Abstract
Objectives
This systematic review aims to improve our knowledge of enablers and barriers to implementing obesity-related anthropometric assessments in clinical practice.
Design
A mixed-methods systematic review.
Data sources
Medline, Embase and CINAHL to November 2021.
Eligibility criteria
Quantitative studies that reported patient factors associated with obesity assessments in clinical practice (general practice or primary care); and qualitative studies that reported views of healthcare professionals about enablers and barriers to their implementation.
Data extraction and synthesis
We used random-effects meta-analysis to pool ratios for categorical predictors reported in ≥3 studies expressed as pooled risk ratio (RR) with 95% CI, applied inverse variance weights, and investigated statistical heterogeneity (I2), publication bias (Egger’s test), and sensitivity analyses. We used reflexive thematic analysis for qualitative data and applied a convergent integrated approach to synthesis.
Results
We reviewed 22 quantitative (observational) and 3 qualitative studies published between 2004 and 2020. All had ≥50% of the quality items for risk of bias assessments. Obesity assessment in clinical practice was positively associated with patient factors: female sex (RR 1.28, 95% CI 1.10 to 1.50, I2 99.8%, mostly UK/USA), socioeconomic deprivation (RR 1.21, 95% CI 1.18 to 1.24, I2 73.9%, UK studies), non-white race/ethnicity (RR 1.27, 95% CI 1.03 to 1.57, I2 99.6%) and comorbidities (RR 2.11, 95% CI 1.60 to 2.79, I2 99.6%, consistent across most countries). Obesity assessment was also most common in the heaviest body mass index group (RR 1.55, 95% CI 0.99 to 2.45, I2 99.6%). Views of healthcare professionals were positive about obesity assessments when linked to patient health (convergent with meta-analysis for comorbidities) and if part of routine practice, but negative about their role, training, time, resources and incentives in the healthcare system.
Conclusions
Our evidence synthesis revealed several important enablers and barriers to obesity assessments that should inform healthcare professionals and relevant stakeholders to encourage adherence to clinical practice guideline recommendations.
Keywords: PRIMARY CARE, Quality in health care, PREVENTIVE MEDICINE, QUALITATIVE RESEARCH
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Study design that allowed a convergent integrated synthesis of evidence from quantitative and qualitative studies on the enablers and barriers to implementing obesity-related anthropometric assessments in clinical practice.
Comprehensive search strategy of major electronic databases and rigorous data extraction and risk of bias assessments.
Conclusive results from several meta-analyses corrected for heterogeneity across studies and convergent with results from rigorous thematic analysis.
Results from meta-analyses were based on observational studies and slightly weakened or inconclusive for some patient factors. Small number of qualitative studies reviewed also limits the applicability of our findings to encourage better adherence to clinical practice guideline recommendations.
Findings might have limited applicability in settings not reviewed, especially in low/middle-income countries.
Introduction
Obesity rates have nearly tripled in most countries since 1975.1 The rising health problems attributable to obesity are undoubtedly challenging health systems worldwide.2 As the first point of contact for most people seeking healthcare services, general practice or primary care (‘clinical practice’) remains at the forefront of efforts to prevent and manage obesity.2 Although a range of evidence‐based guidelines provide recommendations on how to provide effective weight management in clinical practice,3 obesity and related complications remain under diagnosed and poorly treated.4 5 Quality improvements in obesity care would result in significant population health and economic benefits.6–9
Most international guidelines recommend that body mass index (BMI) should be used as a routine measure for diagnosis.3 10 They also recommend that waist circumference (WC) should be considered as an additional measure to assess the risk of developing obesity-related complications.3 There is a growing body of evidence indicating that routine clinical practices for obesity-related anthropometric measures fall short of guideline recommendations and standards.2 Studies have reported that the rate of weight, BMI or WC measurement in clinical practice could be as low as 20%–30%, even in high-income countries.11 12 The reasons for such low adherence rates to these guideline recommendations are likely to vary across countries. For instance, patient factors such as female sex was associated with an increased likelihood of weight recording in the UK11 but not in the Netherlands,13 and was associated with a decreased likelihood of BMI documentation in Australia.12 Cardiovascular disease was associated with an increased likelihood of a weight recording in the Netherlands,13 whereas a reverse association was reported in Australia.12 Furthermore, qualitative research suggests that healthcare professionals report several barriers to implementing obesity-related anthropometric measure in clinical practice such as lack of knowledge and specific training, negative perceptions about its usefulness, clinical importance and acceptability.14 Given the existence of relevant quantitative and qualitative studies, as well as several inconsistencies within this evidence base, this mixed-methods systematic review aims to improve our knowledge of the enablers and barriers to implementing obesity assessments in clinical practice.
Methods
Protocol and registration
We developed the protocol for this systematic review with guidance from previous research,15–17 the Centre for Review and Dissemination’s Guidance for undertaking reviews in healthcare,18 the JBI methodology for mixed-methods systematic reviews using a convergent integrated approach to synthesis and integration,19 and the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols statement.20
Patient and public involvement
This rapid systematic review did not involve patients and the public in the protocol development.
Eligibility
Using modified versions of the Population, Interventions, Comparators and Outcomes framework, we developed two research questions and selected study eligibility criteria (table 1).21
Table 1.
Inclusion criteria for quantitative and qualitative studies
| Parameter | Criteria | |
| Quantitative studies | ||
| P | Population and setting | Adult patients in clinical practice (general practice or primary care) |
| P | Patient factor (independent variable) | Patient factors associated with implementing obesity-related anthropometric assessments such as previous obesity-related anthropometric assessment (eg, weight, waist circumference and BMI); demographic characteristics (eg, age, sex and ethnicity); existing medical conditions (eg, type 2 diabetes, hypertension and hyperlipidaemia) and clinical encounter (eg, reason for appointment) |
| O | Outcome (dependent variable) | Obesity-related anthropometric assessments (eg, weight, BMI, waist circumference and weight-to-hip ratio) |
| Qualitative studies | ||
| P | Population and setting | Healthcare professionals in clinical practice (general practice or primary care) |
| I | Interest | Healthcare professionals’ views (perspectives or experiences) about implementing obesity-related anthropometric assessments in clinical practice |
| Co | Context | Any country worldwide |
BMI, body mass index.
Quantitative research question
What are the patient factors associated with implementing obesity-related anthropometric assessments in clinical practice?
Qualitative research question
What are the views of healthcare professionals about implementing obesity-related anthropometric assessments in clinical practice?
To answer the quantitative research question, we considered observational studies (eg, cohort, cross-sectional, case–control and case series) that reported associations between patient factors (independent variables) and outcomes (dependent variables) in the clinical practice setting (general practice or primary care). For the qualitative research question, we considered qualitative studies that reported on the views of healthcare professionals about enablers and barriers to implementing obesity-related anthropometric assessments in the clinical practice setting. We considered qualitative studies using designs such as phenomenological, ethnographic, grounded theory, historical, case study and action research.
Search strategy, information sources and study selection
The academic liaison librarian (BC) developed our search strategy in consultation with the subject expert (EA). She searched Medline, Embase and CINAHL databases for potentially relevant articles on 25 September 2021. Due to a typographical error for one search term used in Embase, she repeated the search in that database on 25 November 2021. The mixed-methods, quantitative and qualitative search string was adapted from the OVID expert search tool ‘Mixed Methods’ (online supplemental table S1). All records identified were exported from the databases into EndNote V.20 reference manager and duplicate records were removed where possible. All titles and abstracts were first screened for eligibility against the criteria mentioned above. Second, the available full-length reports retrieved from these records were screened for possible inclusion. We considered studies published in English language without any restrictions on the publication date and geographical location. References from included studies were also searched. Reasons why studies identified in the second screen were excluded are available in online supplemental table S2.
bmjopen-2022-063659supp001.pdf (596.6KB, pdf)
Data extraction and risk of bias assessment
We independently extracted key characteristics and assessed the risk of bias of the quantitative (RC, CNS, DL and EA) and qualitative (KP, GM and EA) studies included for review using the JBI’s standardised critical appraisal checklists.22 We used this information to assist our discussion on the strength of the body of evidence following our synthesis of results. For quantitative studies, we sought information about study details, population and setting, patient factors (independent variables), outcomes (obesity-related anthropometric assessments), statistical methods, results/effect estimates and author’s conclusions. For qualitative studies, we sought information about study details, population and setting, study design, aims and methods, main themes and subthemes with explanations, and author’s conclusions.
Effect measures
Results for categorical predictor variables, where the effect was expressed as a ratio relative to a reference category accompanied by a 95% CI, were considered for pooling. These results comprised risk ratios (RRs), rate ratios and ORs with no HRs reported. Results which were only reported as frequency counts were converted to RRs and associated 95% CIs using an appropriate online calculator via the VassarStats website.23
Synthesis methods
To allow pooling of results, we expressed ratios relative to the same or a similar reference category. Where reference categories were swapped (eg, females defined as the reference category instead of males), we corrected the reference category by inverting the ratio (and associated 95% CI) around the null value of ‘1’. Where a numeric variable had been categorised into varying categories, the lowest category was taken as the reference category and the highest category compared with it. Where there was a common reference category but varied comparator categories, the comparator categories were combined using the method by Borenstein et al.24 For example, for the variable ‘race/ethnicity’, as ‘white’ was the common reference category, the results for the various non-white categories were recombined to produce a single ‘non-white’ to ‘white’ ratio. Where a single study presented results separately in independent subgroups (such as separate results for males and females), ratios were first combined using a fixed effects meta-analysis prior to being pooled with results from other studies. Once reference categories, comparator categories and subgroups had been corrected, random-effects meta-analysis was used to pool ratios for predictors reported in three or more studies. To correct for heterogeneity across studies, we applied heterogeneous specific inverse variance weights in these analyses.25 Meta-analysis was only conducted for the BMI assessment outcome as ‘BMI recording’ or ‘BMI diagnosis recording’, which was more commonly reported than alternatives such as WC. Results reported include the pooled ratio with associated 95% CI and p value and the I2 statistic and the p value from the heterogeneity test. Forest plots are used to present commonly reported predictors, while results for other predictors are tabulated.
We used subgroup analyses to explore possible explanations for heterogeneity. This included assessing candidate grouping variables related to what was measured, how the results were summarised and where the studies were conducted. First, studies were stratified according to whether the outcome was the recording of BMI assessment or the recording of BMI as a health diagnosis. Second, as ORs generally overestimate RRs, studies could be stratified according to whether ORs or RRs were presented. Finally, as we assumed that different countries have different healthcare systems and policies, studies were stratified according to country (UK, USA, Australia or ‘other’). Subgroup analyses proceeded when at least two categories of the grouping variable contained at least three studies each. Sensitivity analyses, excluding all studies which failed to achieve 100% ‘yes’ responses on the quality assessment checklist, were conducted to check whether any of the findings were sensitive to study quality.
Reporting bias assessment
Funnel plots were visually reviewed for indications of reporting bias and Egger’s tests were reported for meta-analyses containing 10 or more studies only, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (section 13.3.5.4 tests for funnel plot asymmetry).26
Thematic analysis
We applied the widely used reflexive thematic analysis method by Braun and Clarke to establish findings from the qualitative data.27 Studies were read several times by two authors (GM and KP). Each author extracted the main findings from individual studies. Further, as recommended,27 we spent time individually coding to construct categories from the data. The categories were reviewed to seek potential commonalities and differences between the papers, from which themes were established. The two authors met regularly to review areas of data extraction, coding allocation and theme creation. Ongoing reflexive discussions created a space for mutual understanding and agreement about the overarching themes.
Results
Study selection
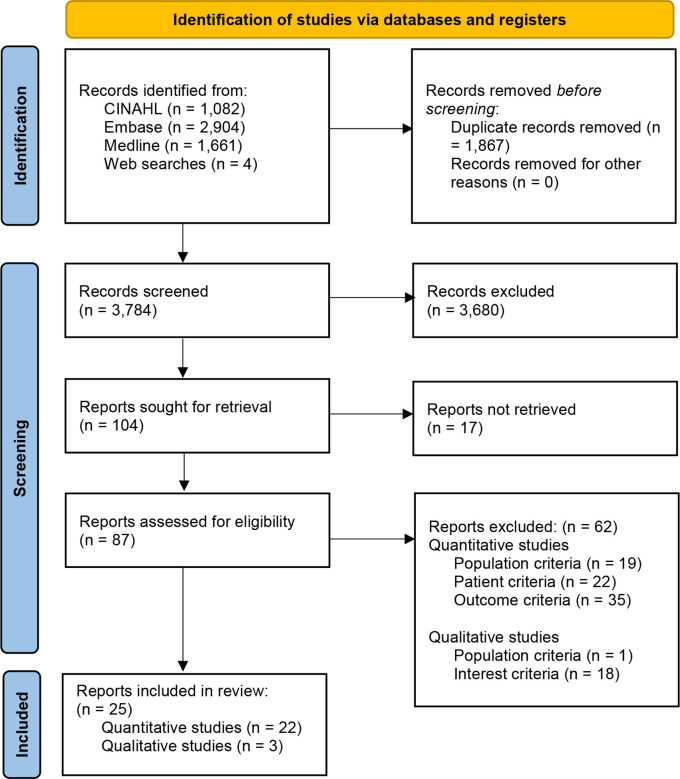
A flow diagram of the study selection process appears below (figure 1). Our search strategy identified 3784 records including four additional studies from other sources after 1867 duplicates were removed. Of these, we excluded 3680 records after the first screening, leaving 104 records for a second screening. After further assessment of 87 reports retrieved, we excluded 62 additional records for reasons summarised below and described in online supplemental table S2.
Figure 1.
Flow diagram of the study selection process.59
Study characteristics
We present a detailed summary of the study characteristics in online supplemental tables S3 and S4. In total, there were 22 quantitative studies (observational)11–13 28–46 and 3 qualitative studies,14 47 48 published between 2004 and 2020. Eight studies were from the UK,11 14 37 39 40 43 46 47 nine from the USA,28 31 32 34 36 41 42 45 48 four from Australia12 33 35 44 and one each from Germany,38 Spain,30 Israel29 and the Netherlands.13 All three qualitative studies included interviews with 7–14 primary care practitioners.14 47 48 All qualitative studies conducted semistructured interviews and thematic analysis to explore healthcare professionals’ views towards WC measurement including identification of possible barriers to carrying out the assessment,14 primary care providers’ perception of WC measurement rejection in primary care48 and primary care providers’ perception of recognition of overweight and obesity.47 Quantitative studies were based on records of patients from primary practices, with sample sizes between 100 and 1000 in 3 studies,28–30 1000 and 10 000 in 6 studies,13 31–35 10 000 and 100 000 in 6 studies,36–41 and greater than 100 000 in 7 studies.11 12 42–46 The patient factors associated with the implementation of obesity-related anthropometric assessment in primary care varied between studies, with sociodemographic factors such as age and sex identified in 16 studies,11–13 28 31 32 34 35 37–39 41–45 ethnicity and/or race identified in 9 studies,11 28 31 32 34 39 41 42 45 and socioeconomic status identified in 4 studies.11 37 39 43 Presence of comorbidities or any specific medical condition was identified to be a patient factor independently associated with the obesity assessment in 20 studies.11–13 28–35 38–46 Six studies identified insurance type as a factor associated with obesity-related anthropometric assessment.31 32 34–36 41 42 Outcomes in studies varied, with 11 studies having BMI ‘measurements’ or ‘recording’ or ‘documentation’ or ‘screening’,12 29 30 36 37 39–42 44 46 4 studies having obesity ‘diagnosis’ or ‘recognition’ or ‘identification’,28 31 38 40 2 studies having weight ‘recording’ or ‘measurement’,11 13 2 studies having overweight/obesity ‘documentation’,32 34 and 1 study each for null BMI recording,43 weight and/or WC measurement,33 ICD-9 (international classification of diseases, ninth revision) codes for overweight/obesity,45 and non-identification of overweight and obesity35 as a dependent variable.
Risk of bias within studies
We present the results of our quality assessment of each study in online supplemental table S5. All four cohort studies had at least 70% of the quality items clearly met,11 37 40 46 with three studies having one to two items unclear.11 40 46 Of the 18 cross-sectional studies, 12 studies had 100% of the quality items clearly met,12 13 29 31–34 39 42–45 and 6 studies had at least 50% of the quality items clearly met,28 30 35 36 38 41 with four studies having one to two items unclear.28 30 35 38 Of the three qualitative studies, two studies had 70%,14 47 and one study had 80%48 of the quality items clearly met.
Findings of meta-analysis
All patient factors potentially associated with obesity assessments as predictors were considered in each quantitative study reviewed (online supplemental table S3). Meta-analyses were conducted on each of the fourteen potential predictors identified, which were reported in at least three studies each (table 2). These were grouped as demographic characteristics (age, sex, race/ethnicity, deprivation index and health insurance status), BMI category, smoking status and comorbidities (number of comorbidities and individual comorbidities such as cardiovascular disease and diabetes). All except one study40 contributed results to at least one of these predictors. All meta-analyses found very high heterogeneity between studies. More detailed descriptions appear below, and additional results are presented in online supplemental section S6.
Table 2.
Summary of meta-analyses which pooled the ratios of BMI assessment by patient groups
| Predictor | Comparison | No of studies | Pooled risk ratio | I2, heterogeneity test, p value |
| Demographics | ||||
| Sex | Female vs male (reference) | 15 | 1.28 (1.10,1.50) | 99.8%, p<0.001 |
| Age | Closest to 65 years vs closest to 30 years (reference) | 12 | 0.90 (0.50,1.63) | 100%, p<0.001 |
| Race/ethnicity | Non-white vs white (reference) | 9 | 1.27 (1.03,1.57) | 99.6%, p<0.001 |
| Deprivation index | Highest deprivation vs least (reference) | 4 | 1.21 (1.18,1.24) | 73.9%, p=0.009 |
| BMI category | Highest BMI vs lowest BMI (reference) | 8 | 1.55 (0.99,2.45) | 99.6%, p<0.001 |
| Smoking status | Current smoker vs never smoker (reference) | 3 | 1.01 (0.90,1.14) | 98.3%, p<0.001 |
| Comorbidities | ||||
| No of comorbidities | Most vs fewest (reference) | 10 | 2.11 (1.60,2.79) | 99.6%, p<0.001 |
| Cardiovascular disease | Present vs absent (reference) | 7 | 0.94 (0.81,1.10) | 98.0%, p<0.001 |
| Diabetes | Present vs absent (reference) | 9 | 1.19 (0.93,1.52) | 99.0%, p<0.001 |
| Dyslipidaemia | Present vs absent (reference) | 6 | 1.12 (0.92,1.37) | 99.5%, p<0.001 |
| Hypertension | Present vs absent (reference) | 10 | 1.17 (0.98,1.40) | 99.5%, p<0.001 |
| Mental illness | Present vs absent (reference) | 3 | 1.16 (0.79,1.70) | 99.6%, p<0.001 |
| Depression | Present vs absent (reference) | 3 | 1.22 (0.85,1.74) | 98.7%, p<0.001 |
BMI, body mass index.
Demographics
Despite the high levels of heterogeneity between studies, the pooled results suggested that female sex, non-white race/ethnicity and socioeconomic deprivation were associated with statistically significant increases in the rate of BMI assessment of 1.2–1.3 fold, and there was no statistically significant evidence of reporting bias (online supplemental sections S6.1–3). There was no evidence of such differences in BMI assessment rates between younger and older age groups.
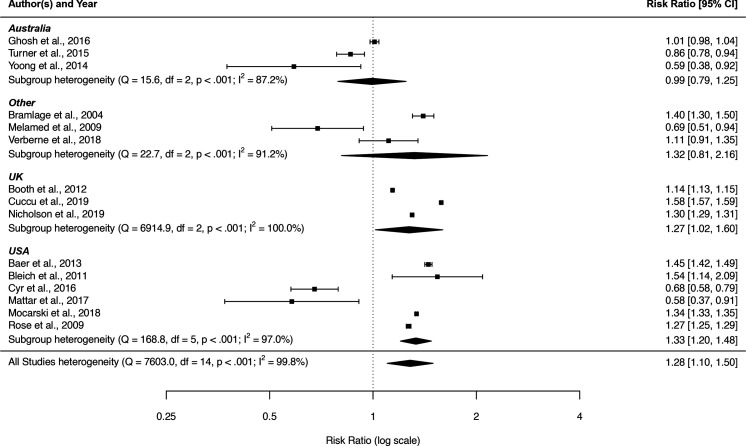
There was statistically significant evidence of increased assessment of BMI among females among studies from the UK and USA but not Australia (figure 2). As would be expected, the pooled OR (11 studies, OR 1.45, 95% CI 1.21 to 1.74, I2 99.5%) were higher than pooled other RRs (4 studies, RR 1.18, 95% CI 1.04 to 1.35, I2 99.7%) (online supplemental section S6 table S6.1). For all other predictors, there were insufficient studies reporting other RRs to allow further investigation of these subgroups. No other statistically significant results arose during the subgroup analysis.
Figure 2.
Forest plot of risk ratios for BMI assessment associated with female relative to male sex (reference) by country regions. BMI, body mass index.
In sensitivity analysis, restricting analysis to studies with the highest quality ratings yielded an increased pooled RR (10 studies, RR 1.45, 95% CI 1.21 to 1.74, I2 99.6%) for sex, but did not alleviate the heterogeneity between studies. The equivalent sensitivity analysis for age category also increased the size of the effect estimate, although still not statistically significant (nine studies, RR 0.69, 95% CI 0.19 to 2.48, I2 100%).
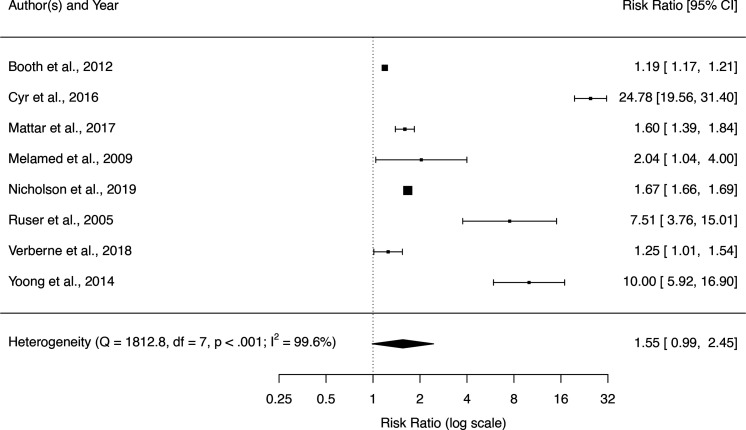
BMI and smoking status
All eight studies reporting results for BMI category found statistically significant effects, but the high heterogeneity yielded a wide CI and lack of statistical significance for the pooled RR (figure 3). Sensitivity analysis using only the studies with the highest quality rating produced a larger effect estimate for the difference between BMI assessment in the higher and lower BMI groups, but the high heterogeneity and lack of statistical significance remained (four studies, RR 2.56, 95% CI 0.45 to 14.6, I2 99.3%) (online supplemental section S6 table S6.6). There was no evidence of difference in BMI assessment between current and never smokers (three studies, RR 1.01, 95% CI 0.90 to 1.14, I2 98.2%) (online supplemental section S6 table S6.7).
Figure 3.
Forest plot of risk ratios for BMI assessment associated with highest relative to lowest (reference) BMI category. BMI, body mass index.
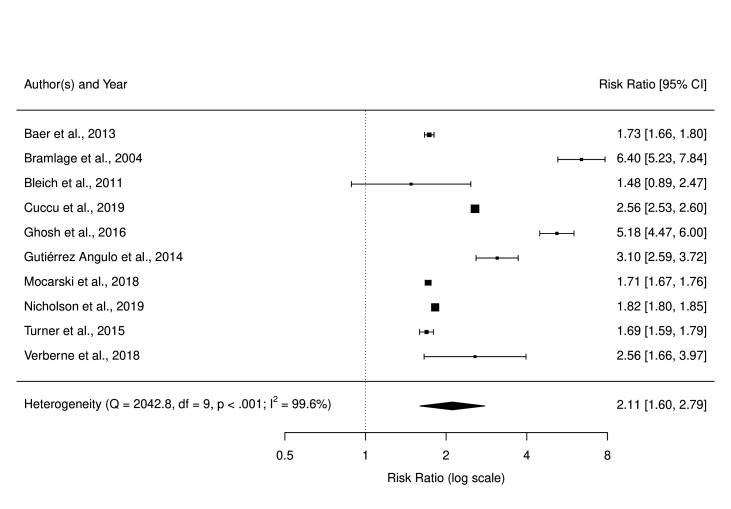
Comorbidities
Despite considerable heterogeneity in measures, methods and outcomes (online supplemental section S6 table S6.8), all 10 studies found that those with the higher comorbidities were more likely to have a BMI assessment recorded, with these results being statistically significant in 9 of the 10 studies (figure 4). Subgroup and sensitivity analyses showed that this association was broadly consistent across outcomes, countries and study quality, with no visual or statistical evidence of publication bias (online supplemental section S6 table S6.8).
Figure 4.
Forest plot of risk ratios for BMI assessment associated with most relative to fewest (reference) number of comorbidities groups. BMI, body mass index.
Pooled ratio of BMI assessment for those with relative to those without each specific comorbidity produced quite uniform results (online supplemental section S6 table S6.8). None of the individual comorbidities had a statistically significant association with BMI assessment and all displayed very high heterogeneity between studies: cardiovascular disease (7 studies, RR 0.94, 95% CI 0.81 to 1.10, I2 98.0%), diabetes (9 studies, RR 1.19, 95% CI 0.93 to 1.51, I2 99.0%), dyslipidaemia (6 studies, RR 1.12, 95% CI 0.92 to 1.37, I2 99.5%), hypertension (10 studies, RR 1.17, 95% CI 0.98 to 1.40, I2 99.5%), mental illness (3 studies, RR 1.16, 95% CI 0.79 to 1.70, I2 99.6%) and depression (3 studies, RR 1.22, 95% CI 0.85 to 1.74, I2 98.7%). However, subgroup analyses found that studies from Australia, unlike those from the UK and USA, had statistically significantly higher BMI assessment for those with comorbidities with lower heterogeneity: diabetes (three studies, RR 1.84, 95% CI 1.75 to 1.93, I2 0%); dyslipidaemia (three studies, RR 1.21, 95% CI 1.08 to 1.36, I2 80.6%) and hypertension (three studies, RR 1.15, 95% CI 1.05 to 1.26, I2 69.4%). Sensitivity analyses, restricting pooling to studies with the higher quality ratings, gave statistically significant evidence of the association between the comorbidity and BMI assessment in dyslipidaemia (four studies, RR 1.21, 95% CI 1.15 to 1.28, I2 57.3%) and hypertension (eight studies, RR 1.26, 95% CI 1.10 to 1.43, I2 97.7%).
Findings of thematic analysis
Three themes were established from our thematic analysis of the qualitative studies: personnel, resources and systemic factors.
Personnel
The theme of personnel factors focused on two subthemes: roles and responsibilities and communications and discomfort. While nurse participants believed that weight assessment and management was part of their professional role, there was ambiguity about this among the medical participants. One General Practitioner (GP) noted “I don’t want to be weighing people every week. I don’t think that’s my role. I think it’s also not a good use of our expertise as generalist doctors. I think we’ve got other things that we could be doing”,47 (p. 7). There were variable views among GPs about their role in obesity prevention. The GPs asserted that patients should retain responsibility for their weight unless they have weight-related health issues: “Patients need to take some responsibility themselves. And if they know that they're carrying a bit of extra weight, they don't need to see a GP necessarily”,47 (p. 7): “I have a responsibility to make them aware that (their weight) is an issue where it’s clearly impacting on their (health). Do I have a responsibility to assist them with that? If they are looking for that assistance. I would have a responsibility to assist them or signpost them to what can assist them”,47 (p. 7). This finding was aligned with another study which found that weight-related measurements were only undertaken if part of routine practice.48 Although GPs and nurses perceived that patients lacked understanding of the health risks associated with increasing waist size, and that WC measurement could motivate patients to make healthy lifestyle changes, they did not routinely carry out this assessment.14
Our thematic analysis highlighted a second subtheme in relation to personnel factors namely: communications and discomfort. Primary care practitioners perceived that patients might feel uncomfortable or embarrassed about having their WC measured.14 Others expressed a preference for discussing weight with the patient within the context of existing, and possibly weight related, health issues47: “So, I have to say that I tend only to (raise weight for discussion) if I see it as relevant to the problem that they've got”,47 (p. 7). They also thought that measuring waist might cause patient discomfort, particularly given the intimate nature of WC measurements,14 48 as a practice nurse highlighted: “It’s personal to go up and start putting your arms around a patient”,14 (p. 365). The need to consider cultural sensitivities was also reported: “Depends on the individual circumstances. Some patients don’t care, but if you’re a Muslim woman and very strict about it you wouldn’t want anybody other than a woman touching you, so it depends on your individual ethnic preferences and your personal preferences as well”,14 (p. 368). This was further reinforced when primary care providers reported their own discomfort when measuring a person’s WC, more so, a person of a different gender to themselves: “five providers shared that obtaining a WCM (abbreviated for WC measurement) was “uncomfortable,” particularly if the patient was “large” and/or the opposite gender of the provider”,48 (p. 686).
Resources
The theme of resources included subthemes associated with time, equipment, costs, knowledge and training. All three qualitative studies referred to the challenges of time for appointments and consultations. One healthcare practitioner stated: “You don’t just take the measurement, you have to explain what it means so in itself it doesn’t take a moment does it, but then you’ve got quite a good length of topic of conversation to explain it”,14 (p. 368). Limited availability of equipment such as tape measures48 and lack of specific training on correct measuring technique14 were other barriers to primary care practitioners for undertaking WC measurements. However, it was noted that “the degree to which HCPs (abbreviated for health care professionals) felt comfortable about WCM appeared to be positively related to the increased experience of measuring waist size and to routine rather than ad hoc use of this measurement and negatively associated with patients being overweight or obese”,14 (p. 369), despite health care professionals noting that they had not received specific training related to implementing WC measurements.14 An additional barrier to obesity-related anthropometric assessments could be that primary care practitioners question the evidence-base for recommended weight management interventions by clinical guidelines: “If someone’s got obesity, I'm kind of stuck. I can give them advice on what to do but I don't feel in many cases, that’s terribly helpful or terribly effective”,47 (p. 7).
Systemic factors
Two studies found systemic factors as barriers to undertaking WC measurements.14 47 One study highlighted the limited human and financial resources offered to primary care services.47 Another referred to the need for greater organisational incentives for undertaking WC measurements.14 Similarly, one primary care practitioner noted that the National Health Service contracts in the UK did not ‘prioritise or incentivise’ weight management within primary care settings.47 However, finance-related issues were not the only systemic factors highlighted. There were concerns about restrictive eligibility criteria for referring to specialised weight management services as summarised: “There was despondency among PCPs (abbreviated for primary care practitioners) that they had nowhere to refer overweight patients when weight was not (yet) impacting on their health, and even when patients had clinical weight issues, they were not eligible for some specialist care”,47 (p. 6). While findings were mainly related to service level issues, primary care practitioners argued that the inclusion of WC measurement within both quality and outcome frameworks could incentivise clinical practice.14
Discussion
We are the first authors to have systematically reviewed, synthesised and integrated the published evidence from quantitative and qualitative studies on the enablers and barriers to implementing obesity-related anthropometric assessments in clinical practice. Our evidence synthesis revealed several important enablers and barriers to obesity assessments that could inform healthcare professionals and relevant stakeholders such as academic institutions, professional bodies and regulatory agencies.
Enablers
We found evidence from our meta-analysis indicating that an obesity assessment is most likely for patients with weight-related complications (‘comorbidities’). This finding was broadly consistent across countries and slightly strengthened among high quality studies (including for ‘dyslipidaemia’ and ‘hypertension’). Similarly, the presence of ‘obesity-related comorbidities’ is reportedly one of the principal reasons cited by healthcare professionals for initiating weight management discussions.49 Although highly variable, we also found evidence to suggest that BMI assessment (‘recording’) was most likely among patients with the highest BMI. Overall, the results of our meta-analyses suggest that both excess weight and weight-related complications encourage healthcare professionals to conduct obesity assessments in high-risk patients.
Convergent with this hypothesis, the findings of our thematic analysis revealed positive views among healthcare professionals about obesity assessments if they suspected that their patient’s excess weight was negatively impacting on their health.47 Healthcare professionals also expressed positive views about obesity assessments if part of routine practice,48 and because they could motivate patients to make healthy lifestyle changes.14 Indeed, frequent self-weighing is associated with favourable weight loss, particularly among those with excess weight.50 This is consistent with findings of a recent systematic review of qualitative studies in which healthcare professionals expressed positive views on the usefulness of routine BMI assessment at every consultation alongside a treatment framework for discussing weight management with patients in primary care.51 Healthcare professionals should consider focusing on the health benefits of obesity assessments for clinical diagnosis and monitoring in all patients with visible signs of obesity, as part of their routine practice.
Findings from our meta-analyses also revealed evidence that obesity assessment was most likely for patients with socioeconomic deprivation in the UK, patients of ‘non-white’ race/ethnicity in the UK and USA, and for female patients, particularly in the UK and USA. These results are likely partially explained by increasing obesity52 and higher clinical encounter rates with socioeconomic disadvantage groupings,53 healthcare professionals being more verbally dominant towards non-White than White patients,54 and a higher prevalence of severe obesity among women than men,55 respectively, in high-income countries. Healthcare professionals should be aware of these potential biases to ensure that they conduct routine obesity assessments in all high-risk patients regardless of their socioeconomic status, race/ethnicity and sex.
Barriers
Our thematic analysis revealed negative attitudes among healthcare professionals about patients with obesity and their role in obesity assessment and weight management, generally. They expressed views that patients, rather than healthcare professionals, should retain responsibility for, and lacked motivation to, address their weight issues.47 Healthcare professionals expressed doubts about their patients’ understanding of health risks associated with the results of obesity assessments.14 Overall, these findings suggest that weight stigma among healthcare professionals is a barrier to obesity assessments.
We found evidence that healthcare professionals expressed negative views about adequate training and equipment for obesity assessments.14 47 48 They expressed negative views on limited access to specialist weight management services and the evidence base for treatments,47 as required after an obesity assessment and diagnosis.3 There were expressions of discomfort about obtaining obesity assessments for patients of the opposite sex,48 which is consistent with previous research showing that patients often preferred to see a healthcare professional of the same-sex.56 Convergent with findings from our meta-analyses for patients with weight-related complications, healthcare professionals expressed apprehension to discuss weight in the absence of suspected health issues.47 A recently validated brief diagnostic screening tool (EOSS-2 Risk Tool) for predicting weight-related complications in patients with excess weight could provide healthcare professionals with a structured framework for further investigations including obesity assessments.57 Finally, healthcare professionals expressed lack of time,14 47 48 increased financial cost implications14 and lack of incentives in the health system14 47 as additional resource and systematic barriers to obesity assessment. Collectively, these findings strengthen the urgency for implementing recommendations to incorporate ‘formal teaching on the causes, mechanisms, and treatments of obesity’ into standard curricula for healthcare professionals by academic institutions, professional bodies, and regulatory agencies.58 It would encourage better adherence to clinical practice guideline recommendations that BMI and WC measurements should be used for routine diagnosis and monitoring.3 10
Limitations
The applicability of our findings to encourage better adherence to clinical practice guideline recommendations is limited because results from meta-analyses were based on observational studies and slightly weakened or inconclusive for some patient factors, whereas only a small number of qualitative studies were reviewed. As the studies reviewed were predominately from the UK and USA, our findings might have limited applicability in other settings, especially in low/middle-income countries. Furthermore, we might have missed relevant studies for inclusion by using a streamlined rapid systematic review approach.
Conclusion
The key findings of our mixed-methods systematic review indicate that obesity-related anthropometric assessment in clinical practice is positively associated weight-related complications, socioeconomic deprivation, ‘non-white’ race/ethnicity and female sex among patients. Views of healthcare professionals were positive about obesity assessments when linked to patient health and if part of routine practice, but negative about their role, training, time, resources and incentives in the healthcare system. To encourage better adherence to clinical practice guideline recommendations, high-income countries should consider incorporating formal teaching of obesity medicine into their academic institutions, professional bodies and regulatory agencies. Future research for developing and testing interventions should consider the enablers and barriers to obesity assessments identified in this study.
Supplementary Material
Footnotes
Twitter: @evanatlantis?lang=en
Contributors: EA and RC were responsible for designing the review protocol, writing the protocol and report, conducting the search, screening potentially eligible studies, extracting data, interpreting results, conducting risk of bias assessments, and updating reference lists. CNS was responsible for conducting the search, screening potentially eligible studies, extracting data, interpreting results, updating reference lists and writing the supplementary. KP and GM were responsible for designing the review thematic analysis protocol, screening potentially eligible studies, extracting qualitative data, interpreting results and updating reference lists. BC was responsible for developing and conducting the search strategy. DL contributed to the design of the review protocol, writing the report, arbitrating potentially eligible studies, conducting risk of bias assessments, and interpreting results. PF was responsible for the meta-analyses including extracting, analysing, writing, and interpreting the results from quantitative data, screening potentially eligible studies and contributed to writing the results and Supplementary. EA is the author responsible for the overall content as the guarantor.
Funding: This pilot work was partially supported by grants from iNova Pharmaceuticals (Australia) (https://inovapharma.com/), in partnership with the National Association of Clinical Obesity Services Incorporated (https://www.nacos.org.au/) and Western Sydney University (https://www.westernsydney.edu.au/) (P00026836).
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: EA was the Founding President, and now serves as the Secretary, of the National Association of Clinical Obesity Services (NACOS). He has received honoraria from Novo Nordisk for speaking and participating at meetings. He has received unrestricted research funding from Novo Nordisk and iNova on behalf of NACOS. RC and CNS have received payments for their contributions through casual employment contracts at Western Sydney University. PF, KP, GM, BC and DL declare no competing financial interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.World Health Organization . Obesity and overweight. Secondary Obesity and overweight, 2021. Available: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2.Wolfenden L, Ezzati M, Larijani B, et al. The challenge for global health systems in preventing and managing obesity. Obes Rev 2019;20 Suppl 2:185–93. 10.1111/obr.12872 [DOI] [PubMed] [Google Scholar]
- 3.Semlitsch T, Stigler FL, Jeitler K, et al. Management of overweight and obesity in primary care-a systematic overview of international evidence-based guidelines. Obes Rev 2019;20:1218–30. 10.1111/obr.12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterson ID, Alfadda AA, Auerbach P, et al. Gaps to bridge: misalignment between perception, reality and actions in obesity. Diabetes Obes Metab 2019;21:1914–24. 10.1111/dom.13752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordmo M, Danielsen YS, Nordmo M. The challenge of keeping it off, a descriptive systematic review of high-quality, follow-up studies of obesity treatments. Obes Rev 2020;21:e12949. 10.1111/obr.12949 [DOI] [PubMed] [Google Scholar]
- 6.Cefalu WT, Bray GA, Home PD, et al. Advances in the science, treatment, and prevention of the disease of obesity: reflections from a diabetes care editors' expert forum. Diabetes Care 2015;38:1567–82. 10.2337/dc15-1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray GA, Frühbeck G, Ryan DH, et al. Management of obesity. Lancet 2016;387:1947–56. 10.1016/S0140-6736(16)00271-3 [DOI] [PubMed] [Google Scholar]
- 8.PwC . Weighing the cost of obesity: a case for action, 2015. Available: https://www.pwc.com.au/publications/healthcare-obesity.html [Accessed 05 Aug 2022].
- 9.Atlantis E, Kormas N, Samaras K, et al. Clinical obesity services in public hospitals in Australia: a position statement based on expert consensus. Clin Obes 2018;8:203–10. 10.1111/cob.12249 [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran D, Atlantis E, Markovic T, et al. Standard baseline data collections in obesity management clinics: a Delphi study with recommendations from an expert panel. Clin Obes 2019;9:e12301. 10.1111/cob.12301 [DOI] [PubMed] [Google Scholar]
- 11.Nicholson BD, Aveyard P, Bankhead CR, et al. Determinants and extent of weight recording in UK primary care: an analysis of 5 million adults' electronic health records from 2000 to 2017. BMC Med 2019;17:222–22. 10.1186/s12916-019-1446-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner LR, Harris MF, Mazza D. Obesity management in general practice: does current practice match guideline recommendations? Med J Aust 2015;202:370–2. 10.5694/mja14.00998 [DOI] [PubMed] [Google Scholar]
- 13.Verberne LDM, Nielen MMJ, Leemrijse CJ, et al. Recording of weight in electronic health records: an observational study in general practice. BMC Fam Pract 2018;19:174. 10.1186/s12875-018-0863-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunkley AJ, Stone MA, Patel N, et al. Waist circumference measurement: knowledge, attitudes and barriers in patients and practitioners in a multi-ethnic population. Fam Pract 2009;26:365–71. 10.1093/fampra/cmp048 [DOI] [PubMed] [Google Scholar]
- 15.Hong QN, Pluye P, Bujold M, et al. Convergent and sequential synthesis designs: implications for conducting and reporting systematic reviews of qualitative and quantitative evidence. Syst Rev 2017;6:61. 10.1186/s13643-017-0454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noyes J, Booth A, Moore G, et al. Synthesising quantitative and qualitative evidence to inform guidelines on complex interventions: clarifying the purposes, designs and outlining some methods. BMJ Glob Health 2019;4:e000893. 10.1136/bmjgh-2018-000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tricco AC, Antony J, Zarin W, et al. A scoping review of rapid review methods. BMC Med 2015;13:224. 10.1186/s12916-015-0465-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akers J. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. Layerthorpe, York: Centre for Reviews and Dissemination, University of York; 2009. [Google Scholar]
- 19.Lizarondo L, Stern C, Carrier J. Chapter 8: Mixed Methods Systematic Reviews. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis: JBI, 2020. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7:16. 10.1186/1472-6947-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.JBI . Critical Appraisal Checklist for Systematic Reviews and Research Syntheses. In: Secondary critical appraisal checklist for systematic reviews and research syntheses, 2020. https://jbi.global/critical-appraisal-tools [Google Scholar]
- 23.VassarStats: website for statistical computation. Secondary VassarStats: website for statistical computation, 2001-2021 RL. Available: http://vassarstats.net/odds2x2.html
- 24.Borenstein M, Hedges LV, Higgins JPT. Introduction to meta-analysis. Wiley, 2021. [Google Scholar]
- 25.Doi SAR, Barendregt JJ, Khan S, et al. Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials 2015;45:130–8. 10.1016/j.cct.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 26.Cochrane Handbook for Systematic Reviews of Interventions . Version 6.2, 2021. In: Julian Higgins JT, ed. Copyright © 2022 The Cochrane Collaboration, 2022. [Google Scholar]
- 27.Braun V, Clarke V. Reflecting on reflexive thematic analysis. Qual Res Sport Exerc Health 2019;11:589–97. 10.1080/2159676X.2019.1628806 [DOI] [Google Scholar]
- 28.Ruser CB, Sanders L, Brescia GR, et al. Identification and management of overweight and obesity by internal medicine residents. J Gen Intern Med 2005;20:1139–41. 10.1111/j.1525-1497.2005.0263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melamed OC, Nakar S, Vinker S. Suboptimal identification of obesity by family physicians. Am J Manag Care 2009;15:619–24. [PubMed] [Google Scholar]
- 30.Gutiérrez Angulo ML, Amenabar Azurmendi MD, Cuesta Solé ML, et al. Prevalence of obesity recorded in primary care. Endocrinol Nutr 2014;61:469–73. 10.1016/j.endoen.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 31.Bleich SN, Pickett-Blakely O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient Educ Couns 2011;82:123–9. 10.1016/j.pec.2010.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cyr PR, Haskins AE, Holt C, et al. Weighty problems: predictors of family physicians documenting overweight and obesity. Fam Med 2016;48:217–21. [PubMed] [Google Scholar]
- 33.Gonzalez-Chica DA, Bowden J, Miller C, et al. Patient-reported GP health assessments rather than individual cardiovascular risk burden are associated with the engagement in lifestyle changes: population-based survey in South Australia. BMC Fam Pract 2019;20:1–10. 10.1186/s12875-019-1066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattar A, Carlston D, Sariol G, et al. The prevalence of obesity documentation in primary care electronic medical records. are we acknowledging the problem? Appl Clin Inform 2017;8:67–79. 10.4338/ACI-2016-07-RA-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoong SL, Carey ML, Sanson-Fisher RW, et al. A cross-sectional study examining Australian general practitioners' identification of overweight and obese patients. J Gen Intern Med 2014;29:328–34. 10.1007/s11606-013-2637-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aleem S, Lasky R, Brooks WB, et al. Obesity perceptions and documentation among primary care clinicians at a rural academic health center. Obes Res Clin Pract 2015;9:408–15. 10.1016/j.orcp.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 37.Booth HP, Prevost AT, Gulliford MC. Epidemiology of clinical body mass index recording in an obese population in primary care: a cohort study. J Public Health 2013;35:67–74. 10.1093/pubmed/fds063 [DOI] [PubMed] [Google Scholar]
- 38.Bramlage P, Wittchen H-U, Pittrow D, et al. Recognition and management of overweight and obesity in primary care in Germany. Int J Obes Relat Metab Disord 2004;28:1299–308. 10.1038/sj.ijo.0802752 [DOI] [PubMed] [Google Scholar]
- 39.Dalton ARH, Bottle A, Okoro C, et al. Implementation of the NHS health checks programme: baseline assessment of risk factor recording in an urban culturally diverse setting. Fam Pract 2011;28:34–40. 10.1093/fampra/cmq068 [DOI] [PubMed] [Google Scholar]
- 40.Emanuel G, Charlton J, Ashworth M, et al. Cardiovascular risk assessment and treatment in chronic inflammatory disorders in primary care. Heart 2016;102:1957–62. 10.1136/heartjnl-2016-310111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose SA, Turchin A, Grant RW, et al. Documentation of body mass index and control of associated risk factors in a large primary care network. BMC Health Serv Res 2009;9:236. 10.1186/1472-6963-9-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baer HJ, Karson AS, Soukup JR, et al. Documentation and diagnosis of overweight and obesity in electronic health records of adult primary care patients. JAMA Intern Med 2013;173:1648–52. 10.1001/jamainternmed.2013.7815 [DOI] [PubMed] [Google Scholar]
- 43.Cuccu Z, Abi-Aad G, Duggal A. Characteristics of patients with body mass index recorded within the Kent integrated dataset (KID). BMJ Health Care Inform 2019;26:e000026. 10.1136/bmjhci-2019-000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghosh A. Depressed, anxious and breathless missing out: weight screening in general practice in a regional catchment of new South Wales. Aust J Rural Health 2016;24:246–52. 10.1111/ajr.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mocarski M, Tian Y, Smolarz BG, et al. Use of international classification of diseases, ninth revision codes for obesity: trends in the United States from an electronic health Record-Derived database. Popul Health Manag 2018;21:222–30. 10.1089/pop.2017.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osborn DPJ, Baio G, Walters K, et al. Inequalities in the provision of cardiovascular screening to people with severe mental illnesses in primary care: cohort study in the United Kingdom thin primary care database 2000-2007. Schizophr Res 2011;129:104–10. 10.1016/j.schres.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 47.McHale CT, Laidlaw AH, Cecil JE. Primary care patient and practitioner views of weight and weight-related discussion: a mixed-methods study. BMJ Open 2020;10:e034023. 10.1136/bmjopen-2019-034023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaynor B, Habermann B, Wright R. Waist circumference measurement diffusion in primary care. J Nurse Pract 2018;14:683–8. 10.1016/j.nurpra.2018.06.002 [DOI] [Google Scholar]
- 49.Rigas G, Williams K, Sumithran P, et al. Delays in healthcare consultations about obesity - Barriers and implications. Obes Res Clin Pract 2020;14:487–90. 10.1016/j.orcp.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 50.Vuorinen A-L, Helander E, Pietilä J, et al. Frequency of self-weighing and weight change: cohort study with 10,000 smart scale users. J Med Internet Res 2021;23:e25529. 10.2196/25529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warr W, Aveyard P, Albury C, et al. A systematic review and thematic synthesis of qualitative studies exploring GPs' and nurses' perspectives on discussing weight with patients with overweight and obesity in primary care. Obes Rev 2021;22:e13151. 10.1111/obr.13151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes J. Tackling obesity; 2021.
- 53.Gordon J, Valenti L, Bayram C, et al. An analysis of general practice encounters by socioeconomic disadvantage. Aust Fam Physician 2016;45:702–5. [PubMed] [Google Scholar]
- 54.Johnson RL, Roter D, Powe NR, et al. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health 2004;94:2084–90. 10.2105/AJPH.94.12.2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief 2020;360:1–8. [PubMed] [Google Scholar]
- 56.Fink M, Klein K, Sayers K, et al. Objective data reveals gender preferences for patients' primary care physician. J Prim Care Community Health 2020;11:215013272096722. 10.1177/2150132720967221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atlantis E, John JR, Fahey PP, et al. Clinical usefulness of brief screening tool for activating weight management discussions in primary cARE (aware): a nationwide mixed methods pilot study. PLoS One 2021;16:e0259220. 10.1371/journal.pone.0259220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med 2020;26:485–97. 10.1038/s41591-020-0803-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063659supp001.pdf (596.6KB, pdf)
Data Availability Statement
No data are available. Not applicable.