Abstract
Cannabis and its natural derivatives have emerged as promising therapeutics for multiple pathological and nonpathological medical conditions. For example, cannabinoids, the most popular and biologically active chemicals in cannabis, aid in many clinical ailments, including pain, inflammation, epilepsy, sleep disturbances or insomnia, multiple sclerosis, anorexia, schizophrenia, neurodegenerative diseases, antinausea, and most importantly, cancer. Despite the comprehensive benefits, certain aspects of cannabis present unique challenges in the medical cannabis landscape. Recent studies have highlighted the inherent challenges associated with cannabinoids' formulation like low solubility, rapid metabolism, poor bioavailability, and erratic pharmacokinetics − all of which contribute to the limited efficacy of cannabinoids. Several efforts are underway to address the bottlenecks and modify the formulations along with the delivery systems to achieve greater solubility/bioavailability, potency, and efficacy in treatment settings while minding the necessary standards for purity associated with the pharmaceutical industry. The current article presents a perspective on (1) a working knowledge of cannabinoids and their mechanisms of action, (2) the landscape of using medicinal cannabis for cancer-related medical conditions along with adversities, (3) current approaches, formulations, and challenges in medicinal cannabis delivery systems (oral, transdermal, pulmonary, and transmucosal), and lastly, (4) emerging approaches to improve delivery systems.
Keywords: Cannabis, Cannabinoids, Cancer, Pain, Nanomedicine, Drug delivery systems
Introduction: What Are Cannabinoids and How Do They Work?
Cannabis and the bioactive derivatives come from either plants (phytocannabinoids) or mammals (endocannabinoids). Cannabis sativa, a member of the Cannabaceae family and one of the oldest known psychoactive plants, has garnered worldwide attention for its medical and recreational uses. Studies on the therapeutic efficacy of cannabis formulations have been ongoing for more than three decades. The therapeutic benefits of cannabis have been ascribed to a diverse set of bioactive chemicals such as cannabinoids, terpenes, and flavonoids, with cannabinoids being the most well-known. Although the pharmacology of most cannabinoids is unknown, 9-tetrahydrocannabinol (9-THC) and cannabidiol (CBD) have been studied as key contributors to the therapeutic effects of cannabis. These two cannabinoids are more abundant in cannabis, with THC having higher cannabimimetic activity than CBD. Furthermore, these two phytocannabinoids have similar chemical structures yet different pharmacological effects [1, 2, 3].
Recent studies have elucidated the molecular mechanisms underlying the phytocannabinoids induced responses and crosstalk with the endocannabinoid signaling (ECS) in humans. Identification of THC in cannabis created a roadmap to characterize cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2), which belong to the G-protein-coupled receptor (GPCR) superfamily [4, 5]. CB1 receptors are distributed and expressed in the brain and peripheral nerve terminals and, upon activation, mediate the central effects and many of the peripheral effects of cannabinoids. The CB1 receptor is also present in the nonneural tissues such as the testis, eye, vascular endothelium, and spleen. Notably, the CB2 receptor is expressed in immune cells, such as lymphocytes (B-cell and T-cell) and macrophages, and organs, such as the spleen, tonsils, and lymph nodes. Collectively, the CB2-driven signaling is responsible for the anti-inflammatory and immunomodulatory effects of cannabis [6, 7, 8].
To date, seven distinct endogenous endocannabinoid receptor ligands have been identified, with the derivatives of N-arachidonoyl ethanolamide (anandamide) and 2-arachidonoylglycerol (2-AG) being the most studied endocannabinoids. These two endocannabinoids elicit physiological responses primarily through the CB1 and CB2 receptors [9, 10]. Similarly, 9-THC, a plant-derived psychotropic cannabinoid ligand, interacts with the CB1 receptor to produce psychoactive effects and engages with the CB2 receptor to produce immune-modulatory effects. On the contrary, CBD is a nonintoxicating cannabinoid with a modest affinity for CB1 and CB2 [11]. Apart from cannabinoid receptors, endocannabinoids and phytocannabinoids have been reported to interact with other groups of receptors, including orphan G-protein-coupled receptor 55 (GPR55), peroxisome proliferator-activated receptors (PPARs), and transient receptor potential vanilloid channel type 1 (TRPV1) [12]. Figure 1 depicts the signal transduction pathways modulated by endocannabinoids and their receptors.
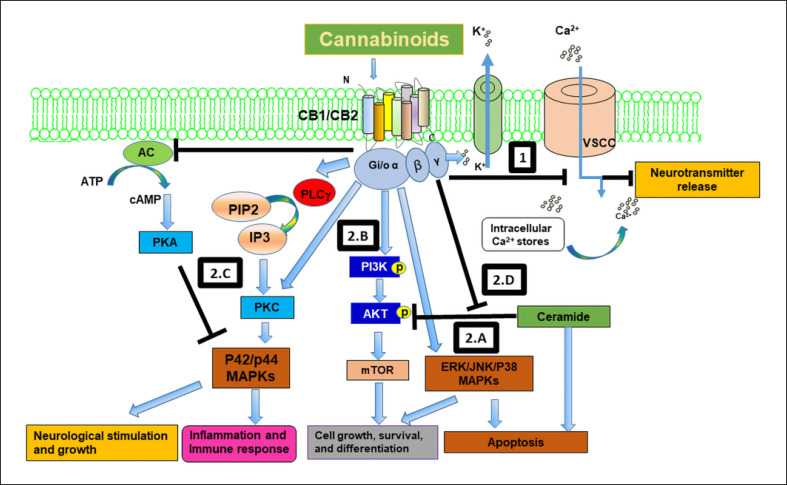
Fig. 1.
The endocannabinoid system regulates a battery of signaling pathways involved in neurological stimulation and growth, inflammation and immune response, control of cell fate, growth differentiation, and apoptosis. (1) Cannabinoids interact with the CB1 receptor to block voltage sensitive Ca2+ channels and activate K+ channels, resulting in neurotransmitter release and effects on learning, locomotion, and memory. (2.A–D) Cannabinoids interact with cannabinoid receptors and modulate several signaling pathways that control cell growth, differentiation, survival, and apoptosis. These pathways include: (2.A) activation of mitogen-activated protein kinases (MAPKs) such as the extracellular-signal-regulated kinase (ERK) and the stress-activated kinases JUN amino-terminal kinase (JNK) and p38 MAPK; (2.B) activation of phosphatidylinositol 3-kinase (PI3K)-a serine/threonine kinase (AKT)-the mammalian target of rapamycin (mTOR) survival pathway; (2.C) activation of p42/p44 MAPKs by inhibiting adenylyl cyclase (AC) and activating protein kinase C (PKC), which control neuronal growth, inflammation, and apoptosis; and (2.D) cannabinoids also promote survival by activating AKT by blocking ceramide induced AKT inhibition.
The effects of the endocannabinoids on the CB receptors-mediated downstream signaling under physiological conditions are well documented [13, 14]. Although the precise mechanism of cannabinoid receptor expression modulation is unknown, studies have revealed a significant association between changes in the ECS activity and a wide variety of diseases, including cancer. CB1 and CB2 receptors are overexpressed in many cancer types [15, 16, 17, 18] and linked to poor clinical outcomes in patients with pancreatic, prostate, ovarian, and colorectal cancers [19, 20, 21]. Interestingly, CB1 and CB2 receptor expression in the normal cells is significantly lower than the cancer cells, suggesting that CB receptors are potential targets for cancer treatment.
Phytocannabinoid-mediated modulation of ECS receptors is a promising therapeutic strategy for cancer and many other diseases. Cannabinoids have shown promise for treating a wide variety of ailments like multiple sclerosis spasticity, chemotherapy-induced nausea and vomiting, sleep and anxiety disorder, and several other clinical conditions [1, 3]. This review article summarizes the evidence for the cannabinoids' palliative and antineoplastic effects in cancer, adverse effects, perceptive delivery methods, and cannabinoids formulations for pain and cancer treatment. Relevant findings have been summarized from public databases (Pubmed and U.S. Patent and Trademark Office) and literature searches ranging from 2000 to 2022.
The Landscape of Cannabinoids in Cancer Treatments
Palliative Care
Until the 1900s, the use of cannabis in oncology was not well documented; however, reports from the early 1970s highlight the palliative effects of cannabinoids in cancer. Cannabinoids and cannabinoid-based formulations were primarily exploited for their analgesic and antiemetic effects as well as alleviating chemotherapy-induced side effects [1]. For example, Sallan et al. [22] reported that THC relieves nausea and vomiting caused by chemotherapy. Subsequently, many other studies have documented the inhibition of chemotherapy-induced nausea and vomiting by THC and its synthetic derivatives [23, 24]. Dronabinol, a synthetic isomer of THC, and nabilone have been approved by the U.S. Food and Drug Administration (FDA) for nausea and vomiting caused by chemotherapy [25, 26]. Dronabinol is now being prescribed for anorexia with weight loss in patients with AIDS [1, 26, 27]. The FDA also approved THC for anorexia in HIV/AIDS [1, 25]. Epidiolex (cannabidiol), an oral CBD solution approved by the FDA in 2018, is prescribed for severe forms of epilepsy occurring in two rare genetic disorders (Dravet syndrome and tuberous sclerosis complex) and one peripartum brain injury condition (Lennox-Gastaut syndrome in both adults and children [25, 28, 29]. For chronic pain and in patients with cancer, oral THC alone or in combination with CBD, or vaporized cannabis, has been shown to reduce neuropathic and cancer pain, as well as enhance self-reported sleep [25, 30]. In mice injected with paclitaxel (side effect includes peripheral neuropathy), CBD pretreatment inhibits paclitaxel-induced peripheral neuropathy [29, 31]. Nabiximols effectively relieve the pain associated with multiple sclerosis, cancer, and rheumatoid arthritis [32, 33]. Interestingly, the effects of oral THC 20 mg were comparable to codeine 120 mg, but with markedly fewer psychological effects, providing a solid basis for using THC as a substitute for opioids [34]. Although the mechanisms underlying the palliative effects of cannabis and cannabinoids are still being investigated, its anti-inflammatory, antiangiogenic, and proapoptotic effects paved the way for exploring its effects on other pathological conditions such as microbial infections, inflammation, and cancer [1, 3].
Cannabinoids are currently being tested in clinical trials for palliative care (for pain and nausea). Furthermore, many recent studies suggest that cannabinoids could also be used as antineoplastic agents. Munson et al. [35] first reported the antineoplastic effects of cannabinoids, such as ∆8-THC, ∆9-THC, and CBD, in lung adenocarcinoma. Subsequently, multiple studies confirmed the antineoplastic activities of cannabinoids in a broad spectrum of cancer cells and tumors, including lung, glioma, breast, pancreatic, prostate, skin, colorectal, thyroid epithelioma, and lymphomas [1, 36, 37]. The cannabinoids that modulate key cell-signaling pathways involved in cell proliferation, cell death, tumor angiogenesis, invasion, and metastasis are depicted in Figure 2 [1, 36]. The most intriguing aspect of cannabinoids activity is that it selectively targets tumor cells but does not affect nonneoplastic cells, implying differential regulation of prosurvival and apoptotic pathways in neoplastic and nonneoplastic cells by cannabinoids. For e.g., cannabinoids induce apoptosis in glioma cells through ceramide but inhibit apoptosis in normal glial cells by activating the CB1 receptor and the PI3K-AKT survival pathway [1, 38, 39].
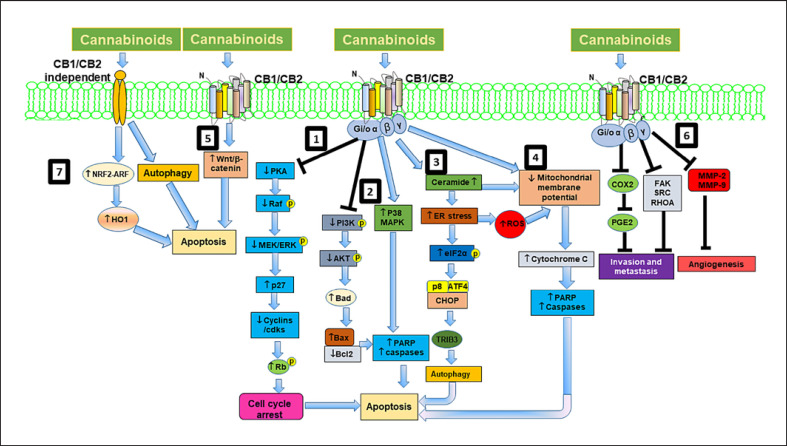
Fig. 2.
Schematic representation of cannabinoid mediated signaling pathways in cancer cells. In cancer cells, cannabinoids engage the CB1 or CB2 receptors to modulate numerous signaling pathways promoting cell cycle arrest, autophagy, and apoptosis and inhibit cell proliferation, invasion, metastasis, and angiogenesis. (1) Cannabinoids inhibit cAMP-dependent protein kinase, which modulates mitogen-activated protein kinases (MAPKs) and leads to stimulation of cyclin kinase inhibitor p27 and inhibition of cell cycle regulatory molecules, resulting in G1 arrest and apoptosis. (2) Cannabinoids also modulate MAPKs and PI3K/Akt pathways and its downstream apoptotic proteins leading to induction of apoptosis. (3) Cannabinoids also stimulate ceramide synthesis via activation of serine palmitoyl transferase (SPT) and upregulate p8, leading to induction of apoptosis through autophagy. (4) Cannabinoids also cause depolarization of the mitochondrial membrane, and caspase activation. (5) Cannabinoids inhibit cancer cell proliferation and apoptosis by modulating Wnt/β-catenin signaling pathway. (6) Cannabinoids inhibit invasion, metastasis, and angiogenesis through modulating COX-2/prostaglandin E2 (PGE2) signaling pathways, focal adhesion kinase (FAK) and RhoA-ROCK pathways and inhibiting MMPs respectively. (7) Cannabinoids also induce cell death via CB1/CB2 receptor-independent mechanisms by activating nuclear factor (erythroid derived 2)-like 2 (Nrf2)/antioxidant responsive element (ARE) pathway and heme oxygenase-1 (HO-1) induction and transcription.
In recent years, cannabinoids have received increased attention as chemosensitizers. Studies suggest that cannabinoids, CBD and/or THC and enhance the sensitivity of traditional anticancer drugs including vinblastine, mitoxantrone, cytarabine, doxorubicin, vincristine, bortezomib, carmustine, doxorubicin temozolomide, and carfilzomib [1, 36]. A combination of gemcitabine and cannabinoids synergistically inhibited the growth of pancreatic adenocarcinoma [40]. Similarly, a combination of temozolomide, THC, and CBD blocked the growth of glioma xenografts [41]. Although there is a wealth of preclinical evidence supporting the anticancer properties of cannabinoids THC and CBD, clinical evidence is still lacking mostly due to regulatory conditions limiting engagement of medical professionals.
Adverse Effects of Cannabinoids in Cancer Treatments
Many therapeutically efficacious drug candidates never make it to the clinical trials because of their adverse effects. Cannabinoids, on the other hand, are regarded as a safe medicine that is well tolerated in animals. In preclinical animal studies, a combination of delta-9-THC marijuana with conventional chemotherapy agents improved the efficacy of the chemotherapeutic drugs with no adverse side effects [1, 3, 42].
Despite the minimal toxicity of cannabinoids, their history of psychotropic effects limits clinical use. Cannabinoids like THC exert their psychoactive effects through the CB1 receptor activation, while cannabinoids targeting the CB2 receptor have reduced psychotropic effects. Selective CB2-receptor activation has been shown to suppress the growth of gliomas, skin carcinomas, and pain in animals without any signs of psychotropic activity. Nonpsychoactive cannabinoids, such as CBD, have a lower affinity for CB1 and CB2 receptors and a higher affinity for noncannabinoid receptors, which may prove helpful in the treatment of various pathological conditions including cancer. Synthesizing cannabinoids, from predetermined genetic strains of that cannabis, that do not penetrate the blood-brain barrier can be another strategy to minimize the psychotropic effects while improving antitumor, pain-relief, and appetite-stimulating qualities.
Current Approaches, Formulations, and Challenges in Medicinal Cannabis Delivery Systems
Cannabinoids' poor bioavailability limits their clinical use. Over the past several decades, various pharmaceutical industries have developed and customized cannabis formulations to improve bioavailability. This section describes the current routes of administration, and formulations of cannabinoids designed to improve bioavailability. Table 1 surveys and summarizes currently available cannabinoids delivery systems.
Table 1.
Currently available cannabinoids delivery systems
| Name | Formulation | Admin route | Disease | References |
|---|---|---|---|---|
| Cannabinoids | Encapsulation within micelles | Oral | [46, 47, 48] | |
|
| ||||
| Arvisol® | CBD | Oral | Rett syndrome, schizophrenia, and epilepsy | [49, 50] |
|
| ||||
| Dronabinol | THC | Syndros as Oral and Marinol as capsule | Pain and nausea | [51, 52] |
|
| ||||
| Nabilone | THC | Oral, capsule | Parkinson's disease | [52, 68, 69] |
|
| ||||
| Epidiolex | CBD | Oral | Dravet syndrome, Lennox-Gastaut syndrome, and severe myoclonic epilepsy | [54, 55, 56] |
|
| ||||
| CardiolRxTM | CBD oil | Oral | Prevent cardiovascular problems in SARS-CoV-2 infected patients | [57, 58] |
|
|
||||
| CBD conjugates | Oral | Cancer | [59] | |
|
| ||||
| TurboCBDTM | CBD in hemp oil | Increasing circulating CBD | [60, 61] | |
|
| ||||
| Nanoemulsion | CBD | Oral | – | [62] |
|
| ||||
| PTL 101 | CBD | Oral | Intractable epilepsy | [61, 63] |
|
| ||||
| Canemes® | CBD cyclodextrins; cannabinoids with sulfo-alkyl-β-CD | Oral | Pain, Parkinson's disease | [68, 69]; [70] |
|
|
||||
| CBD/naproxen | Oral | Acute and chronic pain | [71, 72] | |
|
|
||||
| CBD formulation | Oral | Graft-Versus-Host Disease prevention | [73, 74] | |
|
|
||||
| CBD in self-emulsifying delivery | Oral | Increases bioavailability | [75, 76] | |
|
|
||||
| CBD and THC combination | Oral | Pain-relieving drug | [77, 78] | |
|
| ||||
| AX 1505 | Chewing cum with cannabinoids | Oral | Multiple sclerosis-related pain and spasticity, Parkinson's disease, dementia, restless leg syndrome, and post-herpetic neuralgia | [79, 80, 81] |
|
| ||||
| BCT-521 | Combination of CBD and THC | Oral | Pain management with cancer patients | EudraCT2019-001382-32 |
|
|
||||
| Liquid formulation of CBD: THC combination | Oral | Fibromyalgia | [82] | |
|
| ||||
| THC/CBD (1:1) | Oral | Symptom relief in patients with advanced cancer | [83] | |
|
| ||||
| ZYN001 | Synthetic D-glyceric acid ester prodrug of THC | Transdermal | Fibromyalgia and neuropathic pain | [86] |
|
|
||||
| THC encapsulated in multilayered lipid vesicles | Topical and transdermal | Pain treatment | [87, 88] | |
|
| ||||
| Transcutol®-Neat or Diluted Mixtures | Cannabinoids in a gel formulation | Transdermal | Increased penetration through skin | [91, 92, 93] |
|
|
||||
| Gel containing CBD | Transdermal | Epilepsy, developmental and epileptic encephalopathy, fragile-X syndrome, and osteoarthritis | [94, 95, 96] | |
|
|
||||
| CBD and argan oil combination | Transdermal | Pain and edema associated with arthritic inflammation and rheumatic disorders | [97] | |
|
|
||||
| THC prodrugs | Transdermal | Glaucoma patients with pathologically high intraocular pressure lowered ocular pressure | [94, 95] | |
|
|
||||
| CBD and THC | Inhalation | [101] | ||
|
|
||||
| THC, CBD, and various terpenes | Inhalation | Pain and inflammation | [102, 103] | |
|
|
||||
| THC buccal formulation | Transmucosal | Cancer pain management, chemotherapy-induced nausea, vomiting, anorexia, and weight loss in AIDS patients | [107] | |
|
| ||||
| BRCX014 | Sublingual formulation of CBD | Transmucosal | Cancer treatment | [108] |
|
|
||||
| Nabiximols | The oromucosal spray | Multiple sclerosis spasticity, overrative bladder, and neuropathic pain management in adults with multiple sclerosis | [32, 33, 109] | |
|
|
||||
| Sativex® with temozolomide | Transmucosal | Cancer pain management, muscle stiffness, post-traumatic stress disorder | [112, 113] | |
|
|
||||
| CBD with Poloxamer 407, carboxymethyl cellulose, and starch | Transmucosal | [114] | ||
|
|
||||
| Combination of CBD and THC | Chewing cum | Pain management, multiple sclerosis-associated spasticity, Parkinson's disease, post-herpetic neuralgia, dementia, and other illnesses | [79, 81] | |
|
|
||||
| CBD formulations | Nasal | [90] | ||
|
|
||||
| Numerous cannabis formulations | Nasal | [115] | ||
Oral Administration, Formulations, and Modifications
Many cannabis products are taken orally via transmucosal route. Patients with chronic conditions constantly requiring large quantities of cannabis prefer oral delivery [43, 44]. Findings from the preclinical and clinical studies evaluating oral administration of cannabinoids (THC and CBD) have shown low bioavailability, likely due to slow and erratic absorption and degradation of the cannabinoids by stomach acids [43, 44]. Maximum plasma concentrations are usually obtained after 60–120 min but can last up to 6 h [45]. THC's oral bioavailability is further reduced by its presystemic metabolism in the liver and storage in the adipose tissue for a longer period of time [43, 44].
Another approach to oral administration of cannabinoids is encapsulation within micelles. When cannabinoids were loaded into micelles using nonionic surfactants (e.g., macrogolglycerol hydroxystearate) and delivered as oral aqueous formulations, they demonstrated a 2-fold increase in Cmax and a 1.21-fold greater area under the curve (AUC) in healthy participants. Soft-gelatin capsules containing cannabinoids have also been developed [46, 47, 48]. For gelatin encapsulation, cannabinoids are melted with poloxamers (poloxamer 124 and poloxamer 188 in particular) and then filled into hydroxypropyl methylcellulose.
Echo Pharmaceuticals is developing a CBD pill formulation (Arvisol®) to treat neurological conditions including Rett syndrome, schizophrenia, and epilepsy. The formulation consists of CBD (6.7% w/w), sucrose monolaurate (13.3%), ascorbic acid (0.7%), and lactose), which are combined in the wet granulation process to create the tablets and granules. Pharmacokinetic (PK) studies revealed a significant increase (100-fold) in the AUC of pills compared to CBD powder filled [49, 50].
The bioavailability of currently available cannabinoid pharmacological preparations, Dronabinol (available as a capsule (Marinol) and a solution (Syndros)), nabilone (available as a capsule, in a polyvinylpyrrolidone carrier), and Epidiolex is improved by using adjuvants (like surfactant, solubilizers, cosolvency, hydrotrophy, and novel excipients) and complexation (like cyclodextrins [CDs]) technologies) [43, 44, 51, 52].
The oral bioavailability of cannabinoids can be boosted by combining them with lipids from food. When CBD was taken with alcohol or a high-fat/high-calorie meal, the rate and degree of absorption increased. Lipid/oil-based formulations and gelatin matrix pellets are two effective formulations. Notably, the bioavailability of cannabinoids was significantly increased by a cannabis formulation in sesame oil (a vehicle with long-chain triglycerides, containing 42% oleic, 40% linoleic, and 16% palmitic acids). When THC and CBD are combined with long-chain triglycerides, they can bypass the liver, lowering the risk of presystemic metabolism [53]. In the USA, Epidiolex, as previously stated, was approved in 2018, as an adjuvant treatment for Dravet syndrome, Lennox-Gastaut syndrome, and severe myoclonic epilepsy in children [54, 55, 56]. The FDA recently approved a Phase II/III trial for an oral CBD oil formulation (CardiolRxTM) (developed by Cardiol Therapeutics Inc. [CA]) to prevent cardiovascular problems in hospitalized patients with a confirmed diagnosis of SARS-CoV-2 infection [57, 58].
For the treatment of cancer, preclinical trials using CBD conjugates created by Diverse Biotech Inc. (USA) are now underway [59]. Capsules (TurboCBDTM) with CBD in hemp oil, a high proportion of American ginseng and Ginko Biloba extracts have been proposed by Lexaria Bioscience Corp. (CA) [60]. In this formulation, long-chain fatty acids that are high in oleic acid compositions are associated with CBD using a dehydration process procedure. The presence of long-chain fatty acids, associated with CBD allowed higher concentrations of CBD to enter the circulatory system, perhaps by bypassing first-pass liver metabolism. The clinical trial using TurboCBDTM revealed that the use of TurboCBDTM increased CBD bioavailability by 111 percent as compared to an identical generic 90 mg dose but not with a half dose, according to a clinical trial. The authors also found that TurboCBDTM had a potential advantage over CBD in gelatin matrix pellets, with a Cmax time of 120 min, compared to the 3–3.5 h associated with gelatin matrix pellets. TurboCBDTM is now being tested in clinical research as a dietary supplement [61].
An oral nanoemulsion formulation of CBD containing lactose monohydrate powder, as a base substrate, and highly purified CBD crystals and sunflower oil in a 1:1 ratio has been reported in a recent patent by Docherty and Bunka [62]. MMJ PhytoTech Ltd is developing PTL 101 capsules (oral formulation) for the treatment of intractable epilepsy using Satipharm's Gelpell R product technology (AU) [63]. Briefly, 10–100 mg CBD dissolved in oil was added to a hot gelatin solution, which was then cooled and dried to produce gelatin pellets, and finally placed in hard gastro-resistant capsules. The soluble gelatin polymer was employed to facilitate dispersion by creating an in situ microemulsion, which is expected to improve CBD bioavailability while preventing stomach mucosa irritation. A clinical trial has demonstrated that PTL 101 capsules significantly reduce seizures in pediatric patients with treatment-resistant epilepsy over 12 weeks [61, 62, 63, 64]. When compared to an oromucosal spray, PK research found that CBD delivered during fed conditions caused a significant increase in AUC (134 percent for a 100 mg dose) [65].
Studies have shown that host-guest inclusion complexes of native CD with CBD increase aqueous solubility of CBD. Among the host-guest inclusion complexes at 1:1 (α-CD), 2:1 (β-CD), and 2:1 (γ-CD), complexation of CBD with γ-CD is insensitive to temperature, suggesting that the CBD-γ-CD complex is more stable than the others [66, 67].
Another formulation, developed by AOP Orphan Pharmaceuticals, significantly increased the aqueous solubility and bioavailability of the drug by using randomly methylated β-CD inclusion complexes of nabilone [52]. A pilot Phase II trial with Canemes® for pain was started in Austria in 2016 (EudraCT2015-004227-31), and Phase III development for its use in Parkinson's disease in the elderly is currently ongoing (EudraCT2017-004253-16) [68, 69]. The water solubility of CD-cannabinoid complexes can be further improved by chemically derivatizing β-CD. A patent application has been filed for the complexation of cannabinoids with sulfo-alkyl-β-CD in the presence of Cremophor EL (polyoxyl-35 castor oil) to promote cannabinoid solubility [70].
Another potential approach is chemically modifying CBD and creating prodrugs or conjugates that increase its solubility. Claritas Pharmaceuticals, USA is developing an oral CBD/naproxen combination drug that targets the glycine pain receptor-alpha3 mediated synthesis of the pain-inducing prostaglandin molecule PGE2 (dinoprostone) in the spinal cord to treat acute and chronic pain. Claritas Pharmaceuticals is also working on an intravenous (IV) formulation of CBD/naproxen drug [71, 72]. An oral CBD formulation is being tested for Graft-Versus-Host Disease prevention after allogeneic hematopoietic stem-cell transplantation [73, 74]. Williams et al. [75] have developed five oral CBD formulations; a dose of 30 mg CBD as a solid in suspension in water (code 203); with oil and starch (code 472); an oil solution (code 178); a liquid formulation with quillaia extract (20% CBD, code 340); with gum arabic (5% CBD code 707). CBD with gum arabic (5% CBD code 707) displayed maximum solubility, fastest Tmax, highest Cmax, and AUC. This finding agrees with the available data and literature on oral CBD. Knaub et al. [75, 76] found that higher Cmax and solubility were reached only when CBD was formulated in a self-emulsifying delivery manner (25 mg).
AusCann Group Holdings Ltd, AU, has developed a CBD and THC-containing pain-relieving drug. A randomized, open-label crossover trial is underway in Australia, where 28 fed and healthy volunteers were given capsules containing (ratio 1:1) 2.5 mg and 10 mg formulations of the pain-relieving drug. Two patents applications have been filed for this formulation, which includes solid self-emulsifying compositions containing either a single or a blend of cannabinoids, medium-chain triglycerides, surfactant Cremophor EL, and colloidal anhydrous silica [77, 78].
Axim Biotechnologies Inc., USA is developing a controlled release chewing composition that combines the two cannabinoids (1:1) at a 5 mg dose to treat patients with multiple sclerosis-related pain and spasticity, Parkinson's disease, dementia, restless leg syndrome, and post-herpetic neuralgia. Clinical trials for post-herpetic neuralgia and psychotic illnesses are ongoing in the USA. Patents have been filed for the coadministration of cannabis and opioid agonists and/or antagonists. AX 1505, an analogous orally administrable formulation, is a sustained release, floating-capsule containing cannabinoids developed by Axim Biotechnologies for the treatment of Crohn's disease. Preclinical testing of AX 1505 is in progress [79, 80, 81].
A Phase I/II trial using BCT-521, an oral, fixed-dose combination of CBD and THC for pain management with cancer patients who have already received standard-of-care treatment with opioids, has been completed by Beckley Canopy Therapeutics (UK) (EudraCT2019-001382-32; BCT-521-201). Knop labs (Chile) is working on an oral, fixed-dose, liquid formulation of CBD: THC combination (1 mg THC and 0.45 mg CBD per drop) to treat fibromyalgia. A Phase II trial evaluating this formulation in patients with fibromyalgia is ongoing in Chile [82]. Similarly, Hardy et al. are assessing the efficacy, safety, and acceptability of 1:1 THC/CBD for symptom relief in patients with advanced cancer in an ongoing clinical trial [83].
Transdermal, nasal, inhaled/pulmonary, and oral transmucosal delivery formulations circumvent the issues associated with oral administration by facilitating direct drug uptake and transport into the bloodstream, thereby avoiding hepatic metabolism. These delivery mechanisms will be described in more detail in the following sections.
Transdermal Administration, Formulations, and Modifications
The two types of dermal formulations include transdermal (for systemic effects) and topical formulations (for localized effects in the skin). Transdermal delivery bypasses the first-pass metabolism effect, resulting in increased drug bioavailability. Furthermore, transdermal administration provides continuous medicine release over an extended period while limiting the adverse effects of higher drug concentrations and thus improving patient adherence. Topical application is suitable for localized symptoms observed in dermatological disorders, arthritis, and peripheral neuropathic pain. Local irritation and limited skin penetration of drugs with a hydrophilic structure are the primary drawbacks of this method. Enhancers can be used in transdermal formulations to boost permeant penetration by altering the structure of the stratum corneum, the skin's outer layer, and enhancing permeant solubility. As cannabis is highly lipophilic, its delivery through the skin is challenging. Thus, there is an unmet need for synthesizing cannabis medicines with greater skin permeability [43, 84]. Several groups have developed synthetic cannabinoid prodrugs that are more skin permeable than the parent [43, 85].
ZYN001, a synthetic D-glyceric acid ester prodrug of THC, synthesized by Zynerba Pharmaceuticals Inc. (USA), is administered as a transdermal patch (36 mg dose). Although this formulation underwent Phase I testing in Australia for the treatment of fibromyalgia and neuropathic pain, the drug failed to achieve the desired blood levels of 5–15 ng/mL of THC and was withdrawn [86]. Biphasic multilayered lipid vesicles (MLVs) have also found applications in the delivery of cannabinoids. Altum Pharmaceuticals Inc. developed THC encapsulated in MLVs for topical and transdermal applications in pain treatment. MLVs are typically comprised of concentric lipid bilayers, separated by an aqueous phase, that encapsulate a central aqueous core [87]. In the biphasic MLVs, the core is an emulsion with a continuous aqueous phase and hydrophobic dispersed phase. The region between the lipid bilayers may also consist of an emulsion in the case of biphasic MLVs. Hydrophilic drugs can be dissolved in the aqueous phase and hydrophilic drugs can be encapsulated within the hydrophobic regions of the emulsions, or within the lipid bilayers [88].
CBD transdermal treatments are becoming increasingly popular. Paudel et al. [89] reported preclinical data on the pharmacokinetics of CBD patches. Compared to CBD in a propylene glycol-hydroxyethyl cellulose gel, Transcutol-HP (diethylene glycol monoethyl ether) at 6% CBD in a gel formulation increased skin absorption 3.7-fold [89, 90]. Stinchcomb et al. [91, 92, 93] also developed alternative approaches to improve delta-THC, cannabinol, and CBD transdermal penetration.
Zynerba Pharmaceuticals Inc. (USA) is actively developing a topical, transdermal gel containing CBD to treat epilepsy, developmental and epileptic encephalopathy, fragile-X syndrome, and osteoarthritis [94, 95, 96]. Shemanky et al. [97] reported the beneficial effects of CBD and argan oil combination in the treatment of pain and edema associated with arthritic inflammation and rheumatic disorders. Topical (ocular) administration of THC prodrugs in glaucoma patients with pathologically high intraocular pressure lowered ocular pressure [98, 99]. Linking THC prodrugs to valine, dipeptides, and amino acid-dicarboxylic esters created hydrophilic THC prodrugs, and of these, the THC-Val-HS emulsion and micellar solution formulations showed the best corneal permeability and intraocular pressure-lowering efficacy [98, 99].
Pulmonary Administration, Formulations, and Modifications
Inhalation and smoking cannabis cigarettes are the two most common consumption methods among recreational and therapeutic users. Intrapulmonary cannabis delivery is highly effective, with maximum plasma concentration reaching at 5–10 min (maintained at a steady-state for 3–5 h), rapid distribution to the central nervous system, and higher systemic bioavailability [43, 44, 100]. Inhaled cannabis PK profile is like IV delivered THC, despite having a lower AUC. CBDs PK profile is comparable to THC, regardless of whether taken orally, IV, or inhaled. Furthermore, patients unable to take oral drugs benefit from the pulmonary administration method. Potential disadvantages of pulmonary delivery include chronic cough and bronchitis as a result of inhaling hazardous combustion products [43].
Solowij et al. [101] developed a vaporization method for delivering CBD and THC. Vaporization involves heating cannabis to cannabinoid vaporization temperature (below the combustion point], to avoid the generating combustion by-products. A liquid pad (a removable disc made of tightly packed stainless steel wire mesh) from the Volcano® vaporizer device was used to load the crystalline-form CBD (preliminary experiments), and the ethanolic solutions of CBD (4 or 200 mg) and THC (4 or 8 mg) separately into the vaporizer filling chamber [101]. Tetra Bio-Pharma, USA, is developing a dry, compressed cannabis inhalable formulation standardized in THC, CBD, and various terpenes to treat pain and inflammation. This formulation is currently being tested in clinical trials in Canada and the USA [102, 103]. Multiple reports and patents describing cannabis vaporization systems and nebulization formulations for enhanced cannabinoid bioavailability upon pulmonary administration have been published [104, 105, 106].
Transmucosal Administration, Formulations, and Modifications
Transmucosal dosing is a noninvasive delivery method in which the drug is absorbed into the systemic circulation via the highly vascularized mouth mucosa, bypassing first-pass liver processing. The principal transmucosal routes that have been demonstrated to be more effective than oral dosing include intranasal, oral transmucosal, and rectal [43, 44]. Orally disintegrating tablets, buccal mucoadhesive tablets, films, and patches, sublingual disintegrating thin films, sprays, chewing gum, and lozenges are some of the pharmacological dosage forms of cannabinoids that are available [43, 44].
IntelGenx Technologies Corp, USA, and Tetra Bio-Pharma are working on a THC buccal formulation for cancer pain management, chemotherapy-induced nausea, vomiting, anorexia, and weight loss in AIDS patients. The sublingual THC is an orally dissolving film formulated using IntelGenx technology (AdVersaTM) that improves its solubility, stability, and bioavailability [107]. Diverse Biotech Inc., USA, is preclinically testing an oral, sublingual formulation of CBD (BRCX014) for cancer treatment. A patent application describing the methods for extraction and purification of CBD (>99%) as well as some preliminary clinical testing data from cancer patients treated with standard-of-care medication plus BRCX014 is pending approval [108].
The oromucosal spray formulations of nabiximols (Sativex®) produced by GW Pharmaceuticals plc., UK, have been used to treat various disorders, including multiple sclerosis spasticity, overrative bladder, and neuropathic pain management in adults with multiple sclerosis [32, 33, 109]. In addition, nabiximols have also been used as an adjuvant analgesic in adult cancer patients with persistent background pain receiving the highest tolerated dose of potent opioids [32]. Mouth ulcers, due to the ethanol excipient, are a common adverse effect associated with long-term usage of nabiximols. Discontinuation of nabiximols during long-term follow-up results in multiple sclerosis patients with spasticity was reported to result in physical disability and cognitive impairment [110, 111]. A Phase Ib study of Sativex® with temozolomide in cancer patients with recurrent glioblastoma reported adequate safety and tolerability with no drug-drug interactions [112]. After multiple successful clinical trials, Sativex® is approved in Israel and Canada for neuropathic pain management in multiple sclerosis patients [113]. Other clinical trials with Sativex® include cancer pain management, muscle stiffness, posttraumatic stress disorder, and glioblastoma and are ongoing in several countries [43, 44]. However, in-depth research is needed to establish the optimum responder profile for nabiximols and to comprehensively explore nabiximols potential.
Temtsin-Krayz et al. [114] developed the transmucosal formulations of CBD with Poloxamer 407, carboxymethyl cellulose, and starch. Axim Biotech Inc., USA, is developing a controlled release chewing gum containing a 1:1 combination of CBD and THC that enables oromucosal adsorption. Clinical trials evaluating this formulation for pain management, multiple sclerosis-associated spasticity, Parkinson's disease, post-herpetic neuralgia, dementia, and other illnesses are ongoing. More recently, Axim Biotech has developed chewing gums with microencapsulated cannabinoids, opioid agonists, and opioid antagonists that are controlled released during mastication [79, 81].
Intranasally administered drugs enter the systemic blood circulation via the well-vascularized thin mucosal lining of the nasal cavity and escape the hepatic and intestinal metabolism, resulting in a robust effect. This method is suitable for patients experiencing nausea, vomiting, oral mucositis, and impaired gastrointestinal function. Furthermore, intranasal delivery is painless and noninvasive, preferred over IV injection, and improves patient compliance [43, 44]. Paudel et al. [89] evaluated the intranasal permeation ability of various CBD formulations prepared in polyethylene glycol (PEG) 400 and in a 50:35:15 (v/v) PEG: saline: ethanol solvent system in the presence and absence of permeation enhancers (1% sodium glycocholate or 1% dimethyl-beta-cyclodextrin) using an anesthetized rat model. They found that CBD was absorbed within 10 min of nasal administration with 34–46% bioavailability. On the contrary, the formulation with 100% PEG exhibited lower bioavailability than the other formulations with enhancers [89]. Bryson and Sharma developed numerous cannabis formulations for nasally delivery and a device to provide targeted nasal delivery − these formulations have been patented [115]. While gelatin matrix pellets and lipid/oil-based formulations were initially employed to boost cannabis bioavailability, they could not target cannabis to tumors or diseased sites and had significant adverse effects. Injecting a low dose of cannabis directly into the target site may improve efficacy and reduce adverse side effects. Thus, there is an urgent need to develop cannabis-based nano-formulations for pain management, cancer therapy, and treatment of several other pathological conditions.
Emerging Approaches to Improve Delivery Systems
Many factors contribute to the utility and efficacy of successful delivery systems. Properties such as solubility, drug half-life, metabolism, distribution, and ease of use can all impact whether formulations move forward for clinical use. To this end, many efforts are in place to enhance the properties listed above for improved and practical delivery systems that will support optimal efficacy for medicinal cannabis. The section below describes how current formulations are being subjected to novel approaches and formulations to further improve these delivery systems.
Nanotechnology Approaches for Cannabinoid Delivery
Nanoparticle technology has been widely used in medical applications. Their attractive features are (i) enhanced encapsulation efficiency or drug solubilization capacity for safe and targeted delivery, (ii) presence of surface functional groups that can be modified to improve stability and internalization, (iii) potential for superior pharmacokinetics, increased bioavailability, and minimal clearance from the body, and (iv) controlled, stimuli-responsive, remote actuation, and on-demand drug release properties [43, 44, 116, 117]. Several studies have reported the development of nanoparticle-based delivery systems for cannabis, cannabinoids, and ECS components delivery [118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128]. Cannabinoid delivery systems based on nanoconjugates consist of nano-conjugated cannabinoids in a multifunctional metallic nanocarrier, classified as inorganic, organic, or hybrid nano systems, depending upon the specific requirements and administration route. Encapsulation approaches such as nanoemulsions, dendrimers, micelles, liposomes, and biodegradable polymer nanoparticles for cannabis products increase physical stability, controlled release, targeted, and safe delivery [43, 44, 117]. Many cannabis-based nanoformulations are currently in preclinical or clinical development. Table 2 summarizes prominent nano-based cannabinoids delivery systems.
Table 2.
Nano-based cannabinoids delivery systems
| Drugs | Type | Administration routes | References |
|---|---|---|---|
| Lipid-based nanosized cannabinoids delivery systems | |||
| THC | Liposomes | IV | [129] |
| Terpenes, hemp oil, cannabinoids, or combinations of cannabinoids | Liposome and Micelle | [130] | |
| CBD | Micelle | Oromucosal | [131, 132] |
| Synthetic cannabinoid | Nanomicellar formulation | [120] | |
| THC and CBD | NLC | Nasal | [123, 125] |
| AM251, rimonabant, and URB597 | NLC | Nasal | [122] |
| CB-13 | NLC | Oral | [134] |
| CBD | Lipid nanocapsules | [121, 123] | |
| PTL401 (THC:CBD) | Pro-nano liposphere | Oral | [119, 126] |
| Self-emulsifying drug delivery systems | |||
| Namisol | Self-emulsifying drug delivery | Oral | [138, 139] |
| CBD | Self-emulsifying drug delivery | [76, 140, 141] | |
| THC-CBD-piperine | Emulsifying nanoformulation | [78, 79] | |
| Cannabinoids and terpenes | Nanoemulsion | Oral | [143] |
| CTX01 (CBD) | Nanotherapeutic formulation | [144] | |
| Synthetic CBD | Self-emulsifying drug delivery | Oral | [76, 145] |
| Polymeric nano-formulations | |||
| CB-13 | PLGA | Oral | [147] |
| Cannabinoid | PLGA | IP | [127] |
| THC | PLGA | [148] | |
| CBD | PLGA | [149] | |
Lipid-Based Nanoformulations
Cannabinoids have been successfully developed in nanomicelles, nanoemulsions, and nanostructured lipid carriers (NLCs) for potential medicinal purposes. Pulmonary administration of THC (0.3 mg/mL) in a liposomal formulation containing dipalmitoyl phosphatidylcholine and cholesterol, average size of 300–500 nm, resulted in sustained release with rapid bioavailability of THC [129].
A patent application for stable, fast-acting liposome and micelle formulations containing terpenes, hemp oil, cannabinoids, or combinations of cannabinoids, with stability ranging from a few days (micelles) to several months (liposomes), has been filed [130]. Medlab Clinical, Australia has developed NanoCelleTM, a micelle formulation made from oils, glycerol, and nonionic surfactants, to deliver lipophilic molecules (vitamin D3, statins, testosterone propionate, CBD) across the oral buccal mucosa, bypassing the gastrointestinal (GI) tract [131, 132]. Nanomicellar formulation of synthetic cannabinoid has been shown to inhibit tumor growth and adverse psychoactive effects in a syngeneic mouse model of triple-negative breast cancer [120].
NLCs have great potential as a carrier system for THC and CBD because they use solid particle matrices rather than fluid matrices like emulsions and liposomes and thus can better host and protect drugs from degradation. THC transport from inside to the particle surface is further slowed by the solid particle matrix [43, 133]. Esposito et al. [122] encapsulated cannabinoid drugs including AM251, rimonabant, and URB597 (a fatty acid amide hydrolase inhibitor), in NLCs (100 nm) with enhanced encapsulation efficiency. Furthermore, improvement in mucoadhesive THC-loaded NLC formulation and long-term physicochemical stability appears conceivable [125].
Duran-Lobato et al. [134] developed a promising carrier for the oral administration of CB-13, a cannabinoid with strong therapeutic potential in chronic pain management for which an effective oral treatment is lacking. This formulation consists of either glyceryl dibehenate or glyceryl palmitostearate together with two different surfactants (polysorbate 20 and sodium deoxycholate) using the emulsification-solvent evaporation method. Glyceryl palmitostearate proved to be effective as a lipid matrix in producing cannabinoid-loaded particles with high encapsulation efficiency and storage stability at 4°C [134]. CBD encapsulated into lipid nanocapsules (LNCs) displayed an antitumor effect in cell-based glioma model [121]. Notably, as tiny cannabinoid-decorated LNCs have been shown to improve transport across the blood-brain barrier and increase bioavailability, LNCs represent a promising strategy for the designing and developing novel CNS therapeutics [121, 123]. A potential limitation of LNCs is the lower encapsulation efficiencies. Collectively, these findings suggest that NLC based cannabinoid formulations have the potential to overcome the low solubility, absorption, lipophilicity, and oral bioavailability related challenges.
Self-Emulsifying Drug Delivery Systems
The self-emulsifying delivery systems (SEDDS) have attracted considerable attention as a strategy for cannabinoids to improve dissolution, stability, and bioavailability [43, 44, 135]. SEDDS are isotropic mixtures of oils, surfactants, solvents, and cosolvents/surfactants. They spontaneously disperse upon contact with an aqueous phase, such as gastric or intestinal fluids, under conditions of mild agitation comparable to those found in the GI tract. Resulting in situ droplets preserve the lipophilic active ingredient and solubilize them in the aqueous environment, which facilitates the transport of the active ingredient across the aqueous lumen of the GI tract to the absorptive epithelium surface. The active ingredient then dissociates from the droplets and is transported to the blood or lymphatic vessels via the enterocyte membrane [43, 44, 135, 136]. The performance of the self-emulsifying delivery systems in improving the bioavailability is dependent on the emulsion droplet size distribution. SEDDS, self-micro emulsifying drug delivery systems (SMEDDS), and self-nano emulsifying drug delivery systems (SNEDDS) are all examples of self-emulsifying formulations. SEDDS produces coarse emulsions, whereas SNEDDS and SMEDDS produce nano- and micro-sized emulsions, respectively [135].
Several studies have demonstrated that the incorporating lipids in SNEDDS improve solubility and contribute to the lymphatic absorption of cannabinoids. The absorption of lipid-based nano-formulations in healthy rats was quick, whereas the absorption of oily preparations took longer, lasting 4–6 h [137]. PTL4 is a self-emulsifying-based formulation of THC and CBD (1:1) with polysorbate 20, sorbitan monooleate 80, polyoxyethylene hydrogenated castor oil 40, glyceryl tridecanoate, lecithin, and ethyl lactate [119, 126]. NamisolTM, an oral tablet formulation of dronabinol, was produced using AlitraTM, an emulsifying drug delivery technology. In this method, THC microgranules (size 30 micron) were coated with the surfactant sucrose monolaurate to increase the solubility of THC. One study reported no significant PK changes after oral and sublingual (crushed tablets) administration of NamisolTM [138]. In another Phase II study using NamisolTM in subjects with anorexia, study results indicated a shorter time to reach maximum THC concentration (39–56 min) while maintaining high safety and tolerability [139]. It has been reported that self-emulsifying drug delivery of CBD increases the bioavailability of CBD with a 4.2-fold higher Cmax and a 2.2-fold higher AUC, compared to the reference oromucosal spray Sativex®, a CBD/THC ethanol/propylene glycol solution [140]. Similarly, VESIsorb®, a self-emulsifying drug delivery formulation technology developed by Vesifact AG (Baar, Switzerland), has increased CBD absorption and oral bioavailability in healthy subjects. These findings collectively indicate that cannabis pharmacokinetics are model-dependent with inconsistency between study groups and impacted by the delivery technique, further stressing the need for a standardized CBD oral formulation to lower the significant intra- and inter-subject absorption variability in humans [76, 141].
Piperine, an alkaloid, was also mixed with SNEDDS and orally fed to rats to increase the bioavailability of cannabinoids. When compared to CBD-SNEDDS alone, the oral bioavailability of CBD with piperine dissolved in SNEDDS (once-daily dose) increased by about 2.5 times [142]. A rat model was also utilized to test the effects of advanced pro-nano lipospheres and SNEDDS loaded with natural absorption enhancers on the oral bioavailability of THC and CBD. The addition of different enhancers such as curcumin, resveratrol, and piperine to CBD-THC self-emulsifying systems increased CBD oral bioavailability in vivo, with piperine having the maximum effect [77]. The presence of piperine in the CBD-THC self-emulsifying system slows intestinal processes rather than hepatic first-pass metabolism, which explains the increased bioavailability. THC-CBD-piperine-emulsifying nanoformulation demonstrated higher absorption rates than Sativex® in healthy human volunteers. However, as a cytochrome and glucuronyl transferase inhibitor, piperine can modify the metabolism of several other medicines and has a nonnegligible toxicity [78, 79].
A patent also provides rectal-vaginal and solid oral dosage forms using micro and nanoemulsions of active cannabis components (cannabinoids and terpenes) [143]. Cardiol Therapeutics (Oakville, ON, Canada) is developing a patented CBD nanotherapeutic formulation (CTX01) for subcutaneous delivery to treat heart failure [144]. A study investigated the oral absorption processes of synthetic CBD when given in different oral formulations (powder form, dissolved in sesame oil, and in SNEDDS) in a three-way, blind, crossover single administration. When compared to powder form, CBD in SNEDDS was quickly absorbed by all volunteers, followed by CBD in lipid-based vehicle [145]. Similarly, Knaub et al. [76] orally administered the CBD (used as hemp extract)-containing SEDDS formulation or the same extract diluted with medium-chain triglycerides oil in a randomized, double-blind, crossover study in 16 healthy volunteers under fasting conditions. Compared to the reference formulation, a single oral dosage of CBD-SEDDS increased Cmax and AUC0–8h/AUC0–24h [76].
Polymeric Nano-Formulations
Polymeric drug delivery systems have also been used for enhancing stability and controlled release of cannabis and cannabinoids. Specifically, the polymer poly (lactic-co-glycolic acid) (PLGA) is often utilized for drug encapsulation due to its biocompatibility and biodegradability [43, 146]. CB-13-loaded nanoparticles coated with a range of agents (chitosan, Eudragit RS, vitamin E, and lecithin) for oral administration create nanoparticles with particle sizes ranging from 250 to 350 nm, good entrapment efficiency, and CB-13 nanoparticles release rates improve with vitamin E and lecithin surface modifications [147]. In an ovarian cancer model, intraperitoneal delivery of cannabinoid-loaded PLGA nanoparticles decreased tumor growth [127]. Martin-Banderas developed THC-loaded PLGA nanoparticles (size 290–800 nm) for applications as an anticancer agent. PEG, chitosan, and PEG-chitosan conjugates were used as coating agents with increased encapsulation efficiency [148]. Poly-ε-caprolactone is another polymer extensively utilized in drug delivery systems as it is biocompatible, biodegradable, FDA-approved semi-crystalline aliphatic polyester. Hernán Pérez de la Ossa et al. [149] developed spherical CBD-loaded poly-ε-caprolactone microparticles with a size range of 20–50 nm and high entrapment efficiency.
More recently, the use of nanoparticle-based cannabis in treating cancer and infections caused by microbial biofilms was reported [118, 150]. Formulations based on pro nanodispersion technology enhanced oral cannabis bioavailability in healthy volunteers [119]. Aparicio-Blanco et al. [121] showed that LNCs containing CBD could be used as targeted long-acting carriers for glioma therapy. Although cell membrane-derived cannabinoid delivery systems may offer advantages in terms of delivering biomimetic systems for the efficient administration of cannabis nanomedicines [151], these systems have not been fully developed yet.
Conclusions
Cannabis has fascinating pharmacological properties and could prove to be a viable source of novel medications. Despite the efficacy and safety of cannabis, low water solubility, weak stability, and poor bioavailability represent the significant challenges in therapeutic challenges moving forward with cannabis use in clinics. Finding appropriate delivery systems for cannabis and cannabinoids is another challenge to overcome. Many studies supported by emerging technologies are ongoing to improve the bioavailability of cannabis and its analogs.
Numerous strategies and cannabinoid formulations have been explored, such as modulation of route and medium of cannabinoids administration as well as conjugation and structural modifications of cannabinoids. Cannabinoid nanoformulations offer several advantages like increased efficacy, tumor targeting, lower systemic toxicity, and controlled release at target sites. Pharmaceutical and generic firms have developed tailored cannabis formulations to enhance solubility and bioavailability. Several cannabis formulations are currently undergoing clinical trials to establish efficacy in treating pain and other pathological conditions, and more formulations are in the pipeline.
Most of the cannabinoid formulations are based on pure THC and a mixture of THC and CBD, which have poor water solubility. To circumvent the solubility issue, adjuvants, cyclodextrin, and several other patented technologies have been used in the past. The solubility and bioavailability of cannabinoids are also enhanced by the modifying cannabinoids' chemical structure. Recent reports suggest that combining cannabis with dietary lipids increases cannabis bioavailability by escaping the hepatic and intestinal metabolism. Furthermore, combining cannabis with piperine lowers hepatic and intestinal glucuronidation, allowing them to bypass liver and intestinal metabolism and improve bioavailability.
A drug's bioavailability directs the dosage and mode of administration modification. Oral administration is the most common and popular route of administration, followed by inhalation and transmucosal administration. Transdermal, nasal, inhaled-pulmonary, and oral transmucosal delivery formulations circumvent the bioavailability issues associated with oral administration by facilitating direct uptake of drug into the bloodstream. Nonetheless, all cannabis delivery methods have some restrictions − poor water solubility makes IV administration difficult, orally administered medications are metabolized, resulting in low bioavailability, and inhaled cannabis have an adverse effect on the respiratory system.
Nanotechnology-based cannabis nano-formulations could provide several benefits, including increased efficacy, tumor targeting, and lower systemic toxicity. One formulation does not fit all cannabinoids, so it is essential to develop and optimize specific cannabinoid-based nano-formulations. In our viewpoints, cannabis nano-formulations based on the lipophilic carrier are highly appropriate. Cannabinoid delivery by nano-formulations based on liposomes, polymeric nanoparticles, and others are currently being tested in preclinical studies, with self-emulsifying systems being one of the more fascinating and lucrative techniques. Again, as there are limited new nano-formulations in the market or clinical trials currently, there is a need for extensive preclinical and clinical research to support its development as a drug for cancer therapy and various other conditions. Last but not the least, extensive investigations on cannabinoids and their analogs, efficient delivery systems, and nano-formulations that can enhance the bioavailability, and studies on sub-acute, acute, and chronic toxicity are, however, warranted before translating preclinical findings to the clinic.
Conflict of Interest Statement
The authors declare no conflicts of interest associated with this manuscript. There are no other conflicts of interest or competing financial interests to disclose.
Funding Sources
This work was supported by Department of Biomedical Sciences and Drug Discovery, KOR Life Sciences, Biotechnology.
Author Contributions
This review article was designed by Saeed Khan. The manuscript was written by Manikandan Palrasu, Lillianne Wright, and Saeed Khan. Manikandan Palrasu, Lillianne Wright, and Saeed Khan contributed to the molecular and drug delivery aspects of cannabis. Manish Patel, Lindsey Leech, Scotty Branch, and Shea Harrelson contributed to clinical aspects. The manuscript was revised and approved by Saeed Khan, Manikandan Palrasu, Lillianne Wright, Manish Patel, Lindsey Leech, Scotty Branch, and Shea Harrelson.
Funding Statement
This work was supported by Department of Biomedical Sciences and Drug Discovery, KOR Life Sciences, Biotechnology.
References
- 1.Guzmán M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003 Oct;3((10)):745–755. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- 2.Afrin F, Chi M, Eamens AL, Duchatel RJ, Douglas AM, Schneider J, et al. Can hemp help? Low-THC cannabis and non-THC cannabinoids for the treatment of cancer. Cancers. 2020 Apr 23;12((4)):1033. doi: 10.3390/cancers12041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Śledziński P, Nowak-Terpiłowska A, Zeyland J. Cannabinoids in medicine: cancer, immunity, and microbial diseases. Int J Mol Sci. 2020 Dec 29;((1)):22. doi: 10.3390/ijms22010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346((6284)):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 5.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993 Sep 2;365((6441)):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 6.Svízenská I, Dubový P, Sulcová A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures: a short review. Pharmacol Biochem Behav. 2008 Oct;90((4)):501–511. doi: 10.1016/j.pbb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International union of pharmacology. XXVII Classification of cannabinoid receptors. Pharmacol Rev. 2002 Jun;54((2)):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 8.Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y, et al. Crystal structure of the human cannabinoid receptor CB(1) Cell. 2016 Oct 20;167((3)):750–62.e14. doi: 10.1016/j.cell.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Marzo V, Bifulco M, Petrocellis LD. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004 Sep;3((9)):771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 10.Reggio PH. Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown. Curr Med Chem. 2010;17((14)):1468–1486. doi: 10.2174/092986710790980005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Marzo V, Piscitelli F. The Endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 2015 Oct;12((4)):692–698. doi: 10.1007/s13311-015-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales P, Reggio PH. An update on non-CB(1), non-CB(2) cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res. 2017;2((1)):265–273. doi: 10.1089/can.2017.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J. Cannabis, cannabinoid receptors, and endocannabinoid system: yesterday, today, and tomorrow. Acta Pharmacol Sin. 2019 Mar;40((3)):297–299. doi: 10.1038/s41401-019-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018 Mar 13;19((3)):833. doi: 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashemi M, Bashi S, Zali A. The expression level of cannabinoid receptors type 1 and 2 in the different types of astrocytomas. Mol Biol Rep. 2020 Jul;47((7)):5461–5467. doi: 10.1007/s11033-020-05636-8. [DOI] [PubMed] [Google Scholar]
- 16.Zheng D, Bode AM, Zhao Q, Cho YY, Zhu F, Ma WY, et al. The cannabinoid receptors are required for ultraviolet-induced inflammation and skin cancer development. Cancer Res. 2008 May 15;68((10)):3992–3998. doi: 10.1158/0008-5472.CAN-07-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joosten M, Valk PJM, Jordà MA, Vankan-Berkhoudt Y, Verbakel S, van den Broek M, et al. Leukemic predisposition of pSca-1/Cb2 transgenic mice. Exp Hematol. 2002 Feb;30((2)):142–149. doi: 10.1016/s0301-472x(01)00779-2. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Gómez E, Andradas C, Blasco-Benito S, Caffarel MM, García-Taboada E, Villa-Morales M, et al. Role of cannabinoid receptor CB2 in HER2 pro-oncogenic signaling in breast cancer. J Natl Cancer Inst. 2015 Jun;107((6)):djv077. doi: 10.1093/jnci/djv077. [DOI] [PubMed] [Google Scholar]
- 19.Ramer R, Schwarz R, Hinz B. Modulation of the endocannabinoid system as a potential anticancer strategy. Front Pharmacol. 2019;10:430. doi: 10.3389/fphar.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Xu Y, Zou Y, Zhu L, Dong B, Huang J, et al. Overexpression of cannabinoid receptor 1 promotes renal cell carcinoma progression. Tumour Biol. 2016 Oct 18; doi: 10.1007/s13277-016-5447-6. [DOI] [PubMed] [Google Scholar]
- 21.Milian L, Mata M, Alcacer J, Oliver M, Sancho-Tello M, Martín de Llano JJ, et al. Cannabinoid receptor expression in non-small cell lung cancer Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS One. 2020;15((2)):e0228909. doi: 10.1371/journal.pone.0228909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallan SE, Zinberg NE, Frei E. 3rd Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N Engl J Med. 1975 Oct 16;293((16)):795–797. doi: 10.1056/NEJM197510162931603. [DOI] [PubMed] [Google Scholar]
- 23.Frytak S, Moertel CG, O'Fallon JR, Rubin J, Creagan ET, O'Connell MJ, et al. Delta-9-tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy A comparison with prochlorperazine and a placebo. Ann Intern Med. 1979 Dec;91((6)):825. doi: 10.7326/0003-4819-91-6-825. [DOI] [PubMed] [Google Scholar]
- 24.Chang AE, Shiling DJ, Stillman RC, Goldberg NH, Seipp CA, Barofsky I, et al. Delta-9-Tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. Ann Intern Med. 1979 Dec;91((6)):819. doi: 10.7326/0003-4819-91-6-819. [DOI] [PubMed] [Google Scholar]
- 25.PDQ Integrative, Alternative, and Complementary Therapies Editorial Board . In: PDQ cancer information summaries [Internet] Bethesda (MD): National Cancer Institute (US); 2002. Cannabis and cannabinoids (PDQ®): patient version. [cited 2022 Jan 25]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK65875/ [Google Scholar]
- 26.Tramèr MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic. BMJ. 2001 Jul 7;323((7303)):16. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badowski ME, Yanful PK. Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther Clin Risk Manag. 2018;14:643–651. doi: 10.2147/TCRM.S126849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lattanzi S, Brigo F, Trinka E, Zaccara G, Striano P, Del Giovane C, et al. Adjunctive cannabidiol in patients with Dravet Syndrome: a systematic review and meta-analysis of efficacy and safety. CNS Drugs. 2020 Mar;34((3)):229–241. doi: 10.1007/s40263-020-00708-6. [DOI] [PubMed] [Google Scholar]
- 29.Seltzer ES, Watters AK, MacKenzie DJ, Granat LM, Zhang D. Cannabidiol (CBD) as a promising anti-cancer drug. Cancers. 2020 Oct 30;12((11)):3203. doi: 10.3390/cancers12113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sexton M, Garcia JM, Jatoi A, Clark CS, Wallace MS. The management of cancer symptoms and treatment-induced side effects with cannabis or cannabinoids. JNCI Monogr. 2021 Nov;2021((58)):86–98. doi: 10.1093/jncimonographs/lgab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA, et al. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT1A receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol. 2014 Feb;171((3)):636–645. doi: 10.1111/bph.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichtman AH, Lux EA, McQuade R, Rossetti S, Sanchez R, Sun W, et al. Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J Pain Symptom Manag. 2018 Feb;55((2)):179–88.e1. doi: 10.1016/j.jpainsymman.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Tanasescu R, Constantinescu CS. Pharmacokinetic evaluation of nabiximols for the treatment of multiple sclerosis pain. Expert Opin Drug Metab Toxicol. 2013 Sep;9((9)):1219–1228. doi: 10.1517/17425255.2013.795542. [DOI] [PubMed] [Google Scholar]
- 34.Noyes RJ, Brunk SF, Avery DH, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther. 1975 Jul;18((1)):84–89. doi: 10.1002/cpt197518184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munson AE, Harris LS, Friedman MA, Dewey WL, Carchman RA. Antineoplastic activity of Cannabinoids2. J Natl Cancer Inst. 1975 Sep;55((3)):597–602. doi: 10.1093/jnci/55.3.597. [DOI] [PubMed] [Google Scholar]
- 36.Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for cancer treatment: progress and promise. Cancer Res. 2008 Jan 15;68((2)):339–342. doi: 10.1158/0008-5472.CAN-07-2785. [DOI] [PubMed] [Google Scholar]
- 37.Clark TM. Scoping Review and meta-analysis suggests that cannabis use may reduce cancer risk in the United States. Cannabis Cannabinoid Res. 2021 Oct;6((5)):413–434. doi: 10.1089/can.2019.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gómez del Pulgar T, Velasco G, Sánchez C, Haro A, Guzmán M. De novo-synthesized ceramide is involved in cannabinoid-induced apoptosis. Biochem J. 2002 Apr 1;363((1)):183. doi: 10.1042/0264-6021:3630183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gómez Del Pulgar T, De Ceballos ML, Guzmán M, Velasco G. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2002 Sep 27;277((39)):36527–33. doi: 10.1074/jbc.M205797200. [DOI] [PubMed] [Google Scholar]
- 40.Donadelli M, Dando I, Zaniboni T, Costanzo C, Dalla Pozza E, Scupoli MT, et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011 Apr 28;2((4)):e152. doi: 10.1038/cddis.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torres S, Lorente M, Rodríguez-Fornés F, Hernández-Tiedra S, Salazar M, García-Taboada E, et al. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol Cancer Ther. 2011 Jan;10((1)):90–103. doi: 10.1158/1535-7163.MCT-10-0688. [DOI] [PubMed] [Google Scholar]
- 42.Yasmin-Karim S, Moreau M, Mueller R, Sinha N, Dabney R, Herman A, et al. Enhancing the therapeutic efficacy of cancer treatment with cannabinoids. Front Oncol. 2018;8:114. doi: 10.3389/fonc.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruni N, Della Pepa C, Oliaro-Bosso S, Pessione E, Gastaldi D, Dosio F, et al. Cannabinoid delivery systems for pain and inflammation treatment. Molecules. 2018 Sep 27;23((10)):2478. doi: 10.3390/molecules23102478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stella B, Baratta F, Della Pepa C, Arpicco S, Gastaldi D, Dosio F, et al. Cannabinoid formulations and delivery systems: current and future options to treat pain. Drugs. 2021 Sep;81((13)):1513–1557. doi: 10.1007/s40265-021-01579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018 Nov;84((11)):2477–2482. doi: 10.1111/bcp.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright S, Wilkhu J, GW Research Limited Oral cannabinoid formulations. Patent WO2018150182. 2018 [Google Scholar]
- 47.Wilkhu J, Bender J, GW Research Limited Cannabinoid formulations. Patent WO2018002665. 2018 [Google Scholar]
- 48.Wilkhu J, Bender J, GW Research Limited Oral pharmaceutical formulation comprising cannabinoids and poloxamer. Patent WO2019135077. 2019 [Google Scholar]
- 49.De Vries JA, Fernandez Cid MV, Heredia Lopez AM, Eiroa Martinez CM, Echo Pharmaceuticals B.V. Compressed tablet containing cannabidiol, method for its manufacture and use of such tablet in oral treatment of psychosis or anxiety disorders. Patent WO2015065179A1. 2015 [Google Scholar]
- 50.De Vries JA, Fernandez Cid MV, Heredia Lopez AM, Echo Pharmaceuticals B.V. Granulate containing cannabinoid, method for its manufacture and oral dosage unit comprising such granulate. Patent US20150132400. 2015 [Google Scholar]
- 51.Schussel V, Kenzo L, Santos A, Bueno J, Yoshimura E, de Oliveira Cruz Latorraca C, et al. Cannabinoids for nausea and vomiting related to chemotherapy: overview of systematic reviews. Phytother Res. 2018 Apr;32((4)):567–576. doi: 10.1002/ptr.5975. [DOI] [PubMed] [Google Scholar]
- 52.Peball M, Krismer F, Knaus HG, Djamshidian A, Werkmann M, Carbone F, et al. Non-motor symptoms in Parkinson's disease are reduced by nabilone. Ann Neurol. 2020 Oct;88((4)):712–722. doi: 10.1002/ana.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sengupta A, Roychoudhury SK. Triglyceride composition of sesamum indicum seed oil. J Sci Food Agric. 1976 Feb;27((2)):165–169. doi: 10.1002/jsfa.2740270214. [DOI] [PubMed] [Google Scholar]
- 54.Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017 Aug 17;376((21)):2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 55.Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018 May 17;378((20)):1888–1897. doi: 10.1056/NEJMoa1714631. [DOI] [PubMed] [Google Scholar]
- 56.Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018 Mar 17;391((10125)):1085–1096. doi: 10.1016/S0140-6736(18)30136-3. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen LC, Yang D, Nicolaescu V, Best TJ, Ohtsuki T, Chen SN, et al. Cannabidiol inhibits SARS CoV-2 replication and promotes the host innate immune response. bioRxiv. 2021 Mar 10; doi: 10.1126/sciadv.abi6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parker AB, Hamer A, Cardiol Therapeutics Inc Cannabidiol in patients with COVID-19 and cardiovascular disease or risk factors. Patent NCT04615949. 2021 [Google Scholar]
- 59.Hershberger P, Arlen P, Diverse Biotech Inc Cannabinoid conjugate molecules. WO2020263888. 2020 [Google Scholar]
- 60.Patrician A, Versic-Bratincevic M, Mijacika T, Banic I, Marendic M, Sutlović D, et al. Examination of a new delivery approach for oral cannabidiol in healthy subjects: a randomized, double-blinded, placebo-controlled pharmacokinetics study. Adv Ther. 2019 Nov;36((11)):3196–210. doi: 10.1007/s12325-019-01074-6. [DOI] [PubMed] [Google Scholar]
- 61.Washington ME, Reillo M, Poviva Tea LLC Food and beverage compositions infused with lipophilic active agents and methods of use thereof. Patent WO2015191728. 2015 [Google Scholar]
- 62.Docherty J, Bunka CA, Poviva Corp Nanoemulsion compositions comprising biologically active ingredients. Patent WO2020236798. 2020 [Google Scholar]
- 63.Sacks H, Edvinsson T, GelpellSatipharm AGAG Oral solid cannabinoid formulations, methods for producing and using thereof. Patent WO2017137992. 2017 [Google Scholar]
- 64.Mitelpunkt A, Kramer U, Hausman Kedem M, Zilbershot Fink E, Orbach R, Chernuha V, et al. The safety, tolerability, and effectiveness of PTL-101, an oral cannabidiol formulation, in pediatric intractable epilepsy: a phase II, open-label, single-center study. Epilepsy Behav. 2019 Sep;98((Pt A)):233–237. doi: 10.1016/j.yebeh.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Atsmon J, Heffetz D, Deutsch L, Deutsch F, Sacks H. Single-dose pharmacokinetics of oral cannabidiol following administration of ptl101: a new formulation based on gelatin matrix pellets technology. Clin Pharmacol Drug Dev. 2018 Sep;7((7)):751–758. doi: 10.1002/cpdd.408. [DOI] [PubMed] [Google Scholar]
- 66.Lv P, Zhang D, Guo M, Liu J, Chen X, Guo R, et al. Structural analysis and cytotoxicity of host-guest inclusion complexes of cannabidiol with three native cyclodextrins. J Drug Deliv Sci Technol. 2019;51:337–344. [Google Scholar]
- 67.da Silva AJ, Dos Santos ES. Energetic and thermodynamical aspects of the cyclodextrins-cannabidiol complex in aqueous solution: a molecular-dynamics study. Eur Biophys J. 2020 Oct;49((7)):571–589. doi: 10.1007/s00249-020-01463-8. [DOI] [PubMed] [Google Scholar]
- 68.Viernstein H, Toegel S, Schueller R, Aop Orphan Pharmaceuticals AG Fast disintegrating compositions comprising nabilone and randomly methylated β-cyclodextrin. Patent WO2012069591. 2012 [Google Scholar]
- 69.U.S. National Library of Medicine Nabilone for non-motor symptoms in Parkinson's disease: an open-label study to evaluate long-term safety and efficacy. NCT03773796. 2021 [Google Scholar]
- 70.Kingsley K, Lee S, Greenbaum E, Vireo Health LLC Cannabinoid formulations with improved solubility. Patent US20190030170. 2019 [Google Scholar]
- 71.Jagtap P, Shoken D, Avidan- Shlomovich S, Salzman AL, Beetlebung Pharma Ltd Preparation of cannabinoid derivatives and conjugates as neuroprotectants and for treating pain. Patent WO2019159168. 2019 [Google Scholar]
- 72.Jagtap P, Musa S, Beetlebung Pharma Ltd Synthesis of cannabinoid compounds. Patent WO2020031179. 2020 [Google Scholar]
- 73.U.S. National Library of Medicine An open label, study to evaluate the safety of cannabidiol (CBD) for the prevention of acute graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation [Internet] ACTRN12619000623190. 2021. Available from: https://www.australianclinicaltrials.gov.au/anzctr/trial/ACTRN12619000623190.
- 74.U.S. National Library of Medicine. Cannabidiol for graft versus host disease (GVHD) prophylaxis in allogeneic stem cell transplantation [Internet] NCT0138512. 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT01385124.
- 75.Williams NNB, Ewell TR, Abbotts KSS, Harms KJ, Woelfel KA, Dooley GP, et al. Comparison of five oral cannabidiol preparations in adult humans: pharmacokinetics, body composition, and heart rate variability. Pharmaceuticals. 2021 Jan 6;14((1)):35. doi: 10.3390/ph14010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knaub K, Sartorius T, Dharsono T, Wacker R, Wilhelm M, Schön C, et al. A novel self-emulsifying drug delivery system (SEDDS) based on VESIsorb® formulation technology improving the oral bioavailability of cannabidiol in healthy subjects. Molecules. 2019 Aug 16;((16)):2967. doi: 10.3390/molecules24162967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.MacLeman PDR, Mavlianov M, Auscann Group Holdings Ltd Solid self-emulsifying pharmaceutical compositions comprising cannabinoids. Patent WO2020024009. 2020 [Google Scholar]
- 78.MacLeman PDR, Mavlianov M, Auscann Group Holdings Ltd Free-flowing powder compositions for oral solid dosages containing cannabinoids. Patent WO2020024011. 2020 [Google Scholar]
- 79.Anastassov G, Changoer L, AXIM Biotechnologies, Inc Chewing gum composition comprising cannabinoids and opioid agonists and/or antagonists. Patent WO2018075665A1. 2018 [Google Scholar]
- 80.Changoer L, Anastassov G, AXIM Biotechnologies, Inc Chewing gum composition comprising cannabinoids and nicotine. Patent WO2017189375A1. 2017 [Google Scholar]
- 81.Van Damme PA, Anastassov GE, Mareda Holding B.V. Chewing gum compositions comprising cannabinoids. Patent WO2009120080A1. 2009 [Google Scholar]
- 82.U.S. National Library of Medicine. Phase II Clinical Trial, Use of KL16-012 in women with fibromyalgia refractory to conventional treatment [Internet] NCT04239469. 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04239469.
- 83.Hardy J, Haywood A, Gogna G, Martin J, Yates P, Greer R, et al. Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double-blind, placebo-controlled, randomised clinical trial of efficacy and safety of 1:1 delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) Trials. 2020 Jul 6;21((1)):611. doi: 10.1186/s13063-020-04541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004 Apr 24;328((7446)):991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Casiraghi A, Musazzi UM, Centin G, Franzè S, Minghetti P. Topical administration of cannabidiol: influence of vehicle-related aspects on skin permeation process. Pharmaceuticals. 2020 Oct 23;13((11)):337. doi: 10.3390/ph13110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stinchcomb AL, Golinski MJ, Hammell DC, Howard JL, Alltranz Products of tetrahydrocannabinol, compositions comprising prodrugs of tetrahydrocannabinol and methods of using the same. Patent US20090143462. 2009 [Google Scholar]
- 87.Gruner SM, Lenk RP, Janoff AS, Ostro N J. Novel multilayered lipid vesicles: comparison of physical characteristics of multilamellar liposomes and stable plurilamellar vesicles. Biochemistry. 1985 Jun 4;24((12)):2833–2842. doi: 10.1021/bi00333a004. [DOI] [PubMed] [Google Scholar]
- 88.Doroudian A, Frankham P, Altum Pharmaceuticals Inc Biphasix cannabinoid delivery. Patent WO2018213932. 2018 [Google Scholar]
- 89.Paudel KS, Hammell DC, Agu RU, Valiveti S, Stinchcomb AL. Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev Ind Pharm. 2010 Sep;36((9)):1088–1097. doi: 10.3109/03639041003657295. [DOI] [PubMed] [Google Scholar]
- 90.Osborne DW, Musakhanian J. Skin penetration and permeation properties of transcutol®-neat or diluted mixtures. AAPS PharmSciTech. 2018 Nov;19((8)):3512–3533. doi: 10.1208/s12249-018-1196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stinchcomb AL, Valiveti S, Hammell DC, Ramsey DR. Human skin permeation of Delta8-tetrahydrocannabinol, cannabidiol and cannabinol. J Pharm Pharmacol. 2004 Mar;56((3)):291–297. doi: 10.1211/0022357022791. [DOI] [PubMed] [Google Scholar]
- 92.Stinchcomb AL, Banks SL, Golinski MJ, Howard JL, Hammell DC, AllTranz Inc Cannabidiol prodrugs in topical and transdermal administration with microneedles. Patent WO2011026144A1. 2011 [Google Scholar]
- 93.Stinchcomb AL, Nalluri BN, Alltranz LLC Transdermal delivery of cannabinoids. Patent US20050266061A1. 2005 [Google Scholar]
- 94.Gutterman D, Sebree T, Smith T, Messenheimer J, Zynerba Pharmaceuticals Inc Synthetic transdermal cannabidiol for the treatment of focal epilepsy in adults. Patent US20190083388. 2019 [Google Scholar]
- 95.Bonn-Miller M, Tich N, Gutterman D, Messenheimer J, Sebree T, Zynerba Pharmaceuticals Inc Treatment of fragile X syndrome with cannabidiol. Patent US10213390. 2019 [Google Scholar]
- 96.Messenheimer J, Tich N, Gutterman D, Clauw D, Zynerba Pharmaceuticals Inc Methods of treatment of osteoarthritis with transdermal cannabidiol gel. Patent WO2019034985. 2019 [Google Scholar]
- 97.Shemanski ME. Formulations of argan oil and cannabidiol for treating inflammatory disorders including arthritis. WO2017160923A121. 2017 [Google Scholar]
- 98.Cairns EA, Baldridge WH, Kelly MEM. The endocannabinoid system as a therapeutic target in glaucoma. Neural Plast. 2016;2016:9364091–10. doi: 10.1155/2016/9364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adelli GR, Bhagav P, Taskar P, Hingorani T, Pettaway S, Gul W, et al. Development of a δ9-tetrahydrocannabinol amino acid-dicarboxylate prodrug with improved ocular bioavailability. Invest Ophthalmol Vis Sci. 2017 Apr 1;58((4)):2167. doi: 10.1167/iovs.16-20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney G, et al. Controlled cannabis vaporizer administration: blood and plasma cannabinoids with and without alcohol. Clin Chem. 2015 Jun;61((6)):850–869. doi: 10.1373/clinchem.2015.238287. [DOI] [PubMed] [Google Scholar]
- 101.Solowij N, Broyd SJ, van Hell HH, Hazekamp A. A protocol for the delivery of cannabidiol (CBD) and combined CBD and ∆9-tetrahydrocannabinol (THC) by vaporisation. BMC Pharmacol Toxicol. 2014 Oct 16;15((1)):58. doi: 10.1186/2050-6511-15-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chamberland G, Campbell C, Ringuette R, Bassett JD, Yifrach-Stav O, Rackov A, et al. Cannabis compositions and methods. Patent WO2020124220. 2020 [Google Scholar]
- 103.U.S. National Library of Medicine. Safety and efficacy of inhaled cannabis for the uncontrolled pain relief in patients with advanced cancer (PLENITUDE) [Internet] NCT04042545. 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT04042545.
- 104.Cameron J. Hybrid vapor delivery system utilizing nebulized and non-nebulized elements. WO2016187115A1. 2016 [Google Scholar]
- 105.Raichman Y. Vaporizing devices and methods for delivering a compound using the same. U.S. patent 20180104214A1. 2018 [Google Scholar]
- 106.McCullough T. Methods and devices using cannabis vapors. U.S. Patent 20150223515A1. 2015 [Google Scholar]
- 107.Madwar C, Paiement N, Obeid R, Conway JW, GonzalezLabrada E, IntelGenx Corp Lipophilic active oral flm formulation and method of making the same. Patent WO2020093146. 2020 [Google Scholar]
- 108.Fisher W, Katta S, Arlen P, Diverse Biotech, Inc Cannabinoid preparations and therapeutic uses. Patent WO2019222459. 2019 [Google Scholar]
- 109.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral δ9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011 Jan;57((1)):66–75. doi: 10.1373/clinchem.2010.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boland EG, Bennett MI, Allgar V, Boland JW. Cannabinoids for adult cancer-related pain: systematic review and meta-analysis. BMJ Support Palliat Care. 2020 Mar;10((1)):14–24. doi: 10.1136/bmjspcare-2019-002032. [DOI] [PubMed] [Google Scholar]
- 111.Carotenuto A, Costabile T, De Lucia M, Moccia M, Falco F, Petruzzo M, et al. Predictors of Nabiximols (Sativex®) discontinuation over long-term follow-up: a real-life study. J Neurol. 2020 Jun;267((6)):1737–1743. doi: 10.1007/s00415-020-09739-x. [DOI] [PubMed] [Google Scholar]
- 112.Twelves C, Sabel M, Checketts D, Miller S, Tayo B, Jove M, et al. A phase 1b randomised, placebo-controlled trial of nabiximols cannabinoid oromucosal spray with temozolomide in patients with recurrent glioblastoma. Br J Cancer. 2021 Apr;124((8)):1379–1387. doi: 10.1038/s41416-021-01259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D, et al. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007 Dec 15;133((1)):210–220. doi: 10.1016/j.pain.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 114.Temtsin-Krayz G, Glozman S, Kazhdan P. Pharmaceutical compositions comprising lipophilic compd and water-sol polymers for transmucosal delivery. WO2017072774A1. 2017 [Google Scholar]
- 115.Bryson N, Sharma AC. Nasal cannabidiol compositions. WO2017208072A2. 2017 [Google Scholar]
- 116.Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018 Sep 19;16((1)):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Onaivi ES, Singh Chauhan BP, Sharma V. Challenges of cannabinoid delivery: how can nanomedicine help? Nanomedicine. 2020 Sep;15((21)):2023–2028. doi: 10.2217/nnm-2020-0221. [DOI] [PubMed] [Google Scholar]
- 118.Ngwa W, Kumar R, Moreau M, Dabney R, Herman A. Nanoparticle drones to target lung cancer with radiosensitizers and cannabinoids. Front Oncol. 2017;7:208. doi: 10.3389/fonc.2017.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Atsmon J, Cherniakov I, Izgelov D, Hoffman A, Domb AJ, Deutsch L, et al. PTL401, a new formulation based on pro-nano dispersion technology, improves oral cannabinoids bioavailability in healthy volunteers. J Pharm Sci. 2018 May;107((5)):1423–1429. doi: 10.1016/j.xphs.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 120.Greish K, Mathur A, Al Zahrani R, Elkaissi S, Al Jishi M, Nazzal O, et al. Synthetic cannabinoids nano-micelles for the management of triple negative breast cancer. J Control Release. 2018 Dec 10;291:184–195. doi: 10.1016/j.jconrel.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 121.Aparicio-Blanco J, Sebastián V, Benoit JP, Torres-Suárez AI. Lipid nanocapsules decorated and loaded with cannabidiol as targeted prolonged release carriers for glioma therapy: in vitro screening of critical parameters. Eur J Pharm Biopharm. 2019 Jan;134:126–137. doi: 10.1016/j.ejpb.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 122.Esposito E, Drechsler M, Cortesi R, Nastruzzi C. Encapsulation of cannabinoid drugs in nanostructured lipid carriers. Eur J Pharm Biopharm. 2016 May;102:87–91. doi: 10.1016/j.ejpb.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 123.Aparicio-Blanco J, Romero IA, Male DK, Slowing K, García-García L, Torres-Suárez AI, et al. Cannabidiol enhances the passage of lipid nanocapsules across the blood-brain barrier both in vitro and in vivo. Mol Pharm. 2019 May 6;16((5)):1999–2010. doi: 10.1021/acs.molpharmaceut.8b01344. [DOI] [PubMed] [Google Scholar]
- 124.Yin J, Xiang C, Wang P, Yin Y, Hou Y. Biocompatible nanoemulsions based on hemp oil and less surfactants for oral delivery of baicalein with enhanced bioavailability. Int J Nanomedicine. 2017;12:2923–2931. doi: 10.2147/IJN.S131167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hommoss G, Pyo SM, Müller RH. Mucoadhesive tetrahydrocannabinol-loaded NLC: formulation optimization and long-term physicochemical stability. Eur J Pharm Biopharm. 2017 Aug;117:408–417. doi: 10.1016/j.ejpb.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 126.Cherniakov I, Izgelov D, Domb AJ, Hoffman A. The effect of Pro NanoLipospheres (PNL) formulation containing natural absorption enhancers on the oral bioavailability of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a rat model. Eur J Pharm Sci. 2017 Nov 15;109:21–30. doi: 10.1016/j.ejps.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 127.Fraguas-Sánchez AI, Torres-Suárez AI, Cohen M, Delie F, Bastida-Ruiz D, Yart L, et al. PLGA nanoparticles for the intraperitoneal administration of CBD in the treatment of ovarian cancer: in vitro and in ovo assessment. Pharmaceutics. 2020 May 9;12((5)):439. doi: 10.3390/pharmaceutics12050439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siissalo S, de Waard H, de Jager MH, Hayeshi R, Frijlink HW, Hinrichs WLJ, et al. Nanoparticle formulation of a poorly soluble cannabinoid receptor 1 antagonist improves absorption by rat and human intestine. Drug Metab Dispos. 2013 Aug;41((8)):1557–1565. doi: 10.1124/dmd.112.049585. [DOI] [PubMed] [Google Scholar]
- 129.Hung O, Zamecnik J, Shek PN, Tikuisis P. Pulmonary delivery of liposome-encapsulated cannabinoids. WO2001003668A1. 2001 [Google Scholar]
- 130.Donsky M, Winnicki R. Terpene and cannabinoid liposome and micelle formulations. WO2015068052A2. 2015 [Google Scholar]
- 131.Vitetta L, Hall S. Protection of plant extracts and compounds from degradation. WO2017193169A1. 2017 [Google Scholar]
- 132.Hall SM, Vitetta L, Zhou Y, Rutolo DA, Jr, Coulson SM. Transmucosal and transdermal delivery systems comprising non-ionic surfactant and polyol. WO2016141069A1. 2016 [Google Scholar]
- 133.Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009 Jan 21;366((1–2)):170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 134.Durán-Lobato M, Martín-Banderas L, Lopes R, Gonçalves LMD, Fernández-Arévalo M, Almeida AJ, et al. Lipid nanoparticles as an emerging platform for cannabinoid delivery: physicochemical optimization and biocompatibility. Drug Dev Ind Pharm. 2016;42((2)):190–198. doi: 10.3109/03639045.2015.1038274. [DOI] [PubMed] [Google Scholar]
- 135.Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004 Apr;58((3)):173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 136.Mishra V, Nayak P, Yadav N, Singh M, Tambuwala MM, Aljabali AAA, et al. Orally administered self-emulsifying drug delivery system in disease management: advancement and patents. Expert Opin Drug Deliv. 2021 Mar;18((3)):315–332. doi: 10.1080/17425247.2021.1856073. [DOI] [PubMed] [Google Scholar]
- 137.Izgelov D, Regev A, Domb AJ, Hoffman A. Using the absorption cocktail approach to assess differential absorption kinetics of cannabidiol administered in lipid-based vehicles in rats. Mol Pharm. 2020 Jun 1;17((6)):1979–1986. doi: 10.1021/acs.molpharmaceut.0c00141. [DOI] [PubMed] [Google Scholar]
- 138.Klumpers LE, Beumer TL, van Hasselt JGC, Lipplaa A, Karger LB, Kleinloog HD, et al. Novel Δ(9) -tetrahydrocannabinol formulation Namisol® has beneficial pharmacokinetics and promising pharmacodynamic effects. Br J Clin Pharmacol. 2012 Jul;74((1)):42–53. doi: 10.1111/j.1365-2125.2012.04164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pellikaan HC, Vermeulen PS, Bender JCME, Woerlee GF, Echo Pharmaceuticals B.V. Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances, such as cannabinoids. Patent WO2008033024. 2008 [Google Scholar]
- 140.Cherniakov I, Izgelov D, Barasch D, Davidson E, Domb AJ, Hoffman A, et al. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J Control Release. 2017 Nov 28;266:1–7. doi: 10.1016/j.jconrel.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 141.Liu ZX, Artmann C. Relative bioavailability comparison of different coenzyme Q10 formulations with a novel delivery system. Altern Ther Health Med. 2009 Mar–Apr;15((2)):42–46. [PubMed] [Google Scholar]
- 142.Izgelov D, Domb AJ, Hoffman A. The effect of piperine on oral absorption of cannabidiol following acute vs. chronic administration. Eur J Pharm Sci. 2020 May 30;148:105313. doi: 10.1016/j.ejps.2020.105313. [DOI] [PubMed] [Google Scholar]
- 143.Wallis SW. Therapeutic delivery formulations and systems comprising cannabinoids and terpenes. CA2952335A1. 2017 [Google Scholar]
- 144.Lavasanifar A, Bolton AE, Cardiol Therapeutics Inc Amphiphilic block copolymers, micelles, and methods for treating or preventing heart failure using a cardioactive agent. Patent WO2019113685. 2019 [Google Scholar]
- 145.Izgelov D, Davidson E, Barasch D, Regev A, Domb AJ, Hoffman A, et al. Pharmacokinetic investigation of synthetic cannabidiol oral formulations in healthy volunteers. Eur J Pharm Biopharm. 2020 Sep;154:108–115. doi: 10.1016/j.ejpb.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 146.Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for drug delivery systems. Annu Rev Chem Biomol Eng. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Durán-Lobato M, Muñoz-Rubio I, Holgado MA, Alvarez-Fuentes J, Fernández-Arévalo M, Martín-Banderas L, et al. Enhanced cellular uptake and biodistribution of a synthetic cannabinoid loaded in surface-modified poly(lactic-co-glycolic acid) nanoparticles. J Biomed Nanotechnol. 2014 Jun;10((6)):1068–1079. doi: 10.1166/jbn.2014.1806. [DOI] [PubMed] [Google Scholar]
- 148.Martín-Banderas L, Muñoz-Rubio I, Prados J, Álvarez-Fuentes J, Calderón-Montaño JM, López-Lázaro M, et al. In vitro and in vivo evaluation of Δ9-tetrahidrocannabinol/PLGA nanoparticles for cancer chemotherapy. Int J Pharm. 2015 Jun 20;487((1–2)):205–212. doi: 10.1016/j.ijpharm.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 149.Hernán Pérez de la Ossa D, Ligresti A, Gil-Alegre ME, Aberturas MR, Molpeceres J, Di Marzo V, et al. Poly-ε-caprolactone microspheres as a drug delivery system for cannabinoid administration: development, characterization and in vitro evaluation of their antitumoral efficacy. J Control Release. 2012 Aug 10;161((3)):927–932. doi: 10.1016/j.jconrel.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 150.Singh P, Pandit S, Garnæs J, Tunjic S, Mokkapati VR, Sultan A, et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int J Nanomedicine. 2018;13:3571–3591. doi: 10.2147/IJN.S157958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Varnamkhasti BS, Jafari S, Taghavi F, Alaei L, Izadi Z, Lotfabadi A, et al. Cell-penetrating peptides: as a promising theranostics strategy to circumvent the blood-brain barrier for cns diseases. Curr Drug Deliv. 2020;17((5)):375–386. doi: 10.2174/1567201817666200415111755. [DOI] [PubMed] [Google Scholar]