Summary
Fruit and vegetables are excellent sources of health-promoting bioactive compounds and nutraceuticals. Regular consumption of fruit and vegetables helps prevent the onset and progression of many non-communicable diseases. The Mediterranean diet envisages consumption of healthy vegetables and fruit on a daily basis for maximum health benefits. Traditional use envisages vegetable-based and fruit-based diets, and many studies scientifically proved the beneficial effects of Mediterranean vegetables and fruits. Rich in bioactive phytochemicals, citrus, cucumbers and grapes have antioxidant, anti-inflammatory, antimicrobial, cardioprotective, anti-ageing and anti-cancer properties. Studies indicate that intake of citrus, cucumbers and grapes reduces hypertension, hyperlipidemia, skin problems and infections and improves the health of the cardiovascular and nervous systems. These beneficial effects are mediated by several bioactive molecules present in Mediterranean diet vegetables and fruits, such as citrus, cucumbers and grapes. Indeed, they contains flavones, isoflavones, tannins, polyphenols and many beneficial natural molecules. This review focuses on the bioactive ingredients in citrus fruit, cucumbers and grapes, all components of the Mediterranean diet, and their health effects. A deep understanding of Mediterranean diet’s components, as well as clinical trials to test natural molecules beneficial effects, will permit to further explore the therapeutic potential of the Mediterranean diet in several pathological conditions.
Keywords: Mediterranean diet, Citrus, Cucumber, Grape, Phytochemicals
Citation
How to cite this article: Naureen Z, Dhuli K, Donato K, Aquilanti B, Velluti V, Matera G, Iaconelli A, Bertelli M. Foods of the Mediterranean diet: citrus, cucumber and grape. J Prev Med Hyg 2022;63(suppl.3):E21-E27.https://doi.org/10.15167/2421-4248/jpmh2022.63.2S3.2743
Introduction
The Mediterranean diet is widely accepted to be a very healthy diet. Whole grains, legumes, fruit, vegetables, white meat, fish, nuts and olive oil are all essential components. This diet is beneficial for human health and wellbeing because of its abundance of antioxidants, fibre, vitamins, minerals, phytosterols, probiotics, omega 3 and omega 6 fatty acids and phytosterols.
Citrus fruit is popular in the Mediterranean region. Though not native to the region, it has become an important commodity because of its place in the Mediterranean diet. Various citrus fruits have been used traditionally to treat illnesses in many Asian countries. Due to their medicinal qualities, citrus secondary metabolites such flavonoids, alkaloids, limonoids, coumarins, carotenoids, phenolic acids and essential oils are precious for maintaining human health. Their qualities include cardiovascular and neuroprotective properties and antioxidant, anti-inflammatory and anti-cancer effects [1-15].
The cucumber Cucumis sativus L. (family Cucurbitaceae) is a vegetable widely consumed throughout the world. It has a high water content and relatively few calories, as well as strong antioxidant, lipid-lowering and anti-diabetic effects. By eliminating pockets of toxins and old debris, cucumber has a purifying effect on the body. The skin is nourished by application fresh cucumber juice, which has a calming effect on irritation and reduces edema. Cucumber can also calm the body and reduce the pain of sunburn. The fruit is cooling, hemostatic, tonic and effective against excessive thirst and heat stroke. The seeds are used to treat constipation and have a cooling effect on the body. Cucumber is a source of cucurbitacins, cucumerin A and B, cucumegastigmanes I and II, vitexin, orientin, isoscoparin 2′′-O-(6′′′-(E)-p-coumaroyl) glucoside and apigenin 7-O-(6′′-O-p-coumaroyl glucoside) [16-32].
Grapes are rich in primary and secondary metabolites such as polyphenols. Polyphenols and phenolic acids have strong anti-inflammatory and anti-cancer effects and immunomodulatory properties. Although their distribution varies greatly in the different tissues, the pericarp and seeds of grapes are rich in polyphenols and phenolic acids. The leaves and seeds of the grapevine are tasty and healthy ingredients of Mediterranean cooking.
Citrus phytochemicals and their health-promoting effects
Citrus fruit is a distinctive component of the Mediterranean diet and adds richness and variety and to its health-promoting effects. It has many nutritional and healthy properties. It has been used for centuries to treat illnesses, such as bronchitis, tuberculosis, coughs, colds and menstrual disorders [1]. Citrus fruit is rich in vitamins, minerals and fibre and without sodium, fat and cholesterol. It also contains secondary phytochemicals, such as carotenoids and polyphenols, that prevent non-communicable diseases such as cardiovascular disease and cancer. Citrus fruit is rich in vitamin C which plays a key role in the absorption of inorganic iron, thus helping to alleviate anemia if administered with appropriate medicines [2, 3]. Vitamin C also promotes formation of collagen. Although citrus is not an excellent source of iron or zinc, its vitamin C content mediates release of iron and zinc from other foods, thereby maintaining iron status [2]. Citrus contains cellulose, hemicellulose and pectin which make up 60 to 70% of its total fibre content. Together with lignin, this form of fibre may decrease absorption of glucose after consumption of carbohydrates and reduce reabsorption of bile acids, lowering plasma levels of cholesterol [1-3].
Citrus fruit contains many important secondary metabolites in its peel, pulp, seeds, pressed oil and juice (Tab. I). Their concentrations vary between citrus species and in the various parts of the fruit [1-3]. Hesperidin and flavonoids have been linked to the anti-inflammatory and antioxidant activities of phenolic acids, such as caffeic, chlorogenic and ferulic acids [2]. The compounds responsible for these benefits include coumarins, essential oils (limonene) and flavonoids such naringin, naringenin, hesperidin and nobiletin (auraptene, imperatorin) [1]. Citrus also contains triterpene compounds like limonoids that have been remarked for their anti-cancer properties in animal models (stomach, skin, colon and lung) and in human colon adenocarcinoma cells and human breast cancer cells. Flavonoids extracted from citrus can also lower blood sugar, triglycerides and cholesterol levels. The flavanones hesperidin, naringenin, naringin and neohesperidin, as well as polyethoxylated flavonoids (nobiletin, tangeretin) are responsible for these qualities [1, 2].
Tab. I.
Phytochemicals in the citrus family and their bioactivity.
| Components | Bioactivity | References |
|---|---|---|
| Folate (Folic acid / folacin) | Promotes cell proliferation and red blood cell synthesis, prevents neural tube defects, stabilizes genetic material and may protect against cancer and heart disease. | [4,5] |
| Potassium | Minerals and sodium can retain biological fluids. Low sodium and high potassium levels may lower blood pressure. | [4,5] |
| Dietary fibre | Includes resistant starch, soluble and insoluble polysaccharides, pectin and certain other components. | [5] |
| Boosts intestinal flora and shortens the time food spends in the gut. Some types of fibre help lower blood fat levels. May lower risk of certain malignancies, heart disease and digestive issues including constipation. | ||
| Non-nutrient phytochemicals | ||
| Coumarins Polymethoxyflavones | Anti-obesity effects. Citrus peels are rich in coumarins, a class of polyphenols. Auraptene, the most prevalent coumarin in citrus, is found in grapefruit, trifoliate oranges, sour oranges and particularly Citrus natsudaidai. Products made from citrus fruits like marmalade and grapefruit juice maintain auraptene activity. | [6] |
| Prevent formation of lipid droplets | ||
| Polyphenols | Anti-microbial, anti-viral, anti-allergic, anti-inflammatory, anti-proliferative and anti-carcinogenic activity; astringent flavors. Anthocyanins are responsible for many of the colors of fruit and vegetables. | [5, 7-10] |
| Flavonoids | ||
| Nobelitin Citromitin Tangeretin | Hepatoprotective, anti-obesity and anti-hyperglycemic effects. Prevent D-galactosamine-induced liver damage | [11] |
| Kaempferol Quercetin Apigenin Naringenin | Anti-microbial effects | [12] |
| Cell-cell signaling antagonists, biofilm formation suppressors | ||
| Hesperidin | Anti-allergic effects | [13] |
| Reduces granulocytes degranulation and allergic symptoms | ||
| Limonin Nomilin | Hepatoprotective, anti-obesity and anti-hyperglycemic effects | [14] |
| Improves indicators of liver injury and inflammation | ||
| Essential oils | Anti-microbial effects | [15] |
| Inhibit pathogens and microorganisms of decomposition | ||
| Chimpi | Anti-anxiety effects | [16] |
| Strong anxiolytic effect equivalent to fluoxetine. |
Cucumber phytochemicals and their health-promoting effects
Cucumbers (Cucumis sativus L.) belong to the Cucurbitaceae family and are widely cultivated all over the world. They are eaten raw in salads, fermented (pickled) or cooked [17]. C. sativus, one of the 30 species of cucumbers, has the highest commercial value [18, 19]. The therapeutic potential of C. sativus leaves, fruits and seeds is extensively mentioned in ayurvedic medicine for management of ageing [20, 21]. The cucumber is often used to treat skin problems such as sunburn and eye inflammation. It is thought to revive, cool, heal, soothe and soften irritated skin and to prevent itching. Bioactive substances in several chemical classes have been isolated from this plant. A distinguishing feature of this species are cucurbitacins. Cucumber fruits are 96.4% water, 0.4% protein, 0.1% fat, 2.8% carbohydrate, 0.3% minerals, 0.01% calcium, 0.03% phosphorus, 1.5 mg/100 g iron and 30 IU/100 g vitamin B. They also contain enzymes such as proteases, ascorbate oxidases, succinic dehydrogenase and malic dehydrogenase [19]. They have a significant amount of ascorbic acid [20] while pulp and peel extracts contain lactic acid (7-8% w/w), which has demonstrated antioxidant properties [17]. The tetracyclic cucurbitane nuclear skeleton of cucurbitacins is arbitrarily split into twelve groups that form many cucurbitacins with antiproliferative properties [22].
PHYTOCHEMICALS OF CUCUMBER SEEDS
Cucumber seeds contain compounds such flavonoids, tannins, saponins and steroids [23]. Indeed, ethanol extract of cucumber seeds contained flavonoids, terpenoids, tannins, cardiac glycosides, phenols and carbohydrates [24]. Moreover, methanol extract of cucumber pulp contained tannins, alkaloids, saponins, glycosides, terpenes, phenolics and glycosides [25, 26]. The cotyledons of various varieties of C. sativus seedlings contained cucurbitacins A B, C, D, E and I [27] that have cytotoxic, antitumor, hepatoprotective, anti-inflammatory, antibacterial, anti-helminthic, cardiovascular and anti-diabetic properties [28]. Finally, cucumber contains two newly discovered primary C-glycosyl flavonoid compounds: cucumerin A and cucumerin B. Cucumber leaves and fruits contain many flavonoids, such as vitexin, isovitexin, and orientin, and carotenoids [29-33]. Table II summarized the main phytochemicals present in cucumber plant.
Tab. II.
Phytochemicals in cucumber plant.
| Plant | Part of plant | Class of compounds | References |
|---|---|---|---|
| Cucumber | Seed | Tannins, saponins, terpenoids and steroids | [23, 24] |
| Pulp | Tannins, alkaloids, saponins, glycosides, terpenes, phenolics and glycosides | [25, 26] | |
| Cotyledons | Cucurbitacins A B, C, D, E and I | [27] | |
| Leaves and fruits | Flavonoids, carotenoids | [29-33] |
PHARMACOLOGICAL ACTIVITY OF CUCUMBER PHYTOCHEMICALS
Antioxidant activity
Cucumber (Cucumis sativus L.) contains several free radical scavenging compounds that reduce oxidative stress by reducing superoxide mutase, guaiacol peroxidase, glutathione reductases and ascorbic acid peroxidases [20, 34]. Other antioxidants include butylated hydroxyl anisole (BHA) which is a more effective free radical scavenger than ascorbic acid. Tannins, polyphenols and flavonoids in cucumber also show radical-scavenging effects [34].
Anti-cancer activity
Cucumber contains many compounds with anti-cancer properties [35]. For instance, methanol and acetone extracts of cucumber has shown cytotoxicity against cancer cell lines HeLa and Michigan Cancer Foundation-7. Ethanol extract of cucumber leaves is rich in glycosides, alkaloids, tannins, proteins, amino acids, phytosterols, steroids, terpenoids and saponins; it inhibited the growth of HeLa and HepaG2 cancer cell lines in the MTT assay. Significant anticancer activity was seen at doses of 62.5, 125, 250 and 500 mg. Ethanol extract was more effective against HepG2 than against HeLa. According to the authors, the triterpenoids in the extract were responsible for the anti-cancer activity [35].
Antibacterial and antifungal properties
Ethyl acetate and methanol extracts of cucumber fruit have been reported to have antimicrobial activities, while methanol extract of cucumber seeds is reported to inhibit entero-pathogens S. aureus, P. aeruginosa and E. coli. Volatile cucumber oil has shown antibacterial and antifungal properties against gram-negative and gram-positive bacterial strains [36]. The research demonstrated an effect of C.s. against two major fungi. The authors concluded that C.s. seeds may have antifungal potential [37]. The study showed that ethanol extract of Cucumis sativus Linn. screened positively for antifungal activity against six fungi compared to reference griseofulvin [37].
Cytotoxicity
Ethanol extracts of Cucumis sativus showed cytotoxicity against brine shrimp nauplii in a brine shrimp toxicity assay. Toxicity rates were concentration-dependent. The LC50 and LC90 of the extract against brine shrimp nauplii were 75 μg/ml and 120 μg/ml, respectively [37].
Hepatoprotective activity
Cucumis sativus has been demonstrated to protect against oxidative stress caused by cumene hydroperoxide. Antioxidant and radical-scavenging compounds of Cucumis sativus fruit extract readily cross the cell membrane and protect against intracellular generation of reactive oxygen species. Aqueous extract of Cucumis sativus fruit acts as a hepatoprotective and antioxidant against cumene hydroperoxide-induced hepatotoxicity [38].
Hypoglycemic and hypolipidemic activity
Animal models such as alloxan-induced diabetic rats (AIDRs) have been used to test the hypoglycemic and hypolipidemic effects of cucumber. For instance, ethanol extracts of fruits of the Cucurbitaceae family, including cucumber, white pumpkin and ridge gourd, significantly reduced blood sugar levels in AIDRs. They also lowered the high lipid profiles of these rats. Ridge gourd was shown to significantly improve liver glycogen levels in AIDRs. The study suggested that cucumber fruit extracts may prove beneficial as supplements in the treatment of hyperglycemia and hyperlipidemia, which frequently coexist in diabetic patients. However, additional research is required to screen individual chemical compounds, identify antidiabetic lead compounds and determine their mechanisms of action [39].
Grape phytochemicals and their health-promoting effects
Grapes are grown in many parts of the world but are perhaps best known in California in the United States and in the Mediterranean from Portugal to Lebanon and Syria. Many Mediterranean sites produce magnificent wines, famous for their flavor and bouquet [40, 41]. Grapes contain high concentrations of phenolic acids, flavanols, flavon-3-ols [42], myricetin, peonidin, flavonoids, resveratrol, quercetin, tannins, anthocyanins, cyanidin, ellagic acid and proanthocyanins [41-49]. Besides the phytochemicals in the fruit, pulp, seeds and leaves of the grapevine, the wine derived from grapes also contains polyphenols and antioxidants [49]. The primary polyphenols in wine are resveratrol, anthocyanins, catechins and tannins (proanthocyanidins and ellagitannins) [50, 53]. Red wine and white wine are an integral part of the Mediterranean diet; they have alcohol concentrations of 14% and 11%, respectively, significantly lower than 35%, the alcohol content of spirits [51-53]. The polyphenol component of wine can have great health-protective effects [50-52], whereas white wine has fewer bioactive compounds than red wine; distilled drinks like liquor and spirits have virtually no bioactive compounds [46]. Different health effects are anticipated for alcoholic beverages due to their different chemical compositions.
Like wine, other bioactive elements of the Mediterranean diet, including nuts, fruit and vegetables (which mostly contain flavonoids) and olive oil (mainly hydroxytyrosol, tyrosol and oleocanthal), can also have cardioprotective effects through various synergistic pathways [53]. Besides containing many potential health-promoting phytochemicals (Tab. II), grapes are also rich in potassium, a mineral that maintains the body’s fluid balance and can help lower high blood pressure and reduce the probability of developing heart disease and stroke. Grape seeds are rich in vitamin E, which helps keep the skin supple and moisturized. Grapes contain other components that may help prevent acne and improve the hair by boosting blood flow to the scalp. Resveratrol in grapes can strengthen the immune system, promote wound healing and prevent bacterial infections. Grape resveratrol slows the normal aging process by inhibiting cell death and reducing age-dependent deterioration of cells. Antioxidants such as flavan-3-ols and resveratrol in grapes have been observed to be effective against cancer of the mouth, throat, pancreas, prostate and colon, among others [68, 69]. Being rich in water and fibre, grapes help bowel movements, thus maintaining a healthy digestive system. Grape seed extract has been shown to play an important role in combating irritable bowel syndrome [70], while grape skin is rich in melatonin that plays a role in circadian rhythms [71]. The consumption of certain varieties of grapes, rich in polyphenols and fibre, has been shown to reduce the onset and incidence of cardiovascular disorders [72]. For instance, a randomized, parallel-group, controlled trial with 34 non-smoking adults (13 hypercholesterolemic and 21 normocholesterolemic) who were given supplements of 7.5 g/day grape antioxidant fibre containing 1400 mg polyphenols and 5.25 g dietary fibre for 16 weeks showed a significant reduction in blood pressure and normalisation of lipid profile [73]. Other studies have indicated that grapes and their phytochemical constituents have cardioprotective effects by lowering blood pressure, lipids, platelet function and thrombosis [74].
Conclusions
Vegetable- and fruit-based nutraceuticals have become popular for many pathophysiological conditions and cosmetic uses at traditional and commercial scale. Although the bioactive phytochemicals of these essential ingredients of the Mediterranean diet are backed by much evidence of traditional use, carefully designed clinical trials are required to further explore their therapeutic potential in different pathophysiological conditions.
Acknowledgements
This research was funded by the Provincia Autonoma di Bolzano in the framework of LP 15/2020 (dgp 3174/2021).
Conflicts of interest statement
Authors declare no conflict of interest.
Author's contributions
MB: study conception, editing and critical revision of the manuscript; ZN, Kristjana D, Kevin D, BA, VV, GM, AI: literature search, editing and critical revision of the manuscript. All authors have read and approved the final manuscript.
Figures and tables
Tab. III.
Health benefits of phytochemicals present in grapevine and other sources.
| Grapevine phytochemicals | Other sources | Health benefits | References |
|---|---|---|---|
Anthocyanin: natural non-toxic water-soluble flavonoid pigments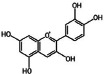
|
Mulberries, black currants, red currants, sweet cherries, blue berries, strawberries, plums, red onion, red raspberries, black chokeberries |
|
[54-57] |
Flavanols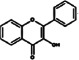
|
Onions, kale, red wine, tea, peaches, berries, tomatoes, lettuce, scallions, broccoli |
|
[57-59] |
Flavan-3-ols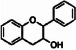
|
White, green, oolong and black tea, apples, purple and red apples, blue berries, strawberries, chocolate |
|
[57, 58, 60] |
Flavones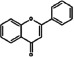
|
Parsley, red peppers, celery, chamomile, peppermint |
|
[57, 58, 61] |
Flavanones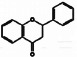
|
Limes, lemons, oranges |
|
[57, 58, 62] |
Isoflavones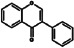
|
Soy, red clover, green tea, split peas, pigeon peas, peanuts, chickpeas, lima beans |
|
[57, 58, 63] |
Phenolic acids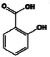
|
Berries, herbs, spices, cocoa, flax seeds, vegetables, olives, coffee, tea |
|
[64] |
Resveratrol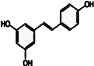
|
Peanuts, berries, cocoa, blueberries, bilberries, cranberries |
|
[65-69] |
References
- [1].Favela-Hernández JM, González-Santiago O, Ramírez-Cabrera MA, Esquivel-Ferriño PC, Camacho-Corona Mdel R. Chemistry and pharmacology of citrus sinensis. Molecules 2016;21:247. https://doi.org/10.3390/molecules21020247 10.3390/molecules21020247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lev X, Zhao S, Ning Z, Zeng H, Shu Y, Tao O, Xiao C, Lu C, Liu Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem Cent J 2015;9:68. https://doi.org/10.1186/s13065-015-0145-9 10.1186/s13065-015-0145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ejaz S, Ejaz A, Matsuda K, Lim CW. Limonoids as cancer chemopreventive agents. J Sci Food Agric 2006;86:339-45. https://doi.org/10.1002/jsfa.2396 10.1002/jsfa.2396 [DOI] [Google Scholar]
- [4].Nutritional and health benefits of citrus fruits. Available at: https://agris.fao.org/agris-search/search.do?recordID=XF2000392903 (Accessed on 08/08/2022).
- [5].Citrus health benefits. Available at: https://dokumen.tips/documents/citrus-health-benefits-health-benefits-of-citrus-fruits-csiro-full-report1.html?page=1 (Accessed on 08/08/2022).
- [6].Hirata T, Fujii M, Akita K, Yanaka N, Ogawa K, Kuroyanagi M, Hongo D. Identification and physiological evaluation of the components from citrus fruits as potential drugs for anti-corpulence and anticancer. Bioorg Med Chem 2009;17:25-8. https://doi.org/10.1016/j.bmc.2008.11.039 10.1016/j.bmc.2008.11.039 [DOI] [PubMed] [Google Scholar]
- [7].Kiani AK, Dhuli K, Anpilogov K, Bressan S, Dautaj A, Dundar M, Beccari T, Ergoren MC, Bertelli M. Natural compounds as inhibitors of SARS-CoV-2 endocytosis: A promising approach against COVID-19. Acta Biomed 2020;91:e2020008. https://doi.org/10.23750/abm.v91i13-S.10520 10.23750/abm.v91i13-S.10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bertelli M, Kiani AK, Paolacci S, Manara E, Kurti D, Dhuli K, Bushati V, Miertus J, Pangallo D, Baglivo M, Beccari T, Michelini S. Hydroxytyrosol: A natural compound with promising pharmacological activities. J Biotechnol 2020;309:29-33. https://doi.org/10.1016/j.jbiotec.2019.12.016 10.1016/j.jbiotec.2019.12.016 [DOI] [PubMed] [Google Scholar]
- [9].Paolacci S, Ergoren MC, De Forni D, Manara E, Poddesu B, Cugia G, Dhuli K, Camilleri G, Tuncel G, Kaya Suer H, Sultanoglu N, Sayan M, Dundar M, Beccari T, Ceccarini MR, Gunsel IS, Dautaj A, Sanlidag T, Connelly ST, Tartaglia GM, Bertelli M. In vitro and clinical studies on the efficacy of α-cyclodextrin and hydroxytyrosol against SARS-CoV-2 infection. Eur Rev Med Pharmacol Sci 2021;25:81-9. https://doi.org/10.26355/eurrev_202112_27337 10.26355/eurrev_202112_27337 [DOI] [PubMed] [Google Scholar]
- [10].Dhuli K, Ceccarini MR, Precone V, Maltese PE, Bonetti G, Paolacci S, Dautaj A, Guerri G, Marceddu G, Beccari T, Michelini S, Bertelli M. Improvement of quality of life by intake of hydroxytyrosol in patients with lymphedema and association of lymphedema genes with obesity. Eur Rev Med Pharmacol Sci 2021;25:33-42. https://doi.org/10.26355/eurrev_202112_27331 10.26355/eurrev_202112_27331 [DOI] [PubMed] [Google Scholar]
- [11].Akachi T, Shiina Y, Ohishi Y, Kawaguchi T, Kawagishi H, Morita T, Mori M, Sugiyama K. Hepatoprotective effects of flavonoids from shekwasha (Citrus depressa) against D-galactosamine-induced liver injury in rats. J Nutr Sci Vitaminol (Tokyo). 2010;56:60-7. https://doi.org/10.3177/jnsv.56.60 10.3177/jnsv.56.60 [DOI] [PubMed] [Google Scholar]
- [12].Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J Appl Microbiol 2010;109:515-27. https://doi.org/10.1111/j.1365-2672.2010.04677.x 10.1111/j.1365-2672.2010.04677.x [DOI] [PubMed] [Google Scholar]
- [13].Murata K, Takano S, Masuda M, Iinuma M, Matsuda H. Anti-degranulating activity in rat basophil leukemia RBL-2H3 cells of flavanone glycosides and their aglycones in citrus fruits. J Nat Med 2013;67:643-6. https://doi.org/10.1007/s11418-012-0699-y 10.1007/s11418-012-0699-y [DOI] [PubMed] [Google Scholar]
- [14].Ono E, Inoue J, Hashidume T, Shimizu M, Sato R. Anti-obesity and anti-hyperglycemic effects of the dietary citrus limonoid nomilin in mice fed a high-fat diet. Biochem Biophys Res Commun 2011;410:677-81. https://doi.org/10.1016/j.bbrc.2011.06.055 10.1016/j.bbrc.2011.06.055 [DOI] [PubMed] [Google Scholar]
- [15].Espina L, Somolinos M, Lorán S, Conchello P, García D, Pagán R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food control 2011;22:896-902. https://doi.org/10.1016/j.foodcont.2010.11.021 10.1016/j.foodcont.2010.11.021 [DOI] [Google Scholar]
- [16].Ito A, Shin N, Tsuchida T, Okubo T, Norimoto H. Antianxiety-like effects of chimpi (dried citrus peels) in the elevated open-platform test. Molecules 2013;18:10014-23. https://doi.org/10.3390/molecules180810014 10.3390/molecules180810014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sotiroudis G, Melliou E, Sotiroudis TG, Chinou I. Chemical analysis, antioxidant and antimicrobial activity of three Greek cucumber (Cucumis sativus) cultivars. J Food Biochem 2010;34:61-78. https://doi.org/10.1111/j.1745-4514.2009.00296.x 10.1111/j.1745-4514.2009.00296.x [DOI] [Google Scholar]
- [18].Peter KV, Abraham Z. Biodiversity in horticultural crops. New Delhi: Daya publishing house; 2007. [Google Scholar]
- [19].Kapoor LD. CRC handbook of Ayurvedic medicinal plants. Florida: CRC Press LLC; 1990. [Google Scholar]
- [20].Mukherjee PK, Maity N, Nema NK, Sarkar BK. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011;19:64-73. https://doi.org/10.1016/j.phymed.2011.10.003 10.1016/j.phymed.2011.10.003 [DOI] [PubMed] [Google Scholar]
- [21].Anonymous. Ayurvedic Pharmacopoeia of India, The Controller of Publication. New Delhi: National Institute of Science Communication and Information Resources (NISCAIR); 2001 [Google Scholar]
- [22].Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep 2005;22:386-99. https://doi.org/10.1039/b418841c. Epub 2005 4. Erratum in: Nat Prod Rep 2005;22:794-5. PMID: 16010347. https://doi.org/10.1039/b418841c 10.1039/b418841c [DOI] [PubMed] [Google Scholar]
- [23].Tuama AA, Mohammed AA. Phytochemical screening and in vitro antibacterial and anticancer activities of the aqueous extract of Cucumis sativus. Saudi J Biol Sci 2019;26:600-4. https://doi.org/10.1016/j.sjbs.2018.07.012 10.1016/j.sjbs.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Begum HA, Asad F, Sadiq A, Mulk S, Ali K. 44. Antioxidant, antimicrobial activity and phytochemical analysis of the seeds extract of Cucumis sativus Linn. Pure Appl Biol 2019. January;8:433-41. 10.19045/bspab.2018.700202 [DOI] [Google Scholar]
- [25].Saidu AN, Oibiokpa FI, Olukotun IO. Phytochemical screening and hypoglycemic effect of methanolic fruit pulp extract of Cucumis sativus in alloxan-induced diabetic rats. J Med Plant Res 2014;8:1173-8. https://doi.org/10.5897/JMPR2014.5506 10.5897/JMPR2014.5506 [DOI] [Google Scholar]
- [26].Hakim AR, Saputri R. Identifikasi senyawa kimia ekstrak etanol mentimun (Cucumis sativus L.) dan ekstrak etanol nanas (Ananas comosus (L) Merr). J Pharm Sci 2017;04:34-8. 10.20527/jps.v4i1.5753 [DOI] [Google Scholar]
- [27].Rice CA, Rymal KS, Chambliss OL, Johnson FA. Chromatographic and mass spectral analysis of cucurbitacins of three Cucumis sativus cultivars. J Agric Food Chem 1981;29:194-6. https://doi.org/10.1021/jf00103a051 10.1021/jf00103a051 [DOI] [Google Scholar]
- [28].Alghasham AA. Cucurbitacins - a promising target for cancer therapy. Int J Health Sci (Qassim) 2013;7:77-89. https://doi.org/10.12816/0006025 10.12816/0006025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McNally DJ, Wurms KV, Labbé C, Quideau S, Bélanger RR. Complex C-glycosyl flavonoid phytoalexins from Cucumis sativus. J Nat Prod 2003;66:1280-3. https://doi.org/10.1021/np030150y 10.1021/np030150y [DOI] [PubMed] [Google Scholar]
- [30].Kai H, Baba M, Okuyama T. Two new megastigmanes from the leaves of Cucumis sativus. Chem Pharm Bull (Tokyo) 2007;55:133-6. https://doi.org/10.1248/cpb.55.133 10.1248/cpb.55.133 [DOI] [PubMed] [Google Scholar]
- [31].Abou-Zaid MM, Lombardo DA, Kite GC, Grayer RJ, Veitch NC. Acylated flavone C-glycosides from Cucumis sativus. Phytochemistry 2001;58:167-72. https://doi.org/10.1016/s0031-9422(01)00156-x 10.1016/s0031-9422(01)00156-x [DOI] [PubMed] [Google Scholar]
- [32].Krauze-Baranowska M, Cisowski W. Flavonoids from some species of the genus Cucumis. Biochem Syst Ecol 2001;29:321-4. https://doi.org/10.1016/s0305-1978(00)00053-3 10.1016/s0305-1978(00)00053-3 [DOI] [PubMed] [Google Scholar]
- [33].Kumar D, Kumar S, Singh J, Narender Rashmi, Vashistha B, Singh N. Free Radical Scavenging and Analgesic Activities of Cucumis sativus L. Fruit Extract. J Young Pharm 2010;2:365-8. https://doi.org/10.4103/0975-1483.71627 10.4103/0975-1483.71627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gill NS, Garg M, Bansal R, Sood S, Muthuraman A, Bali M, Sharma PD. Evaluation of antioxidant and antiulcer potential of Cucumis sativus L. seed extract in rats. Asian J Clin Nutr 2009;1:131-8. https://doi.org/10.3923/ajcn.2009.131.138 10.3923/ajcn.2009.131.138 [DOI] [Google Scholar]
- [35].Swaminathan G, Sundaram RS, Mamatha M, Vaijayanthimala P. Evaluation of in vitro anticancer activity of Cucumis sativus Linn leaves. IJRPP 2015;4:223-9. [Google Scholar]
- [36].Ankita S, Kaur P, Gupta R. Phytochemical screening and antimicrobial assay of various seeds extracts of Cucurbitaceae Family. Int J Appl Biol Pharm 2012;3:401-9. [Google Scholar]
- [37].Mallik J, Akhter R. Phytochemical screening and in-vitro evaluation of reducing power, cytotoxicity and anti-fungal activities of ethanol extracts of Cucumis sativus. Int J Pharm Biol 2012;3:555-60. [Google Scholar]
- [38].Heidari H, Kamalinejad M, Eskandari M. Hepatoprotective activity of Cucumis sativus against cumene hydroperoxide induced-oxidative stress. Res Pharm Sci 2012;7:S936-S939. [Google Scholar]
- [39].Sharmin R, Khan MRI, Akhtar MA, Alim A, Islam MA, Anisuzzaman ASM, Ahmed M. Hypoglycemic and hypolipidemic effects of cucumber, white pumpkin and ridge gourd in alloxan induced diabetic rats. J Sci Res 2013;5:161-70. https://doi.org/10.3329/jsr.v5i1.10252 10.3329/jsr.v5i1.10252 [DOI] [Google Scholar]
- [40].Liang N, Kitts DD. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015;8:16. https://doi.org/10.3390/nu8010016 10.3390/nu8010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Teixeira A, Eiras-Dias J, Castellarin SD, Gerós H. Berry phenolics of grapevine under challenging environments. Int J Mol Sci 2013;14:18711-39. https://doi.org/10.3390/ijms140918711 10.3390/ijms140918711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yuzuak S, Ballington J, Xie DY. HPLC-qTOF-MS/MS-based profiling of flavan-3-ols and dimeric proanthocyanidins in berries of two muscadine grape hybrids FLH 13-11 and FLH 17-66. Metabolites 2018;8:57. https://doi.org/10.3390/metabo8040057 10.3390/metabo8040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Šuković D, Knežević B, Gašić U, Sredojević M, Ćirić I, Todić S, Mutić J, Tešić Ž. Phenolic profiles of leaves, grapes and wine of grapevine variety vranac (Vitis vinifera L.) from Montenegro. Foods 2020;9:138. https://doi.org/10.3390/foods9020138 10.3390/foods9020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mattivi F, Guzzon R, Vrhovsek U, Stefanini M, Velasco R. Metabolite profiling of grape: flavonols and anthocyanins. J Agric Food Chem 2006;54:7692-702. https://doi.org/10.1021/jf061538c 10.1021/jf061538c [DOI] [PubMed] [Google Scholar]
- [45].Schoedl K, Schuhmacher R, Forneck A. Studying the polyphenols of grapevine leaves according to age and insertion level under controlled conditions. Sci Hortic 2012;141:37-41.. https://doi.org/10.1016/j.scienta.2012.04.014 10.1016/j.scienta.2012.04.014 [DOI] [Google Scholar]
- [46].He F, Liang NN, Mu L, Pan QH, Wang J, Reeves MJ, Duan CQ. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012;17:1571-601. https://doi.org/10.3390/molecules17021571 10.3390/molecules17021571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pantelić MM, Zagorac DČ, Davidović SM, Todić SR, Bešlić ZS, Gašić UM, Tešić ŽL, Natić MM. Identification and quantification of phenolic compounds in berry skin, pulp and seeds in 13 grapevine varieties grown in Serbia. Food Chem 2016;211:243-52. https://doi.org/10.1016/j.foodchem.2016.05.051 10.1016/j.foodchem.2016.05.051 [DOI] [PubMed] [Google Scholar]
- [48].de Freitas V, Mateus N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal Bioanal Chem 2011;401:1463-73. https://doi.org/10.1007/s00216-010-4479-9 10.1007/s00216-010-4479-9 [DOI] [PubMed] [Google Scholar]
- [49].Gómez-Brandón M, Lores M, Insam H, Domínguez J. Strategies for recycling and valorization of grape marc. Crit Rev Biotechnol 2019;39:437-50. https://doi.org/10.1080/07388551.2018.1555514 10.1080/07388551.2018.1555514 [DOI] [PubMed] [Google Scholar]
- [50].Klatsky AL. Alcohol and cardiovascular diseases: where do we stand today? J Intern Med 2015;278:238-50. https://doi.org/10.1111/joim.12390 10.1111/joim.12390 [DOI] [PubMed] [Google Scholar]
- [51].Matsumoto C, Miedema MD, Ofman P, Gaziano JM, Sesso HD. An expanding knowledge of the mechanisms and effects of alcohol consumption on cardiovascular disease. J Cardiopulm Rehabil Prev 2014;34:159-71. https://doi.org/10.1097/HCR.0000000000000042 10.1097/HCR.0000000000000042 [DOI] [PubMed] [Google Scholar]
- [52].Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean Diet; a Literature Review. Nutrients 2015;7:9139-53. https://doi.org/10.3390/nu7115459 10.3390/nu7115459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Caimi G, Carollo C, Presti RL. Diabetes mellitus: oxidative stress and wine. Curr Med Res Opin 2033;19:581-6. https://doi.org/10.1185/030079903125002324 10.1185/030079903125002324 [DOI] [PubMed] [Google Scholar]
- [54].Bakuradze T, Tausend A, Galan J, Groh IA, Berry D, Tur JA, Marko D, Richling E. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic Res 2019;53:1045-55. https://doi.org/10.1080/10715762.2019.1618851 10.1080/10715762.2019.1618851 [DOI] [PubMed] [Google Scholar]
- [55].Tsuda T. Dietary anthocyanin‐rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res 2012;56:159-70. https://doi.org/10.1002/mnfr.201100526 10.1002/mnfr.201100526 [DOI] [PubMed] [Google Scholar]
- [56].Camire ME, Chaovanalikit A, Dougherty MP, Briggs J. Blueberry and grape anthocyanins as breakfast cereal colorants. J Food Sci 2002;67:438-41. https://doi.org/10.1111/j.1365-2621.2002.tb11425.x 10.1111/j.1365-2621.2002.tb11425.x [DOI] [Google Scholar]
- [57].Kiani AK, Falsini B, Ziccardi L, Gusso E, Mangialavori D, Allegrini F, Colao E, Bertelli M. Flavonoid supplements increase neurotrophin activity to modulate inflammation in retinal genetic diseases. Acta Biomed 2020;91:e2020014. https://doi.org/10.23750/abm.v91i13-S.10683 10.23750/abm.v91i13-S.10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mastroiacovo D, Kwik-Uribe C, Grassi D, Necozione S, Raffaele A, Pistacchio L, Righetti R, Bocale R, Lechiara MC, Marini C, Ferri C. Cocoa flavanol consumption improves cognitive function, blood pressure control and metabolic profile in elderly subjects: the Cocoa, Cognition and Aging (CoCoA) Study – a randomized controlled trial. Am J Clin Nutr 2015;101:538-48. https://doi.org/10.3945/ajcn.114.092189 10.3945/ajcn.114.092189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Aron PM, Kennedy JA. Flavan-3-ols: Nature, occurrence and biological activity. Mol Nutr Food Res 2008;52:79-104. https://doi.org/10.1002/mnfr.200700137 10.1002/mnfr.200700137 [DOI] [PubMed] [Google Scholar]
- [60].Zuiter AS. Proanthocyanidin: Chemistry and Biology: From Phenolic Compounds to Proanthocyanidins. Reference Module in Chem Eng Sci 2014. https://doi.org/10.1016/b978-0-12-409547-2.11046-7 10.1016/b978-0-12-409547-2.11046-7 [DOI] [Google Scholar]
- [61].Farooqui AA, Farooqui T. Importance of fruit and vegetable-derived flavonoids in the mediterranean diet. In: Role of the mediterranean diet in the brain and neurodegenerative diseases. 2018:417-27. https://doi.org/10.1016/b978-0-12-811959-4.00027-4 10.1016/b978-0-12-811959-4.00027-4 [DOI] [Google Scholar]
- [62].Wong MCY, Emery PW, Preedy VR, Wiseman H. Health benefits of isoflavones in functional foods? Proteomic and metabonomic advances. Inflammopharmacology 2008;16:235-9. https://doi.org/10.1007/s10787-008-8023-x 10.1007/s10787-008-8023-x [DOI] [PubMed] [Google Scholar]
- [63].Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol Rep 2019;24:e00370. https://doi.org/10.1016/j.btre.2019.e00370 10.1016/j.btre.2019.e00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem 2002;50:3337-40. https://doi.org/10.1021/jf0112973 10.1021/jf0112973 [DOI] [PubMed] [Google Scholar]
- [65].Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. Resveratrol, pterostilbene and piceatannol in vaccinium berries. J Agric Food Chem 2004;52:4713-9. https://doi.org/10.1021/jf040095e 10.1021/jf040095e [DOI] [PubMed] [Google Scholar]
- [66].Sanders TH, McMichael RW, Jr, Hendrix KW. Occurrence of resveratrol in edible peanuts. J Agric Food Chem 2000;48:1243-6. https://doi.org/10.1021/jf990737b 10.1021/jf990737b [DOI] [PubMed] [Google Scholar]
- [67].Hurst WJ, Glinski JA, Miller KB, Apgar J, Davey MH, Stuart DA. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J Agric Food Chem 2008;56:8374-8. https://doi.org/10.1021/jf801297w 10.1021/jf801297w [DOI] [PubMed] [Google Scholar]
- [68].Almatroodi SA, Almatroudi A, Alsahli MA, Rahman AH. Grapes and their bioactive compounds: role in health management through modulating various biological activities. Pharmacogn J 2020;12:1455-62. https://doi.org/10.5530/pj.2020.12.200 10.5530/pj.2020.12.200 [DOI] [Google Scholar]
- [69].Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med.1995;155:381-6. [PubMed] [Google Scholar]
- [70].Wang H, Xue Y, Zhang H, Huang Y, Yang G, Du M, Zhu MJ. Dietary grape seed extract ameliorates symptoms of inflammatory bowel disease in IL 10‐deficient mice. Mol Nutr Food Res 2013;57:2253-7. https://doi.org/10.1002/mnfr.201300146 10.1002/mnfr.201300146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mercolini L, Mandrioli R, Raggi MA. Content of melatonin and other antioxidants in grape‐related foodstuffs: measurement using a MEPS‐HPLC‐F method. J Pineal Res 2012;53:21-8. https://doi.org/10.1111/j.1600-079X.2011.00967.x 10.1111/j.1600-079X.2011.00967.x [DOI] [PubMed] [Google Scholar]
- [72].Rasines-Perea Z, Teissedre PL. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017;22:68. https://doi.org/10.3390/molecules22010068 10.3390/molecules22010068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Jiménez JP, Serrano J, Tabernero M, Arranz S, Díaz-Rubio ME, García-Diz L, Goñi I, Saura-Calixto F. Effects of grape antioxidant dietary fiber in cardiovascular disease risk factors. Nutrition 2008;24:646-53. https://doi.org/10.1016/j.nut.2008.03.012 10.1016/j.nut.2008.03.012 [DOI] [PubMed] [Google Scholar]
- [74].Zhao CN, Meng X, Li Y, Li S, Liu Q, Tang GY, Li HB. Fruits for prevention and treatment of cardiovascular diseases. Nutrients 2017;9:598. https://doi.org/10.3390/nu9060598 10.3390/nu9060598 [DOI] [PMC free article] [PubMed] [Google Scholar]


