Summary
Oral health is one of the necessary preludes to the overall quality of life. Several medical procedures and therapies are available to treat oral diseases in general and periodontal diseases in particular, yet caries, periodontitis, oral cancer, and oral infections remain a global concern. Natural molecules, with their anti-oxidant, anti-inflammatory, and anti-microbic properties, are one of the main sources of oral health and dental health care, and should be supplemented to exploit their beneficial effects. A possible way to improve the intake of these molecules is adhering to a diet that is rich in fruits, vegetables, and probiotics, which has many beneficial properties and can improve overall health and wellbeing. The Mediterranean diet, in particular, provides several beneficial natural molecules, mainly because of the precious nutrients contained in its typical ingredients, mainly plant-based (olives, wine, citrus fruits, and many more). Its beneficial effects on several diseases and in increasing the overall wellbeing of the population are currently being studied by physicians. Among its nutrients, polyphenols (including, among other molecules, lignans, tannins, and flavonoids) seem to be of outmost importance: several studies showed their anticariogenic properties, as well as their effects in decreasing the incidence of non-communicable diseases. Therefore, plant-derived molecules – such as polyphenols – and probiotics – such as Lactobacillus reuteri – have shown a significant potential in treating and curing oral diseases, either alone or in combination, owing to their antioxidant and antimicrobial properties, respectively.
Keywords: Mediterranean diet, Polyphenols, Oral health, Lactobacillus reuteri
Citation
How to cite this article: Naureen Z, Medori MC, Dhuli K, Donato K, Connelly ST, Bellinato F, Gisondi P, Bertelli M. Polyphenols and Lactobacillus reuteri in oral health. J Prev Med Hyg 2022;63(suppl.3):E246-E254. https://doi.org/10.15167/2421-4248/jpmh2022.63.2S3.2767
Introduction
Plant-based foods, such as the typical fruits and vegetables of Mediterranean diet, are rich in many important phytochemicals that confer several health benefits. Among these, an important role is played by Polyphenols, a group of chemicals having at least one phenol moiety, which are particularly beneficial for human health [1]. Dietary polyphenols are a diverse group of phytochemicals, having approximately 8000 types of phenolic structures that are naturally present in cereals, fruits, vegetables, and beverages; based on the number of phenolic rings they contain, they can be classified into five subclasses, which are: phenolic acids, lignans, tannins, stilbenes, and flavonoids (Fig. 1, Tab. I) [2]. Epidemiological studies have shown that polyphenols – more specifically flavonoids – have strong antioxidant, anti-inflammatory, anti-cancerous, and anticariogenic properties [3], which can reduce the severity and incidence of non-communicable diseases such as diabetes, cancer, and cardiovascular problems.
Fig. 1.
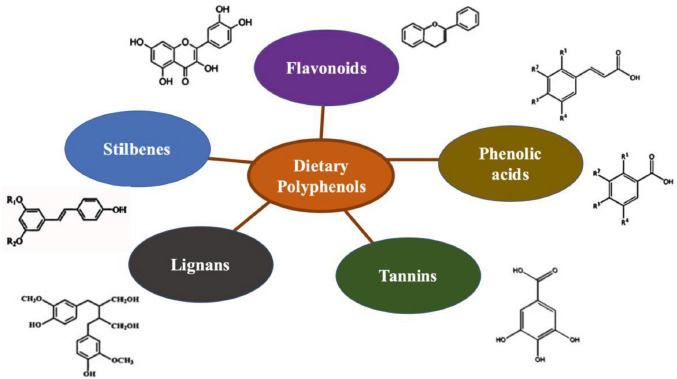
Classification of dietary polyphenols.
Tab. I.
Classes and properties of polyphenols.
| Polyphenols | Subclasses and examples | Food sources | Health benefits | References |
|---|---|---|---|---|
| Phenolic acids | Benzoic acid and cinnamic acid derivatives (e.g. caffeic acid) | Cherries, black radish, onion, kiwi, berries, coffee | Antimicrobial, anticancer, anti-inflammatory, anti-mutagenic | [22] |
| Flavonoids | Flavonols (e.g. quercetin) | Leek, ginger, broccoli, onion, leafy greens, berries, tea | Antioxidant and radical-scavenging activities, inhibiting cell migration of hepatocyte growth factor-induced medulloblastoma | [23] |
| Flavanols (e.g. catechins) | Grapes, chocolate, red wine, cocoa, apricots, black beans, green tea | Antioxidants, antimalarial, anticancer, antiviral, anti-inflammatory, anti-allergenic, UV protective | [24] | |
| Flavanones (e.g. hesperetin) | Citrus fruits (orange, grapefruit, lemon) and their juices | Antioxidative e anti-inflammatory. Ameliorate memory impairment and Aβ pathology | [25] | |
| Flavones (e.g. luteolin) | Oregano, celery, parsley, capsicum pepper | Neuroprotective, cardioprotective, antioxidant, anti-inflammatory, antiallergic | [26] | |
| Isoflavonones (e.g. genistein) | Milk, tofu, soy, tempeh miso | Anti-cancerous activity by inhibiting DNA topoisomerases and tyrosine kinases and inducing apoptosis, modulating PI3K/Akt and Wnt/β-catenin signal conduction | [27] | |
| Anthocyanidins (e.g. delphinidin) | Aubergine, red cabbage, rhubarb, red wine, black grapes, berries, cherries | Antioxidative, antidiabetic, anti-inflammatory | [28] | |
| Tannins | Dense tannins (e.g. pro-cyanidins) | Cocoa, chocolate, apples, grapes | Prevention, delay in the onset and treatment of cardiovascular diseases | [29] |
| Hydrolyzable tannins (e.g., gallotannins) | Mango, pomegranate | Anti-inflammatory activity | [30] | |
| Stilbenes | Resveratrol | Grapes, wine | Cardioprotective, antioxidant, antiplatelet, anti-inflammatory, anticancer activities, lowering blood glucose levels | [31] |
| Phenolic alcohols | Hydroxytyrosol | Olive | Antioxidant, anti-inflammatory, anticancer, protecting skin and eyes | [32, 33] |
PHENOLIC ACIDS
Abundantly present in fruits and vegetables, phenolic acids are derivatives of cinnamic and benzoic acids. Hydroxycinnamic acids (such as ferulic, p-coumaric, caffeic, rosmarinic, chlorogenic, and sinapic acids) are more common than hydroxybenzoic acids, which for example are contained in high amounts in black radish, onions, and red fruits [4]; hydroxybenzoic acids include gallic, syringic, vanillic, protocatechuic, and salicylic acids. The major dietary sources of phenolic acids include cocoa, wholegrains, fruits, nuts, coffee, and beer [5]. Observational studies have shown an inverse relationship between phenolic acid consumption and non-communicable diseases, such as metabolic syndrome [6], type-2 diabetes [7], hypertension [8], and non-alcoholic fatty liver disease [9]. In addition, phenolic acid consumption had a profound effect in the management of depressive disorders and in sleep quality [10, 11]. However, studies related to cardiovascular effects of these phenolic acids are scarce.
FLAVONOIDS
Flavonoids are perhaps the most powerful antioxidants naturally present in plants. They can be structurally distinguished by the presence of a di-phenyl-propane moiety (C6-C3-C6) and can be further classified into isoflavones, flavones, flavanones, flavonols, anthocyanidins, and flavanols [12]. Flavonoids have strong antioxidant, antimutagenic, anti-proliferative (against tumor cells), cardioprotective, radio-protective, antiatherosclerosis, and antimicrobial properties. In addition, these molecules also help in maintaining hormonal balance in menopausal women [13-15]. Among them, quercetin and genistein manifest a highly beneficial property of inhibiting ATP binding to tyrosine kinases, thus preventing proliferative diseases like cancer and psoriasis [15].
STILBENES
This class of flavonoids is present in low amounts in various food sources, but some of its members are widely studied because their intake brings a myriad of health benefits [16]. For instance, resveratrol, which is contained in high quantities in red grapes and grape juice (both fermented and non-fermented), manifests antioxidant, anti-inflammatory, antibacterial, and anticancer properties [17, 18]. Besides being a strong antioxidant, resveratrol also has antidiabetic and cardioprotective activity and, because of its several molecular targets, its usage is very promising in the development of novel remedial approaches against metabolic syndrome, atherosclerosis, ischemic heart disease, and heart failure [19].
TANNINS
Tannins are complex, water-soluble phenolic compounds derived from phenolic acids. They have a strong free radical-scavenging capability, which gives them antimutagenic and antibacterial activities. An example of powerful anticarcinogenic tannins are Ellagitannins derivatives, such as Ellagic acid, which can be found in many fruits and nuts like cranberries, strawberries, red grapes, raspberries, pomegranates, peaches, walnuts, and pecans [20].
LIGNANS
Lignans are diphenolic compounds, defined as phytoestrogens, which are derived from phenolic acids by the dimerization of two cinnamic acid residues. Lignans confer several health benefits, such as lowering the risk of cancer and cardiovascular diseases. In women, lignans help in alleviating the symptoms of menopause and osteoporosis. Wholegrain cereals and seeds – such as linseed, flaxseed and legumes – are rich sources of lignans [21].
Potential Effects of Polyphenols on Health
Polyphenols manifest a wide variety of health benefits, owing to their antioxidant, immunomodulator, anti-inflammatory, and radical-scavenging properties. For instance, resveratrol, curcumin, and epigallocatechin gallate (EGCG) have neuroprotective properties against neurodegenerative diseases (e.g. Alzheimer’s-like diseases and dementia). Furthermore, these polyphenols inhibit the neurotoxic effects of the beta-amyloid protein that accumulated due to Alzheimer’s disease [34, 35]. Moreover, the iron-chelating effects of EGCG, ginkgetin, curcumin, ginsenosides, and myricetin prevent neurotoxicity, thus protecting against Alzheimer’s, Parkinson’s, and Huntington’s [35, 36].
Besides that, phenolic compounds manifest strong anti-inflammatory properties against systemic and localized inflammation by mitigating the cytokine pathway and reducing oxidative stress [37]. For instance, flavonoid-rich foods and resveratrol reduce inflammation, lower blood pressure, reduce platelet activity, block cholesterol oxidation, reduce LDL, and improve ventricular health, thereby preventing cardiovascular diseases [38].
The free radical-scavenging activity of Flavonoids such as catechins, flavanols, flavones, anthocyanins, flavanones, and isoflavones, prevents cellular growth in tumours, thereby decreasing the risk of oncogenesis [39]. Polyphenols have been observed to be beneficial in breast, endometrial, colon, prostate, and epithelial cancer [40].
Several polyphenols can inhibit lipid, starch, and protein digestion in the gastrointestinal tract by binding to the respective digestive enzymes, thus inhibiting it [41]. For instance, anthocyanins slow down the digestion of starch and can regulate and alter glucose transport, thus providing better glycaemic control in type 2 diabetes [42, 43]. Curcumin, catechins, and resveratrol exhibit anti-obesogenic effects by reducing inflammation, inhibiting lipogenesis, oxidating adipocyte and increasing energy expenditure, thus resulting in enhanced weight loss and improved weight maintenance [41].
In addition to that, polyphenols have been indicated in wound healing, but perhaps the most apparent effect of polyphenols is maintaining oral health. Several researchers have reported health benefits of polyphenols in relieving periodontal diseases, maintaining oral health, and preventing oral cancer.
Polyphenols in the oral cavity
Oral mucosa is continuously under food- and environment-mediated oxidative stress. Polyphenols come in contact to the oral cavity directly and, due to their antioxidant and antimicrobial properties, prevent several diseases of the oral cavity, from infections to cancers [44]. In addition, polyphenols can be used as “processing cofactors” to enhance the mechanical and functional characteristics of the biomaterials that are used in dental tissue engineering [45]. For instance, grape seed extract, which is rich in proanthocyanidins (PAs), has been used in various dental applications, e.g. resin-dentin binding, because of its dual action of cross-linking with the collagen and of inhibiting the metalloprotein [46].
Polyphenols make stable complexes with proline-rich proteins and histatins in the oral cavity, which remain stable during their course from the oral cavity to the gastrointestinal tract and so on [47].
Polyphenols play multiple roles in dental diseases: for example, some polyphenol hydroxyls are very reactive, while others are very protective against microorganisms, yet another group acts as disinfectants by producing hydrogen peroxide and inhibiting bacterial proteins and enzymes [48]. This antimicrobial activity of polyphenols is concentration-dependent: for instance, at low concentration polyphenols interfere with specific sites, thus inhibiting the enzymes, whereas at high concentrations they cause enzyme denaturation [49]. In addition, polyphenols such as flavonoids affect the bacterial membrane permeability by interacting with membrane proteins, enzymes, and lipids, thus mediating loss of macromolecules, protons and ions [50].
Polyphenols in Oral cancer
Oral cancer is one of the most important medical issues and necessitates the development of effective strategies to reduce its incidence and mortality rates. Polyphenols are well known for their anti-cancerous activities against various types of cancers, based on their capability to inhibit enzymes and tumour development [51]. They are also known for their roles in preventing oxidative stress and DNA damage, modulating carcinogens metabolism and inhibiting DNA adduct formation [52-54]. For instance, catechins extracted from tea induce apoptosis, arrest cell growth, inhibit metalloproteinase synthesis, reduce metastasis risk by inhibiting the invasion and proliferation in both oral lekoplakia cell lines and in oral cancer [54, 55].
Two different case control studies, conducted in Uruguay and Italy, have reported an inverse relationship between flavonoids consumption and risk of oral cancer [56]. The Italian study demonstrated a significant inverse relationship of flavanones, flavonols, and total flavonoids, with risk of oral cancers, while this is not the case with isoflavones, anthocyanidins, flavan-3-ols, and flavones [57]. This indicates that different types of polyphenols have different effects on the risk and development of oral cancers. For instance, some polyphenols are particularly useful in human papillomavirus-mediated oral cancers because they considerably reduce the development of HPV-induced cancers by inhibiting DNA adduct formation and reducing cell proliferation, as well as preventing the invasion of affected cells into unaffected cells, exhibiting cytotoxic activity, apoptosis induction, and cell differentiation [58].
Polyphenols in Dental Caries and Periodontal Diseases
Containing over 700 bacterial species, the oral cavity is one of the most complex microbial ecosystems of the human body [59]. Integrated in an extracellular matrix of polysaccharides, the dental biofilm formed on hard and soft tissues of the oral cavity (containing food debris, epithelial cells, proteins, enzymes, and microbial cells) is the source of dental caries and periodontal diseases – the two main problems of oral cavity of bacterial origin [60]. For instance, the cariogenic Streptococcus mutans and Streptococcus sobrinus ferment sugars contained in food particles stuck to the teeth or other parts of the oral cavity, resulting in the production of organic acids that reduce the pH to 5.5, demineralising tooth enamel and thus causing dental caries. Dental caries is the most common infectious, multifactorial disease that results in the dissolution of tooth enamel [61]. Therefore, fruits and vegetables (and their extracts) that are rich in polyphenols not only reduce the number of harmful pathogens in the oral cavity, but also maintain oral hygiene (Tab. II).
Tab. II.
Beneficial effects of polyphenols in oral cavity pathogens.
| Polyphenols/food sources | Beneficial effects | References |
|---|---|---|
| Tea Polyphenols | Inhibition of glucosyltransferase (GTF), acid production, adherence to hard surfaces | [62] |
| Polymeric polyphenols | Inhibition of Streptococcus mutans polysaccharide synthesis | [63] |
| Extracts of unfermented cocoa, epicatechin, red grape seed, and green tea | Bacteriostatic against S. mutans, inhibit acid production, and reduce adherence of the bacterium against glass | [64] |
| Tannins from grapes | Salivary alpha-amylase inhibition in humans | [65] |
| Cocoa flavonols | Enhance interleukin 5 secretion by peripheral blood mononuclear cells and trigger IgA production against S. mutans | [66] |
| Oolong tea extract and its polyphenols | Significant reductions in caries and plaque development in rats infected with S. mutans | [67] |
| Barley coffee, coffee, wine, and tea | High consumption accompanies lower Lactobacilus sp. and S. mutans in plaque and saliva, thus lowering dental plaque scores | [68] |
| Oolong tea extract | Cariostatic activity against S. sorbinus in the oral cavity | [69] |
| Hydroxytyrosol from olives and olive tree | Reduces the viral load of the oral and nasal cavity mucosa during SARS-CoV-2 infections | [70-73] |
Periodontal diseases are the diseases of the tissue that supports and surrounds the teeth; they can manifest episodically. Periodontal diseases can be further classified into gingivitis and periodontitis, which result in the inflammation of the free gingiva and the progressive destruction of all of the tooth-supporting tissue, including periodontal ligament and alveolar bone, respectively. These diseases follow a pattern of active destruction, latency and healing periods, and are dependent on accumulation of gram-negative anaerobes in the subgingival region and immune-destructive response of the host.
Dietary polyphenols play an important role in preventing the disequilibrium between oxidative stress and antioxidant activities in the oral cavity, thereby preventing periodontal tissue destruction. This implicates the effectiveness of these dietary phytochemicals in fighting periodontal diseases, especially when the oral cavity is exposed to oxidative stress from environment and food sources. For instance, tea polyphenols enhance the antioxidant capacity of saliva if the (green or black) tea is held in the mouth for 2-3 minutes. Localised application of fruit extracts prevents biofilm formation and reduces pocket depth. Furthermore, increased phagocytic activity of polymorphonuclear leucocytes in the gingival crevicular fluid was observed upon daily intake of two fresh grapefruits for 2 weeks. The effects of dietary polyphenols on oral cavity diseases are presented in Figure 2.
Fig. 2.
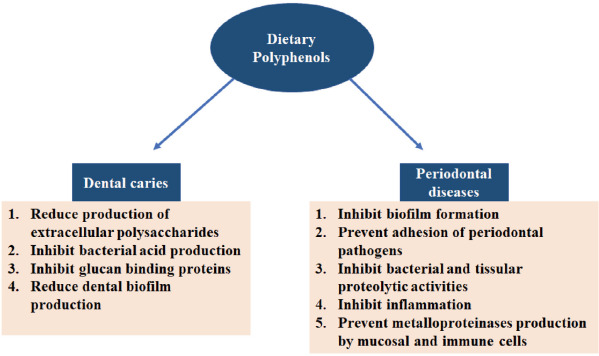
Effect of dietary polyphenols on dental caries and periodontal diseases.
Perhaps the most widely studied polyphenols in connection to periodontal conditions are those from various kinds of tea: they not only enhance the proliferation of human periodontal ligament fibroblasts, but also inhibit virulence manifestation of the periodontic anaerobic pathogen Porphyromonas gingivalis. Therefore, regular consumption of polyphenol-rich diets presents an effective method of fighting periodontal diseases.
Lactobacillus reuteri in oral health
Lactobacillus reuteri (L. reuteri) is one of the most widely studied bacteria, having a huge repertoire of beneficial effects on human health. It colonizes a variety of niches in human body such as the oral cavity, the gastrointestinal tract, the skin and the urinary tract [74]. Owing to its antimicrobial activities (production of antimicrobial organic acids, ethanol, and reuterin), L. reuteri can inhibit the colonization of pathogenic microbes in its vicinity and even reshape the microbial communities in the host [75]. Second, L. reuteri can have beneficial impact on the immune system of the host. For instance, it has strong immunomodulatory and anti-inflammatory properties, as manifested by the synthesis of pro-inflammatory cytokines and promotion of regulatory T cell growth and function and strengthening of the intestinal barrier, which prevents transfers of gut microbiota from the lumen to tissues [76]. As periodontal diseases are caused by bacteria inhabiting these niches, one of the basic methods of prevention and cure is to decrease the pathogen load by scaling and root planning (SRP), involving the probiotic bacteria. Alternatively, deploying probiotic bacterial strains as a method of biological control of pathogenic ones holds promise in treating the periodontal diseases. Many studies have shown the efficacy of the probiotic bacterium Lactobacillus reuteri Prodentis (LrP) as a useful therapeutic supplement, as part of periodontal maintenance regime post-intervention. This not only replenishes the oral cavity with useful bacteria, but also decreases the harmful ones. For instance, daily oral administration of L. reuteri strains – DSM 17938 and PTA 5289 – in human subjects for 12 weeks resulted in changes in oral microbiota in a randomized controlled trial, while keeping the bacterial species richness unaltered throughout the duration of the trial [77]. In addition, oral L. reuteri treatment suppressed the growth of periodontal pathogens in the subgingival microbiota [78]. Studies have also shown that Lactobacillus reuteri Prodentis can sustainably increase the population of beneficial bacteria in the oral cavity, thus restoring the natural oral flora lost during infection [79].
Several in vitro studies showed L. reuteri’s inhibitory effects on periodontopathogens, which are likely due to its by-products: an example is reuterin, a non-protein broad-spectrum antibiotic that can inhibit the growth of a variety of gram-positive/negative bacteria, yeast, and fungi [80]. Many periodontopathogens, such as P. gingivalis ATCC 33277, P. intermedia ATCC 25611, and F. nucleatum ATCC 25586, are effectively inhibited by L. reuteri ATCC PTA 5289, except for A. actinomycetemcomitans ATCC 33384 [81].
Both live L. reuteri PTA 5289 and DSM 17938 and their CFS show inhibition on P. gingivalis ATCC 33277 and F. nucleatum ATCC 25586; however, only the live form of the two L. reuteri inhibited the growth of A. actinomycetemcomitans ATCC 29522 in vitro [82, 83]. Another subspecies, L. reuteri ATCC 55730, inhibited the growth of F. nucleatum ATCC 10953, P. gingivalis ATCC 33277, and A. actinomycetemcomitans ATCC 33384, also preventing the mortality of HOK cells infected with periodontal pathogens [84].
Exopolysaccharide (EPS) is a substance that L. reuteri DSM 17938 produces to enhance in adherence to epithelial cells and to compete with pathogenic bacteria for adhesion sites [85]. The release of IL-6 triggered by F. nucleatum in KB cells was shown to be suppressed by L. reuteri KCTC 3594. L. reuteri was used in clinical trials to inhibit P. gingivalis, supragingival plaque, subgingival plaque, and P. intermedia in saliva [86, 87]. In patients with peri-implant mucositis, L. reuteri DSM 17938 and PTA 5289 for peri-implant diseases could only reduce the load of P. gingivalis [88]. In animal investigations, it was discovered that live L. reuteri DSM 17938 and PTA 5289 improved immune reactions and increased hemocyte density in Galleria mellonella infected with P. gingivalis ATCC 33277 [81, 82]. The immunomodulatory properties of L. reuteri may help to manage the imbalance between MMP and TIMP or to inhibit the effectiveness of pro-inflammatory cytokines, which may minimize the inflammation and degeneration of periodontal tissues [89]. This implicates that L. reuteri has a repertoire of beneficial traits that can be deployed to successfully manage periodontal pathogens and subgingival microbiota.
Conclusion
The benefits of polyphenols and probiotics in management and cure implicate that these molecules should be given due consideration in terms of clinical evaluation. Although there are numerous in vitro and in vivo studies demonstrating the potential benefits of polyphenols in oral health, strong evidence from well-designed clinical trials is still lacking. On the other hand, L. reuteri is already well known for its probiotic effects and bio-antagonism against oral pathogens. It would be interesting to see how these two can act synergistically in combating oral diseases and maintaining oral health.
Acknowledgements
This research was funded by the Provincia Autonoma di Bolzano in the framework of LP 15/2020 (dgp 3174/2021).
Conflicts of interest statement
Authors declare no conflict of interest.
Author's contributions
MB: study conception, editing and critical revision of the manuscript; ZN, MCM, Kristjana D, Kevin D, STC, FB, PG: literature search, editing and critical revision of the manuscript. All authors have read and approved the final manuscript.
Figures and tables
References
- [1].Di Meo F, Valentino A, Petillo O, Peluso G, Filosa S, Crispi S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int J Mol Sci 2020;21:2564. https://doi.org/10.3390/ijms21072564 10.3390/ijms21072564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds–nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric 2000;80:1094-117. https://doi.org/10.1002/(SICI)1097-0010(20000515)80:7<1094::AID-JSFA569>3.0.CO;2-1 [DOI] [Google Scholar]
- [3].Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front Nutr 2018;5:87. https://doi.org/10.3389/fnut.2018.00087 10.3389/fnut.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lafay S, Gil-Izquierdo A. Bioavailability of phenolic acids. Phytochem Rev 2008;7:301-11. https://doi.org/10.1007/s11101-007-9077-x 10.1007/s11101-007-9077-x [DOI] [Google Scholar]
- [5].Román GC, Jackson RE, Gadhia R, Román AN, Reis J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol (Paris) 2019;175:724-41. https://doi.org/10.1016/j.neurol.2019.08.005 10.1016/j.neurol.2019.08.005 [DOI] [PubMed] [Google Scholar]
- [6].Sohrab G, Hosseinpour-Niazi S, Hejazi J, Yuzbashian E, Mirmiran P, Azizi F. Dietary polyphenols and metabolic syndrome among Iranian adults. Int J Food Sci Nutr 2013;64:661-7. https://doi.org/10.3109/09637486.2013.787397 10.3109/09637486.2013.787397 [DOI] [PubMed] [Google Scholar]
- [7].Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X, Kong M, Li L, Zhang Q, Liu Y, Chen H. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016;21:1374. https://doi.org/10.3390/molecules21101374 10.3390/molecules21101374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alam MA. Anti-hypertensive effect of cereal antioxidant ferulic acid and its mechanism of action. Front Nutr 2019;6:121. https://doi.org/10.3389/fnut.2019.00121 10.3389/fnut.2019.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Salomone F, Godos J, Zelber-Sagi S. Natural antioxidants for non-alcoholic fatty liver disease: molecular targets and clinical perspectives. Liver Int 2016;36:5-20. https://doi.org/10.1111/liv.12975 10.1111/liv.12975 [DOI] [PubMed] [Google Scholar]
- [10].Naveen S, Siddalingaswamy M, Singsit D, Khanum F. Anti-depressive effect of polyphenols and omega-3 fatty acid from pomegranate peel and flax seed in mice exposed to chronic mild stress. Psychiatry Clin Neurosci 2013;67:501-8. https://doi.org/10.1111/pcn.12100 10.1111/pcn.12100 [DOI] [PubMed] [Google Scholar]
- [11].Godos J, Ferri R, Castellano S, Angelino D, Mena P, Del Rio D, Caraci F, Galvano F, Grosso G. Specific dietary (poly) phenols are associated with sleep quality in a cohort of Italian adults. Nutrients 2020;12:1226. https://doi.org/10.3390/nu12051226 10.3390/nu12051226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lima GP, Vianello F, Corrêa CR, Campos RA, Borguini MG. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr Sci 2014:1065-82. https://doi.org/10.4236/fns.2014.511117 10.4236/fns.2014.511117 [DOI] [Google Scholar]
- [13].Segura Campos MR. Bioactive compounds, 1st Edition. Woodhead Publishing; 2019. https://doi.org/10.1016/C2017-0-02265-6 10.1016/C2017-0-02265-6 [DOI] [Google Scholar]
- [14].Kiani AK, Falsini B, Ziccardi L, Gusson E, Mangialavori D, Allegrini F, Colao E, Bertelli M. Flavonoid supplements increase neurotrophin activity to modulate inflammation in retinal genetic diseases. Acta Biomed 2020;91:e2020014. https://doi.org/10.23750/abm.v91i13-S.10683 10.23750/abm.v91i13-S.10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huntley AL. The health benefits of berry flavonoids for menopausal women: cardiovascular disease, cancer and cognition. Maturitas 2009;63:297-301. https://doi.org/10.1016/j.maturitas.2009.05.005 10.1016/j.maturitas.2009.05.005 [DOI] [PubMed] [Google Scholar]
- [16].Błaszczyk A, Sady S, Sielicka M. The stilbene profile in edible berries. Phytochem Rev 2019;18:37-67. https://doi.org/10.1007/s11101-018-9580-2 10.1007/s11101-018-9580-2 [DOI] [Google Scholar]
- [17].Bertelli AA, Das DK. Grapes, wines, resveratrol, and heart health. Journal of cardiovascular pharmacology 2009;54:468-76. https://doi.org/10.1097/FJC.0b013e3181bfaff3 10.1097/FJC.0b013e3181bfaff3 [DOI] [PubMed] [Google Scholar]
- [18].Perrone D, Fuggetta MP, Ardito F, Cottarelli A, De Filippis A, Ravagnan G, De Maria S, Lo Muzio L. Resveratrol (3, 5, 4’-trihydroxystilbene) and its properties in oral diseases. Exp Ther Med 2017;14:3-9. https://doi.org/10.3892/etm.2017.4472 10.3892/etm.2017.4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rauf A, Imran M, Suleria HA, Ahmad B, Peters DG, Mubarak MS. A comprehensive review of the health perspectives of resveratrol. Food Funct 2017;8:4284-305. https://doi.org/10.1039/c7fo01300k 10.1039/c7fo01300k [DOI] [PubMed] [Google Scholar]
- [20].Basli A, Belkacem N, Amrani I. Health benefits of phenolic compounds against cancers. Phenolic compounds-biological activity. Available at: https://www.intechopen.com/chapters/54035. Accessed on: 07/07/2022.
- [21].Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009;2:270-8. https://doi.org/10.4161/oxim.2.5.9498 10.4161/oxim.2.5.9498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol Rep 2019;24:e00370. https://doi.org/10.1016/j.btre.2019.e00370 10.1016/j.btre.2019.e00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Labbé D, Provençal M, Lamy S, Boivin D, Gingras D, Béliveau R. The flavonols quercetin, kaempferol, and myricetin inhibit hepatocyte growth factor-induced medulloblastoma cell migration. J Nutr 2009;139:646-52. https://doi.org/10.3945/jn.108.102616 10.3945/jn.108.102616 [DOI] [PubMed] [Google Scholar]
- [24].Bae J, Kim N, Shin Y, Kim S-Y, Kim Y-J. Activity of catechins and their applications. Biomed Dermatol 2020;4:8. https://doi.org/10.1186/s41702-020-0057-8 10.1186/s41702-020-0057-8 [DOI] [Google Scholar]
- [25].Alam F, Mohammadin K, Shafique Z, Amjad ST, Asad MHHB. Citrus flavonoids as potential therapeutic agents: A review. Phytother Res 2022;36:1417-41. https://doi.org/10.1002/ptr.7261 10.1002/ptr.7261 [DOI] [PubMed] [Google Scholar]
- [26].Özcan Ö, Aldemir O, Karabulut B. Flavones (apigenin, luteolin, chrysin) and their importance for health. Mellifera 2020;20:16-27. [Google Scholar]
- [27].Senft D, Ronai ZE. Adaptive stress responses during tumor metastasis and dormancy. Trends in Cancer 2016;2:429-42. https://doi.org/10.1016/j.trecan.2016.06.004 10.1016/j.trecan.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Saulite L, Jekabsons K, Klavins M, Muceniece R, Riekstina U. Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes. Phytomedicine 2019;53:86-95. https://doi.org/10.1016/j.phymed.2018.09.029 10.1016/j.phymed.2018.09.029 [DOI] [PubMed] [Google Scholar]
- [29].Schroeter H, Heiss C, Spencer JP, Keen CL, Lupton JR, Schmitz HH. Recommending flavanols and procyanidins for cardiovascular health: current knowledge and future needs. Mol Aspects Med 2010;31:546-57. https://doi.org/10.1016/j.mam.2010.09.008 10.1016/j.mam.2010.09.008 [DOI] [PubMed] [Google Scholar]
- [30].Kiss AK, Piwowarski JP. Ellagitannins, gallotannins and their metabolites-the contribution to the anti-inflammatory effect of food products and medicinal plants. Curr Med Chem 2018;25:4946-67. https://doi.org/10.2174/0929867323666160919111559 10.2174/0929867323666160919111559 [DOI] [PubMed] [Google Scholar]
- [31].Kuršvietienė L, Stanevičienė I, Mongirdienė A, Bernatonienė J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016;52:148-55. https://doi.org/10.1016/j.medici.2016.03.003 10.1016/j.medici.2016.03.003 [DOI] [PubMed] [Google Scholar]
- [32].Martínez L, Ros G, Nieto G. Hydroxytyrosol: Health benefits and use as functional ingredient in meat. Medicines 2018;5:13. https://doi.org/10.3390/medicines5010013 10.3390/medicines5010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dhuli K, Ceccarini MR, Precone V, Maltese PE, Bonetti G, Paolacci S, Dautaj A, Guerri G, Marceddu G, Beccari T, Michelini S, Bertelli M. Improvement of quality of life by intake of hydroxytyrosol in patients with lymphedema and association of lymphedema genes with obesity. Eur Rev Med Pharmacol Sci 2021;25:33-42. https://doi.org/10.26355/eurrev_202112_27331 10.26355/eurrev_202112_27331 [DOI] [PubMed] [Google Scholar]
- [34].Orgogozo JM, Dartigues JF, Lafont S, Letenneur L, Commenges D, Salamon R, Renaud S, Breteler MB. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol 1997;153:185-92. [PubMed] [Google Scholar]
- [35].Di Meo F, Valentino A, Petillo O, Peluso G, Filosa S, Crispi S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int J Mol Sci 2020;21:2564. https://doi.org/10.3390/ijms21072564 10.3390/ijms21072564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jiang H, Wang J, Rogers J, Xie J. Brain iron metabolism dysfunction in Parkinson’s Disease. Mol Neurobiol 2017;54:3078-101. https://doi.org/10.1007/s12035-016-9879-1 10.1007/s12035-016-9879-1 [DOI] [PubMed] [Google Scholar]
- [37].Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci 2016;8:33-42. https://doi.org/10.1016/j.cofs.2016.02.002 10.1016/j.cofs.2016.02.002 [DOI] [Google Scholar]
- [38].Khurana S, Venkataraman K, Hollingsworth A, Piche M, Tai TC. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients 2013;5:3779-827. https://doi.org/10.3390/nu5103779 10.3390/nu5103779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen YM, Li HB. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016;8:515. https://doi.org/10.3390/nu8080515 10.3390/nu8080515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fujiki H, Sueoka E, Watanabe T, Suganuma M. Primary cancer prevention by green tea, and tertiary cancer prevention by the combination of green tea catechins and anticancer compounds. J Cancer Prev 2015;20:1-4. https://doi.org/10.15430/JCP.2015.20.1.1 10.15430/JCP.2015.20.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang S, Sun Z, Dong S, Liu Y, Liu Y. Molecular interactions between (–)-epigallocatechin gallate analogs and pancreatic lipase. PLoS ONE 2014;9:e111143. https://doi.org/10.1371/journal.pone.0111143 10.1371/journal.pone.0111143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xiao JB, Hogger P. Dietary polyphenols and type 2 diabetes: current insights and future perspectives. Curr Med Chem 2015;22:23-38. https://doi.org/10.2174/0929867321666140706130807 10.2174/0929867321666140706130807 [DOI] [PubMed] [Google Scholar]
- [43].Zhang B, Deng Z, Ramdath DD, Tang Y, Chen PX, Liu R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem 2015;172:862-72. https://doi.org/10.1016/j.foodchem.2014.09.144 10.1016/j.foodchem.2014.09.144 [DOI] [PubMed] [Google Scholar]
- [44].Kharouf N, Haikel Y, Ball V. Polyphenols in Dental Applications. Bioengineering 2020;7:72. https://doi.org/10.3390/bioengineering7030072 10.3390/bioengineering7030072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shavandi A, Bekhit AEA, Saeedi P, Izadifar Z, Bekhit AA, Khademhosseini A. Polyphenol uses in biomaterials engineering. Biomaterials 2018;167:91-106. https://doi.org/10.1016/j.biomaterials.2018.03.018 10.1016/j.biomaterials.2018.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Al-Ammar A, Drummond JL, Bedran-Russo AK. The use of collagen cross-linking agents to enhance dentin bond strength. J Biomed Mater Res B Appl Biomater 2009;91:419-24. https://doi.org/10.1002/jbm.b.31417 10.1002/jbm.b.31417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Skopec MM, Hagerman AE, Karasov WH. Do salivary proline-rich proteins counteract dietary hydrolysable tannin in laboratory rats? J Chem Ecol 2004;30:1679-92. https://doi.org/10.1023/b:joec.0000042395.31307.be 10.1023/b:joec.0000042395.31307.be [DOI] [PubMed] [Google Scholar]
- [48].Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents 2005;26:343-56. https://doi.org/10.1016/j.ijantimicag.2005.09.002 10.1016/j.ijantimicag.2005.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fung DYC, Taylor S, Kahan J. Effects of butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) on growth and aflatoxin production of Aspergillus flavus. J Food Saf 1977;1:39-51. https://doi.org/10.1111/j.1745-4565.1977.tb00258.x 10.1111/j.1745-4565.1977.tb00258.x [DOI] [Google Scholar]
- [50].Tamba Y, Ohba S, Kubota M, Yoshioka H, Yamazaki M. Single GUV method reveals interaction of tea catechin (-)-epigallocatechin gallate with lipid membranes. Biophys J 2007;92:3178-94. https://doi.org/10.1529/biophysj.106.097105 10.1529/biophysj.106.097105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Galati G, O’Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med 2004;37:287-303. https://doi.org/10.1016/j.freeradbiomed.2004.04.034 10.1016/j.freeradbiomed.2004.04.034 [DOI] [PubMed] [Google Scholar]
- [52].Hsu S, Lewis JB, Borke JL, Singh B, Dickinson DP, Caughman GB, Athar M, Drake L, Aiken AC, Huynh CT, Das BR, Osaki T, Schuster GS. Chemopreventive effects of green tea polyphenols correlate with reversible induction of p57 expression. Anticancer Res 2001;21:3743-8. [PubMed] [Google Scholar]
- [53].Khafif A, Schantz SP, Al-Rawi M, Edelstein D, Sacks PG. Green tea regulates cell cycle progression in oral leukoplakia. Head and Neck 1998;20:528-34. https://doi.org/10.1002/(sici)1097-0347(199809)20:6<528::aid-hed7>3.0.co;2-3 [DOI] [PubMed] [Google Scholar]
- [54].Masuda M, Suzui M, Weinstein IB. Effects of Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res 2001;7:4220-9. [PubMed] [Google Scholar]
- [55].Yang CS, Lambert JD, Sang S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch Toxicol 2009;83:11-21. https://doi.org/10.1007/s00204-008-0372-0 10.1007/s00204-008-0372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].De Stefani E, Ronco AL, Mendilaharsu M, Deneo-Pellegrini H. Diet and risk of cancer of the upper aerodigestive tract—II. Nutrients Oral Oncol 1999;35:22-6. https://doi.org/10.1016/S1368-8375(98)00061-X 10.1016/S1368-8375(98)00061-X [DOI] [PubMed] [Google Scholar]
- [57].Rossi M, Garavello W, Talamini R, Negri E, Bosetti C, Dal Maso L, Lagiou P, Tavani A, Polesel J, Barzan L, Ramazzotti V, Franceschi S, La Vecchia C. Flavonoids and the risk of oral and pharyngeal cancer: A case-control study from Italy. Cancer Epidemiol Biomark Prev 2007;16:1621-5. https://doi.org/10.1158/1055-9965.EPI-07-0168 10.1158/1055-9965.EPI-07-0168 [DOI] [PubMed] [Google Scholar]
- [58].Petti S, Scully C. Polyphenols, oral health and disease: a review. J Dent 2009;37:413-23. https://doi.org/10.1016/j.jdent.2009.02.003 10.1016/j.jdent.2009.02.003 [DOI] [PubMed] [Google Scholar]
- [59].Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005;43:5721-32. https://doi.org/10.1128/JCM.43.11.5721-5732.2005 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Marsh PD. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol 2005;326:7-15. https://doi.org/10.1111/j.1600-051X.2005.00790.x 10.1111/j.1600-051X.2005.00790.x [DOI] [PubMed] [Google Scholar]
- [61].Marsh PD. Dental plaque as a microbial biofilm. Caries Res 2004;38:204-11. https://doi.org/10.1159/000077756 10.1159/000077756 [DOI] [PubMed] [Google Scholar]
- [62].Hattori M, KUSUMOTO IT, NAMBA T, ISHIGAMI T, HARA Y. Effect of tea polyphenols on glucan synthesis by glucosyltransferase from Streptococcus mutans. Chem Pharm Bull (Tokyo) 1990;38:717-20. https://doi.org/10.1248/cpb.38.717 10.1248/cpb.38.717 [DOI] [PubMed] [Google Scholar]
- [63].Ren Z, Chen L, Li J, Li Y. Inhibition of Streptococcus mutans polysaccharide synthesis by molecules targeting glycosyltransferase activity. J Oral Microbiol 2016;8:31095. https://doi.org/10.3402/jom.v8.31095 10.3402/jom.v8.31095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Smullen J, Koutsou GA, Foster HA, Zumbé A, Storey DM. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007;41:342-9. https://doi.org/10.1159/000104791 10.1159/000104791 [DOI] [PubMed] [Google Scholar]
- [65].Kandra L, Gyémánt G, Zajácz Á, Batta G. Inhibitory effects of tannin on human salivary α-amylase. Biochem Biophys Res Commun 2004;319:1265-71. https://doi.org/10.1016/j.bbrc.2004.05.122 10.1016/j.bbrc.2004.05.122 [DOI] [PubMed] [Google Scholar]
- [66].Andújar I, Recio MC, Giner RM, Ríos J. Cocoa polyphenols and their potential benefits for human health. Oxid Med Cell Longev 2012;2012:906252. https://doi.org/10.1155/2012/906252 10.1155/2012/906252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ooshima T, Minami T, Aono W, Izumitani A, Sobue S, Fujiwara T, Kawabata S, Hamada S. Oolong tea polyphenols inhibit experimental dental caries in SPF rats infected with mutatis streptococci. Caries Res 1993;27:124-9. https://doi.org/10.1159/000261529 10.1159/000261529 [DOI] [PubMed] [Google Scholar]
- [68].Lolayekar N, Shanbhag C. Polyphenols and oral health. RSBO 2012;9:74-84. [Google Scholar]
- [69].Sasaki H, Matsumoto M, Tanaka T, Maeda M, Nakai M, Hamada S, Ooshima T. Antibacterial activity of polyphenol components in oolong tea extract against Streptococcus mutans. Caries Res 2004;38:2-8. https://doi.org/10.1159/000073913 10.1159/000073913 [DOI] [PubMed] [Google Scholar]
- [70].Naureen Z, Capodicasa N, Paolacci S, Anpilogov K, Dautaj A, Dhuli K, Camilleri G, Connelly ST, Gasparetto A, Bertelli M. Prevention of the proliferation of oral pathogens due to prolonged mask use based on α-cyclodextrin and hydroxytyrosol mouthwash. Eur Rev Med Pharmacol Sci 2021;25:74-80. https://doi.org/10.26355/eurrev_202112_27336 10.26355/eurrev_202112_27336 [DOI] [PubMed] [Google Scholar]
- [71].Paolacci S, Ergoren MC, De Forni D, Manara E, Poddesu B, Cugia G, Dhuli K, Camilleri G, Tuncel G, Kaya Suer H, Sultanoglu N, Sayan M, Dundar M, Beccari T, Ceccarini MR, Gunsel IS, Dautaj A, Sanlidag T, Connelly ST, Tartaglia GM, Bertelli M. In vitro and clinical studies on the efficacy of α-cyclodextrin and hydroxytyrosol against SARS-CoV-2 infection. Eur Rev Med Pharmacol Sci 2021;25:81-9. https://doi.org/10.26355/eurrev_202112_27337 10.26355/eurrev_202112_27337 [DOI] [PubMed] [Google Scholar]
- [72].Paolacci S, Kiani AK, Shree P, Tripathi D, Tripathi YB, Tripathi P, Tartaglia GM, Farronato M, Farronato G, Connelly ST, Ceccarini MR, Coatto M, Ergoren MC, Sanlidag T, Dautaj A, Bertelli M. Scoping review on the role and interactions of hydroxytyrosol and alpha-cyclodextrin in lipid-raft-mediated endocytosis of SARS-CoV-2 and bioinformatic molecular docking studies.. Eur Rev Med Pharmacol Sci 2021;25:90-100. https://doi.org/10.26355/eurrev_202112_27338 10.26355/eurrev_202112_27338 [DOI] [PubMed] [Google Scholar]
- [73].Ergoren MC, Paolacci S, Manara E, Dautaj A, Dhuli K, Anpilogov K, Camilleri G, Suer HK, Sayan M, Tuncel G, Sultanoglu N, Farronato M, Tartaglia GM, Dundar M, Farronato G, Gunsel IS, Bertelli M, Sanlidag T. A pilot study on the preventative potential of alpha-cyclodextrin and hydroxytyrosol against SARS-CoV-2 transmission. Acta Biomed 2020;91(13-S):e2020022. https://doi.org/10.23750/abm.v91i13-S.10817 10.23750/abm.v91i13-S.10817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mu Q, Tavella VJ, Luo XM. Role of Lactobacillus reuteri in human health and diseases. Front Microbiol 2018;9:757. https://doi.org/10.3389/fmicb.2018.00757 10.3389/fmicb.2018.00757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Greifová G, Májeková H, Greif G, Body P, Greifová M, Dubničková M. Analysis of antimicrobial and immunomodulatory substances produced by heterofermentative Lactobacillus reuteri. Folia Microbiol 2017;62:515-24. https://doi.org/10.1007/s12223-017-0524-9 10.1007/s12223-017-0524-9 [DOI] [PubMed] [Google Scholar]
- [76].Thomas CM, Saulnier DM, Spinler JK, Hemarajata P, Gao C, Jones SE, Grimm A, Balderas MA, Burstein MD, Morra C, Roeth D, Kalkum M, Versalovic J. FolC2-mediated folate metabolism contributes to suppression of inflammation by probiotic Lactobacillus reuteri. Microbiologyopen 2016;5:802-18. https://doi.org/10.1002/mbo3.371 10.1002/mbo3.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Romani Vestman N, Chen T, Lif Holgerson P, Öhman C, Johansson I. Oral Microbiota shift after 12-week supplementation with Lactobacillus reuteri DSM 17938 and PTA 5289; a randomized control trial. PLoS One 2015;10:e0125812. https://doi.org/10.1371/journal.pone.0125812 10.1371/journal.pone.0125812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Iniesta M, Herrera D, Montero E, Zurbriggen M, Matos AR, Marín MJ, Sánchez-Beltrán MC, Llama-Palacio A, Sanz M. Probiotic effects of orally administered Lactobacillus reuteri-containing tablets on the subgingival and salivary microbiota in patients with gingivitis. A randomized clinical trial. J Clin Periodontol 2012;39:736-44. https://doi.org/10.1111/j.1600-051X.2012.01914.x 10.1111/j.1600-051X.2012.01914.x [DOI] [PubMed] [Google Scholar]
- [79].Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, Haytac MC. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol 2013;40:1025-35. https://doi.org/10.1111/jcpe.12155 10.1111/jcpe.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lacroix C. Protective cultures, antimicrobial metabolites and bacteriophages for food and beverage biopreservation, 1st Edition. Zurich ET: Woodhead Publishing; 2011. [Google Scholar]
- [81].Jansen PM, Abdelbary MM, Conrads G. A concerted probiotic activity to inhibit periodontitis-associated bacteria. Plos One 2021;16:e0248308. https://doi.org/10.1371/journal.pone.0248308 10.1371/journal.pone.0248308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Geraldo BM, Batalha MN, Milhan NV, Rossoni RD, Scorzoni L, Anbinder AL. Heat-killed Lactobacillus reuteri and cell-free culture supernatant have similar effects to viable probiotics during interaction with Porphyromonas gingivalis. J Periodontal Res 2020;55:215-20. https://doi.org/10.1111/jre.12704 10.1111/jre.12704 [DOI] [PubMed] [Google Scholar]
- [83].Santos TA, Scorzoni L, Correia R, Junqueira JC, Anbinder AL. Interaction between Lactobacillus reuteri and periodontopathogenic bacteria using in vitro and in vivo (G. mellonella) approaches. Pathog Dis 2020;78:ftaa044. https://doi.org/10.1093/femspd/ftaa044 10.1093/femspd/ftaa044 [DOI] [PubMed] [Google Scholar]
- [84].Moman R, O’Neill CA, Ledder RG, Cheesapcharoen T, McBain AJ. Mitigation of the toxic effects of periodontal pathogens by candidate probiotics in oral keratinocytes, and in an invertebrate model. Front Microbiol 2020;11:999. https://doi.org/10.3389/fmicb.2020.00999 10.3389/fmicb.2020.00999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kšonžeková P, Bystrický P, Vlčková S, Pätoprstý V, Pulzová L, Mudroňová D, Kubašková T, Csank T, Tkáčiková Ľ. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr Polym 2016;141:10-9. https://doi.org/10.1016/j.carbpol.2015.12.037 10.1016/j.carbpol.2015.12.037 [DOI] [PubMed] [Google Scholar]
- [86].Kang MS, Lim HS, Kim SM, Lee HC, Oh JS. Effect of Weissella cibaria on Fusobacterium nucleatum-induced interleukin-6 and interleukin-8 production in KB cells. J Bacteriol 2011;41:9-18. https://doi.org/10.4167/jbv.2011.41.1.9 10.4167/jbv.2011.41.1.9 [DOI] [Google Scholar]
- [87].Invernici MM, Salvador SL, Silva PH, Soares MS, Casarin R, Palioto DB, Souza SL, Taba M, Jr, Novaes AB, Jr, Furlaneto FA, Messora MR. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: a randomized clinical trial. J Clin Periodontol 2018;45:1198-210. https://doi.org/10.1111/jcpe.12995 10.1111/jcpe.12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Galofré M, Palao D, Vicario M, Nart J, Violant D. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. J Periodontal Res 2018;53:378-90. https://doi.org/10.1111/jre.12523 10.1111/jre.12523 [DOI] [PubMed] [Google Scholar]
- [89].İnce G, Gürsoy H, İpçi ŞD, Cakar G, Emekli-Alturfan E, Yılmaz S. Clinical and biochemical evaluation of lozenges containing Lactobacillus reuteri as an adjunct to non-surgical periodontal therapy in chronic periodontitis. J Periodontol 2015;86:746-54. https://doi.org/10.1902/jop.2015.140612 10.1902/jop.2015.140612 [DOI] [PubMed] [Google Scholar]


