Abstract
The major surface glycoprotein (gp63) of Leishmania amazonensis is a metalloprotease implicated in the infection of mammalian macrophages. The expression of gp63 and its participation in this infection were further examined by modulating the level of this molecule in a virulent gp63-abundant wild-type clone. Promastigotes were transfected with gp63 genes cloned into a Leishmania-specific vector in two different orientations, leading to the expression of gp63 sense and antisense RNAs. With increasing selective pressure, cell surface gp63 was increasingly augmented in the transfectants with sense transcripts and suppressed to a very low level in those with antisense transcripts. Thus, the expression of gp63 from chromosomal, repetitive genes is not stringently regulated at the protein level and can be substantially reduced by episomal antisense transcription of a single copy. The transfectants differed significantly only in the level of gp63, thereby allowing specific evaluation of this molecule in leishmanial infection of macrophages in vitro. Kinetic studies of infection in vitro indicate that gp63 plays a role not only in the binding of this parasite to these macrophages but also in its intramacrophage survival and replication.
Trypanosomatid protozoan Leishmania organisms live alternately as extracellular promastigotes in the insect vector and as intralysosomal amastigotes in macrophages, causing leishmaniasis (7, 31). Multiple determinants are clearly involved in this type of intracellular parasitism (9). Among them, the surface glycoconjugates have been extensively studied and implicated as Leishmania virulence factors (9, 26, 30).
The major surface glycoprotein (gp63) of Leishmania spp. is a zinc protease (3). It has been suggested that gp63 functions in multiple steps in the infection of macrophages by the promastigotes of Leishmania amazonensis (9, 10). The wild-type cells of this and other species expressing abundant gp63 are more virulent than the gp63-deficient variants obtained after long-term culture (17, 33). Overexpressing gp63 by extrachromosomal complementation of such deficient variants increases to a certain extent their binding to macrophages (19) and intracellular survival (21).
In the present study, the endogenous level of gp63 in L. amazonensis was modulated specifically by episomal expression of sense and antisense gp63 RNA. This was accomplished by transfecting a virulent clone with a copy of the gp63 gene placed in correct or reverse orientation in a Leishmania-specific expression vector. Varying the selective pressures resulted in a wide margin of up- and down-regulation of gp63, significantly affecting the ability of these transfectants to infect macrophages in vitro. The results obtained provide further evidence for a role for gp63 in both the binding of the parasites to macrophages and their intracellular survival and replication.
MATERIALS AND METHODS
Cells.
L. amazonensis (LV78) promastigotes of virulent clone 12-1 were cultured at 25°C in medium 199–10% heat-inactivated fetal bovine serum (HIFBS)–25 mM HEPES (pH 7.4). These cells express abundant gp63 (17). Macrophages of the J774 lines (J774G8 or J774A1) were cultured at 35°C in HEPES-buffered RPMI 1640 medium (pH 7.4) with 20% HIFBS. Similar results were obtained by using either line. Additional cells used were resident peritoneal macrophages collected from C57BL/6 mice. They were cultured under the same conditions as J774 cells.
Plasmid constructs.
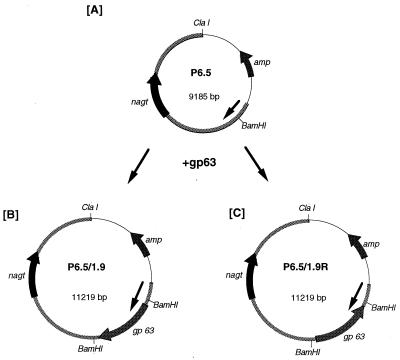
The Leishmania-specific vector P6.5 (Fig. 1A), designed on the basis of previous work (15, 16, 18), will be described elsewhere in detail. Briefly, P6.5 was constructed from an ∼7.5-kb Leishmania DNA in pBluescript or pBS-HC7.5 (20), in which the upstream p36 gene was replaced with a BamHI site for cloning and the downstream gene (nagt), encoding N-acetylglucosamine-1-phosphate transferase, was kept as the selective marker for tunicamycin (TM) resistance (18). A copy of the 1.9-kb gp63 gene from L. amazonensis (22) was inserted into the cloning site in two different orientations with reference to that of nagt (Fig. 1B and C). The plasmids were expanded in Escherichia coli and isolated with a Maxi kit (Qiagen).
FIG. 1.
Expression of sense and antisense gp63 transcripts with a Leishmania-specific vector in L. amazonensis. (A) P6.5, a Leishmania-specific vector (see text for its origin). (B) P6.5/1.9, a plasmid carrying the gp63 gene cloned at a BamHI site in P6.5 in the correct orientation. (C) P6.5/1.9R, a plasmid carrying the gp63 gene cloned in P6.5 in the reverse orientation. Arrowed bars in circles represent functional genes and the direction of their transcription: amp, ampicillin resistance gene of pBluescript; nagt, gene of ∼1.4 kb encoding N-acetylglucosamine-1-phosphate transferase; gp63, gene of ∼1.9 kb encoding gp63 of L. amazonensis. Thick lines of the circles including nagt represent the L. amazonensis DNA portion of P6.5 (∼6.2 kb). Thin lines of the circles represent pBluescript. The arrow inside each circle indicates the direction of Leishmania DNA transcription.
Transfection of Leishmania.
Promastigotes grown to late log or stationary phase were transfected with ∼20 μg of the following plasmids: vector alone (P6.5) and P6.5 with the gp63 gene in the correct orientation (P6.5/1.9) and in the reverse orientation (P6.5/1.9R). Cells were transfected by electroporation with a Gene Pulser (Bio-Rad) under conditions described previously (15, 18). Transfectants were allowed to recover in drug-free medium for 24 h and then were selected for resistance to TM at 5, 10, and 20 μg/ml. Three sets of transfectants were independently obtained in separate experiments using cryopreserved cells of clone 12-1 and those of the same clone in continuous culture. Each set consisted of transfectants with P6.5, P6.5/1.9, and P6.5/1.9R. Similar results were obtained from all three sets (see below).
Northern blot analysis.
Total RNA was isolated with hot phenol for Northern blot analysis as previously described (15). Blots were probed with oligonucleotides complementary to sense and antisense strands of the gp63 gene at nucleotides 559 to 573, i.e., 5′ACCTTGAAGTACCTGCACACGTCGG3′ and 5′CCGACGTGTGCAGGTACTTCAAGGT3′, specific to sense and antisense RNA transcripts, respectively. Probes were labeled with [γ-32P]dCTP by using terminal transferase according to manufacturer's instructions (Promega).
Western blot and concanavalin A binding analyses.
Proteins resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were transferred to a nitrocellulose membrane (Schleicher & Schuell) for Western blot analysis with rabbit antiserum raised against gp63 at 1:10,000 dilution (10). Anti-P36 antiserum was simultaneously included to assess the constitutively expressed P36 (20) levels. The secondary antibody used was peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma). Immunoblots were exposed to X-ray films after reaction with ECL reagents for chemiluminescence (Amersham). The relative amounts of gp63 expressed by different cells were evaluated by laser densitometry (model 2202 Ultrascan; LKB), which was used to measure band intensities with reference to that of P36 in each sample.
Concanavalin A affinity binding of gp63 was carried out with Nonidet P-40-soluble fractions of biosynthetically labeled promastigotes essentially as described previously for immunoprecipitation with protein A-Sepharose (16, 17), except that different affinity beads were used in the absence of antiserum.
Flow cytometry and confocal microscopy for cell surface gp63.
Cells used for these experiments were fixed at 4°C in 0.5% glutaraldehyde in phosphate-buffered saline (pH 7.4) for 15 min, followed by extensive washing in the same buffer. These fixed cells maintained their morphological integrity, as verified by microscopic observation. They were incubated with a 1:100 dilution of rabbit anti-gp63 antiserum, washed, and then incubated with a 1:25 dilution of fluorescein isothiocyanate-labeled goat anti-rabbit immunoglobulin G (Sigma). These cells were washed three times before examination.
For flow cytometry, these cells were examined in a Coulter XL flow cytometer to compare their mean channel intensities. Untreated cells and those treated with the secondary antibodies alone were used as controls. Each experimental population was then mapped by using a two-parameter histogram of forward-angle light scatter versus side scatter. The mapped population was then analyzed for log green fluorescence (FL1) by using a single-parameter histogram.
For fluorescence microscopy, cell samples were each suspended in mounting medium (polyvinyl alcohol-diazabicyclo [2.2.2] octane) and placed under a coverslip on a glass slide. Imaging was accomplished with an Olympus Fluoview confocal microscope equipped with a ×100 oil objective (numerical aperture, 1.35) and a 488-nm excitation source. Detector levels were adjusted to the appropriate range, and identical settings were used for imaging the experimental samples presented.
Macrophage binding assay.
The binding of promastigotes to macrophages was assayed essentially as described previously (19), except that promastigotes were labeled biosynthetically with a mixture of [35S]methionine and [35S]cysteine (specific activity = ∼1,000 μCi/μM) (Amersham) instead of [3H]leucine. Briefly, promastigotes at early stationary phase were radiolabeled at 1 to 2 μCi/ml overnight and chased in complete medium for 3 to 4 h until no radioactivity was detected in the culture medium with or without trichloroacetic acid precipitation. Labeled cells were washed twice and resuspended in serum-free medium 199. Aliquots of 107 labeled promastigotes/100 μl were each added to 106 macrophages on glass coverslips as monolayers. After incubation at 35°C for 5, 10, and 20 min, samples in triplicate were withdrawn and rinsed extensively with medium 199 to remove unbound promastigotes. Samples were then digested with Protosol (NEN) and subjected to scintillation counting. Radioactivity was converted into cell numbers based on the labeling efficiency of individual cell samples determined separately.
In vitro assay for intramacrophage survival and replication.
The J774 macrophages were infected with L. amazonensis promastigotes at a host-to-parasite ratio of 1:10. Subsequently, the infection in each culture was continuously monitored quantitatively by periodic sampling for microscopic counting. By using this in vitro system, it was possible to estimate the net increase of the total intracellular parasites starting from a known number of promastigotes as the initial inoculum (8, 16). Specifically, 4 × 106 macrophages were mixed with 4 × 107 stationary-phase promastigotes in 4 ml of medium to begin the infection at 35°C. The level of infection was quantitated at the time of medium renewal, i.e., every 3 to 4 days. An aliquot of about 15 μl from each culture was sacrificed for microscopic counting of macrophages and parasites. The remainder was centrifuged, resuspended in fresh medium, and returned to the original culture flask. The total number of intracellular parasites per culture was estimated at each time point as the total number of macrophages × the percentage of infected cells × the average number of intracellular parasites/macrophage. The percentage of infected cells and the average number of intracellular parasites per cell were tallied microscopically by continuous examination of consecutive areas in a given infected culture until the infected cells examined reached at least 100. The infection experiments were repeated six times.
RESULTS
Transcription of gp63 sense and antisense RNAs.
Transfectants emerged in all cases after selection and subsequently grew in medium with TM up to 20 μg/ml. The integrity of all plasmids in these transfectants was confirmed by Southern blot analysis with specific probes and by restriction mapping or sequencing (data not shown).
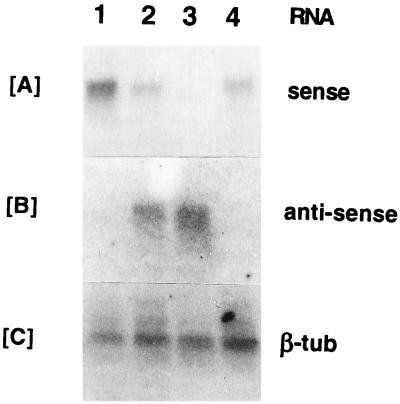
Total RNA was isolated from the transfectants exposed to increasing selective pressures, i.e., 0 to 20 μg of TM/ml. Northern blot analysis of these samples with specific oligonucleotides (see Materials and Methods) revealed that they contained sense and antisense gp63 transcripts at different levels, as expected. The sense RNA was more abundant in the transfectants with the gp63 gene in the correct orientation (P6.5/1.9) (Fig. 2A, lane 1) than in those with vector alone (lane 4) or with the gp63 gene in the reverse orientation (P6.5/1.9R) (lanes 2 and 3; see below). Only transfectants with P6.5/1.9R expressed antisense transcripts (Fig. 2B), and expression increased in intensity with TM concentrations from 0 (lane 2) to 20 μg/ml (lane 3). Concomitantly, the sense transcripts in the same set of samples appeared to decrease accordingly (compare lanes 2 and 3 in Fig. 2A), resulting apparently from the sense-antisense RNA duplex formation. The signals for the beta-tubulin probe among different samples were comparable (Fig. 2C). The results obtained clearly demonstrate episomal transcription of sense and antisense mRNA, which significantly augments and reduces the endogenous species, respectively. The relative levels of up- and down-regulation of the endogenous species cannot be adequately assessed because of the inherent differences in labeling and hybridization efficiency between different probes used.
FIG. 2.
Northern blot analysis of transfectants for gp63 sense and antisense transcripts. Total RNA samples from different transfectants are shown. Lane 1, P6.5/1.9; lanes 2 and 3, P6.5/1.9R; lane 4, P6.5 alone. All transfectants were grown in 20 μg of TM/ml, except the sample from P6.5/1.9R in lane 2, which was grown in drug-free medium. Probes used are oligonucleotides specific to sense gp63 transcripts (A), oligonucleotides for antisense gp63 transcripts (B), and the beta-tubulin (β-tub) gene of L. amazonensis (C).
Modulation of gp63 by episomal sense and antisense transcripts.
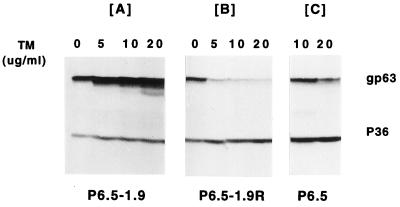
Western blot analysis of these transfectants showed that the expression of the sense and antisense RNA affected the level of gp63 but not that of other proteins, i.e., p36 (Fig. 3). With increasing selective pressures of TM from 0 to 20 μg/ml, the level of gp63 gradually increased in P6.5/1.9 transfectants (Fig. 3A) and decreased to negligible levels, in contrast to what was found for p36, in P6.5/1.9R transfectants (Fig. 3B). These changes in the level of gp63 are significant compared to those seen in the controls, i.e., transfectants with P6.5 alone selected for resistance to 10 to 20 μg of TM/ml (Fig. 3C). Selection of the transfectants with P6.5/1.9 at the highest drug pressure, i.e., 20 μg of TM/ml resulted in the appearance of a faint band with increased mobility (Fig. 3A, lane 20). This band of ∼54 kDa is similar in size to the deglycosylated gp63 reported previously (22) and was absent from the same samples affinity bound to concanavalin A-Sepharose (data not shown). The appearance of such deglycosylated species may result from overproduction of the gp63 peptides beyond the cellular capacity of N glycosylation (13, 22). In the transfectants with P6.5/1.9R, gp63 was dramatically reduced already at the lowest drug pressure of 5 μg of TM/ml but trace levels persisted even at increased selective pressures up to 20 μg of TM/ml (Fig. 3B). Thus, the expression of sense transcripts augments the endogenous level of gp63; in contrast, the expression of antisense RNA effectively inhibits the translation of endogenous gp63 RNA, thereby reducing this protein to an insignificant level. Densitometry of gp63 with reference to p36 intensity (Fig. 3, lanes 10 and 20) revealed the following ratio of banding intensity: ∼150 (P6.5/1.9F):8 (P6.5):1 (P6.5/1.9R).
FIG. 3.
Western blot analysis of gp63 sense and antisense transfectants. Transfectants were grown in 0, 5, 10, and 20 μg of TM/ml. The blots were probed with antiserum (1:10,000 dilution) against gp63. p36 revealed by anti-p36 antiserum (1:10,000 dilution) was used for the loading control.
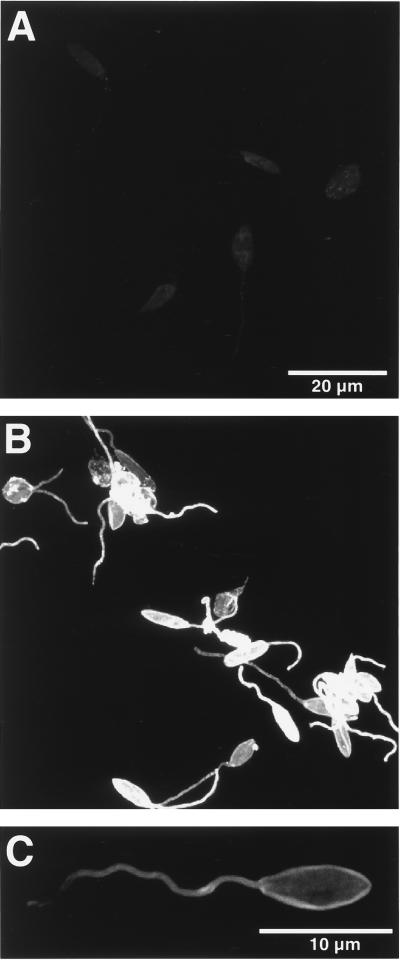
The observed differences in the levels of gp63 between P6.5/1.9 and P6.5/1.9R transfectants were reflected in their cell surface staining intensities. These were initially determined by immunofluorescence imaging of glutaraldehyde-fixed cells. Cells so treated became impermeable to antibodies, thereby limiting positive signals to gp63 on the cell surface. The fluorescence signal intensity was much lower in P6.5/1.9R cells than in P6.5/1.9 cells. Because of the large difference in staining intensity between the two, it became necessary to set the detection parameters so that the signal intensity of the P6.5/1.9R cells was just above the background levels (Fig. 4A) to prevent the image of the P6.5/1.9 cells from being too oversaturated at the same detector settings (Fig. 4B). Heterogeneous cell staining intensity was apparent in the P6.5/1.9 cells. By altering the detector setting to provide the optimal dynamic range for imaging P6.5/1.9 cells, it became evident that gp63 immunostaining was localized to the cell surface (Fig. 4C).
FIG. 4.
Confocal fluorescence microscopy of transfectants for cell surface gp63. Glutaraldehyde-fixed cells were treated first with anti-gp63 antiserum and then with fluorescein isothiocyanate-conjugated secondary antibodies; those treated with normal serum and/or with the secondary antibodies alone served as controls (see Materials and Methods for details). (A) P6.5/1.9R cells viewed at settings to reduce the images to the background level; (B) P6.5/1.9 cells with intensive signals for gp63 viewed under the same settings; (C) surface localization of gp63 in a P6.5/1.9 cell revealed under appropriate settings.
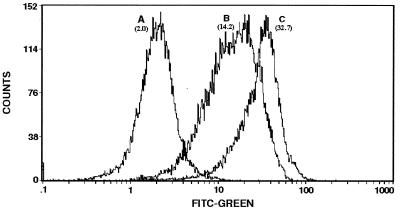
Flow cytometric analysis of the transfectants provided comparative measurements for the relative levels of their surface gp63. Analysis of 25,000 cells each from the three populations yielded mean channel fluorescence intensities (MCFI). The ratio calculated from these MCFI is as follows: 1 (P6.5/1.9R):7 (P6.5):16.5 (P6.5/1.9) (Fig. 5, peaks A to C). In three separate experiments, the readings for P6.5/1.9 cells were consistently much higher than those for P6.5/1.9R cells, maintaining their wide margin of difference, while those for the controls (P6.5 cells and untransfected cells) varied among different samples by a wider range. The results obtained thus indicate that up-regulation and down-regulation of gp63 by sense and antisense transcription produce significant differences in its surface expression on otherwise identical cells.
FIG. 5.
Flow cytometry of transfectants showing different levels of surface gp63. Cells used were those fixed with glutaraldehyde and treated as described in the legend to Fig. 4. Fluorescence-activated cell sorter analysis of 25,000 cells for each population was done for green fluorescence with a Coulter XL flow cytometer with the accompanying software programs (See Materials and Methods for details). Peaks A to C, samples of P6.5/1.9R, P6.5, and P6.5/1.9 cells, respectively. The numbers in brackets are MCFI. FITC, fluorescein isothiocyanate.
Contribution of gp63 to leishmanial infection of macrophages.
Infection of macrophages by the transfectants with different levels of gp63 was assessed in vitro, specifically, their binding to macrophages and their subsequent intracellular survival and replication.
(i) Promastigote-macrophage binding.
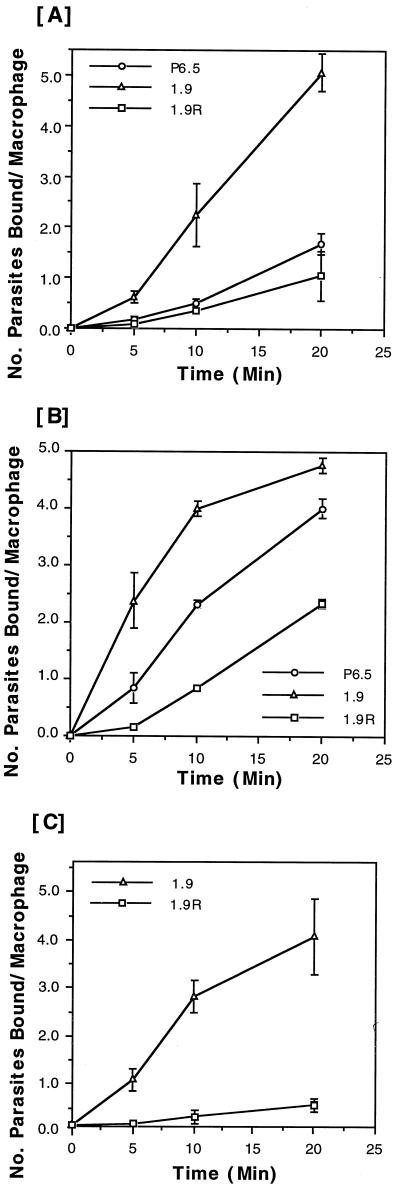
The binding of these transfectants to J774 macrophages followed linear kinetics in most cases, as assessed at three time points up to 20 min in each experiment. The results from all six independent experiments with these transfectants ranked their macrophage-binding efficiencies as follows: P6.5/1.9 > P6.5 > P6.5/1.9R. Two sets of representative results are presented (Fig. 6A and B), showing that the binding kinetics displayed two patterns among the six independent experiments. The binding of P6.5/1.9 cells differs from that of P6.5/1.9R cells by 5- to 15-fold at the first time point of 5 min, and the difference was narrowed to 2- to 10-fold at the last time point of 20 min (see Discussion for the variations seen). Similar results were obtained in two separate experiments using resident peritoneal macrophages for binding with P6.5/1.9 and P6.5/1.9R cells. In both cases, the binding of the two transfectants followed linear kinetics, resulting in a four- to eightfold difference at the last time point of 20 min (Fig. 6C).
FIG. 6.
Binding of different transfectants expressing variable amounts of gp63 to macrophages. The binding of transfectants to macrophages was assayed at 5, 10, and 20 min, as described in Materials and Methods. Radioactive counts were normalized to the number of parasites bound per macrophage based on a standard linear curve of cell numbers versus values of radioactivity in counts per minute. (A and B) J774G8 macrophages. Results are based on two separate sets of representative data from six different experiments. (C) C57BL/6 peritoneal macrophages. Results are based on one representative experiment of two separate experiments. Samples at each time point are in triplicate.
(ii) Intramacrophage survival and replication of the parasites.
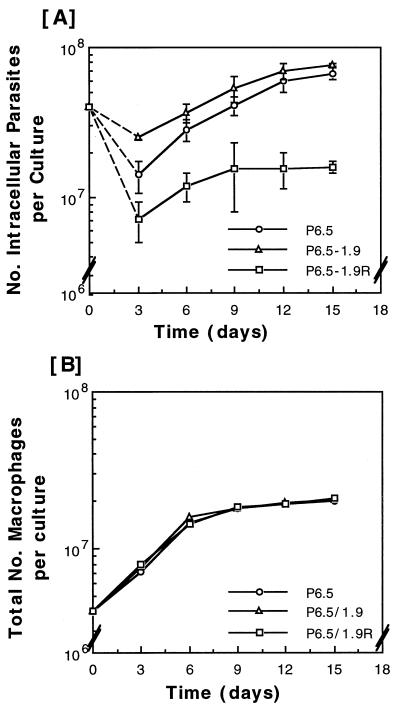
These activities of the transfectants were assessed with the in vitro system by using J774 cells adequate for quantitating the total number of intracellular parasites per flask every 3 to 4 days (see Materials and Methods). Results of six independent experiments showed that the infectivity was always the highest with P6.5/1.9 cells, somewhat lower with P6.5 cells, and lowest with P6.5/1.9R cells (Fig. 7A), consistent with the levels of their gp63 observed (Fig. 3 to 5). At the first time point of day 3 after infection, the total number of intracellular parasites was lower than the original inoculum in all cases (Fig. 7A). This is not unexpected since some promastigotes failed to survive intracellularly and others, in small numbers, not taken up by the macrophages eventually perished extracellularly. At this time point, the differences among the three transfectants are evident (Fig. 7A), consistent with the differences seen in their binding to macrophages at the 20-min point (Fig. 6). With further incubation up to 15 days, the survival and replication of both P6.5/1.9 and P6.5 cells increased exponentially, while that of P6.5/1.9R cells leveled off after an initial small rise from day 3 to day 9. The difference between P6.5 or P6.5/1.9 cells and P6.5/1.9R cells became widened to almost fivefold from that time point until the conclusion of the study on day 15 (Fig. 7A). Throughout this period, the J774 cells grew equally well in all cultures, irrespective of the transfectants used (Fig. 7B).
FIG. 7.
Intramacrophage survival and replication of various transfectants with different levels of gp63 in J774 lines. Infection was carried out in triplicate. Each began with 4 × 107 promastigotes and 4 × 106 macrophages in a 25-cm2 flask. The infectivity of these parasites was assayed by quantitating the total number of intracellular parasites in each flask every 3 to 4 days after incubation (see Materials and Methods). The value for each time point was obtained from triplicate samples. Total numbers of intracellular parasites (A) and growth of J774 macrophages (B) are shown. The square on the vertical axis of panel A indicates the size of the inoculum (4 × 107 promastigotes) with which the infection in each culture began. The dashed lines trace the net decrease in number of the parasites from the inoculum added on day 0 to day 3, when the intracellular parasites were first enumerated. This loss was taken to indicate extracellular and intracellular degeneration or killing of the parasites in this closed in vitro system.
Gp63 thus contributes to the binding of L. amazonensis promastigotes to J774 macrophages, as indicated by the correlation of their gp63 levels with the binding kinetics. The widened difference between P6.5 or P6.5/1.9 cells and P6.5/1.9R cells in infection with time strongly suggests that gp63 is also required for the intramacrophage survival and/or replication of these parasites.
DISCUSSION
The infection of mammalian macrophages by Leishmania involves the binding of the parasites to the macrophage surface, their entry into these phagocytes, and their subsequent survival and replication intralysosomally. The early interaction at the host-parasite interface thus appears to be critical for this type of parasitism, involving surface molecules of Leishmania, such as gp63.
Earlier, we have examined the functional significance of gp63 in such an infection by using the L. amazonensis-J774 murine macrophage in vitro system (10, 16, 17, 19, 21). One advantage of this in vitro system is the ease with which the promastigotes infect macrophages, followed by their successful differentiation into amastigotes and the continuous replication of the latter intracellularly (8). This system compares favorably with other host-parasite combinations for the quantitation of promastigote-macrophage binding and the subsequent intracellular survival and replication of the differentiated amastigotes. It is adequate for evaluating the intrinsic ability of the parasites to achieve intracellular parasitism in macrophages at the basal level of no specific activation.
In this in vitro system, gp63-deficient avirulent variants of L. amazonensis (stock 250) were shown previously to perform less efficiently than gp63-abundant virulent variants (stock 12) in both macrophage-binding and intramacrophage survival and replication assays (17). Genetic complementation of these deficient variants restored their gp63 expression to the virulent wild-type level but only partially recovered their infectivity to macrophages (19, 21). The simplest explanation for this observation is that these avirulent variants (stock 250) may have additional defects in gp63 or elsewhere which cannot be genetically rescued efficiently with the copy of the gp63 gene used (see below for further discussion).
In the present study, we were able to examine the functional significance of gp63 in vitro more specifically. The specificity is made possible by transfecting cloned cells separately with identical constructs containing the same gp63 gene, but in two different orientations (Fig. 1), leading to the expression of specific sense and antisense transcripts (Fig. 2). In the respective transfectants, the sense and antisense transcripts produced modulate the levels of gp63 accordingly, but not those of p36 proteins from a different chromosomal gene (Fig. 3). Thus, the two sets of transfectants appear to differ only in the level of their gp63, and this difference can be optimized simply by manipulating the selective conditions used. The most dramatic and consistent differences seen under optimal conditions are differences in total gp63 (Fig. 3) and cell surface gp63 (Fig. 4 and 5) expression levels between the sense and antisense transfectants. This is clearly indicated by the quantitative data presented, even though such data cannot be adequately compared due to the limitations inherent to the different techniques used. Notably, overexpression of the sense transcripts up-regulates gp63 at the protein level in cells already known to produce this molecule in abundance (17, 29). Thus, gp63 is not stringently regulated translationally in wild-type cells. However, excessive production of the peptides results in the emergence of nonglycosylated species, consistent with the previous finding that this protein is posttranslationally regulated by N glycosylation (22). More significantly, the gp63 from multicopy chromosomal genes in gp63-abundant cells is reduced to a negligible level (Fig. 3B, lanes 5, 10, and 20) by overexpressing the antisense transcripts episomally from a single gene sequence. Thus, the antisense suppression appears to achieve a substantial “knockout” of gp63 proteins, regardless of their origins, which may be from different clusters of the gene family. Selection for the gp63-negative phenotype this way is procedurally simpler than knockouts of gene clusters by homologous recombination (14). The antisense knockouts obtained appear to be as specific as the genetic knockouts and are certainly more so than the spontaneously generated gp63-deficient variants used in the previous studies (17, 29). A small amount of gp63 in the antisense transfectants is still visible at the optimal selective conditions used (Fig. 3B, lane 20; Fig. 5, peak A). The incompleteness of antisense suppression inherent to this approach is likely to account for this. It is possible, although unlikely, that the residual gp63 may come from mRNA species of the genes very divergent in sequence from the copy used to generate the antisense transcripts.
By using the transfectants thus obtained, we demonstrated the dual functions of gp63, i.e., in the binding of L. amazonensis promastigotes to macrophages and the intracellular survival and replication of the parasites in J774 cells, more conclusively than before. The transfectants with their gp63 up- and down-regulated specifically as described above differ significantly in both phenotypes, viz., binding to both J774 cells (Fig. 6A and B) and peritoneal macrophages (Fig. 6C), and intracellular survival and replication in the cell line (8). Preliminary data from work still in progress showed that infection of human peripheral blood monocytes with the gp63 sense and antisense transfectants produced results similar to those presented in Fig. 7A (unpublished data). The actual differences between these transfectants may well be greater, since reversal of the antisense effects is expected from release of the selective pressure necessary to avoid drug toxicity to macrophages (34). This is especially true in the assays for intracellular survival and replication, since they require a prolonged period of incubation (Fig. 7A). The transient nature of the antisense suppression in the absence of selection dissuaded us from attempts to undertake in vivo studies that require prolonged infection of laboratory animals with these transfectants. Recently, gp63 genes were knocked out to near completion from a strain of Leishmania major, which was found to differ from the wild type, but only to a small extent, in both in vitro and in vivo infectivity (14). Further study is needed to determine whether this inconsistency may result from differences in the species, host-parasite combinations, assay conditions, or methodologies used.
How gp63 mediates the dual functions of infection in this in vitro system is of interest for further discussion. This molecule has long been implicated as a ligand of the promastigotes for their receptor-mediated endocytosis by macrophages (6, 27, 28). Recent evidence suggests that two different domains of gp63 are involved in this event: its catalytic domain generates C3bi for the initial attachment of promastigotes to the complement receptor and its RGD-equivalent domain reinforces this attachment to the fibronectin receptor to effect their final engulfment by the macrophages (5). The concept of multiple ligand-receptor interactions (9) involved in this event, however, precludes the expectation that endocytosis of promastigotes can be inhibited completely by knocking out gp63 alone. In the present study, the residual macrophage-binding activities of the transfectants and their differences in different experiments (Fig. 6) can be accounted for by the presence of other ligands, such as lipophosphoglycan (1, 30), and by their variable levels of expression. Whether both ligands mediate the binding of promastigotes in this system to the complement receptors (2, 4, 12, 24, 32) or to other receptors is a subject of interest for further investigation. Clear evidence indicating that depletion of gp63 from promastigotes not only hinders their binding to the macrophages but also affects their intracellular fate adversely is presented. The depression of the total intracellular parasite number per culture seen on day 3 in the order of P6.5/1.9 cells ≥ P6.5 cells > P6.5/1.9R cells (Fig. 7A) is reflective of disparity in initial binding and thus intracellular entry. The quantitative data obtained subsequently showed an exponential rise in the number of the intracellular parasites, except those depleted of gp63, viz., P6.5/1.9R cells (Fig. 7A). The number of these cells plateau from days 9 to 15. Microscopic observations of these cultures during the enumeration of intracellular parasites revealed that they differentiated successfully but that the differentiated amastigotes failed to survive or to replicate. The later events thus appear to require the association of gp63 with promastigotes during the early phase of their infection. Down-regulation of gp63 during promastigote-to-amastigote differentiation (10, 23, 25) is a gradual event, taking 5 to 6 days to reach the basal level for L. amazonensis (unpublished data). Further investigation is needed to determine if gp63 simply serves a protective function (10, 29) or acts in another capacity on the macrophages to ensure parasite survival during this period. In a mammalian host, the substrates for gp63, which acts as a zinc protease, appear to include serum factors, e.g., C3b (4, 10), and macrophage factors, e.g., MARCKS (myristoylated alanine-rich C-kinase substrate) (11). The molecular interactions of gp63 with these and other factors in the immediate surroundings of promastigotes during their infection of macrophages are of interest for further investigation.
ACKNOWLEDGMENTS
This work was supported by a grant (AI 20486) from the National Institutes of Health to K.-P.C. D.A.P. was supported by a Temple Foundation Award from the Alzheimer's Association.
We thank Jerome Franklin Sah for reviewing the manuscript.
REFERENCES
- 1.Beverley S M, Turco S J. Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol. 1998;6:35–40. doi: 10.1016/S0966-842X(97)01180-3. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell J M, Ezekowitz R A, Roberts M B, Channon J Y, Sim R B, Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985;162:324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvier J, Schneider P, Etges R. Leishmanolysin: surface metalloproteinase of Leishmania. Methods Enzymol. 1995;248:614–633. doi: 10.1016/0076-6879(95)48039-0. [DOI] [PubMed] [Google Scholar]
- 4.Brittingham A, Morrison C J, McMaster W R, McGwire B S, Chang K-P, Mosser D M. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J Immunol. 1995;155:3102–3111. [PubMed] [Google Scholar]
- 5.Brittingham A, Chen G, McGwire B S, Chang K-P, Mosser D. Interaction of leishmania gp63 with cellular receptors for fibronectin. Infect Immun. 1999;679:4477–4484. doi: 10.1128/iai.67.9.4477-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang K-P, Fong D, Bray R. Biology of Leishmania and leishmaniasis. In: Chang K-P, Bray R, editors. Leishmaniasis. Vol. 1. New York, N.Y: Elsevier; 1985. pp. 1–30. [Google Scholar]
- 7.Chang K-P. Leishmaniasis. Encycl Hum Biol. 1997;5:275–281. [Google Scholar]
- 8.Chang K-P. Human cutaneous Leishmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980;209:1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- 9.Chang K-P, Chaudhuri G, Fong D. Molecular determinants of Leishmania virulence. Annu Rev Microbiol. 1990;44:499–529. doi: 10.1146/annurev.mi.44.100190.002435. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhuri G, Chaudhuri M, Pan A, Chang K-P. Surface acid proteinase (gp63) of Leishmania mexicana. A metalloenzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. J Biol Chem. 1989;264:7483–7489. [PubMed] [Google Scholar]
- 11.Corradin S, Ransijn A, Corradin G, Roggero M A, Schmitz A A P, Schneider P, Mauel J, Vergeres G. MARCKS-related protein (MRP) is a substrate for the Leishmania major surface protease leishmanolysin (gp63) J Biol Chem. 1999;274:25411–25418. doi: 10.1074/jbc.274.36.25411. [DOI] [PubMed] [Google Scholar]
- 12.Ezekowitz R A, Sim R B, Hill M, Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984;159:244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funk V A, Jardim A, Olafson R W. An investigation into the significance of the N-linked oligosaccharides of Leishmania gp63. Mol Biochem Parasitol. 1994;63:23–35. doi: 10.1016/0166-6851(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 14.Joshi P B, Sacks D L, Modi G, McMaster W R. Targeted gene deletion of Leishmania major genes encoding developmental stage-specific leishmanolysin (GP63) Mol Microbiol. 1998;27:519–530. doi: 10.1046/j.1365-2958.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 15.Kawazu S I, Lu H-G, Chang K-P. Stage-independent splicing of transcripts from two heterogeneous neighboring genes in Leishmania amazonensis. Gene. 1997;196:49–59. doi: 10.1016/s0378-1119(97)00190-x. [DOI] [PubMed] [Google Scholar]
- 16.Kink J A, Chang K-P. Biological and biochemical characterization of tunicamycin-resistant Leishmania mexicana: mechanism of drug resistance and virulence. Infect Immun. 1987;55:1692–1700. doi: 10.1128/iai.55.7.1692-1700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kink J A, Chang K-P. N-Glycosylation as a biochemical basis for virulence in Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1988;27:181–190. doi: 10.1016/0166-6851(88)90037-0. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Chang K-P. The 63-kilobase circular amplicon of tunicamycin-resistant Leishmania amazonensis contains a functional N-acetylglucosamine-1-phosphate transferase gene that can be used as a dominant selectable marker in transfection. Mol Cell Biol. 1992;12:4112–4122. doi: 10.1128/mcb.12.9.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Chang K-P. Extrachromosomal genetic complementation of surface metalloproteinase (gp63)-deficient Leishmania increases their binding to macrophages. Proc Natl Acad Sci USA. 1992;89:4991–4995. doi: 10.1073/pnas.89.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Chang K-P. Identification by extrachromosomal amplification and overexpression of a zeta-crystallin/NADPH-oxidoreductase homologue constitutively expressed in Leishmania spp. Mol Biochem Parasitol. 1994;66:201–210. doi: 10.1016/0166-6851(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 21.McGwire B, Chang K-P. Genetic rescue of surface metalloproteinase (gp63)-deficiency in Leishmania amazonensis variants increases their infection of macrophages at the early phase. Mol Biochem Parasitol. 1994;66:345–347. doi: 10.1016/0166-6851(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 22.McGwire B S, Chang K-P. Posttranslational regulation of a Leishmania HEXXH metalloprotease (gp63). The effects of site-specific mutagenesis of catalytic, zinc binding, N-glycosylation, and glycosyl phosphatidylinositol addition sites on N-terminal end cleavage, intracellular stability, and extracellular exit. J Biol Chem. 1996;271:7903–7909. doi: 10.1074/jbc.271.14.7903. [DOI] [PubMed] [Google Scholar]
- 23.Medina-Acosta E, Karess R E, Schwartz H, Russell D G. The promastigote surface protease (gp63) of Leishmania is expressed, but differentially processed and localized in the amastigote stage. Mol Biochem Parasitol. 1989;37:263–273. doi: 10.1016/0166-6851(89)90158-8. [DOI] [PubMed] [Google Scholar]
- 24.Mosser D M, Edelson P J. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. Nature. 1987;327:329–331. doi: 10.1038/327329b0. [DOI] [PubMed] [Google Scholar]
- 25.Roberts S C, Wilson M E, Donelson J E. Developmentally regulated expression of a novel 59-kDa product of the major surface protease (Msp or gp63) gene family of Leishmania chagasi. J Biol Chem. 1995;270:8884–8892. doi: 10.1074/jbc.270.15.8884. [DOI] [PubMed] [Google Scholar]
- 26.Russell D G. Biology of the Leishmania surface: with particular reference to the surface proteinase, gp63. Protoplasma. 1994;181:191–201. [Google Scholar]
- 27.Russell D G, Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986;136:2613–2620. [PubMed] [Google Scholar]
- 28.Russell D G, Wright S D. Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. J Exp Med. 1988;168:279–292. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seay M B, Heard P L, Chaudhuri G. Surface Zn-proteinase as a molecule for defense of Leishmania mexicana amazonensis promastigotes against cytolysis inside macrophage phagolysosomes. Infect Immun. 1996;64:5129–5137. doi: 10.1128/iai.64.12.5129-5137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turco S J, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 31.Wilson M E. Leishmaniasis. Curr Opin Infect Dis. 1993;6:331–341. [Google Scholar]
- 32.Wilson M E, Hardin K K. The major concanavalin A-binding surface glycoprotein of Leishmania donovani chagasi promastigotes is involved in attachment to human macrophages. J Immunol. 1988;141:265–272. [PubMed] [Google Scholar]
- 33.Wilson M E, Hardin K K, Donelson J E. Expression of the major surface glycoprotein of Leishmania donovani chagasi in virulent and attenuated promastigotes. J Immunol. 1989;143:678–684. [PubMed] [Google Scholar]
- 34.Zhang W W, Matlashewski G. Loss of virulence in Leishmania donovani deficient in an amastigote-specific protein, A2. Proc Natl Acad Sci USA. 1997;94:8807–8811. doi: 10.1073/pnas.94.16.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]