Summary
A clinical research requires a systematic approach with diligent planning, execution and sampling in order to obtain reliable and validated results, as well as an understanding of each research methodology is essential for researchers. Indeed, selecting an inappropriate study type, an error that cannot be corrected after the beginning of a study, results in flawed methodology. The results of clinical research studies enhance the repertoire of knowledge regarding a disease pathogenicity, an existing or newly discovered medication, surgical or diagnostic procedure or medical device. Medical research can be divided into primary and secondary research, where primary research involves conducting studies and collecting raw data, which is then analysed and evaluated in secondary research. The successful deployment of clinical research methodology depends upon several factors. These include the type of study, the objectives, the population, study design, methodology/techniques and the sampling and statistical procedures used. Among the different types of clinical studies, we can recognize descriptive or analytical studies, which can be further categorized in observational and experimental. Finally, also pre-clinical studies are of outmost importance, representing the steppingstone of clinical trials. It is therefore important to understand the types of method for clinical research. Thus, this review focused on various aspects of the methodology and describes the crucial steps of the conceptual and executive stages.
Keywords: Study design, Clinical research, Observational studies, Experimental studies, Bias
Citation
How to cite this article: Kiani AK, Naureen Z, Pheby D, Henehan G, Brown R, Sieving P, Sykora P, Marks R, Falsini B, Capodicasa N, Miertus S, Lorusso L, Dondossola D, Tartaglia GM, Ergoren MC, Dundar M, Michelini S, Malacarne D, Bonetti G, Donato K, Medori MC, Beccari T, Samaja M, Connelly ST, Martin D, Morresi A, Bacu A, Herbst KL, Kapustin M, Stuppia L, Lumer L, Farronato G, Bertelli M. Methodology for clinical research. J Prev Med Hyg 2022;63(suppl.3):E267-E278. https://doi.org/10.15167/2421-4248/jpmh2022.63.2S3.30
Introduction
According to epistemologists, who study the nature, origin and scope of knowledge, epistemic justification, the rationality of belief and related issues [1], there are six ways to obtain knowledge:
authoritarianism;
mysticism;
rationalism and empiricism;
pragmatism;
scepticism.
Rationalism and empiricism, pragmatism and scepticism may be within the scope of the scientific method, whereas authoritarianism and mysticism are clearly pseudoscience or anti-science [2]. Science is characterized by systematic observation and experimentation, inductive and deductive reasoning, and the formation and testing of hypotheses and theories. The details of how these are carried out can vary greatly, but these characteristics are sufficient to distinguish scientific activity from non-science [3-8]
The choice and selection of a particular methodology depends on factors such as the hypothesis to investigate, the research question or statement of the problem, the objectives, the nature of the study, the study population and controls, intervention and variables [9-12]. The reliability and validity of the results therefore depend on an overall study design having well-defined objectives, reproducible methodology, diligent data collection and analysis to minimise errors and bias, and efficient reporting of the findings [9, 12]. Selecting an appropriate methodology is therefore essential to obtain valid results, and an understanding of research methodology is essential for researchers.
Medical research can be broadly categorised into primary and secondary research. Primary research involves conducting studies and collecting raw data that is then analysed and evaluated in secondary research [13]. Primary research can be further classified into three types as shown in Figure 1: basic or laboratory studies, also known as preclinical studies, clinical research, and epidemiological research. Both clinical and epidemiological research involve observational and experimental methods. Clinical research investigates the effects of specific interventions on individuals, while epidemiological research studies the causes and distribution of disease or mortality in human populations, especially the effects of exposure to single or multiple environmental agents [14]. Similar in essence, clinical research methods differ somewhat, depending on the type of study. Type is an integral element of study design and depends on the research question to answer. It should be specified before the start of any study [15]. Selecting an inappropriate study type results in flawed methodology, and if it occurs after commencement of the study, it is an error that cannot corrected.
Fig. 1.
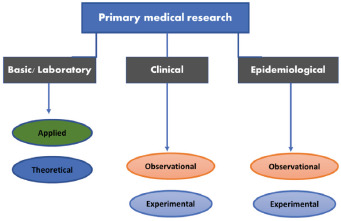
Types of primary medical research.
Stages of clinical research
A clinical research project consists broadly of two stages: planning and action [16].
PLANNING STAGE
The planning stage consists of all the preliminary paperwork and search of the literature done before starting actual research. It includes identifying the problem, reviewing the literature, developing a research question, formulating a hypothesis, determining the type of study, selecting a study design, identifying the target/study population, and seeking informed consent to participation. It also includes establishing collaborations with experts and determining the overall feasibility of the proposed work [9, 16].
Before beginning the scientific investigation, the researchers should decide the data collection strategy, sampling techniques and statistical analysis. After choosing a working hypothesis and reformulating it as null and alternative hypotheses, the next step is to decide the type of study required to answer the research question and an appropriate method to implement it.
ACTION STAGE
This stage includes the actionable research, implementation of the method in coherence with the theoretical concept, randomisation, blinding, application of sampling techniques, data collection and statistical analysis [10, 11].
Classification of clinical research
Depending on the study design, clinical research can in principle be categorised as either quantitative or qualitative [9]. Further classification of clinical research methods may be based on data collection techniques and the direction of causality being investigated, as illustrated for example by time relationships. Clinical research can be classified as either descriptive or analytical, as illustrated in Figure 2 [9, 12, 17-20].
Fig. 2:
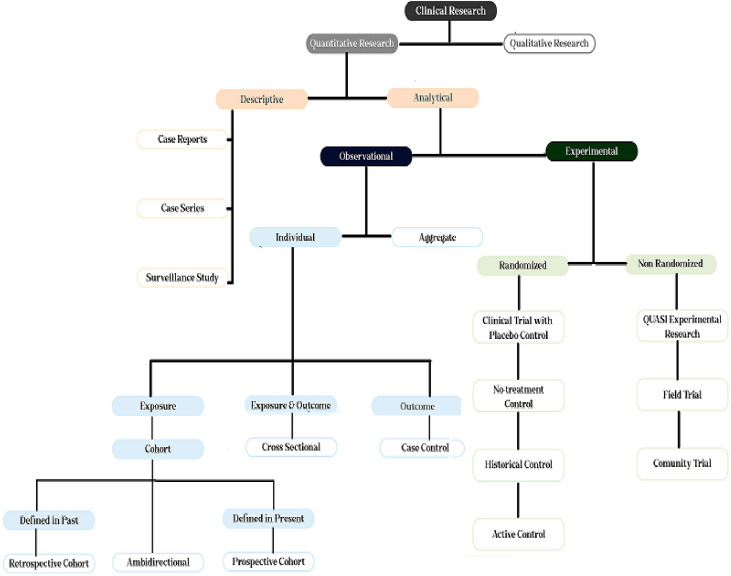
Classification of clinical research.
DESCRIPTIVE RESEARCH
Descriptive studies record and report unusual or new events, e.g. the prevalence of a disease or syndrome in a family, and correlate the events with possible explanations. This type of research is neither randomized nor pre-designed, and is presented as a case report, case series or surveillance study.
Case Reports
These are reports of individual patients with particular clinical characteristics. Such reports present baseline characteristics recorded and evaluated for single patients, compared with population values. Sometimes these studies may consist of observations recorded for administration of a certain treatment to an individual. They are essentially hypothesis generating, opening the way for more rigorous studies of an experimental nature [12].
Case series
Case series may include examination of successive clinical cases having common characteristics. They may, for example, present observations from patients exposed to a particular drug or group of drugs at regular intervals, and may include former histories of patients having similar outcomes, to detect possible cause-effect relationships.
Surveillance studies
This type of study involves continuous monitoring of disease occurrence in a population. Information related to a health problem of interest is collected in databases, analysed over a time period and inferences are made based on observed correlations.
ANALYTICAL/EXPLANATORY STUDIES
The most significant difference between descriptive and analytical studies is the presence in the latter of control groups that enable comparative evaluations to be made. Analytical clinical studies can be further classified into experimental (intervention) studies and observational (non-intervention) studies.
Observational studies
Observational studies are non-intervention studies in which patients are prescribed a specified therapy based on diagnosis and therapeutic need. They include therapeutic, prognostic, observational drug studies, secondary data analyses, case series and single case reports, and may be retrospective, prospective or ambidirectional [21]. In non-intervention studies, “knowledge from the treatment of persons with drugs in accordance with the instructions for use specified in their registration is analysed using epidemiological methods” [21]. “Diagnosis, treatment and monitoring are not performed according to a previously specified study protocol, but exclusively according to medical practice” [21].
Observational studies involve collecting data pertaining to study participants in their natural or real-world environments. They are usually diagnostic and prognostic studies, with a cross-sectional approach to data collection. The comparative-effectiveness study is the hallmark of non-experimental research [22], and involves comparison of comparable groups to interpret outcome effects. Such studies are also known as benchmarking-controlled trials because of the element of peer comparison [22].
Observational studies can be broadly categorised into individual and aggregate studies.
Aggregate observation studies
Individual level data aggregated by geographic area, year or any other parameter is termed aggregate data. Aggregate studies are conducted to record observations on pandemics and epidemics of communicable diseases and their treatment regimens, for example aggregate data on COVID-19 in a particular country, or the occurrence and effective treatment of malaria and its relapse in a particular geographical area. Data pertaining to non-communicable diseases is also aggregated in the same way to generate insights into the distribution of diseases in specified populations, as for example in cancer registries [23, 24].
Individual observation studies
Individual studies are based on disaggregated individual results and involve analysis to estimate differences between subgroups. In individual observational studies, subjects are observed individually and then gathered in groups based on outcomes or exposures or both. Based on grouping criteria, individual observational studies may take the form of case-control, cohort or cross-sectional studies.
-
Case-control studies
Individual observational studies that involve grouping of subjects based on selected outcomes are termed case-control studies. In these studies, the exposure experience of the case group (subjects with the outcome of interest) is compared with that of the control group (subjects without the outcome), for instance occurrence or non-occurrence of renal failure in diabetic patients or heart attacks in hypertensive patients. The design of such studies is retrospective and evaluates possible associations between exposures and outcome. They are quick and inexpensive to perform, and the results are expressed as odds ratios (OR) and risk ratio/relative risk. Case control studies enable multiple exposure variables to be examined for a given outcome, but they do not allow correlation of sequential causes and effects with the outcome [12].
-
Cohort studies
In this type of study subjects are grouped based on exposure. Cohort studies enable multiple outcomes to be studied for a given exposure. The exposure is well-defined, but the outcome may vary, thus providing an opportunity to monitor many outcomes of a single exposure [12]. Cohort studies can be retrospective, where the cohorts are defined on the basis of a past exposure, or prospective, where the cohorts are defined by a current exposure.
-
Retrospective cohort studies
In retrospective cohort observational studies, the researchers look back in time at archived or self-reported data in order to compare outcomes in exposed and non-exposed patients. The two groups are identified retrospectively and studied prospectively. This type of study is quick and inexpensive [25, 26], but is prone to recall-bias [27].
-
Prospective cohort studies
A prospective cohort study is a longitudinal cohort study in which cohorts differing in exposure to the factors being studied are followed up at predetermined time intervals to determine the effect on outcomes. This type of study helps to determine associations between a particular exposure and outcomes. For rare outcomes, large numbers of subjects and long follow-up periods are required, so such studies tend to be very expensive. In addition, if randomization and blinding are not conducted properly, the chances of bias and confounders increases [26].
-
-
Cross-sectional studies
Cross-sectional studies have transverse study design and involve concurrent assessment of exposures and outcomes without any follow-up. These studies are essentially based on surveys, and are therefore appropriate for determining prevalence but cannot shed light on causation [12, 26].
Experimental studies
Experimental studies are intervention studies, and include preclinical trials on animals as well as clinical trials in humans. In these studies, the effect of an intervention is compared with that of another intervention or a placebo. Interventions studied may include, for example, use of medical devices, surgical, physical or psychotherapeutic procedures, psychosocial interventions, rehabilitation measures, acupuncture, physiotherapy training or diet [1, 14]. Experimental studies mostly aim to compare outcomes of treatment procedures in a group of patients exhibiting minimal internal differences. To avoid bias, patients are randomly allocated to treatment and control groups. Different countries have different procedures and legal and ethical requirements governing the conduct of such studies. For instance, the United Kingdom Medicines and Medical Devices Act 2021 requires that studies using medical devices be registered by the relevant authorities. In the European Union, interventional studies must be conducted in accordance with the binding rules of Good Clinical Practice (GCP) [28]. In Germany, vaccine studies are considered to be intervention studies and are conducted as clinical studies according to the AMG [13]. Likewise, drug studies must seek approval from ethical committees. Informed consent must be obtained from the patient and an ethically defensible control group included. The control group is given another treatment regimen and/or placebo and should enable the central questions of the study to be answered [28].
Some experimental studies in biomedical research may focus on possible biomarkers, such as enzymes or genes, on evaluation of imaging techniques, such as magnetic resonance imaging and computed tomography, or on techniques such as gene sequencing in order to find correlations between genotypes and phenotypes. The development of statistical tests and mathematical models may also be regarded as experimental studies. Generally, the design of biomedical studies should be based on their purpose and objectives [13].
Design of experimental studies
The design of an experimental study depends on the type of information sought, the objectives of the study and the ultimate application. Designs can be characterized by interventions on selected groups of the study population under controlled environmental conditions compared with a control group without any interventions. The main designs employed in experimental studies are randomised controlled trials and non-randomised clinical trials, also known as quasi-experimental studies [9, 12, 26].
Non-randomized studies
In non-randomised studies, the study population is selected on the basis of pre-determined selection criteria; it is not randomized with respect to treatment(s) but is prescribed treatment based on the course of the disease. In many experimental studies involving surgical intervention which is only appropriate for particular patient groups, randomization is either not possible or not ethical. Generally, phase IV of a clinical trial has non-randomized design. Non-randomised studies can be further categorised as:
-
Quasi-experiment
The investigator assigns exposure to the intervention as in a randomized controlled trial, but the subjects are not randomized [12].
-
Field trial
These are large scale studies of therapeutic interventions, for example the efficacy of COVID-19 vaccines in combatting COVID-19. Many samples are required to determine efficacy, particularly when the incidence of a particular disease in the population is low [26].
-
Community trial
In these trials, treatments are allocated to a community group. For instance, the effect of fluoridation of water was tested by exposing some communities to fluoride and comparing outcomes with those in unexposed communities.
Randomized controlled trials
Randomised controlled trials (RCTs) are trials in which the subjects are randomly assigned to experimental and control groups. The experimental group is given the treatment that is being tested and the control group is given an alternative treatment or a placebo or no treatment at all. Most experimental clinical studies are RCTs, and the subjects are either healthy volunteers or patients. After a new drug passes a pre-clinical trial, it is tested via RCTs. Various aspects of the RCT require careful consideration before the trial begins, for example study design, patient population, control group selection, randomization, sampling, blinding or open labelling of treatments and outcomes [12, 26].
-
Study design
Study design is an important prerequisite for the success of the study. Randomised controlled trials commonly use parallel group design, matched pairs and cross-over designs [29].
-
Parallel group design
This design requires large number of subjects/patients who are enrolled, followed up and observed for outcomes on a parallel basis over a period of time.
-
Matched pairs
In this design, patients are matched for different variables. Matched subjects are assigned at random to intervention or control groups. Although this type of design is difficult to conduct, it helps overcome the influence of confounding variables on outcomes.
-
Crossover design
This design is used for drugs having reversible and transient effects. The effects of two interventions, administered sequentially, are assessed. The number of patients required is smaller than for the other designs [29].
-
-
Patient population
In RCTs, the patient population is selected on the basis of predetermined selection criteria. This selection is carried out to avoid confounding variables and should be based on predefined inclusion and exclusion criteria. Withdrawal criteria, indicating the circumstances under which subjects should be withdrawn from the trial, should also be predefined.
-
Inclusion criteria
The criteria for selection of subjects (patients or healthy volunteers) are based on age, body mass index, gender, ethnicity, prognostic factors and diagnostic admission criteria. They are used to select the subjects and then randomly assign them to various treatments for comparison of outcomes [26, 30].
-
Exclusion criteria
These are criteria for excluding subjects from a particular trial, for example severity of disease, concurrent medication, allergies, underlying health conditions and many more [30].
Withdrawal criteria
-
These indicate situations in which the trial is terminated for particular subjects and specify how and when the subjects should be withdrawn from the study. When subjects are withdrawn, they are no longer subject to follow-up.
-
c. Control group
Perhaps the most important factor in any scientific research is identification and determination of a control group. Without successful deployment of a control group, a study cannot be authentic. Randomised controlled trials can include placebo, no-treatment, historical or active controls [26].
-
Placebo control
A placebo is a fake or inert version of the drug under evaluation, with no pharmacological effect. Placebos help overcome any psychological impact of drug dispensing on disease progression, allowing the investigator to estimate the effectiveness of a treatment free from confounding psychological factors. However, placebo controls in drug research and sham surgery are ethically controversial, especially in cases where an effective treatment exists.
-
No treatment controls
This is the least preferred type of control, where subjects are not given anything by way of treatment, not even a placebo. Such controls serve as a neutral reference group for the experimental groups receiving the treatment under investigation. This approach avoids bias due to psychological factors that may influence outcomes.
-
Historical control
In some studies, concurrent controls are dispensed with and only historical control data is used. This is done specifically for studies involving rare diseases with high mortality. In such circumstances, withholding treatment from a control group would raise very considerable ethical implications [9]. Historical controls are controls used in previous studies. They help reduce the overall cost of the study, making drug developers more likely to invest. Historical controls also make enrolment in rare disease trials more feasible by reducing the number of patients required.
-
-
d. Randomization
Randomization is the optimal method of allocating subjects to the therapy arms of a trial. Random assignment of subjects to the treatment and control groups ensures equal distribution of all variables and confounding factors, such as genetic variabilities, risk factors and comorbidities, in all groups, thereby alleviating bias. Randomization is intended to ensure comparability between the groups, and it reduces the chance of allocating a specific therapy to patients with a particularly favourable prognosis. Randomization is carried out using random number tables, mathematical algorithms for pseudorandom number generation, physical randomization devices such as coins and cards, or sophisticated devices such as electronic random number indicator equipment [9-12, 26].
The main randomization techniques used in RCTs are simple randomization, cluster randomization and stratified randomization [31].
-
Simple randomization
Randomization involving a single sequence of random assignments is known as simple randomization. It randomizes patients selected on the basis of selection criteria to various treatment groups.
-
Cluster randomization
Cluster-randomized trials are used to compare treatments that are allocated to clusters (groups) of subjects, rather than to individuals. Groups of patients matching the selection criteria are randomly assigned to the group receiving the treatment or to a control group. Randomised controlled trials are used to evaluate complex interventions.
-
Stratified randomization
This is a two-step procedure. As the name indicates, the subjects entering the clinical trial are first grouped in strata (groups) based on clinical features that might affect the outcome of their condition, and then undergo intra-group randomization to assign them to various treatment groups.
-
-
e. Sampling method
Sampling is the process of selecting a sample population from the target population. Sampling allows information to be obtained about the target population based on statistical analysis of a subset of the population, without any need to investigate the characteristics of every individual in the target population [32]. Sampling techniques are broadly categorised into probability and non-probability sampling, as shown in Figure 3.
-
Probability sampling
In this sampling technique, every element of the population has an equal chance of being selected. This helps create a sample truly representative of a given population [22]. Types of probability sampling techniques are:
-
Fig. 3.
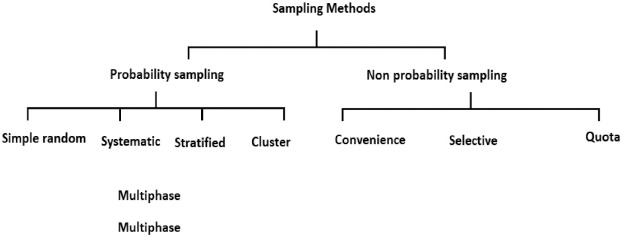
Sampling methods in clinical research.
Simple random sampling
In this type of sampling every experimental unit has an equal chance of being selected during sampling.
Systematic sampling
This sampling is used where a complete and up-to-date sampling frame is available. The first experimental unit is selected randomly, while the rest are selected randomly based on a predesigned pattern.
Stratified sampling
In this method the study population is divided into strata according to age, gender etc. and then sampling is carried out from these strata.
Cluster sampling
In this method the study population is divided into clusters and these clusters rather than individuals are taken as sampling units. The clusters are then randomly selected for inclusion in the study.
Multistage sampling
Multistage random sampling is conducted at several stages within population clusters. This sampling method is usually applied to large nationwide surveys.
Multiphase sampling
The sampling is conducted in two or more phases. In the first phase some data is collected from the whole sample and in the second, data is collected from a subset of the original sample.
-
Non-probability sampling
In this type of sampling technique, not all experimental units get an equal chance of being selected [22]. A non-representative sample which does not produce generalizable results is a possible result. Different types of non-probability sampling are:
Convenience sampling
This sampling is based on the convenience of the investigator.
Purposive/judgemental/selective/subjective sampling
This type is based on the judgement of the investigator.
Quota sampling
This method of sampling is used in studies involving interviews and is based on the judgment of the interviewers, depending on characteristics such as sex and physical status.
-
f. Blinding
Blinding is defined as “concealing or masking the assignment of subjects to a study group from the participants of the study, i.e., patients/subjects, observers and researchers”. Randomised clinical trials may be blinded or non-blinded [9, 12, 26].
-
Non-blinded experiments
Non-blinded experiments are also known as open-label studies. In this type of study, all participating patients, physicians, observers and researchers know the treatment used. This may result in bias, but is unavoidable where hiding a treatment raises ethical concerns. For instance, it is unethical to hide the treatment regime from patients with cancer, AIDS or organ failure. Patients may also be allowed to select the drug brand themselves.
-
Blinded experiments
In these experiments the blinding is done at the start of the experiment. Blinding can be single, double or triple.
-
Single-blind trials
The subjects (patients or healthy volunteers) do not know whether they are in the intervention or the placebo group.
Double-blind trials
Neither the subjects nor the researcher knows who has been assigned to the control and the test groups. Only the observer knows to which group the subjects have been assigned.
Triple-blind trials
In triple blind RCTs, personal or intentional bias is eliminated by none of the study participants (subjects, observer, researcher) knowing the label or nature of the treatment administered.
The information identifying treatment and subjects in double- and triple-blind experiments is held by another party and only made available to the researcher at the end of the trial.
Prospective, randomized, open-label, blinded-endpoint (PROBE) trials
Randomized controlled trials can be conducted as PROBE trials in which patients are randomly assigned to different treatment regimens and both patients and researchers are aware of the treatments administered. The PROBE trial is much easier to conduct than double- or triple-blind or doubled-blind placebo-controlled design, as it enables trials to be performed in conditions that resemble real-world practice. It is also economical and simplifies patient enrolment. However, it imposes certain conditions to avoid the bias associated with open label trials. PROBE designs are endpoint blinded, as the observer is unaware of the treatment being used. Since the subjects and researchers know the treatments, potential bias can be avoided by using so-called hard endpoints as primary endpoints. However, the results obtained by PROBE are less reliable than those obtained by double- or triple-blind studies [33].
-
g. Treatment considerations
Another important prelude to a successful clinical study is the selection of treatment dosage, form, frequency, route of administration and concurrent medications for the test and active control groups. A drug may be available in various doses and in forms such as tablet, capsule or injectable. Since these factors affect the plasma concentrations and effects of the drug, and ultimately the outcome; all these factors, except dose and frequency, are maintained constant throughout the study. If necessary, the dose and frequency of the drug may be changed gradually and sequentially. If the treatments involve more than one drug, their pharmacokinetic and pharmacodynamic interactions are kept under observation while determining dosage, in order to avoid any influence of these interactions on outcomes [9, 12, 33]. Another important consideration in treatment selection is patient compliance, since non-compliance may have adverse effects on outcome.
-
h. Outcome measures
Since the objectives of a clinical study indicate the possible outcomes, this is borne in mind in selecting the methods of monitoring and the data required for recording the outcomes of interest. In clinical experiments, outcomes are assessed in terms of efficacy endpoints, i.e. primary endpoints and surrogate or secondary endpoints. Primary endpoints are measures specified by the researcher at the start of the study in order to verify or refute the hypothesis, whereas surrogate endpoints are specified before commencement of the study but can be modified during its course. For instance, in an experiment estimating the efficacy of an antihypertensive drug, the primary endpoint would be to see whether or not the treatment reduces cardiovascular events, while a surrogate endpoint could be its ability to reduce blood pressure [26]. Many primary and secondary endpoints are prespecified before beginning a study. However, the main primary endpoint is the quality of life afforded by a particular treatment for individuals in the study group.
-
Bias, chicanery and confounders
Bias is distortion of outcomes due to introduction of errors, voluntarily or involuntarily, at different stages of the research, e.g. the stages of design, population selection, calculation of number of samples, data entry and statistical analysis. Several types of bias can occur during clinical research (Tab. I).
-
Tab. I.
Types of bias in clinical research.
| Type of Bias | Description |
|---|---|
| Investigator bias | Conscious or unconscious preference given to one group over another by the investigator |
| Evaluator bias | Introduced when an investigator making endpoint-variable measurements favours one group over another. Common with subjective endpoints |
| Performance bias/ set of Hawthorne effects | Introduced when participants know their allocation to a particular group and change their response or behaviour during a particular treatment |
| Selection bias | Introduced when samples (individuals or groups) are selected for data analysis without proper randomization; includes admission bias and non-response bias, in which case the sample is not representative of the population |
| Ascertainment or information bias | Errors in measurement or classification of patients; includes diagnostic bias and recall bias |
| Allocation bias | Systematic differences in the allocation of participants to treatment groups and comparison groups, when the investigator knows which treatment is going to be allocated to the next eligible participant |
| Confirmation bias | Information is processed in a manner consistent with someone’s belief |
| Belief bias | The strength of arguments is judged on the basis of the plausibility of their conclusions rather than how strongly they support that conclusion. |
| Expectation bias | Introduced during publication by a personal preference for positive results over negative results when the results deviate from expected outcomes |
| Detection bias | Systematic errors in observation of outcomes in different groups results in detection bias when outcomes in one group are not as vigilantly sought as in the other. |
| Attrition bias/loss-to-follow-up bias | Preferential loss-to-follow-up in a particular group leads to attrition bias. |
| Commercial bias | Introduced for commercial reasons in the form of advertising or economic pressure on editors, particularly in studies involving new medical devices and drugs |
Chicanery
Chicanery involves deliberate unethical changes to interventions, results and the data of patients. Copying data from other sources is also classified as chicanery.
Confounders
Confounders are factors, other than those being studied, that can affect an outcome parameter. These factors are not directly relevant to the research question but may possibly alter the outcomes [10, 11]. For example, while studying the effect of hypertension on renal failure, diabetes could be a confounder as it also affects kidney function. It is therefore essential to take all potential confounders into consideration when designing a study. If known, confounders can be controlled for by selection constraints or statistical adjustments, such as stratification and mathematical modelling, during study design. Various strategies are used during data analysis to adjust for confounders; these include stratified analysis using the Mantel-Haenszel method, a matched design approach, data restriction and model fitting using regression techniques [34].
Bias, chicanery and confounders can be avoided by randomization and blinding. The randomized controlled and blinded clinical trial with case number planning is therefore accepted as the gold standard for evaluating the efficacy and safety of drugs and therapeutic regimes [35].
-
i. Validity
The results of a clinical trial are said to be valid if the differences observed between the study and control groups are real and not influenced by bias or confounders (internal validity) and are applicable to a broader population (external validity). Placebo-controlled, double-blinded, randomised clinical trials have high internal validity, while external validity can be increased by broadening the eligibility criteria for enrolling subjects [36].
Preclinical studies for the development of biomedical products
Pre-clinical (or laboratory) studies form the basis of clinical trials. To reduce the time for, and to improve the chances of approval of a new drug, the choice of an appropriate preclinical model is of utmost importance. Preclinical studies evaluate the pharmacodynamics, pharmacokinetics and toxicology of a drug in in vitro and in vivo settings. Clinical trials are conducted when preclinical studies have demonstrated the efficacy and safety of a new drug. The results of clinical trials can improve preclinical studies and vice versa. Nonetheless, only a small fraction of drugs that pass the preclinical evaluation criteria are selected for clinical trials, and only a few are approved for use in humans, so optimization of standard preclinical procedures to mimic the complexity of human disease mechanisms is urgently needed [37].
In summary, preclinical studies involve the use of various in vivo and in vitro models and computer designs to evaluate the efficacy and safety of a new drug.
IN VITRO MODELS (CELL STUDIES)
Advances in cell culture technology have made it possible to test new drugs on cell lines grown in vitro. These studies may involve testing of drugs on human or animal cancer cells [38].
IN VIVO MODELS (ANIMAL STUDIES)
Drugs that prove effective in vitro are then tested in vivo in live animals to ensure their safety in living systems. Animal models, and their critical validation, are of great importance in minimizing unpredicted adverse effects of a drug in clinical trial phases. Animal models are carefully selected on the basis of their advantages and limitations and on the objectives of the study, in order to mimic pathophysiological conditions in humans [38]. The validity of animal models is increased by following the relevant guidelines and standards in designing a study. Three types of models are used in preclinical studies:
Homologous models
Homologous models are animals which have the same causes, symptoms, and treatments of a particular disease that humans would have.
Isomorphic models
These animals have same symptoms and treatments of a particular disease as humans, but the cause may be different.
Predictive models
These models are only like humans in some aspects of a particular disease; however they provide useful information about the mechanisms of disease features.
IN SILICO MODELS (COMPUTER STUDIES)
In silico models are based on computer simulations that complement or precede in vitro and in vivo studies. They predict how a drug might behave in these subsequent studies. In-silico studies require expertise in biochemistry, molecular biology, cheminformatics, and bioinformatics [38].
Pre-clinical studies provide useful information about the behaviour and safety of drugs. However, drugs do not necessarily behave in the same way in humans as they do in animal models. For example, human subjects and mice models differ sharply in absorption, processing, and excretion of certain drugs. Unexpected side-effects may therefore occur in humans that do not occur in animal models. Drugs which show promising outcomes in preclinical studies are then approved for testing in human subjects by regulatory authorities such as the Food and Drug Administration (FDA) in the US [37, 38].
Design, performance, and monitoring of clinical trials
Once preclinical studies on a new drug are completed and promising results are achieved, the next stage in biomedical research is testing the safety, efficacy and reproducibility of the drug’s action on humans through clinical trials. Clinical trials are considered to be a safe and dependable method of evaluating the efficacy of a treatment. Clinical trials may be therapeutic or preventive [37-39].
THERAPEUTIC TRIALS
These trials are conducted to test experimental treatments, combinations of new or existing drugs, and new surgical interventions.
PREVENTIVE TRIALS
These trials test the efficacy of interventions (drugs, vaccines) in preventing diseases and their outcomes.
In general, clinical trials aim to enhance the repertoire of information related to an intervention or lifestyle regime that might prove beneficial for patient management or treatment. They are designed to develop and test new diagnostic methods or treatments and their effects on humans, or new uses of existing diagnostic methods or treatment. They also help identify the most cost-effective and risk-free diagnostic methods or treatments. Randomized controlled trials are conducted to compare the safety and efficacy of two or more interventions in humans, and can often be based on clinical equipoise. Their phases [26] are shown in Table II.
Tab. II.
Phases of a randomized controlled trial of a drug.
| Phases | Aim | Number of participants |
|---|---|---|
| Phase 0 | To check the
|
a few volunteers |
| Phase I | To check
|
20-80 healthy volunteers or patients in an advanced stage of disease |
| Phase II | To assess
|
Hundreds of volunteers |
| Phase III | To
|
Hundreds to thousands of volunteers |
| Phase IV | To collect more information on
|
Hundreds of thousands of volunteers |
Good clinical practice: guidelines and requirements
Clinical trials are the gold standard for evaluating the superiority or similarity of new drugs or surgical procedures with respect to existing ones. As clinical trials involve testing on humans, their design and conduct require careful planning, diligent execution and enormous resources to comply with regulations set by the regulatory authorities so that robust results can be attained. The good clinical practice (GCP) guidelines published by the International Council of Harmonization (ICH) is an international ethical standard that ensures that the design, conduct, performance, monitoring, auditing, recording, analysis and reporting of clinical trials takes place according to established values. It also ensures the reliability and precision of reported data, and protects the rights, integrity and privacy of subjects participating in a trial [28, 31]. Protection of the safety, wellbeing and rights of human subjects participating in a clinical trial is consistent with the principles of the Declaration of Helsinki [40] and with the ethical principles formulated by the World Medical Association [41]. The requirements for conducting clinical trials in the European Union, including GCP and good manufacturing practice and their respective inspections, are implemented in the Clinical Trial Directive (Directive 2001/20/EC) and the Good Clinical Practice Directive (Directive 2005/28/EC) [31].
The responsibility for GCP lies with all participants in the trial, from the site staff to the subjects and the ethical and monitoring committees. The roles and responsibilities of GCP participants are shown in Table III.
Tab. III.
Clinical trial participants and their role in good clinical practice.
| Participants | Role |
|---|---|
| Regulatory authorities | Review clinical data and conduct inspections for GCP and good manufacturing practice |
| Sponsor | Institution/organization responsible for initiation, management and finance of clinical trial |
| Project monitor | Monitors the project and is appointed by the sponsor |
| Investigator | Team leader responsible for conducting trial at trial site |
| Trial site pharmacist | In charge of maintaining, storing and dispensing drugs |
| Patients | Human subjects |
| Ethical review committee for the protection of subjects | Institutional or national regulatory authorities ensuring safety, well-being and protection of human subjects |
| Committee to monitor large trials | Overseas sponsors, drug companies |
Conclusion
The planning and execution of clinical research is of vital importance for the advancement of medical science. The validity of clinical research findings depends on a variety of factors, such as study design, sampling techniques and statistical analysis. Choosing an appropriate study design requires detailed knowledge of the types of clinical study, the situations where they are applied and the possible outcomes, so that a methodology befitting the hypothesis is adopted. Careful implementation of study design eliminates the chances of bias, provides quality assurance of the data collected and increases the validity of the results, adding value to the findings. Successful preclinical studies, basic research and pilot scale intervention studies pave the way for more sophisticated clinical trials. Randomised, double-blind clinical trials with case number planning are accepted as the gold standard for evaluating the efficacy and safety of drugs and therapeutic regimes and in evaluating the superiority or similarity of new drugs or surgical procedures to existing ones. As clinical trials involve testing on humans, their design and conduct require careful planning, diligent execution and enormous resources to comply with the rules set by the regulatory authorities, necessary to achieve robust results.
Acknowledgements
This research was funded by the Provincia Autonoma di Bolzano in the framework of LP 15/2020 (dgp 3174/2021).
Conflicts of interest statement
Authors declare no conflict of interest.
Author's contributions
MB: study conception, editing and critical revision of the manuscript; AKK, DP, GH, RB, Paul S, Peter S, RM, BF, NC, SM, LL, DD, GMT, MCE, MD, SM, Daniele M, GB, KD, MCM, TB, MS, STC, Donald M, AM, AB, KLH, MK, LS, LL, GF: literature search, editing and critical revision of the manuscript. All authors have read and approved the final manuscript.
Figures and tables
Contributor Information
INTERNATIONAL BIOETHICS STUDY GROUP:
Derek Pheby, Gary Henehan, Richard Brown, Paul Sieving, Peter Sykora, Robert Marks, Benedetto Falsini, Natale Capodicasa, Stanislav Miertus, Lorenzo Lorusso, Gianluca Martino Tartaglia, Mahmut Cerkez Ergoren, Munis Dundar, Sandro Michelini, Daniele Malacarne, Tommaso Beccari, Michele Samaja, Matteo Bertelli, Donald Martin, Assunta Morresi, Ariola Bacu, Karen L. Herbst, Mykhaylo Kapustin, Liborio Stuppia, Ludovica Lumer, and Giampietro Farronato
References
- [1].Pepperell Montague W. The ways of knowing; or, The methods of philosophy. London: G. Allen & Unwin Ltd. 1st ed. New York: The Macmillan Company; 1925. [Google Scholar]
- [2].Goldstein M, Goldstein IF. How we know: an exploration of the scientific process. 1st ed. New York: Plenum Press; 1979. [Google Scholar]
- [3].Hepburn B, Hanne A. Scientific Method. In: Zalta EN, ed. The Stanford Encyclopedia of Philosophy. Available at: https://plato.stanford.edu/index.html. Accessed on: 30/02/2022. [Google Scholar]
- [4].Popper K. The Logic of Scientific Discovery. 2nd ed. London: Routledge; 2002. [Google Scholar]
- [5].Hugh G. Gauch Jr. Scientific method in practice, 1st ed. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- [6].Achinstein P. Science rules: a historical introduction to scientific methods. 1st ed. Baltimore: The Johns Hopkins University Press; 2004. [Google Scholar]
- [7].Gower B. Scientific Method. 1st ed. London: Routledge; 1996. https://doi.org/10.4324/9780203046128 10.4324/9780203046128 [DOI] [Google Scholar]
- [8].Carey SS. A beginner’s guide to scientific method. 4th ed. Belmont: Wadsworth Cengage Learning; 2011. [Google Scholar]
- [9].Garg R. Methodology for research I. Indian J Anaesth 2016;60:640. https://doi.org/10.4103/0019-5049.190619 10.4103/0019-5049.190619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Prasad K. Fundamentals of evidence based medicine. 1st ed. New Delhi: Springer India; 2014. https://doi.org/10.1007/978-81-322-0831-0 10.1007/978-81-322-0831-0 [DOI] [Google Scholar]
- [11].Lewsey J. Medical Statistics: a guide to data analysis and critical appraisal. Ann R Coll Surg Eng 2006;88:600-3. https://doi.org/10.1308/003588406X117098 10.1308/003588406X117098 [DOI] [Google Scholar]
- [12].Ozhan Caparlar C, Donmez A. What is scientific research and how can it be done? Turk J Anaesthesiol Reanim 2016;44:212-8. https://doi.org/10.5152/TJAR.2016.34711 10.5152/TJAR.2016.34711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Röhrig B, du Prel JB, Wachtlin D, Blettner M. Types of study in medical research. Dtsch Arztebl Int 2009;106:262-8. https://doi.org/10.3238/arztebl.2009.0262 10.3238/arztebl.2009.0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Frérot M, Lefebvre A, Aho S, Callier P, Astruc K, Aho Glélé LS. What is epidemiology? Changing definitions of epidemiology 1978-2017. PLoS One 2018;13:e0208442. https://doi.org/10.1371/journal.pone.0208442 10.1371/journal.pone.0208442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schröder W, Nickel S. Research Data Management as an Integral part of the research process of empirical disciplines using landscape ecology as an example. Data Sci J 2020;19. https://doi.org/ 10.5334/dsj-2020-026 10.5334/dsj-2020-026 [DOI] [Google Scholar]
- [16].Chew BH. Planning and conducting clinical research: the whole process. Cureus 2019. https://doi.org/10.7759/cureus.4112 10.7759/cureus.4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].del Mar C, Hoffmann TC. A guide to performing a peer review of randomised controlled trials. BMC Med 2015;13:248. https://doi.org/10.1186/s12916-015-0471-8 10.1186/s12916-015-0471-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sessler DI, Imrey PB. Clinical Research Methodology 2. Anesth Analg 2015;121:1043-51. https://doi.org/10.1213/ANE.0000000000000861 10.1213/ANE.0000000000000861 [DOI] [PubMed] [Google Scholar]
- [19].Stanley K. Design of randomized controlled trials. Circulation 2007;115:1164-9. https://doi.org/10.1161/CIRCULATIONAHA.105.594945 10.1161/CIRCULATIONAHA.105.594945 [DOI] [PubMed] [Google Scholar]
- [20].Levin KA. Study design VII. Randomised controlled trials.Evid Based Dent 2007;8:22-3. https://doi.org/10.1038/sj.ebd.6400473 10.1038/sj.ebd.6400473 [DOI] [PubMed] [Google Scholar]
- [21].NIS Definitions. Available at: https://www.chcuk.co.uk/non-interventional-studies/nis-definitions/. Accessed on: 26/06/2022.
- [22].Elfil M, Negida A. Sampling methods in clinical research; an educational review. Emerg (Tehran) 2017;5:e52. [PMC free article] [PubMed] [Google Scholar]
- [23].Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, Ferlay J. Cancer Incidence in five continents, Volume XI. IARC Scientific Publication 2021. Available at: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Cancer-Incidence-In-Five-Continents%C2%A0Volume-XI-2021. Accessed on: 26/06/2022.
- [24].Pheby DF, Etherington DJ. Improving the comparability of cancer registry treatment data and proposals for a new national minimum dataset. J Public Health Med 1994;16:331-40. [PubMed] [Google Scholar]
- [25].Sedgwick P. Retrospective cohort studies: advantages and disadvantages, BMJ 2014;348:g1072-g1072. https://doi.org/10.1136/bmj.g1072 10.1136/bmj.g1072 [DOI] [PubMed] [Google Scholar]
- [26].Gandhi P. Clinical research methodology, Ind J Pharm Edu Res 2011;45:199-209. [Google Scholar]
- [27].Sedgwick P. What is recall bias? BMJ 2012;344:e3519-e3519. https://doi.org/10.1136/bmj.e3519 10.1136/bmj.e3519 [DOI] [Google Scholar]
- [28].Pieterse H, Diamant Z. Good clinical practice in clinical interventional studies. Eur Clin Respir J 2014;1:26422. https://doi.org/10.3402/ecrj.v1.26422 10.3402/ecrj.v1.26422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nair B. Clinical trial designs. Indian Dermatol Online J 2019;10:193. https://doi.org/10.4103/idoj.IDOJ_475_18 10.4103/idoj.IDOJ_475_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Patino CM, Ferreira JC. Inclusion and exclusion criteria in research studies: definitions and why they matter. J Bras Pneumol 2018;44:84. https://doi.org/10.1590/s1806-37562018000000088 10.1590/s1806-37562018000000088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lim CY, In J. Randomization in clinical studies. Korean J Anesthesiol 2019;72:221-32. https://doi.org/10.4097/kja.19049 10.4097/kja.19049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Suresh G, Suresh K, Thomas S. Design, data analysis and sampling techniques for clinical research. Ann Indian Acad Neurol 2011;14:287. https://doi.org/10.4103/0972-2327.91951 10.4103/0972-2327.91951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Smith DH, Neutel JM, Lacourcière Y, Kempthorne-Rawson J. Prospective, randomized, open-label, blinded-endpoint (PROBE) designed trials yield the same results as double-blind, placebo-controlled trials with respect to ABPM measurements. J Hypertens 2003;21:1291-8. https://doi.org/10.1097/00004872-200307000-00016 10.1097/00004872-200307000-00016 [DOI] [PubMed] [Google Scholar]
- [34].Mann H, Djulbegovic B. Choosing a control intervention for a randomised clinical trial. BMC Med Res Methodol 2003;3:7. https://doi.org/10.1186/1471-2288-3-7 10.1186/1471-2288-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Misra S. Randomized double blind placebo control studies, the ‘Gold Standard’ in intervention based studies. Indian J Sex Transm Dis AIDS 2012;33:131. https://doi.org/10.4103/0253-7184.102130 10.4103/0253-7184.102130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sedgwick P. External and internal validity in clinical trials. BMJ 2012;344:e1004-e1004. https://doi.org/10.1136/bmj.e1004 10.1136/bmj.e1004 [DOI] [Google Scholar]
- [37].Arjmand B, Payab M, Goodarzi P. Biomedical Product Development: Bench to Bedside. 1st ed. Cham: Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-35626-2 10.1007/978-3-030-35626-2 [DOI] [Google Scholar]
- [38].Honek J. Preclinical research in drug development. Medical Writing 2017;26:5-8. [Google Scholar]
- [39].Sarzotti-Kelsoe M, Cox J, Cleland N, Denny T, Hural J, Needham L, Ozaki D, Rodriguez-Chavez IR, Stevens G, Stiles T, Tarragona-Fiol T, Simkins A. Evaluation and recommendations on good clinical laboratory practice guidelines for phase I-III clinical trials. PLoS Med 2009;6:e1000067. https://doi.org/10.1371/journal.pmed.1000067 10.1371/journal.pmed.1000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Declaration of Helsinki Medical Research Involving Human Subjects, 1964. Available at: https://www.wma.net/wht-we-do/medical-ethics/declaration-of-helsinki. Accessed on: 26/06/2022.
- [41].Medical ethics Deontology, Codes of Practice, Guidelines Professionalism. Available at: https://www.wma.net/what-we-do/medical-ethics. Accessed on: 26/06/ 2022.


