Abstract
Introduction
Cannabidiol (CBD) is a widely utilized nonpsychoactive cannabinoid available as an over-the-counter supplement, a component of medical cannabis, and a prescriptive treatment of childhood epilepsies. In vitro studies suggest CBD may inhibit a number of drug-metabolizing enzymes, including carboxylesterase 1 (CES1). The aim of this study was to evaluate effect of CBD on the disposition of the CES1 substrate methylphenidate (MPH).
Methods
In a randomized, placebo-controlled, crossover study, 12 subjects ingested 750 mg of CBD solution, or alternatively, a placebo solution twice daily for a 3-day run-in period followed by an additional CBD dose (or placebo) and a single 10 mg dose of MPH and completed serial blood sampling for pharmacokinetic analysis. MPH and CBD concentrations were measured by liquid chromatography with tandem mass spectrometry.
Results
The C<sub>max</sub> (mean ± CV) for the CBD group and placebo group was 13.5 ± 43.7% ng/mL and 12.2 ± 36.4% ng/mL, respectively. AUC<sub>inf</sub> (ng/mL*h) for the CBD group and placebo group was 70.7 ± 32.5% and 63.6 ± 25.4%, respectively. The CBD AUC<sub>0-8h</sub> (mean ± CV) was 1,542.2 ± 32% ng/mL*h, and C<sub>max</sub> was 389.2 ± 39% ng/mL. When compared to MPH only, the geometric mean ratio (CBD/control, 90% CI) for AUC<sub>inf</sub> and C<sub>max</sub> with CBD co-administration was 1.09 (0.89, 1.32) and 1.08 (0.85, 1.37), respectively.
Discussion/Conclusion
Although the upper bound of bioequivalence was not met, the mean estimates of AUC and C<sub>max</sub> ratios were generally small and unlikely to be of clinical significance.
Keywords: Cannabidiol, Drug interaction, Carboxylesterase, Methylphenidate, Carboxylesterase 1, Cannabis
Introduction
The use of cannabis for medical purposes is rapidly expanding in the USA. As of 2021, 36 states and the District of Colombia have some form of approved medical cannabis (MC) and at least 18 states permit the recreational use of cannabis [1]. In general, MC refers to any physician-recommended preparation from the cannabis plant that patients use to treat medical/psychiatric conditions or symptoms. Cannabis contains >500 unique chemical components and at least 125 constituents classified as cannabinoids (CBs) [2]. The most abundant and well studied of these are ∆9-tetrahydrocannabinol (THC) and cannabidiol (CBD) [3], and these are generally associated with the putative therapeutic actions of MC. While THC produces the psychoactive effects and other physiological effects which are believed to be mediated by partial agonism of CB1 receptors in the CNS [3], CBD does not appreciably bind to CB1 or CB2 receptors and has low or absent abuse potential. Both CBs are generally components of MC and have been reported to have therapeutic potential individually or in various combinations/ratios. A multitude of MC products and dosing routes are available including “edibles,” oils, cannabis cartridges (for vaporization), and cannabis flower [4]. Essentially, all US states with MC programs have specified conditions for which MC can be legally used that typically include chronic conditions and symptoms such as chronic pain, nausea/vomiting, muscle spasms, inflammatory conditions, epilepsy, Parkinson's disease, HIV/AIDS, cancer, and others which are often inadequately or only partially relieved by conventional therapeutics. The chronic and resistant nature of these conditions often necessitates the use of multiple medications that will frequently be used concurrently with MC or CBD [5]. This scenario presents a risk for potential DDIs, yet the risks of cannabis-DDIs are incompletely understood. Regarding CBD specifically, recent changes in US laws have led to drastic increases in the production and sale of hemp-derived products across the USA [6]. Thus, nonprescriptive CBD is widely available to the public in the form of MC as well as a myriad of products and formulations marketed as alternative treatments. In vitro inhibition studies suggest that CBD may inhibit multiple drug-metabolizing enzymes including CYP3A4/5, CYP2D6, CYP2C19, CYP2B6, CYP2C9, and others [7]. DDIs between CBD and non-CYP enzymes have undergone little study although the results of a recent in vitro investigation suggested significant inhibition of the major liver esterase carboxylesterase 1 (CES1) by THC and CBD at clinically relevant concentrations [8]. Furthermore, Gaston and associates (2017) [9] reported that adding CBD to an existing regimen of anticonvulsants resulted in significant dose-dependent increases in the plasma concentration of the anticonvulsant rufinamide, an agent whose primary biotransformation pathway is catalyzed by CES1 [10]. Taken together, these findings suggest a potential for CBD to inhibit the metabolism of medications largely reliant on CES1 for biotransformation. The present study, conducted in healthy subjects, assessed the potential influence of multiple doses of CBD on the pharmacokinetics of the known CES1 substrate [11], methylphenidate (MPH).
Materials and Methods
Choice of CBD Formulation and Dose for Assessment
Although thousands of commercially produced CBD products are presently available for purchase in the USA, the regulation and quality control can be questionable and several recent reports have documented that CBD containing products purchased from retail outlets can be at significant variance with their labeled claims of CBD content with some containing significant amounts of THC and others adulterated with synthetic CBs [12, 13]. Accordingly, with subject safety and accuracy in dosing for this assessment in mind, we utilized the FDA-approved Epidiolex® brand of CBD solution in the study. Epidiolex® is a nonsynthetic form of CBD extracted from cannabis which is manufactured under strict guidelines governing its purity and labeled content. The recommended maintenance dosage of Epidiolex® for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome ranges from 2.5 mg/kg twice daily (5 mg/kg/day) to a maximum of 10 mg/kg twice daily (20 mg/kg/day). For the present study, a fixed dose of 750 mg twice daily was chosen which generally represents the higher end of dosing for nonobese adults. Greenwich Biosciences Inc (Carlsbad, CA, USA) generously donated Epidiolex® CBD (single lot source) as well as an Epidiolex® placebo solution in support of this investigation.
Study Subjects
Prospective study subjects were recruited with a goal of 12 subjects (6 males and 6 females) completing the entire protocol. The study protocol and informed consent document, inclusive of Health Insurance Portability and Accountability Act language, were approved by the University of Florida Investigational Review Board (NCT04603391). All subjects provided written informed consent. The study participants were required to be healthy nonsmoking adults (ages 21–45) as determined by a history and physical exam as well as basic laboratory monitoring indices including serum electrolyte and liver function tests (LFTs), urinalysis, and complete blood count. Participating females of child-bearing potential were required to have a negative urine pregnancy test prior to enrollment and avoid pregnancy during study participation. Female subjects were tested prior to each scheduled study day to provide additional assurance. Apart from oral contraceptives, no other prescription or over-the-counter medications were permitted. These restrictions also extended to nutritional supplements, vitamins, and “energy drinks.” All subjects were required to have a BMI between 18.5 and 28 kg/m2 (inclusive) and to have no history of any surgical or medical condition that might interfere with drug absorption, distribution, metabolism, or excretion. The presence of a known allergy, hypersensitivity, or adverse reaction to CBD or cannabis, sesame seed oil, or MPH was considered exclusion criteria. Additionally, all subjects underwent a urine THC screening to exclude the use of cannabis outside of the study protocol which could spuriously contribute CBD to the assessment.
Study Design and Drug Administration
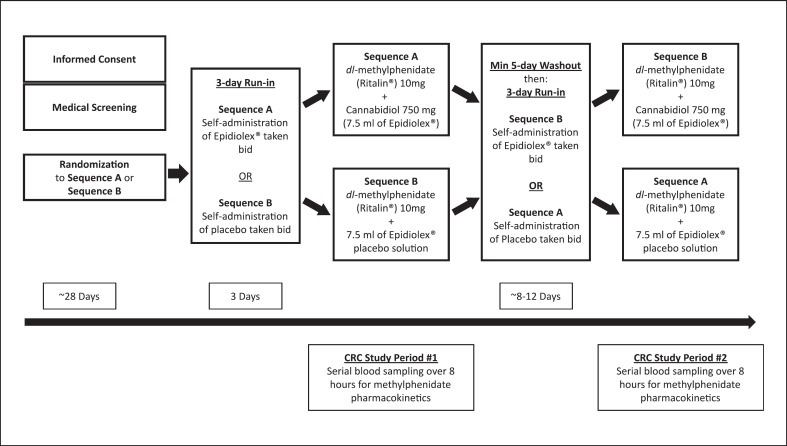
Two pharmacokinetic study visits took place at the University of Florida Clinical Research Center (CRC) following a 3-day run-in of either CBD 750 mg, given as 7.5 mL of CBD solution 100 mg/mL of CBD, or depending on randomization, 7.5 mL of CBD placebo solution containing no CBD, to be taken twice per day. The subject's doses were drawn up in labeled pre-filled oral syringes by the UF Investigational Drug Service and provided to subjects for self-administration over the 3-day run-in period (Fig. 1). Subjects were asked to keep and return the empty oral syringes as an indication of protocol adherence/compliance. Following an overnight fast, subjects arrived at the UF CRC at approximately 8:00 a.m., the morning of each of the two pharmacokinetic sampling visits. Each of the visits lasted approximately 8 h and was separated by a minimum 5-day wash-out period. For those randomized to CBD initially, the 3-day lead-in of placebo dosing would result in a minimum 8-day wash-out of any previous exposure. After check-in, an indwelling venous catheter was placed into each subject's arm to facilitate serial blood sampling. Subjects were fed a standardized breakfast (bagel, cream cheese, and orange juice) consumed over approximately 30 min. After breakfast, the subjects were administered one 10 mg tablet of dl-MPH and either CBD 750 mg, administered as 7.5 mL of 100 mg/mL CBD solution, or 7.5 mL of Epidiolex® placebo solution containing no CBD, matching their respective 3-day run-in randomization. Subjects were then provided 240 mL of room temperature water and asked to drink it in its entirety. A standardized lunch was provided to the subjects approximately 4 h post-dosing. The lunch consisted of a choice of frozen entrée (Healthy Choices®), and drinks were water, juice, or soda. The composition and amount of food consumed for both meals were recorded by nursing staff, and identical meals were provided on the subsequent study visit. Vital signs including blood pressure, pulse, and temperature were recorded at the 0-h timepoint as well as 4-h timepoint. Additionally, a LFT was obtained following the visit in which CBD was administered.
Fig. 1.
Clinical study design.
Blood Sample Collection and Processing
A total of nine blood samples (∼10 mL each) were collected via an indwelling venous catheter over the 8-h study period during the respective study days. Specific timepoints of blood collection occurred immediately prior to the CBD/placebo administration (0-h timepoint), and at 0.5, 1, 1.5, 2, 3, 4, 6, and 8 h post dose. Blood samples were collected using 10-mL gray-topped tubes (BD Vacutainer; Becton Dickinson, Franklin Lakes, NJ, USA) containing sodium oxalate to prevent clotting and sodium fluoride to prevent the hydrolysis of MPH. The blood samples were then stored on ice until centrifugation at 4°C. Each ∼10 mL of whole blood sample yielded ∼5 mL of plasma following centrifugation. Plasma samples were then split into duplicate sets, labeled, and stored at −70°C until LC-MS/MS analysis for MPH and CBD.
Analytical Methods
Drugs, Chemicals, and Standards
dl-MPH 10 mg tablets were from a single lot source and manufactured by American Health Packaging (Columbus, OH, USA). The urine THC screening tests were Easy@Home® Single Panel Testing (Burr Ridge, IL, USA). The analytical reference standard of dl-MPH HCl and the internal standard (IS) (+/−)-threo-MPH-D4 HCl were purchased from Cerilliant Corp. (Round Rock, TX, USA). CBD and d3-CBD were from Cayman Chemical (Ann Arbor, MI, USA). Liquid chromatography-mass spectrometry grade methanol, acetonitrile, and formic acid were all purchased from Fischer Scientific (Fair Lawn, NJ, USA). All other chemicals were of high analytical grade and available commercially.
MPH Analysis
Determinations of dl-MPH plasma concentrations were achieved using LC-MS/MS assay largely based upon a previously validated method [14]. In brief, 500 µL sodium carbonate, pH 11.0, containing 100 ng/ml d4-dl-MPH (IS) was added to 500 µL of plasma. Next, 2 mL of a solution of 4:1 butyl chloride/acetonitrile was added and the mixture was vortex-mixed for 20 s and then centrifuged at 2,000 g for 5 min. The organic phase (∼1 mL) containing the analytes of interest was then transferred to clean glass tubes. Samples were then evaporated to dryness under a gentle stream of nitrogen. Prior to LC-MS/MS analysis, the remaining residue was reconstituted with 100 µL of mobile phase (methanol:1% formic acid), 5 µL of which was injected per sample. The analysis was performed on a Shimadzu HPLC system (Shimadzu, Tokyo, Japan) including two pumps (LC-10ATvp), an autosampler (SIL-10ADvp), and system controller (SCL-10Avp) coupled to an Applied Biosystems-Sciex API 3000 triple quadrupole mass spectrometer (Foster City, CA, USA). Measurement of dl-MPH was achieved by running samples through an Inertsil ODS-4 3 µm C18 column (100A 50 × 2.1 mm, 3 µm, Tokyo, Japan). A gradient method with a flow rate of 0.25 mL/min was utilized of 8-min duration as follows; 20% of methanol was held for 0.5 min, with a linear increase to 80% in 6 min, and held for 0.5 min, and then changed back to 20% in 0.5 min and held for another 0.5 min, balanced with 0.1% formic aid in water. The MS was operated in positive ion mode using turbo electrospray ionization. The following parameters were used for the MS analysis: curtain gas, 12 psi; nebulizer gas (gas 1), 12 psi; CAD gas, 4 psi; TurboIonSpray (IS) voltage, 5,500 V; entrance potential, 10 V; collision cell exit potential, 15 V; declustering potential, 31 V; collision energy, 30 eV for both m/z: 234.0→84; m/z: 238.2→88.1; source temperature, 350°C; and dwell time, 100 ms. The following transitions were monitored in the multiple reaction monitoring mode MPH 234.0→84 and d4-dl-MPH (IS), m/z: 238.2→88.1. Data were acquired and analyzed by AB Sciex Analyst software, version 1.4.2 (AB Sciex, Toronto, Canada). The lower limit of quantification was 0.25 ng/mL for dl-MPH. Calibration curves were linear over the range of 0.25–40 ng/mL for all analytes (r2 > 0.99).
CBD Analysis
Plasma samples were analyzed by LC-MS/MS utilizing a modification of a previously described liquid-liquid extraction method [15]. In brief, 0.5 mL of plasma was aliquoted and mixed with 0.5 mL water containing 50 ng/mL d3-CBD as the IS. The mixture was then extracted with 2 mL butyl chloride/acetonitrile (4:1, vol/vol), vortex-mixed for 20 s. After centrifuging at 2,000 g for 5 min at room temperature, the organic phase was transferred to clean glass tubes and evaporated to dryness under a stream of nitrogen. The residue was reconstituted with 100 µL of mobile phase for LC-MS/MS analysis. The LC-MS/MS instrument and analytical software were exactly as described above for dl-MPH analysis. Chromatographic separation was achieved using a C18 reverse phase column (Gemini, 50 × 2.00 mm, 5 μm; Phenomenex Inc., Torrance, CA, USA). A gradient method was employed with 5 mM ammonium acetate in water (pH = 9) as the aqueous phase and methanol as the organic phase delivered at a flow rate of 0.375 mL/min. The gradient method commenced using a 30% aqueous phase and 70% organic phase being maintained for 3 min, was then linearly changed to a 5% aqueous phase and 95% organic phase over 4.5 min, and was held for 1 min. At 9 min, there was a switch back to 30% aqueous phase and 70% organic phase for a total run time of 9.5 min. Mass spectrometric analysis was performed via electrospray ionization in the positive mode. The following transitions were monitored for CBD: m/z: 315.2→193, d3-CBD: m/z: 318→196. The lower limit of quantification was 25 ng/mL for CBD. Calibration curves were linear over the range of 25–1,600 ng/mL for all analytes (r2 > 0.99).
Pharmacokinetic Analysis of MPH and CBD
The pharmacokinetics of MPH and CBD were evaluated using noncompartmental analysis using SAS® 9.4 (Cary, NC, USA). Peak concentration (Cmax) and the time to Cmax (Tmax) were reported as observed. The terminal elimination rate constant (λz) was estimated by linear least-squares regression of the terminal portion of the plasma concentration (on natural logarithmic scale)-time curve, and the elimination half-live (t1/2) was then calculated using the formula t1/2 = 0.693/λz. The area under the plasma concentration-time curve from time 0 to infinity (AUCinf) for these single-dose assessments and AUC0-8h were calculated according to the linear trapezoidal rule. The apparent clearance (CL/F) for MPH was calculated using the formula dose/AUCinf. Additionally, geometric mean ratios (GMRs) of the pharmacokinetic parameters for MPH were compared between the two exposure conditions, i.e., MPH with CBD versus MPH with placebo. An appraisal of DDI was determined following FDA guidance (https://www.fda.gov/media/143849/). In brief, if the 90% confidence interval (CI) of GMR on AUCinf and Cmax between the CBD group and placebo group extend beyond the limits of 0.8–1.25, the result was interpreted to represent a DDI.
Results
Research Subjects
Twelve healthy volunteers (6 men and 6 women) aged 21–44 years (26.7 ± 6.5 years; weight, 65.8 ± 14.1 kg; mean ± SD) completed the entire protocol and had evaluable pharmacokinetic data. Overall, CBD and placebo were well tolerated. Two subjects were discontinued from or withdrew from the study, one subject receiving CBD during the 3-day run-in who experienced the common side effect of nausea, and the other withdrew due to personal scheduling conflicts. No subject experienced any unanticipated adverse events related to any study-related procedures. No vital signs were recorded outside normal parameters during the study nor differed significantly from those recorded during their baseline screening visit. Additionally, there were no abnormalities or significant changes in LFTs from baseline screening values and those following CBD exposure.
MPH Pharmacokinetics
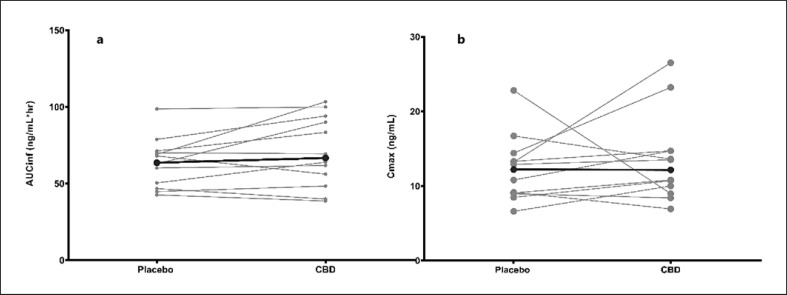
The pharmacokinetic parameters for MPH with or without CBD co-administration are summarized in Table 1. Differences in Cmax and AUC between the placebo and CBD study phases are depicted in Figure 2. When compared to MPH administered alone, the GMRs (90% CI) for AUCinf and Cmax with CBD co-administration were 1.09 (0.89, 1.32) and 1.08 (0.85, 1.37), respectively. The 90% CI of AUCinf and Cmax GMR were outside the range of 80–125% DDI criteria (Fig. 3; Table 1).
Table 1.
Pharmacokinetic parameters of MPH with and without CBD co-administration
| Parameters | CBD group |
Placebo group |
GMR (CBD group/placebo group) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | %CV | range | mean | %CV | range | CI (90%) | |||||
| Cmax, ng/mL | 13.5 | 43.7 | 6.9 | 26.5 | 12.2 | 36.4 | 6.6 | 22.8 | 1.08 | 0.85 | 1.37 |
| AUCinf, ng/mL*h | 70.7 | 32.5 | 38.6 | 103.3 | 63.6 | 25.4 | 42.6 | 98.6 | 1.09 | 0.89 | 1.32 |
| AUC0-8h, ng/mL*h | 55.7 | 29.8 | 31.6 | 80.7 | 49.6 | 25.3 | 30.7 | 74.3 | |||
| t1/2, h | 3.17 | 21.70 | 2.24 | 4.52 | 3.12 | 27.5 | 1.76 | 4.54 | |||
| tmax, h | 1.25a | – | 0.50 | 3.00 | 1.75a | – | 0.50 | 3.00 | |||
CV, coefficient of variation; CI, confidence interval.
tmax was expressed as median and range.
Fig. 2.
Cmax and AUC difference between the placebo and CBD study phases. The bold points and line represent the average change between the placebo and the CBD phases.
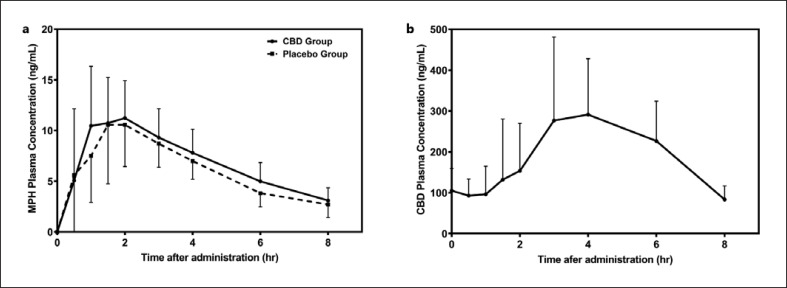
Fig. 3.
Mean MPH (a) and CBD (b) plasma concentration versus time curves for all subjects (n = 12). Error bars represent SD.
CBD Pharmacokinetics
The pharmacokinetic parameters for CBD from the CBD treatment group are summarized in Table 2. Major pharmacokinetic parameters were consistent with those published for adults dosed with CBD solution (Epidiolex®) [16]. A mean concentration-time profile of MPH as well as CBD is provided in Figure 3. It can be appreciated that the median Tmax of CBD is significantly longer than that of MPH. Among all 12 subjects, the CBD AUC0-8h (mean ± CV) was 1,542.2 ± 32% ng/mL*h, Cmax was 389.2 ± 39% ng/mL, and the minimum plasma concentration (Cmin) was 49.1 ± 49% ng/mL.
Table 2.
Pharmacokinetic parameters of CBD
| Parameters | Mean | SD | %CV | Range | |
|---|---|---|---|---|---|
| Cmax, ng/mL | 389.2 | 153.2 | 39 | 177.0 | 648.0 |
| AUC0-8h, ng/mL*h | 1,542.2 | 488.0 | 32 | 737.3 | 2,331.0 |
| Cmin, ng/mL | 49.1 | 24.0 | 49 | 6.7 | 84.9 |
| Caverage, ng/mL | 192.8 | 61.0 | 32 | 92.2 | 291.4 |
| tmax, h | 4a | – | – | 1.00 | 6.00 |
tmax was expressed as median and range.
Discussion
The serine hydrolase CES1 is a major drug-metabolizing enzyme accounting for up to 95% of total hydrolytic activity in the liver [17]. CES1 catalyzes the metabolism of a wide range of therapeutic drugs, pesticides, environmental pollutants, and endogenous substrates including those containing ester, thioester, carbamate, and amide functional groups as well as endogenous esters like cholesteryl esters and triacylglycerols [18]. Examples of CES1 substrates are found among essentially every major therapeutic drug class. CES1-catalyzed biotransformation can be a key step in the deactivation and clearance of a pharmacologically active drug (e.g., dl-MPH de-esterified into its major inactive metabolite ritalinic acid) [19], or it can catalyze the conversion of a prodrug into its active form (e.g., oseltamivir converted to oseltamivir carboxylate) [20]. There are both an increasing recognition of significant gene-drug interactions associated with CES1 variants and catalytic inefficiency [18, 21] and the potential drug-drug, botanical-drug, and drug-alcohol interactions resulting from CES1 inhibition [18, 22, 23]. In the present study, we assessed the influence of multiple doses of orally administered CBD on the pharmacokinetics of MPH in healthy subjects and measured both CBD and MPH concentrations. To our knowledge, this is the first clinical DDI study specifically assessing the influence of any CB or conventional medication on CES1 activity. Furthermore, this study incorporated a standardized, naturally derived formulation of CBD in a placebo-controlled study design and measured the plasma concentrations of both the hypothesized “victim” drug (i.e., dl-MPH) and overall exposures to the evaluated “perpetrator” compound (i.e., CBD). Although MPH bioequivalence criteria (i.e., GMR) were not met between the two study conditions, the observed change in MPH exposures with CBD co-administration was a relatively small magnitude and viewed as lacking clinical significance.
We have previously developed physiologically based pharmacokinetic models and predicted a minor interaction (AUC of MPH increased by 55%) between MPH and CBD after administration of multiple doses of 10 mg/kg twice daily CBD [8]. The interaction was presumed to be mediated by inhibition of CES1 by CBD and would be dose dependent. Although the administered doses of CBD in this study were viewed as fairly robust, given the relatively long half-life of CBD, the current study did not assess the DDI potential at steady-state conditions for CBD. A more significant DDI may also be possible at the presence of additional factors which may increase CBD exposure or when baseline CES1 activity is influenced by other factors. For example, nonprescriptive CBD use is very common among self-reported heavy users of recreational cannabis [24], and accordingly, exposure to CBD and CBs could be significantly higher and more protracted than that assessed in our study. A further consideration in cannabis users is that the major CB THC has also been shown to be a potent inhibitor of CES1 in vitro which might potentially produce additive inhibitory effects on the enzyme [25].
In conclusion, the short-term co-administration of CBD at a clinically relevant dose had minimal effect on the pharmacokinetics of MPH. Thus, concomitant ingestion of CBD at the dose evaluated is not likely to result in a significant DDI with MPH and, potentially, other CES1 substrates.
Statement of Ethics
The study protocol and informed consent document, inclusive of Health Insurance Portability and Accountability Act language, were approved by the University of Florida Investigational Review Board (NCT04603391). All subjects provided written informed consent.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was made possible by a grant from the State of Florida Consortium for Medical Marijuana Clinical Outcomes Research.
Author Contributions
Study design: John S. Markowitz and Yuli Qian. Experimental design: John S. Markowitz and Yuli Qian. Recruitment, screening, and assessment of research subjects: Brandon O. Klee, Ludmila De Faria, and John S. Markowitz. Analysis of data: Qingchen Zhang, Philip W. Melchert, Yuli Qian, Reginald F. Frye, and John S. Markowitz. Preparation of the manuscript: John S. Markowitz, Ludmila De Faria, Qingchen Zhang, Philip W. Melchert, Reginald F. Frye, Brandon O. Klee, and Yuli Qian.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Epidiolex® and Epidiolex® Placebo were generously donated by Greenwich Biosciences, Inc.
Funding Statement
This study was made possible by a grant from the State of Florida Consortium for Medical Marijuana Clinical Outcomes Research.
References
- 1.Levinsohn EA, Hill KP. Clinical uses of cannabis and cannabinoids in the United States. J Neurol Sci. 2020 Apr 15;411:116717. doi: 10.1016/j.jns.2020.116717. [DOI] [PubMed] [Google Scholar]
- 2.Radwan MM, Chandra S, Gul S, ElSohly MA. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules. 2021 May 8;26((9)):2774. doi: 10.3390/molecules26092774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lafaye G, Karila L, Blecha L, Benyamina A. Cannabis, cannabinoids, and health. Dialogues Clin Neurosci. 2017 Sep 30;19((3)):309–316. doi: 10.31887/DCNS.2017.19.3/glafaye. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use - basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy. 2018 Feb;52:87–96. doi: 10.1016/j.drugpo.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Caputi TL, Humphreys K. Medical marijuana users are more likely to use prescription drugs medically and nonmedically. J Addict Med. 2018 Aug;12((4)):295–299. doi: 10.1097/ADM.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 6.McGregor IS, Cairns EA, Abelev S, Cohen R, Henderson M, Couch D, et al. Access to cannabidiol without a prescription: a cross-country comparison and analysis. Int J Drug Policy. 2020 Nov;85:102935. doi: 10.1016/j.drugpo.2020.102935. [DOI] [PubMed] [Google Scholar]
- 7.Qian Y, Gurley BJ, Markowitz JS. The potential for pharmacokinetic interactions between cannabis products and conventional medications. J Clin Psychopharmacol. 2019 Oct;39((5)):462–471. doi: 10.1097/JCP.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 8.Qian Y, Markowitz JS. Prediction of carboxylesterase 1 (CES1)-mediated in vivo drug interaction between methylphenidate and cannabinoids using static and physiologically based pharmacokinetic models. Drug Metab Dispos. 2022 Jan;50((7)):968–979. doi: 10.1124/dmd.121.000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP, UAB CBD Program Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017 Sep;58((9)):1586–1592. doi: 10.1111/epi.13852. [DOI] [PubMed] [Google Scholar]
- 10.T Williams E, Eric Carlson J, George Lai W, Nancy Wong Y, Yoshimura T, J Critchley D, et al. Investigation of the metabolism of rufinamide and its interaction with valproate. Drug Metab Lett. 2011 Dec;5((4)):280–289. doi: 10.2174/187231211798472511. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z, Murry DJ, Sanghani SP, Davis WI, Kedishvili NY, Zou Q, et al. Methylphenidate is stereoselectively hydrolyzed by human carboxylesterase CES1A1. J Pharmacol Exp Ther. 2004 Aug;310((2)):469–476. doi: 10.1124/jpet.104.067116. [DOI] [PubMed] [Google Scholar]
- 12.Gurley BJ, Murphy TP, Gul W, Walker LA, ElSohly M. Content versus label claims in cannabidiol (CBD)-containing products obtained from commercial outlets in the state of Mississippi. J Dietary Supplements. 2020;17((5)):599–607. doi: 10.1080/19390211.2020.1766634. [DOI] [PubMed] [Google Scholar]
- 13.Miller OS, Elder EJ, Jones KJ, Gidal BE. Analysis of cannabidiol (CBD) and THC in nonprescription consumer products: implications for patients and practitioners. Epilepsy Behav. 2022 Feb;127:108514. doi: 10.1016/j.yebeh.2021.108514. [DOI] [PubMed] [Google Scholar]
- 14.Zhu HJ, Patrick KS, Markowitz JS. Enantiospecific determination of dl-methylphenidate and dl-ethylphenidate in plasma by liquid chromatography-tandem mass spectrometry: application to human ethanol interactions. J Chromatogr B. 2011 Apr 1;879((11–12)):783–788. doi: 10.1016/j.jchromb.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González-Mariño I, Thomas KV, Reid MJ. Determination of cannabinoid and synthetic cannabinoid metabolites in wastewater by liquid-liquid extraction and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry. Drug Test Anal. 2018 Jan;10((1)):222–228. doi: 10.1002/dta.2199. [DOI] [PubMed] [Google Scholar]
- 16.Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018 Nov;32((11)):1053–1067. doi: 10.1007/s40263-018-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Zou L, Jin Q, Hou J, Ge G, Yang L. Human carboxylesterases: a comprehensive review. Acta Pharmaceutica Sinica B. 2018 Sep;8((5)):699–712. doi: 10.1016/j.apsb.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Her L, Zhu HJ. Carboxylesterase 1 and precision pharmacotherapy: pharmacogenetics and nongenetic regulators. Drug Metab Dispos. 2020 Mar;48((3)):230–244. doi: 10.1124/dmd.119.089680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu HJ, Appel DI, Peterson YK, Wang Z, Markowitz JS. Identification of selected therapeutic agents as inhibitors of carboxylesterase 1: potential sources of metabolic drug interactions. Toxicology. 2010 Apr 11;270((2–3)):59–65. doi: 10.1016/j.tox.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Zhu HJ, Markowitz JS. Activation of the antiviral prodrug oseltamivir is impaired by two newly identified carboxylesterase 1 variants. Drug Metab Dispos. 2009 Feb;37((2)):264–267. doi: 10.1124/dmd.108.024943. [DOI] [PubMed] [Google Scholar]
- 21.Zhu HJ, Patrick KS, Yuan HJ, Wang JS, Donovan JL, DeVane CL. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. The Am J Hum Genet. 2008 Jun;82((6)):1241–1248. doi: 10.1016/j.ajhg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian Y, Markowitz JS. Natural products as modulators of CES1 activity. Drug Metab Dispos. 2020 Oct;48((10)):993–1007. doi: 10.1124/dmd.120.000065. [DOI] [PubMed] [Google Scholar]
- 23.Parker RB, Hu ZY, Meibohm B, Laizure SC. Effects of alcohol on human carboxylesterase drug metabolism. Clin Pharmacokinet. 2015 Jun;54((6)):627–638. doi: 10.1007/s40262-014-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunbar MS, Seelam R, Tucker JS, Firth CL, Pedersen ER, Klein DJ, et al. Patterns and correlates of cannabidiol product and marijuana co-use in a sample of U.S young adults. Addict Behav. 2022 Mar;126:107185. doi: 10.1016/j.addbeh.2021.107185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian Y, Wang X, Markowitz JS. In vitro inhibition of carboxylesterase 1 by major cannabinoids and selected metabolites. Drug Metab Dispos. 2019 May;47((5)):465–472. doi: 10.1124/dmd.118.086074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.