Abstract
Neonicotinoid is a new class of systemic insecticides that are selectively toxic to insects. However, cases of human toxicity have been reported. A man in his 60s, who worked as a pest control operator (which required the use of thiamethoxam), presented with fever and headache. We investigated the levels of thiamethoxam and clothianidin in the blood and urine. Our results suggested that chronic thiamethoxam intoxication was caused by occupational inhalation exposure and environmental pollution. After cessation of insecticide use, the patient remained asymptomatic but had persistent oral dysesthesia and postural finger tremor, even at undetectable levels of thiamethoxam and clothianidin. This case report is the first to describe human thiamethoxam intoxication after occupational inhalation exposure. When similar symptoms are encountered and a history of insecticide use is confirmed, clinicians should consider the diagnosis of neonicotinoid intoxication.
Keywords: General practice / family medicine, Exposures, Occupational and environmental medicine
Background
Neonicotinoid insecticides are systemic insecticides—the most widely used alternative to organophosphorus insecticides in pest management.1–3 Neonicotinoid insecticides have selective toxicity to insects as agonists of α4β2 nicotinic acetylcholine receptors.4 Seven neonicotinoid insecticides were commercially available worldwide—imidacloprid, acetamiprid, nitenpyram, thiacloprid, clothianidin, thiamethoxam and dinotefuran. However, in response to the recent evidence supporting that neonicotinoids were lethal to pollinator insects, such as wild bees and honeybees, the European Union started banning clothianidin, imidacloprid, and thiamethoxam in member countries. In 2018, the French government passed a law banning the use of the following five neonicotinoids that were previously approved for agricultural use: clothianidin, imidacloprid, thiamethoxam, acetamiprid and thiacloprid.5 On the contrary, some countries, such as Denmark, have not banned the use of several neonicotinoids owing to the lack of available alternatives.6 Although neonicotinoids were considered harmless to the human body, cases of human exposure and toxicity have been reported in recent years.7–12 Severe neonicotinoid intoxication leads to respiratory, cardiovascular and neurological symptoms, eventually causing death.7–10 According to the literature review, the use of imidacloprid accounted for 94% of the neonicotinoid intoxication cases. The remaining 6% of intoxication cases resulted from the use of acetamiprid (5%) and clothianidin (1%).7 Most cases of neonicotinoid intoxication are caused by environmental exposure or self-poisoning and present with acute symptoms.7–12 Thiamethoxam intoxication due to occupational inhalational exposure has not been reported. Herein, we report a case of chronic thiamethoxam intoxication related to occupational inhalation exposure due to usage of inappropriate gas mask.
Case presentation
A man in his 60s presented with low-grade fever for 30 days, and headaches and abdominal pain for 7 days. He experienced low-grade fever and notable sweating every summer for 5 years. One year prior to presentation, he experienced burning pain in the oral mucosa (mainly the tongue and lips), which was exacerbated by eating hot foods. Chills, cough, shortness of breath, haemoptysis, vomiting, decreased appetite, dysgeusia, diarrhoea, genitourinary symptoms, fatigue and weight loss were not reported.
The patient had a history of hypertension and obstructive sleep apnoea syndrome managed by continuous positive airway pressure (CPAP) therapy. His only medication was candesartan. He smoked one pack per day for >30 years and occasionally consumed alcohol. He did not use herbal remedies, illegal drugs or supplements. The patient had no known drug allergies and no history of recent travel or contact with sick people. He worked as a pest control operator, specifically for termites and wasps, but had not recently acquired insect bites or experienced dermal contact incidents.
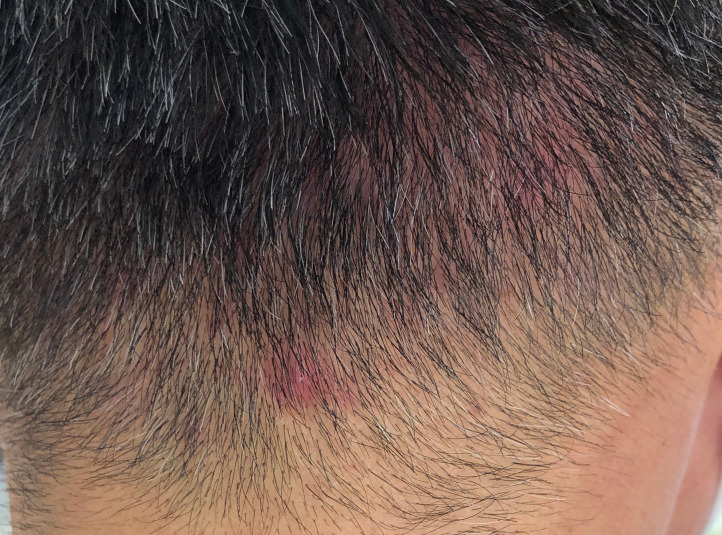
Physical examination showed a body temperature of 38.3°C, a blood pressure of 153/83 mm Hg, a heart rate of 102 beats/min and oxygen saturation of 97% while breathing ambient air. Erythematous papules with a perifollicular pustule appeared on the temporal and posterior regions of the patient’s head, which was consistent with the characteristics of folliculitis (figure 1). The oral cavity had no abnormal findings; however, the burning sensation on the lips and tongue was consistent with the characteristics of oral dysesthesia. Cardiac examination showed absence of murmurs. The lungs were clear upon auscultation. Bowel sounds were normal. Rectal examination revealed an enlarged, non-tender prostate. Results of the neurological examination were unremarkable, except for postural finger tremors.
Figure 1.
The patient had erythematous papules with a perifollicular pustule on the posterior head.
The white cell count was 7.2 ×109/L, with 71% neutrophils, 22.6% lymphocytes, 5.7% monocytes and 0.7% eosinophils. The hemoglobin level was 141 g/L and the platelet count was 184 ×109/L. The total protein level was 7.4 g/dL, the albumin level was 5.1 g/dL, the blood urea nitrogen level was 9.1 mg/dL and the creatinine level was 0.77 mg/dL. The serum sodium level was 141 mmol/L, the potassium level was 4.2 mmol/L and the bicarbonate level was 14 mmol/L. The alkaline phosphatase level was 142 U/L, the alanine aminotransferase level was 12 U/L, the aspartate aminotransferase level was 29 U/L, the total bilirubin level was 1.6 mg/dL, the direct bilirubin level was 0.5 mg/dL and the lactate dehydrogenase level was 247 U/L. The C reactive protein level was 0.03 mg/dL. Urinalysis revealed no abnormal findings. Sputum staining, PCR and interferon-gamma release assay for tuberculosis showed negative results. Results of antibody-based screening tests for hepatitis B, hepatitis C, HIV and syphilis were negative. The levels of antinuclear antibodies, antineutrophilic cytoplasmic antibodies and serum ACE were normal. The levels of vitamin B1, vitamin B12, folic acid, iron, zinc and copper were normal. ECG showed sinus rhythm at 100 beats/min, with normal voltage and no ST-segment elevation or depression. CT of the head, chest and abdomen revealed no abnormal findings. Upper gastrointestinal endoscopy and colonoscopy revealed no malignant findings.
During history taking, the patient’s occupational history was evaluated, which revealed that he had been working as a pest control operator for 30 years. Furthermore, he had been using thiamethoxam, a neonicotinoid insecticide, to kill termites and wasps for >10 years. In addition, he used a large amount of neonicotinoid insecticides every spring and summer. PubMed was searched using a combination of the following terms: “neonicotinoid”, “fever” and “headache”. An article titled ‘Detection of chloropyridinyl neonicotinoid insecticide metabolite 6-chloronicotinic acid in the urine: six cases with subacute nicotinic symptoms’ was found during the search.11 Further search was carried out using the key terms “neonicotinoid” and “poisoning”, which identified various publications that presented the clinical features of neonicotinoid syndrome, most of which were reported in patients who ingested the chemical to die by suicide.
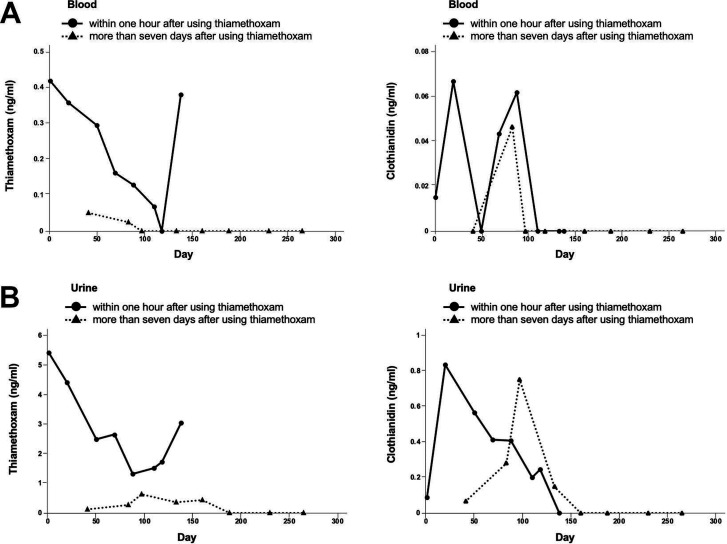
In our case, the patient was suspected of experiencing thiamethoxam intoxication presenting with fever and headache. Therefore, the thiamethoxam levels in blood and urine were measured immediately after using thiamethoxam insecticides, and the patient’s symptoms and thiamethoxam levels were monitored. Thiamethoxam intoxication was confirmed by measurement of thiamethoxam levels in the blood and urine sample (0.42 ng/mL and 5.43 ng/mL, respectively) immediately after exposure to thiamethoxam. Blood and urine samples collected more than 7 days after thiamethoxam exposure showed residual levels (0.051 ng/mL and 0.12 ng/mL, respectively). Thiamethoxam was also detected in sweat, clean regular clothes, unused work clothes and CPAP headgear (103 ng/mL, 85 ng/mL, 21 ng/mL and 4.4 ng/mL, respectively). Clothianidin, a metabolite of thiamethoxam, was detected in the blood and urine samples (0.015 ng/mL and 0.09 ng/mL, respectively) immediately after using thiamethoxam insecticides.
To identify the reason for thiamethoxam intoxication, we found a notably dirty gas mask that the patient has been using for >5 years.
Treatment
The patient underwent supportive care and was followed up in our outpatient clinic. He was instructed to use a new gas mask, and advised to implement appropriate protective measures when using thiamethoxam insecticides. His symptoms and the levels of thiamethoxam and clothianidin in blood and urine were monitored for over 270 days.
Outcome and follow-up
Although fever, headache and abdominal pain subsided at the 30-day follow-up, thiamethoxam and clothianidin continued to be detected in his samples despite receiving proper guidance on the use of these insecticides; therefore, the use of insecticides was eventually discontinued at the 150-day follow-up. Consequently, thiamethoxam and clothianidin levels reduced, and they were no longer detected until the 270-day follow-up (figure 2). Oral dysesthesia and postural finger tremor persisted despite the undetectable levels of thiamethoxam and clothianidin. Folliculitis also gradually improved after changing the CPAP headgear. He remained asymptomatic except for oral dysesthesia and postural finger tremor that persisted until the 270-day follow-up, then, the outpatient follow-up was discontinued. One year after the 270-day follow-up, the patient was followed up again to assess his symptoms. We found that his postural finger tremor and oral dysesthesia had resolved. The patient continuously avoided the use of neonicotinoid insecticides and replaced his CPAP mask with a new one.
Figure 2.
This figure shows the levels of thiamethoxam and clothianidin in blood (A) and urine (B) with a 270-day follow-up. Thiamethoxam and clothianidin were measured just after using thiamethoxam, and more than 7 days after using thiamethoxam. The use of thiamethoxam was eventually discontinued at 150-day follow-up.
Discussion
Neonicotinoid insecticides are absorbed through ingestion, dermal contact and inhalation, and act as agonists of the nicotinic acetylcholine receptor, which influences the central nervous system and autonomic nervous system. The toxin levels remained high for up to 15 hours post-ingestion, but it eventually reduced below detection levels in 42 hours.8 This result shows that poisoned patients may present with dizziness, drowsiness, disorientation, coma, diaphoresis, mydriasis, tachycardia and elevated blood pressure. Stimulation of the cardiovascular system may induce the occurrence of cardiovascular symptoms. Oral ingestion can result in oral ulcers, nausea, vomiting, dysphagia and abdominal pain.7 Significant toxicity of neonicotinoid insecticides can occur after ingesting a large amount of this chemical.9 The clinical features varied between case reports, which might depend on the dosage and method of exposure.7 ‘Neonicotinic symptoms’ comprised six subjective symptoms (headache, general fatigue, palpitations, chest pain, abdominal pain, muscle pain or muscle weakness or muscle spasm, and cough) and three objective symptoms (postural finger tremor, recent memory loss and fever). Postural finger tremors did not improve in some patients.12
The acute symptoms (such as headache, abdominal pain, postural finger and fever) observed in our patient were consistent with those previously reported in patients with acute neonicotinoid poisoning.7 However, folliculitis and oral dysesthesia were unique to this case. The occurrence of folliculitis was directly linked to the use of a CPAP headgear with contaminated contact areas, and gradual improvement in the symptoms of folliculitis was noted after headgear replacement, thus suggesting frequent dermal contact with thiamethoxam may have caused folliculitis. The aetiopathogenesis of oral dysesthesia is generally unclear; however, it may be associated with trigeminal small-fibre neuropathy.13 In this patient, oral dysesthesia was possibly caused by chronic thiamethoxam intoxication from using contaminated CPAP mask and gas mask. The neonicotinoid-related pathogenesis of folliculitis and oral dysesthesia requires further elucidation.
Adequate protective equipment, including hat, goggles, gloves, apron and boots, should be recommended to reduce exposure to neonicotinoid insecticides during spraying.14 This case showed that inappropriate use of a gas mask caused thiamethoxam intoxication, and cessation of insecticide use eventually resulted in undetectable levels of thiamethoxam in samples. All poisoned patients should undergo skin decontamination and removal of contaminated clothes as neonicotinoid insecticides can be absorbed via inhalation and dermal contact. In our case, thiamethoxam was detected in the blood and urine more than 7 days after thiamethoxam exposure, as well as in sweat, clothes and CPAP headgear, which implied chronic thiamethoxam intoxication due to environmental pollution.
Although one human case of acute kidney injury was reported due to oral ingestion of thiamethoxam,15 this case report is the first to describe the symptoms of thiamethoxam intoxication due to occupational inhalational exposure; the patient was followed up for more than 8 months to monitor the symptoms and levels of thiamethoxam and clothianidin. In patients who work as pest control operators, clinicians should assess for history of neonicotinoid use and consider the diagnosis of neonicotinoid intoxication.
Patient’s perspective.
I had heard that it was a pesticide that was harmless to the body, but I was surprised to see such symptoms. I am very grateful for the diagnosis.
Learning points.
Although neonicotinoid is a new class of systemic insecticides that are selectively toxic to insects, cases of human toxicity have also been reported.
Neonicotinic symptoms comprised six subjective symptoms (headache, general fatigue, palpitations, chest pain, abdominal pain, muscle pain or muscle weakness or muscle spasm, and cough) and three objective symptoms (postural finger tremor, recent memory loss and fever).
Folliculitis and oral dysesthesia might also be caused by thiamethoxam intoxication.
Acknowledgments
The authors wish to thank Yasuhiro Osugi, Toyota Regional Medical Center, Toyota, Aichi, Japan; and Yoshihiro Terasawa, Kuchinotsu Hospital, Nagasaki, Japan, for their kind support. The authors also wish to thank Antaa QA, a physician-to-physician question resolution platform, for connecting specialised doctors.
Footnotes
Contributors: TN and TT cared for the patient and wrote the report. YI performed the concentration measurement. GI provided professional advice. All authors read and approved the final version of the report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Jeschke P, Nauen R, Schindler M, et al. Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 2011;59:2897–908. 10.1021/jf101303g [DOI] [PubMed] [Google Scholar]
- 2.Simon-Delso N, Amaral-Rogers V, Belzunces LP, et al. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res Int 2015;22:5–34. 10.1007/s11356-014-3470-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonmatin J-M, Giorio C, Girolami V, et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res Int 2015;22:35–67. 10.1007/s11356-014-3332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol 2005;45:247–68. 10.1146/annurev.pharmtox.45.120403.095930 [DOI] [PubMed] [Google Scholar]
- 5.Jactel H, Verheggen F, Thiéry D, et al. Alternatives to neonicotinoids. Environ Int 2019;129:423–9. 10.1016/j.envint.2019.04.045 [DOI] [PubMed] [Google Scholar]
- 6.Sonne C, Alstrup AKO. Denmark defies EU neonicotinoid ban. Science 2019;363:938. 10.1126/science.aaw6754 [DOI] [PubMed] [Google Scholar]
- 7.Lin P-C, Lin H-J, Liao Y-Y, et al. Acute poisoning with neonicotinoid insecticides: a case report and literature review. Basic Clin Pharmacol Toxicol 2013;112:282–6. 10.1111/bcpt.12027 [DOI] [PubMed] [Google Scholar]
- 8.Mohamed F, Gawarammana I, Robertson TA, et al. Acute human self-poisoning with imidacloprid compound: a neonicotinoid insecticide. PLoS One 2009;4:e5127. 10.1371/journal.pone.0005127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phua DH, Lin CC, Wu M-L, et al. Neonicotinoid insecticides: an emerging cause of acute pesticide poisoning. Clin Toxicol 2009;47:336–41. 10.1080/15563650802644533 [DOI] [PubMed] [Google Scholar]
- 10.Huang N-C, Lin S-L, Chou C-H, et al. Fatal ventricular fibrillation in a patient with acute imidacloprid poisoning. Am J Emerg Med 2006;24:883–5. 10.1016/j.ajem.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 11.Taira K, Aoyama Y, Kawakami T, et al. [Detection of chloropyridinyl neonicotinoid insecticide metabolite 6-chloronicotinic acid in the urine: six cases with subacute nicotinic symptoms]. Chudoku Kenkyu 2011;24:222–30. [PubMed] [Google Scholar]
- 12.Marfo JT, Fujioka K, Ikenaka Y, et al. Relationship between urinary N-Desmethyl-Acetamiprid and typical symptoms including neurological findings: a prevalence case-control study. PLoS One 2015;10:e0142172. 10.1371/journal.pone.0142172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauria G, Majorana A, Borgna M, et al. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain 2005;115:332–7. 10.1016/j.pain.2005.03.028 [DOI] [PubMed] [Google Scholar]
- 14.Calumpang SM, Medina MJ. Applicator exposure to imidacloprid while spraying mangoes. Bull Environ Contam Toxicol 1996;57:697–704. 10.1007/s001289900246 [DOI] [PubMed] [Google Scholar]
- 15.Ramanathan S, Kumar M S, Sanjeevi G, et al. Thiamethoxam, a neonicotinoid poisoning causing acute kidney injury via a novel mechanism. Kidney Int Rep 2020;5:1111–3. 10.1016/j.ekir.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]