Abstract
Introduction
Influenza virus infection is associated with high morbidity and mortality, and so additional therapeutic strategies to reduce the burden for healthcare systems are needed. Statins, by virtue of their anti-inflammatory and immunomodulatory effects, have been hypothesized as capable of influencing the host’s response against the influenza virus. The aim of this meta-analysis was to assess the effect of ongoing statin treatment on susceptibility to influenza virus infection and on influenza-associated mortality.
Material and methods
Studies investigating the impact of statin treatment on influenza prevalence and mortality were searched for in the PubMed-Medline, Scopus, ISI Web of Knowledge, Embase, Proquest, OVID, EBSCO, and CINAHL databases (up to 8 November 2021). Fixed- and random-effects models and the generic inverse variance method were used for quantitative data synthesis.
Results
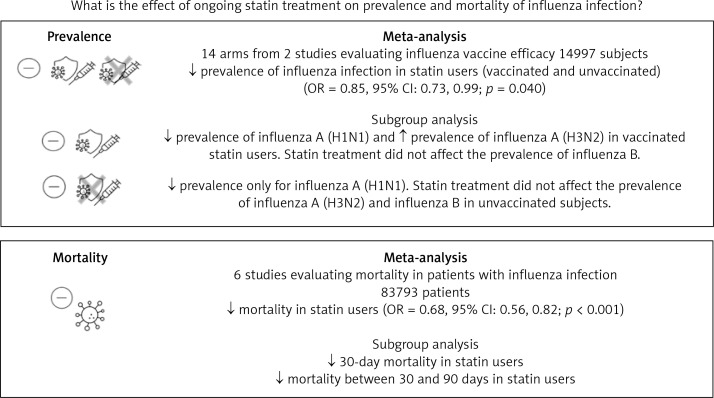
In the meta-analysis of 14 arms of 2 eligible studies, including 14,997 flu-vaccinated and unvaccinated patients, treatment with statins was associated with a reduction of influenza virus prevalence (odds ratio (OR) = 0.85, 95% confidence interval (CI): 0.73–0.99; p = 0.040). No significant effect of statins on the susceptibility to influenza infection was observed in the distinct communities of either vaccinated or unvaccinated subjects. Among 9 arms of 6 eligible studies, including 87,204 patients, the use of statins among patients with influenza was associated with a reduced mortality (OR = 0.68, 95% CI: 0.56, 0.82; p < 0.001). This result was confirmed for both 30-day mortality since influenza infection diagnosis (OR = 0.61, 95% CI: 0.47, 0.80; p <0.001) and for up to 90-day mortality (OR = 0.74, 95% CI: 0.55, 1.00; p = 0.042).
Conclusions
Reduced influenza prevalence and increased survival from influenza infection was observed in patients on ongoing statin treatment. Further research is needed to define the possible role of statins as adjunctive therapy in patients with influenza infection.
Keywords: statins, influenza, immunomodulatory effects, vaccine, mortality, prevalence
Introduction
The influenza virus infection is responsible for a large number of deaths worldwide, especially among elderly patients, and it is a significant burden on healthcare systems [1]. The current coronavirus disease 2019 (COVID-19) pandemic has made even more urgent the need of measures aimed at reducing the overload of healthcare facilities related to influenza virus outbreaks.
The severity of the clinical complications of influenza infection is partly attributable to a dysregulation of the host’s immune response, characterized by an abnormal release of inflammatory cytokines, which may finally culminate in a fatal outcome [2, 3]. Despite vaccine campaigns, antiviral drug therapies, and supportive therapies implemented for the prevention and treatment of influenza virus infection, morbidity and mortality related to the infection are still remarkably high [4]. In addition, resistance of various viral strains to commonly used antiviral drugs is very frequent [5, 6]. Unfortunately, there are no new classes of drugs that have convincingly demonstrated to ameliorate the clinical outcomes of influenza infection. For this reason, the search for new therapeutic strategies could have a major impact on public health. Targeting the virus and the associated host’s immune response has been proposed as a potential, inexpensive, and readily available adjunctive therapy to improve patients’ outcomes and reduce the burden of severe influenza diseases [7]. Among these strategies, statins may play a crucial role.
Statins are the centrepiece of cholesterol lowering therapy and are among the most effective drugs in the prevention of cardiovascular diseases. Beyond their lipid lowering effect, several other activities including anti-inflammatory and immunomodulatory effects have been attributed to statins [8–21]. Leukocyte-endothelial interaction, expression of MHC class II antigens, inflammatory gene transcription, and heme oxygenase expression have been proposed as possible targets for the effects of statins on the immune response and inflammatory cascade [22].
A great deal of scientific literature has explored the relationship between the use of statins and a broad spectrum of infectious diseases, in both human and animal models, including bacterial, parasitic, fungal, and viral infections [23] including influenza. Although the immuno-modulatory effect of statins could theoretically weaken the host’s defence against influenza infection, the attenuation of an exaggerated inflammatory response may beneficially affect the clinical manifestation of the disease and may improve treatment response and patient survival. Several studies have investigated the potential role of statins in preventing influenza infection, in reducing its clinical severity, and in improving its clinical outcomes [24–33]. However, inconsistent results have been released so far, with some studies reporting efficacy [28–30, 32, 33] and other studies reporting negative results [24–27, 30, 31]. Also, some studies have suggested reduced protection from influenza vaccination in statin users due to the immunomodulatory effects of statins [34, 35], although the possible role of these drugs in decreasing vaccination efficacy has not been fully elucidated.
In this meta-analysis the effect of statin on susceptibility to influenza infection in vaccinated and unvaccinated subjects was assessed. In addition, the effects of ongoing statin treatment on influenza associated mortality have been examined.
Material and methods
Search strategy
The guidelines of the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement were followed [36]. The review was not registered prior to the literature search. The PubMed-Medline, Scopus, ISI Web of Knowledge, Embase, Proquest, OVID, EBSCO, Cochrane library, and CINAHL databases were searched using the following search terms in titles and abstracts: (‘statin’ OR ‘atorvastatin’ OR ‘rosuvastatin’ OR ‘simvastatin’ OR ‘pitavastatin’ OR ‘lovastatin’ OR ‘fluvastatin’ OR ‘pravastatin’ OR ‘Hydroxymethylglutaryl-CoA reductase inhibitors’ OR ‘HMG-CoA reductase inhibitors) AND (‘influenza’ OR ‘flu’ OR ‘vaccine’ OR ‘vaccination’ OR ‘vaccines’ OR ‘community-acquired pneumonia’) AND (‘outcome’ OR ‘mortality’ OR ‘prevalence’ OR ‘vaccine effectiveness’ OR ‘effectiveness’). The wild-card term ‘*’ was used to increase the sensitivity of the search strategy. A manual search of the reference lists of identified studies was also performed to find additional relevant studies. The search was limited to articles published in English language. The literature was searched from inception to 8 November 2021.
Study selection
Original studies were included if they met the following inclusion criteria: (1) investigating the impact of statin treatment on either influenza prevalence or influenza mortality among influenza-vaccinated or unvaccinated subjects; (2) observational, retrospective, cohort or case-control study design; and (3) presentation of sufficient information on influenza prevalence or mortality within 30 days or within 90 days since influenza infection diagnosis. Exclusion criteria were as follows: (1) non-clinical studies; (2) lack of sufficient information on influenza prevalence or mortality.
Data extraction
The following data were collected from eligible studies: (1) first author’s name; (2) year of publication; (3) study location; (4) study design; (5) number of participants in the statin and control groups; and (6) odds ratio (OR) of influenza prevalence or mortality. Two authors reviewed the papers, and disagreements were resolved through discussion and consultation with a third author.
Quality assessment
The quality of studies included in this meta-analysis was assessed according to the Cochrane criteria. Risk of bias was evaluated according to the Cochrane instructions [37].
Statistical analysis and summary of synthesis
Meta-analyses were performed on the extracted and evaluated epidemiological data for dichotomous outcome variables, which included factors associated with the presence of influenza infection and influenza-related death. Pooled OR and 95% confidence intervals (CI) of the prevalence of influenza infection and of influenza mortality were calculated for statin users compared to nonusers. An OR of less than 1 indicated reduced odds of influenza in statin users or a protective effect of statin against mortality outcomes. An OR of more than 1 indicated a detrimental effect of statin use. Forest plots were obtained to indicate the effect of the pooled estimates of OR, using either fixed or random models with the inverse variance (IV) in the sub-groups of vaccinated and unvaccinated subjects, in different influenza strains, and in overall analysis. Finally, we tested the significance of the estimates separately.
We assessed heterogeneity using the I2 measure and the Cochran Q-statistic within or between study designs. The null hypothesis was the absence of heterogeneity; if rejected, a random model was used to calculate pooled estimates. Assessment of publication bias was evaluated by designing a funnel plot and calculating Egger’s and Begg’s (with continuity correction) tests. The null hypothesis was that there was no publication bias in the study. STATA version 16.0 (Stata Corp, College Station, TX) was used for statistical analysis.
Results
The flow diagram of study selection process is summarized in Figure 1. The main characteristics of the studies included in the meta-analyses are shown in Table I.
Figure 1.
Flowchart of the study selection process
Table I.
Characteristics of eligible studies included in the meta-analysis
| Studies | Year of Publication | Study period | Multi-/single centre | Country/region | Study design | Patients | Number of statin user | Men among statin users | Ethnicity among statin users | Number of statin non-user | Men among statin non-users | Ethnicity of statin non-users | Included endpoint |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortensen [24] | 2005 | 1999–2002 | Multi | USA | Retrospective cohort study | Hospitalized with pneumonia | 110 | 84% | n.a. | 787 | 78% | n.a. | 30-d mortality |
| Frost [25] | 2007 | 1992–2003 | Multi | USA | Case-control | Hospitalized with pneumonia/influenza | 19,058 | 54% | n.a. | 57,174 | 52% | n.a. | In-hospital mortality |
| Vandermeer [27] | 2012 | 2007–2008 | Multi | USA | Retrospective cohort study | Hospitalized with influenza | 1013 | 49% | Hispanic 4% White 69% Black 13% Asian 3% Others/Unknown 10% |
2030 | 41% | Hispanic 6% White 60% Black 20% Asian 3% Others/Unknown 12% |
30-d mortality |
| Lee [29] | 2015 | 2000–2011 | Multi | China | Prospective cohort study | Hospitalized with influenza | 336 | n.a. | n.a. | 2313 | n.a. | n.a. | 30-d mortality 60-d mortality |
| McLean [30] | 2016 | 2004–2015 | Multi | USA | Prospective cohort study | Acute Respiratory Illness | 1165 | 34% | White 97% Others 3% |
2120 | 33% | White 96% Others 4% |
Vaccine Efficacy |
| Havers [32] | 2018 | 2011–2017 | Multi | USA | Retrospective cohort study | Acute Respiratory Infection | 3,359 | 46% | Hispanic 4% White 85% Black 4% Others/Unknown 7% |
8333 | 35% | Hispanic 4% White 84% Black 5% Others/Unknown 6% |
Vaccine Efficacy |
| Atamna [31] | 2019 | 2012–2017 | Single | Israel | Retrospective cohort study | Hospitalized with influenza | 188 | 55% | n.a. | 388 | 46% | n.a. | 30-d mortality 90-d mortality |
| Pawelka [33] | 2020 | 2017–2018 | Single | Austria | Retrospective cohort study | Hospitalized with influenza | 115 | n.a | n.a. | 281 | n.a. | n.a. | 30-d mortality 90-d mortality |
n.a. – not available.
Use of statins and prevalence of influenza in vaccine and non-vaccine recipients
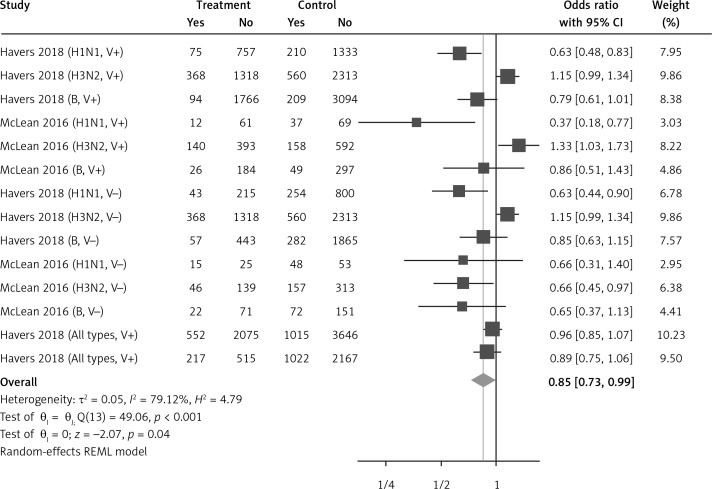
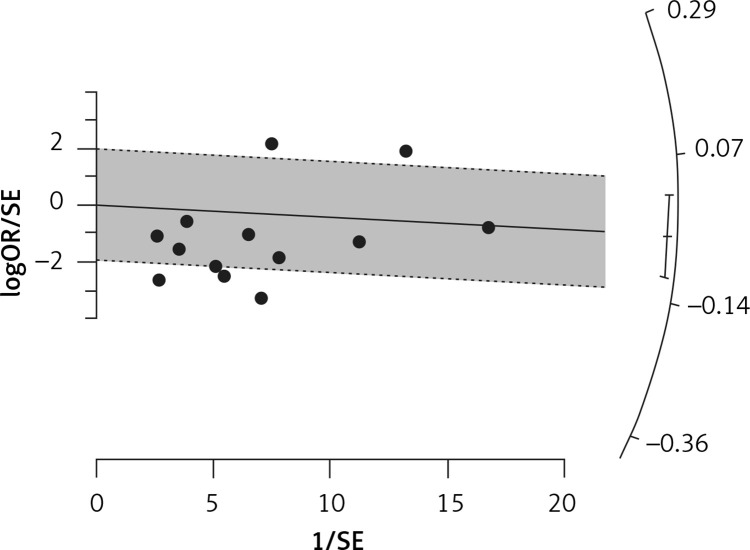
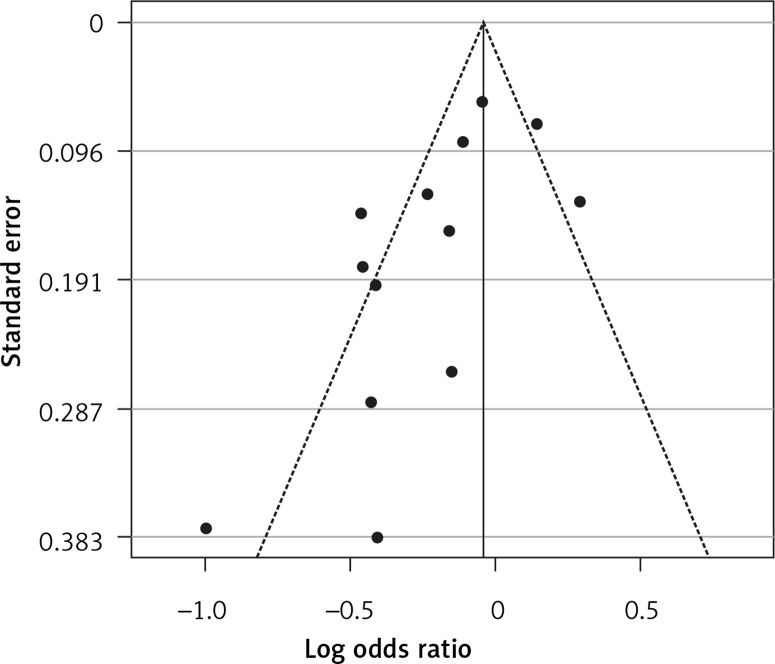
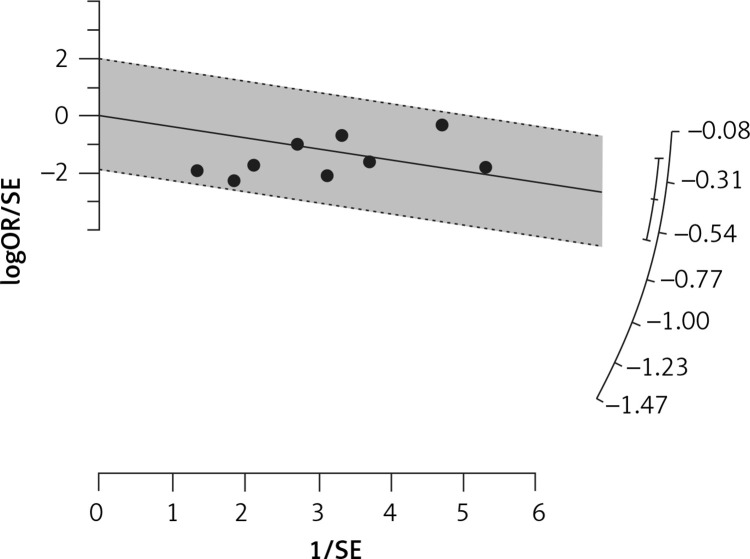
Fourteen arms from 2 studies involving 14,997 subjects were eligible for the analysis of the effect of statin use on influenza prevalence, which was performed for both vaccine recipients and non-vaccinated subjects. Prevalence studies included a variety of H1N1, H3N2, and B influenza vaccines. Also, in the study of Havers [32], an investigation was performed on all influenza types and subtypes, which was added in the final analysis. The analysis was conducted by using a random inverse variance model due to heterogeneity of variance (I2 = 79.12%, p < 0.001) (Figures 2 and 3). Moreover, there was no publication bias at the level of 1% according to the Egger’s test (p = 0.018) and no bias by Begg’s test with continuity correction (p = 0.071) (Figure 4). A significant association between statin use and influenza prevalence was observed (OR = 0.85, 95% CI: 0.73, 0.99; p = 0.040) (Figure 2). This result was robust in leave-one-out sensitivity analysis.
Figure 2.
Forrest plot for odds ratio of the use of statins and influenza outcome analysis in the community of vaccine and non-vaccine recipients
Figure 3.
Radial plot for heterogeneity of the use of statins and influenza outcome analysis in the community of vaccine and non-vaccine recipients
Figure 4.
Funnel plot for verification of publication bias of the use of statins and influenza outcome analysis in the community of vaccine and non-vaccine recipients
Use of statins and the prevalence of influenza in vaccinated people
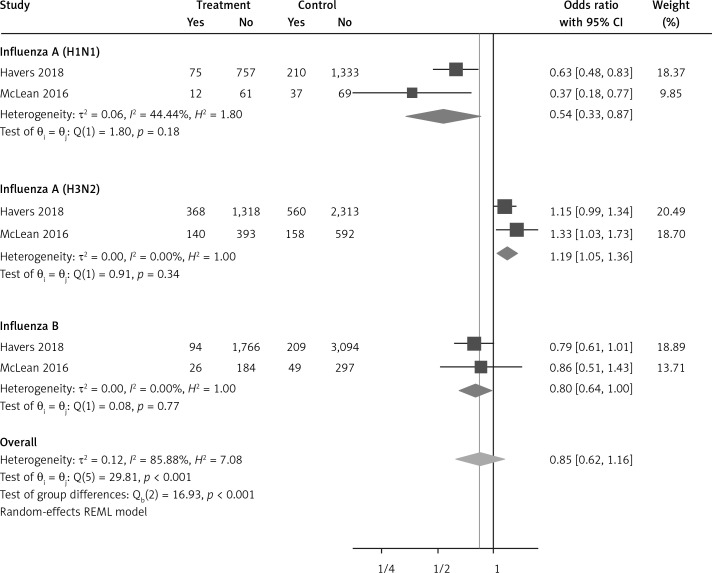
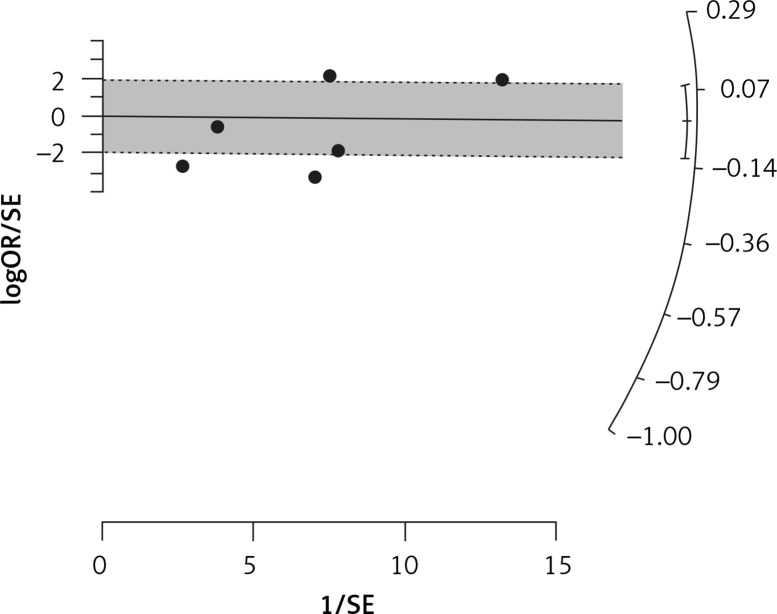
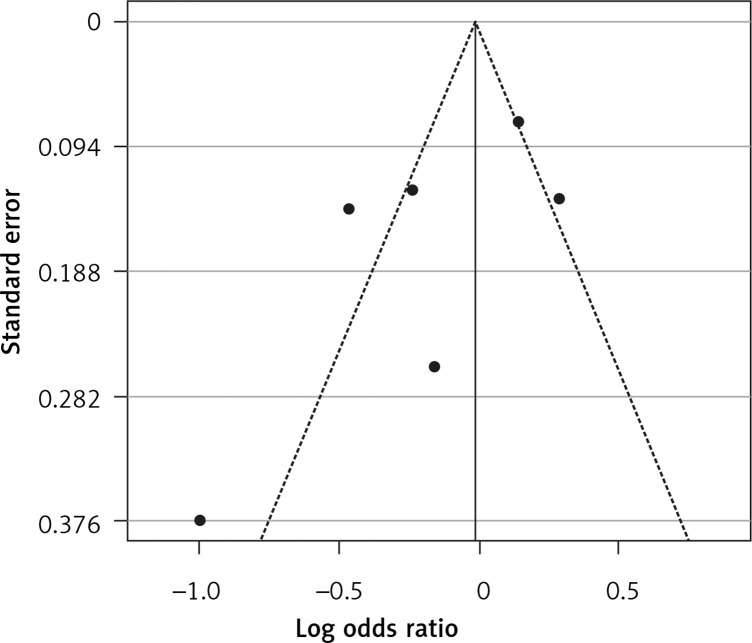
Six arms of 2 eligible studies were included in the analysis of the effect of ongoing statin treatment on the prevalence of influenza infection, in the sub-group of vaccine recipients. Heterogeneities within and between study designs were obtained for H1N1 (I2 = 44.4%, p = 0.180), H3N2 (I2 = 0.0%, p = 0.340), B (I2 = 0.0%, p = 0.774), and overall (I2 =83.2%, p < 0.001) (Figure 5). Therefore, analysis was performed by using a random model (Figure 6). Also, no publication bias was observed according to Egger’s test (p = 0.176) and Begg’s test with continuity correction (p = 0.260) (Figure 7). According to the results, random effect analysis was applied. No significant association was observed between statin use and influenza prevalence (OR = 0.85, 95% CI: 0.62, 1.16; p = 0.282). This result was robust in leave-one-out sensitivity analysis. Subgroup analysis upon the type of influenza showed that statin use was associated with reduced prevalence of H1N1 influenza infection and increased prevalence of H3N2 (Figure 5). Statin treatment did not affect the prevalence of influenza B (Figure 5).
Figure 5.
Forrest plot for odds ratio of the use of statins and influenza outcome analysis in the community of vaccine recipients
Figure 6.
Radial plot for heterogeneity of the use of statins and influenza outcome analysis in the community of vaccine recipients
Figure 7.
Funnel plot for verification of publication bias of the use of statins and influenza outcome analysis in the community of vaccine recipients
Use of statins and the prevalence of influenza in unvaccinated people
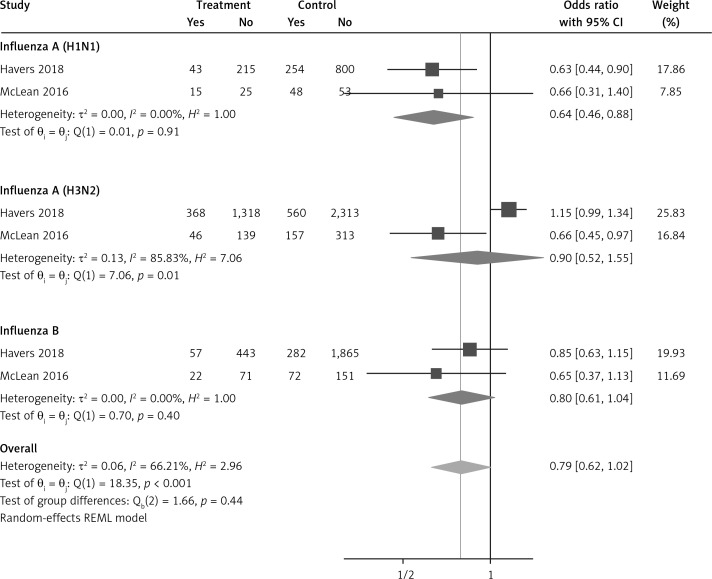
Six arms from 2 eligible studies involving 9630 subjects were included in the analysis of the effect of statin use on influenza prevalence in unvaccinated subjects. Heterogeneities within and between study designs were obtained for H1N1 (I2 = 0.0%, p = 0.910), H3N2 (I2 = 85.8%, p = 0.0.010), B (I2 = 0.0%, p = 0.403), and overall (I2 = 72.8%, p = 0.003) (Figure 8). A random model was applied to obtain the estimates (Figure 9). There was no publication bias at the level of 1% according to Egger’s test (p = 0.019) and no bias by Begg’s test with continuity correction (p = 0.707) (Figure 10). Our results showed that pooling effect sizes resulted in no significant association between statin use and influenza prevalence (OR = 0.79, 95% CI: 0.62, 1.02; p = 0.084). In the leave-one-out sensitivity analysis, the pooled result was sensitive to one of the studies [33], the removal of which led to a significant result (OR = 0.73, 95% CI: 0.60, 0.85; p = 0.0004). In the subgroup analysis, the result was significant only for H1N1 (OR = 0.64, 95% CI: 0.46, 0.88; p = 0.006) (Figure 8).
Figure 8.
Forrest plot for odds ratio of the use of statins and influenza outcome analysis in the community of non-vaccine recipients
Figure 9.
Radial plot for heterogeneity of the use of statins and influenza outcome analysis in the community of non-vaccine recipients
Figure 10.
Funnel plot for verification of publication bias of the use of statins and influenza outcome analysis in the community of non-vaccine recipients
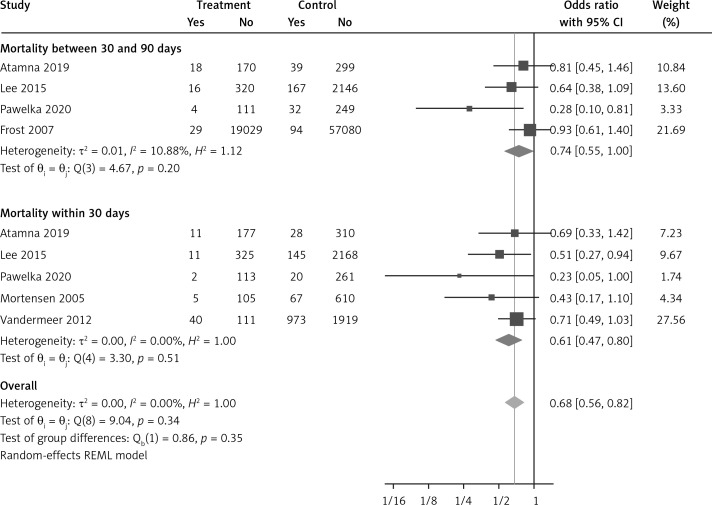
The effect of statins on mortality outcome
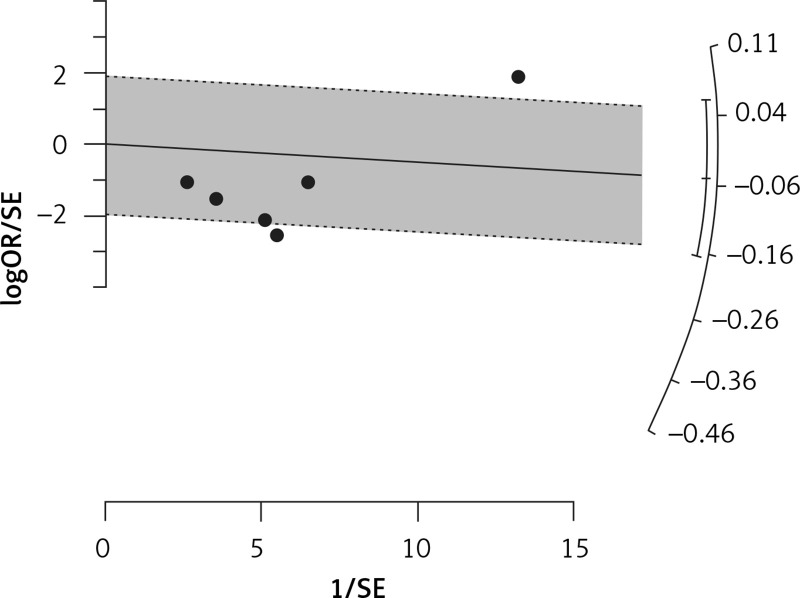
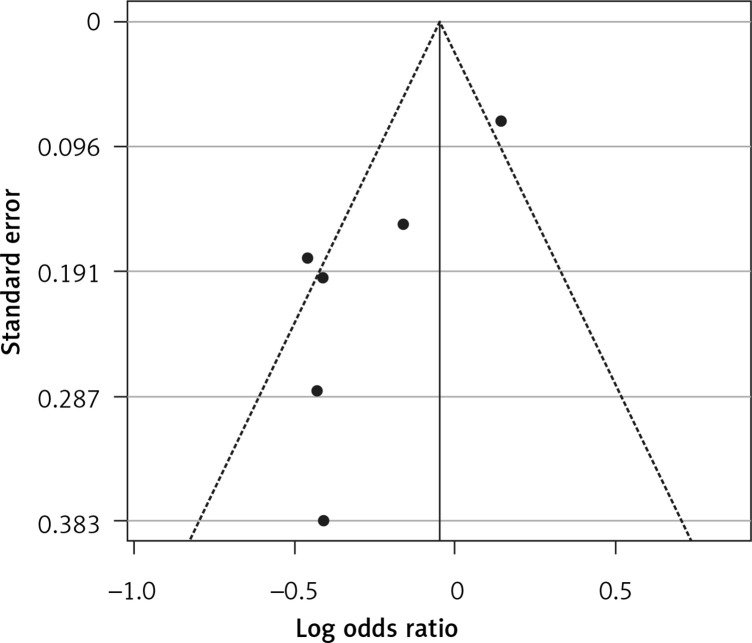
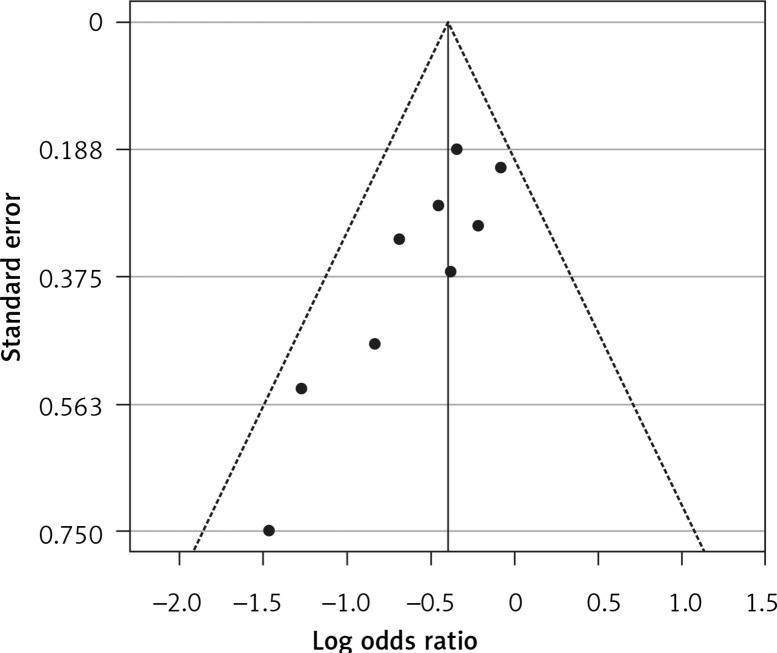
Six eligible studies, involving 83,793 patients with influenza infection, were used to analyse the effects of the statins on mortality. Deaths were divided into 2 categories of within 30-day mortality (also including in hospital mortality) and up to 90-day mortality. Heterogeneities within and between study designs were obtained for mortality within 30 days (I2 = 0.0%, p = 0.509), up to 90 days (I2 = 35.7%, p = 0.198), and overall (I2 = 11.5%, p = 0.339) (Figure 11). Therefore, the use of a fixed model for analysis was confirmed (Figure 12). While the Egger’s test confirmed the publication bias (p = 0.007), there was no bias at the 1% level according to Begg’s test with continuity correction (p = 0.029) (Figure 13).
Figure 11.
Forrest plot for odds ratio of mortality event due to using statins in the community of influenza patients
Figure 12.
Radial plot for heterogeneity of mortality event due to using statins in the community of influenza patients
Figure 13.
Funnel plot for verification of publication bias of mortality event due to using statins in the community of influenza patients
Subgroup analysis showed a significant effect for the pooled estimate of OR on mortality both within 30 days (OR = 0.61, 95% CI: 0.47, 0.80; p < 0.001) and up to 90 days (OR = 0.74, 95% CI: 0.55, 1.00; p = 0.042). Furthermore, the results of overall inverse variance pooled OR analysis displayed a significant effect of statin treatment on the survival of patients with influenza infection (OR = 0.68, 95% CI: 0.56, 0.82; p < 0.001) (Figure 11). This result was robust in the leave-one-out sensitivity analysis.
Discussion
Three main results can be derived from our meta-analysis. First, an overall reduction in the prevalence of influenza among people who are on statin treatment. Second, an inconsistent effect of statins on influenza prevalence depending on the different influenza virus strains. Third, the association between statin use and increased survival among patients with influenza infection (Figure 14).
Figure 14.
Summary
In our meta-analysis of data from 14 arms of 2 multi-centre studies, including almost 15,000 vaccinated and unvaccinated patients, treatment with statins was associated with a 14% reduction (OR = 0.87; p = 0.032) of influenza prevalence. The protective impact of statins on the development of influenza infection could be related to their effectiveness in favourably modulating the immune host’s response to the infection, thus limiting illness severity. This could lead statin users to manifest less severe symptoms, limiting their access to healthcare facilities and reducing the chance of diagnosing influenza infection. In this regard, the anti-inflammatory and immunomodulatory role of statins has long been recognized [8, 9]. A reduction in the activation of antigen-presenting cells, through lower expression of CD40/CD40L, has been attributed to statins [8]. Also, statins have been shown to reduce circulating levels of pro-inflammatory cytokines and inflammatory markers [38]. Other immunomodulatory effects of statins include the inhibition of T-cell activation, their function, and cytotoxicity [39]. On the whole, these effects may support a role for statins in mitigating the harmful effects of various infectious diseases, including influenza [23, 40, 41]. Furthermore, an immune-regulating effect may be suggested also for other lipid-lowering drugs. A link between treatment with PCSK9 inhibitors and immunity may be hypothesized because high levels of PCSK9 might have a detrimental effect on immune host response and survival during infections [42]. In addition, ezetimibe has been shown to have immunomodulatory properties by affecting CD4+ T-cell function [43]. In addition to the anti-inflammatory and immunomodulatory effects of statins, other unmeasured factors might explain the negative association between statin use and the prevalence of influenza. In this regard, it has been suggested that cholesterol in the virion envelope is crucial in the fusion process of influenza virus, and a reduced cholesterol concentration in the host’s cell membrane could interfere with the entry of the virus into the cell [44].
Our findings showing protection with statin use against the development of influenza infection are of extreme importance in the light of the divergent results of some studies suggesting a reduced protection from influenza vaccination among statin users [30, 34, 35]. In these studies, the immunomodulatory effects of statins have been invoked to promote reduced antibody response resulting from influenza vaccination and a consequent reduced vaccine effectiveness [34]. However, in the present meta-analysis, no significant effect of statins on the overall susceptibility to influenza infection was observed among vaccinated subjects, thus mitigating concerns about the potential predisposing effect of statins on the risk of developing influenza infection.
Our analysis suggests a possible difference in the influence of statin treatment in preventing infections from different influenza virus subtypes. We found that statin users seem to receive greater protection against H1N1 strains, irrespective of vaccination status; thus, treatment with statins was associated with a reduced prevalence of H1N1 infection by 46% in vaccinated subjects and by 36% in unvaccinated ones. Conversely, based on our data, the prevalence of H3N2 influenza infection was higher in vaccinated subjects taking statins as compared to statin nonusers, whereas no difference in the prevalence of H3N2 infection was found, in relation to statin use, among unvaccinated subjects. The differential effect of statins on the likelihood to be infected by different viral subtypes is unclear. It was observed that post-vaccination influenza antibody titres were lower in statin users versus nonusers, and this effect was more pronounced for the H3N2 subtype than for H1N1 and for influenza B [35]. However, caution should be applied in the interpretation of the impact of statins on different influenza virus strains in that this finding comes from only 2 observational studies with some limitations [30, 32]. In both studies, the numbers of influenza AH1N1 and influenza B cases were much smaller than for the H3N2 subtype. Also, possible confounding factors, such as patients’ demographic characteristics, comorbidities, or adherence to statin therapy, may not have been evaluated accurately.
Another remarkable result of our study concerns the influence of ongoing statin treatment on influenza survival. The use of statins by patients with laboratory-confirmed influenza infection was associated with a 32% reduction in mortality (OR = 0.68, p < 0.001). The improved survival of statin users was observed both in the short term (within 30 days) and in the medium term (up to 90 days) from the time of influenza infection diagnosis. Several reasons, related both to statin cholesterol-lowering and pleiotropic effects, could be behind this finding. As already mentioned, reduced cholesterol concentration has been associated with reduced influenza virus infectivity [36]. Moreover, the possible efficacy of statins in reducing complications related to influenza virus infection could be linked to a modulation of the host’s inflammatory response. Also, a possible protective role of statins in acute lung injury, mediated by a reduced lung vascular permeability and inflammation, has been suggested [45]. Finally, influenza infection has been associated with an increased incidence of acute coronary syndrome and death from cardiovascular diseases [46]. As further evidence that influenza virus infection significantly affects cardiovascular prognosis, in the IAMI trial [47], influenza vaccination administered shortly after myocardial infarction or in high-risk stable coronary heart disease was associated with a lower risk of a composite of all-cause death, MI, or stent thrombosis, and a lower risk of all-cause and cardiovascular death, compared to placebo. Consequently, our observation of a reduced influenza mortality in statin users could be related to the beneficial effect of statins on cardiovascular prognosis.
Other studies not included in this meta-analysis investigating the role of statins in influencing the prognosis of patients with influenza infection deserve to be mentioned. In the study by Brett et al. [26], the association between pre-admission statin use and severe outcome of patients with influenza infection was not statistically significant. Contrarily, in hospitalized patients with laboratory-confirmed influenza, Atamna et al. [31] reported that statin use was more than 50% lower among those transferred to intensive care units. In the study by Knight et al. [28], statin use was associated with an increase in subsequent cardiovascular disease events (OR = 2.66, 95% CI: 1.83, 3.87; p < 0.0001) in inpatients with confirmed respiratory viral infection with prior history of cardiovascular disease.
The favourable prognostic impact of statin use in patients with influenza may have additional explanations related to the so-called “healthy user bias”. Statins may be preferentially prescribed to healthier patients, and their use is often avoided in patients with short life expectancy or significant concurrent illness [48]. Statin use has been associated with increased use of preventive services and adherence to healthier protective lifestyle [49, 50]. Altogether, this could have led to an overestimation of the positive effect of statins on patients’ survival.
Among the strengths of this study, it should be noted that large and multi-centre studies in which the diagnosis of influenza was laboratory-confirmed were included in the analysis. This study also has several limitations that need to be mentioned. The first lies in the observational nature of the collected data, which does not allow the causality of the relationship between the use of statins and the occurrence of influenza and its complications to be defined. Another limitation lies in the methodological differences between the studies included in the meta-analysis, in terms of enrolled population, diagnostic methods, and treatments. In addition, in some studies, data on comorbidities, adherence to drug therapy, and vaccination status were partial, thus leading to potential underestimation of their confounding effect on the observed associations. Moreover, the evaluation of different viral strains in the same analysis could have produced an additional potential confounding factor. Also, it should be mentioned that differences in sample sizes of the different studies included in this meta-analysis could have partly influenced the results. In this regard, the effect size on mortality within 30 days is mainly derived by the study by Vandermeer et al. [27], with a relative weight of 27%.
In conclusion, ongoing statin treatment was associated with a reduced susceptibility to influenza infection and with increased survival of patients infected with influenza. Based on the results of our study, statins, which are inexpensive and easily available medications, might represent a beneficial adjunct therapy able to mitigate clinical manifestations and improve survival of patients with influenza virus infection. Also, it might be viewed as a preventive therapy. At the moment, the evidence is not sufficient to implement the use of statins with these specific indications, while the practical advice to continue statin treatment in patients with influenza infection is further supported by our findings. Further studies are needed to define the differential influence of statins on the susceptibility to different influenza virus subtypes and to investigate the prospective impact of statins on influenza infection occurrence and prognosis.
Acknowledgments
Amir Vahedian-Azimi and Massimo R. Mannarino equally contributed as the first author.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391: 1285-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12: 1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung C, Poon LLM, Lau AS, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 2002; 360: 1831-7. [DOI] [PubMed] [Google Scholar]
- 4.Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza – the need for a universal influenza vaccine. N Engl J Med 2018; 378: 7–9. [DOI] [PubMed] [Google Scholar]
- 5.Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360: 953-6. [DOI] [PubMed] [Google Scholar]
- 6.Baz M, Abed Y, Papenburg J, Bouhy X, Hamelin MÈ, Boivin G. Emergence of oseltamivir-resistant pandemic H1N1 virus during prophylaxis. N Engl J Med 2009; 361: 2296-7. [DOI] [PubMed] [Google Scholar]
- 7.Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis 2008; 8: 571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schonbeck U. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation 2004; 109 (21 Suppl 1): II-18-26. [DOI] [PubMed] [Google Scholar]
- 9.Zeiser R. Immune modulatory effects of statins. Immunology 2018; 154: 69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vahedian-Azimi A, Mohammadi SM, Beni FH, et al. Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: a systematic review and meta-analysis. Arch Med Sci 2021; 17: 579-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banach M, Dinca M, Ursoniu S. Lipid blood pressure meta-analysis collaboration group. A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials investigating the effects of statin therapy on plasma lipid concentrations in HIV-infected patients. Pharmacol Res 2016; 111: 343-56. [DOI] [PubMed] [Google Scholar]
- 12.Mollazadeh H, Tavana E, Fanni G, et al. Effects of statins on mitochondrial pathways. J Cachexia Sarcopenia Muscle 2021; 12: 237-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouhpeikar H, Delbari Z, Sathyapalan T, Simental-Mendía LE, Jamialahmadi T, Sahebkar A. The effect of statins through mast cells in the pathophysiology of atherosclerosis: a review. Curr Atheroscler Rep 2020; 22: 19. [DOI] [PubMed] [Google Scholar]
- 14.Serban C, Sahebkar A, Mikhailidis D, Rysz J, Watts G, Banach M. Association between statin use and plasma d-dimer levels – a systematic review and meta-analysis of randomized controlled trials. Atherosclerosis 2015; 241: e199. [DOI] [PubMed] [Google Scholar]
- 15.Afshari AR, Mollazadeh H, Henney NC, Jamialahmad T, Sahebkar A. Effects of statins on brain tumors: a review. Semin Cancer Biol 2021; 73: 116-33. [DOI] [PubMed] [Google Scholar]
- 16.Bahrami A, Bo S, Jamialahmadi T, Sahebkar A. Effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors on ageing: molecular mechanisms. Ageing Res Rev 2020; 58: 101024. [DOI] [PubMed] [Google Scholar]
- 17.Dehnavi S, Kiani A, Sadeghi M, et al. Targeting AMPK by statins: a potential therapeutic approach. Drugs 2021; 81: 923-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koushki K, Shahbaz SK, Mashayekhi K, et al. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin Rev Allergy Immunol 2021; 60: 175-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakour N, Ruscica M, Hadizadeh F, et al. Statins and C-reactive protein: in silico evidence on direct interaction. Arch Med Sci 2020; 16: 1432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahebkar A, Serban C, Ursoniu S, et al.; Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group . The impact of statin therapy on plasma levels of von Willebrand factor antigen. Systematic review and meta-analysis of randomised placebo-controlled trials. Thromb Haemost 2016; 115: 520-32. [DOI] [PubMed] [Google Scholar]
- 21.Serban C, Sahebkar A, Ursoniu S, et al. A systematic review and meta-analysis of the effect of statins on plasma asymmetric dimethylarginine concentrations. Sci Rep 2015; 5: 9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehrbod P, Omar AR, Hair-Bejo M, Haghani A, Ideris A. Mechanisms of action and efficacy of statins against influenza. Biomed Res Int 2014; 2014: 872370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parihar SP, Guler R, Brombacher F. Statins: a viable candidate for host-directed therapy against infectious diseases. Nat Rev Immunol 2019; 19: 104-17. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The effect of prior statin use on 30-day mortality for patients hospitalized with community-acquired pneumonia. Respir Res 2005; 6: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest 2007; 131: 1006-12. [DOI] [PubMed] [Google Scholar]
- 26.Brett SJ, Myles P, Lim WS, et al. Pre-admission statin use and in-hospital severity of 2009 pandemic influenza A (H1N1) disease. PLoS One 2011; 6: e18120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandermeer ML, Thomas AR, Kamimoto L, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis 2012; 205: 13-9. [DOI] [PubMed] [Google Scholar]
- 28.Knight S, Miller III RR, Bair T, et al. Statin use at time of respiratory viral infection in patients with prior history of cardiovascular disease and risk of subsequent cardiovascular events. J Am Coll Cardiol 2015; 65: A1444. [Google Scholar]
- 29.Lee N, Leo YS, Cao B, et al. Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. Eur Respir J 2015; 45: 1642-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLean HQ, Chow BDW, Van Wormer JJ, King JP, Belongia EA. Effect of statin use on influenza vaccine effectiveness. J Infect Dis 2016; 214: 1150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atamna A, Babitch T, Bracha M, et al. Statins and outcomes of hospitalized patients with laboratory-confirmed 2017–2018 influenza. Eur J Clin Microbiol Infect Dis 2019; 38: 2341-8. [DOI] [PubMed] [Google Scholar]
- 32.Havers FP, Chung JR, Belongia EA, et al. Influenza vaccine effectiveness and statin use among adults in the United States, 2011–2017. Clin Infect Dis 2019; 68: 1616-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawelka E, Karolyi M, Daller S, et al. Influenza virus infection: an approach to identify predictors for in-hospital and 90-day mortality from patients in Vienna during the season 2017/18. Infection 2020; 48: 51-6. [DOI] [PubMed] [Google Scholar]
- 34.Omer SB, Phadke VK, Bednarczyk RA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213: 1216-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Black S, Nicolay U, Del Giudice G, Rappuoli R. Influence of statins on influenza vaccine response in elderly individuals. J Infect Dis 2016; 213: 1224-8. [DOI] [PubMed] [Google Scholar]
- 36.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd, Chichester, UK: 2008. [Google Scholar]
- 38.Tousoulis D, Psarros C, Demosthenous M, Patel R, Antoniades C, Stefanadis C. Innate and adaptive inflammation as a therapeutic target in vascular disease. J Am Coll Cardiol 2014; 63: 2491-502. [DOI] [PubMed] [Google Scholar]
- 39.Bu D, Griffin G, Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr Opin Lipidol 2011; 22: 165-70. [DOI] [PubMed] [Google Scholar]
- 40.Reiner Ž, Hatamipour M, Banach M, et al. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci 2020; 16: 490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radenkovic D, Chawla S, Pirro M, Sahebkar A, Banach M. Cholesterol in relation to COVID-19: should we care about it? J Clin Med 2020; 9: 1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paciullo F, Fallarino F, Bianconi V, Mannarino MR, Sahebkar A, Pirro M. PCSK9 at the crossroad of cholesterol metabolism and immune function during infections. J Cell Physiol 2017; 232: 2330-8. [DOI] [PubMed] [Google Scholar]
- 43.Shaw SM, Najam O, Khan U, Yonan N, Williams SG, Fildes JE. Ezetimibe and atorvastatin both immunoregulate CD4+ T cells from cardiac transplant recipients in vitro. Transpl Immunol 2009; 21: 179-82. [DOI] [PubMed] [Google Scholar]
- 44.Sun X, Whittaker GR. Role for influenza virus envelope cholesterol in virus entry and infection. J Virol 2003; 77: 12543-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singla S, Jacobson JR. Statins as a novel therapeutic strategy in acute lung injury. Pulm Circ 2012; 2: 397-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren-Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis 2009; 9: 601-10. [DOI] [PubMed] [Google Scholar]
- 47.Fröbert O, Gotberg M, Erlinge D, et al. Influenza vaccination after myocardial infarction: a randomized, double-blind, placebo-controlled, multicenter trial. Circulation 2021; 144: 1476-84. [DOI] [PubMed] [Google Scholar]
- 48.Glynn R, Schneeweiss S, Wang P, Levin R, Avorn J. Selective prescribing led to overestimation of the benefits of lipid-lowering drugs. J Clin Epidemiol 2006; 59: 819-28. [DOI] [PubMed] [Google Scholar]
- 49.Patrick AR, Shrank WH, Glynn RJ, et al. The association between statin use and outcomes potentially attributable to an unhealthy lifestyle in older adults. Value Heal 2011; 14: 513-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banach M, Burchardt P, Chlebus K, et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch Med Sci 2021; 17: 1447-547. [DOI] [PMC free article] [PubMed] [Google Scholar]