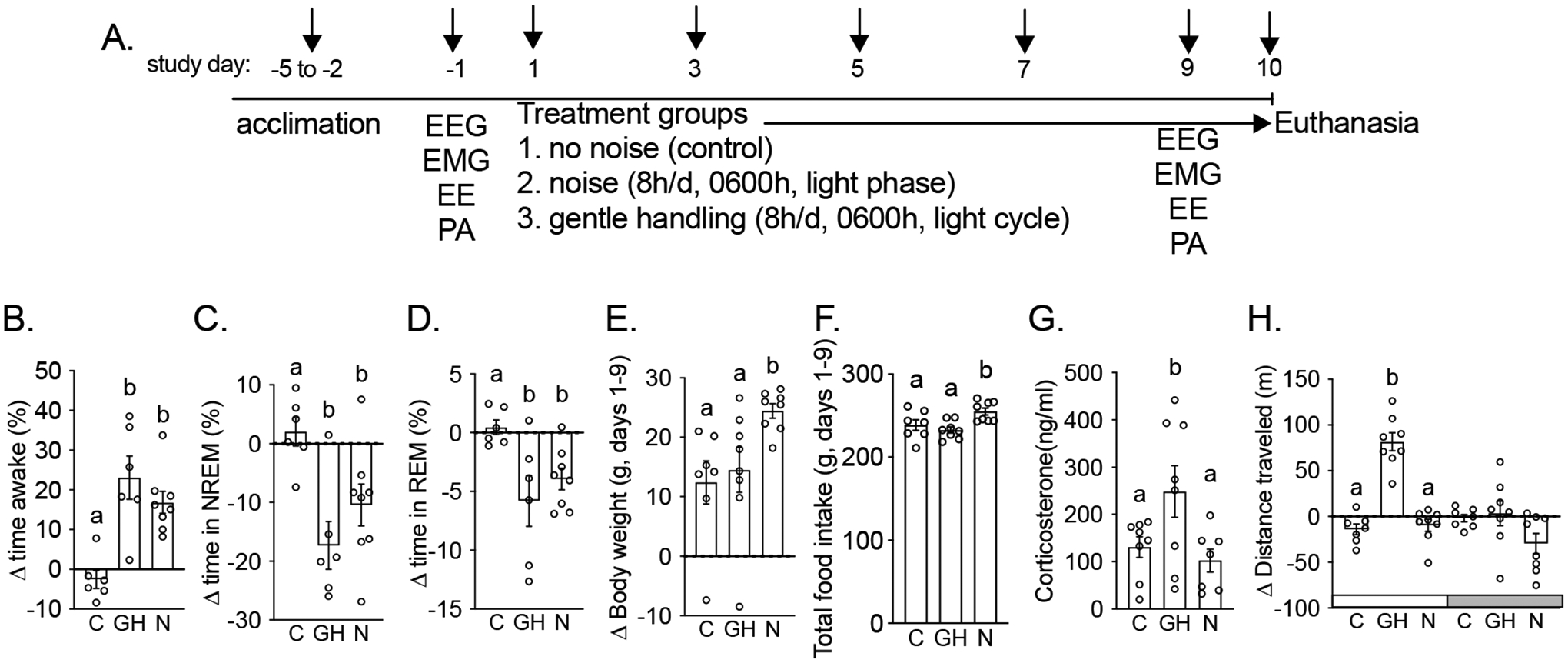
(A) Experimental design. Change in time spent (B) awake or asleep [C-D: non-rapid eye movement (NREM) sleep and REM sleep], (E-F) cumulative weight gain and food intake during the 9d treatment, (G) plasma corticosterone, and (H) change in physical activity in male rats that slept undisturbed (control (C)) or had sleep disruption (8h/d during the lightphase) for 9-days due to gentle handling (GH) or noise (N). Panel A: arrows indicate bodyweight and food intake measurements prior to beginning treatments at 0600h. Different letters indicate treatments were significantly different (P < 0.05). Data expressed as mean±SEM. n=7–8/group.
For studies 2–3, bodyweight and food intake (corrected for uneaten food) were measured every 48h during the treatment (30 min. before noise began). Rats were euthanized (1100h) and plasma corticosterone was measured.
Data analysis
Alpha was 0.05, normality was verified with the Shapiro-Wilk test and t-tests were two-tailed. Data were analyzed by paired-tests (study 1), two-factor ANOVA (study 2) or ANOVA (study 3) followed by post-hoc t-tests corrected for the false discovery rate (two-stage step-up method of Benjamini, Krieger and Yekutieli) [6] when the higher order terms were significant (Prism 8.01, GraphPad, Prism). The independent variables were noise (presence, absence) and time-period (lightphase, darkphase) for study 2 and treatment (control, gentle handling or noise) for study 3. The primary outcomes were time in vigilance states (study 1) and weight gain (studies 2–3). The secondary outcomes were change in bodyweight (study 1) and food intake, PA and stress assessed by corticosterone (studies 2–3). Sample sizes were based on prior studies [18, 22].
RESULTS
Noise during the rodent’s active period (darkphase) does not reduce sleep or affect weight gain.
We first tested whether darkphase noise affected vigilance states. As expected, darkphase noise (8h/1d) did not significantly alter waketime or sleeptime. (Fig. 1B). The lack of effect of darkphase noise on time in vigilance states was due to significantly more wakeepisodes and NREMSepisodes that were significantly shorter in duration, which together led to more transitions between vigilance states (Fig. 1B–E) during darkphase noise. Weight gain, food intake and PA were not significantly different in the presence or absence of darkphase noise (Fig. 1F–H). Together, these data confirm that darkphase noise during the rodent’s active period does not alter vigilance states or weight gain.
Unlike lightphase noise, 9-days of noise during the darkphase failed to stimulate weight gain and food intake despite a similar stress response assessed by corticosterone
We next tested the hypothesis that unlike 9-days of lightphase noise [8, 18], 9-days of darkphase noise would not stimulate weight gain or food intake and that stress, assessed by corticosterone levels, would be similar between rats exposed to lightphase and darkphase noise. Lightphase noise significantly increased weight gain and food intake compared to its control group (no noise lightphase, Fig. 1J–K). In contrast, darkphase noise had no significant effect on weight gain or food intake compared to its control group (no noise darkphase, Fig. 1J–K). The main effects and interaction were not significant for corticosterone levels (Fig. 1L). Together, these data show that 9-days of noise during the rodent’s inactive period (lightphase) stimulates weight gain and food intake.
SD induced by noise during the lightphase is less stressful than SD induced by gentle handling and only noise increases weight gain
Finally, we tested whether the method used to cause SD during the lightphase influenced weight gain and whether this effect was due to altered food intake, PA and stress assessed by corticosterone levels. Since gentle handling had no effect on weight gain [2, 26], we hypothesized that in contrast to lightphase noise [8, 18], lightphase gentle handling would not stimulate weight gain or food intake and would increase PA and corticosterone [16, 27], compared to both the control and noise groups.
Both SD methods (lightphase noise and gentle handling) caused SD as they significantly increased waketime and reduced NREMStime and REMStime compared to the control group (Fig. 2B–D). However, lightphase noise significantly increased weight gain and food intake compared to both gentle handling and control (Fig. 2E–F). Finally, gentle handling significantly increased corticosterone and lightphase PA compared to both the noise and control groups (Fig. 2F–G).
DISCUSSION
Different experimental methods that cause SD in rodents have contradictory effects on bodyweight and food intake [2, 3, 8, 12, 17, 18, 22, 26, 31]. Still it’s unknown whether these results are due to SD alone, the stress associated with SD, or the approach used to cause SD per se [19]. We reported that SD induced by lightphase noise increased weight gain and food intake [8, 18, 22]. To determine whether these effects were due to SD or the stress associated with the noise, here we compared the effect of noise during the lightphase or darkphase (as a way of controlling for the stress during SD) on bodyweight and food intake in male rats. We also determined the contribution of the method of SD on these endpoints by comparing noise to gentle handling. Noise during the lightphase is a validated method od SD, but after showing that noise during the darkphase did not change vigilance states, we report two primary results. First, unlike lightphase noise, darkphase noise and lightphase gentle handling failed to stimulate weight gain or food intake. Second, despite similar corticosterone levels between lightphase and darkphase noise, corticosterone was greater during lightphase gentle handling compared to lightphase noise. Together, these results suggest that stress assessed by corticosterone was not the primary factor contributing to weight gain and food intake during SD induced by lightphase noise.
We aimed to control for stress associated with the reduction in sleeptime during SD induced by noise by exposing rats to darkphase noise. We reasoned that darkphase noise was an appropriate control for two reasons. First, nocturnal rodents spend more awake and active during the darkphase. Second, others have controlled for stress during SD by exposing rodents to SD methods during the darkphase [20, 21]. Those studies showed that while lightphase gentle handling reduced sleep, increased markers of the unfolded protein response [20] and impaired cognition; darkphase gentle handling ameliorated impaired cognition [21] and markers of the unfolded protein response [20]. In contrast to the reduction in sleeptime and increase in waketime during 9-days of lightphase noise [8, 22], here we show that darkphase noise (8h/1d) failed to increase waketime or reduce sleeptime. These data support using darkphase noise as a control intervention to test the primary hypothesis.
We reasoned that if stress rather than the SD due to lightphase noise was the primary factor contributing to greater weight gain and food intake during SD induced by noise [8, 18], then darkphase noise should also stimulate weight gain and food intake compared to undisturbed sleep. In contrast, if SD rather than stress was the primary factor stimulating elevated weight gain and food intake during lightphase noise, then darkphase noise would not stimulate weight gain and food intake when compared to undisturbed sleep. Consistent with prior studies [8, 18], we show that lightphase noise stimulated weight gain and food intake while darkphase noise did not. This occurred despite the similar corticosterone levels during both lightphase and darkohase noise. Thus, these data demonstrate that male rats exposed to darkphase noise failed to significantly gain weight or eat more despite having a similar physiological stress response as assessed by a single measurement of plasma corticosterone when compared to male rats exposed to lightphase noise. Hence, we conclude that SD rather than stress, assessed by corticosterone, was the primary factor promoting elevated weight gain and food intake during SD induced by lightphase noise.
We acknowledge the possibility that 9-days of darkphase noise was insufficient to change bodyweight. Also, habilitation to chronic stress occurs [4], which underscores the importance of measuring multiple indicators of stress (i.e., glucocorticoids, catecholamines, etc.). before and multiple times during a stress response and testing conclusions about stress in different models [11]. The similar corticosterone levels between the control and lightphase noise groups reported here could be due to habituation in response to chronic stress among the rats exposed to lightphase noise. Moreover, our conclusions require testing and validation in female rats to explore potential sex differences.
We measured corticosterone as a marker of stress since experimental SD increases corticosterone [1, 2, 19, 24, 27, 29, 30]. Corticosterone was measured once to avoid repeated blood sampling and to reduce the stress associated with non-specific handling and procedures that could confound other endpoints (i.e., weight gain and food intake). The significant increase in corticosterone that occurred with gentle handling and the non-significant increase with noise during both circadian phases indicate that both SD methods are stressors, which concurs with other methods that deprive, disrupt or fragment sleep [19, 24, 27, 29, 30]. However, the non-significant increase in corticosterone during lightphase noise suggests that this SD method is less stressful than gentle handling. Nevertheless, differences between the physical and auditory stimuli used in these SD methods (gentle handling and noise, respectively) or inherent differences in PA [10, 16, 27] may have contributed to the observed differences in plasma corticosterone. Despite the complex effects of stress on bodyweight [23], these data further suggest that differences in stress caused by SD methods might explain their contradictory effects on bodyweight in rats. However, this conclusion remains tentative until other methods are available to separate stress from SD experimentally [19].
We examined how different factors (i.e., food intake, PA, vigilance states, and stress) might explain the differential effects of stimuli causing SD (i.e., gentle handling and noise) on bodyweight [2, 3, 8, 12, 17, 18, 22, 26, 31]. Here, in male rats exposed to lightphase gentle handling or noise, differences in food intake, PA, and stress assessed by corticosterone, but not in vigilance states contribute to the opposing effects of these SD methods on bodyweight. Unlike noise which decreased PA in rats [8, 22], gentle handling stimulated PA. Thus, elevated PA during lightphase gentle handling here may have caused SD. We acknowledge that methods of SD may alter bodyweight by mechanisms unexplored here (e.g., components of total energy expenditure). Yet, the failure of SD by gentle handling to stimulate weight gain was likely due to the combination of greater energy expenditure due to elevated PA combined with a non-significant reduction in food intake. Overall, these data suggest that differences in factors that contribute to energy balance contribute to previously reported differences in the effect of these methods of SD on bodyweight.
CONCLUSION
Overall, these data show that by controling for stress during SD, weight gain and food intake due to SD induced by noise during the inactive lightphase were not driven by stress, as assessed by corticosterone. Therefore, conclusions about the relationship between stress, SD and bodyweight are intrinsically linked to the method of SD utilized in these studies. Together, these data suggest that lightphase noise in male rats increases weight gain and food intake by inducing SD and that these effects are not driven by stress (assessed by corticosterone). These results are significant because they provide additional evidence that the noise-induced method of SD may provide a more accurate translational model for testing clinical indications of insomnia-induced obesity.
HIGHLIGHTS.
Weight gain during noise-induced sleep disruption is not driven by stress assessed by corticosterone.
Sleep loss due to gentle handling is more stressful than noise exposure.
Noise exposure and gentle handling have opposing effects on energy balance.
ACKNOWLEDGEMENTS
Declarations of interest: none. The authors declare no conflicts of interest. The authors thank D.Dominguez, L.Troeger, D.Scali, K.Savage, and S.Wren for technical assistance. Dr. Coborn is now employed by Novo Nordisk Inc. and participated in the studies while at the University of Arizona. Funding for this research was supported by the National Institutes of Health [NS099468-01A1], a grant Fondecyt Regular [1200578], and the U.S. Department of Agriculture [ARZT-1372540-R23-131]. Funding sources had no involvement in the study design or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of competing interests: none
REFERENCES
- [1].Baratta AM, Buck SA, Buchla AD, Fabian CB, Chen S, Mong JA, Pocivavsek A, Sex Differences in Hippocampal Memory and Kynurenic Acid Formation Following Acute Sleep Deprivation in Rats, Sci Rep 8 (2018) 6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barclay JL, Husse J, Bode B, Naujokat N, Meyer-Kovac J, Schmid SM, Lehnert H, Oster H, Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork, PLoS One 7 (2012) e37150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barf RP, Van Dijk G, Scheurink AJ, Hoffmann K, Novati A, Hulshof HJ, Fuchs E, Meerlo P, Metabolic consequences of chronic sleep restriction in rats: changes in body weight regulation and energy expenditure, Physiol Behav 107 (2012) 322–328. [DOI] [PubMed] [Google Scholar]
- [4].Barnum CJ, Blandino P Jr., Deak T, Adaptation in the corticosterone and hyperthermic responses to stress following repeated stressor exposure, J Neuroendocrinol 19 (2007) 632–642. [DOI] [PubMed] [Google Scholar]
- [5].Basner M, McGuire S, WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Effects on Sleep, Int J Environ Res Public Health 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Benjamini Y, Hochberg Y, Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing, JSTOR 57 (1995) 289–300. [Google Scholar]
- [7].Buckley TM, Schatzberg AF, On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders, J Clin Endocrinol Metab 90 (2005) 3106–3114. [DOI] [PubMed] [Google Scholar]
- [8].Coborn JE, Lessie RE, Sinton CM, Rance NE, Perez-Leighton CE, Teske JA, Noise-induced sleep disruption increases weight gain and decreases energy metabolism in female rats, Int J Obes (Lond) 43 (2019) 1759–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cramer J, Therming Jorgensen J, Sorensen M, Backalarz C, Laursen JE, Ketzel M, Hertel O, Jensen SS, Simonsen MK, Brauner EV, Andersen ZJ, Road traffic noise and markers of adiposity in the Danish Nurse Cohort: A cross-sectional study, Environ Res 172 (2019) 502–510. [DOI] [PubMed] [Google Scholar]
- [10].De Nys L, Anderson K, Ofosu EF, Ryde GC, Connelly J, Whittaker AC, The effects of physical activity on cortisol and sleep: A systematic review and meta-analysis, Psychoneuroendocrinology 143 (2022) 105843. [DOI] [PubMed] [Google Scholar]
- [11].Du Preez A, Eum J, Eiben I, Eiben P, Zunszain PA, Pariante CM, Thuret S, Fernandes C, Do different types of stress differentially alter behavioural and neurobiological outcomes associated with depression in rodent models? A systematic review, Front Neuroendocrinol 61 (2021) 100896. [DOI] [PubMed] [Google Scholar]
- [12].Everson CA, Gilliland MA, Kushida CA, Pilcher JJ, Fang VS, Refetoff S, Bergmann BM, Rechtschaffen A, Sleep deprivation in the rat: IX. Recovery, Sleep 12 (1989) 60–67. [PubMed] [Google Scholar]
- [13].Foraster M, Eze IC, Vienneau D, Schaffner E, Jeong A, Heritier H, Rudzik F, Thiesse L, Pieren R, Brink M, Cajochen C, Wunderli JM, Roosli M, Probst-Hensch N, Long-term exposure to transportation noise and its association with adiposity markers and development of obesity, Environ Int 121 (2018) 879–889. [DOI] [PubMed] [Google Scholar]
- [14].Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ, Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review, Psychoneuroendocrinology 62 (2015) 301–318. [DOI] [PubMed] [Google Scholar]
- [15].Jafari Z, Kolb BE, Mohajerani MH, Chronic traffic noise stress accelerates brain impairment and cognitive decline in mice, Exp Neurol 308 (2018) 1–12. [DOI] [PubMed] [Google Scholar]
- [16].Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T Jr., Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior, Gen Comp Endocrinol 156 (2008) 210–217. [DOI] [PubMed] [Google Scholar]
- [17].Martins PJ, D’Almeida V, Nobrega JN, Tufik S, A reassessment of the hyperphagia/weight-loss paradox during sleep deprivation, Sleep 29 (2006) 1233–1238. [DOI] [PubMed] [Google Scholar]
- [18].Mavanji V, Teske JA, Billington CJ, Kotz CM, Partial sleep deprivation by environmental noise increases food intake and body weight in obesity-resistant rats, Obesity (Silver Spring) 21 (2013) 1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Meerlo P, Sgoifo A, Suchecki D, Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity, Sleep Med Rev 12 (2008) 197–210. [DOI] [PubMed] [Google Scholar]
- [20].Naidoo N, Giang W, Galante RJ, Pack AI, Sleep deprivation induces the unfolded protein response in mouse cerebral cortex, J Neurochem 92 (2005) 1150–1157. [DOI] [PubMed] [Google Scholar]
- [21].Palchykova S, Winsky-Sommerer R, Tobler I, Sleep deprivation in the dark period does not impair memory in OF1 mice, Chronobiol Int 26 (2009) 682–696. [DOI] [PubMed] [Google Scholar]
- [22].Parrish JB, Teske JA, Acute partial sleep deprivation due to environmental noise increases weight gain by reducing energy expenditure in rodents, Obesity (Silver Spring) 25 (2017) 141–146. [DOI] [PubMed] [Google Scholar]
- [23].Razzoli M, Bartolomucci A, The Dichotomous Effect of Chronic Stress on Obesity, Trends Endocrinol Metab 27 (2016) 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rechtschaffen A, Bergmann BM, Sleep deprivation in the rat: an update of the 1989 paper, Sleep 25 (2002) 18–24. [DOI] [PubMed] [Google Scholar]
- [25].Schwierin B, Borbely AA, Tobler I, Sleep homeostasis in the female rat during the estrous cycle, Brain Res 811 (1998) 96–104. [DOI] [PubMed] [Google Scholar]
- [26].Sternthal HS, Webb WB, Sleep deprivation of rats by punitive and non punitive procedures, Physiol Behav 37 (1986) 249–252. [DOI] [PubMed] [Google Scholar]
- [27].Tartar JL, Ward CP, Cordeira JW, Legare SL, Blanchette AJ, McCarley RW, Strecker RE, Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open field test of anxiety while increasing plasma corticosterone levels, Behav Brain Res 197 (2009) 450–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tomiyama AJ, Stress and Obesity, Annu Rev Psychol 70 (2019) 703–718. [DOI] [PubMed] [Google Scholar]
- [29].Tufik S, Andersen ML, Bittencourt LR, Mello MT, Paradoxical sleep deprivation: neurochemical, hormonal and behavioral alterations. Evidence from 30 years of research, An Acad Bras Cienc 81 (2009) 521–538. [DOI] [PubMed] [Google Scholar]
- [30].van Dalfsen JH, Markus CR, The influence of sleep on human hypothalamic-pituitary-adrenal (HPA) axis reactivity: A systematic review, Sleep Med Rev 39 (2018) 187–194. [DOI] [PubMed] [Google Scholar]
- [31].Wang Y, Carreras A, Lee S, Hakim F, Zhang SX, Nair D, Ye H, Gozal D, Chronic sleep fragmentation promotes obesity in young adult mice, Obesity (Silver Spring) 22 (2014) 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]


