Abstract
Introduction
Various pharmacological treatments are available for preterm infants with patent ductus arteriosus (PDA), but their risks and benefits are controversial. This study aimed to identify the best treatment for PDA using network meta-analysis (NMA) and risk-benefit assessment (RBA).
Methods
Relevant randomized controlled trials (RCTs) were identified from MEDLINE, Scopus, and the Cochrane Library. RCTs were eligible if they were studied for preterm or low birth weight infants with presymptomatic PDA and hemodynamically significant PDA (hsPDA). The outcomes were PDA closure for a benefit and the composite risk outcome of adverse effects (AEs) for risk. An NMA was used to estimate the treatment effects of benefit and risk. The RBA helped to incorporate the risk and benefits of multiple treatments. Then, an incremental risk-benefit ratio was calculated by dividing the incremental risk by benefit using data from NMA, and they were jointly simulated using Monte Carlo methods. Finally, net clinical benefit (NCB) probability curves were constructed at varying acceptability thresholds.
Results
Seventy RCTs with hsPDA were eligible considering 13 different interventions, but data on presymptomatic PDA were not enough for pooling. The clustered ranking plot from NMA indicated that 3 interventions (i.e., high-dose oral ibuprofen, standard-dose oral acetaminophen, and standard-dose oral ibuprofen) yielded high PDA closure and low AE. These three treatments and additional commonly used indomethacin were considered in the RBA. Given an acceptable threshold of 25% or having one AE out of four PDA closures, high-dose oral ibuprofen had a 36% chance of having the highest NCB, followed by standard-dose oral acetaminophen (27%), and oral ibuprofen (23.7%). Subgroup analysis indicated that the chances of having the highest NCB of GA ≥28 weeks were similar to that of all available studies. The best for GA <28 weeks, no data for high-dose oral ibuprofen, was standard-dose oral acetaminophen, followed by standard-dose oral ibuprofen.
Conclusions
Trade-off RBA indicated that high-dose oral ibuprofen might be the best treatment for preterm, GA ≥28 weeks, with hsPDA followed by the standard-dose oral acetaminophen and ibuprofen. Preferably, optimal high doses, postnatal age to start treatment, and long-term outcomes are needed to study in the future.
Keywords: Patent ductus arteriosus, Preterm, Hemodynamically significant patent ductus arteriosus, Risk-benefit analysis, Indomethacin, Ibuprofen, Acetaminophen
Introduction
The ductus arteriosus is a vessel connecting the pulmonary artery and the proximal descending aorta, which spontaneously closes within 24–72 h after birth [1]. However, the closure may be delayed or fail in preterm infants, especially in extremely low birth weight (LBW) infants (<1,000 g) or with gestational age (GA) <28 weeks [2]. A persistent ductus arteriosus can cause significant flow shunting from the descending aorta to the pulmonary artery and may result in serious complications, including congestive heart failure, necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), chronic lung disease (CLD), and death [3, 4, 5, 6].
Treatments for patent ductus arteriosus (PDA) range from observation and pharmacological treatment to surgery. Recently, many pharmacological regimens have been available, including cyclooxygenase (COX) inhibitors (i.e., indomethacin and ibuprofen) and acetaminophen. Choosing the best treatment option can be difficult. For example, the treatment may not only have high benefits but also have high serious adverse events (SAEs), or it may have low benefits and also have low SAE. A few network meta-analyses (NMA) have been recently published, considering both benefits (PDA closure) and risks (e.g., death, NEC, and IVH), but they did not bring the two together [7, 8]. Therefore, it is difficult to balance risks with benefits. The decision may mainly depend on the decision maker's perception. Then, methods that can offer a more transparent approach are required.
Risk-benefit analysis (RBA) has been proposed recently by incorporating the risk and benefits of multiple treatments from individual RCTs or NMA-RCTs [9]. An incremental risk-benefit ratio (IRBR) is estimated, which is analogous to the incremental cost-effectiveness ratio (ICER). Benefit refers to effectiveness, and risk refers to SAEs [10, 11]. The Monte Carlo simulations are applied to simultaneously estimate the probability of net benefit over different preference thresholds [12]. Therefore, we conducted an NMA and RBA to identify the best treatment of PDA in preterm neonates.
Materials and Methods
Search Terms and Strategies
We constructed search terms and strategies to identify relevant studies from the Cochrane Database, MEDLINE, and Scopus up to May 31, 2021. Additional reference lists of the selected studies were checked. The review protocol was registered with PROSPERO (CRD42016046261).
Study Selection
We included RCTs if they met the following criteria:
Enrolled preterm neonates (GA <37 weeks) and/or LBW infants (<2,500 g) with either presymptomatic or hsPDA.
Compared any pair with any dose/route of the following interventions: indomethacin, ibuprofen, acetaminophen, and placebo.
Reported any of the following outcomes: PDA closure, death, CLD or bronchopulmonary dysplasia (BPD), IVH, NEC, retinopathy of prematurity (ROP), acute renal failure, and liver failure.
PDA was classified as hsPDA if preterm neonates had significant hemodynamic change, confirmed by echocardiography and/or clinical symptoms of congestive heart failure (i.e., active precordium and/or worsening respiratory status); otherwise, they were classified as presymptomatic PDA. For multiple reports, the one with the most complete data was used. We excluded studies that compared the same interventions, total mg/course, and route of administration, although subjects received the treatment at a different interval or duration. In addition, studies that diagnosed hsPDA based on only clinical data or defined hsPDA based on calculated scores from echocardiographic data were also excluded.
Data Extraction
Data were extracted independently by four authors (S.A.-O.V., C.O., and P.N.) using a standardized extraction form consisting of characteristics of studies/patients, interventions (i.e., dosages, interval, duration, route), and type of treatments and outcomes based on intention-to-treat analysis. Disagreements were resolved by consensus with the fourth author (A.T). Relevant missing data were sought by emailing the authors of the studies.
Intervention and Outcomes
Treatment regimens were categorized according to types, administration, and total dosages (milligrams/course) [13]. A total of 15 interventions with standard and high total doses/courses are defined in Table 1.
Table 1.
Abbreviation of treatment groups
| Treatment abbreviation | Types of treatment | Total doses* |
|---|---|---|
| Plac | Placebo or conservative treatment | – |
| SOI | A standard dose of oral indomethacin | ≤0.6 |
| HOI | A high dose of oral indomethacin | >0.6 |
| SII | A standard dose of intravenous indomethacin | ≤0.6 |
| HII | A high dose of intravenous indomethacin | >0.6 |
| SOB | A standard dose of oral ibuprofen | ≤20 |
| HOB | A high dose of oral ibuprofen | >20 |
| SIB | A standard dose of intravenous ibuprofen | ≤20 |
| HIB | A high dose of intravenous ibuprofen | >20 |
| SOA | A standard dose of oral acetaminophen | ≤180 |
| HOA | A high dose of oral acetaminophen | >180 |
| SIA | A standard dose of intravenous acetaminophen | ≤180 |
| HIA | A high dose of intravenous acetaminophen | >180 |
| SIIdrip | A standard dose and continuous infusion of indomethacin | ≤0.6 |
| SIBdrip | A standard dose and continuous infusion of ibuprofen | ≤20 |
The unit of total doses = mg/kg.
The benefit outcome was PDA closure, which was defined as ductal closure confirmed by echocardiography and/or clinical criteria after receiving the first course of treatments. The composite risk outcome was used instead of multiple risk outcomes to summarize data for NMA and the net clinical benefit (NCB). The composite outcome enables the incorporation of a flexible framework for assessing the risk-benefit analysis [14]. The composite risk outcomes were defined as the maximum number of adverse events among seven moderate to SAEs reported from individual studies, including mortality during the initial hospitalization, BPD (needed to supplement oxygen at 36 weeks after conception), severe IVH grade ≥III [15, 16], severe ROP stage ≥III [17, 18], severe NEC stage ≥IIb [19], renal failure (serum creatinine >1.5 mg/dL, or >132 μmol/L, and/or urine output <0.5 mL/kg/h) [20], and liver failure (ALT and AST >2 times the upper boundary of the normal range) [21]. The clinical severity of these SAEs was equally weighed.
Statistical Analysis
An NMA was performed to compare relative treatment effects (i.e., risk ratios [RRs]) with placebo using a multivariate meta-analysis with a consistency model [22]. The inconsistency was checked based on a design-by-treatment interaction model using a global χ2 test. A loop-specific method was also used to estimate the inconsistency factor (IF) and the ratio of two odds ratios (ROR). In addition, characteristics of patients, interventions, outcomes, and study designs were further explored if there was any evidence of inconsistency. The probability of being the best treatment was estimated using the surface under the cumulative ranking curve (SUCRA) from the frequentist approach. A cluster ranking plot was constructed considering both benefits and risks; the larger the SUCRA value, the higher the rate of PDA closure, and the lower the SAE rate. Interventions from NMA, which had high benefits and low composite risks (in the right upper quadrant of the clustered ranking plot), and commonly used intervention (indomethacin) were included in the RBA.
For the RBA, we estimated values of incremental risk (ΔR) and incremental benefit (ΔB) using “network setup” and specified the treatment effects using the risk difference compared with Plac (a base case). These ΔR and ΔB were further jointly simulated using Monte Carlo methods with 1,000 replications, assuming normal distributions for both ΔR and ΔB. The simulation randomly selected values from each specified distribution, allowing the joint uncertainty of the risks and benefits [23]. The IRBR was calculated by dividing ΔR by ΔB [24, 25]. A risk-benefit plane (RBP) was constructed, assigning ΔR and ΔB on Y-axis and X-axis, respectively, with varying risk-benefit acceptability thresholds (RBAT, µ) [24]. IRBRs falling in the right-lower quadrant indicate a dominant intervention or more benefit with less risk; IRBRs falling in the right upper quadrant provide higher benefit but with higher risk. Next, the risk-benefit acceptability curves (RBACs) were further constructed by estimating the percentages of the simulated IRBRs falling below and to the right of a threshold line [26]. These curves represent the probability that each intervention has the chance of being net-beneficial relative to placebo across a range of RBAT. However, the RBACs did not allow a comparison of all interventions at the same time. Thus, the NCB for each comparison with placebo of the 1,000 simulations was simultaneously calculated. Subsequently, the proportions in which each intervention had the highest NCB across a range of thresholds were estimated and plotted in net clinical benefit probability curves. This curve shows the treatment which is most likely to have the best risk-benefit profile at a given threshold value [9, 27]. A subgroup analysis was performed by GA <28 and ≥28 weeks. Additionally, sensitivity analysis was performed, including only a study reporting both PDA closure and risk outcomes. All analyses were performed using STATA version 16.0 (StataCorp LP, College Station, TX, USA), and the simulations were done using Microsoft Excel 2019 (Microsoft Corp., Seattle, WA, USA). A two-tailed p value <0.05 was considered statistically significant. The sequence of the complete analysis is shown in eTable 1.1 (for all online suppl. material, see www.karger.com/doi/10.1159/000526318).
Quality of Evidence
The risk of bias assessment was independently assessed by four authors (S.E., S.A.-O.V., C.O, and P.N.) using the Cochrane Collaboration tool [28]. Six domains were assessed: selection, performance, detection, attrition, reporting, and other biases. Each item was classified as low, high, or unclear risk of bias. Disagreements were resolved by consensus with a fifth author (A.T). The confidence in the results of NMA was graded using the CINeMA software. Six domains were assessed, including within-study-variation, reporting bias, indirectness, imprecision, heterogeneity, and inconsistency [29].
Results
Study Characteristics
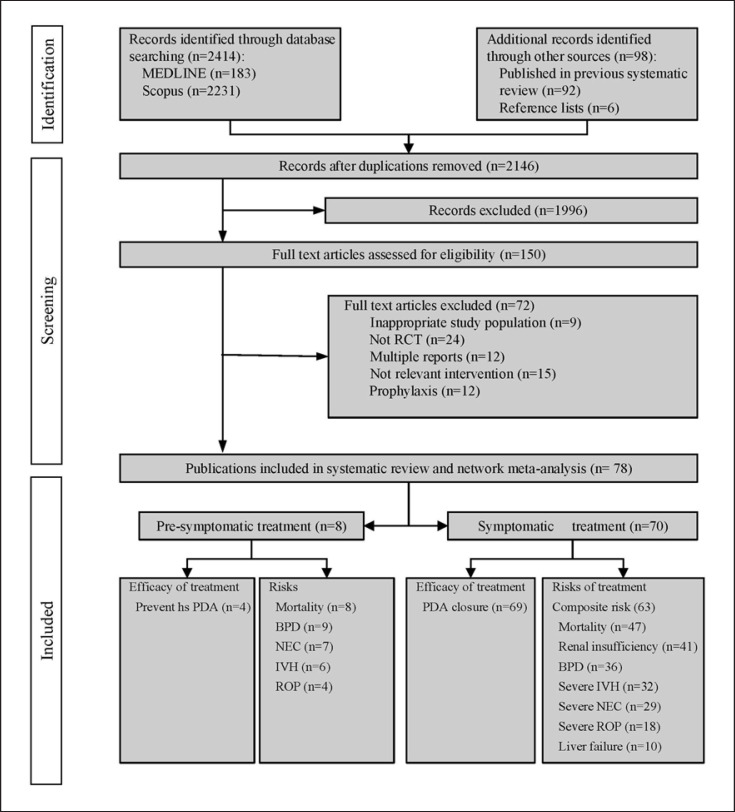
A total of 2,414 individual RCTs were identified from MEDLINE and Scopus additional 92 RCTs from systematic reviews and 6 studies from reference lists. After screening the titles and abstracts, 150/2,146 studies met our inclusion criteria. A total of 78 RCTs were eligible for pooling consisting of 8 [30, 31, 32, 33, 34, 35, 36, 37] and 70 [38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107] for presymptomatic and hsPDA (shown in Fig. 1). The summary of searching was in online eTable 2.1–2.3; the reasons for exclusion after full-text screening were in online eTable 3.1. We analyzed only hsPDA because of a small number of presymptomatic studies (shown in online eTable 3.2). Data for a high dose of oral indomethacin (HOI) and a high dose of intravenous acetaminophen (HIA) were unavailable, leaving 13 out of 15 interventions for analysis. Among these, the common comparators were SII (n = 2,294) and SOB (n = 2,180); 3 studies [46, 81, 108] had three arms, one with SII-SOB-HOB and the others with SII-SIB-SIA and SOI-SOB-SIA. The mean GAs and BWs varied from 25 to 33.5 weeks and 736–2,455.74 g, respectively. All RCTs prescribed treatments at the postnatal age of ≤14 days (mean age range = 4–277 h), except in 1 study where the mean age was 411 h. PDA diameters ranged from 1.39 to 2.85 mm (shown in Table 2). All studies used echocardiographic criteria (e.g., PDA size, LA/AO, and/or reverse diastolic flow in the descending aorta) for the diagnosis of hsPDA, and 30/70 studies also had additional clinical criteria (e.g., tachycardia, bounding pulse, continuous murmur, or hepatomegaly).
Fig. 1.
Study selection process.
Table 2.
Describe characteristics of included studies for hsPDA
| Study and year of publication | Country | Intervention | n | Diagnosis of hsPDA | GA, weeks | BW, g | PDA size, mm | Age at treatment, h | Dose, mg/kg/dose | Duration, h | Total doses, mg/kg/course |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adamska et al. 2005 [38] | Turkey | SII | 19 | Echo | 27.6±2 | 1,003±192 | N/A | 48 | 0.2-0.2-0.2 | 72 | 0.6 |
|
|
|||||||||||
| SIB | 16 | 27.7±1.8 | 1,074±264 | 10-5-5 | 72 | 20 | |||||
|
| |||||||||||
| Akisu et al. 2001 [40] | Turkey | SOI | 11 | Echo | 31.9±1.3 | 1,645±190 | N/A | 3.5±0.6 | 0.2-0.2-0.2 | 36 | 0.6 |
|
|
|||||||||||
| SOB | 12 | 32.1±1.2 | 1,706±187 | 10-5-5 | 72 | 20 | |||||
|
| |||||||||||
| Al-lawama et al. 2018 [41] | Jordan | HOB | 9 | Echo & clinical | 28.0 (25.0–35.0) | 1,192±269 | N/A | 72–120 | 10-10-10 | 72 | 30 |
|
|
|||||||||||
| SOA | 13 | 28.0 (23.0–32.0) | 1,059±386 | 10 every 6 h | 72 | 120 | |||||
|
| |||||||||||
| Aly et al. 2007 [42] | Egypt | SII | 9 | Echo | 31.2±2.5 | 1,521±398 | 2.3 | 48–168 | 0.2-0.2-0.2 | 36 | 0.6 |
|
|
|||||||||||
| SOB | 12 | 32.9±1.6 | 1,884±485 | 2.1 | 10-5-5 | 72 | 20 | ||||
|
| |||||||||||
| Bagheri et al. 2016 [43] | Iran | HOB | 80 | Echo | 31.7±2.2 | 1,643±58 | >1.5 | 82.1±51.0 | 20-10-10 | 72 | 40 |
|
|
|||||||||||
| SOA | 80 | 31.53±2.3 | 1,646±59 | 68.4±30.7 | 15 every 6 h | 72 | 180 | ||||
|
| |||||||||||
| Bagnoli et al. 2013 [44] | Italy | Plac | 67 | Echo | 27.0±4.0 | 1,197±835 | >1.5 | 72–96 | – | – | – |
|
|
|||||||||||
| SIB | 67 | 27.0±2.5 | 989±326 | 10-5-5 | 72 | 20 | |||||
|
| |||||||||||
| Balachander et al. 2018 [45] | India | SOB | 55 | Echo & clinical | 31.5±2.9 | 1,513±415 | 2.4 | 79.2±38.4 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SOA | 55 | 31.5±2.9 | 1,535±408 | 2.4 | 79.2±38.4 | 15 every 6 h | 48 | 120 | |||
|
| |||||||||||
| Cheng et al. 2012 [46] | Philippines | SII | 10 | Echo | 31.2±2.2 | 1,435±413 | 2.8 | 60–80 | 0.2–0.1–0.1 | 36 | 0.4 |
|
|
|||||||||||
| SOB | 10 | 32.9±2.2 | 1,250±321 | 2.0 | 10-5-5 | 36 | 20 | ||||
|
|
|||||||||||
| HOB | 10 | 31.1±3.6 | 1,250±448 | 2.1 | 10-10-10 | 36 | 30 | ||||
|
| |||||||||||
| Cherif et al. 2008 [47] | Tunisia | SOB | 32 | Echo | 29.3±1.2 | 1,227±188 | 2.6 | 48–96 | 10 for each dose, ECHO before next dose | 45.6 | 19 |
|
|
|||||||||||
| SIB | 32 | 28.3±1.1 | 1,198±158 | 2.5 | 10 for each dose, ECHO before next dose | 45.6 | 19 | ||||
|
| |||||||||||
| Chotigeat et al. 2003 [48] | Thailand | SII | 15 | Echo & clinical | 29.9±2.9 | 1,434±421 | 2.3 | 3.5±1.6 | 0.2-0.2-0.2 | 36 | 0.6 |
|
|
|||||||||||
| SOB | 15 | 30.8±2.3 | 1,412±354 | 1.7 | 6.0±2.4 | 10-5-5 | 72 | 20 | |||
|
| |||||||||||
| Christmann et al. 2002 [49] | Netherlands | SII | 14 | Echo & clinical | 30.8±0.5 | 1,424±150 | N/A | 120.0±33.6 | 0.2–0.1–0.1 | 36 | 0.4 |
|
|
|||||||||||
| SIIdrip | 18 | 29.4±0.5 | 1,150±77 | 96.0±16.8 | 0.011 mg/kg/h cont infusion | 36 | 0.4 | ||||
|
| |||||||||||
| Dang et al. 2013 [50] | China | SOB | 80 | Echo | 30.9±2.2 | 1,531±454 | 2.4 | 89.0±3.8 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SOA | 80 | 31.2±1.8 | 1,592±349 | 2.4 | 77.3±3.4 | 15 every 6 h | 72 | 180 | |||
|
| |||||||||||
| Dani et al. 2012 [52] | Italy | SIB | 47 | Echo | 26±1.7 | 835±215 | >1.5 | 12–24 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| HIB | 48 | 25.6±1.8 | 781±225 | 20-10-10 | 72 | 40 | |||||
|
| |||||||||||
| Dash et al. 2015 [53] | India | SII | 39 | Echo | 28.9±2.6 | 1,027±262 | 2.1 | 15.9±11.8 | 0.2-0.2-0.2 | 72 | 0.6 |
|
|
|||||||||||
| HOA | 38 | 28.5±2.7 | 989±299 | 2.0 | 14.7±8.4 | 15 every 6 h | 168 | 420 | |||
|
| |||||||||||
| Ding et al. 2014 [55] | China | Plac | 37 | Echo | 30.2±1.5 | 1,469±448 | N/A | >24 | – | – | – |
|
|
|||||||||||
| SOB | 35 | 10-5-5 | 72 | 20 | |||||||
|
| |||||||||||
| El Farrash et al. 2018 [56] | Egypt | SOB | 30 | Echo | 31.7±1.9 | 1,740±470 | 2.5 | 188.4±143.0 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SOA | 30 | 30.5±1.6 | 1,530±560 | 2.2 | 145.2±126.2 | 15 every 6 h | 72 | 180 | |||
|
| |||||||||||
| El-Mashad et al. 2017 [57] | Egypt | SII | 100 | Echo & clinical | 26.0±2.1 | 1,100±140 | 2.7 | 74.4±122.4 | 0.2-0.2-0.2 | 36 | 0.6 |
|
|
|||||||||||
| SIB | 100 | 25.0±2.1 | 1,000±120 | 2.8 | 76.8±100.8 | 10-5-5 | 72 | 20 | |||
|
|
|||||||||||
| SIA | 100 | 26.0±1.9 | 1,100±130 | 2.7 | 64.8±105.6 | 15 every 6 h | 72 | 180 | |||
|
| |||||||||||
| Erdeve et al. 2012 [58] | Turkey | SOB | 40 | Echo | 26.4±1.1 | 892±117 | >1.5 | 48–96 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SIB | 40 | 26.3±1.3 | 872±123 | 10-5-5 | 72 | 20 | |||||
|
| |||||||||||
| Fakhraee et al. 2007 [59] | Iran | SOI | 18 | Echo | 30.9±2.0 | 1,522±358 | >1.5 | 74.4±14.4 | 0.2-0.2-0.2 | 72 | 0.6 |
|
|
|||||||||||
| SOB | 18 | 31.5±1.4 | 1,658±387 | 84.0±12.0 | 10-5-5 | 72 | 20 | ||||
|
| |||||||||||
| Fesharaki et al. 2012 [60] | Iran | SOB | 30 | Echo | 30.9 | 1,324 | N/A | 72–120 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| HOB | 30 | 29.8 | 1,300 | 15–7.5–7.5 | 72 | 30 | |||||
|
| |||||||||||
| Gersony et al. 1983 [61] | USA | Plac | 281 | Echo & clinical | 29.32 | 1,109 | N/A | 0–336 | – | – | – |
|
|
|||||||||||
| SII | 140 | 0.2-0.1-0.1 for age 2–7 days 0.2-0.25-0.25 for age ≥8 d | 72 | 0.4–0.7 | |||||||
|
| |||||||||||
| Ghanem et al. 2010 [63] | Saudi Arabia | Plac | 33 | Echo | 28.9±2.7 | 1,047±403 | 2.2 | 57.6±21.6 | – | – | – |
|
|
|||||||||||
| SOB | 33 | 28.8±2.8 | 1,035±353 | 2.3 | 60.0±14.4 | 10 for each dose, ECHO before next dose | 34.8 | 12.25 | |||
|
| |||||||||||
| Gimeno et al. 2005 [64] | Spain | SII | 24 | Echo | 28.5 (27.0–30.0) | 1,206±513 | N/A | 72 | 0.2-0.2-0.2 | 36 | 0.6 |
|
|
|||||||||||
| SIB | 23 | 28.0 (24.0–31.0) | 1,169±490 | 10-5-5 | 72 | 20 | |||||
|
| |||||||||||
| Gokmen et al. 2011 [65] | Turkey | SOB | 54 | Echo | 28.5±1.9 | 1,170±297 | >1.5 | 48–96 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SIB | 54 | 28.7±2.1 | 1,205±366 | 10-5-5 | 72 | 20 | |||||
|
| |||||||||||
| Hammerman and Aramburo 1990 [66] | USA | SII | 19 | Echo & clinical | 27.0±7.0 | 1,040±394 | N/A | 240.0±120.0 | 0.2-0.2-0.2 | 72 | 0.6 |
|
|
|||||||||||
| HII | 20 | 28.0±3.0 | 1,099±435 | 216.0±96.0 | 0.2-0.2-0.2-0.2-0.2 | 120 | 10 | ||||
|
| |||||||||||
| Hammerman et al. 1995 [67] | Israel | SII | 9 | Echo | 29.0±2.0 | 1,200±300 | N/A | N/A | 0.2-0.1-0.1 | 36 | 0.4 |
|
|
|||||||||||
| SIIdrip | 9 | 28.0±2.0 | 1,100±200 | 0.011 mg/kg/h cont infusion | 36 | 0.396 | |||||
|
| |||||||||||
| Hammerman et al. 2008 [68] | Israel | SIB | 31 | Echo | 27.8±2.6 | 1,100 | 2.0 | 108.0 (55.2–184.0) | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SIIdrip | 33 | 27.8±2.8 | 1,060 | 2.0 | 88.8 (60.0–132.0) | 0.017 mg/kg/h | 36 | 0.612 | |||
|
| |||||||||||
| Härkin et al. 2016 [69] | Finland | Plac | 25 | Echo & clinical | 28.3±2.1 | 1,120±430 | 1.4 | 24 | – | – | – |
|
|
|||||||||||
| SIA | 23 | 28.4±2.4 | 1,220±340 | 1.6 | 20 for 1st dose followed by 7.5 every 6 h | 120 | 140 | ||||
|
| |||||||||||
| Hoxha et al. 2013 [70] | Albania | SOB | 44 | Echo | 29.9 | 1,295 | 1.9 | 48–96 | 10 for each dose, ECHO before next dose | 24–48 | 11.7 |
|
|
|||||||||||
| SIB | 36 | 29.3 | 1,289 | 2.1 | 10 for each dose, ECHO before next dose | 24–48 | 12.8 | ||||
|
| |||||||||||
| Kappa et al. 1983 [71] | Finland | Plac | 14 | Echo & clinical | 32.5±3.1 | 2,119±625 | N/A | 18 (6.5–68) | - | - | - |
|
|
|||||||||||
| SOI | 13 | 32.9±2.8 | 2,072±801 | 0.2 for each dose, clinical evaluation before next dose | 24–48 | 0.28 | |||||
|
| |||||||||||
| Khuwuthyakorn et al. 2018 [72] | Thailand | SOI | 17 | Echo & clinical | 28.0 (25.0–30.0) | 930 (510–1,370) | 2.9 | 60.0 (23.0–504.0) | 0.2-0.1-0.1 for age<2 d 0.2-0.2-0.2 for age 2–7 days 0.2-0.25-0.25 for age>7 d | 36 | 0.6 |
|
|
|||||||||||
| SOB | 15 | 29.0 (24.0–32.0) | 950 (520–1,360) | 2.8 | 64.0 (24.0–332.0) | 10-5-5 | 72 | 20 | |||
|
| |||||||||||
| Kluckow et al. 2014 [73] | Australia | Plac | 48 | Echo & clinical | 26.0±1.4 | 876±203 | >1.8 mm at age 3–5 h, >1.6 mm at age 6–8 h, >1.3 mm at age 9–12 h | 9.1±3.4 | - | - | |
|
|
|||||||||||
| SII | 44 | 26.0±1.4 | 892±205 | 8.3±2.9 | 0.2–0.1–0.1 | 72 | : | ||||
|
| |||||||||||
| Krauss et al. 1989 [74] | USA | Plac | 15 | Echo & clinical | N/A | 1,022±58 | N/A | 72–96 | - | - | - |
|
|
|||||||||||
| SII | 12 | N/A | 1,183±77 | 0.2-0.2-0.2 | 72 | 0.6 | |||||
|
| |||||||||||
| Lago et al. 2002 [76] | Italy | SII | 81 | Echo | 29.0±3.0 | 1,214±427 | N/A | 48–72 | 0.2-0.2-0.2 | 72 | 0.6 |
|
|
|||||||||||
| SIB | 94 | 28.0±2.0 | 1,126±412 | 10-5-5 | 36 | 20 | |||||
|
| |||||||||||
| Lago et al. 2014 [77] | Italy | SIB | 56 | Echo | 27.4±2.7 | 1,027±346 | 2.5 | 79.2±24.0 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SIBdrip | 56 | 27.3±2.1 | 1,012±315 | 2.4 | 64.8±16.8 | 0.416–0.208–0.208 mg/kg/h | 72 | 20 | |||
|
| |||||||||||
| Lee et al. 2008 [78] | Korea | SII | 18 | Echo & clinical | 29.4±2.6 | 1,290±360 | >1.5 | 93.6±43.2 | 0.2-0.2-0.2 | 36 | 0.6 |
|
|
|||||||||||
| SOB | 16 | 30.2±3.0 | 1,480±560 | 93.6±33.6 | 10-5-5 | 72 | 20 | ||||
|
| |||||||||||
| Lin et al. 2017 [80] | Taiwan | SII | 75 | Echo & clinical | 26.3±1.6 | 812±160 | 1.9 | 79.2±33.6 | 0.2–0.1–0.1 | 72 | 0.4 |
|
|
|||||||||||
| SIB | 75 | 26.2±1.7 | 801±153 | 1.8 | 76.8±48.0 | 10-5-5 | 72 | 20 | |||
|
| |||||||||||
| Lin et al. 2012 [79] | China | Plac | 32 | Echo & clinical | 30.8±2.3 | 1,350±221 | >1.5 | 20.0±5.0 | 0 | ||
|
|
|||||||||||
| SOB | 32 | 31.2±2.4 | 1,301 ±260 | 23.0±4.0 | 10-5-5 | 72 | 20 | ||||
|
| |||||||||||
| Merritt et al. 1981 [82] | USA | Plac | 13 | Echo & clinical | N/A | ≤1,350 | >1.2 | 48.8 | – | – | – |
|
|
|||||||||||
| SII | 12 | 167.4 | 0.2 for each dose, clinical & ECHO before next dose | 36 | 0.3 | ||||||
|
| |||||||||||
| Mosca et al. 1997 [83] | Italy | SII | 8 | Echo | 28.0 (25.0–30.0) | 820 (600–1,390) | N/A | 29.0 (5.0–120.0) | 0.2–0.1–0.1 | 72 | 0.6 |
|
|
|||||||||||
| SIB | 8 | 29.0 (37.0–31.0) | 855 (620–1,620) | 24.0 (10.0–53.0) | 10-5-5 | 72 | 20 | ||||
|
| |||||||||||
| Mullet et al. 1982 [84] | USA | Plac | 23 | Echo & clinical | 29.5 | 1,212 | N/A | 180.0 | – | – | – |
|
|
|||||||||||
| SOI | 24 | 30.1 | 1,237 | 177.6 | 0.2–0.2 | 48 | 0.4 | ||||
|
| |||||||||||
| Nestrud et al. 1980 [85] | Ark | Plac | 11 | Echo & clinical | 28.1±2.0 | 1,189±376 | N/A | 482.4±400.8 | – | – | – |
|
|
|||||||||||
| SOI | 12 | 30.8±1.8 | 1,287±325 | 345.6±240.0 | 0.2 for each dose, clinical & ECHO before next dose | 64.8 | 0.54 | ||||
|
| |||||||||||
| Neu et al. 1981 [86] | USA | Plac | 10 | Echo & clinical | 29.3±0.6 | 1,142±80 | N/A | 218.4 | – | – | – |
|
|
|||||||||||
| SOI | 11 | 0.25–0.25 | 48 | 0.5 | |||||||
|
| |||||||||||
| Oncel et al. 2014 [88] | Turkey | SOB | 45 | Echo & clinical | 27.3±2.1 | 973±224 | 2.2 | 48–96 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SOA | 45 | 27.3±1.7 | 931±217 | 2.4 | 15 every 6 h | 72 | 180 | ||||
|
| |||||||||||
| Osborn et al. 2003 [89] | Australia | Plac | 35 | Echo | 26.9±0.3 | 1,002±49 | >1.6 | 4.3 (2.0–12.0) | – | – | – |
|
|
|||||||||||
| SII | 35 | 26.7±0.3 | 958±43 | 0.2 for each dose, ECHO before next dose | 24–48 | 0.2–0.4 | |||||
|
| |||||||||||
| Patel et al. 2000 [90] | UK | SII | 15 | Echo & clinical | 26.7 (23.2–30.0) | 838 (458–1,377) | N/A | 228.0±125.0 | 0.2-0.1-0.1 for age 2–7 days 0.2-0.25-0.25 for age ≥8 d | 36 | 0.6 |
|
|
|||||||||||
| SIB | 18 | 26 (23.9–35.0) | 790 (620–2,780) | 234.0±96.0 | 10-5-5 | 72 | 20 | ||||
|
| |||||||||||
| Pezzati et al. 1999 [91] | Italy | SII | 8 | Echo | 29.5±2.6 | 1,277±440 | N/A | 33.2±5.4 | 0.2-0.1-0.1 | 72 | 0.6 |
|
|
|||||||||||
| SIB | 9 | 29.1±2.1 | 1,151±426 | 31.9±4.5 | 10-5-5 | 72 | 20 | ||||
|
| |||||||||||
| Pourarian et al. 2008 [92] | Iran | SOI | 10 | Echo & clinical | 33.2±3.1 | 1,720±630 | N/A | 153.6 (120.0–192.0) | 0.2 for each dose, ECHO before next dose | 72 | 0.2–0.6 |
|
|
|||||||||||
| SOB | 10 | 31.3±4.4 | 1,860±402 | 132.0 (96.0–168.0) | 10-5-5, ECHO before next dose | 72 | 10–20 | ||||
|
| |||||||||||
| Pourarian et al. 2015 [93] | Iran | SOB | 32 | Echo | 31.3±2.1 | 1,493±346 | N/A | 72–168 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| HOB | 33 | 30.0±2.6 | 1,339±524 | 20–10–10 | 72 | 40 | |||||
|
| |||||||||||
| Rudd et al. 1983 [95] | UK | Plac | 15 | Echo & clinical | 29.0±1.7 | 1,170±211 | N/A | 264±194.4 | – | – | – |
|
|
|||||||||||
| SOI | 15 | 28.9±1.2 | 1,105±251 | 244.8±127.2 | 0.2 for each dose, ECHO before next dose | 40.8 | 0.34 | ||||
|
| |||||||||||
| Salama et al. 2008 [96] | Gatar | SII | 20 | Echo & clinical | 27.8±2.8 | 1,050±440 | 2.6 | 170.4±45.6 | 0.2-0.2-0.2 | 72 | 0.6 |
|
|
|||||||||||
| SOB | 21 | 27.7±2.5 | 1,094±480 | 2.5 | 194.4±36.0 | 10-5-5 | 72 | 20 | |||
|
| |||||||||||
| Su et al. 2003 [98] | Taiwan | SII | 31 | Echo | 28.2±2.4 | 1,109±244 | >1.5 | 117.6 (48.0–168.0) | 0.2-0.2-0.2 | 36 | 0.6 |
|
|
|||||||||||
| SIB | 32 | 28.7±2.2 | 1,134±200 | 98.4 (48.0–168.0) | 10-5-5 | 72 | 20 | ||||
|
| |||||||||||
| Su et al. 2008 [97] | Taiwan | SII | 59 | Echo & clinical | 25.0 (23.0–28.0) | 762 (540–980) | N/A | 8.0 (3.0–24.0) | 0.2-0.1-0.1 for age<48 h 0.2-0.2-0.2 for age>48 h ECHO before next dose | 66 | 0.38 |
|
|
|||||||||||
| SIB | 60 | 25.0 (23.0–28.0) | 825 (550–990) | 8.0 (4.0–21.0) | 10-5-5, ECHO before next doses | 54 | 16.25 | ||||
|
| |||||||||||
| Tammela et al. 1999 [100] | Finland | SII | 31 | Echo & clinical | 27.9±2.3 | 1,154±388 | N/A | 103.2 (28.8–480.0) | 0.2-0.1-0.1 | 72 | 0.6 |
|
|
|||||||||||
| HII | 30 | 27.3±1.9 | 1,094±298 | 74.4 (24.0–168.0) | 0.1 × 7 doses | 168 | 0.7 | ||||
|
| |||||||||||
| VanOvermeire et al. 1997 [102] | Belgium | SII | 20 | Echo & clinical 6 | 28.7±1.9 | 1,210±360 | 2.5 | 74.4±12.0 | 0.2-0.2-0.2 | 36 | 0.6 |
|
|
|||||||||||
| SIB | 20 | 29.0±2.4 | 1,270±450 | 2.6 | 76.8±9.6 | 10-5-5 | 72 | 20 | |||
|
| |||||||||||
| VanOvermeire et al. 2000 [103] | Belgium | SII | 74 | Echo & clinical 6 | 29.0±2.1 | 1,230±380 | 2.5 | 74.4±12.0 | 0.2-0.2-0.2 | 36 | 0.6 |
|
|
|||||||||||
| SIB | 74 | 29.0±2.3 | 1,230±390 | 2.5 | 74.4±14.4 | 10-5-5 | 72 | 20 | |||
|
| |||||||||||
| Yadav et al. 2014 [104] | India | SOI | 35 | Echo | 30.3±3.1 | 1,380±450 | >1.5 | 240.0±146.16 | 0.2-0.2-0.2 for age<2–7 days 0.2-0.25-0.25 for age>7 d | 72 | 0.6 |
|
|
|||||||||||
| SOB | 48 | 29.7±3.2 | 1,440±450 | 236.0±144.9 | 10-5-5 | 72 | 20 | ||||
|
| |||||||||||
| Yanagi et al. 1981 [105] | USA | Plac | 9 | Echo | 30.4±1.0 | 1,500±200 | N/A | 223.2±36.0 | – | – | – |
|
|
|||||||||||
| SOI | 8 | 29.4±1.0 | 1,200±100 | 249.6±72.0 | 0.2-0.2-0.2 (only phase I) | 72 | 0.6 | ||||
|
| |||||||||||
| Yang et al. 2016 [106] | China | SOB | 43 | Echo & clinical | 33.4±2.1 | 2,091 ±657 | 1.8 | 139.2±48.0 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SOA | 44 | 33.6±2.1 | 2,219±606 | 2.1 | 153.6±43.2 | 15 every 6 h | 72 | 180 | |||
|
| |||||||||||
| Yeh et al. 1981 [107] | USA | Plac | 27 | Echo & clinical | 30.2±2.3 | 1,167±354 | N/A | 261.6±146.4 | – | – | – |
|
|
|||||||||||
| SII | 28 | 31.5±2.3 | 1,233±408 | 213.1±1272 | 0.3-0.3-0.3, clinical evaluation 43.2 before next dose | 0.54 | |||||
|
| |||||||||||
| Dani et al. 2020 [51] | Italy | SIB SIA | 52 58 | Echo | 28.4±2.0 28.2±1.4 | 1,068±278 1,022±266 | N/A | 46±16 46±15 | 10-5-5 15 every 6 h | 72 72 | 20 180 |
|
| |||||||||||
| Davidson et al. 2020 [54] | USA | SII | 21 | Echo | 25.3±1.8 | 756±241 | 2.9±0.7 | 6.5 (4,9.3) | 0.2-0.2-0.2 for age 2–7 days 0.2-0.25-0.25 for age>7 days | 36 | 0.6 |
|
|
|||||||||||
| SIA | 17 | 25.7±1.4 | 785±203 | 2.7±0.7 | 8.0 (7,11) | 15 every 6 h | 72 | 180 | |||
|
| |||||||||||
| Ahranjani et al. 2020 [39] | Iran | SOB | 25 | Echo | GA <37 weeks | BW <2,599 g | N/A | <15 days | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SIA | 25 | 10 every 6 h | 72 | 120 | |||||||
|
| |||||||||||
| Ghaderian et al. 2019 [62] | Iran | SOB | 20 | Echo & clinical | 30.8±1.9 | 1,230±182 | ≥1.5 | <14 days | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SOA | 20 | 30.4±2.1 | 1,126±200 | 15 every 6 h | 72 | 180 | |||||
|
| |||||||||||
| Kumar et al. 2020 [75] | India | SOB | 80 | Echo & clinical | 28.7±1.7 | 1,129±268 | 2.1 (1.9–2.5) | 48–72 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SOA | 81 | 28.7±1.6 | 1,167±249 | 2.3 (1.8–2.6) | 15 every 6 h | 72 | 180 | ||||
|
| |||||||||||
| Meena et al. 2020 [81] | India | SOI | 35 | Echo & clinical | 31.8±2.3 | 1,410±320 | 1.8±0.3 | 260±102 | 0.2-0.1-0.1 for age<2 days 0.2-0.2-0.2 for age 2–7 days | 36 | 0.6 |
|
|
|||||||||||
| SOB | 35 | 31.4±1.7 | 1,340±220 | 1.9±0.7 | 258±135 | 10-5-5 | 72 | 20 | |||
|
|
|||||||||||
| SIA | 35 | 32.1 ±2.0 | 1,440±340 | 1.8±0.4 | 216±82 | 15 every 6 h | 72 | 180 | |||
|
| |||||||||||
| Oboodi et al. 2020 [87] | Iran | SOB | 70 | Echo & clinical | 31.1±2.4 | 1,354±333 | ≥1.5 | 130±57 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SIA | 70 | 31.0±2.9 | 1,334±513 | 128±96 | 15 every 6 h | 72 | 180 | ||||
|
| |||||||||||
| Rahman et al. 2020 [94] | Indonesia | SOB | 11 | Echo & clinical | 34.9±1.4 | 1,904±315 | 3.1±1.1 | 192±146 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SOA | 11 | 33.7±1.7 | 1,723±171 | 2.6±0.9 | 185±137 | 15 every 6 h | 72 | 180 | |||
|
| |||||||||||
| Sung et al. 2020 [99] | Korea | Plac | 72 | Echo | 26.7±2.0 | 915±243 | 2.5±0.6 | 202±60 | – | ||
|
|
|||||||||||
| SOB | 74 | 26.8±2.1 | 893±256 | 2.5±0.5 | 199±55 | 10-5-5 | 72 | 20 | |||
|
| |||||||||||
| Tauber et al. 2020 [101] | USA | SIB | 5 | Echo | 26.3±2.3 | 916±300 | N/A | 192±120 | 10-5-5 | 72 | 20 |
|
|
|||||||||||
| SIA | 5 | 26.2±1.4 | 736±240 | 168±72 | 15 every 6 h | 72 | 180 | ||||
n, number of sample size; BW, birth weight; cont, continuous; d, day; DAO, descending aorta; g, gram; GA, gestational age; PDA, patent ductus arteriosus; mm, millimeters; h, hours; mg, milligram; Kg, kilogram; Plac, placebo; SOI, a standard dose of oral indomethacin; HOI, a standard dose of oral indomethacin; SII, a standard dose of intravenous indomethacin; HII, a high dose of intravenous indomethacin; SMA, superior mesenteric artery; SOB, A standard dose of oral ibuprofen; HOB, A high dose of oral ibuprofen; SIB, A standard dose of intravenous ibuprofen; HIB, A high dose of intravenous ibuprofen; SOA, A standard dose of oral acetaminophen; HOA, A high dose of oral acetaminophen; SIA, A standard dose of intravenous acetaminophen; HIA, A high dose of intravenous acetaminophen; MPA, main pulmonary artery; SIIdrip, a standard dose and continuous infusion of indomethacin; SIBdrip, A standard dose and continuous infusion of ibuprofen; wks, weeks.
Inconsistency Assumption
Sixty-nine and sixty-three studies provided data for PDA closure and composite risk outcomes. The global inconsistency was tested, which suggested no evidence of inconsistency for PDA closure and composite risk (p values = 0.140. and 0.972); see online eTable 4.1. The loop-specific method indicated that comparisons of SOI-SIA, HOB-SOA, and SOB-SIB for PDA closure from Plac-SOI-SIA, SOB-HOB-SOA, and SII-SOB-SIB loops had significant IF; see online eTable 4.2 and online eFigure 4.1. Then, the characteristics of patients were compared among these three comparisons (see online eTable 4.3–4.5), indicating that most characteristics were not much different except age at treatment between SOI and SIA, i.e., about 8 versus 4 days. The loop-specific composite risk showed no statistically significant inconsistency; see online eTable 4.6 and online eFig. 4.2.
Risk of Bias and GRADE
Most studies were at low risk of bias for all items, except blinding participants and researchers (shown in online eTable 5.1; online eFig. 5.1). All studies were described as randomized, but 27 of 70 studies did not blind clinicians and patients. One of the main reasons was the different routes of administration. Adjusted funnel plots of PDA closure and composite risk were inspected visually to assess publication bias. They showed no substantial asymmetry (online eFig. 5.2). The majority of the confidence rating from GRADE was low-quality evidence for both PDA closure (47/78 comparisons) and composite risk outcomes (61/78 comparisons). The downgraded scores were mainly from imprecision and some from heterogeneity. For the comparisons included in RBA, i.e., HOB & Plac, SOA & Plac, SOB & Plac, and SII & Plac, the quality of evidence for PDA closure outcome was high while the evidence for composite risk outcome was moderate for SOB & Plac, SII & Plac, and low for the rest (online eTable 6.1–6.2).
Benefit Outcome
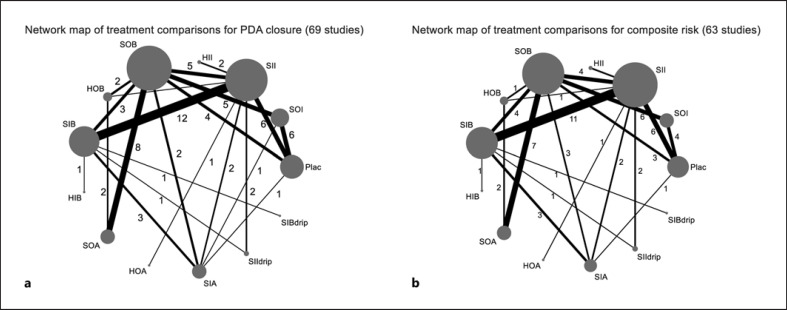
PDA closure data were extracted from 69 of 70 RCTs (n = 5,118), considering 13/15 interventions where their data were available. The direct meta-analysis and league table results are described in online eTable 7.1–7.2. NMA was performed, and a network map was shown in Figure 2a. The results from the league table indicated that all interventions showed a significantly higher PDA closure rate than placebo; the highest treatment effect was HOB; 2.26 (1.64, 3.10), followed by HIB; 2.26 (1.27, 4.01) and SOA; 2.03 (1.57, 2.63). From SUCRAs, the best treatment for PDA closure was HOB, followed closely by HIB, SOA, and SOB, with SUCRAs of 83.0, 75.7, 69.6, and 62.1, respectively.
Fig. 2.
Network maps of pooling relative treatment benefits and risks. Two network maps for PDA closure (a), and composite risk outcomes (b). Each node indicates a treatment modality, and node sizes are proportional to the number of NMA publications. Line thickness is proportional to the number of NMAs.
Risk Outcomes
Data on composite risk for the individual study were extracted from seven SAEs; see online eTable 8.1. Sixty-three studies with 13 interventions were analyzed in NMA compared with Plac (shown in Fig. 2b). Pairwise comparisons and league tables are shown in online eTable 8.2–8.3. SUCRAs for minimum SAE were estimated and showed that HOB had the highest probability of being the best or the lowest SAEs with SUCRA of 80.0, followed by SOA, SOB, and HII with a SUCRAs of 76.5, 70.1, and 59.8, respectively.
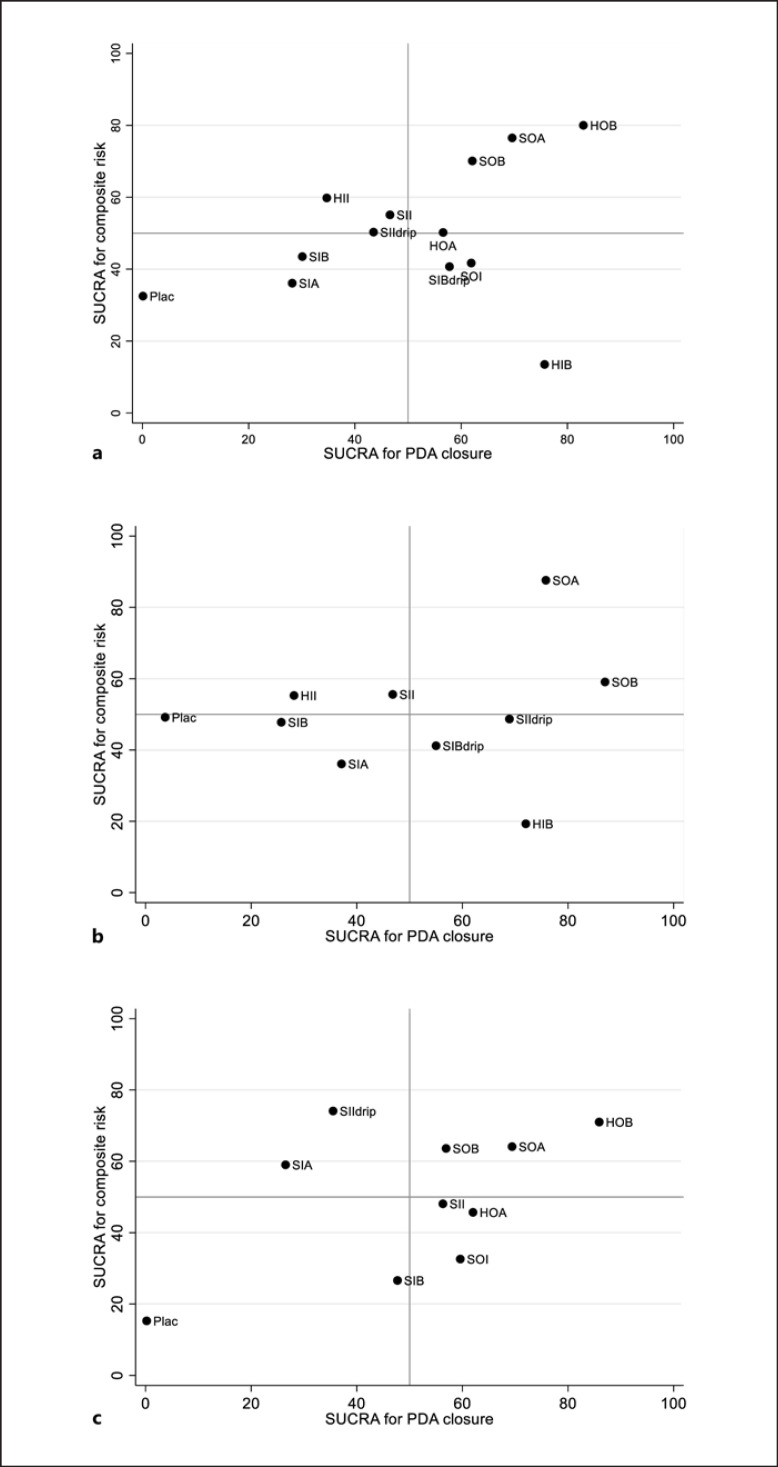
Cluster Rank
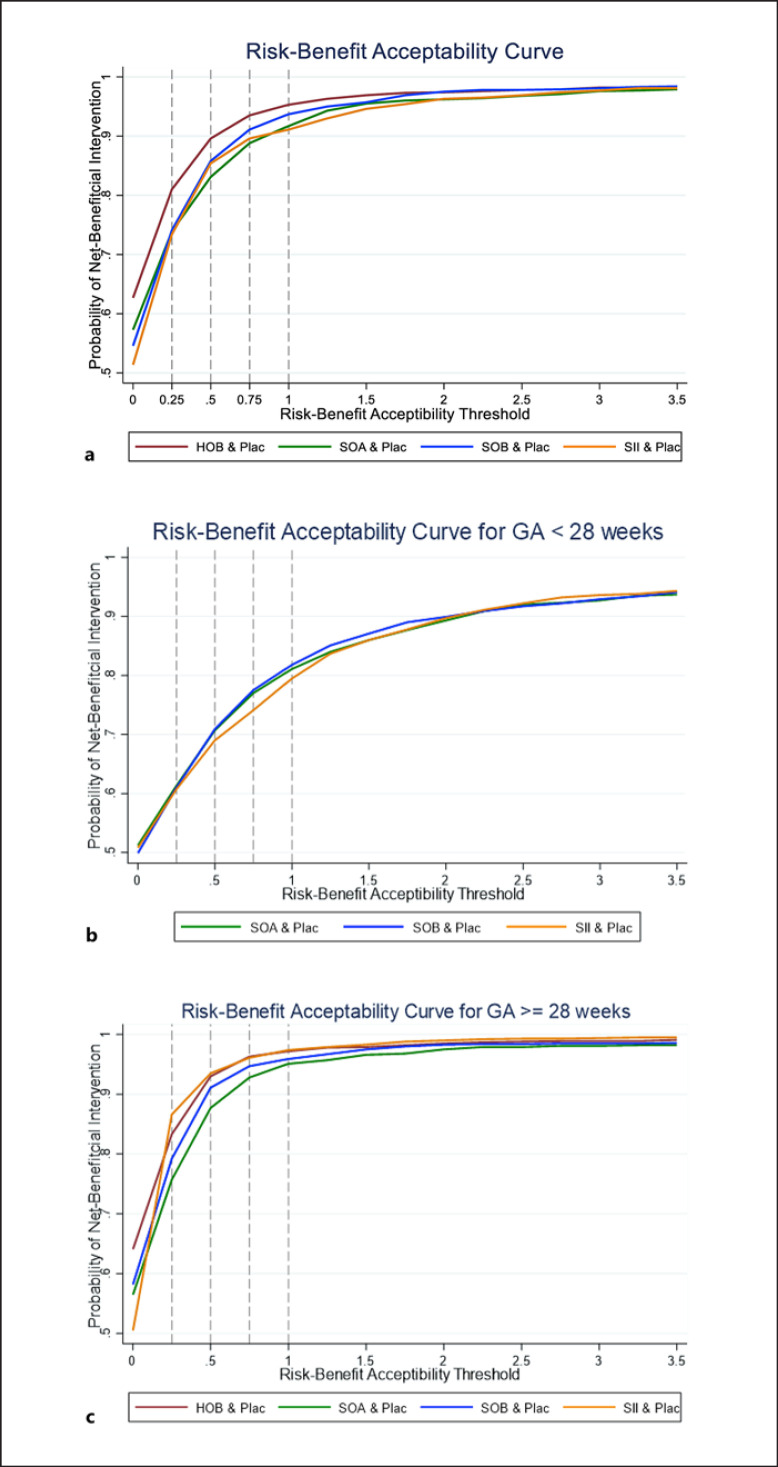
Cluster rank was constructed by plotting the SUCRAs of PDA closure on the x-axis and the composite risk on the y-axis (shown in Fig. 3); higher SUCRA values reflect higher PDA closure and lower SAEs. The cluster ranking plot can be divided into four quadrants; 3 interventions HOB, SOA, and SOB fell in the right upper dominant quadrant, meaning they had high PDA closure and low SAEs. These three treatments, plus the commonly used SII regimen, were further considered in RBA. HIB fell in the right lower quadrant indicating higher PDA closure but high SAEs.
Fig. 3.
Clustered ranking plots for the relative ranking of treatment for the efficacy of PDA closure and composite risk outcomes in network meta-analysis. a For all studies, b For GA <28 weeks, c For GA ≥28 weeks. Clustered ranking plots for the medical treatments of PDA based on the SUCRA. The larger the SUCRA, the higher the rate of PDA closure, and the lower the rate of adverse effects.
Risk-Benefit Analysis
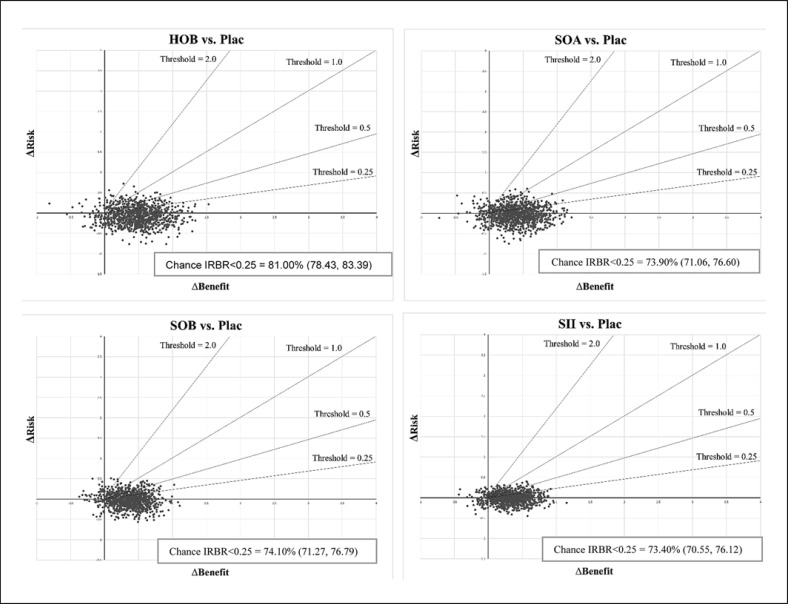
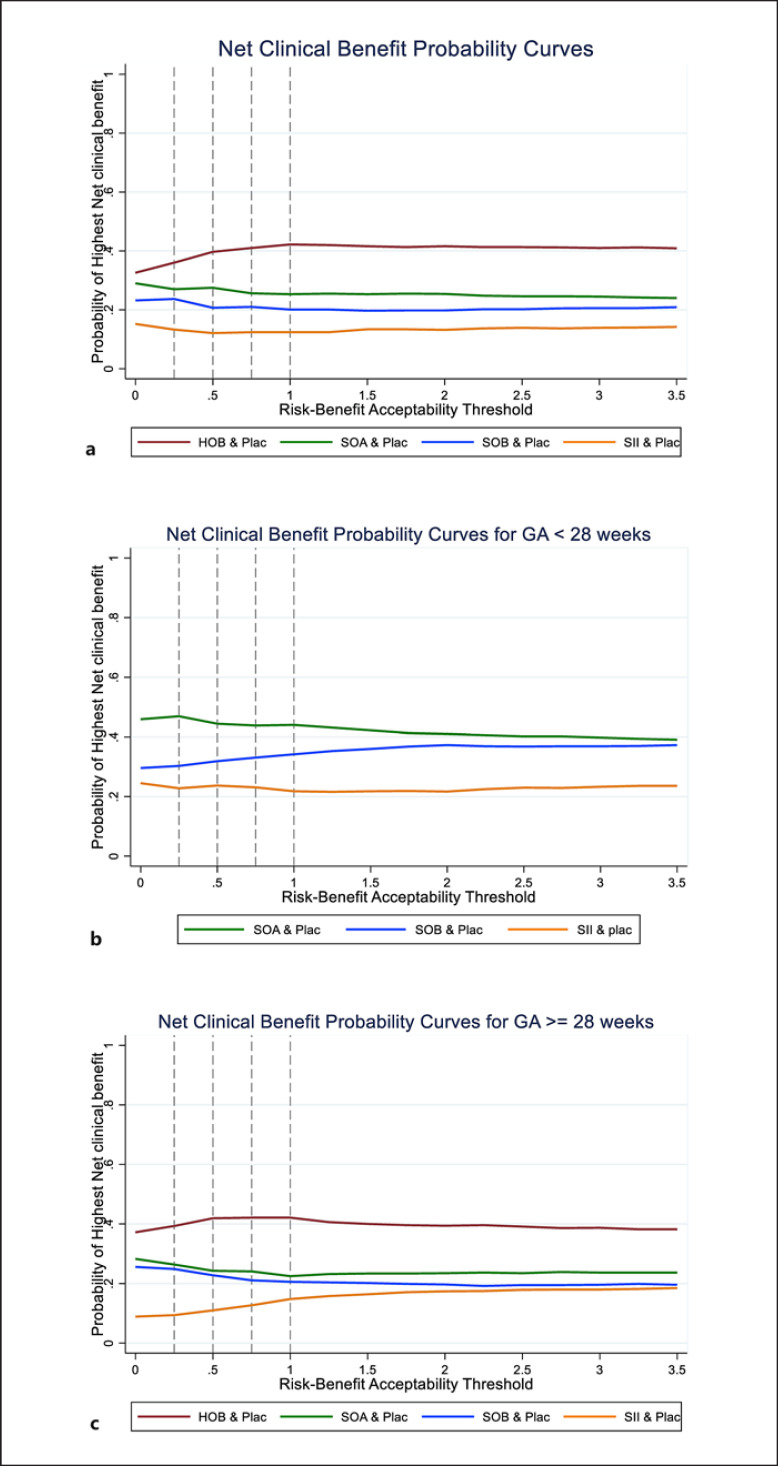
ΔR and ΔB of four treatments, compared with Placebo, were estimated and then pooled across RCTs. The point estimate of ΔR and ΔB were jointly simulated and fell in the SE quadrant, which yielded the point estimated IRBRs of −0.139 (−2.68, 2.40), −0.075 (−0.599, 0.449), −0.057 (−2.518, 2.404), and −0.009 (−0.217, 0.199) for HOB, SOA, SOB, and SII, respectively (shown in online eTable 9.1). These convey that the interventions have greater benefits and less risk than Plac. Next, Monte Carlo was run, allowing the joint uncertainty of the risks and benefits, and they were plotted on RBPs with varying thresholds from 0.25 to 2.0 (Fig. 4). The chance that IRBRs were less than µ was shown in online eTable 9.2. RBACs were plotted (Fig. 5a). However, these curves do not allow a comparison of all treatments simultaneously. Then, curves of the highest NCB of each treatment were constructed (Fig. 6a). HOB was the treatment with the highest probability of having the best NCB. Suppose decision-makers accept one additional SAE to obtain four PDA closures (threshold of 0.25); HOB was the treatment with the highest probability (36%), followed by SOA (27%) and SOB (23.7%) (see online eTable 9.3).
Fig. 4.
The risk-benefit acceptability planes of HOB, SOA, SOB, and SII compared with placebo. The horizontal bar represents the 95% CI for the difference in benefits of PDA closure, and the vertical bar is the 95% CI for the difference in the probability of the composite risk outcomes. The ● marks the point estimate of the risk-benefit ratio. Dashed lines are the risk-benefit acceptability threshold of 0.25, 0.5, 0.75, 1.0, and 2.0.
Fig. 5.
Risk-benefit acceptability curves for the treatment of PDA in preterms compared with placebo at any risk-benefit acceptability threshold (µ). a For all studies. b For GA <28 weeks. c For GA ≥28 weeks. The reference value is shown as the vertical dashed line with varying thresholds of 0.25, 0.5, 0.75, and 1.
Fig. 6.
Net clinical benefit probability curves compared with placebo at any risk-benefit acceptability threshold (µ). a For all studies. b For GA <28 weeks. c For GA ≥28 weeks. The reference value is shown as the vertical dashed line with varying thresholds of 0.25, 0.5, 0.75, and 1.
Subgroup Analysis
Subgroup analysis by GA <28 (n = 1,749) and ≥28 weeks (n = 3,398) were performed. Eighteen and 17 studies reported PDA closure and composite risk in GA <28 weeks, and 51 and 46 studies for those GA ≥28 weeks. The mean birth weights of these corresponding GA groups were 937.08 ± 118.80 and 1,332.78 ± 270.92. There was no evidence of inconsistency for all sub-pooling as for the global χ2 tests; see online eTable 4.1. Data for HOB was not available for GA <28 weeks. RBAC Results of subgroups were similar to overall results (shown in Fig. 5b, c; online eFig. 10.1–10.2; online eTable 10.1–10.2), in which HOB, SOA, and SOB were the three ranks for GA ≥28 weeks. For GA <28 weeks, SOA was the top, followed by SOB. Moreover, the results of the highest probabilities of NCB for GA <28 weeks (see Fig. 6b) and GA ≥28 weeks (see Fig. 6c) were similar to RBAC.
Sensitivity Analysis
A sensitivity analysis was performed to assess the robustness of RBA by excluding 6 studies from PDA closure and considering only 63 studies reported both PDA closure and composite risk. Results were robust to the main analysis, i.e., HOB, SOA, and SOB fell in the right upper dominant quadrant of the clustered ranking plot. In addition, the treatment with the highest probability of having the best NCB for GA <28 and ≥28 weeks were similar to the main analysis (see online eTable 11.1–11.2; online eFig. 11.1–11.4).
Discussion
We applied NMA and RBA for the treatment of hsPDA in preterm neonates to summarize the data and incorporate risks into benefits that facilitate decision-making. An NMA indicated that HOB, SOA, and SOB seemed to be the 3 best treatments, which all had lower complications and higher benefits than placebo. Likewise, given the risk-benefit threshold from 0.25 to 3.5, HOB is the best choice to have the highest probability of NCB, followed by SOA and SOB. Treatment with HIB might gain high benefits but also increase the risk of SAEs. The probabilities of being the best treatment for GA ≥28 weeks were the same trends as the results from including all studies. However, HOB was not available for the smaller GA group.
Recently, there has been a considerable debate about whether or not to treat PDA in preterm. Although some studies have demonstrated the association between PDA in preterm with increased mortality and morbidities, e.g., CLD and NEC [3, 4, 5, 6], a paucity of information shows short-term adverse effects after treatment. Individual variability may play an essential role in deciding the treatment, and the target population which responds and benefits from the treatment is paramount. Many are interested in treating symptomatic PDA, which widely uses echocardiography to demonstrate significant shunting via PDA. Although the long-term outcomes of treatment have not been clearly identified, therapeutic closure of PDA seems to be accepted in current practice.
From our review, the mean BWs of most studies were classified as VLBW (less than 1,500 gm), and most treatments were started 3 days after birth. Similarly, in real-world clinical practice, the spontaneous closure rate is nearly 70% within 3 days after birth [109]. The infants were clinically observed and received treatment after that. Indomethacin and ibuprofen are widely used for PDA closure in preterm neonates. Their effectiveness is based on the role of prostaglandin in ductal constriction via the inhibition of COX enzymes. Our study found that both HOB and SOB had a higher rate of PDA closure than intravenous indomethacin, and incorporating risk into PDA closure also indicated a higher NCB of ibuprofen. Total doses of HOB ranged from 30 to 40 mg/kg/course; 10–20 mg/kg, followed by 7.5–10 mg/kg administered every 12–24 h for a total of 3 doses, compared with 20 mg/kg/course for SOB; 10 mg/kg, followed by 5 mg/kg administered every 12–24 h for a total of 3 doses. Although ibuprofen has been reported to associate with the risk of NEC and renal insufficiency [110], the RBA conveys that the benefit outweighed the risk. However, HOB for preterm with a smaller GA group was undetermined due to unavailable data.
Acetaminophen, which acts on the peroxidase site of prostaglandin H2 synthesis, has been increasingly used because of the contraindication from those two COX inhibitors. However, many are uncertain about its effectiveness and risks for first-line therapy compared with COX inhibitors. The standard dose of acetaminophen might be an alternative for hsPDA closure, with a dosage of 15 mg/kg every 6 h for 3 days or a total dose of 180 mg/kg/course. Enteral acetaminophen seems to have more treatment effects than the parenteral route. The reason to explain this may be from the steadier plasma levels of the drug administered orally, similar to the oral use of ibuprofen [111]. Liver toxicity from acetaminophen was considered for composite risk, but no data on long-term side effects, e.g., neurodevelopment, were reported. Therefore, more information is needed.
Nowadays, the continuous infusion has been an interesting issue of increasing effectiveness and reducing adverse effects. From our research, all included studies of SIIdrip and SIBdrip were compared with standard bolus doses [49, 67, 68, 77]. 36-h continuous infusion for SIIdrip, and 24-h continuous infusion of SIBdrip had more stable blood flow velocities in cerebral, renal, and mesenteric vessels. As a result, there might be the question of whether the continuous infusion of the standard dose is more favored in terms of risk and benefit assessment. However, results from the clustered ranking plot, SIIdrip and SIBdrip did not lie in the right upper dominant quadrant. Additionally, compared with standard bolus doses, SIIdrip and SIBdrip had no more benefits and fewer risks. However, summarized data of SIIdrip and SIBdrip were from only 3 studies (n = 114) and 1 study (n = 112), respectively. Further trial studies concerning continuous infusion are required.
Several NMAs were conducted to summarize the efficacy and safety of medical treatment for hsPDA. Two large NMAs [7, 8] were published. The first NMA included 67 RCTs covering 10 interventions [14]. They found that HOB (i.e., 15–20 mg/kg followed by 7.5–10 mg/kg administered every 12–24 h for 3 doses) was the best for hsPDA closure, followed by HIB and oral acetaminophen. However, there was no significant difference in the odds of adverse effects. The second NMA was recently published, combining data from 64 RCTs with 24 observational studies [8]. These two NMAs are different comparing from our NMA in some respects: we did not include studies comparing the same intervention and route with equal dosages/course [49, 77, 112, 113, 114], although the studies compared the different duration of administration (e.g., short vs. prolonged durations) or the same treatment with/without additional one medication (e.g., SII vs. SII and furosemide, or SII vs. SII and dopamine). This is because interventions from our study were grouped from the total dose/course. Additionally, we used data based on intention-to-treat instead of per-protocol approaches to maximize validity. A composite SAE was considered instead of individual adverse events [14], and RR and RD were used to estimate relative treatment effects instead of odds ratios. As a result, although the highest-ranked treatment for PDA closure was similar to Mitra's study [7], the second and third-ranked therapies were different; SOA and SOB for our study but HIB and oral acetaminophen for Mitra's study. Finally, we applied RBA to incorporate the composite risks and benefits, which have not been performed to date in the previous NMAs. Therefore, this should prove more helpful to clinicians in decision-making.
Strengths and Limitations
This is the first study of PDA treatment in preterm, which combined the estimations between NMA and RBA. Our summarization represents the treatment of hsPDA from various NICUs worldwide. Many of these studies had high methodologic quality. Besides, this approach allowed summarizing the joint distribution of benefits and risks and provided a transparent benefit-risk outcome for treating hsPDA in preterm. However, there are some limitations: the uncertain appropriate starting time for treatment since there are various postnatal age treatments and some inconsistencies, the lack of data on HOB for small GA <28 weeks, and long-term SAEs.
Conclusion
Our study suggested that the best treatment, trading off risks and benefits with the various degrees of acceptability thresholds, in terms of PDA closure and SAEs was HOB, followed by SOA and SOB, particularly for the newborns GA ≥28 weeks. Whereas SOA, followed by SOB, might be the best for newborns GA <28 weeks. However, further well-designed studies are needed to define the optimal high doses, appropriate postnatal age for treatment, and long-term outcomes to ensure efficacy and safety in clinical practice.
Statement of Ethics
The paper is exempt from Ethics Committee approval as this is a systematic review using data from published literature, and no additional patient data were collected.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This present work was not supported by any organization.
Author Contributions
All the authors contributed to this study's conception and design. S.E. searched for and selected studies, performed data extraction, statistical analyses and risk of bias assessment, and drafted the manuscript. A.T. designed the review methodology, assisted in statistical analyses and interpretation, critically revised the manuscript, and supervised all study parts. O.P. assisted in the risk-benefit analysis and critically revised the manuscript. S.A.-O.V. performed data extraction and risk of bias assessment, critically revised the manuscript, and supervised the clinical content. P.N. performed data extraction and risk of bias assessment. J.A. critically commented and revised the manuscript. C.O., the corresponding author, performed data extraction and risk of bias assessment, critically revised the manuscript, supervised clinical content, controlled the decision to publish, and attested that all listed authors met authorship criteria. All the authors approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgments
We thank all staff from the Department of Clinical Epidemiology and Biostatistics, Faculty of Medicine, Ramathibodi Hospital, for their assistance in research coordination.
Funding Statement
This present work was not supported by any organization.
References
- 1.Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36((2)):123–129. doi: 10.1053/j.semperi.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madan JC, Kendrick D, Hagadorn JI, Frantz ID, 3rd, National Institute of Child Health and Human Development Neonatal Research Network Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics. 2009;123((2)):674–681. doi: 10.1542/peds.2007-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler U, Schulte F, Cholewa D, Nelle M, Schaefer SC, Klimek PM, et al. Outcome in neonates with necrotizing enterocolitis and patent ductus arteriosus. World J Pediatr. 2016;12((1)):55–59. doi: 10.1007/s12519-015-0059-6. [DOI] [PubMed] [Google Scholar]
- 4.Evans N, Kluckow M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75((3)):F183–6. doi: 10.1136/fn.75.3.f183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunha GS, Mezzacappa-Filho F, Ribeiro JD. Risk factors for bronchopulmonary dysplasia in very low birth weight newborns treated with mechanical ventilation in the first week of life. J Trop Pediatr. 2005;51((6)):334–340. doi: 10.1093/tropej/fmi051. [DOI] [PubMed] [Google Scholar]
- 6.Noori S, McCoy M, Friedlich P, Bright B, Gottipati V, Seri I, et al. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123((1)):e138–44. doi: 10.1542/peds.2008-2418. [DOI] [PubMed] [Google Scholar]
- 7.Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki AA, et al. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA. 2018;319((12)):1221–1238. doi: 10.1001/jama.2018.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marconi E, Bettiol A, Ambrosio G, Perduca V, Vannacci A, Troiani S, et al. Efficacy and safety of pharmacological treatments for patent ductus arteriosus closure: a systematic review and network meta-analysis of clinical trials and observational studies. Pharmacol Res. 2019;148:104418. doi: 10.1016/j.phrs.2019.104418. [DOI] [PubMed] [Google Scholar]
- 9.Lazo-Langner A, Rodger MA, Barrowman NJ, Ramsay T, Wells PS, Coyle DA. Comparing multiple competing interventions in the absence of randomized trials using clinical risk-benefit analysis. BMC Med Res Methodol. 2012;12:3. doi: 10.1186/1471-2288-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien BJ, Drummond MF, Labelle RJ, Willan A. In search of power and significance: issues in the design and analysis of stochastic cost-effectiveness studies in health care. Med Care. 1994;32((2)):150–163. doi: 10.1097/00005650-199402000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Willan AR, O'Brien BJ, Cook DJ. Benefit-risk ratios in the assessment of the clinical evidence of a new therapy. Contr Clin Trials. 1997;18((2)):121–130. doi: 10.1016/s0197-2456(96)00092-x. [DOI] [PubMed] [Google Scholar]
- 12.Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335((10)):701–707. doi: 10.1056/NEJM199609053351003. [DOI] [PubMed] [Google Scholar]
- 13.Young TE. Neofax 2011. Montvale, NJ: Thomson Reuters; 2011. [Google Scholar]
- 14.Miyahara S, Ramchandani R, Kim S, Evans SR, Gupta A, Swindells S, et al. Applying a risk-benefit analysis to outcomes in tuberculosis clinical trials. Clin Infect Dis. 2020;70((4)):698–703. doi: 10.1093/cid/ciz784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papile L-A, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1, 500 gm. J Pediatr. 1978;92((4)):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 16.Volpe JJ. Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol. 1998;5((3)):135–151. doi: 10.1016/s1071-9091(98)80030-2. [DOI] [PubMed] [Google Scholar]
- 17.An international classification of retinopathy of prematurity The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984;102((8)):1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 18.International Committee for the Classification of Retinopathy of Prematurity The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123((7)):991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 19.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187((1)):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012;24((2)):191–196. doi: 10.1097/MOP.0b013e32834f62d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson R, Roberts EA. Identification of neonatal liver failure and perinatal hemochromatosis in Canada. Paediatr Child Health. 2001;6((5)):248–250. doi: 10.1093/pch/6.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8((10)):e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briggs AH. Handling uncertainty in cost-effectiveness models. PharmacoEconomics. 2000;17((5)):479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 24.Lynd LD, O'Brien BJ. Advances in risk-benefit evaluation using probabilistic simulation methods: an application to the prophylaxis of deep vein thrombosis. J Clin Eidemiol. 2004;57((8)):795–803. doi: 10.1016/j.jclinepi.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Evans SR, Follmann D. Using outcomes to analyze patients rather than patients to analyze outcomes: a step toward pragmatism in benefit: risk evaluation. Stat Biopharm Res. 2016;8((4)):386–393. doi: 10.1080/19466315.2016.1207561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynd LD, O'Brien BJ. Advances in risk-benefit evaluation using probabilistic simulation methods: an application to the prophylaxis of deep vein thrombosis. J Clin Epidemiol. 2004;57((8)):795–803. doi: 10.1016/j.jclinepi.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18((2 Suppl l)):S68–80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020;16((1)):e1080. doi: 10.1002/cl2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aranda JV, Clyman R, Cox B, Van Overmeire B, Wozniak P, Sosenko I, et al. A randomized, double-blind, placebo-controlled trial on intravenous ibuprofen L-lysine for the early closure of nonsymptomatic patent ductus arteriosus within 72 hours of birth in extremely low-birth-weight infants. Am J Perinatol. 2009;26((3)):235–245. doi: 10.1055/s-0028-1103515. [DOI] [PubMed] [Google Scholar]
- 31.Hammerman C, Strates E, Valaitis S. The silent ductus: its precursors and its aftermath. Pediatr Cardiol. 1986;7((3)):121–127. doi: 10.1007/BF02424985. [DOI] [PubMed] [Google Scholar]
- 32.Krueger E, Mellander M, Bratton D, Cotton R. Prevention of symptomatic patent ductus arteriosus with a single dose of indomethacin. J Pediatr. 1987;111((5)):749–754. doi: 10.1016/s0022-3476(87)80262-7. [DOI] [PubMed] [Google Scholar]
- 33.Mahony L, Carnero V, Brett C, Heymann MA, Clyman RI. Prophylactic indomethacin therapy for patent ductus arteriosus in very-low-birth-weight infants. N Engl J Med. 1982;306((9)):506–510. doi: 10.1056/NEJM198203043060903. [DOI] [PubMed] [Google Scholar]
- 34.Rhodes PG, Ferguson MG, Reddy NS, Joransen JA, Gibson J. Effects of prolonged versus acute indomethacin therapy in very low birth-weight infants with patent ductus arteriosus. Eur J Pediatr. 1988;147((5)):481–484. doi: 10.1007/BF00441971. [DOI] [PubMed] [Google Scholar]
- 35.Sangtawesin C, Sangtawesin V, Lertsutthiwong W, Kanjanapattanakul W, Khorana M, Ayudhaya JKN. Prophylaxis of symptomatic patent ductus arteriosus with oral ibuprofen in very low birth weight infants. J Med Assoc Thai. 2008;91((Suppl 3)):S28–34. [PubMed] [Google Scholar]
- 36.Sosenko IRS, Fajardo MF, Claure N, Bancalari E. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr. 2012;160((6)):929–35.e1. doi: 10.1016/j.jpeds.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 37.Weesner KM, Dillard RG, Boyle RJ, Block SM. Prophylactic treatment of asymptomatic patent ductus arteriosus in premature infants with respiratory distress syndrome. South Med J. 1987;80((6)):706–708. doi: 10.1097/00007611-198706000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Adamska E, Helwich E, Rutkowska M, Zacharska E, Piotrowska A. Comparison of the efficacy of ibuprofen and indomethacin in the treatment of patent ductus arteriosus in prematurely born infants. Med Wieku Rozwoj. 2005;9((3 Pt 1)):335–354. [PubMed] [Google Scholar]
- 39.Ahranjani BM, Dalili H, Nashtifani ZH, Shariat M, Khorgami M. The comparison between intravenous acetaminophen versus oral ibuprofen in preterm newborns with patent ductus arteriosus: a clinical trial. Acta Med Iranica. 2020:631–636. [Google Scholar]
- 40.Akisu M, Ozyurek A, Dorak C, Parlar A, Kultursay N. Enteral ibuprofen versus indomethacin in the treatment of patent ductus arteriosus in preterm newborn infants. Cocuk Sagligi Ve Hastalikari Dergisi. 2001;44((1)):56–60. [Google Scholar]
- 41.Al-lawama M, Alammori I, Abdelghani T, Badran E. Oral paracetamol versus oral ibuprofen for treatment of patent ductus arteriosus. J Int Med Res. 2018;46((2)):811–818. doi: 10.1177/0300060517722698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aly H, Lotfy W, Badrawi N, Ghawas M, Abdel-Meguid IE, Hammad TA. Oral Ibuprofen and ductus arteriosus in premature infants: a randomized pilot study. Am J Perinatol. 2007;24((5)):267–270. doi: 10.1055/s-2007-976550. [DOI] [PubMed] [Google Scholar]
- 43.Bagheri MM, Niknafs P, Sabsevari F, Torabi MH, Bahman Bijari B, Noroozi E, et al. Comparison of oral acetaminophen versus ibuprofen in premature infants with patent ductus arteriosus. Iran J Pediatr. 2016;26((4)):e3975. doi: 10.5812/ijp.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagnoli F, Rossetti A, Messina G, Mori A, Casucci M, Tomasini B. Treatment of patent ductus arteriosus (PDA) using ibuprofen: renal side-effects in VLBW and ELBW newborns. J Matern Fetal Neonatal Med. 2013;26((4)):423–429. doi: 10.3109/14767058.2012.733775. [DOI] [PubMed] [Google Scholar]
- 45.Balachander B, Mondal N, Bhat V, Adhisivam B, Kumar M, Satheesh S, et al. Comparison of efficacy of oral paracetamol versus ibuprofen for PDA closure in preterms - a prospective randomized clinical trial. J Matern Fetal Neonatal Med. 2018:1–6. doi: 10.1080/14767058.2018.1525354. [DOI] [PubMed] [Google Scholar]
- 46.Cheng DD, Ortiz EE, Angtuaco JL. A single-blind randomized controlled trial comparing the efficacy of two doses of oral ibuprofen with intravenous indomethacin in terms of ductus arteriosus closure among premature infants with patent ductus arteriosus: a phase 2A clinical trial. Acta Med Philippina. 2013;47((1)):51–55. [Google Scholar]
- 47.Cherif A, Khrouf N, Jabnoun S, Mokrani C, Amara MB, Guellouze N, et al. Randomized pilot study comparing oral ibuprofen with intravenous ibuprofen in very low birth weight infants with patent ductus arteriosus. Pediatrics. 2008;122((6)):e1256–61. doi: 10.1542/peds.2008-1780. [DOI] [PubMed] [Google Scholar]
- 48.Chotigeat U, Jirapapa K, Layangkool T. A comparison of oral ibuprofen and intravenous indomethacin for closure of patent ductus arteriosus in preterm infants. J Med Assoc Thai. 2003;86((Suppl 3)):S563–9. [PubMed] [Google Scholar]
- 49.Christmann V, Liem KD, Semmekrot BA, van de Bor M. Changes in cerebral, renal and mesenteric blood flow velocity during continuous and bolus infusion of indomethacin. Acta paediatrica. 2002;91((4)):440–446. doi: 10.1080/080352502317371698. [DOI] [PubMed] [Google Scholar]
- 50.Dang D, Wang D, Zhang C, Zhou W, Zhou Q, Wu H. Comparison of oral paracetamol versus ibuprofen in premature infants with patent ductus arteriosus: a randomized controlled trial. PLoS One. 2013;8((11)):e77888. doi: 10.1371/journal.pone.0077888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dani C, Lista G, Bianchi S, Mosca F, Schena F, Ramenghi L, et al. Intravenous paracetamol in comparison with ibuprofen for the treatment of patent ductus arteriosus in preterm infants: a randomized controlled trial. Eur J Pediatr. 2021;180((3)):807–816. doi: 10.1007/s00431-020-03780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dani C, Vangi V, Bertini G, Pratesi S, Lori I, Favelli F, et al. High-dose ibuprofen for patent ductus arteriosus in extremely preterm infants: a randomized controlled study. Clin Pharmacol Ther. 2012;91((4)):590–596. doi: 10.1038/clpt.2011.284. [DOI] [PubMed] [Google Scholar]
- 53.Dash SK, Kabra NS, Avasthi BS, Sharma SR, Padhi P, Ahmed J. Enteral paracetamol or intravenous indomethacin for closure of patent ductus arteriosus in preterm neonates: a randomized controlled trial. Indian Pediatr. 2015;52((7)):573–578. doi: 10.1007/s13312-015-0677-z. [DOI] [PubMed] [Google Scholar]
- 54.Davidson JM, Ferguson J, Ivey E, Philip R, Weems MF, Talati AJ. A randomized trial of intravenous acetaminophen versus indomethacin for treatment of hemodynamically significant PDAs in VLBW infants. J Perinat. 2021;41((1)):93–99. doi: 10.1038/s41372-020-0694-1. [DOI] [PubMed] [Google Scholar]
- 55.Ding YJ, Han B, Yang B, Zhu M. NT-proBNP plays an important role in the effect of ibuprofen on preterm infants with patent ductus arteriosus. Eur Rev Med Pharmacol Sci. 2014;18((18)):2596–2598. [PubMed] [Google Scholar]
- 56.El-Farrash RA, El Shimy MS, El-Sakka AS, Ahmed MG, Abdel-Moez DG. Efficacy and safety of oral paracetamol versus oral ibuprofen for closure of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Maternal-Fetal Neonatal Med. 2018:1–8. doi: 10.1080/14767058.2018.1470235. [DOI] [PubMed] [Google Scholar]
- 57.El-Mashad AER, El-Mahdy H, El Amrousy D, Elgendy M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. Eur J Pediatr. 2017;176((2)):233–240. doi: 10.1007/s00431-016-2830-7. [DOI] [PubMed] [Google Scholar]
- 58.Erdeve O, Yurttutan S, Altug N, Ozdemir R, Gokmen T, Dilmen U, et al. Oral versus intravenous ibuprofen for patent ductus arteriosus closure: a randomised controlled trial in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2012;97((4)):F279–83. doi: 10.1136/archdischild-2011-300532. [DOI] [PubMed] [Google Scholar]
- 59.Fakhraee SH, Badiee Z, Mojtahedzadeh S, Kazemian M, Kelishadi R. Comparison of oral ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9((5)):399–403. [PubMed] [Google Scholar]
- 60.Fesharaki HJ, Nayeri FS, Asbaq PA, Amini E, Sedaqat M. Different doses of ibuprofen in the treatment of patent ductus arteriosus: a randomized clinical trial. Tehran Univ Med J. 2012;70((8)):488–493. [Google Scholar]
- 61.Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr. 1983;102((6)):895–906. doi: 10.1016/s0022-3476(83)80022-5. [DOI] [PubMed] [Google Scholar]
- 62.Ghaderian M, Barekatain B, Dardashty AB. Comparison of oral acetaminophen with oral ibuprofen on closure of symptomatic patent ductus arteriosus in preterm neonates. J Res Med Sci. 2019;24((1)):96. doi: 10.4103/jrms.JRMS_197_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghanem S, Mostafa M, Shafee M. Effect of oral ibuprofen on patent ductus arteriosus in premature newborns. J Saudi Heart Assoc. 2010;22((1)):7–12. doi: 10.1016/j.jsha.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gimeno Navarro A, Cano Sanchez A, Fernandez Gilino C, Carrasco Moreno J, Izquierdo Macian I, Gutierrez Laso A, et al. Ibuprofen versus indomethacin in the treatment of patent ductus arteriosus in preterm infants. An Pediatr. 2005;63((3)):212–218. doi: 10.1157/13078483. [DOI] [PubMed] [Google Scholar]
- 65.Gokmen T, Erdeve O, Altug N, Oguz SS, Uras N, Dilmen U. Efficacy and safety of oral versus intravenous ibuprofen in very low birth weight preterm infants with patent ductus arteriosus. J Pediatr. 2011;158((4)):549–54.e1. doi: 10.1016/j.jpeds.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Hammerman C, Aramburo MJ. Prolonged indomethacin therapy for the prevention of recurrences of patent ductus arteriosus. J Pediatr. 1990;117((5)):771–776. doi: 10.1016/s0022-3476(05)83342-6. [DOI] [PubMed] [Google Scholar]
- 67.Hammerman C, Glaser J, Schimmel MS, Ferber B, Kaplan M, Eidelman AI. Continuous versus multiple rapid infusions of indomethacin: effects on cerebral blood flow velocity. Pediatrics. 1995;95((2)):244–248. [PubMed] [Google Scholar]
- 68.Hammerman C, Shchors I, Jacobson S, Schimmel MS, Bromiker R, Kaplan M, et al. Ibuprofen versus continuous indomethacin in premature neonates with patent ductus arteriosus: is the difference in the mode of administration? Pediatr Res. 2008;64((3)):291–297. doi: 10.1203/PDR.0b013e31817d9bb0. [DOI] [PubMed] [Google Scholar]
- 69.Härkin P, Härmä A, Aikio O, Valkama M, Leskinen M, Saarela T, et al. Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. J Pediatr. 2016;177:72–7.e2. doi: 10.1016/j.jpeds.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 70.Hoxha A, Kola E, Kuneshka N, Tushe E. Oral versus intravenous ibuprofen for the early closure of patent ductus arteriosus in low birth weight preterm infants. Eur Med Health Pharm J. 2013;6 [Google Scholar]
- 71.Kaapa P, Lanning P, Koivisto M. Early closure of patent ductus arteriosus with indomethacin in preterm infants with idiopathic respiratory distress syndrome. Acta Paediatrica Scandinavica. 1983;72((2)):179–184. doi: 10.1111/j.1651-2227.1983.tb09693.x. [DOI] [PubMed] [Google Scholar]
- 72.Khuwuthyakorn V, Jatuwattana C, Silvilairat S, Tantiprapha W. Oral indomethacin versus oral ibuprofen for treatment of patent ductus arteriosus: a randomised controlled study in very low-birthweight infants. Paediatr Int Child Health. 2018;38((3)):187–192. doi: 10.1080/20469047.2018.1483566. [DOI] [PubMed] [Google Scholar]
- 73.Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014;99((2)):F99–F104. doi: 10.1136/archdischild-2013-304695. [DOI] [PubMed] [Google Scholar]
- 74.Krauss AN, Fatica N, Lewis BS, Cooper R, Thaler HT, Cirrincione C, et al. Pulmonary function in preterm infants following treatment with intravenous indomethacin. Am J Dis Child. 1989;143((1)):78–81. doi: 10.1001/archpedi.1989.02150130088021. [DOI] [PubMed] [Google Scholar]
- 75.Kumar A, Gosavi RS, Sundaram V, Oleti TP, Krishnan A, Kiran S, et al. Oral paracetamol vs oral ibuprofen in patent ductus arteriosus: a randomized, controlled, noninferiority trial. J Pediatr. 2020;222:79–84.e2. doi: 10.1016/j.jpeds.2020.01.058. [DOI] [PubMed] [Google Scholar]
- 76.Lago P, Bettiol T, Salvadori S, Pitassi I, Vianello A, Chiandetti L, et al. Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: a randomised controlled trial. Eur J Pediatr. 2002;161((4)):202–207. doi: 10.1007/s00431-002-0915-y. [DOI] [PubMed] [Google Scholar]
- 77.Lago P, Salvadori S, Opocher F, Ricato S, Chiandetti L, Frigo AC. Continuous infusion of ibuprofen for treatment of patent ductus arteriosus in very low birth weight infants. Neonatology. 2014;105((1)):46–54. doi: 10.1159/000355679. [DOI] [PubMed] [Google Scholar]
- 78.Lee SJ, Kim JY, Park EA, Sohn S. The pharmacological treatment of patent ductus arteriosus in premature infants with respiratory distress syndrome: oral ibuprofen vs. indomethacin. Korean J Pediatr. 2008;51((9)):956–963. [Google Scholar]
- 79.Lin XZ, Chen HQ, Zheng Z, Li YD, Lai JD, Huang LH. Therapeutic effect of early administration of oral ibuprofen in very low birth weight infants with patent ductus arteriosus. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14((7)):502–505. [PubMed] [Google Scholar]
- 80.Lin YJ, Chen CM, Rehan VK, Florens A, Wu SY, Tsai ML, et al. Randomized trial to compare renal function and ductal response between indomethacin and ibuprofen treatment in extremely low birth weight infants. Neonatology. 2017;111((3)):195–202. doi: 10.1159/000450822. [DOI] [PubMed] [Google Scholar]
- 81.Meena V, Meena DS, Rathore PS, Chaudhary S, Soni JP. Comparison of the efficacy and safety of indomethacin, ibuprofen, and paracetamol in the closure of patent ductus arteriosus in preterm neonates: a randomized controlled trial. Ann Pediatr Cardiol. 2020;13((2)):130–135. doi: 10.4103/apc.APC_115_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merritt TA, Harris JP, Roghmann K, Wood B, Campanella V, Alexson C, et al. Early closure of the patent ductus arteriosus in very low-birth-weight infants: a controlled trial. J Pediatr. 1981;99((2)):281–286. doi: 10.1016/s0022-3476(81)80479-9. [DOI] [PubMed] [Google Scholar]
- 83.Mosca F, Bray M, Lattanzio M, Fumagalli M, Tosetto C. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. J Pediatr. 1997;131((4)):549–554. doi: 10.1016/s0022-3476(97)70060-x. [DOI] [PubMed] [Google Scholar]
- 84.Mullett MD, Croghan TW, Myerberg DZ, Krall JM, Neal WA. Indomethacin for closure of patent ductus arteriosus in prematures. Clin Pediatr. 1982;21((4)):217–220. doi: 10.1177/000992288202100404. [DOI] [PubMed] [Google Scholar]
- 85.Nestrud RM, Hill DE, Arrington RW, Beard AG, Dungan WT, Lau PY, et al. Indomethacin treatment in patent ductus arteriosus. Dev Pharmacol Ther. 1980;1((2–3)):125–136. [PubMed] [Google Scholar]
- 86.Neu J, Ariagno RL, Johnson JD, Pitlick PT, Cohen RS, Beets CL, et al. A double blind study of the effects of oral indomethacin in preterm infants with patent ductus arteriosus who failed medical management. Pediatric pharmacol. 1981;1((3)):245–249. [PubMed] [Google Scholar]
- 87.Oboodi R, Najib KS, Amoozgar H, Pourarian S, Moghtaderi M, Mehdizadegan N, et al. Positive tendency toward synchronous use of acetaminophen and ibuprofen in treating patients with patent ductus arteriosus. Turk Kardiyol Dernegi Arsivi. 2020;48((6)):605–612. doi: 10.5543/tkda.2020.03902. [DOI] [PubMed] [Google Scholar]
- 88.Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N, Oguz SS, et al. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr. 2014;164((3)):510–4.e1. doi: 10.1016/j.jpeds.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 89.Osborn DA, Evans N, Kluckow M. Effect of early targeted indomethacin on the ductus arteriosus and blood flow to the upper body and brain in the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2003;88((6)):F477–F482. doi: 10.1136/fn.88.6.F477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patel J, Roberts I, Azzopardi D, Hamilton P, Edwards AD. Randomized double-blind controlled trial comparing the effects of ibuprofen with indomethacin on cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatr Res. 2000;47((1)):36–42. doi: 10.1203/00006450-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 91.Pezzati M, Vangi V, Biagiotti R, Bertini G, Cianciulli D, Rubaltelli FF. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. J Pediatr. 1999;135((6)):733–738. doi: 10.1016/s0022-3476(99)70093-4. [DOI] [PubMed] [Google Scholar]
- 92.Pourarian S, Pishva N, Madani A, Rastegari M. Comparison of oral ibuprofen and indomethacin on closure of patent ductus arteriosus in preterm infants. East Mediterr Health J. 2008;14((2)):360–365. [PubMed] [Google Scholar]
- 93.Pourarian S, Takmil F, Cheriki S, Amoozgar H. The effect of oral high-dose ibuprofen on patent ductus arteriosus closure in preterm infants. Am J Perinatol. 2015;32((12)):1158–1163. doi: 10.1055/s-0035-1551671. [DOI] [PubMed] [Google Scholar]
- 94.Rahman MA, Utamayasa IKA, Cahyono A. The comparison between acetaminophen and ibuprofen effectiveness for ductus arterious closure therapy in premature infants. J Int Dental Med Res. 2020;13((2)):704–707. [Google Scholar]
- 95.Rudd P, Montanez P, Hallidie-Smith K, Silverman M. Indomethacin treatment for patent ductus arteriosus in very low birthweight infants: double blind trial. Arch Dis Child. 1983;58((4)):267–270. doi: 10.1136/adc.58.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salama H, Alsisi A, Al-Rifai H, Shaddad A, Samawal L, Habboub L, et al. A randomized controlled trial on the use of oral ibuprofen to close patent ductus arteriosus in premature infants. J Neonatal Perinat Med. 2008;1((3)):153–158. [Google Scholar]
- 97.Su BH, Lin HC, Chiu HY, Hsieh HY, Chen HH, Tsai YC. Comparison of ibuprofen and indometacin for early-targeted treatment of patent ductus arteriosus in extremely premature infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93((2)):F94–9. doi: 10.1136/adc.2007.120584. [DOI] [PubMed] [Google Scholar]
- 98.Su PH, Chen JY, Su CM, Huang TC, Lee HS. Comparison of ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants. Pediatr Int. 2003;45((6)):665–670. doi: 10.1111/j.1442-200x.2003.01797.x. [DOI] [PubMed] [Google Scholar]
- 99.Sung SI, Lee MH, Ahn SY, Chang YS, Park WS. Effect of nonintervention vs oral ibuprofen in patent ductus arteriosus in preterm infants: a randomized clinical trial. JAMA Pediatrics. 2020;174((8)):755–763. doi: 10.1001/jamapediatrics.2020.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tammela O, Ojala R, Iivainen T, Lautamatti V, Pokela ML, Janas M, et al. Short versus prolonged indomethacin therapy for patent ductus arteriosus in preterm infants. J Pediatr. 1999;134((5)):552–557. doi: 10.1016/s0022-3476(99)70239-8. [DOI] [PubMed] [Google Scholar]
- 101.Tauber KA, King R, Colon M. Intravenous acetaminophen vs intravenous ibuprofen to close a patent ductus arteriosus closure: a pilot randomized controlled trial. Health Sci Rep. 2020;3((3)):e183. doi: 10.1002/hsr2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van Overmeire B, Follens I, Hartmann S, Creten WL, Van Acker KJ. Treatment of patent ductus arteriosus with ibuprofen. Arch Dis Child Fetal Neonatal Ed. 1997;76((3)):F179–84. doi: 10.1136/fn.76.3.f179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Overmeire B, Smets K, Lecoutere D, Van de Broek H, Weyler J, Degroote K, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343((10)):674–681. doi: 10.1056/NEJM200009073431001. [DOI] [PubMed] [Google Scholar]
- 104.Yadav S, Agarwal S, Maria A, Dudeja A, Dubey NK, Anand P, et al. Comparison of oral ibuprofen with oral indomethacin for PDA closure in Indian preterm neonates: a randomized controlled trial. Pediatr Cardiol. 2014;35((5)):824–830. doi: 10.1007/s00246-014-0861-2. [DOI] [PubMed] [Google Scholar]
- 105.Yanagi RM, Wilson A, Newfeld EA, Aziz KU, Hunt CE. Indomethacin treatment for symptomatic patent ductus arteriosus: a double-blind control study. Pediatrics. 1981;67((5)):647–652. [PubMed] [Google Scholar]
- 106.Yang B, Gao X, Ren Y, Wang Y, Zhang Q. Oral paracetamol vs. oral ibuprofen in the treatment of symptomatic patent ductus arteriosus in premature infants: a randomized controlled trial. Exp Ther Med. 2016;12((4)):2531–2536. doi: 10.3892/etm.2016.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yeh TF, Luken JA, Thalji A, Raval D, Carr I, Pildes RS. Intravenous indomethacin therapy in premature infants with persistent ductus arteriosus: a double-blind controlled study. J Pediatr. 1981;98((1)):137–145. doi: 10.1016/s0022-3476(81)80560-4. [DOI] [PubMed] [Google Scholar]
- 108.El-Mashad AER, El-Mahdy H, El Amrousy D, Elgendy M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. Eur J Pediatr. 2017;176((2)):233–240. doi: 10.1007/s00431-016-2830-7. [DOI] [PubMed] [Google Scholar]
- 109.Ding Y, Wang X, Wu Y, Li H, Xu J, Wang X. Effects of prophylactic oral ibuprofen on the closure rate of patent ductus arteriosus in premature infants. Medicine. 2018;97((37)):e12206. doi: 10.1097/MD.0000000000012206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pranata R, Yonas E, Vania R, Prakoso R. The efficacy and safety of oral paracetamol versus oral ibuprofen for patent ductus arteriosus closure in preterm neonates: a systematic review and meta-analysis. Indian Heart J. 2020;72((3)):151–159. doi: 10.1016/j.ihj.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amitai Y, Hammerman C, Batash D, Keidar R, Baram S, Goldman M, et al. Pharmacokinetics of oral ibuprofen for patent ductus arteriosus closure in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2012;97((1)):F76. doi: 10.1136/fetalneonatal-2011-301204. [DOI] [PubMed] [Google Scholar]
- 112.Hammerman C, Patent ductus arteriosus Clinical relevance of prostaglandins and prostaglandin inhibitors in PDA pathophysiology and treatment. Clin Perinatol. 1995;22((2)):457–479. [PubMed] [Google Scholar]
- 113.Lee J, Rajadurai VS, Tan KW, Wong KY, Wong EH, Leong JYN. Randomized trial of prolonged low-dose versus conventional-dose indomethacin for treating patent ductus arteriosus in very low birth weight infants. Pediatrics. 2003;112((2)):345–350. doi: 10.1542/peds.112.2.345. [DOI] [PubMed] [Google Scholar]
- 114.Rennie JM, Cooke RW. Prolonged low dose indomethacin for persistent ductus arteriosus of prematurity. Arch Dis Child. 1991;66((1 Spec No)):55–58. doi: 10.1136/adc.66.1_spec_no.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.