Summary
Mediterranean people, which follows a diet rich in minimally-processed plant-based foods, are believed to live longer and healthier lives than many other populations in the Western world. Epidemiological and clinical data suggest that the Mediterranean diet has beneficial effects for several chronic diseases, such as cardiovascular diseases, obesity, cancer and diabetes. Although the mechanisms of action of the Mediterranean diet are not completely clear, the synergistic effects of a number of its components and their bioactive phytochemicals exert antioxidant, anti-inflammatory, anti-microbial and anti-cancer effects. The Mediterranean diet includes daily consumption of whole cereals, fruit, vegetables and legumes in moderate proportions, weekly consumption of white meat in low to moderate proportions and occasionally sweets and chocolates in small amounts. Since olive oil is the main lipids source, it has special significance for health. Healthy fruit and vegetables, rich in phytochemicals, are a major proportion of this diet and contribute to the overall nutritional value and bioactivity of its components. Here we review the nutritional and health benefits of wheat germ, tomatoes, olives and chili pepper, items at the base of Mediterranean diet food pyramid that provides beneficial molecules, such as polyphenols, vitamins and flavonoids, and exert anti-inflammatory, anti-microbial and anti-oxidative actions.
Keywords: Mediterranean diet, Flavonoids, Wheat germ, Chili pepper, Tomatoes, Olives
Citation
How to cite this article: Naureen Z, Dhuli K, Donato K, Aquilanti B, Velluti V, Matera G, Iaconelli A, Bertelli M. Foods of the Mediterranean diet: tomato, olives, chili pepper, wheat flour and wheat germ. J Prev Med Hyg 2022;63(suppl.3):E4-E11. https://doi.org/10.15167/2421-4248/jpmh2022.63.2S3.2740
Introduction
The Mediterranean diet is currently considered one of the healthiest diets in the world [1-3]. It features daily intake of nuts, vegetables, fruit, olives, olive oil, legumes and whole grains. Rich in antioxidants, fiber, vitamins, minerals, phytosterols, probiotics, omega 3 and omega 6 fatty acids, it has value for human health and wellbeing [1-3]. Mediterranean diet is a term coined for the dietary pattern of the people of the southern European countries in 1960s. This population was observed to have a high adult life expectancy, and low rate of diet-related chronic diseases and of certain cancers. The food pattern is typically represented in the form of a pyramid [1]. Indeed, regular consumption of whole grains, vegetables, fruit and legumes with plenty of olive oil and moderate weekly consumption of white meat and low consumption of red meat were the main features of their diet. Although it is still unclear why this diet affords health benefits, epidemiological and clinical studies have produced much evidence that it has lipid-lowering effects, scavenges free radicals thereby reducing oxidative stress, suppresses inflammation and platelet aggregation, restricts specific amino acids thereby inhibiting nutrient-sensing pathways, modulates and regulates growth factors and hormones involved in oncogenesis, and shapes the gut microbiota and corresponding metabolites, thereby influencing overall metabolic health [1-5]. Further research is needed to define the specific effects Mediterranean diet’s nutrients, and how their modification can modulate the microbiome, energy intake and expenditure, thus influencing cells, tissue and organ health during aging.
In addition, the Mediterranean diet is abundant in fresh fruit and vegetables and relies heavily on olive oil as lipid source. Low to moderate consumption of dairy products, red meat, fish, seafood, poultry and eggs [1-3] is another feature. Various studies report that portion-controlled consumption of the components of the diet is beneficial in preventing non-communicable diseases, including chronic and inflammatory diseases such as obesity [4-6], cardiovascular disease [7], diabetes [8, 9], metabolic syndrome [4] and cancer [10] and has a positive impact on autoimmune diseases [11, 12]. The diet has been observed to reduce inflammation and enhance mobility and vitality in patients with rheumatoid arthritis [12] and to decrease the risk of other autoimmune diseases including multiple sclerosis [11]. Among the several foods typical of the Mediterranean diet, wheat, tomatoes, chili pepper and olives proved to have high beneficial effects by many studies. Thus, in this review we focused on the beneficial properties of these specific foods.
Whole grain cereals
Since ancient times, cereals have remained the major staple food across the globe. Cereals are an important content of every diet, providing carbohydrates, dietary fiber and bioactive molecules with antioxidant, anti-cancer and anti-thrombotic action [13, 14]. Cereals and their derivatives, such as bread and pasta, contribute 55-60% of the total calories of the Mediterranean diet and are therefore indicated at the base of the food pyramid [15]. Unlike refined cereals, whole grain cereals maintain bran, germ, thus containing several bioactive molecules that promote health and mitigate metabolic disorders [16].
WHOLE WHEAT
Wheat is not only an important cash crop but also one of the most widely consumed staple foods in the world. Besides being a major source of starch, dietary fiber and energy, wheat provides substantial amounts of protein (60-70% of which is gluten), B vitamins, carotenoids, phenolic acids, benzoxazinoids, tocopherols, alkyl-resorcinol, phytosterols, biogenic amines and lignans [13-15]. The high fiber content of whole wheat helps digestion and bowel movements, thereby decreasing the risk of intestinal cancer [17]. Phenolic compounds, vitamin E and carotenoids with antioxidant properties help scavenge free radicals, thereby protecting against their deleterious effects.
Wheat polyphenols and antioxidants prevent or decrease the development of several chronic diseases, among which colon and breast cancer, type 2 diabetes and cardiovascular diseases [18-21]. Different varieties of bread wheat and wild-type wheat have been explored for their chemical constituents and bioactive compounds. Some bioactive compounds, such as phenols, antioxidants and vitamin E, are present in almost all types of wheat, however wild-type wheat contains more bioactive compounds. For instance, a comparative study of Triticum monococcum ssp. Monococcum, Triticum aestivum (bread wheat) and Triticum durum Desf. (durum wheat) revealed that the first had significantly higher total phenolic, ferulic acid and p-coumaric acid contents than bread and durum wheat, indicating its antioxidative activities and potential health benefits in reducing and preventing cardiovascular disease, diabetes and cancer, in addition to its valuable nutritional properties [22].
The main antioxidants of wheat grain are terpenoids and phenolic acids, such as hydroxycinnamic acid derivatives. They are not present in white wheat flour, while they are concentrated mainly in the bran and germ of wheat [21]. Examples are synapic and p-coumaric acids and dehydrodimers and dehydrotrimers of ferulic acid [23]. The phenols are bound to the cell wall of the bran by ester bonds. The highest antioxidant activity occurs in the aleurone layer of the wheat grain [24]. Other antioxidants in wheat bran are flavonoids, carotenoids (mainly lutein) and lignans [25, 26]. In addition to this, wheat contain many vitamins. Indeed, wheat is rich of vitamin B1 (thiamin), B2 (riboflavin), B3 (niacin), B6 (pyridoxine) and B9 (folate) [27].
WHEAT GERM
Wheat germ is usually removed with the milling process, although it is rich in nutrients [17]. Indeed, what germ contains a high amount of proteins and carbohydrates, as well as water and lipids. Moreover, as well as whole wheat, wheat germ contains bioactive molecules such as antioxidants (e.g., flavonoids, polyphenols, tocopherols, tocotrienol i.e. vitamin E), carotenoids, plant sterols and biogenic amines [18] (Tab. I).
Tab. I.
Classification of bioactive phytochemicals in whole wheat and wheat germ.
| Classification | Bioactive compounds | Bioactivity | References |
|---|---|---|---|
| Polyamines | Putrescine, spermine, and spermidine | Healthy metabolic function, antioxidants | [18, 19] |
| Phenolic acids | Hydroxy benzoic acid and hydroxy cinnamic acid derivatives e.g., γ-oryzanol, ferulic acid | Antioxidants, lower lipid absorption, anti-cancer, anti-inflammatory | [22] |
| Tocopherol | Alpha tocopherols and tocotrienols | Cardioprotective | [23] |
| Carotenoids | Carotenoids, lutein | Anti-cancer, anti-proliferative | [24] |
| Phenolic lipids | Alkylresorcinols such as 5-alkenylresorcinol | Antioxidant, anti-genotoxic and cytostatic activities | [22] |
Biogenic amines have several beneficial effects for human health, especially on fat metabolism, blood pressure and neurotransmitters regulation, but they can cause food poisoning if assumed in excess [19, 20]. Examples are putrescine, spermine, spermidine and histamine. Indeed, they are used as industrial food quality parameters in the Chemical Quality Index [19]. Several research studies proved that these polyamines have an antioxidant activity, preventing damages to cellular membrane and nucleic acids [18]. Wheat germ is rich in polyamine, especially if compared to other foodstuffs [21].
Another significant bioactive compound of wheat is gamma-oryzanol, a phenolic compound with antioxidant activity. Moreover, it participates in lipid metabolism, lowering lipid uptake and improving blood lipid levels [22]. Gamma-oryzanol have been tested in various diseases, such as diabetes, hyperlipidemia and cancer, for its beneficial effects [23-27].
Vegetables and fruit
Bran cereal fiber, fruit, vegetables and tea have a special place in the Mediterranean diet as they inhibit the onset and progress of cardiovascular disease and cancer by virtue of their antioxidant, anti-genotoxic, anti-inflammatory properties.
TOMATOES
Tomatoes both raw and cooked are extensively consumed in the Mediterranean region. Tomatoes are low in fats, rich in vitamins A and C, folate, potassium, carotenoids and polyphenols [28]. Dietary consumption of ripe red tomatoes (rich in polyphenols such as lycopene, flavanones and flavones and carotenoids such as phytoene, β-carotene and lycopene) has beneficial chemoprotective, anti-inflammatory, anti-genotoxic and anti-proliferative effects [29]. Tomato has been studied for its biologically active compounds (Tab. II).
Tab. II.
Bioactive compounds of tomato and their health effects.
| Classification | Bioactive compounds | Bioactivity | References |
|---|---|---|---|
| Phenolic acids | Caffeic, chlorogenic, synapic, p-coumaric and ferulic acids | Reduction of oxidative stress | [30, 31] |
| Flavonoids | Quercetin, rutin, kaempferol, chlorogenic acid and naringenin | Antioxidant, anti-cancer | [32, 33] |
| Carotenoids | Lycopene, α-carotene, β-carotene, γ-carotene, δ-carotene, phytoene, phytofluene, neurosporene and lutein | Antioxidant | [34] |
| Vitamins | A, B, C and E | Antioxidants, radical scavenging | [35] |
| Glycoalkaloids | α-tomatine and dihydroxy tomatine | Toxic to pathogens | [36] |
Rich in phytochemicals with anti-proliferative, anti-mutagenic, anti-cancer, anti-inflammatory, and anti-oxidative properties, tomatoes inhibit the onset and progression of chronic diseases, such as cardiovascular disease and cancer. For instance, lycopene and vitamins C and E reduce oxidative stress and the risk of chronic diseases [34, 35]. Specifically, blood concentrations of lycopene are reported to be inversely correlated to the incidence of heart disease [37]. Tomato intake is also inversely correlated with atherosclerosis by virtue of its anti-inflammatory properties [38]. Lipophilic compounds in tomatoes modulate LDLs and the corresponding atherogenic processes in endothelial cells, thereby reducing the risk of cardiovascular disease and atherosclerosis [39].
Tomato products have shown protective effects against lung and prostate cancer [40, 41]. This may be because polyphenols and carotenoids prevent tumorigenesis by impeding the initiation and progression of cancer [34]. Moreover, flavonoids such as quercetin foster chromatin remodeling, thereby inhibiting epigenetic changes that promote cancer [34].
Because tomatoes are rich in carotenoids, tomato consumption promotes intercellular signaling pathways, modulates immune reactions, regulates the cell cycle, induces apoptosis, and interacts with many physiological systems, thereby playing an important role in protecting against chronic diseases [42]. One of the most studied carotenoids of tomato is lycopene. It is stably released during cooking when the plant cell wall is disrupted by heating [43].
In the Mediterranean diet, absorption of lycopene, a non-polar compound, is favored by olive oil. Since lycopene has many useful properties, it has been extensively studied. Figure 1 summarizes some interesting studies on lycopene bioactivity.
Fig. 1.
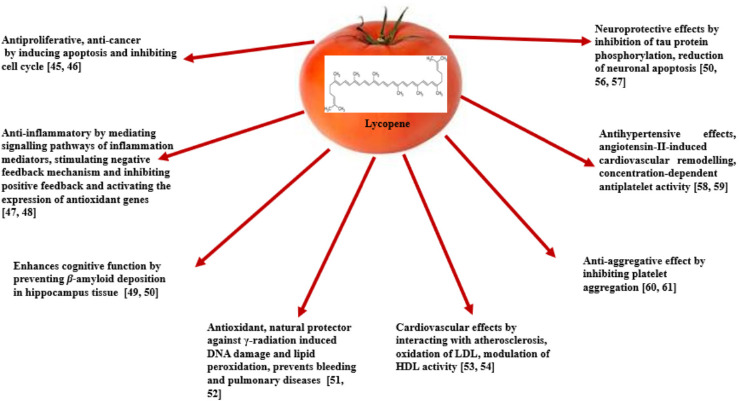
Health benefits of lycopene.
OLIVES
Olives trees, olives and olive oils have special significance in the Mediterranean region, providing an important food and nutrient source for the indigenous populations of the region. The fruit of the olive tree is processed to obtain table olives which are consumed daily in a moderate amount. Olives contain nutritional and non-nutritional components such as carbohydrates, proteins, lipids, minerals, water and phenols [62]. Phenolic compounds occur in almost all parts of olives in different concentrations. The major phenolic compounds are acids, alcohols, flavonoids and secoiridoids [63, 62]. The hydroxytyrosol derivatives, ligstroside, oleuropein and verbascoside are the most abundant biologically active phenolic alcohols of the olive fruit along with tyrosols [63]. They are produced by hydrolysis of oleuropein in the unripe fruit, removing the typical bitter taste of untreated olives [64, 65]. Besides being precursors of hydroxytyrosol, ligstroside and verbascoside, oleuropein has cardioprotective, anti-hypertensive, antioxidant and anti-inflammatory properties [66]. Hydroxytyrosol has been proposed for the treatment of diseases, such as lymphedema and COVID-19 [67-71]. Olive fruits are also rich in flavonoids such as quercetin-3-rutinoside, apigenin-7-rutinoside, apigenin-7-glucoside, luteolin-7-glucoside and luteolin-5-glucoside [72].
Since the phenolic compounds of olive fruits are high in hydroxytyrosol and flavonoids, they have anti-carcinogenic, anti-proliferative and antioxidant activity [73]. Hydroxytyrosol has been shown to scavenge free radicals and activate glutathione reductase and superoxide dismutase [74, 75]. It also has anti-inflammatory properties through inhibition of prostaglandin E, nitric oxide and inflammatory cytokines release [76]. It plays a cardioprotective role by reducing oxidative stress [19, 64], and has cancer preventing properties, significantly suppressing tumor proliferation in skin and breast tissue [77]. Hydroxytyrosol, oleuropein, ligstroside and verbascoside are also useful in the treatment of osteoarthritis [78], in neuroprotection and for wound healing (Fig. 2) [79].
Fig. 2.
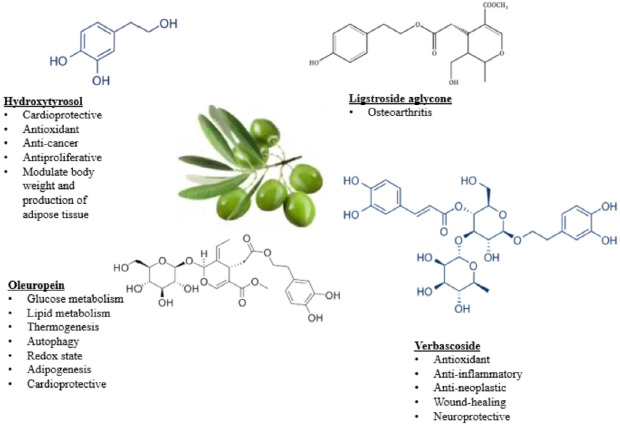
Nutraceutical effects of hydroxytyrosol and its derivatives in olives.
CHILI PEPPER
Epidemiological data regarding chili pepper, a significant constituent of the Mediterranean diet, is still scarce. Chili pepper (Capsicum annuum) belongs to the highly diverse and globally distributed genus Capsicum of the Solanaceae family, in which domesticated species such as C. baccatum, C. pubescens, C. chinense and C. frutescens are also present. Although chili pepper is best known for its pungent flavor, it has significant amounts of carotenoids, provitamin A, vitamins K, E and C and phenolic compounds such as capsaicinoids, quercetin and luteolin (Fig. 3), which act synergistically to confer antioxidant, anti-inflammatory, anti-cancer, anti-microbial, antiseptic and immunomodulatory properties [80-82]. This makes chili pepper particularly useful in scavenging free radicals and promoting good health.
Fig. 3.
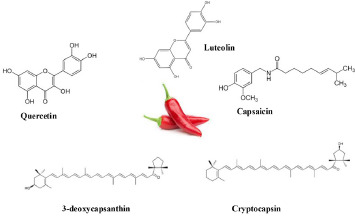
Major phytochemicals of chili pepper.
Chili pepper have been used for the treatment and prevention of several conditions, such as toothache, wound healing, sore throats, cough, parasitic infections and rheumatism. Creams containing capsaicin and capsicum are commercially available for intractable pain and HIV-linked neuropathy [82]. Antioxidant carotenoids (α- and β- carotenes), vitamin C and pro-vitamin A in capsicum peppers support immune function and combat inflammation, thereby easing rheumatism, asthma attacks and arthritis [83]. Chili pepper has been studied for its biologically active compounds (Tab. III).
Tab. III.
Biologically active metabolites of chili pepper.
| Biological activity | Metabolites | Target cell/organism/disorder | Mechanism | References |
|---|---|---|---|---|
| Anti-proliferative | Quercetin | Glioblastoma multiforme | Regulates several proteins participating in cell signal transduction | [84] |
| Antimicrobial | Capsaicin | Bacillus, Micrococcus sp., E. coli, Pseudomonas sp., Citrobacter sp., Salmonella typhimurium, Pseudomonas aeruginosa | Affects membrane stability | [85, 86] |
| Antiviral | Capsaicin | Guinea pig cutaneous herpes simplex virus | Disrupts virus-neuron connections | [87] |
| Insecticidal | Capsicum frutescens extracts | A. aegypti mosquitoes | Extract acts as repellent | [88] |
| Anthelmintic and larvicidal | Capsicum annuum leaf extract essential oils | Cercaria of Schistosoma mansoni | Kills larvae | [89] |
| Cardiovascular effects | Capsaicin (10-300 μg/kg) | Mongrel dogs | Temporary increase in mean systemic blood pressure | [90] |
| Anti-inflammatory | Carotenoids | Egg albumin-induced inflammation of rat hind paw | Reduces inflammation | [91] |
| Capsaicin | Osteoarthritis | Pain relief | [92] |
Conclusion
The Mediterranean diet is rich in fresh fruit, vegetables and cereals that not only are economical but a healthy choice for a long life. These ingredients at the base of the pyramid are recommended for daily consumption. Rich in phytochemicals such as vitamins, carotenoids, flavonoids and phenols, these dietary items may be responsible for the beneficial effects of the Mediterranean diet. Indeed, the Mediterranean population is believed to live longer and healthier than many other population in the Western world, with low incidence of non-communicable diseases such as obesity, cardiovascular diseases and cancer. It therefore stands to reason that the Mediterranean diet holds promise for healthy people with a healthy lifestyle and also for people with chronic health conditions.
Acknowledgements
This research was funded by the Provincia Autonoma di Bolzano in the framework of LP 15/2020 (dgp 3174/2021).
Conflicts of interest statement
Authors declare no conflict of interest.
Author's contributions
MB: study conception, editing and critical revision of the manuscript; ZN, Kristjana D, Kevin D, BA, VV, GM, AI: literature search, editing and critical revision of the manuscript. All authors have read and approved the final manuscript.
Figures and tables
References
- [1].Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 1995;61:1402S-6S. https://doi.org/10.1093/ajcn/61.6.1402S 10.1093/ajcn/61.6.1402S [DOI] [PubMed] [Google Scholar]
- [2].Pocovi-Gerardino G, Correa-Rodríguez M, Callejas-Rubio JL, Ríos-Fernández R, Martín-Amada M, Cruz-Caparros MG, Rueda-Medina B, Ortego-Centeno N. Beneficial effect of Mediterranean diet on disease activity and cardiovascular risk in systemic lupus erythematosus patients: a cross-sectional study. Rheumatology 2021;60:160-9. https://doi.org/10.1093/rheumatology/keaa210 10.1093/rheumatology/keaa210 [DOI] [PubMed] [Google Scholar]
- [3].Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean diet; a literature review. Nutrients 2015;7:9139-53. https://doi.org/10.1093/ajcn/61.6.1402S 10.1093/ajcn/61.6.1402S [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Babio N, Bulló M, Basora J, Martínez-González MA, Fernández-Ballart J, Márquez-Sandoval F, Molina C, Salas-Salvadó J, Nureta-PREDIMED Investigators . Adherence to the Mediterranean diet and risk of metabolic syndrome and its components. Nutr Metab Cardiovasc Dis 2009;19:563-70. https://doi.org/10.1016/j.numecd.2008.10.007 10.1016/j.numecd.2008.10.007 [DOI] [PubMed] [Google Scholar]
- [5].Estruch R. Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc 2010;69:333-40. https://doi.org/10.1016/j.numecd.2008.10.007 10.1016/j.numecd.2008.10.007 [DOI] [PubMed] [Google Scholar]
- [6].Martínez-González MA, García-Arellano A, Toledo E, Salas-Salvadó J, Buil-Cosiales P, Corella D, Covas MI, Schröder H, Arós F, Gómez-Gracia E, Fiol M, Ruiz-Gutiérrez V, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Muñoz MA, Wärnberg J, Ros E, Estruch R, PREDIMED Study Investigators . A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One 2012;7:e43134. https://doi.org/10.1371/journal.pone.0043134 10.1371/journal.pone.0043134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rosato V, Temple NJ, La Vecchia C, Castellan G, Tavani A, Guercio V. Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur J Nutr 2019;58:173-91. https://doi.org/10.1007/s00394-017-1582-0 10.1007/s00394-017-1582-0 [DOI] [PubMed] [Google Scholar]
- [8].Agnoli C, Sieri S, Ricceri F, Giraudo MT, Masala G, Assedi M, Panico S, Mattiello A, Tumino R, Giurdanella MC, Krogh V. Adherence to a Mediterranean diet and long-term changes in weight and waist circumference in the EPIC-Italy cohort. Nutr Diabetes 2018;8:22. https://doi.org/10.1038/s41387-018-0023-3 10.1038/s41387-018-0023-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open 2015;5:e008222. https://doi.org/10.1136/bmjopen-2015-008222 10.1136/bmjopen-2015-008222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients 2017;9:1063. https://doi.org/10.3390/nu9101063 10.3390/nu9101063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sedaghat F, Jessri M, Behrooz M, Mirghotbi M, Rashidkhani B. Mediterranean diet adherence and risk of multiple sclerosis: a case-control study. Asia Pac J Clin Nutr 2016;25:377-84. https://doi.org/10.6133/apjcn.2016.25.2.12 10.6133/apjcn.2016.25.2.12 [DOI] [PubMed] [Google Scholar]
- [12].Forsyth C, Kouvari M, D'Cunha NM, Georgousopoulou EN, Panagiotakos DB, Mellor DD, Kellett J, Naumovski N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: a systematic review of human prospective studies. Rheumatol Int 2018;38:737-47. https://doi.org/10.1007/s00296-017-3912-1 10.1007/s00296-017-3912-1 [DOI] [PubMed] [Google Scholar]
- [13].Forum O.B.O.T.H., Ross AB, van der Kamp JW, King R, Lê KA, Mejborn H, Seal CJ, Thielecke F. Perspective: A Definition for Whole-Grain Food Products—Recommendations from the Healthgrain Forum. Adv Nutr 2017;8:525-31. https://doi.org/10.3945/an.116.014001. 10.3945/an.116.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cavazos A, De Mejia EG. Identification of Bioactive Peptides from Cereal Storage Proteins and Their Potential Role in Prevention of Chronic Diseases. Compr Rev Food Sci Food Saf 2013;12:364-80. https://doi.org/10.1111/1541-4337.12017 10.1111/1541-4337.12017 [DOI] [PubMed] [Google Scholar]
- [15].Brites C. Dimensions of Mediterranean Diet: World cultural Heritage. Universidade do Algarve, Faro, Portugal, 2015. Cereals in the context of the Mediterranean Diet 2015, pp. 181-95. [Google Scholar]
- [16].De Jong N, Hoendervangers CT, Bleeker JK, Ocké MC. The opinion of Dutch dietitians about functional foods. J Hum Nutr Diet 2004;17:55-62. https://doi.org/10.1046/j.1365-277x.2003.00498.x 10.1046/j.1365-277x.2003.00498.x [DOI] [PubMed] [Google Scholar]
- [17].Huang T, Xu M, Lee A, Cho S, Qi L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med 2015;13:1-9. https://doi.org/10.1186/s12916-015-0294-7 10.1186/s12916-015-0294-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jangam GB, Badole SL. Polyphenols in Human Health and Disease. In: Watson RR, Preedy VR, Zibadi S, eds. London: Elsevier Inc. 2014. [Google Scholar]
- [19].Visioli F, Borsani L, Galli C. Diet and prevention of coronary heart disease: the potential role of phytochemicals. Cardiovasc Res 2000;47:419-25. https://doi.org/10.1016/s0008-6363(00)00053-5 10.1016/s0008-6363(00)00053-5 [DOI] [PubMed] [Google Scholar]
- [20].Dembinska-Kiec A, Mykkänen O, Kiec-Wilk B, Mykkänen H. Antioxidant phytochemicals against type 2 diabetes. Br J Nutr 2008;99:ES109-17. https://doi.org/10.1017/S000711450896579X 10.1017/S000711450896579X [DOI] [PubMed] [Google Scholar]
- [21].Lv J, Yu L, Lu Y, Niu Y, Liu L, Costa J, Yu LL. Phytochemi-cal compositions, and antioxidant properties, and antipro-liferative activities of wheat flour. Food Chem 2012;135:325-31. https://doi.org/10.1016/j.foodchem.2012.04.141 10.1016/j.foodchem.2012.04.141 [DOI] [PubMed] [Google Scholar]
- [22].Şahin Y, Yıldırım A, Yücesan B, Zencirci N, Erbayram Ş, Gürel E. Phytochemical content and antioxidant activity of einkorn (Triticum monococcum ssp. monococcum), bread (Triticum aestivum L.), and durum (Triticum durum Desf.) wheat. Progr Nutr 2017;19:450-9. https://doi.org/10.23751/pn.v19i4.5847 10.23751/pn.v19i4.5847 [DOI] [Google Scholar]
- [23].Laddomada B, Caretto S, Mita G. Wheat bran phenolic acids: Bioavailability and stability in whole wheat-based foods. Molecules 2015;20:15666-85. https://doi.org/10.3390/molecules200915666 10.3390/molecules200915666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mateo Anson N, van den Berg R, Havenaar R, Bast A, RMM Haenen G. Ferulic acid from aleurone determines the antioxidant potency of wheat grain (Triticum aestivum L.). J Agric Food Chem 2008;56:5589-94. https://doi.org/10.1021/jf800445k 10.1021/jf800445k [DOI] [PubMed] [Google Scholar]
- [25].Adom KK, Sorrells ME, Liu RH. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J Agric Food Chem 2005;53:2297-306. https://doi.org/10.1021/jf048456d 10.1021/jf048456d [DOI] [PubMed] [Google Scholar]
- [26].Qu H, Madl RL, Takemoto DJ, Baybutt RC, Wang W. Lignans are involved in the antitumor activity of wheat bran in colon cancer SW480 cells. J Nutr 2005;135:598-602. https://doi.org/10.1093/jn/135.3.598 10.1093/jn/135.3.598 [DOI] [PubMed] [Google Scholar]
- [27].Liaqat H, Kim KJ, Park SY, Jung SK, Park SH, Lim S, Kim JY. Antioxidant effect of wheat germ extracts and their antilipidemic effect in palmitic acid-induced steatosis in HepG2 and 3T3-L1 cells. Foods 2021;10:1061. https://doi.org/10.3390/foods10051061 10.3390/foods10051061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tan H, Thomas-ahner JM, Grainger EM, Wan L, Francis DM, Schwartz SJ, Clinton SK. Tomato-based food products for prostate cancer prevention: what have we learned? Cancer Metastasis Rev 2010;29:553-68. https://doi.org/10.1007/s10555-010-9246-z 10.1007/s10555-010-9246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chaudhary P, Sharma A, Singh B, Nagpal AK. Bioactivities of phytochemicals present in tomato. J Food Sci Technol 2018;55:2833-49. https://doi.org/10.1007/s13197-018-3221-z 10.1007/s13197-018-3221-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Singh B, Singh JP, Kaur A, Singh N. Phenolic composition and antioxidant potential of grain legume seeds: a review. Food Res Int 2017;101:1-16. https://doi.org/10.1016/j.foodres.2017.09.026 10.1016/j.foodres.2017.09.026 [DOI] [PubMed] [Google Scholar]
- [31].Singh B, Singh JP, Kaur A, Singh N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: a review. Food Chem 2018;261:75-86. https://doi.org/10.1016/j.foodchem.2018.04.039 10.1016/j.foodchem.2018.04.039 [DOI] [PubMed] [Google Scholar]
- [32].Sharma A, Kaur M, Katnoria JK, Nagpal AK. Polyphenols in food: cancer prevention and apoptosis induction. Curr Med Chem 2018;25:4740-57. https://doi.org/10.2174/0929867324666171006144208 10.2174/0929867324666171006144208 [DOI] [PubMed] [Google Scholar]
- [33].Tomas M, Beekwilder J, Hall RD, Sagdic O, Boyacioglu D, Capanoglu E. Industrial processing versus home processing of tomato sauce: effects on phenolics, flavonoids and in vitro bioaccessibility of antioxidants. Food Chem 2017;220:51-8. https://doi.org/10.1016/j.foodchem.2016.09.201 10.1016/j.foodchem.2016.09.201 [DOI] [PubMed] [Google Scholar]
- [34].Martí R, Leiva-Brondo M, Lahoz I, Campillo C, Cebolla-Cornejo J, Roselló S. Polyphenol and l-ascorbic acid content in tomato as influenced by high lycopene genotypes and organic farming at different environments. Food Chem 2018;239:148-56. https://doi.org/10.1016/j.foodchem.2016.09.201 10.1016/j.foodchem.2016.09.201 [DOI] [PubMed] [Google Scholar]
- [35].Watada AE, Aljlenbach BB, Worthington JT. Vitamins A and C in ripe tomatoes as affected by stage of ripeness at harvest and by supplementary ethylene. J Food Sci 1976;41:856-8. https://doi.org/10.1111/j.1365-2621.1976.tb00738_41_4.x 10.1111/j.1365-2621.1976.tb00738_41_4.x [DOI] [Google Scholar]
- [36].Friedman M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α -tomatine, and tomatidine in pure form and in fresh and processed tomatoes. Agric Food Chem 2013;61:9534-50. https://doi.org/10.1021/jf402654e 10.1021/jf402654e [DOI] [PubMed] [Google Scholar]
- [37].Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am J Clin Nutr 2004;79:47-53. https://doi.org/10.1093/ajcn/79.1.47 10.1093/ajcn/79.1.47 [DOI] [PubMed] [Google Scholar]
- [38].Hazewindus M, Haenen GR, Weseler AR, Bast A. Protection against chemotaxis in the anti-inflammatory effect of bioactives from tomato ketchup. PLoS ONE 2014;9:e114387. https://doi.org/10.1371/journal.pone.0114387 10.1371/journal.pone.0114387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Viuda-Martos M, Sanchez-Zapata E, Sayas-Barberá E, Sendra E, Pérez-Álvarez JA, Fernández-López J. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: a review. Crit Rev Food Sci Nutr 2014;54:1032-49. https://doi.org/10.1080/10408398.2011.623799 10.1080/10408398.2011.623799 [DOI] [PubMed] [Google Scholar]
- [40].Hwang ES, Bowen PE. Cell cycle arrest and induction of apoptosis by lycopene in LNCaP human prostate cancer cells. J Med Food 2004;7:284-9. https://doi.org/10.1089/jmf.2004.7.284 10.1089/jmf.2004.7.284 [DOI] [PubMed] [Google Scholar]
- [41].Palozza P, Serini S, Boninsegna A, Bellovino D, Lucarini M, Monastra G, Gaetani S. The growth-inhibitory effects of tomatoes digested in vitro in colon adenocarcinoma cells occur through down regulation of cyclin D1, Bcl-2 and Bcl-xL. Br J Nutr 2007;98:789-95. https://doi.org/10.1017/S0007114507746883 10.1017/S0007114507746883 [DOI] [PubMed] [Google Scholar]
- [42].Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res 2007;55:207-16. https://doi.org/10.1016/j.phrs.2007.01.012 10.1016/j.phrs.2007.01.012 [DOI] [PubMed] [Google Scholar]
- [43].Fernández-garcía E, Carvajal-lérida I, Jarén-galán M, Garrido-fernández J, Pérez-gálvez A, Hornero-méndez D. Carotenoids bioavailability from foods: from plant pigments to efficient biological activities. Food Res Int 2012;46:438-50. https://doi.org/10.1016/j.foodres.2011.06.007 10.1016/j.foodres.2011.06.007 [DOI] [Google Scholar]
- [44].Amir H, Karas M, Giat J, Danilenko M, Levy R, Yermiahu T, Sharoni Y. Lycopene and 1, 25-dihydroxyvitamin d3 cooperate in the inhibition of cell cycle progression and induction of differentiation in hl-60 leukemic cells. Nutr Cancer 1999;33:105-12. https://doi.org/10.1080/01635589909514756 10.1080/01635589909514756 [DOI] [PubMed] [Google Scholar]
- [45].Kelkel M, Schumacher M, Dicato M, Diederich M. Antioxidant and anti-proliferative properties of lycopene. Free radical research. 2011;45:925-40. https://doi.org/10.3109/10715762.2011.564168 10.3109/10715762.2011.564168 [DOI] [PubMed] [Google Scholar]
- [46].Fornelli F, Leone A, Verdesca I, Minervini F, Zacheo G. The influence of lycopene on the proliferation of human breast cell line (MCF-7). Toxicol In Vitro 2007;21:217-223. https://doi.org/10.1016/j.tiv.2006.09.024 10.1016/j.tiv.2006.09.024 [DOI] [PubMed] [Google Scholar]
- [47].Hung CF, Huang TF, Chen BH, Shieh JM, Wu PH, Wu WB. Lycopene inhibits TNF-α-induced endothelial ICAM-1 expression and monocyte-endothelial adhesion. Eur J Pharmacol 2008;586:275-82. https://doi.org/10.1016/j.tiv.2006.09.024 10.1016/j.tiv.2006.09.024 [DOI] [PubMed] [Google Scholar]
- [48].Rafi MM, Yadav PN, Reyes M. Lycopene inhibits LPS-induced proinflammatory mediator inducible nitric oxide synthase in mouse macrophage cells. J Food Sci 2007;72:S069-S074. https://doi.org/10.1111/j.1750-3841.2006.00219.x 10.1111/j.1750-3841.2006.00219.x [DOI] [PubMed] [Google Scholar]
- [49].Liu CB, Wang R, Yi YF, Gao Z, Chen YZ. Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer’s disease rats. J Nutr Biochem 2018;53:66-71. https://doi.org/10.1016/j.jnutbio.2017.10.014 10.1016/j.jnutbio.2017.10.014 [DOI] [PubMed] [Google Scholar]
- [50].Yu L, Wang W, Pang W, Xiao Z, Jiang Y, Hong Y. Dietary lycopene supplementation improves cognitive performances in tau transgenic mice expressing P301L mutation via inhibiting oxidative stress and tau hyperphosphorylation. J Alzheimers Dis 2017;57:475-82. https://doi.org/10.3233/JAD-161216 10.3233/JAD-161216 [DOI] [PubMed] [Google Scholar]
- [51].Bai SK, Lee SJ, Na HJ, Ha KS, Han JA, Lee H, Kim YM. [beta]-carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-[kappa] B activation. Exp Mol Med 2005;37:323. https://doi.org/10.1038/emm.2005.42 10.1038/emm.2005.42 [DOI] [PubMed] [Google Scholar]
- [52].Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. Lycopene as a natural protector against γ-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes in vitro. Biochim Biophys Acta 2007;1770:659-65. https://doi.org/10.1016/j.bbagen.2006.11.008 10.1016/j.bbagen.2006.11.008 [DOI] [PubMed] [Google Scholar]
- [53].Thies F, Masson LF, Rudd A, Vaughan N, Tsang C, Brittenden J, Simpson WG, Duthie S, Horgan GW, Duthie G. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: a randomized controlled trial. Am J Clin Nutr 2012;95:1013-22. https://doi.org/10.3945/ajcn.111.026286 10.3945/ajcn.111.026286 [DOI] [PubMed] [Google Scholar]
- [54].Gajendragadkar PR, Hubsch A, Mäki-Petäjä KM, Serg M, Wilkinson IB, Cheriyan J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: a randomised controlled trial. PloS one 2014;9:e99070. https://doi.org/10.1371/journal.pone.0099070 10.1371/journal.pone.0099070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen D, Huang C, Chen Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed Pharmacother 2019;111:791-801. https://doi.org/10.1016/j.biopha.2018.12.151 10.1016/j.biopha.2018.12.151 [DOI] [PubMed] [Google Scholar]
- [56].Prema A, Janakiraman U, Manivasagam T, Thenmozhi AJ. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson’s disease in mice. Neurosci Lett 2015;599:12-9. https://doi.org/10.1016/j.neulet.2015.05.024 10.1016/j.neulet.2015.05.024 [DOI] [PubMed] [Google Scholar]
- [57].Chen W, Mao L, Xing H, Xu L, Fu X, Huang L, Huang D, Pu Z, Li Q. Lycopene attenuates Aβ1–42 secretion and its toxicity in human cell and Caenorhabditis elegans models of Alzheimer disease. Neurosci Lett 2015;608:28-33. https://doi.org/10.1016/j.neulet.2015.10.009 10.1016/j.neulet.2015.10.009 [DOI] [PubMed] [Google Scholar]
- [58].Saini RK, Rengasamy KR, Mahomoodally FM, Keum YS. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol Res 2020;155:104730. https://doi.org/10.1016/j.phrs.2020.104730 10.1016/j.phrs.2020.104730 [DOI] [PubMed] [Google Scholar]
- [59].Li N, Wu X, Zhuang W, Xia L, Chen Y, Wu C, Rao Z, Du L, Zhao R, Yi M, Wan Q. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem 2021;343:128396. https://doi.org/10.1016/j.foodchem.2020.128396 10.1016/j.foodchem.2020.128396 [DOI] [PubMed] [Google Scholar]
- [60].Mozos I, Stoian D, Caraba A, Malainer C, Horbańczuk JO, Atanasov AG. Lycopene and vascular health. Front Pharmacol 2018;9:521. https://doi.org/10.3389/fphar.2018.00521 10.3389/fphar.2018.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Böhm V. Lycopene and heart health. Mol Nutr Food Res 2012;56:296-303. https://doi.org/10.1002/mnfr.201100281 10.1002/mnfr.201100281 [DOI] [PubMed] [Google Scholar]
- [62].Boskou D. Olive oil: chemistry and technology. New York: AOCS Publishing; 2006. [Google Scholar]
- [63].Ryan D, Robards K. Critical Review. Phenolic compounds in olives. Analyst 1998;123:31R-44R. https://doi.org/10.1039/A708920A 10.1039/A708920A9581017 [DOI] [Google Scholar]
- [64].Visioli F, Galli C. Olive oil: more than just oleic acid. Am J Clin Nutr 2000;72:853. https://doi.org/10.1093/ajcn/72.3.853 10.1093/ajcn/72.3.853 [DOI] [PubMed] [Google Scholar]
- [65].Waterman E, Lockwood B. Active components and clinical applications of olive oil. Altern Med Rev 2007;12. [PubMed] [Google Scholar]
- [66].Omar SH. Cardioprotective and neuroprotective roles of oleuropein in olive. Saudi Pharm J 2010;18:111-21. https://doi.org/10.1016/j.jsps.2010.05.005 10.1016/j.jsps.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dhuli K, Ceccarini MR, Precone V, Maltese PE, Bonetti G, Paolacci S, Dautaj A, Guerri G, Marceddu G, Beccari T, Michelini S, Bertelli M. Improvement of quality of life by intake of hydroxytyrosol in patients with lymphedema and association of lymphedema genes with obesity. Eur Rev Med Pharmacol Sci 2021;25:33-42. https://doi.org/10.26355/eurrev_202112_27331 10.26355/eurrev_202112_27331 [DOI] [PubMed] [Google Scholar]
- [68].Paolacci S, Ergoren MC, De Forni D, Manara E, Poddesu B, Cugia G, Dhuli K, Camilleri G, Tuncel G, Kaya Suer H, Sultanoglu N, Sayan M, Dundar M, Beccari T, Ceccarini MR, Gunsel IS, Dautaj A, Sanlidag T, Connelly ST, Tartaglia GM, Bertelli M. In vitro and clinical studies on the efficacy of α-cyclodextrin and hydroxytyrosol against SARS-CoV-2 infection. Eur Rev Med Pharmacol Sci 2021;25,81-9. https://doi.org/10.26355/eurrev_202112_27337 10.26355/eurrev_202112_27337 [DOI] [PubMed] [Google Scholar]
- [69].Paolacci S, Kiani AK, Shree P, Tripathi D, Tripathi YB, Tripathi P, Tartaglia GM, Farronato M, Farronato G, Connelly ST, Ceccarini MR, Coatto M, Ergoren MC, Sanlidag T, Dautaj A, Bertelli M. Scoping review on the role and interactions of hydroxytyrosol and alpha-cyclodextrin in lipid-raft-mediated endocytosis of SARS-CoV-2 and bioinformatic molecular docking studies. European review for medical and pharmacological sciences Eur Rev Med Pharmacol Sci 2021;25:90-100. https://doi.org/10.26355/eurrev_202112_27338 10.26355/eurrev_202112_27338 [DOI] [PubMed] [Google Scholar]
- [70].Bertelli M, Kiani AK, Paolacci S, Manara E, Dautaj A, Beccari T, Michelini S. Molecular pathways involved in lymphedema: Hydroxytyrosol as a candidate natural compound for treating the effects of lymph accumulation. J Biotechnol 2020;308:82-6. https://doi.org/10.1016/j.jbiotec.2019.11.017 10.1016/j.jbiotec.2019.11.017 [DOI] [PubMed] [Google Scholar]
- [71].Ergoren MC, Paolacci S, Manara E, Dautaj A, Dhuli K, Anpilogov K, Camilleri G, Suer HK, Sayan M, Tuncel G, Sultanoglu N, Farronato M, Tartaglia GM, Dundar M, Farronato G, Gunsel IS, Bertelli M, Sanlidag T. A pilot study on the preventative potential of alpha-cyclodextrin and hydroxytyrosol against SARS-CoV-2 transmission. Acta Biomed 2020;91:e2020022. https://doi.org/10.23750/abm.v91i13-S.10817 10.23750/abm.v91i13-S.10817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Amiot MJ, Fleuriet A, Macheix JJ. Importance and evolution of phenolic compounds in olive during growth and maturation. J Agric Food Chem 1986;34:823-6. https://doi.org/10.1021/jf00071a014 10.1021/jf00071a014 [DOI] [Google Scholar]
- [73].Bernini R, Merendino N, Romani A, Velotti F. Naturally occurring hydroxytyrosol: synthesis and anticancer potential. Curr Med Chem 2013;20:655-70. https://doi.org/10.2174/092986713804999367 10.2174/092986713804999367 [DOI] [PubMed] [Google Scholar]
- [74].Bertelli M, Kiani AK, Paolacci S, Manara E, Kurti D, Dhuli K, Bushati V, Miertus J, Pangallo D, Baglivo M, Beccari T, Michelini S. Hydroxytyrosol: A natural compound with promising pharmacological activities. J Biotechnol 2020;309:29-33. https://doi.org/10.1016/j.jbiotec.2019.12.016 10.1016/j.jbiotec.2019.12.016 [DOI] [PubMed] [Google Scholar]
- [75].Giordano E, Dávalos A, Visioli F. Chronic hydroxytyrosol feeding modulates glutathione-mediated oxido-reduction pathways in adipose tissue: A nutrigenomic study. Nutr Metab Cardiovasc Dis 2014;24:1144-50. https://doi.org/10.1016/j.numecd.2014.05.003 10.1016/j.numecd.2014.05.003 [DOI] [PubMed] [Google Scholar]
- [76].Zhang X, Cao J, Zhong L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn Schmiedebergs Arch Pharmacol 2009;379:581-6. https://doi.org/10.1007/s00210-009-0399-7 10.1007/s00210-009-0399-7 [DOI] [PubMed] [Google Scholar]
- [77].Kimura Y, Sumiyoshi M. Olive leaf extract and its main component oleuropein prevent chronic ultraviolet B radiation-induced skin damage and carcinogenesis in hairless mice. J Nutr 2009;139:2079-86. https://doi.org/10.3945/jn.109.104992 10.3945/jn.109.104992 [DOI] [PubMed] [Google Scholar]
- [78].de Andrés MC, Meiss MS, Sánchez-Hidalgo M, González-Benjumea A, Fernández-Bolaños JG, Alarcón-de-la-Lastra C, Oreffo RO. Osteoarthritis treatment with a novel nutraceutical acetylated ligstroside aglycone, a chemically modified extra-virgin olive oil polyphenol. J Tissue Eng 2020;11:2041731420922701. https://doi.org/10.1177/2041731420922701 10.1177/2041731420922701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Alipieva K, Korkina L, Orhan IE, Georgiev MI. Verbascoside--a review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol Adv 2014;32:1065-76. https://doi.org/10.1016/j.biotechadv.2014.07.001 10.1016/j.biotechadv.2014.07.001 [DOI] [PubMed] [Google Scholar]
- [80].Reilly CA, Crouch DJ, Yost GS, Fatah AA. Determination of capsaicin, dihydrocapsaicin, and nonivamide in self-defense weapons by liquid chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry. J Chromatogr A 2001;912:259-67. https://doi.org/10.1016/s0021-9673(01)00574-x 10.1016/s0021-9673(01)00574-x [DOI] [PubMed] [Google Scholar]
- [81].Reyes-Escogido Mde L, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E. Chemical and pharmacological aspects of capsaicin. Molecules 2011;16:1253-70. https://doi.org/10.3390/molecules16021253 10.3390/molecules16021253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Batiha GE, Alqahtani A, Ojo OA, Shaheen HM, Wasef L, Elzeiny M, Ismail M, Shalaby M, Murata T, Zaragoza-Bastida A, Rivero-Perez N. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. Int J Mol Sci 2020;21:5179. https://doi.org/10.3390/ijms21155179 10.3390/ijms21155179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Santos LS, Fernandes CC, Dias AL, Souchie EL, Miranda ML. Phenolic compounds and antifungal activity of ethyl acetate extract and methanolic extract from Capsicum chinense Jacq. ripe fruit. Braz J Biol 2022;84. https://doi.org/10.1590/1519-6984.258084 10.1590/1519-6984.258084 [DOI] [PubMed] [Google Scholar]
- [84].Tavana E, Mollazadeh H, Mohtashami E, Modaresi SMS, Hosseini A, Sabri H, Soltani A, Javid H, Afshari AR, Sahebkar A. Quercetin: A promising phytochemical for the treatment of glioblastoma multiforme. Biofactors 2020;46:356-66. https://doi.org/10.1002/biof.1605 10.1002/biof.1605 [DOI] [PubMed] [Google Scholar]
- [85].Careaga M, Fernández E, Dorantes L, Mota L, Jaramillo ME, Hernandez-Sanchez H. Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. International Int J Food Microbiol 2003;83:331-5. https://doi.org/10.1016/s0168-1605(02)00382-3 10.1016/s0168-1605(02)00382-3 [DOI] [PubMed] [Google Scholar]
- [86].Shayan S, Saeidi S. Antibacterial and antibiofilm activities of extract Capsicum annuum L on the growth and biofilm formation of common pathogenic strains. IJSBAR 2013;5:513-8. [Google Scholar]
- [87].Bourne N, Bernstein DI, Stanberry LR. Civamide (cis-capsaicin) for treatment of primary or recurrent experimental genital herpes. Antimicrob Agents Chemother 1999;43:2685-8. https://doi.org/10.1128/AAC.43.11.2685 10.1128/AAC.43.11.2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kollmannsberger H, Rodríguez‐Burruezo A, Nitz S, Nuez F. Volatile and capsaicinoid composition of ají (Capsicum baccatum) and rocoto (Capsicum pubescens), two Andean species of chile peppers. J Sci Food Agric 2011;91:1598-611. https://doi.org/10.1002/jsfa.4354 10.1002/jsfa.4354 [DOI] [PubMed] [Google Scholar]
- [89].Frischkorn CG, Frischkorn HE, Carrazzoni E. Cercaricidal activity of some essential oils of plants from Brazil. Naturwissenschaften 1978;65:480-3. https://doi.org/10.1007/BF00702834 10.1007/BF00702834 [DOI] [PubMed] [Google Scholar]
- [90].KWON YI, Apostolidis E, Shetty K. Evaluation of pepper (Capsicum annuum) for management of diabetes and hypertension. J Food Biochem 2007;31:370-85. https://doi.org/10.1111/j.1745-4514.2007.00120.x 10.1111/j.1745-4514.2007.00120.x [DOI] [Google Scholar]
- [91].Jolayemi AT, Ojewole JA. Comparative anti-inflammatory properties of capsaicin and ethyl acetate extract of Capsicum frutescens Linn [Solanaceae] in rats. Afr Health Sci 2013;13:357-61. https://doi.org/10.4314/ahs.v13i2.23 10.4314/ahs.v13i2.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Persson MS, Stocks J, Walsh DA, Doherty M, Zhang W. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage 2018;26:1575-82. https://doi.org/10.1016/j.joca.2018.08.008 10.1016/j.joca.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


