Abstract
Introduction
Non-cirrhotic treatment-naive hepatitis C patients infected with genotype 1 can be treated with ledipasvir/sofosbuvir (LDV/SOF) for 8 weeks, but in practice this regimen is frequently extended up to 12 weeks at least in part due to insufficient real-world data supporting shortening of treatment. The aim of our study was to compare 8- and 12-week regimens’ efficacy in patients eligible for 8-week therapy in a real-world setting.
Material and methods
Data of HCV genotype 1 infected patients treated with LDV/SOF between 2015 and 2018 included in the EpiTer-2 database were analyzed with respect to patients’ characteristics and length of treatment.
Results
Among a total of 1718 patients treated with LDV/SOF, 679 were included in the analysis, 238 (35%) received 8-week regimen, whereas 441 were treated for 12 weeks although they fulfilled the criteria for a shorter course. The majority of patients were infected with genotype 1b (89%) and demonstrated minimal fibrosis (55%). The 12-week regimen was assigned significantly more frequently to patients with comorbidities, concomitant medications and advanced liver fibrosis. The sustained virologic response rate was similar after 8 (98%) and 12 (97%) weeks of therapy according to intent-to-treat analysis and reached 99% in both groups after exclusion of patients lost to follow-up.
Conclusions
We confirmed high effectiveness regardless of treatment duration with LDV/SOF in non-cirrhotics infected with HCV genotype 1 eligible for the 8-week regimen according to the current label. This real-world study also demonstrated no need for addition of ribavirin (RBV) in this population and showed that shortening of treatment significantly improves the safety profile of LDV/SOF medication.
Keywords: hepatitis C, sofosbuvir, genotype 1, ledipasvir, treatment-naïve
Introduction
According to recent data from the World Health Organization (WHO) approximately 71 million people globally are infected with hepatitis C virus (HCV) [1]. Since chronic hepatitis C (CHC) is responsible for severe complications, including cirrhosis and hepatocellular carcinoma, resulting in 400 000 deaths each year worldwide, early diagnosis and antiviral therapy are essential to reduce HCV-related morbidity and mortality. Introduction of regimens based on direct acting antivirals (DAA) significantly improved treatment efficacy and safety. One of the first approved DAA based regimens was a combination of ledipasvir and sofosbuvir (LDV/SOF) administered with or without RBV, which demonstrated excellent sustained virologic response (SVR) rates in clinical trials, particularly with regard to HCV genotype (GT) 1, the most prevalent GT worldwide and the most frequent among the population included in this study [2–4].
According to the Summary of Product Characteristics (SmPC) patients infected with GT 1 without liver cirrhosis should be treated with LDV/SOF without RBV for 12 weeks, but based on results of the clinical trial ION-3 a shorter 8-week course can be considered for those who are treatment-naïve [5]. According to the previous European Association for the Study of the Liver (EASL) guidelines, the duration of treatment could be shortened to 8 weeks if the baseline HCV RNA level is below 6 million IU/ml, but this recommendation was not listed in SmPC or in the most recent EASL and national HCV management guidelines [6–10]. Although non-cirrhotic, treatment-naïve patients infected with GT 1 can be treated with LDV/SOF for 8 weeks, in practice this regimen is frequently extended up to 12 weeks possibly at least in part due to insufficient real-world data supporting shortening of treatment. There are few published studies carried out in American patients, black or infected mostly with GT 1a, who present a different response profile compared to European Caucasians usually infected with GT 1b.
The aim of our study was to compare 8- and 12-week regimens’ effectiveness in central European patients eligible for 8-week therapy from a large real-world experience study.
Material and methods
We analyzed data of HCV genotype 1 infected patients treated with LDV/SOF between 1st November 2015 and 31st March 2018 in 22 Polish hepatology centers. Data were derived from EpiTer-2, an observational, investigator-initiated, manufacturer-independent study, assessing antiviral treatment of HCV infected patients in routine clinical practice. The study was supported by the Polish Association of Epidemiologists and Infectiologists. Patients were treated within the reimbursed therapeutic program of the National Health Fund and in line with recommendations of the Polish Group of Experts for HCV (PGE HCV) [7, 8]. The analyzed study population was confined to patients treated for 8 weeks and those who were eligible to receive an 8-week regimen but were assigned to 12 weeks of LDV/SOF. According to national reimbursement regulations based on SmPC and PGE HCV recommendations treatment-naïve patients without cirrhosis were eligible for 8-week therapy, and the requirement of baseline HCV RNA below 6 million IU/ml was not obligatory. Among 6228 patients included in the EpiTer-2 database, treated for chronic HCV infection in the analyzed period, a total of 1718 patients infected with GT 1 received LDV/SOF, including 1074 non-cirrhotic patients.
As shown in Table I, final retrospective analysis was conducted on data from 679 patients who fulfilled criteria for 8-week therapy. In this population 238 patients received the 8-week regimen, whereas remaining 441 patients were assigned to the 12-week regimen. In the 12-week subpopulation 79 patients (18%) received RBV; the majority were infected with genotype 1b (89%) and demonstrated minimal fibrosis (55%). Only 5% of patients were infected with HCV GT 1a and the remaining 6% were identified as GT 1 without subgenotyping. Advanced fibrosis (F3) but without cirrhosis was reported in 21% of patients.
Table I.
Baseline characteristics of patients eligible for 8-week regimen of LDV/SOF ± RBV treated for 8 or 12 weeks
| Parameter | 8 weeks n = 238 | 12 weeks n = 441 | P-value |
|---|---|---|---|
| Gender, female/male, n (%) | 166 (70)/72 (30) | 231 (52)/210 (48) | < 0.001 |
| Age [years] mean ± SD (min.–max.): | 46 ±15 (19–82) | 52±15 (19–81) | < 0.001 |
| Female | 48 ±15 (19–82) | 54±15 (19–80) | < 0.001 |
| Male | 42 ±14 (24–74) | 50±15 (20–81) | < 0.001 |
| BMI mean ± SD (min.–max.) | 25 ±4 (16–41) | 26±4 (16–42) | 0.03 |
| Comorbidities, n (%): | |||
| Any comorbidity | 108 (45) | 311 (70.5) | < 0.001 |
| Hypertension | 56 (24) | 185 (42) | < 0.001 |
| Diabetes | 14 (5.6) | 51 (11.6) | 0.02 |
| Renal disease | 8 (3.4) | 46 (10.5) | 0.001 |
| Autoimmune diseases | 9 (3.5) | 8 (1.8) | 0.12 |
| Non-HCC tumors | 4 (1.7) | 11 (2.5) | 0.59 |
| Other | 78 (33) | 227 (51.5) | < 0.001 |
| Concomitant medications, n (%) | 92 (39) | 306 (69) | < 0.001 |
| HCV genotype, n (%): | 0.01 | ||
| 1 | 7 (2.9) | 33 (7.5) | |
| 1a | 8 (3.4) | 26 (5.9) | |
| 1b | 223 (93.7) | 382 (86.6) | |
| Liver fibrosis, n (%): | < 0.001 | ||
| F0 | 2 (0.9) | 0 | |
| F1 | 205 (86.1) | 164 (37.2) | |
| F2 | 27 (11.3) | 135 (30.6) | |
| F3 | 3 (1.3) | 142 (32.2) | |
| F4 | 0 | 0 | |
| No data | 1 (0.4) | 0 | |
| History of liver transplantation, n (%) | 0 | 16 (3.4) | 0.001 |
| History of hepatocellular carcinoma, n (%) | 0 | 8 (1.8) | 0.06 |
| Liver fibrosis assessment, n (%): | |||
| Biopsy | 31 (13) | 61 (13.8) | 0.81 |
| TE | 150 (63) | 315 (71.4) | 0.03 |
| SWE | 56 (23.6) | 65 (14.8) | 0.006 |
| ARFI | 0 | 0 | – |
| No data | 1 (0.4) | 0 | – |
| HIV coinfection, n (%) | 6 (2.5) | 35 (7.9) | 0.004 |
| HBV coinfection, HBsAg-positive, n (%) | 1 (0.4) | 7 (1.6) | 0.27 |
| ALT [IU/l], mean ± SD | 59 ±49 | 73 ±67 | 0.001 |
| Bilirubin [mg/dl], mean ± SD | 0.57 ±0.33 | 0.72 ±0.46 | < 0.001 |
| Albumin [g/dl], mean ± SD | 4.1 ±0.4 | 4.0 ±0.4 | 0.10 |
| Albumin < 3 g/dl, n (%) | 0 | 4 (0.9) | 0.30 |
| Creatinine [mg/dl], mean ± SD | 0.84 ±0.26 | 0.85 ±0.3 | 0.41 |
| Hemoglobin [g/dl], mean ± SD | 14.0 ±1.6 | 14.2 ±1.7 | 0.05 |
| Platelets [× 1000/µl], mean ± SD | 234 ±75 | 210 ±78 | < 0.001 |
| Platelets <100 000/µl, n (%) | 2 (0.8%) | 29 (6.6%) | < 0.001 |
| HCV RNA [× 106 IU/ml], mean ± SD | 1.5 ±1.1 | 2.9 ±6.7 | < 0.001 |
| HCV RNA > 6 × 106 IU/ml], n (%) | 12 (5) | 49 (11.1) | 0.008 |
LDV – ledipasvir, SOF – sofosbuvir, RBV – ribavirin, SD – standard deviation, BMI – body mass index, HCC – hepatocellular carcinoma, HCV – hepatitis C virus, F – fibrosis, TE – transient elastography, SWE – shear wave elastography, ARFI – acoustic radiation force impulse, HIV – human immunodeficiency virus, HBV – hepatitis B virus, HBsAg – hepatitis B surface antigen, ALT – alanine aminotransferase, HCV RNA – hepatitis C virus ribonucleic acid.
The decision on the length of treatment was at the discretion of the treating physician. Demographic, clinical, virologic and safety data were collected retrospectively through the treatment and post-treatment period and submitted online using a questionnaire administered by Tiba sp. z o.o. The efficacy end point was a sustained virologic response (SVR) defined as undetectable serum HCV RNA at least 12 weeks after the end of treatment. Safety evaluations included monitoring adverse events (AEs), serious AEs, deaths and laboratory abnormalities on treatment and during the follow-up period until SVR assessment. The therapy course modifications and discontinuations were also documented.
Statistical analysis
The results were expressed as mean ± standard deviation or n (%). P values of < 0.05 were considered to be statistically significant. Comparisons between groups were performed with analysis of non-parametric tests. The significance of differences was calculated using Fisher’s exact or χ2 test for categorical variables and by the Mann-Whitney U test for continuous variables. Statistical analyses were performed using GraphPad Prism 5.1 (GraphPad Software, Inc., La Jolla, CA).
Results
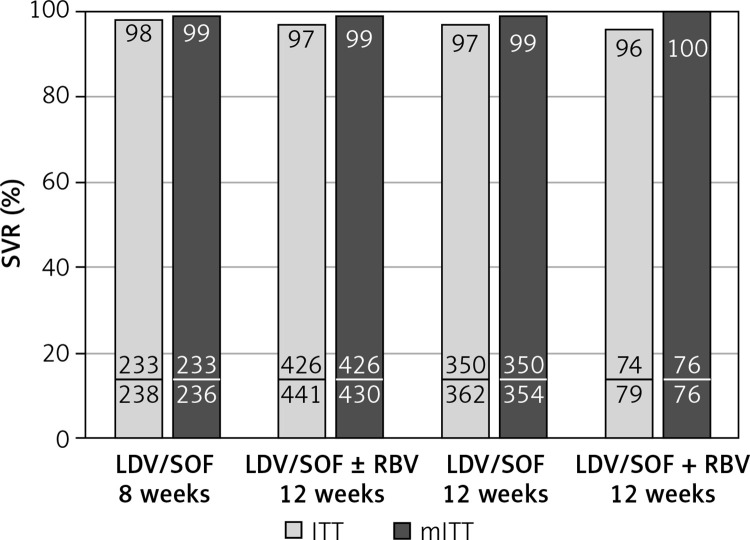
Baseline characteristics demonstrated that the 8-week regimen was administered more frequently to younger and female patients (Table I). The 12-week regimen was assigned significantly more frequently to patients with comorbidities, concomitant medications, advanced liver fibrosis, and HIV coinfected (Table I). Baseline viral load below 6 000 000 IU/ml was observed significantly more frequently in patients treated for 8 (95%) than 12 (89%) weeks. Overall, a sustained virologic response was achieved by 97% of patients. The SVR rate was similar after 8 and 12 weeks of therapy according to intent-to-treat analysis (98% vs. 97%) and reached 99% in both groups after exclusion of patients lost to follow-up and calculated as modified ITT (mITT) (Figure 1). As shown in Figure 1, among those treated for 12 weeks addition of RBV did not affect efficacy.
Figure 1.
Treatment effectiveness (SVR rate) of LDV/SOF with and without RBV administered for 8 or 12 weeks, calculated according ITT and mITT analysis; no statistically significant differences were noticed between particular groups
Of the 20 patients who did not achieve SVR, 7 were non-responders, whereas 13 were lost to follow-up, and 2 of them had a detectable viral load at the end of treatment. All 7 non-responders were infected with GT1b and completed the treatment course as scheduled; 3 of them were treated for 8 weeks and four for 12 weeks. In the 8-weeks arm all non-responders were females with a baseline viral load below 6 million IU/ml, and presented minimal or moderate liver fibrosis; one of them was a relapser to previous triple therapy with daclatasvir (Table II). In the 12-week arm all 4 non-responders were treatment-naïve with a baseline viral load below 6 million IU/ml; two of them had advanced liver fibrosis (F3) (Table III).
Table II.
Characteristics of 3 non-responders to 8-week LDV/SOF regimen
| Patient | Regimen | Genotype | History of previous therapy | Fibrosis | Baseline HCV RNA × 106 IU/ml | EOT | Comment |
|---|---|---|---|---|---|---|---|
| Female 1 | LDV/SOF | 1B | DCV + IFN + RBV | 1 | 1.34 | TND | Relapser after treatment with DCV + IFN + RBV |
| Female 2 | LDV/SOF | 1B | Treatment-naïve | 1 | 0.08 | TD | None |
| Female 3 | LDV/SOF | 1B | Treatment-naïve | 2 | 2.03 | TND | None |
LDV – ledipasvir, SOF – sofosbuvir, HCV RNA – hepatitis C virus ribonucleic acid, EOT – end of treatment, DCV – daclatasvir, IFN – interferon, RBV – ribavirin, TND – target not detected, TD – target detected.
Table III.
Characteristics of 4 non-responders to 12-week LDV/SOF±RBV regimens
| Patient | Regimen | Genotype | History of previous therapy | Fibrosis | Baseline HCV RNA × 106 IU/ml | EOT | Comment |
|---|---|---|---|---|---|---|---|
| Male 1 | LDV/SOF | 1B | Treatment-naïve | 3 | 2.87 | TND | Advanced fibrosis |
| Male 2 | LDV/SOF | 1B | Treatment-naïve | 3 | 0.62 | TD | Advanced fibrosis |
| Female 1 | LDV/SOF | 1B | Treatment-naïve | 2 | 0.20 | TND | None |
| Female 2 | LDV/SOF | 1B | Treatment-naïve | 2 | 0.46 | TND | None |
LDV – ledipasvir, SOF – sofosbuvir, HCV RNA – hepatitis C virus ribonucleic acid, EOT – end of treatment, TND – target not detected, TD – target detected.
Treatment modification was documented in 7 patients, mainly treated with RBV in the form of its dose reduction due to anemia. Therapy was permanently discontinued in 3 other patients treated for 12 weeks; one of them was lost to follow-up and two achieved SVR (Table IV).
Table IV.
Treatment course, modification and discontinuation, and safety data according to regimen
| Parameter | 8 weeks no RBV n = 238 | 12 weeks no RBV n = 362 | 12 weeks + RBV n = 79 |
|---|---|---|---|
| Treatment course, n (%): | |||
| Therapy discontinuation | 0 | 2 (0.6) | 1 (1.3) |
| Therapy modification | 1 (0.4) | 1 (0.3) | 5 (6.3) |
| Patients with at least one AE, n (%) | 7 (3) | 52 (14.4) | 32 (40.5)# |
| Serious adverse events* | 0 | 1 | 1 |
| Most common AEs (> 2%), n (%): | |||
| Weakness/fatigue | 1 (0.4) | 16 (4.4) | 14 (17.7)# |
| Sleep disorder: | 0 | 5 (1.4) | 2 (2.5) |
| Headache | 2 (0.8) | 10 (2.8) | 3 (4) |
| Anemia | 0 | 2 (0.5) | 6 (8) |
| Death in treatment course** | 2 | 0 | 0 |
p < 0.001 vs. LDV/SOF without RBV.
Stomach cancer, stroke.
Not related to antiviral therapy: after bone-marrow transplantation in patient with aplastic anemia, unknown reason.
RBV – ribavirin, AE – adverse event.
Overall, 91 (13%) patients experienced at least one adverse event, mainly from the group treated for 12 weeks with RBV. However, in the majority adverse events (AE) were mild and the most common were weakness/fatigue, headache, sleep disorders and anemia. As shown in Table IV, AEs and weakness/fatigue were significantly more frequent in patients treated with RBV. Two serious adverse events (SAE) were documented in patients treated for 12 weeks. Two deaths were noted in the 8-week regimen group, but they were assessed as not related to antiviral medication.
Discussion
A shortened treatment course of the LDV/SOF regimen has been available since registration in 2015 based on the results of the ION-3 study [5]. According to SmPC this option was recommended for use in treatment-naïve patients infected with GT 1 without liver cirrhosis [8]. Although the LDV/SOF combination has been largely evaluated in clinical trials and real-life populations, surprisingly a literature search provided only a few real-world studies that compared the effectiveness of 8- versus 12-week regimens of LDV/SOF in patients eligible for 8-week therapy. Moreover, these few studies were carried out in American populations, which are not representative for European patients. Marcus et al. [11] demonstrated similar effectiveness of both regimens in black patients, who usually demonstrate a different response profile to HCV treatment compared to Caucasians. Similar data were also obtained by Curry et al. [12], but patients were infected mostly with GT 1a, typical for the American population, in contrast to GT 1b, predominant in Europe. The presented real-world study demonstrates for the first time a very high SVR rate of 99% regardless of LDV/SOF treatment for either 8 or 12 weeks in European, Caucasian patients infected with GT 1b, who should be treated according to guidelines and SmPC for 8 weeks.
High effectiveness and no difference between 8- and 12-week regimens correspond to results obtained in the ION-3 registration clinical trial and real-world studies which did not compare length of treatment effectiveness in those eligible for 8-week therapy [5, 13–17]. However, according to the study by Backus et al. [16] carried out in the real-world setting of USA patients infected with GT 1 and viral load below 6 million IU/ml the SVR rate was significantly lower among those treated for 8 weeks (93%) than those receiving therapy for 12 weeks (97%). The presented results support the observations made by our team a few years ago in the Harvest study, which demonstrated a 100% SVR rate in a small group of patients treated with 8 weeks of LDV/SOF before implementation of the shortened regimen to SmPC [17]. The currently presented data clearly demonstrate a lack of any effect of high viral load on the effectiveness of LDV/SOF treatment, because all 61 patients with a baseline HCV RNA level exceeding 6 million IU/ml finally achieved SVR regardless of the treatment duration. This observation from the real-world setting supports SmPC and expert guidelines based on clinical trials with respect to criteria of possible shortening of treatment to 8 weeks regardless of baseline viral load [6–10].
In this study we also demonstrated that addition of RBV to 12 weeks of LDV/SOF did not improve the effectiveness of treatment, but significantly increased the rate of adverse events to 40% compared to 14% in the non-RBV group. LDV/SOF without ribavirin demonstrated a favorable safety and tolerability profile irrespective of treatment duration. This results support conclusions of ION-1 and ION-2, registration clinical studies [18, 19]. Therapy was well tolerated, especially in the group of patients treated for 8 weeks, where we did not report any SAEs, and only 3% patients had mild AE during the treatment. The only 2 deaths were observed in the 8-week regimen arm, but they were not related to the treatment of HCV infection. A similar safety profile of LDV/SOF was achieved in clinical studies, which proved favorable outcomes for 8-weeks use of LDV/SOF in treatment-naive non-cirrhotic patients [20].
Administration of LDV/SOF extended to 12 weeks in 441 patients was caused by lack of 8-week option in the reimbursement protocol at the beginning of the study period and then possible fear of ineffectiveness among physicians. Therefore the 12-week regimen was more often prescribed for potentially difficult to treat patients with advanced fibrosis (F3), co-morbidities and infected with GT 1a. Physicians also recognized HIV-coinfected patients as difficult to treat, which is no longer true because all 41 such patients included in this study achieved SVR regardless of treatment duration and RBV administration, which was also documented in the recent study by Vega et al. [21]. None of the 16 patients who underwent liver transplantation received the 8-week regimen, but according to the study by Kwok et al. [22] even in this difficult-to-treat population there is no need to extend therapy. Therefore at this moment based on the clinical trials and real-world experience it is difficult to indicate among genotype 1 infected non-cirrhotics which patients need extension of the treatment with LDV/SOF. Unfortunately with currently available data we are not able to compare the effectiveness between arms in patients with advanced liver fibrosis (F3), because a large majority received the 12-week regimen, but it is worth mentioning that all 3 such patients included in the 8-week arm achieved SVR.
Among non-responders to the 8-week regimen, there was only one whose failure could be explained by previous unsuccessful exposure to daclatasvir administered with IFN and RBV, but it was not proven by the RAS testing before re-treatment with LDV/SOF. In 2 other cases of failure of the 8-week regimen, we were not able to identify a possible reason for relapse after treatment termination, but due to the low baseline viral load in these patients the most probable explanation could be non-adherence to prescribed therapy.
Our study has several limitations associated with the non-randomized nature, retrospective observational design resulting with possible insufficient documentation of minor adverse events, electronic data capture resulting in potential physician bias and data entry errors. The strength of our study is the large number of patients enrolled in numerous sites reflecting the central European HCV population treated in routine medical practice. Moreover it is worth mentioning the low rate of patients lost to follow-up (2%).
In conclusion, we confirmed 99% effectiveness of both 8- and 12-week regimens of LDV/SOF in non-cirrhotic, HCV genotype 1 (mostly 1b) infected patients eligible for 8-week therapy according to current label and expert recommendations. This real-world study also demonstrated no need of RBV addition and no effect of major baseline factors, including viral load, on the treatment effectiveness in this population. Moreover, we showed that shortening of therapy significantly improved the safety profile of LDV/SOF medication.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.WHO Global Hepatitis Report 2017 (accessed on 21 September 2018).
- 2.Polaris Observatory HCV Collaborators . Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2: 161-76. [DOI] [PubMed] [Google Scholar]
- 3.Flisiak R, Pogorzelska J, Berak H, et al. Prevalence of HCV genotypes in Poland – the EpiTer study. Clin Exp Hepatol 2016; 2: 144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leszczyszyn-Pynka M, Ciejak P, Maciejewska K, et al. Hepatitis C coinfection adversely affects the life expectancy of people living with HIV in northwestern Poland. Arch Med Sci 2018; 14: 554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowdley KV, Gordon SC, Reddy KR, et al.; ION-3 Investigators . Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370: 1879-88. [DOI] [PubMed] [Google Scholar]
- 6.EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatology 2017; 66: 153-94. [DOI] [PubMed] [Google Scholar]
- 7.EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatology 2018; 69: 461-511. [DOI] [PubMed] [Google Scholar]
- 8.Harvoni Summary of Product Characteristics Gilead.
- 9.Halota W, Flisiak R, Boroń-Kaczmarska A, et al. Recommendations for the treatment of hepatitis C issued by the Polish Group of HCV Experts – 2016. Clin Exp Hepatol 2016; 2: 27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halota W, Flisiak R, Juszczyk J, et al. Recommendations for the treatment of hepatitis C in 2017. Clin Exp Hepatol 2017; 3: 47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus JL, Hurley LB, Chamberland S, et al. No difference in effectiveness of 8 vs 12 weeks of ledipasvir and sofosbuvir for treatment of hepatitis C in black patients. Clin Gastroenterol Hepatol 2018; 16: 927-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curry MP, Tapper EB, Bacon B, et al. Effectiveness of 8- or 12-weeks of ledipasvir and sofosbuvir in real-world treatment-naïve, genotype 1 hepatitis C infected patients. Aliment Pharmacol Ther 2017; 46: 540-8. [DOI] [PubMed] [Google Scholar]
- 13.Buggisch P, Vermehren J, Mauss S, et al. Real-world effectiveness of 8-week treatment with ledipasvir/sofosbuvir in chronic hepatitis C. J Hepatol 2018; 68: 663-71. [DOI] [PubMed] [Google Scholar]
- 14.Kowdley KV, Sundaram V, Jeon CY, et al. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C virus infection. Hepatology 2017; 65: 1094-103. [DOI] [PubMed] [Google Scholar]
- 15.Hezode C. Treatment of hepatitis C: results in real life. Liver Int 2018; 38 Suppl 1: 21-7. [DOI] [PubMed] [Google Scholar]
- 16.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology 2016; 64: 405-14. [DOI] [PubMed] [Google Scholar]
- 17.Flisiak R, Łucejko M, Mazur W, et al. Effectiveness and safety of ledipasvir/sofosbuvir ± ribavirin in the treatment of HCV infection: the real-world HARVEST study. Adv Med Sci 2017; 62: 387-92. [DOI] [PubMed] [Google Scholar]
- 18.Afdhal N, Zeuzem S, Kwo P, et al. ION-1 Investigators . Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370: 1889-98. [DOI] [PubMed] [Google Scholar]
- 19.Afdhal N, Reddy KR, Nelson DR, et al.; ION-2 Investigators . Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370: 1483-93. [DOI] [PubMed] [Google Scholar]
- 20.Andres J, Lott S, Qureshi K. Eight-week outcomes of ledipasvir/sofosbuvir in noncirrhotic treatment-naive patients with hepatitis C: analysis of pharmacy-based data. J Manag Care Spec Pharm 2018; 24: 23-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vega AD, Hynicka LM, Claeys K, Chua JV, Heil EL. Effectiveness of 8 weeks of ledipasvir/sofosbuvir for hepatitis C in HCV-HIV-coinfected patients. Antivir Ther 2019; 24: 11-7. [DOI] [PubMed] [Google Scholar]
- 22.Kwok RM, Ahn J, Schiano TD, et al. Sofosbuvir plus ledipasvir for recurrent hepatitis C in liver transplant recipients. Liver Transpl 2016; 22: 1536-43. [DOI] [PubMed] [Google Scholar]