Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening syndrome involving excessive immune activation. It can be primary (familial) or secondary (triggered by infection, malignancy, or rheumatological disease).
This is a case of a previously healthy 43-year-old African American woman who presented with fever and confusion. The patient was eventually diagnosed with pulmonary aspergillosis and responded well to antifungal therapy. She met the diagnostic criteria of HLH-2004 trial for hemophagocytic lymphohistiocytosis. She also fulfilled the 2019 classification criteria for systemic lupus erythematosus (SLE) without the classical signs and symptoms of SLE.
HLH management includes supportive management, treatment of underlying condition, and immunosuppressive treatment. Etoposide and dexamethasone are commonly used treatments for HLH; however, underlying active infection can limit the treatment options. In our case, the patient was treated with steroids and hydroxychloroquine. Her condition gradually improved and she recovered without complications.
Based on our literature review, we encountered six cases of HLH secondary to Aspergillosis with a mean age of approximately 47 years. The diagnosis of HLH is often delayed because of nonspecific presentation. Early identification and treatment are crucial to improve the survival rate.
Keywords: neurological signs and symptoms, secondary hlh, immune-mediated inflammatory disorder, pulmonary aspergillosis, hemophagocytic lymphohistiocytosis (hlh)
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening syndrome of inflammation and tissue injury secondary to excessive activation of the immune system. It has a wide range of nonspecific symptoms that make the diagnosis difficult. Familial and sporadic cases were reported. The disease can affect any age, but it is more common in the pediatric population.
The pathogenesis is believed to be secondary to the failure of normal elimination of activated macrophages by cytotoxic lymphocytes and natural killer cells leading to poor macrophage function, production of large amounts of cytokines, and phagocytosis of host cells which cause excessive inflammation and tissue injury. Cytokines can be found elevated in HLH, including interferon gamma, chemokine CXCL9, tumor necrosis factor alpha (TNF alpha), interleukin-6, interleukin-10, interleukin-12, and soluble interleukin-2 receptor (CD25) [1-4].
HLH can be triggered by infections, malignancies, and rheumatological disorders. Infectious triggers include viruses (Epstein-Barr virus (EBV), cytomegalovirus (CMV), parvovirus, herpes simplex virus, varicella-zoster virus, measles virus, human herpes virus 8, H1N1 influenza virus, and human immunodeficiency virus (HIV)), bacteria, parasites and fungi. Mutations in genes that cause familial hemophagocytic lymphohistiocytosis (FHL) are commonly found in younger patients. Several genes have been detected in familial cases, including STX11, PRF1, and UNC13D. The disease may be isolated or recurrent. Recurrent HLH usually occurs with a genetic predisposition [5].
Case presentation
We present a case of a 43-year-old African-American female with no significant past medical or surgical history who was brought to the hospital after being found confused.
Vital signs were significant for a temperature of 39.1°C. The physical examination revealed bilateral basal and mid-lung crackles. Initial laboratory tests showed low neutrophil, red blood cell, and platelet counts. Hemoglobin was low and serum creatinine was elevated. Ferritin was significantly increased. She also had elevated aspartate aminotransferase (Table 1).
Table 1. laboratory tests.
Neut: neutrophils, Hb: hemoglobin, RBC: red blood cell, Plt: platelet, S.creatinine: serum creatinine, ALT: alanine transaminase, AST: aspartate aminotransferase, TGL: triglycerides, LDH: lactate dehydrogenase, ANA: antinuclear antibody, ab: antibodies, anti-RNP: antinuclear ribonucleoprotein
| Laboratory tests | On the day of presentation | Two days after presentation | Reference range |
| Neut | 1,000/µl | 1,300/µl | 2500-8000/µl |
| Hb | 8 g/dl | 8.5 g/dl | 11.6-15 g/dl |
| RBC | 2.82 million/ µl | 2.79 million/ µl | 3.92-5.13 million/µl |
| Plt | 34,000/ µl | 31,000/ µl | 150.000-450.000/µl |
| S. Creatinine | 1.33 mg/dl | 0.94 mg/dl | 24-336 ng/ml |
| Ferritin | 5,838 µg/l | 6,432 µg/l | 0.59-1.04 mg/dl |
| ALT | 40 IU/l | 32 IU/l | 19-25 IU/L |
| AST | 250 IU/l | 160 IU/l | 10-40 IU/L |
| Additional laboratory tests two days after presentation | |||
| TGL | 221 mg/dl | <150 mg/dl | |
| LDH | 872 IU/l | <140 IU/l | |
| ANA | 1:80 | ≤1:40 | |
| D-Dimer | >2500 ng/ml | 220-500 ng/ml | |
| Anti-RNP ab | Positive | Lab did not specify | |
| Anti-Smith ab | 8 U/ml | 0-7 U/ml | |
| C3 | 80 mg/dl | 88-201 mg/dl | |
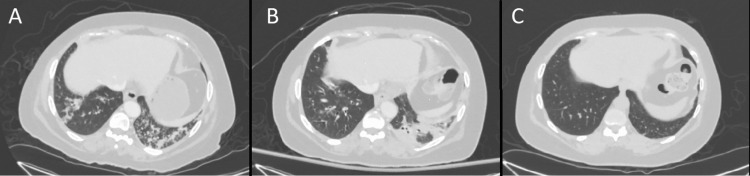
A computerized tomography (CT) scan of the chest, abdomen and pelvis was performed and showed evidence of bilateral pneumonia (Figure 1A). The patient also underwent brain imaging (including CT scan, magnetic resonance imaging (MRI), and magnetic resonance angiogram (MRA)) to evaluate her confusion, and these tests were unremarkable for any acute changes.
Figure 1. CT chest.
CT: computerized tomography scan; A: CT chest upon presentation showing bilateral pneumonia with the largest consolidation in the left lower lobe, B: follow-up CT chest a few days after presentation showing persistence of bilateral pneumonia, C: follow-up CT chest two months after presentation showing resolution of bilateral pneumonia.
Blood and urine cultures were taken, and she was started empirically on intravenous broad-spectrum antibiotics (cefepime and azithromycin). Sputum culture showed normal respiratory flora. Blood and urine cultures remained negative, but she continued to be febrile, and a repeated CT scan of the chest five days later showed the persistence of bilateral pneumonia (Figure 1B).
Two days later, laboratory tests showed consistently low WBC, RBC and platelet counts. She also had elevated triglycerides, lactate dehydrogenase (LDH), and D-dimer levels. Antinuclear antibody (ANA) came back positive and C3 level was low. Antinuclear ribonucleoprotein (anti-RNP) and anti-Smith antibodies were positive (Table 1).
Given the lack of improvement on empiric antibiotics, the decision was made for bronchoscopy, and the bronchoalveolar lavage culture was positive for Aspergillus fumigatus. The patient was started on intravenous micafungin and her fever resolved, micafungin was switched afterward to oral voriconazole (plan to complete six months of therapy).
She fulfilled the 2019 classification criteria for systemic lupus erythematosus (SLE) (positive ANA of 1:80, with additive criteria: fever, leukopenia, thrombocytopenia, positive anti-Smith antibody, low C3, and confusion/delirium), so she was started on hydroxychloroquine (400 mg daily).
Hemophagocytic lymphohistiocytosis (HLH) was suspected because of the significant elevation of ferritin, pancytopenia, fever, and elevated triglyceride levels. The H-score for HLH was calculated and showed a 45% probability of HLH. She fulfilled four out of eight criteria of the HLH-2004 trial, which was not sufficient to diagnose HLH. Bone marrow biopsy and soluble IL-2 receptor alpha (CD25) testing were recommended. CD25 level was elevated at 2,577 (532-1891 pg/ml). Bone marrow biopsy was unsuccessful. With an elevated CD25 level, the patient fulfilled five out of eight criteria of the HLH-2004 trial diagnostic criteria and she was diagnosed with hemophagocytic lymphohistiocytosis secondary pulmonary aspergillosis. She was started on intravenous methylprednisolone which was switched later to oral prednisone. For leukopenia, the hematologist started Granix® (granulocyte colony-stimulating factor - Teva Pharmaceutical Industries Ltd., North Wales, USA) and her leukopenia resolved.
Extensive infectious work-up (for CMV, urine Legionella antigen, COVID-19 test, respiratory panel, viral hepatitis panel, and EBV) was unremarkable. The patient was followed by multidisciplinary medical teams (rheumatology, hematology, infectious disease and pulmonology teams). She continued to improve gradually and was discharged after a month of hospital stay.
On a follow-up visit (one month after discharge) with rheumatology and infectious disease specialists, pancytopenia was resolved, inflammatory markers normalized, and C3 level was normal. She had no recurrence of her neurological or respiratory symptoms. Two-month follow-up thoracic CT scan showed a resolution of bilateral pneumonia (Figure 1C). The patient was sent for genetic testing and natural killer cell activity test; unfortunately, she did not have the tests done and did not follow up afterward.
Discussion
We describe the case of a previously healthy woman who developed HLH secondary to pulmonary aspergillosis. HLH can develop secondary to infections, including fungal infections. The patient did not have a known environmental exposure to aspergillosis. She also did not have a known immunodeficiency status; however, the possible underlying autoimmune rheumatological disease can be a precipitating factor to develop the invasive infection.
Neurological symptoms can be the predominant manifestation of HLH, including changes in mental status, seizures, nerve palsies, focal weakness, and encephalitis, with up to one-third of HLH cases presenting with neurological manifestations [6]. Our case presented with confusion. Pulmonary involvement has been reported in up to 40% of patients, and can include acute respiratory distress syndrome (ARDS) or infections. Our patient showed evidence of bilateral pneumonia upon presentation [7].
Diagnosis of HLH can be determined by HLH-2004 trial criteria, which include either confirmation of HLH-associated genetic mutation or the presence of five of eight diagnostic criteria (Table 2) [8]. While evidence of hemophagocytosis can support HLH, it is not required to make the final diagnosis.
Table 2. Diagnostic criteria of HLH-2004 trial.
[8]
HLH: hemophagocytic lymphohistiocytosis
| Molecular identification of an HLH-associated gene mutation: | Or | 5 of 8 of the following criteria: |
| Children require documentation of homozygosity or compound heterozygosity for HLH-associated gene mutations | 1. Fever | |
| 2. Splenomegaly | ||
| 3. Cytopenia affecting 2 lines | ||
| Adults: heterozygosity may be sufficient if they have clinical findings associated with HLH | a. Hemoglobin <9 g/dl | |
| b. Platelets <100 k/μl | ||
| c. Neutrophils <1.0 x109/l | ||
| 4.Hypertriglyceridemia and/or hypofibrinogenemia | ||
| a. Triglycerides > 265 mg/dl | ||
| b. Fibrinogen<150 mg/dl | ||
| 5. Hemophagocytosis in bone marrow, spleen, liver or lymph nodes | ||
| 6. Low or absent natural killer cell activity | ||
| 7. Ferritin > 500 ng/ml | ||
| 8. CD25 >2400 U/mL |
In our case, the diagnosis was made after fulfilling five of the eight HLH-2004 trial criteria, including fever ≥38.5°C, pancytopenia, elevated triglyceride level, highly elevated ferritin level, and elevated soluble CD25 (soluble interleukin-2 receptor alpha) level.
The patient also tested positive for ANA, anti-Smith, and anti-RNP antibodies, with no classical signs and symptoms of connective tissue disease. She fulfilled the 2019 classification criteria for SLE. SLE is a known risk factor for HLH, and the possible underlying rheumatological disease could have predisposed the patient to excessive immune activation in response to invasive infection.
High fever with markedly elevated ferritin can raise suspicion for adult-onset Still's disease; however, the absence of rash and joint involvement makes the diagnosis unlikely in our case.
HLH treatment includes supportive therapy and treatment of the underlying triggering conditions (infection, malignancy, or rheumatological disease). In severe cases, treatment with immunosuppressive drugs is indicated, these include dexamethasone, etoposide, intravenous immunoglobulin (IVIG) therapy, anakinra (interleukine-1 receptor antagonist) and other immunosuppressive medications. Given the invasive pulmonary aspergillosis in our case, the decision was made to treat her with IV steroids together with hydroxychloroquine, which helped to improve her condition.
Based on our literature review, we encountered six cases of HLH secondary to aspergillosis with a mean age of ~47 years (Table 3) [8-13].
Table 3. Cases of HLH secondary to Aspergillus infection.
BAL: brohchoalveolar lavage, BM: bone marrow, HLH: hemophagocytic lymphohistiocytosis, NK: natural killer cells, ANA: antinuclear antibodies,
| Case | Age In years | Presentation | ferritin | BM biopsy | BAL | Other significant laboratory tests | Diagnosis | Treatment | Outcome |
| Karakosta et al., 2021 [8] | 68 | Fever and respirator distress | 4062 ng/ml then increased to 23,863 ng/ml | histiocytosis | Revealed Aspergillus flavus | Elevated Triglycerides and pancytopenia | Acquired Hemophagocytic lymphohistiocytosis secondary to invasive pulmonary aspergillosis and Turicella Otitidis bacteremia | Broad spectrum antibiotics, Itraconazole and IV Hydrocortisone | death |
| Schouten MJG et al., 2017 [9] | 38 | Respiratory failure and fever | 2961 ng/ml | Not done | positive for influenza-A and aspergillus fumigatus | Low NK activity | HLH secondary to influenza and invasive pulmonary aspergillosis | -Anakinra (interleukin-1 receptor antagonist), Antiviral (oseltamivir), Antifungal (amphotericin-B and voriconazole) and Interferon-gamma | death |
| Umekawa et al., 2020 [10] | 54 | Fever, dyspnea and skin rash | 14.790 ng/ml | not done | Revealed Aspergillus fumigatus | ANA 1:80, elevated CD25 3,970 pg/ml and Decreased NK cell activity | Secondary Hemophagocytic Lymphohistiocytosis Associated with Invasive pulmonary Aspergillosis and the patient was eventually diagnosed with dermatomyositis | IVIG and antifungal therapy | Improved then the patient developed recurrence of secondary HLH associated with disseminated tuberculosis |
| Paul M, et al., 2022 [11] | 68 | Fever, Acute respiratory distress and Impaired consciousness | 141,918 ng/ml | hemophagocytosis | BAL was not done, the patient had positive serum galactomannan | Elevated triglycerides | HLH secondary to mucormycosis and aspergillosis coinfection | Etoposide, steroid therapy, Tocilizumab and Antifungal therapy | death |
| Gandotra A, et al., 2022 [12] | 47 | Fever and respiratory distress in a liver transplant recipient who was recovering from severe COVID-19 infection | 27,717 ng/ml | hemophagocytosis | Not done | Sputum culture was positive for acid fast bacilli and sputum fungal culture was positive for Aspergillus Flavus | Invasive pulmonary Aspergillosis and Tuberculosis complicated by HLH in sequelae of COVID-19 in a liver transplant recipient | IVIG, Antitubercular therapy and Antifungal therapy | recovered |
| Bello, A et al., 2018 [13] | 9 | Fever with respiratory distress | 14,000 ng/ml | Not done | Not done | Elevated triglycerides | Aspergillus induced HLH in child with acute lymphoblastic leukemia | Antifungal therapy | recovered |
On her follow-up appointment after discharge, she was sent for HLH genetic analysis which tests for many genes involved in the pathogenesis of primary HLH, she did not have the test done (possibly due to the cost), and she did not follow up afterward. Genetic testing can help to determine the risk of HLH recurrence, and the future need for hematopoietic cell transplant. If the patient has a genetic predisposition for HLH, this indicates testing of other family members for these genes, keeping in mind that the chance of finding a gene mutation is higher in the younger patients. In a study of 175 adults (age range, 18 to 75 years), only 14 percent had gene mutations [14]. Gene defects include mutations at FHL loci which code for cytotoxic granule formation and release pathway (PRF1/Perforin, UNC13D/Munc13-4, etc.), gene defects also include mutations that cause congenital immunodeficiency syndromes which are associated with an increased risk of HLH (Griscelli syndrome/RAB27A mutation, Chediak-Higashi syndrome/CHS1/LYST mutation, etc.) [15-18].
Conclusions
HLH is a syndrome of intense immune activation and is a life-threatening condition that usually presents with non-specific symptoms. Without a high index of suspicion, diagnosis may be delayed, which can increase the mortality rate. Testing for fungal infection is an important part of secondary HLH workup. We also recommend checking the ferritin level whenever HLH is suspected since it is considered as a hallmark of HLH and has been considered in the literature as prognostic biomarker for high mortality. Finally, bone marrow biopsy can help to confirm HLH but is not necessary for the diagnosis.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Regulation of type I interferon responses. Ivashkiv LB, Donlin LT. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pathogenesis of haemophagocytic lymphohistiocytosis. Aricò M, Danesino C, Pende D, Moretta L. Br J Haematol. 2001;114:761–769. doi: 10.1046/j.1365-2141.2001.02936.x. [DOI] [PubMed] [Google Scholar]
- 3.Elevated soluble interleukin-2 receptor in childhood hemophagocytic histiocytic syndromes. Komp DM, McNamara J, Buckley P. https://doi.org/10.1182/blood.V73.8.2128.2128. Blood. 1989;73:2128. [PubMed] [Google Scholar]
- 4.Early diagnostic and prognostic significance of a specific Th1/Th2 cytokine pattern in children with haemophagocytic syndrome. Tang Y, Xu X, Song H, et al. Br J Haematol. 2008;143:84–91. doi: 10.1111/j.1365-2141.2008.07298.x. [DOI] [PubMed] [Google Scholar]
- 5.How I treat hemophagocytic lymphohistiocytosis. Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. https://doi.org/10.1182/blood-2011-03-278127. Blood. 2011;118:4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Central nervous system involvement in hemophagocytic lymphohistiocytosis: a single-center experience. Jovanovic A, Kuzmanovic M, Kravljanac R, Micic D, Jovic M, Gazikalovic S, Pasic S. Pediatr Neurol. 2014;50:233–237. doi: 10.1016/j.pediatrneurol.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Adult haemophagocytic syndrome. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Lancet. 2014;383:1503. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 8.Acquired hemophagocytic lymphohistiocytosis in a critically ill patient with invasive pulmonary aspergillosis and turicella otitidis bacteraemia: a case report. Karakosta P, Aslanidis T, Flioni EN, Agaliadou-Dioritou U. https://e-journal.gr/en/acquired-hemophagocytic-lymphohistiocytosis-in-a-critically-ill-patient-with-invasive-pulmonary-aspergillosis-and-turicella-otitidis-bacteraemia-a-case-report/ Gr E-J Periop Med. 2021;20:61–66. [Google Scholar]
- 9.Hemophagocytic lymphohistiocytosis in a patient with influenza and invasive pulmonary aspergillosis - a case report. Schouten MJG, van der Hoeven JG. Clin Case Rep Rev 3. 2017 [Google Scholar]
- 10.Secondary hemophagocytic lymphohistiocytosis associated with invasive pulmonary aspergillosis. Umekawa S, Evans T. Critical Care Medicine. 2020;48:254. [Google Scholar]
- 11.Invasive mucormycosis and aspergillosis coinfection associated with post-COVID-19 pneumonia in a tertiary care hospital. Paul M, Sasidharan J, Taneja J, Chatterjee K, Abbas SZ, Chowdhury V, Das A. Med Mycol J. 2022;63:59–64. doi: 10.3314/mmj.21-00019. [DOI] [PubMed] [Google Scholar]
- 12.Invasive pulmonary aspergillosis and tuberculosis complicated by hemophagocytic lymphohistiocytosis - sequelae of COVID-19 in a liver transplant recipient. Gandotra A, Mehtani R, Premkumar M, et al. J Clin Exp Hepatol. 2022;12:1007–1011. doi: 10.1016/j.jceh.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Successful management of aspergillus induced hemophagocytic lymphohistiocytosis in a child with underlying acute lymphoblastic leukemia. Bello A, Cortez R. Clin lymphom myelom leukem. 2018;18:187. [Google Scholar]
- 14.Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Zhang K, Jordan MB, Marsh RA, et al. Blood. 2011;118:5794–5798. doi: 10.1182/blood-2011-07-370148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spectrum of perforin gene mutations in familial hemophagocytic lymphohistiocytosis. Göransdotter Ericson K, Fadeel B, Nilsson-Ardnor S, et al. Am J Hum Genet. 2001;68:590–597. doi: 10.1086/318796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Feldmann J, Callebaut I, Raposo G, et al. Cell. 2003;115:461. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 17.Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Ménasché G, Pastural E, Feldmann J, et al. Nat Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 18.The accelerated phase of Chediak-Higashi syndrome. An expression of the virus-associated hemophagocytic syndrome? Rubin CM, Burke BA, McKenna RW, et al. Cancer. 1985;56:524. doi: 10.1002/1097-0142(19850801)56:3<524::aid-cncr2820560320>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]