Abstract
Introduction
Noise-induced hearing loss is one of the most prevalent causes of hearing impairment and occupational diseases. Although multiple factors lead to noise-induced hearing loss, prevention and protection strategies remain limited. Studies in the past decade have employed antioxidants, especially N-acetyl-cysteine, to prevent noise-induced hearing loss. Therefore, this systematic review and meta-analysis of randomized controlled trials evaluated the effect of N-acetyl-cysteine on the prevention of noise-induced hearing loss.
Material and methods
This systematic review and meta-analysis included relevant studies from the Cochrane Library, EMBASE, PubMed, ScienceDirect, Scopus, and Web of Science by using related terms. The study only included randomized controlled trials in meta-analyses and assessed the quality of the identified randomized controlled trials by using the Cochrane Risk of Bias tool. Two authors extracted and calculated data on characteristics and hearing threshold. The results are presented as weighted mean difference (WMD) with 95% confidence interval (CI).
Results
This study identified five randomized controlled trials that randomized 1,115 patients into N-acetyl-cysteine and control groups. The meta-analysis evidenced that N-acetyl-cysteine has greater protective effects against hearing threshold shifts than the control in the 0 to 4 kHz (WMD = –3.39, 95% CI: –6.56 to –0.22) and 0 to 6 kHz (MD = –3.49, 95% CI: –6.57 to –0.41) subgroups.
Conclusions
The present review and meta-analysis recommends that N-acetyl-cysteine may be considered as an option for protective therapy for noise-induced hearing loss. Nonetheless, larger randomized controlled trials are requisite for further investigation and verification.
Keywords: N-acetyl-cysteine, antioxidants, noise-induced hearing loss
Introduction
Noise-induced hearing loss is one of the most prevalent causes of hearing impairment and occupational diseases, and no effective biological protection or cure is currently available. Based on a 2011–2012 Centers for Disease Control and Prevention study involving hearing tests and interviews with participants, it was estimated that 40 million adults (24%) have hearing loss in one or both ears from exposure to loud noise [1]. Nearly 17% of teenagers (ages 12–19 years) have features of their hearing test suggestive of noise-induced hearing loss in one or both ears, based on the data between 2005 and 2006 [2]. Worldwide, 16% of disabling hearing loss in adults is due to occupational noise, ranging from 7% to 21% in various subregions [1]. Firearms and some industrial equipment can generate very high levels of impulse noise, which is known to cause sensorineural hearing loss.
Although multiple factors lead to the occurrence of noise-induced hearing loss, the prevention and protection strategies remain limited. Previous studies have demonstrated that physical or mechanical mechanisms cause noise-induced hearing loss [3, 4]; however, recently, the role of metabolic mechanisms has been introduced [5–8]. Recent studies have revealed that noise causes oxidative stress and the generation of reactive oxygen species and free radicals, leading to apoptosis and hair cell or neuronal death [5, 9, 10]. Glutathione (GSH) is a principal cochlear antioxidant in the liver and many other organs. It reduces cochlear injury after noise exposure, possibly because of its role in preventing free radical damage [11]. When excessive amounts of reactive oxygen species or free radicals are released, the natural endogenous defenses can be overwhelmed, resulting in the reduction of GSH and possible cell injury or death [9]. When GSH cellular levels are reduced, noise-induced damage to the cochlea intensifies and thresholds increase [11, 12]. Consequently, therapeutic interventions have focused on the administration of exogenous sources of antioxidants or the stimulation of endogenous antioxidants to prevent noise-induced ototoxicity.
Of the antioxidants that have been investigated, N-acetyl-cysteine, a precursor and limiting factor in the synthesis of GSH, has been demonstrated to protect the inner ear from oxidative damage in the laboratory. Furthermore, N-acetyl-cysteine has been a safe and effective Federal Drug Administration-approved oral treatment for acetaminophen overdose for over 20 years, whereas its most commonly reported adverse effects for seldom-used IV formulations are rash and itchiness. The beneficial effects of N-acetyl-cysteine in preventing cell damage or death from noise exposure in animal models (chinchillas and rats) offer promise for using antioxidant agents as a protective therapy against noise-induced cochlear damage in humans [9, 11]. However, N-acetyl-cysteine has only been tested in combination with corticosteroids for treating sudden deafness. Whether antioxidants protect humans from noise-induced hearing loss remains clinically controversial.
Therefore, the effect of N-acetyl-cysteine alone on noise-induced hearing loss is still unknown. In this study, we conducted a meta-analysis of prospective randomized controlled trials to analyze whether noise-induced hearing loss could be significantly improved by the prophylactic oral administration of N-acetyl-cysteine.
Material and methods
This systematic review and meta-analysis reports methods and outcomes according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13, 14]. Because this study used published data, the study was exempt from institutional review board approval.
Search and study selection
The searches for relevant studies that examined the role of N-acetyl-cysteine in preventing noise-induced hearing loss were conducted using the following relevant terms and medical subheadings: N-acetyl-cysteine, acetylcysteine, Acetin, NAC, noise induced hearing loss, noise induced deafness, sound induced hearing loss, sound induced deaf, and sound induced deafness in the Cochrane Library (involving the Central Register of Controlled Trials), EMBASE, PubMed (involving MEDLINE), ScienceDirect, Scopus, and Web of Science. Two authors completed the systematic literature searches by using both free-text words and medical subject headings (MeSH for PubMed and Emtree for EMBASE) with Boolean algebras and without restrictions of language or publication date (Supplementary Table SI). The final search was completed on February 9th 2019.
Two authors (Liu and Kang) screened the returned citation records, and the third author (Zhang) resolved any disagreement in screening categorization between the two authors. The inclusion criteria for the title and abstract screening were as follows: (i) patients had noise-induced hearing loss and (ii) patients received N-acetyl-cysteine medication. The exclusion criteria for further and full-text screening were as follows: (i) not a randomized controlled trial, (ii) incomplete study report (conference report), and (iii) relevant document.
Quality assessment of included studies
In this systematic review, we assessed the quality of the included studies by using the Cochrane Risk of Bias tool. This appraisal tool recommends that randomized controlled trials should be judged on the basis of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. The tool comprises seven items, namely random sequence generation, allocation concealment, blinding of participants and personnel, blinding of assessment, incomplete outcome data, selective reporting, and other sources of bias. The two authors judged all the included randomized controlled trials by using the Cochrane Risk of Bias tool individually. In case of any disagreement, the third author participated in the discussion and resolved it.
Data extraction and analysis
In this systematic review, we extracted information on trials, patient characteristics, and hearing threshold changes. The data on hearing threshold changes were extracted and checked by the two authors independently. They identified and double-checked the meaning of the data and calculated hearing threshold changes when articles provided relevant information on the baseline and endpoint values of the hearing threshold. The authors estimated the standard deviation (SD) based on the sample size (standard error (SE) = SD/√N), when the trials reported mean and SE. Moreover, the authors estimated mean and SD, when the trials presented the median with the minimum value and maximum value only [15].
Results
Literature search and selection
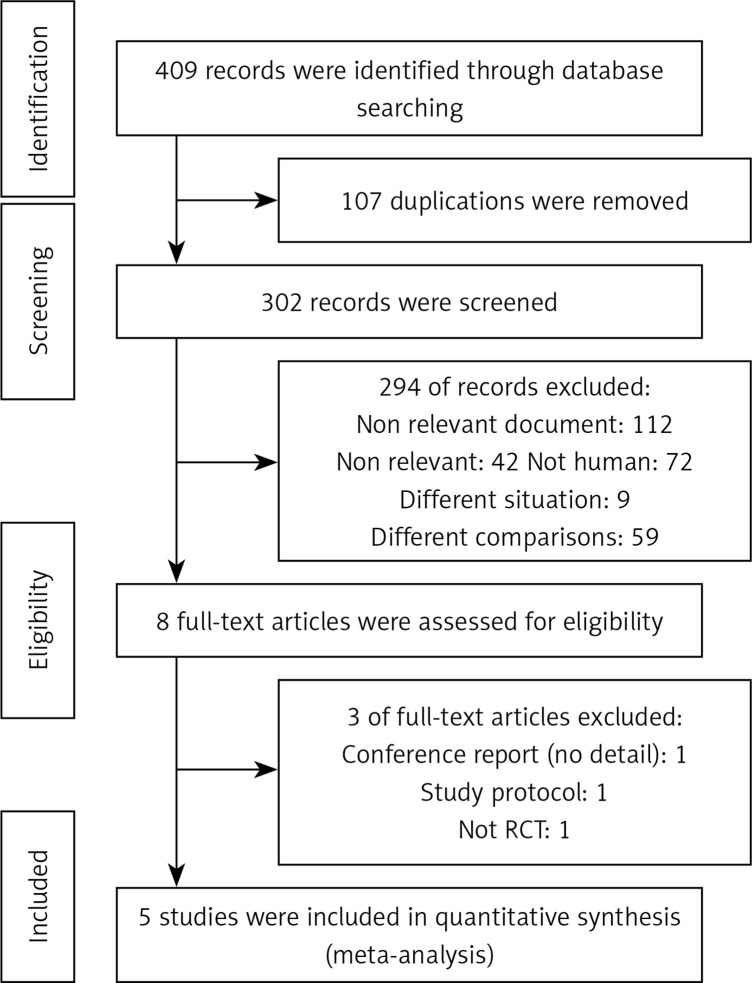
The comprehensive search identified 409 records, of which 62, 51, 172, 53, and 71 were from Embase, PubMed (including MEDLINE), ScienceDirect (previous version, 2018), Scopus, and Web of Science respectively. In the duplicating step, 107 records were removed, of which 83 were systematically removed using EndNote software and 24 were manually removed by the authors. In the title and abstract screening step, 294 records did not meet the inclusion criteria and were excluded. In the full-text screening step, three records were excluded because they were a conference report, study protocol, and not a randomized controlled trial, respectively (Figure 1). Furthermore, we also searched Cochrane Library, and found two Cochrane systematic reviews. In the Controlled Register of Trials (CENTRAL), we found 10 trial registries.
Figure 1.
Study identification diagram according to PRISMA
Characteristics of included studies
This systematic review and meta-analysis identified five randomized controlled trials that randomized 1,115 patients into N-acetyl-cysteine and control groups [16–20]. The characteristics of the trials are presented in Table I, including the study location, inclusion years, sample size, age, patient sex, dosage of NAC, noise exposure, side effects, follow-up period, and loss to follow-up. In the five randomized controlled trials, one was from China [17], one was from Iran [16], one was from Taiwan [20], and two were from the United States [18, 19]. The randomized controlled trials recruited young to middle-aged participants (approximately 19 to 42 years old). The minimum dosage of NAC was 900 mg/day, and the maximum dosage was 2700 mg/day. The trials found few side effects, and they lost few participants to follow-up (< 20%).
Table I.
Characteristics of the included randomized controlled trials
| Item | Doosti et al. (2014) [16] | Ge et al. (2011) [17] | Kopke et al. (2015) [18] | Kramer et al. (2006) [19] | Lin et al. (2010) [20] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAC | Control | NAC | Control | NAC | Control | NAC | Control | NAC | Control | |
| Location | Iran | China | USA | USA | Taiwan | |||||
| Inclusion years | 2013.02–2013.03 | NR | 2004.03–2004.10 | NR | NR | |||||
| Sample size | 17 | 17 | 223 | 140 | 317 | 317 | 15 | 16 | 25 | 28 |
| Age | 39.38 ±6 | 39.62 ±4 | 27.3 ±6.5 | 19.43 | 19.82 | 19–29 | 40 ±11.6 | 42.1 ±9.6 | ||
| Sex (male) | NR | NR | 100% | 100% | NR | NR | NR | NR | 100% | 100% |
| NAC dosage | 1200 mg/day | 1200 mg/day | 2700 mg/day | 900 mg/time | 1200 mg/day | |||||
| Exposure: | ||||||||||
| Hz | NR | NR | NR | NR | NR | NR | NR | NR | 31.5 Hz to 8 kHz | 31.5 Hz to 8 kHz |
| Bd | > 85 | > 85 | NR | NR | 2.2 ±2 | 2.2 ±2 | 92.5–102.8 | 92.5–102.8 | 88.4–88.8 | 88.8–89.4 |
| Side effect | NR | NR | 0 (0%) | 0 (0%) | 26.7% | 27.4% | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Follow-up period | 15 days | 15 days | NR | NR | NR | NR | 2 h | 2 h | 42 days | 42 days |
| Loss to follow-up | 1 (6%) | 1 (6%) | 0% | 0% | 277 (12.6%) | 289 (8.83%) | 0% | 0% | 0% | 0% |
NAC – N-acetyl-cysteine, NR – not reported.
Quality of included evidence
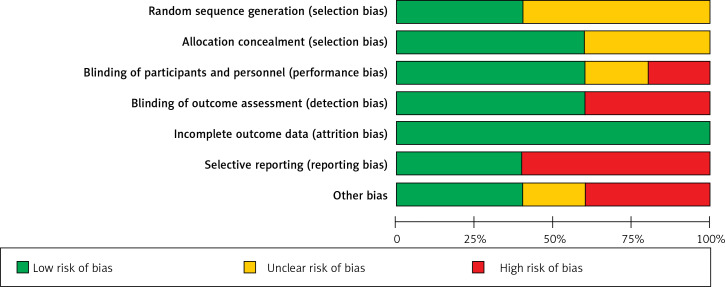
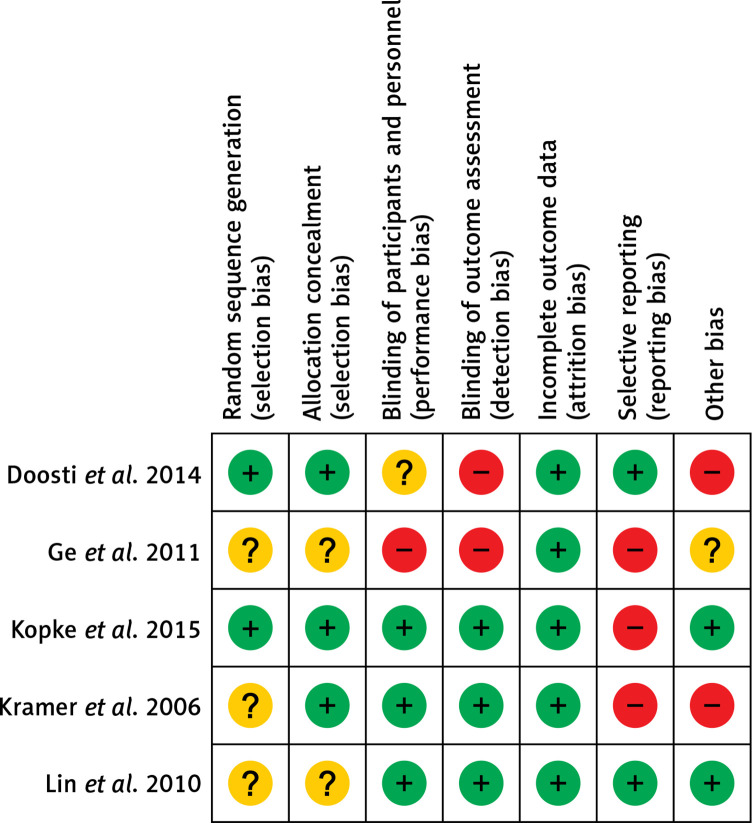
The quality of the five included randomized controlled trials was individually evaluated, and the results are presented in Figure 2. The only study from China had a high risk of bias for three items (blinding of participants and personnel, blinding of assessment, and selective reporting) [17], and the other two studies had a high risk of bias for two items [16, 19]. Another trial from the United States had only reporting bias [18], and another randomized controlled trial from Taiwan had no risk of bias, according to the Cochrane Risk of Bias tool [20]. In summary, the quality of the included studies was fair, except if attrition bias was considered. More than 50% of the randomized controlled trials were assessed to be of unclear or high risk of selection bias, reporting bias, and other bias. Furthermore, 50% of the included trials were assessed to be of unclear or high risk of performance bias and detection bias (Figure 3).
Figure 2.
Risk of bias of the included trials
Figure 3.
Summary of risk of bias
Differences in hearing threshold changes
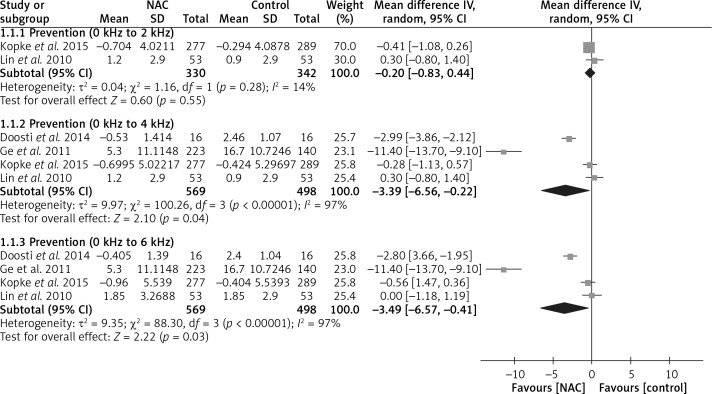
Four included randomized controlled trials with 1,067 patients reported hearing threshold changes [16–18, 20]. The evidence of pooled data from two trials showed no significant differences in mean hearing threshold changes between the N-acetyl-cysteine and control groups at 0 to 2 kHz, and the weighted mean difference (WMD) was –0.20 (95% confidence interval (CI): –0.83 to 0.44, I 2 = 14%). However, the evidence indicates that N-acetyl-cysteine has greater protective effects against hearing threshold shifts than the control in both the 0 to 4 kHz and 0 to 6 kHz subgroups, and the WMDs were −3.39 (95% CI: –6.56 to –0.22, I 2 = 97%) and −3.49 (95% CI: –6.57 to –0.41, I 2 = 97%), respectively (Figure 4).
Figure 4.
Forest plot of meta-analysis for mean hearing threshold changes
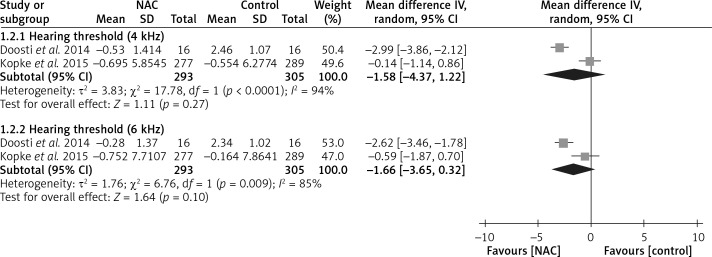
Two included trials reported hearing thresholds that were separated by Hz interval [16, 18]. They analyzed the hearing thresholds of 598 patients randomized to two groups at 4 and 6 kHz. Although no significant difference was found in hearing threshold changes between the N-acetyl-cysteine and control groups in both the 4 kHz (WMD = –1.58, 95% CI: –4.37 to 1.22, I 2 = 94%) and 6 kHz (WMD = –1.66, 95% CI: –3.65 to 0.32, I 2 = 85%) subgroups, the forest plot revealed the trend that the outcomes favor N-acetyl-cysteine in both subgroups (Figure 5). Moreover, the trend in the 6 kHz subgroup was more favorable toward N-acetyl-cysteine than that in the 4 kHz subgroup.
Figure 5.
Forest plot of meta-analysis for hearing threshold changes
Discussion
Contribution of N-acetyl-cysteine to noise-induced hearing loss
Many mechanisms are involved in noise-induced cochlear injury, including oxidative stress, inflammation, and glutamate excitotoxicity [21]. They can induce apoptosis in cochlear cells, leading to hearing loss. Because free oxygen radicals can influence the cells in the human body through oxidative stress [21], antioxidants are considered to be effective against this condition. GSH, an antioxidant, can protect human body cells from damage due to reactive oxygen species [21]. It can reduce cochlear injury caused by noise exposure and can be considerably beneficial in alleviating noise-induced hearing loss [22]. N-acetyl-cysteine, an antioxidant containing drug, has been reported to be effective and protective against damage due to noise exposure in an animal model [23]; however, data on its effectiveness in humans are still limited [16–20]. N-acetyl-cysteine increases the synthesis of intracellular GSH and directly scavenges free oxygen radicals [24, 25], thus preventing cochlear cells from undergoing apoptosis. However, no sufficient evidence is available to determine the specific frequencies or hearing thresholds that are protected by N-acetyl-cysteine among patients exposed to noise.
Evidence derived from the present meta-analysis reveals that N-acetyl-cysteine might protect middle to high hearing thresholds (0 to 4 kHz or 0 to 6 kHz), but it might have no significant effect on low hearing thresholds (0 to 2 kHz). For certain hearing thresholds, a trend of the benefits provided by N-acetyl-cysteine was evidenced. This condition may be associated with the trend observed in this study, which indicates that a higher damage level caused by noise may lead to a more apparent protective effect by N-acetyl-cysteine. The protective effects on low noise frequencies might be limited, because a previous study indicated that the outer ear (or ear canal) and the mechanical properties of the middle ear are most affected by noise of approximate frequencies of 4 kHz [26].
Moreover, the present review and meta-analysis has some other limitations. First, the result showed a wide range of noise frequencies but could not differentiate between certain levels of actual noise exposure, which may be a critical limitation. Second, one of the included trials involving loud noise in nightclubs presented a definition of noise exposure different from those in the other included randomized controlled trials [19]. Third, because of the different ranges of noise exposure across the included trials, obtaining consistent results was difficult. Fourth, the recruited participants of the three included randomized controlled trials were male, with a military background, and across a narrow age range [16–18, 20]. Therefore, the range of noise exposure that was protectable by N-acetyl-cysteine could not be determined. Fourth, the diverse frequencies of hearing threshold in each randomized controlled trial administration posed a challenge. This made it difficult to analyze randomized controlled trials satisfactorily. In addition, all the included randomized controlled trials involving the use of antioxidants for hearing loss protection had short follow-up periods. Therefore, the long-term effectiveness of N-acetyl-cysteine cannot be proven. Thus, additional randomized controlled trials involving larger numbers of individuals exposed to a wider range of noise and different dose regimens are warranted to verify the effectiveness of N-acetyl-cysteine in hearing loss protection.
In conclusion, this is the first systematic review and meta-analysis on hearing loss protection by using N-acetyl-cysteine. The major finding of this review and meta-analysis is that N-acetyl-cysteine may act as an antioxidant for the protection of noise-induced hearing loss, especially around middle to high hearing thresholds (0 to 4 kHz or 0 to 6 kHz). Moreover, in the five included randomized controlled trials, few complications were observed among the participants treated with N-acetyl-cysteine. This systematic review and meta-analysis suggests that N-acetyl-cysteine may be considered as an option of protective therapy for noise-induced hearing loss. Nonetheless, larger randomized controlled trials are required for further investigation and verification.
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
References
- 1.Nelson DI, Nelson RY, Concha-Barrientos M, Fingerhut M. The global burden of occupational noise-induced hearing loss. Am J Industrial Med 2005; 48: 446-58. [DOI] [PubMed] [Google Scholar]
- 2.Henderson E, Testa MA, Hartnick C. Prevalence of noise-induced hearing-threshold shifts and hearing loss among US youths. Pediatrics 2011; 127: 39-46. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins JE Jr, Johnsson LG, Stebbins WC, Moody DB, Coombs SL. Hearing loss and cochlear pathology in monkeys after noise exposure. Acta Otolaryngol 1976; 81: 337-43. [DOI] [PubMed] [Google Scholar]
- 4.Mulroy MJ, Henry WR, McNeil PL. Noise-induced transient microlesions in the cell membranes of auditory hair cells. Hear Res 1998; 115: 93-100. [DOI] [PubMed] [Google Scholar]
- 5.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear 2006; 27: 1-19. [DOI] [PubMed] [Google Scholar]
- 6.Lim DJ, Melnick W. Acoustic damage of the cochlea: a scanning and transmission electron microscopic observation. Arch Otolaryngol 1971; 94: 294-305. [DOI] [PubMed] [Google Scholar]
- 7.Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol 2000; 1: 243-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slepecky N. Overview of mechanical damage to the inner ear: noise as a tool to probe cochlear function. Hear Res 1986; 22: 307-21. [DOI] [PubMed] [Google Scholar]
- 9.Kopke R, Allen KA, Henderson D, Hoffer M, Frenz D, Van de Water T. A radical demise. Toxins and trauma share common pathways in hair cell death. Ann N Y Acad Sci 1999; 884: 171-91. [DOI] [PubMed] [Google Scholar]
- 10.Lynch ED, Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov Today 2005; 10: 1291-8. [DOI] [PubMed] [Google Scholar]
- 11.Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res 2003; 966: 265-73. [DOI] [PubMed] [Google Scholar]
- 12.Yamasoba T, Nuttall AL, Harris C, Raphael Y, Miller JM. Role of glutathione in protection against noise-induced hearing loss. Brain Res 1998; 784: 82-90. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: 2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006-12. [DOI] [PubMed] [Google Scholar]
- 15.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doosti A, Lotfi Y, Moossavi A, Bakhshi E, Talasaz AH, Hoorzad A. Comparison of the effects of N-acetyl-cysteine and ginseng in prevention of noise induced hearing loss in male textile workers. Noise Health 2014; 16: 223-7. [DOI] [PubMed] [Google Scholar]
- 17.Ge Z, Ma S, Jia X, Song L. Study of protective effects on noise-induced hearing loss using N-acetyl-cysteine. J Clin Otorhinolaryngol Head Neck Surg 2011; 25: 1040-1. [PubMed] [Google Scholar]
- 18.Kopke R, Slade MD, Jackson R, et al. Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: a randomized clinical trial. Hear Res 2015; 323: 40-50. [DOI] [PubMed] [Google Scholar]
- 19.Kramer S, Dreisbach L, Lockwood J, et al. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J Am Acad Audiol 2006; 17: 265-78. [DOI] [PubMed] [Google Scholar]
- 20.Lin CY, Wu JL, Shih TS, et al. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res 2010; 269: 42-7. [DOI] [PubMed] [Google Scholar]
- 21.Sakat MS, Kilic K, Bercin S. Pharmacological agents used for treatment and prevention in noise-induced hearing loss. Eur Arch Otorhinolaryngol 2016; 273: 4089-101. [DOI] [PubMed] [Google Scholar]
- 22.Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res 2000; 878: 163-73. [DOI] [PubMed] [Google Scholar]
- 23.Bielefeld EC, Kopke RD, Jackson RL, Coleman JK, Liu J, Henderson D. Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Otolaryngol 2007; 127: 914-9. [DOI] [PubMed] [Google Scholar]
- 24.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Rad Biol Med 1989; 6: 593-7. [DOI] [PubMed] [Google Scholar]
- 25.Gillissen A, Jaworska M, Orth M, et al. Nacystelyn, a novel lysine salt of N-acetylcysteine, to augment cellular antioxidant defence in vitro. Respir Med 1997; 91: 159-68. [DOI] [PubMed] [Google Scholar]
- 26.Pierson LL, Gerhardt KJ, Rodriguez GP, Yanke RB. Relationship between outer ear resonance and permanent noise-induced hearing loss. Am J Otolaryngol 1994; 15: 37-40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.