Abstract
Introduction
Minimal change disease (MCD), a common cause of primary nephrotic syndrome that accounts for 10%–15% of all primary nephrotic syndrome cases in adults, is frequently associated with malignant lymphoma. However, studies on MCD associated with prostate cancer are scarce.
Case Presentation
A 73-year-old male with prostate cancer was referred to our department with hypoalbuminemia and severe proteinuria while waiting for prostatectomy. We diagnosed the patient with nephrotic syndrome and performed a renal biopsy. Renal pathological findings were consistent with those of MCD. The clinical course suggested an association between prostate cancer and MCD as our patient achieved complete remission of MCD after receiving androgen deprivation and radiation therapy for prostate cancer without the use of glucocorticoids or other immunosuppressants.
Discussion
Although MCD can be associated with solid tumors, MCD associated with prostate cancer is very rare. The current case is the first to directly raise the possibility that secondary MCD may develop due to prostate cancer in some patients.
Keywords: Nephrotic syndrome, Proteinuria, Minimal change disease, Prostate cancer, Secondary glomerular diseases
Introduction
Minimal change disease (MCD) is a more common cause of primary nephrotic syndrome in children than in adults, and it accounts for 10–15% of all primary nephrotic syndrome cases in adults [1]. The cause of MCD is considered to be related to immune system dysfunction, specifically T cell dysregulation; however, other causative factors remain unclear [2]. Although less common, MCD diagnosis has also been reported in patients with malignancies [1]. MCD is frequently associated with malignant lymphoma, lending support to the hypothesis that MCD is related to T cell dysregulation [3]. However, MCD has been very rarely reported in association with solid cancers. In one case report of a patient diagnosed with prostate cancer and MCD within a short time frame, MCD was treated using prednisolone [4]. Herein, we present the first case of a patient who exhibited a decrease in proteinuria following the initiation of treatment for prostate cancer, suggesting that MCD is induced by prostate cancer.
Case Presentation
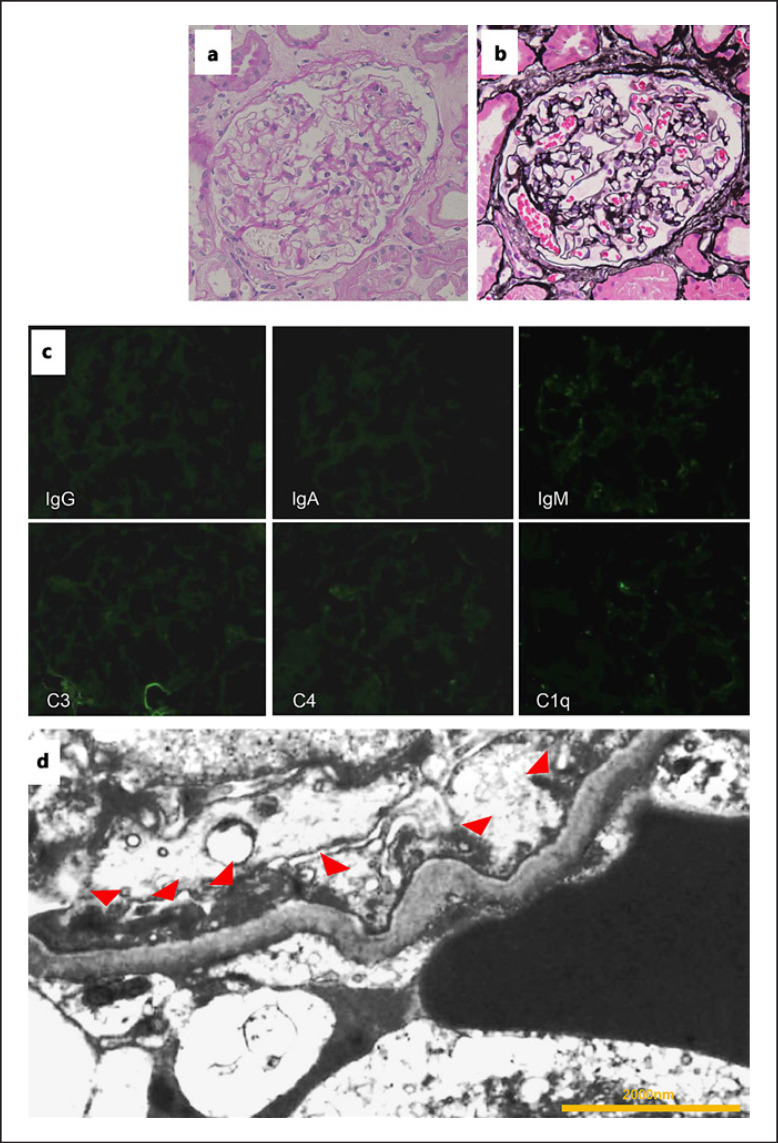
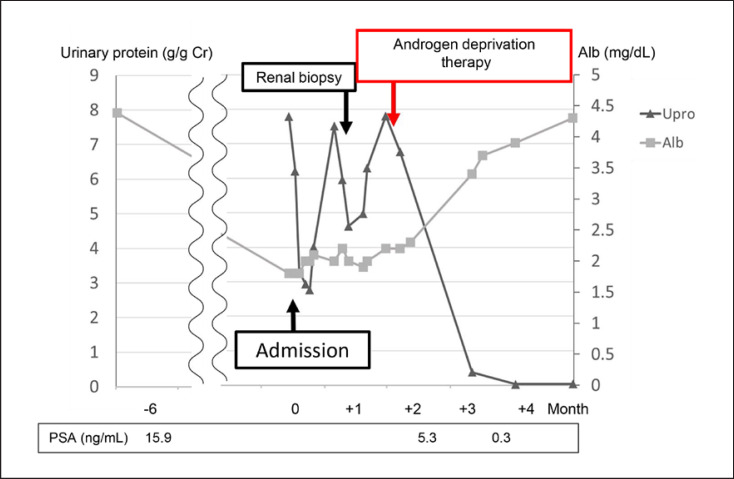
A 73-year-old man was referred to our urology department with a high prostate-specific antigen (PSA) level (15.9 ng/mL; age-adjusted PSA reference range: ≤4 ng/mL) [5]. He underwent a prostate biopsy, which resulted in a diagnosis of prostate cancer. There were no metastases to other organs. Prostatic biopsy revealed stage IIA adenocarcinoma (cT2bN0M0) with a Gleason score of 7 (3 + 4) in 2 of the 12 specimens. A few days before hospitalization for prostatectomy, he experienced bilateral lower limb edema. Upon admission, he was found to have hypoalbuminemia and proteinuria (urine stick test result of 3+); therefore, surgery was postponed and he was referred to our department. We diagnosed nephrotic syndrome due to severe low serum albumin levels (1.8 g/dL) and nephrotic-range proteinuria (7.79 g/g creatinine; Table 1). His serum creatinine level was 1.12 mg/dL. He was receiving angiotensin II receptor antagonist and statin treatments for hypertension and hyperlipidemia, respectively, prior to hospitalization. An annual medical checkup performed 3 months earlier indicated no protein in the urine. He had mild dyspnea, and his serum D-dimer levels were high (9.6 μg/mL); therefore, chest and abdominal contrast computed tomographic (CT) scans were performed, which revealed pulmonary embolism. No abnormality other than pulmonary embolism was found. Antithrombotic therapy with rivaroxaban was administered, and diuretic therapy was initiated to treat the edema. Follow-up contrast-enhanced CT performed 1 month later revealed that the embolus had dissolved, and renal biopsy was performed. He was administered antithrombotic therapy for a total of 3 months, excluding the period of renal biopsy. Renal pathology showed 21 glomeruli. Light microscopy analyses revealed unremarkable pathology with no glomerular lesions (shown in Fig. 1a). Immunofluorescence studies showed no particular deposits of immunoglobulin G (IgG), IgA, IgM, C3, C4, and C1q (shown in Fig. 1b). Electron microscopy revealed diffuse effacement of foot processes (shown in Fig. 1c). There were no electron dense deposits in the mesangial, subepithelial, and subendothelial areas of the capillary wall, including the basement membrane. Owing to nephrotic syndrome, the patient refused surgery and chose combined androgen blockade and radiation therapy for prostate cancer. High-dose steroid therapy for MCD was considered but not started because treatment for prostate cancer was initiated. Improvement in peripheral edema was observed with the use of diuretics, and MCD secondary to prostate cancer was considered the diagnosis. Soon after the initiation of combined androgen blockade therapy, his PSA levels declined, and the patient achieved complete remission of MCD in 2 months without the need for glucocorticoid treatment (shown in Fig. 2).
Table 1.
Laboratory findings at first admission
| Patient values | Reference | Interpretation | |
|---|---|---|---|
| Blood test | |||
| WBC, /µL | 8,700 | 3,900–9,800 | Normal |
| Lymphocyte, % | 22 | 18–59 | Normal |
| Hb, g/dL | 15.3 | 13.5–17.6 | Normal |
| Plt, /µL | 23.3 ×104 | 13.1 ×104 to 36.2 ×104 | Normal |
| CRP, mg/dL | 0.1 | 0–0.3 | Normal |
| TP, mg/dL | 3.9 | 6.7–8.3 | Decreased |
| LDH, U/L | 277 | 245–115 | Elevated |
| Alb, mg/dL | 1.8 | 4–5 | Decreased |
| Cr, mg/dL | 1.12 | 0.61–1.04 | Elevated |
| LDL-chol, mg/dL | 303 | 7–139 | Elevated |
| D-dimer, μg/mL | 9.6 | 0–1 | Elevated |
| FDP, μg/mL | 21.3 | 0–5 | Elevated |
| MPO-ANCA, IU/mL | – | <0.5 | Normal |
| PR3-ANCA, IU/mL | – | <0.5 | Normal |
| IgG, mg/dL | 536 | 820–1,740 | Decreased |
| IgA, mg/dL | 174 | 90–400 | Normal |
| IgM, mg/dL | 87 | 31–200 | Normal |
| Cryoglobulin | – | + or − | Normal |
| Kappa free light chain, mg/L | 24.3 | 3.3–19.4 | Elevated |
| Lambda free light chain, mg/L | 22.2 | 5.7–26.3 | Normal |
| Kappa/lambda ratio | 1.09 | 0.26–1.65 | Normal |
| BNP, pg/mL | 47.3 | 0–18.4 | Elevated |
| Urinary test | |||
| Urinary RBC | 100/HPF (isomorphic) | <1/HPF | Elevated |
| Urinary protein, g/g Cr | 7.79 | <0.15 | Elevated |
| Urinary protein (24 h collection), mg/day | 7,230 | 21–120 | Elevated |
| Selectivity index | 0.129 | <0.2 (high selectivity) | High selectivity |
| Bence Jones protein | – | + or − | Normal |
Alb, albumin; BNP, brain natriuretic peptide; Cr, creatinine; CRP, C-reactive protein; FDP, fibrin degradation product; Hb, hemoglobin; LDH, lactate dehydrogenase; LDL-chol, low-density lipoprotein cholesterol; MPO-ANCA, myeloperoxidase antineutrophil cytoplasmic antibodies; Plt, platelet; PR3-ANCA, proteinase 3 antineutrophil cytoplasmic antibodies; TP, total protein; RBC, red blood cell; WBC, white blood cell.
Fig. 1.
Pathological findings. a Optical microscopy reveals no change in the glomeruli (periodic acid-Schiff staining; magnification, ×400). b No changes are observed in the glomerular basement membrane (periodic acid-methenamine silver staining; magnification, ×400). c Immunofluorescence studies show no particular staining. d Electron microscopy shows foot process effacement (red arrow heads). There are no electron dense deposits (magnification, ×7,000).
Fig. 2.
Clinical course. Note the decreases in the PSA level and proteinuria soon after the initiation of androgen deprivation therapy. PSA, prostate-specific antigen.
Discussion
MCD is a well-known cause of nephrotic syndrome in children, but adult-onset MCD is less frequent [1]. Malignancy-related MCD, less commonly reported than the idiopathic form, is often associated with malignant lymphoma [3, 6]. We have herein summarized the first case of a patient who presented with MCD associated with prostate cancer. Although one review article suggested prostatic carcinoma as a cause of secondary MCD [7], to the best of our knowledge, no cases of MCD caused by prostate cancer have been reported to date.
The MCD diagnosis is based on the clinical presentation of nephrotic syndrome and foot process effacement as determined using electron microscopy. Distinguishing between primary and secondary MCDs via laboratory examination or pathological findings alone is difficult, and we need to diagnose them based on the disease course and clinical presentation. The possibility of spontaneously remitting primary MCD should be considered in the current patient because spontaneous MCD remission before the initiation of corticosteroid treatment for MCD has been reported previously [8]. The clinical course of our patient suggested a strong association between prostate cancer and MCD. First, prostate cancer preceded MCD. Second, the patient did not harbor medical risks of MCD other than prostate cancer. Third, the time between the diagnosis and remission of MCD was relatively short (<3 months). The time required to achieve complete remission of MCD tends to be longer in older patients, even with the use of steroid therapy [9]. Furthermore, the time required to achieve MCD remission is longer and the recovery rate is lower in untreated adult patients [8]. A study comparing untreated patients with those receiving steroids showed that the time for proteinuria to decrease to <1 g/day was >3.5 months from the time of diagnosis in untreated patients [10]. Fourth, urinary protein remission occurred immediately after the initiation of prostate cancer treatment and was consistent with a decline in tumor markers. A previous study has reported that cancer therapy often results in the remission of secondary MCD [7]. Androgen deprivation therapy may increase the risk of acute kidney injury [11], and the use of androgen deprivation therapy for proteinuria reduction and nephroprotection has not been reported. The present case fulfilled the first three of the following four criteria for the diagnosis of paraneoplastic nephrotic syndrome proposed by previous studies: (1) no evidence of other etiology, (2) nephrotic syndrome developing 6 months before or after the diagnosis of malignancy, (3) a decrease in proteinuria in association with cancer treatment, and (4) association of tumor recurrence with an increase in proteinuria [12, 13]. These findings suggest that MCD is secondary to prostate cancer. In patients who fail to achieve proteinuria remission with anticancer treatment, prednisone administration should be considered.
Vascular endothelial growth factor (VEGF) is a key mediator of angiogenesis and a therapeutic target in cancer [14]. Importantly, VEGF signaling maintains glomerular endothelial integrity [14], and anti-VEGF drugs used as anticancer therapeutics are known to be associated with MCD, focal segmental glomerulosclerosis, and thrombotic microangiopathy [14]. Not only the inhibition but also the induction of VEGF signaling results in glomerulomegaly [14]. Interestingly, high levels of VEGF in the serum and prostate tissue of patients with prostate cancer have been reported [15]. To evaluate the role of VEGF in the association of secondary MCD with cancer, future studies should determine whether tumor cells can affect local autocrine VEGF signaling in the glomeruli.
Recent reports demonstrating immune system dysfunction in patients with prostate cancer provide new insights into the association between MCD and prostate cancer [16, 17]. Previous studies have shown that MCD is related to T cell dysregulation [1]. Specifically, the pathogenesis of MCD has been proposed to result from an imbalance between the Th1 and Th2 responses and a possible predominance of the Th2 response in patients with MCD [18]. The involvement of T cells is also a characteristic of MCD secondary to cancer. Cancer, most frequently lymphoma, can be associated with secondary MCD, which suggests a relationship between T cells and secondary MCD induced by cancer [3]. Changes in immune responses are also associated with cancer progression, and the involvement of T cell dysregulation has been reported [19, 20]. Evidence suggests that Th2 cytokine production is superior to Th1 cytokine production in patients with prostate cancer [16]. A recent study reported an increase in serum CD4+/CD8+ T cells in patients with prostate cancer and demonstrated that these cells exhibited enhanced Th2 cytokine production [17]. This change in immune responses is consistent with the hypothesis that MCD is associated with a Th2-mediated immune response [1]. Thus, changes in immune responses, mainly T cells, due to prostate cancer may provide an opportunity to identify the pathogenesis of MCD associated with prostate cancer. The association between prostate cancer and MCD warrants further investigation.
In conclusion, the present case report suggests that MCD can be associated with prostate cancer and remission may be achieved by cancer treatment. Limited studies reported MCD remission with anticancer therapy without the use of corticosteroids in patients with solid cancers, such as prostate cancer [7, 13], and chemotherapy might have included corticosteroids in some of these patients [13]. Moreover, corticosteroids are often initiated early in patients with nephrotic syndrome because of uncontrollable edema and the rare consideration of an association between solid tumors and MCD. The possibility of secondary MCD due to prostate cancer should be considered in patients with MCD and prostate cancer. We do not have strong evidence to recommend PSA measurement in all elderly patients with MCD because the frequency of prostate cancer associated with MCD is unclear; however, it is necessary to check for symptoms of prostate cancer such as frequent urination and hematuria in patients presenting with MCD. Additional considerations in elderly patients with MCD should include ultrasonography or CT imaging, which would also be useful for screening other malignancies. In case of resistance to glucocorticoid therapy or recurrence, secondary MCD should be suspected, and further screening for cancer, including PSA measurement, may be considered. The current case illustrates that the possibility of a secondary cause, including cancer, should be considered for determining the therapeutic approach and for the prognosis of adult patients with MCD. The treatment of cancer should be considered as the initial approach before initiating steroid therapy as MCD may respond to the treatment of the underlying cause; such an approach would prevent the administration of high-dose glucocorticoids and the occurrence of its associated side effects. The present case suggests that the evaluation of MCD in adult male patients should include the consideration of prostate cancer as a potential cause. Future studies are warranted to confirm the association between MCD and prostate cancer observed in the present case.
Statement of Ethics
This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The Ethics Committee of the Yokohama City Minato Red Cross Hospital decided that ethics approval was not required because this single case report was not an experimental study or clinical trial. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. No personal identifying information is included in this manuscript.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was used for this study.
Author Contributions
Draft manuscript and study design: Yuta Nakano. Data acquisition: Yuta Nakano and Hajime Fujisawa. Data analysis: Yuta Nakano, Mariko Yoshida, Naohiro Muraki, Kouhei Sugita, and Saori Ishihara. Pathological evaluation: Jiro Kumagai. Supervision and mentorship: Hajime Fujisawa.
Data Availability Statement
All data are included in this article.
Acknowledgements
We thank all members of the Department of Nephrology, Yokohama City Minato Red Cross Hospital, Japan, for their helpful discussions regarding this work.
Funding Statement
No funding was used for this study.
References
- 1.Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12:332–345. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertelli R, Bonanni A, Di Donato A, Cioni M, Ravani P, Ghiggeri GM. Regulatory T cells and minimal change nephropathy: in the midst of a complex network. Clin Exp Immunol. 2016;183:166–174. doi: 10.1111/cei.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cambier JF, Ronco P. Onco-nephrology: glomerular diseases with cancer. Clin J Am Soc Nephrol. 2012;7:1701–1712. doi: 10.2215/CJN.03770412. [DOI] [PubMed] [Google Scholar]
- 4.Korbet SM, Whittier WL. Management of adult minimal change disease. Clin J Am Soc Nephrol. 2019;14:911–913. doi: 10.2215/CJN.01920219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito K, Yamamoto T, Kubota Y, Suzuki K, Fukabori Y, Kurokawa K, et al. Usefulness of age-specific reference range of prostate-specific antigen for Japanese men older than 60 years in mass screening for prostate cancer. Urology. 2000;56:278–282. doi: 10.1016/s0090-4295(00)00613-0. [DOI] [PubMed] [Google Scholar]
- 6.Audard V, Larousserie F, Grimbert P, Abtahi M, Sotto J-J, Delmer A, et al. Minimal change nephrotic syndrome and classical Hodgkin's lymphoma: report of 21 cases and review of the literature. Kidney Int. 2006;69:2251–2260. doi: 10.1038/sj.ki.5000341. [DOI] [PubMed] [Google Scholar]
- 7.Glassock RJ. Secondary minimal change disease. Nephrol Dial Transplant. 2003;18((Suppl 6)):vi52–8. doi: 10.1093/ndt/gfg1060. [DOI] [PubMed] [Google Scholar]
- 8.Black DA, Rose G, Brewer DB. Controlled trial of prednisone in adult patients with the nephrotic syndrome. Br Med J. 1970;3:421–426. doi: 10.1136/bmj.3.5720.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto R, Imai E, Maruyama S, Yokoyama H, Sugiyama H, Nitta K, et al. Incidence of remission and relapse of proteinuria, end-stage kidney disease, mortality, and major outcomes in primary nephrotic syndrome: the Japan Nephrotic Syndrome Cohort Study (JNSCS) Clin Exp Nephrol. 2020;24:526–540. doi: 10.1007/s10157-020-01864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coggins CH. Adult minimal change nephropathy: experience of the collaborative study of glomerular disease. Trans Am Clin Climatol Assoc. 1986;97:18–26. [PMC free article] [PubMed] [Google Scholar]
- 11.Lameire N. Nephrotoxicity of recent anti-cancer agents. Clin Kidney J. 2014;7:11–22. doi: 10.1093/ckj/sft135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JY, Yong TY, Kuss BJ, Klebe S, Kotasek D, Barbara JA. Malignant pleural mesothelioma with associated minimal change disease and acute renal failure. Ren Fail. 2010;32:1012–1015. doi: 10.3109/0886022X.2010.502275. [DOI] [PubMed] [Google Scholar]
- 13.Masuda S, Koizumi K, Moriya H, Nishino T, Uojima H, Tazawa T, et al. Secondary minimal change disease due to pancreatic cancer improved by chemotherapy. Intern Med. 2021;60:251–257. doi: 10.2169/internalmedicine.5499-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estrada CC, Maldonado A, Mallipattu SK. Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J Am Soc Nephrol. 2019;30:187–200. doi: 10.1681/ASN.2018080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera-Pérez J, Monter-Vera MDR, Barrientos-Alvarado C, Toscano-Garibay JD, Cuesta-Mejías T, Flores-Estrada J. Evaluation of VEGF and PEDF in prostate cancer: a preliminary study in serum and biopsies. Oncol Lett. 2018;15:1072–1078. doi: 10.3892/ol.2017.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filella X, Alcover J, Zarco MA, Beardo P, Molina R, Ballesta AM. Analysis of type T1 and T2 cytokines in patients with prostate cancer. Prostate. 2000;44:271–274. doi: 10.1002/1097-0045(20000901)44:4<271::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Bohner P, Chevalier MF, Cesson V, Rodrigues-Dias S-C, Dartiguenave F, Burruni R, et al. Double positive CD4+CD8+ T cells are enriched in urological cancers and favor T helper-2 polarization. Front Immunol. 2019;10:622. doi: 10.3389/fimmu.2019.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanai T, Shiraishi H, Yamagata T, Ito T, Odaka J, Saito T, et al. Th2 cells predominate in idiopathic steroid-sensitive nephrotic syndrome. Clin Exp Nephrol. 2010;14:578–583. doi: 10.1007/s10157-010-0330-z. [DOI] [PubMed] [Google Scholar]
- 19.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellström M, Egevad L, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 20.Shafer-Weaver KA, Anderson MJ, Stagliano K, Malyguine A, Greenberg NM, Hurwitz AA. Cutting edge: tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J Immunol. 2009;183:4848–4852. doi: 10.4049/jimmunol.0900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in this article.