Abstract
Introduction
Cannabinoids including cannabidiol (CBD) have attracted enormous interest as bioactive ingredients for various dermatological and/or cosmeceutical uses. However, topical applications of cannabinoids might be limited without a fundamental understanding of their skin permeability. Herein, we aimed to evaluate the skin permeability of CBD and its topical formulations using artificial skin membrane assays. The solubility and stability of CBD in various surfactants that are commonly used in topical applications were also evaluated.
Methods
CBD and two CBD-incorporated topical formulations (cream and gel) were prepared for this study. Computational predictions (SwissADME and DERMWIN™) and the parallel artificial membrane permeability assay (PAMPA) were used to evaluate the skin permeability of CBD isolate. The Franz cell diffusion (in vitro release testing) assay was used to evaluate the skin permeability of CBD formulations. The solubility and stability of CBD in surfactants were assessed by high-performance liquid chromatography and mass spectrometry analysis.
Results
CBD isolate showed favorable skin permeability in the SwissADME and DERMWIN™ predictions (−Log Kp of 3.6 and 5.7 cm/s, respectively) and PAMPA (−LogPe value of 5.0 at pH of 6.5 and 7.4). In addition, CBD had higher solubility (378.4 μg/mL) in surfactant Tween 20 as compared to its solubility in polyisobutene. In an acidic environment (pH 5 and 6), Tween 20 maintained the CBD content at 81% and 70% over 30 days, respectively. CBD in the formulations of cream and gel also had moderate skin permeability in the Franz cell diffusion assay.
Conclusion
Data from artificial membrane-based assays support that CBD is a skin permeable cannabinoid and the permeability and stability of its formulations may be influenced by several factors such as surfactant and pH environment. Findings from our study suggest that CBD may have suitable skin permeability for the development of dermatological and/or cosmeceutical applications but further studies using in vivo models are warranted to confirm this.
Keywords: Cannabidiol, Skin permeability, Parallel artificial membrane permeability assay, Franz cell diffusion, Stability
Introduction
The skin is the largest organ covering the human body [1]. It is composed of various layers of cells that together act as a barrier to keep unwanted biological and chemical substances from permeating and entering the body [2]. The avascular outermost layer of the skin, called the epidermis, is comprised of keratinocytes, Merkel cells, melanocytes, and Langerhans cells which participate in the protection against inflammation and immunological responses [2, 3, 4]. The most superficial layer of the epidermis is the stratum corneum, which is the most difficult layer to permeate and is the rate-limiting step in the penetration process. Below the epidermis is the vascular dermis layer, which contains additional cell types such as fibroblasts but also free nerve endings which play a significant role in pain sensation [2, 3]. Based on the skin's physiological structure, suitable formulations for skincare products can be designed to deliver cosmeceuticals to a specific location such as muscles or blood vessels. In addition, transdermal formulations can facilitate the absorption and systemic circulation of active ingredients to achieve their biological effects [5].
Plants-based natural products have been historically used as active components in various topical formulations. Among these natural products, phytocannabinoids including cannabidiol (CBD) have emerged as trending ingredients in skincare products for the management of various skin conditions [6]. The use of CBD as a cosmeceutical ingredient is supported by several preclinical studies showing that CBD may exert promising biological activities including anti-inflammatory, antioxidant, and anti-apoptotic effects in skin cells [3, 7, 8, 9, 10, 11]. For instance, it has been reported that CBD showed antioxidant activity by protecting epidermal cells from ultraviolet light-induced oxidative stress [7]. However, CBD's cosmeceutical applications may be limited by its physico-chemical characteristics including unfavorable solubility and stability in certain environments. For instance, the addition of surfactants may be required to overcome the low solubility of CBD for certain formulations [12], and the incorporation of CBD into different formulations may make it susceptible to aerobic oxidation, resulting in its undesirable color change [13]. Therefore, further understanding of CBD's physico-chemical properties including its stability, solubility, and permeability in the presence of surfactants is critical for the development of CBD-based skincare products. Several studies have explored CBD's skin permeability in different pharmaceutical models. For instance, an in vitro diffusion model using human skin cells showed that CBD (as in a formulation consisting of aqueous propylene glycol, ethanol, and Brij 98) had the highest skin permeation profile (up to 300 nM) [14]. Furthermore, a study using a modified Franz diffusion assay with human epidermis demonstrated that CBD's permeability can be greatly affected by vehicle solutions. A mixture of propylene glycol/water (80/20; v/v) was the best vehicle solution for enhancing CBD's skin permeation rate and skin retention [15]. In addition, a novel formulation (a CBD-loaded O/A microemulsion microemulgel) was developed to enhance the locally acting performance of CBD for dermatological applications [16]. Additionally, a recent study analyzed the levels of CBD in several cannabis-infused consumer products, which revealed that many commercial products contain little to no detectable CBD [17]. This study also showed that CBD in these products may not penetrate the skin depending on their formulation. To date, CBD's skin permeability in cosmetic formulations is not well characterized. Herein, we aimed to evaluate the permeability of CBD (isolate in surfactants) and its topical formulations using membrane-based in vitro models including the parallel artificial membrane permeability assay (PAMPA) and the Franz cell diffusion (in vitro release testing [IVRT]) assay.
Materials and Methods
Chemicals
CBD (purity 99.46%) was obtained from DB Labs (Las Vegas, NV, USA). Materials used for the PAMPA experiments including PAMPA sandwich, buffers, and reference compounds including warfarin, piroxicam, verapamil, and progesterone were purchased from Pion Inc. (Billerica, MA, USA). Cellulose and Strat-M® membranes were purchased from Millipore Sigma (Burlington, MA, USA). Supor PES membranes (pore size 0.1 and 0.45 μM) were purchased from Pall Corporation (Port Washington, NY, USA). Silky Cream Base (BAS-SLKC-01), natural gel base (BAS-NATGE-01), polyisobutene (PIB), mineral oil, phytosqualane, and propylene glycol were purchased through MakingCosmetics (Redmond, WA, USA). Polysorbate 20 NF (Tween 20) was purchased from Letco Medical (Decatur, AL, USA). Polysorbate 80 (Tween 80) was purchased through Fisher Scientific (Waltham, MA, USA). Sodium lauryl sulfate (SLS) was purchased from Gallipot (St. Paul, MN, USA). Simugel and phytosqualane were kindly provided by Seppic (Fairfield, NJ, USA).
In silico Skin Permeability Prediction
Two computational methods, namely, SwissADME and DERMWINTM, were used to predict the skin permeability of CBD using the previously reported method [18, 19]. Skin permeability (Log Kp) and other solubility constants including the partition coefficient between n-octanol and water (Log Po/w) and molar solubility in water (Log S) from estimated aqueous solubility (ESOL) were determined.
Parallel Artificial Membrane Permeability Assay
The skin permeability of CBD isolate in aqueous and in various surfactants was evaluated using a skin membrane-based in vitro model. The PAMPA was completed following Pion's suggested procedures in the instruction manual, version 4 [18, 19]. Briefly, the stock solution of CBD isolate and the reference compounds including warfarin, piroxicam, verapamil, and progesterone were prepared in DMSO to reach a concentration of 10 mM. Next, pH (6.5 and 7.4)-adjusted buffer solution (1 mL) was added to wells in the PAMPA plate, and samples (5 µL; at 50 µM) were added and thoroughly mixed with buffer solution. Then, samples (200 µL) were added to the donor (bottom) plate in the Pion sandwich model and the acceptor sink buffer (200 µL) was added to each well of the acceptor plate. The sandwich model was incubated at room temperature and kept stirring using a stir bar (Pion Inc.) for 4 h. After incubation, the UV absorbance of solutions in the donor and acceptor plates was measured on the SpectraMax plate reader connected to a computer operating the PAMPA software to obtain the −LogPe value. To assess the PAMPA results measuring the skin permeability of CBD in various surfactants, similar procedures were used for the assay but mass spectrometry (MS), instead of UV absorbance, was used to quantify the CBD content using the methods described below.
Quantification of CBD Using High-Performance Liquid Chromatography and LC-MS Analysis
The levels of CBD present in the solubility and stability assays were determined by high-performance liquid chromatography (HPLC) analysis. Prior to the HPLC analysis, samples containing lipophilic surfactants were prepared using a C-18 solid-phase extraction (SPE; 100 mg) column from Agilent Technologies (Santa Clara, CA, USA). The HPLC analysis was performed on a Thermo Scientific instrument (Dionex UltiMate 3000, Waltham, MA, USA) with a C18 column (Thermo C18; 250 × 4.6 mm, 5 μm), and a solvent system consisting of aqueous formic acid (0.05%; A) and acetonitrile (B) was used as the mobile phases eluting via an isocratic method with 20% of A at a flow rate of 0.6 mL/min for 9 min. Based on the concentrations of CBD in different experimental models, for instance, in the stability assay versus the PAMPA and IVRT assay, two analytical methods were used to determine the CBD levels. In the stability experiments, the CBD content was monitored by a diode-array detector at a wavelength of 210 nm and the relative quantity of CBD was measured using the area under the curve using standard linear regression (online suppl. Fig. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000526769). The levels of CBD solubilized in different surfactants following the PAMPA were quantified by LC-MS analysis (Thermo Scientific ISQ system paired with a Dionex HPLC system). LC-MS analysis was performed with a Thermo C18 column (250 × 4.6 mm, 5 μm) and a solvent system consisting of 0.05% formic acid in water (A)/acetonitrile (B) using a 15-min isocratic method at 80% B and a flow rate of 0.6 mL/min.
Solubility and Stability of CBD in PIB and Surfactants
Oil-based carrier PIB and surfactants including polysorbate 20 (Tween 20), polysorbate 80 (Tween 80), and SLS were evaluated as a solubilizer of CBD into aqueous solution. In a falcon tube (Sigma, Burlington, MA, USA), CBD (5 mg) and surfactant (at a final concentration of 10%; w/w) were mixed with phosphate buffer saline (PBS; 12 mL). Solutions were thoroughly mixed and placed still at room temperature for 24 h. CBD in each solution was extracted with an SPE column (100 mg; Agilent Technologies) eluted with methanol for further HPLC analysis. The stability of CBD in Tween 20 aqueous solution (10%; w/w) at different pH conditions was determined by the HPLC analysis. CBD solutions (200 μg/mL) at different pH conditions (pH = 4, 5, 6, and 7) were prepared and kept at room temperature for 30 days. The concentration of CBD in each solution at different timepoints (day 1, 7, 14, and 30) was measured by HPLC analysis with a linear regression based on area under the curve (online suppl. Fig. S1 and S3).
Preparation of CBD in Cream and Gel Formulations
Two creams were used to evaluate CBD permeability in the solution. The first, a commercially available cream (obtained from MakingCosmetics Inc.; www.makingcosmetics.com), was a silky cream base (BAS-SLKC-01). CBD isolate powder (1% w/w) was added directly to this base and dispersed using a homogenizer for 2.5 min. The second cream was formulated using ingredients from the compounding laboratory at the College of Pharmacy at the University of Rhode Island. The formulated cream (referred to as in-house formulation) contained equal parts propylene glycol, phytosqualane, and mineral oil to comprise 50% (w/w) of the total formulation. CBD isolate powder (1% w/w) was added directly to the propylene glycol, mixed thoroughly, and added to the other oils. Next, surfactant SIMULGEL (7%) was added to the lipophilic phase mixture, followed by the addition of deionized water (42%). The cream was homogenized for 2.5 min. Each formulation was inspected to ensure a smooth texture and no signs of phase separation. Formulations were then centrifuged 9 times at 5,000 rpm in 5-min intervals to confirm no phase separation. Both formulations did not show any separation of the 2 phases after centrifugation.
Franz Cell Diffusion Assay
The Franz cell diffusion assay was performed using the reported method with minor modifications [20]. One day prior to the assay, the receptor buffer (3% surfactant; w/w; in PBS) was prepared in 50 mL falcon tubes and pre-warmed in a water bath (Bransonic® CPXH Ultrasonic Baths; Danbury, CT, USA) at 32°C overnight. Tween 20 showed the best solubility for CBD (online suppl. Fig. S3–S5) and was selected as a suitable surfactant. Depending on membrane brand and type, cellulose acetate membranes were hydrated 1 day prior to running the assay by submerging the membrane in PBS in a beaker with parafilm (Bemis Company, Neenah, WI, USA) covering the top [21]. On the day of the assay, the Franz cell water bath was pre-warmed to 32°C for 30 min prior to beginning. After the water had been warmed, the pre-soaked membranes were applied to the apparatus using forceps. Next, 12 mL of acceptor buffer was added via a glass pipette into each Franz cell. Pure CBD (dissolved in a vehicle solvent of sterile-filtered DMSO; 1 mL) or 200 mg of the formulation was added using a pipette for pure compound and a syringe (PermeGear, Hellertown, PA, USA) for formulations. The tops of the cells were clamped and covered in parafilm to prevent evaporation. At each given timepoint, 600 µL of the sample was removed from the spout of the Franz apparatus, transferred into an Eppendorf tube (Sigma, Burlington, MA), and replaced with 600 µL of new acceptor buffer.
The IVRT Data Analysis
The IVRT experiments were performed using the reported method with minor modifications [22]. Each sample (600 μL) taken from the acceptor well was subjected to the IVRT analysis. Surfactant from the sample was first removed by extraction with a C-18 SPE column prior to the analysis of CBD content using the aforementioned LC-MS method. Elution time was compared with that of the standard curve (online suppl. Fig. S4). A linear regression of CBD standards was calculated (R2 = 0.9759), and concentrations of CBD were interpolated. For the initial timepoint collected in the Franz cell assay, the calculated IVRT was determined using the formula: drug concentration/1,000 × 12. For each timepoint following, the calculation was adjusted to account for the addition of buffer in the acceptor well using the following equation: (drug concentration × 12 + 0.6 × [sum of sample concentrations]/1,000).
Results
CBD Isolate Shows Favorable Skin Permeability in the Computational Prediction, PAMPA, and Franz Cell Diffusion Model
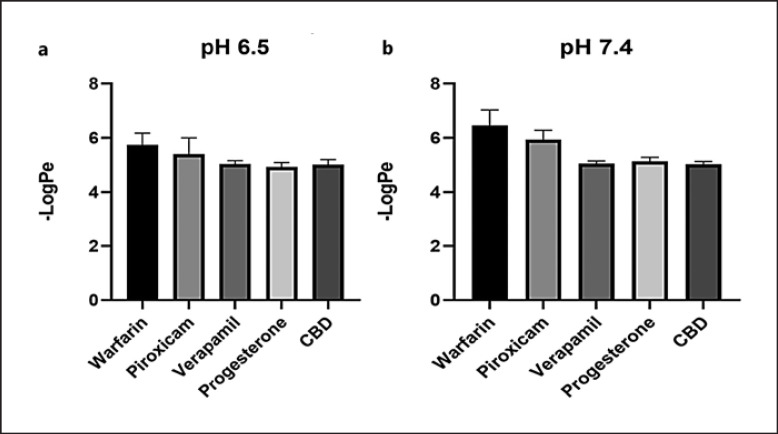
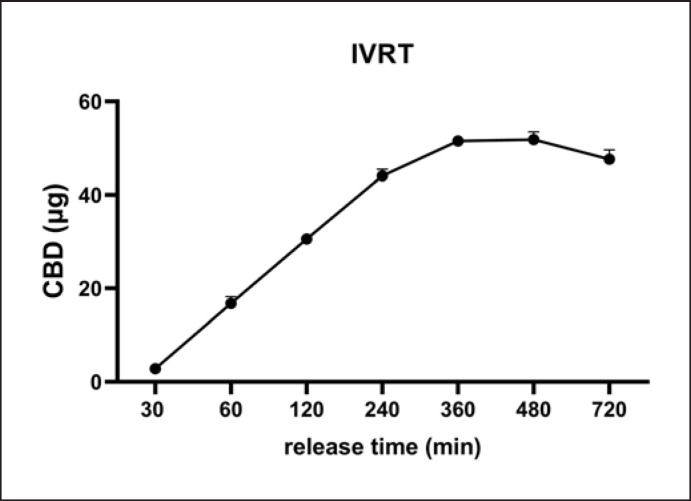
The theoretical skin permeability of CBD isolate was first evaluated by a computational model SwissADME and DERMWINTM. CBD's −Log Kp, a theoretical indicator of skin permeability, was predicted to be 3.6 and 5.7 cm/s by the SwissADME and DERMWINTM model, respectively, suggesting that CBD is skin permeable. This prediction was further validated by experimental data obtained from the PAMPA. CBD had a −LogPe value of 5.0, whereas the reference standards including warfarin (low permeability), piroxicam (medium permeability), and verapamil and progesterone (high permeability; see their computational-based skin permeability in online suppl. Table S1) had −LogPe values of 5.75, 5.41, 5.04, and 4.93 at pH 6.5, respectively (Fig. 1a). The CBD −LogPe value was similar to that of the highly permeable standard compounds. In a slightly basic condition (pH 7.4), CBD's −LogPe was 5.0, which is similar to verapamil's (5.05) and progesterone's (5.13) (Fig. 1b). Data from the PAMPA showed that CBD had similar −LogPe values as compared to known skin permeable compounds including verapamil and progesterone at pH 6.5 and 7.4, suggesting that CBD may have favorable skin permeability. This was further supported by data obtained from the Franz cell diffusion assay (IVRT) showing that the CBD was able to penetrate the Franz cell membrane and was detected in the diffusion compartment. CBD was detectable at 30 min and gradually increased in concentration to 16.80, 30.59, and 44.08 μg/mL at 60, 120, and 240 min, respectively (Fig. 2). The CBD content reached a plateau from 360 to 720 min at 47.6–51.8 μg/mL, respectively. Data from the artificial membrane-based assays suggest that CBD isolate may be able to penetrate the skin barrier. We next sought to evaluate the skin permeability of CBD in topical formulations (i.e., creams). However, given that CBD is a relatively lipophilic compound with low solubility in aqueous solutions [12], it is critical to use proper surfactants to solubilize CBD for its topical formulations. Herein, the solubility and stability of CBD in several surfactants were studied.
Fig. 1.
Permeability of CBD evaluated by the PAMPA model. The PAMPA sandwich was created with the donor (bottom), artificial skin membrane, and skin sink buffer as the acceptor (top). CBD was incubated in the PAMPA sandwich for 1 h at room temperature, and then, the UV profiles of the donor and acceptor plates were read on the SpectraMax plate reader connected to the PAMPA software to calculate the −LogPe values. CBD shows passive permeability in the skin PAMPA model (n = 6). The passive permeability of CBD was evaluated at two pH conditions 6.5 (a) and 7.4 (b).
Fig. 2.
In vitro permeability profile of CBD isolate obtained by using the Franz cell diffusion IVRT assay (n = 3). Surfactant from the sample was first removed by extraction with a C-18 SPE column prior to the analysis of CBD content using the LC-MS method.
CBD Is Soluble and Stable in Surfactant Tween 20
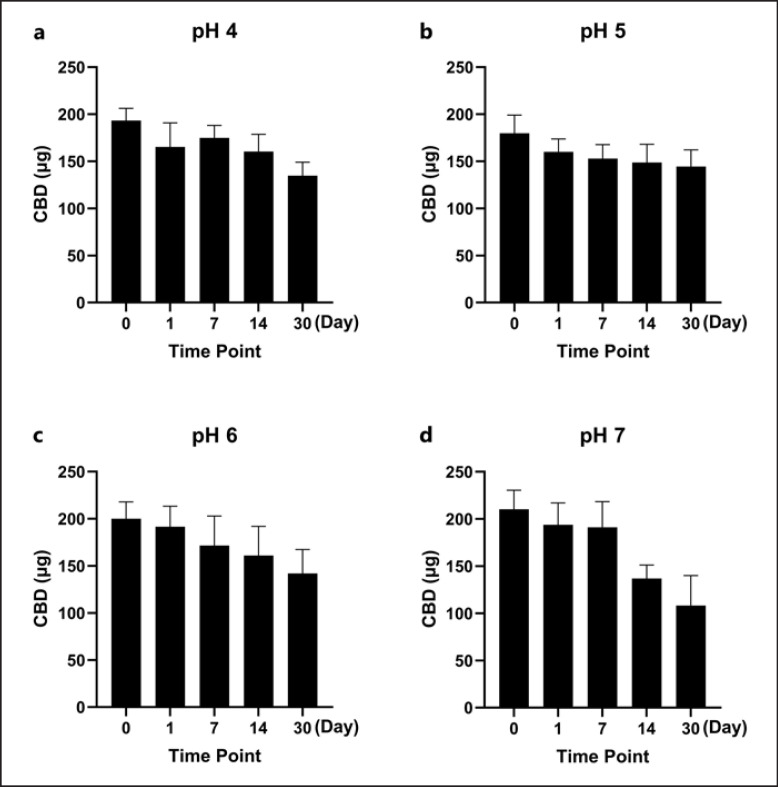
The solubility of CBD in the oil carrier PIB (in PB and PBS) and a panel of surfactants (at 10% w/w in the final solution) including Tween 20, Tween 80, and SLS was evaluated (Fig. 3). CBD was more soluble in surfactants Tween 20, Tween 80, and SLS with a concentration of 378.4, 388.0, and 399.8 μg/mL, respectively, and less soluble in PIB with PBS or PB with a concentration of 47.3 and 55.7 μg/mL, respectively. Tween 20 was selected as the surfactant for further evaluations of CBD's stability at different pH conditions as it is a common surfactant used in various topical formulations. In the presence of Tween 20, CBD had minor degradation up to a period of 7 days as its level decreased by 9.6, 15.0, 14.3, and 9.1% at pH 4, 5, 6, and 7, respectively (Fig. 4). The degradation became significant by 14 days as the CBD level was reduced to 17.1–34.9% at different pH conditions. Notably, CBD at moderate acidic conditions (pH 5 and 6) maintained the highest level of stability with concentrations of 81% and 70%, respectively. In contrast, CBD in pH 7 solution showed the highest degradation over the 30-day period as its level was reduced to 50%. We further evaluated whether CBD solubilized in Tween 20 at different pH conditions can be detected in the PAMPA. The LC-MS analysis showed that CBD was detectable in the acceptor wells with concentrations of 32.5, 27.5, 39.0, and 41.3 μg/mL at pH 4, 5, 6, and 7, respectively. These data suggest that CBD in the presence of Tween 20 was more skin permeable at pH 6 and 7 (Fig. 5).
Fig. 3.
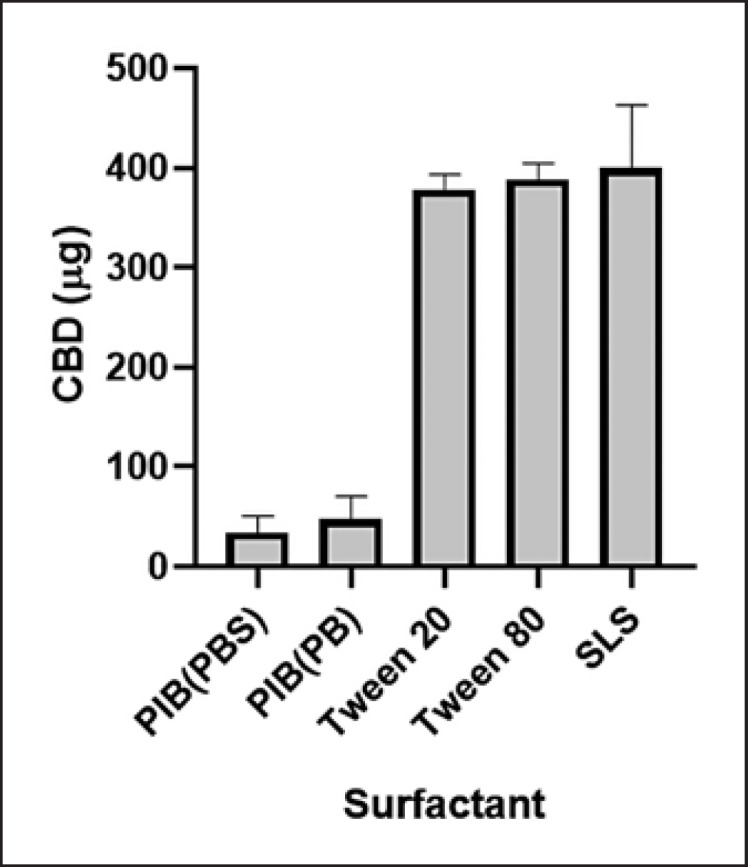
Solubility of CBD in various surfactants including PIB (in PBS and PB), Tween 20, Tween 80, and SLS. The concentrations are expressed as mean ± standard error (n = 3). CBD (5 mg) and different surfactants (at a final concentration of 10%; w/w) were mixed with PBS (12 mL). Solutions were thoroughly mixed and placed still at room temperature for 24 h. CBD in each solution was extracted with a SPE column (100 mg), and the CBD content was determined by the HPLC analysis.
Fig. 4.
a–d Stability of CBD in surfactant Tween 20 at various pH conditions (pH = 4, 5, 6, and 7). The stability of the CBD-surfactant solution was evaluated using a LC-MS method, and CBD degradation was monitored by measuring the accumulation of breakdown products. The CBD content was expressed as percent of cumulative degradation over a 30-day timespan, and values are expressed as mean ± standard error (n = 3).
Fig. 5.
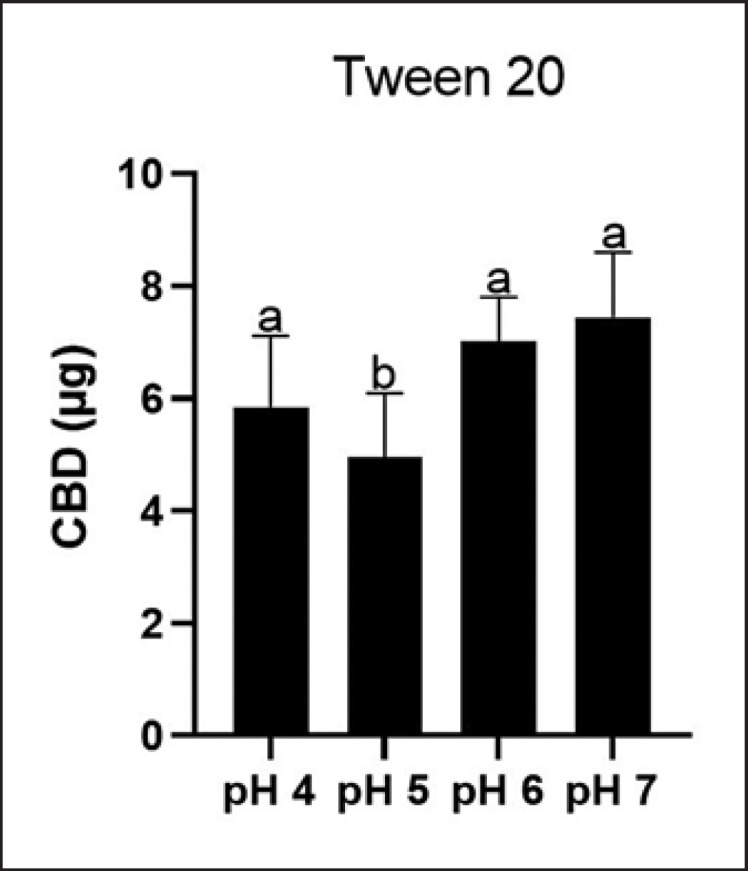
Permeability of CBD in surfactant Tween 20 at different pH conditions (pH = 4, 5, 6, and 7) determined by the PAMPA model. Data are expressed as mean ± standard error (n = 3). Statistical significance was determined by analysis of variance (ANOVA), and significant differences of CBD content at different pH conditions are shown with different letters.
CBD in the Cream Formulations Is Detectable by the Franz Cell Diffusion Assay
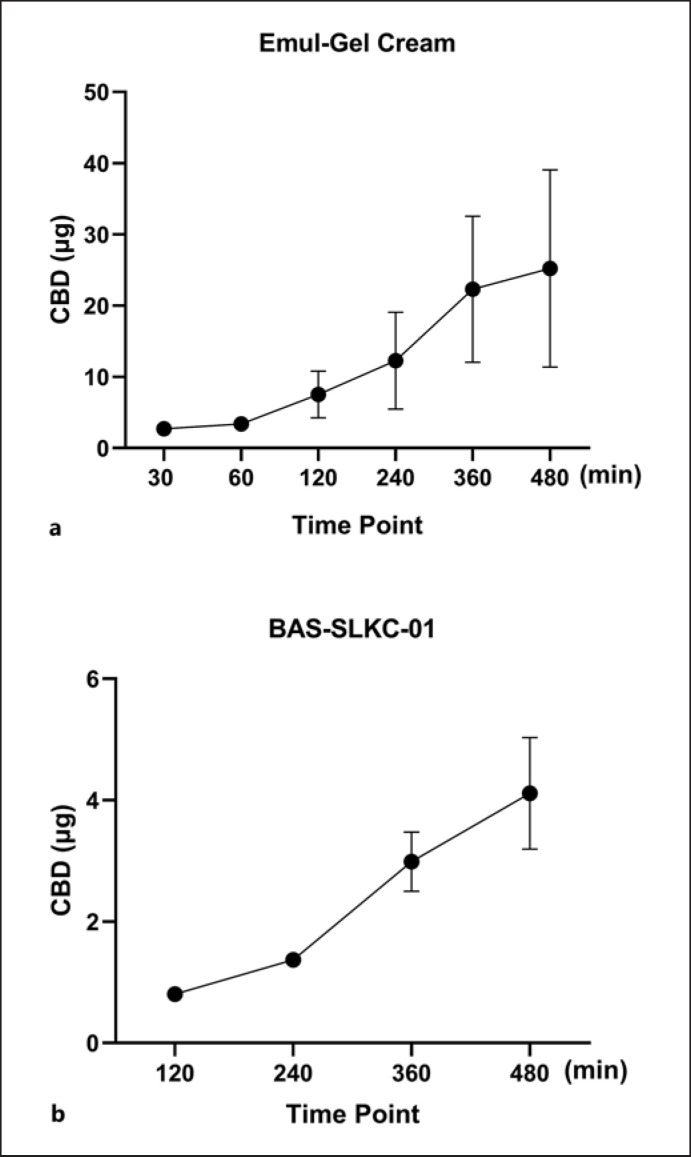
The skin permeability of CBD in a commercial cream and an in-house formulated cream was assessed using the Franz cell diffusion assay. Prior to the testing, a membrane compatibility test with Supor PES membranes was conducted to confirm that the membranes did not interfere with the release of CBD (online suppl. Fig. S5). CBD in the in-house made cream formulation showed feasible skin permeability as CBD was detected in the diffusion compartment at the timepoint of 30 min. The CBD content increased from 2.7 to 25.2 μg/mL from 30 to 480 min, respectively (Fig. 6). The CBD content in the IVRT for the BAS-SLKC-01 cream formulation was much lower as CBD in the diffusion compartment was not detected until the timepoint of 120 min (0.8 μg/mL), and then gradually increased to 1.4, 3.0, and 4.1 μg/mL at 240, 360, and 480 min, respectively.
Fig. 6.
In vitro release profiles of CBD in two cream formulations obtained by the Franz cell diffusion assay (n = 3). The in vitro release profiles of CBD are tested in two formulations including the in-house (a) and BAS-SLKC-01 creams (b), and the CBD content in each cram was determined by the LC-MS method.
Discussion
CBD's extensive use in cosmetics necessitates further evaluations of its stability and permeability through the skin. Cannabinoids are susceptible to UV degradation, chemical rearrangements, and oxidation [23]. Lindholst determined that when organic solvents, such as ethanol and chloroform, are used, both temperature and light exposure influence the stability of cannabinoids [24]. However, most of the published studies that are available have been completed using the resin forms of Cannabis or their alcohol extracts. In skincare, alcohol extracts with solvent present and pure resin are rarely used. Rather, the lipophilic cannabinoids would be incorporated into aqueous-based products using surfactants or simply added to creams without surfactants. It is well established that light and temperature play the most significant role in degradation [24, 25]. We found that in a PBS and 10% w/w Tween 20 solution, which is more relevant in skincare formulations than alcoholic preparations, CBD shows the most degradation at pH 6 and pH 7 compared to pH 4 and pH 5 (Fig. 4). Many skincare products are already formulated to be slightly acidic, often using ingredients such as citric acid to maintain the barrier function of the acid mantle on the skin [26]. In our current study, CBD was evaluated with multiple in vitro assays to evaluate the passive permeability of CBD through biomimetic skin membranes. The first assay employed was the PAMPA model, which evaluated CBD's permeability, and compared it to the reference compounds with known skin permeability and pharmacokinetics (online suppl. Table S1). CBD showed a similar permeability profile to the endogenous steroid progesterone suggesting that CBD is moderately permeable through the skin.
Conceptually similar to PAMPA, the Franz cell diffusion assay was employed to determine the rate at which CBD in various formulations penetrated synthetic skin membranes using the IVRT assay with a cellulose acetate membrane. Although skin-tissue-derived membranes may be a preferred model, their physiological conditions can be affected by various factors including the differences in age, race, and site of skin. Thus, in our current study, an artificial membrane, i.e., the cellulose acetate membrane representing the general biological features of the skin with desired feasibility and reproducibility [27], was selected to study the skin permeability of CBD formulations. This method was also used in a recently reported study showing that CBD's concentration and skin permeability in various cannabis-infused consumer products can be contrasting due to their formulations [17]. Thus, it is crucial to analyze the skin permeability of CBD using the IVRT assay with a cellulose acetate membrane in topical formulations. When comparing formulations of CBD in a commercially available formulation (i.e., BAS-SLKC-01; purchased from MakingCosmetics Inc.) and an in-house made formulation (i.e., Emul Gel cream), the IVRT indicated the release of CBD in the in-house formulation was much higher compared to the BAS-SLKC-01 cream (Fig. 6). The observed difference in permeability may be due to the nonuniformity of CBD in the formulation. The permeability of CBD may have been influenced by the presence of penetration-enhancing oils in the lipophilic phase of the Emul Gel cream. In addition, propylene glycol, a commonly used excipient in various formulations, has been reported to enhance the transdermal permeability of many drugs [28]. Data from the IVRT experiments with two CBD cream formulations suggest that the permeability of CBD can be greatly influenced by ingredients in the oil phase (e.g., coconut, almond, jojoba, and avocado oils) and the manufacturing process (i.e., incorporating CBD in the pre-formulated cream vs. adding CBD in the excipients) [29], which is crucial for the optimization of CBD's formulation for topical applications. Surfactants in different pH conditions also play a pivotal role in CBD's skin permeability in topical recipes and formulations. As we observed in the PAMPA, CBD's permeability can be affected by adding surfactants at varying pH environments (Fig. 1, 4). Findings from the current study showed that CBD is skin permeable in the PAMPA model. Furthermore, CBD may penetrate the skin barrier in cream formulations as supported by findings from the Franz cell diffusion assay. However, these findings are based on experiments using artificial skin membranes, which may not reflect the physiological conditions of human skin tissue warranting further studies using in vivo models to confirm.
Conclusion
CBD isolate showed moderate skin permeability in the PAMPA and Franz cell diffusion assays. CBD is stable in surfactants including Tween 20 at slightly acidic conditions (pH = 5 and 6). Therefore, CBD may maintain its stability in topical products by keeping the product cool, away from light, and at slightly lower pH conditions. Lipophilic creams might be considered a favorable base for compounding CBD into different formulations. However, previous studies have shown that hydrophilic gels incorporating CBD via specific vehicles are effective in terms of permeation and CBD retention on the skin [15]. These formulations should also be further explored. Additionally, nanotechnology-based and solid-state formulations may prove as innovative and effective in terms of CBD delivery to the skin [15, 30]. Further studies are warranted to assess CBD's permeability using models with human skin tissue. Findings from the current study provide critical information for the development of topical products containing CBD.
Statement of Ethics
No ethics approval is required for this study as no human subjects or animal experiments were involved in this study.
Conflict of Interest Statement
Navindra P. Seeram serves on the Advisory Board of Alluvion Brands, LLC (Warwick, RI, USA), as a consultant for the biological evaluations of phytocannabinoids. Hang Ma received funding from a research contract between URI Research Foundation and Alluvion Brands LLC. The other authors declare no conflicts of interest.
Funding Sources
Hang Ma received funding from a research contract between URI Research Foundation and Alluvion Brands LLC. No other external funding received for this study.
Author Contributions
Riley D. Kirk and Toyosi Akanji conducted the experiments, analyzed data, and wrote the manuscript draft. Huifang Li assisted data analysis and manuscript preparation. Jie Shen supervised the Franz cell diffusion experiments. Saleh Allababidi supervised the formulation experiments. Navindra P. Seeram, Matthew J. Bertin, and Hang Ma conceived, designed the overall project, and edited the manuscript.
Data Availability Statement
Raw data obtained in this study are available from the corresponding authors upon reasonable request. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgments
Spectrophotometric data were obtained from equipment and services located at the RI-INBRE Centralized Research Core Facility at the University of Rhode Island in Kingston, RI, USA. The RI-INBRE Core Facility is funded by the Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institute of Health under Grant No. P20GM103430.
Riley D. Kirk and Toyosi Akanji: equal contribution.
Funding Statement
Hang Ma received funding from a research contract between URI Research Foundation and Alluvion Brands LLC. No other external funding received for this study.
References
- 1.Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K. Skin tissue engineering-in vivo and in vitro applications. Clin Plast Surg. 2012;39((1)):33–58. doi: 10.1016/j.cps.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Tadicherla S, Berman B. Percutaneous dermal drug delivery for local pain control. Ther Clin Risk Manag. 2006;2((1)):99–113. [PMC free article] [PubMed] [Google Scholar]
- 3.Bíró T, Tóth BI, Haskó G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009 Aug 1;30((8)):411–420. doi: 10.1016/j.tips.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abels C, Soeberdt M. Can we teach old drugs new tricks?-Repurposing of neuropharmacological drugs for inflammatory skin diseases. Exp Dermatol. 2019 Sep 1;28((9)):1002–1009. doi: 10.1111/exd.13987. [DOI] [PubMed] [Google Scholar]
- 5.Ruela ALM, Perissinato AG, Lino MEd S, Mudrik PS, Pereira GR. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz J Pharm Sci. 2016 Jul 1;52((3)):527–544. [Google Scholar]
- 6.Jhawar N, Schoenberg E, Wang JV, Saedi N. The growing trend of cannabidiol in skincare products. Clin Dermatol. 2019 May–Jun;37((3)):279–281. doi: 10.1016/j.clindermatol.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Jastrząb A, Gęgotek A, Skrzydlewska E. Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells. 2019 Aug 3;8((8)):827. doi: 10.3390/cells8080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 2015 Apr 1;23((7)):1377–1385. doi: 10.1016/j.bmc.2015.01.059. [DOI] [PubMed] [Google Scholar]
- 9.Pucci M, Rapino C, Di Francesco A, Dainese E, D'Addario C, Maccarrone M. Epigenetic control of skin differentiation genes by phytocannabinoids. Br J Pharmacol. 2013 Oct;170((3)):581–591. doi: 10.1111/bph.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Ma H, Slitt AL, Seeram NP. Inhibitory effect of cannabidiol on the activation of NLRP3 inflammasome is associated with its modulation of the P2X7 receptor in human monocytes. J Nat Prod. 2020;83((6)):2025–2029. doi: 10.1021/acs.jnatprod.0c00138. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Li H, Xu F, Jiang X, Ma H, Seeram NP. Cannabidiol protects human skin keratinocytes from hydrogen-peroxide-induced oxidative stress via modulation of the caspase-1–IL-1β axis. J Nat Prod. 2021;84((5)):1563–1572. doi: 10.1021/acs.jnatprod.1c00083. [DOI] [PubMed] [Google Scholar]
- 12.Park C, Zuo J, Somayaji V, Lee BJ, Löbenberg R. Development of a novel cannabinoid-loaded microemulsion towards an improved stability and transdermal delivery. Int J Pharm. 2021 Jul 15;604:120766. doi: 10.1016/j.ijpharm.2021.120766. [DOI] [PubMed] [Google Scholar]
- 13.Caprioglio D, Mattoteia D, Pollastro F, Negri R, Lopatriello A, Chianese G, et al. The oxidation of phytocannabinoids to cannabinoquinoids. J Nat Prod. 2020 May 22;83((5)):1711–1715. doi: 10.1021/acs.jnatprod.9b01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinchcomb AL, Valiveti S, Hammell DC, Ramsey DR. Human skin permeation of delta8-tetrahydrocannabinol, cannabidiol and cannabinol. J Pharm Pharmacol. 2004;56((3)):291–297. doi: 10.1211/0022357022791. [DOI] [PubMed] [Google Scholar]
- 15.Casiraghi A, Musazzi UM, Centin G, Franzè S, Minghetti P. Topical administration of cannabidiol: influence of vehicle-related aspects on skin permeation process. Pharmaceuticals. 2020 Oct 23;13((11)):337. doi: 10.3390/ph13110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanti G, Grifoni L, Bergonzi MC, Antiga E, Montefusco F, Caproni M, et al. Development and optimisation of biopharmaceutical properties of a new microemulgel of cannabidiol for locally-acting dermatological delivery. Int J Pharm. 2021 Sep 25;607:121036. doi: 10.1016/j.ijpharm.2021.121036. [DOI] [PubMed] [Google Scholar]
- 17.Quiñones R, Moreno S, Smythers AL, Sullins C, Pijor H, Brown G, et al. Quantification of cannabis in infused consumer products and their residues on skin. ACS Pharmacol Transl Sci. 2022 Jul 18;5((8)):642–651. doi: 10.1021/acsptsci.2c00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He H, Li H, Akanji T, Niu S, Luo Z, Li D, et al. Synthesis and biological evaluations of oleanolic acid indole derivatives as hyaluronidase inhibitors with enhanced skin permeability. J Enzyme Inhib Med Chem. 2021;36((1)):1665–1678. doi: 10.1080/14756366.2021.1956487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Xu Y, Kirk RD, Li H, Li D, DaSilva NA, et al. Inhibitory effects of skin permeable glucitol-core containing gallotannins from red maple leaves on elastase and their protective effects on human keratinocytes. J Funct Foods. 2020 Dec 1;75:104208. [Google Scholar]
- 20.Ng SF, Rouse JJ, Sanderson FD, Meidan V, Eccleston GM. Validation of a static Franz diffusion cell system for in vitro permeation studies. AAPS PharmSciTech. 2010 Sep 15;11((3)):1432–1441. doi: 10.1208/s12249-010-9522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benavides E, Manjarres S, Salamanca CH, Barrera-Ocampo A. Rabbit ear membranes as an interesting alternative for permeability tests in the preformulation stages of cosmetic products. Cosmetics. 2020 May 18;7((2)):35. [Google Scholar]
- 22.Dong Y, Qu H, Pavurala N, Wang J, Sekar V, Martinez MN, et al. Formulation characteristics and in vitro release testing of cyclosporine ophthalmic ointments. Int J Pharma. 2018 Jun 10;544((1)):254–264. doi: 10.1016/j.ijpharm.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Grafström K, Andersson K, Pettersson N, Dalgaard J, Dunne SJ. Effects of long term storage on secondary metabolite profiles of cannabis resin. Forensic Sci Int. 2019 Aug 1;301:331–340. doi: 10.1016/j.forsciint.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 24.Lindholst C. Long term stability of cannabis resin and cannabis extracts. Aust J Forensic Sci. 2010 Sep 1;42((3)):181–190. [Google Scholar]
- 25.Pacifici R, Marchei E, Salvatore F, Guandalini L, Busardò FP, Pichini S. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin Chem Lab Med. 2018 Mar 28;56((4)):94–96. doi: 10.1515/cclm-2017-0758. [DOI] [PubMed] [Google Scholar]
- 26.Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19((6)):296–302. doi: 10.1159/000094670. [DOI] [PubMed] [Google Scholar]
- 27.Haq A, Dorrani M, Goodyear B, Joshi V, Michniak-Kohn B. Membrane properties for permeability testing: skin versus synthetic membranes. Int J Pharm. 2018 Mar 25;539((1–2)):58–64. doi: 10.1016/j.ijpharm.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Neupane R, Boddu SHS, Renukuntla J, Babu RJ, Tiwari AK. Alternatives to biological skin in permeation studies: current trends and possibilities. Pharmaceutics. 2020 Feb 13;12((2)):152. doi: 10.3390/pharmaceutics12020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad A, Ahsan H. Lipid-based formulations in cosmeceuticals and biopharmaceuticals. Biomed Dermatol. 2020 May 6;4((1)):1–10. [Google Scholar]
- 30.Millar SA, Maguire RF, Yates AS, O'sullivan SE. Towards Better Delivery of Cannabidiol (CBD) Pharmaceuticals. 2020 Sep 1;13((9)):219. doi: 10.3390/ph13090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
Raw data obtained in this study are available from the corresponding authors upon reasonable request. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.