Abstract
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma in which immunochemotherapy, with or without high-dose therapy, and autologous stem cell transplantation remain standard frontline therapies. Despite their clear efficacy, patients inevitably relapse and require subsequent therapy. In this review, we discuss the key therapeutic approaches in the management of relapsed MCL, covering in depth the data supporting the use of covalent Bruton tyrosine kinase (BTK) inhibitors at first or subsequent relapse. We describe the outcomes of patients progressing through BTK inhibitors and discuss the mechanisms of covalent BTKi resistance and treatment options after covalent treatment with BTKi. Options in this setting may depend on treatment availability, patient's and physician's preference, and the patient's age and comorbidity status. We discuss the rapid recent development of anti-CD19 chimeric antigen receptor T-cell therapy, as well as the utility of allogenic stem cell transplantation and novel therapies, such as noncovalent, reversible BTK inhibitors; ROR1 antibody drug conjugates; and bispecific antibodies.
Mantle cell lymphoma (MCL) is a distinct subtype of B-cell non-Hodgkin lymphoma (NHL), with hallmarks of repeated remission, relapse, and resistance to therapy. The management of refractory or relapsed (R/R) MCL remains challenging, but has seen a paradigm shift from chemoimmunotherapy to targeted therapies and the current era of cellular therapy, marked by the success of chimeric antigen receptor (CAR) T-cell treatment. Herein, we attempt to summarize the recent treatment advances in R/R MCL.
Widely applied, standard frontline approaches include cytotoxic and anti-CD20 monoclonal antibody (mAb) therapy. Patients ≤65 years are commonly offered autologous stem-cell transplantation (auto-SCT)1, 2 consolidation after anti-CD20 mAb and high-dose cytarabine (HDAC)-based induction. Older patients or those with comorbidities precluding auto-SCT may be offered immunochemotherapy, including R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone + rituximab),3, 4 R-B (bendamustine+rituximab),5 and VR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone).6, 7 Prospective2, 3 and retrospective evidence8 suggests rituximab maintenance can be reasonably applied after both auto-SCT and immunochemotherapy induction in patients who do not undergo auto-SCT. Recent trials have analyzed noncytotoxic regimens. Although lenalidomide-based treatment,9, 10 Bruton tyrosine kinase inhibitor (BTKi)–based treatment,11 B-cell lymphoma (BCL2i) inhibitor-lenalidomide combinations,12 and BCL2i-BTKi13 combinations all provide great promise as frontline treatment, none have been licensed to date. Despite these developments, patients are not cured by frontline therapy, and a pattern of continued relapse has been observed.
Clinical assessment at relapse
At relapse, patients ideally should undergo another biopsy and a full restaging to assess histological transformation, and TP53 status, and assessment of symptoms, organ function, comorbidity status, and social support structure. A full discussion regarding therapeutic options, clinical trials, and supportive palliative care should be reasonably individualized. Several important biological and clinical prognostic markers are relevant. Although most of the data regarding biological markers are studied in more depth in patients given frontline treatments, important prognostic biological markers at relapse include TP53 mutations, high tumor proliferation (Ki67%), blastoid or pleomorphic histological transformation, and complex karyotype (CK). Studies spanning chronological cohorts have documented that progression within 24 months (POD24) leads to inferior overall survival (OS)14, 15, 16 and is also specific to individual therapy.17
TP53
TP53 (tumor protein 53) aberrations in MCL are strongly associated with therapy resistance, aggressive clinical behavior, and negative prognosis.18, 19, 20 These aberrations include 17p deletion, revealed by fluorescence in situ hybridization; TP53 deletions/mutations, detected by next-generation sequencing; and p53 protein overexpression, determined by immunohistochemistry. Currently, there is little evidence that routinely available therapy can overcome the negative prognosis of TP53 aberrancy in MCL, with the possible exception of anti-CD19 CAR T-cell therapy. Clinical outcome data for patients with TP53 mutations are discussed further for each therapy. In contrast to chronic lymphocytic leukemia (CLL), the role of the deletion of 17p is more unclear in MCL, with mixed outcomes reported and some suggestion that patients with 17p deleted fare better than those with TP53 mutations.
Ki67
Ki-67 is a nuclear protein associated with cellular proliferation and is an independent prognostic factor in patients with MCL. Ki-67 levels are subclassified by fairly crude immunohistochemical staining percentages with poor interobserver reproducibility and the potential for nonrepresentative sampling. Patients are classified as low (<30%), moderate (30% to 50%), and high (≥50%) risk.
Blastoid/pleomorphic variants
By morphologic classification, MCL is defined as either classic or aggressive (pleomorphic and blastoid variants). Pleomorphic and blastoid MCLs represent 10% to 20% of patients at diagnosis20, 21 and typically follow an aggressive, treatment-resistant course, with resultant poor survival.
CK
In addition to the characteristic and pathological t(11;14)(q13;q32) translocation, ≥3 chromosomal aberrations define CK in MCL. Greenwell et al described the prognostic importance of CK, albeit from the frontline setting. Outcomes of 53 patients with newly diagnosed MCL with CK were compared with 221 without CK.22 Progression-free survival (PFS) (1.9 vs 4.4 years; P < .01) and OS (4.5 vs 11.6 years; P < .01) were significantly inferior in patients with CK compared with those without. Although CK often coexists with other high-risk features, in this study, CK remained independently predictive of OS (hazard ratio [HR], 1.98; P = .02).
Covalent BTK inhibition
Constitutive activation of B-cell receptor (BCR) signaling is essential for the survival and proliferation of malignant B cells including those in MCL. BTK is an essential component of the BCR signaling pathway, and numerous oral BTKis have been developed to block this pathway in indolent B-cell malignancies.23 Since the clinical development of ibrutinib, the first-in-class covalent oral BTKi, this therapeutic class has become the cornerstone in R/R MCL management (Table 1). One-hundred eleven heavily pretreated patients received ibrutinib monotherapy in the pivotal phase 2 trial.24 The computed tomography (CT)-based overall response rate (ORR) of 68% (complete remission [CR] 21%) was unparalleled at the time of initial publication. The median PFS was 13.9 months and grade 3 to 4 toxicities were uncommon. The subsequent randomized phase 3 RAY trial25 proved ibrutinib monotherapy to be superior in terms of response rates, PFS, and toxicity profile to the mammalian target of rapamycin inhibitor temsirolimus, with the ibrutinib arm performing similarly to the previous phase 2 trial. A pooled analysis19 (n = 370) has provided further understanding regarding the depth and durability of response according to the line of therapy in which ibrutinib is used. ORR (1 prior line 77.8% vs >1 prior line 66.8%), CR rate (1 prior line 36.4% vs >1 prior line 22.9%), median PFS (1 prior line 25.4 months vs >1 prior line 10.3 months), and median OS (1 prior line not reached vs >1 prior line 22.5 months), were all superior when ibrutinib was used at first relapse rather than at subsequent relapses. Although these subgroups are challenging to compare directly, these findings have led to the widespread adoption of BTKi monotherapy at first relapse. Ibrutinib is associated with some well-described toxicities. From the pooled analysis, 80% had grade ≥3 treatment-emergent adverse events (AEs). The most commonly observed were neutropenia (17.0%), thrombocytopenia (12.4%), pneumonia (12.7%), anemia (10.0%), atrial fibrillation (AF) or flutter (6.2%), and hypertension (5.1%).19
Table 1.
Prospective trials evaluating covalent BTKi monotherapy and covalent BTKi combinations in R/R MCL
| Treatment | Reference | Study | N | Drug class(es) added | Median age, y | Median prior lines (range) | High risk MIPI | Response | Median PFS (mo; 95% CI) | Key grade 3/4 adverse events ≥10% |
|---|---|---|---|---|---|---|---|---|---|---|
| Covalent BTK inhibitor monotherapy | ||||||||||
| Ibrutinib | 14 | Phase 2 | 111 | — | 68 | 3 (1-5) | 49% | ORR 68%; CR 21% | 13.9 (7.0-NE) | Neutropenia 16%; thrombocytopenia 11% |
| Ibrutinib | 25 | Phase 3 | 139 | — | 67 | 2 (1-9) | 22% | ORR 72%; CR 19% | 14.6 (10.4-NE) | Neutropenia 13% |
| Ibrutinib | 19 | Pooled analysis | 370 | — | 68 | 2 (1-9) | 32% | ORR 70%; CR 27% | 12.5 (9.8-16.6) | Neutropenia 17%; thrombocytopenia 12.4%; pneumonia 12.7%; anemia 10.0% |
| Acalabrutinib | 27,28 | Phase 2 | 124 | — | 68 | 2 (1-2) | 17% | ORR 81%; CR 40% | 22 (16.6-33.3) | Neutropenia 12%; anemia 12% |
| Zanubrutinib | 30,31 | Phase 2 | 86 | — | 60.5 | 2 (1-4) | 38.4% | ORR 83.7%; CR 77.9% | 33 (19.4-NE) | Neutropenia 18.6%; infection 18.6%; pneumonia 12.8% |
| Zanubrutinib | 32 | Phase 1/2 | 32 | — | 70.5 | 1 (1-4) | 31.3% | ORR 90.6%; CR 31.3% | 21.1 mo (13.2–NE) | Infections 18.8%; anemia 12.5% |
| Covalent BTK inhibitor-based combinations | ||||||||||
| Ibrutinib-rituximab | 26 | Phase 2 | 50 | Anti-CD20 mAb | 67 | 3 (1-9) | 12% | ORR 88%; CR 44% | Median NR; 15-mo PFS 69% (57%–84%) | Atrial fibrillation 12% |
| Ibrutinib-lenalidomide-rituximab | 36 | Phase 2 | 50 | Immunomodulatory agent; anti-CD20 mAb | 69 | 2 (1-7) | 46% | ORR 76%; CR 56% | 16.0 (13.7-20.5) | Neutropenia 36%; infection 22%; cutaneous 14%; gastrointestinal 12%; thrombocytopenia 12% Vascular 10% |
| Ibrutinib-venetoclax | 40 | Phase 2 | 24 | BCL2 inhibitor | 68 | 2 (0-6) | 75% | ORR 71%; CR 71% | 29 (13-NR) | Neutropenia 33%; thrombocytopenia 17%; diarrhea 12%; anemia 12% |
| Ibrutinib-venetoclax-obinutuzumab | 13 | Phase ½ | 24 | Type 2 glycoengineered; anti-CD20 mAb; BCL2 inhibitor | 66 | 1 (1-3) | 29% | ORR 71%; CR 67%; uMRD 71.5%; (10/14) | Median NR; 2-y PFS 69.5% (52.9-91.4) | Neutropenia 71%; thrombocytopenia 54%; diarrhea 12% |
| Ibrutinib-bortezomib | 37 | Phase 1/2 | 55 | Proteosome inhibitor | 71 | 1 (1-5) | Not known | ORR 87.3%; CR 44% | 18.6 (12.4-NR) | G4 hematotoxicity 16.4%; G3 infections 25% Otherwise not available |
| Ibrutinib-umbralisib | 38 | Phase 2 | 21 | PI3K-δ/casein kinase 1ε inhibitor | 68 | 2 (2-3) | Not known | ORR 67%; CR 27% | 10.5 | Infection 17%*; neutropenia 12%; bruising 12%; diarrhea 10% |
| Ibrutinib-palbociclib | 39 | Phase 1 | 27 | CDK4/6 inhibitor | 69 | 1 (1-5) | 26% | ORR 67%; CR 37% | Median NR; 2-y PFS 59.4% (37.9%-80.9%) | Neutropenia 41%; thrombocytopenia 30%; hypertension 15%; febrile neutropenia 15%; pneumonia 11% |
BCL2, B-cell lymphoma 2; CDK4/6, cyclin-dependent kinase 4 and 6; G, grade; MIPI, mantle cell lymphoma international prognostic index; NR, not reached; PI3K, phosphoinositide 3-kinase; uMRD, undetectable minimal residual disease.
AEs include 21 patients with CLL.
There are no published or ongoing clinical trials comparing BTKi monotherapy with immunochemotherapy in R/R MCL. Indirect comparisons of ibrutinib with bendamustine-rituximab, R-BAC (rituximab-bendamustine-cytarabine) and other therapy in the retrospective, multicenter MANTLE-FIRST study14 suggest that ibrutinib may result in superior OS in patients with POD24 (POD24, n = 127; reference HR, 1.0; ibrutinib: HR 2.41 for R-B; HR 2.78 for R-BAC). Survival outcomes were not different by treatment group in those without POD24.
Although ibrutinib provides durable disease control for many patients, there remains significant subpopulations who both respond poorly and/or progress earlier on ibrutinib monotherapy. From pooled data, 13.9% (n = 20) of 144 patients receiving ibrutinib monotherapy had a mutated TP53, and their median PFS was only 4 months.19 Patients with blastoid morphology had a median PFS of 5 months,19 and patients with R/R MCL with a high tumor proliferation index (Ki67% ≥ 50%) had an inferior PFS when receiving rituximab-ibrutinib in a prospective phase 2 trial compared with patients with a Ki67 <50%.26 Additional poor risk factors with ibrutinib at first relapse include increasing age, poor performance status, and high simplified mantle cell lymphoma international prognostic index, and POD24.17
Acalabrutinib and zanubrutinib are both highly selective, second-generation oral covalent BTKis licensed in R/R MCL. One-hundred twenty-four patients received acalabrutinib monotherapy in a less heavily pretreated (median 2 prior lines) MCL cohort compared with the pivotal ibrutinib trial.27 The positron emission tomography–based ORR and CR rates were 81% and 40%, respectively. The final results28 demonstrated an overall median PFS of 22 months with impressive safety (AF), 2.4%; hypertension, 4.0%; treatment-emergent, and AEs resulting in discontinuation, 12.1%). Interestingly, the ORR and PFS were no different when compared with prior lines. A pooled safety analysis29 of 1040 acalabrutinib-treated patients across a range of B-cell malignancy trials has also demonstrated a favorable safety profile. Common AEs were headache (38%), diarrhea (37%), upper respiratory tract infection (22%), and contusion (22%). AF occurred in 4%, hypertension in 8%, and AEs leading to discontinuation of treatment in 9%.
Zanubrutinib was approved by the US Food and Drug Administration (FDA) after the pivotal phase 2 trial of 86 patients with R/R MCL performed in China. Initial results showed a positron emission tomography–based ORR of 84% and a CR rate of 68.6%.30 Longer follow-up noted a median PFS of 33 months. No AF or grade ≥3 cardiac AEs were recorded,31 with neutropenia and infection the most prevalent grade ≥3 toxicity (both 18.6%). Intriguingly, 15 patients with a TP53 mutation in this prospective trial investigating zanubrutinib monotherapy had a documented ORR of 80% (95% CI, 51.9-95.7) and a median PFS of 14.7 months (95% CI, 2.9-NE).31 Given the lack of compelling biologic rationale for superior efficacy in this subset of patients for zanubrutinib over ibrutinib, this observation requires confirmation in larger studies.
Currently, no randomized trials have compared efficacy and safety of ibrutinib, zanubrutinib,32 and acalabrutinib in R/R MCL. It remains unclear whether the differences in ORR and CR rates across these phase 2 populations are significant, particularly given the variation in radiological assessment methodology and different baseline patient risk profiles. Recent mature randomized data33 (ELEVATE RR) have shown, however, that acalabrutinib has noninferior efficacy and a safety profile superior to that of ibrutinib in high-risk (17p and/or 11q deletion) R/R CLL. The cumulative incidence of AF/flutter, hypertension, arthralgia, diarrhea, bleeding, pneumonitis, and toxicity-related discontinuation were all significantly lower with acalabrutinib. A similar pattern of improved toxicity profile for zanubrutinib compared with ibrutinib have been seen in ASPEN34 and interim analysis of ALPINE35 randomized trials in Waldenstrom macroglobulinemia and non–high-risk R/R CLL, respectively. Differences in AF rates compared with ibrutinib in ASPEN and ALPINE are particularly striking. Although no randomized data have been analyzed to determine toxicity differences between second-generation BTKis and ibrutinib in R/R MCL, there are accumulating data to suggest their safety profile is superior. Where ibrutinib, zanubrutinib, and acalabrutinib are all available, therapeutic choice could be reasonably individualized based on the specific toxicity profiles of each agent.
BTKi-based combinations
Given the overall favorable efficacy and safety profile of covalent BTKis, multiple attempts have been made to improve the outcomes documented with monotherapy. Published trials have combined ibrutinib with numerous other active agents in MCL, such as proteosome inhibitors, BCL2is, and immunomodulatory agents. To date, all studies are nonrandomized phase 1/2 studies of modest size (typically, N = 20-50). Lenalidomide-rituximab,36 rituximab,26 bortezomib,37 umbralisib,38 palbociclib,39 venetoclax,40 and venetoclax-obintuzumab13 have all been combined with ibrutinib (Table 1). The toxicity and efficacy prolife of these combinations are challenging to cross compare, and it is unclear whether the results would be altered by the use of second- or subsequent-generation BTKis in place of ibrutinib. With those caveats, one can observe that the addition of lenalidomide increases cutaneous and gastrointestinal AEs, and the addition of obinutuzumab to ibrutinib-venetoclax increases the rate of grade ≥3 neutropenia and thrombocytopenia. Broadly, rates of grade ≥3 infection are higher with combination strategies compared with ibrutinib monotherapy. Although some studies demonstrate a relatively favorable efficacy profile and particularly encouraging CR rates in small subgroups with a TP53 mutation with R-ibrutinib-lenalidomide36 (n = 11; ORR, 73%; CR, 64%) and ibrutinib-venetoclax40 (n = 12, TP53 mutation or deletion ORR, 50%; all CR), none were licensed at the time of this writing, and the patient numbers are too small to draw strong conclusions. To our knowledge, only venetoclax-based combinations have been taken forward into pivotal randomized phase 3 trials in R/R MCL at present. SYMPATICO (https://clinicaltrials.gov, NCT03112174) is testing whether adding venetoclax to ibrutinib provides survival benefit over ibrutinib alone. The trial has completed accrual, and results are awaited.
Other approved therapies in R/R MCL
Although covalent BTKi have dominated the R/R MCL space during recent years, lenalidomide, bortezomib, and temsirolimus are all therapeutic options licensed before the covalent BTKi era. Lenalidomide resulted in overall response rates of 40% (CR/complete response unconfirmed 5%) in 170 SCT-unfit patients with R/R MCL,41 following a median of 2 prior lines (range, 1-3) and a minority achieving durable remissions in the SPRINT trial. The median duration of response was 16.1 months (95% CI, 9.5-20.0) with lenalidomide and there was a significantly improved PFS compared with investigator's choice (median, 8.7 vs 5.2 months; P = .004). Bortezomib has demonstrated an overall response rate of 32% (CR/complete response unconfirmed 8%) in the phase 2 PINNACLE trial42 and is generally well tolerated. Although licensed,43 temsirolimus was significantly inferior to ibrutinib in the RAY randomized trial and has a limited role.
CNS MCL
Central nervous system (CNS) involvement in MCL is rare and catastrophic (median OS, 3-6 months). Data are scarce, and there have been no prospective trials to guide management and treatment of CNS MCL relapse. Approximately 4% develop CNS relapse44, 45 with Ki-67 ≥30% the risk factor associated with CNS relapse by multivariable analysis.46 Historical management strategies have for the most part been extrapolated from other aggressive B-cell NHL and include high-dose antimetabolites such as high-dose methotrexate (HD-MTX) and HDAC, intrathecal chemotherapy, combined chemoradiotherapy, or radiotherapy alone. Lenalidomide, ibrutinib, rituximab, and acalabrutinib are all reported to penetrate the blood-brain barrier. Prospective trials suggest safe delivery and efficacy of lenalidomide47 or ibrutinib48, 49 in diffuse large B-cell lymphoma (DLBCL). A recent international collaborative series50 (n = 84) has suggested that ibrutinib results in superior OS compared with BBB-penetrating chemotherapy in BTKi-naive patients with MCL with CNS relapse. There was no apparent reduction in CNS relapse rates among patients treated with hyperfractionated cyclophosphamide, vincristine, pegylated liposomal doxorubicin, and dexamethasone alternating with methotrexate/cytarabine + rituximab (R-hyper-CVAD/MA), suggesting that high-dose antimetabolites are not effective prophylaxis.46 Results of prospective studies with patients randomly assigned to induction regimens with and without BTKi may inform whether these agents reduce CNS relapse; however, the rarity of the event would result in the usual issue of statistical power. Our favored approach in light of these recent data in BTKi-naive patients with CNS MCL relapse is ibrutinib. In patients who experience CNS progression while receiving a BTKi, the optimal therapy is unknown. CAR T-cell therapy had demonstrable activity in small patient cohorts with primary and secondary CNS DLBCL51, 52 and activity in a single MCL case in the TRANSCEND trial.53
Outcomes, biology, and therapy for covalent BTKi-resistant patients with MCL
The outcome of patients with progression following ibrutinib was initially reported to be poor, with a response rates to the next therapy of 26% to 32% and median OS of 6 to 8 months.54, 55 The poor outcomes described are attributable to the more heavily treated nature of the patients included. In more contemporary series of patients treated at first relapse, the median OS for patients experiencing disease progression while receiving ibrutinib was even worse (1.7 months).17 However, 57% were unsuitable for any subsequent therapy, a finding reported in other datasets.56 This result highlights that disease progression after ibrutinib is often highly aggressive, and patients are often too frail to receive further therapy. Among patients who received subsequent therapy, the median OS was 11.6 months, with the most promising outcomes in 23 R-BAC–treated patients (median OS, 14.0 months).17 The minority who discontinued ibrutinib while in remission to undergo allogenic stem cell transplantation (allo-SCT) had the most favorable outcomes (median OS not reached).57 Data regarding outcomes of patients who experience progression after zanubrutinib30, 32 and acalabrutinib58 monotherapy have not yet been specifically described.
The mechanisms of BTKi resistance are increasingly well described. In CLL, most patients with ibrutinib resistance acquire mutations in the C481S BTK binding pocket or PLCG2.59 However, in ibrutinib-resistant MCL, BTK C481S mutations are infrequent, and PLCG2 mutations are not observed. Activation of the NF-κB pathway via mutations in TRAF2, BIRCS3, and MAP3K14 have been described in ibrutinib-resistant MCL.60 Activated CARD1161 and CCND162 mutations also confer ibrutinib resistance in some patients with MCL. Others have highlighted the potential role of CXCR4 and FAK upregulation leading to alterations in the MCL tumor microenvironment: increasing cell adhesion–mediated drug resistance63 and metabolic64 and transcriptional65 reprogramming leading to ibrutinib resistance. Further work has demonstrated the complex intratumoral heterogeneity with 17q gain, BIRC5/surviving upregulation, and dampening of CD8+ T cells as a potential mechanism of BTKi-resistance.66
The current optimal management of patients who are refractory to covalent BTKis remains unknown, and there are still relatively few published data sets (Table 2). As use of covalent BTKis is being explored in earlier lines of therapy, the number of patients resistant to covalent BTKi grows, and their management presents one of the most pressing questions in contemporary practice. Wang et al described outcomes after various lenalidomide-based approaches in 58 patients who were ibrutinib resistant or intolerant.67 Patients received lenalidomide monotherapy (n = 13), lenalidomide-rituximab (n = 11), or other combinations (n = 34) with a resultant ORR of 15%, 27%, and 35% respectively. The median duration of response was 20 weeks, and the safety profile was consistent with that expected from lenalidomide in a heavily pretreated MCL population.
Table 2.
Selected studies assessing outcomes of patient who received therapy for MCL after relapsing while treated with BTKi
| Treatment | Reference | Study | N | Median age, y | High risk* | Median prior lines therapy | Response to prior BTKi | Response to treatment | Transplant consolidation | Outcomes, mo |
|---|---|---|---|---|---|---|---|---|---|---|
| Assorted (lenalidomide 26%, cytarabine 18%, bendamustine 16%, bortezomib 10%) | 54 | Retrospective multicenter† | 73 | 67 | 48% | 4 (1-11) | ORR 50% CR 11%; median DOI 4.7 m | ORR 26% CR 7% | 5 (6.8%) | Median OS 5.8 |
| Lenalidomide ± anti-CD20 ± chemotherapy | 67 | Observational multicenter‡ | 58 | 71 | NA | 4 (1-13) | ORR 45% CR 14%; median DOI 4.3 mo | ORR 29% CR 14% | NA | Median DOR 5 |
| Venetoclax monotherapy | 68 | Retrospective multicenter | 20 | 69 | 55% | 3 (2-5) | ORR 55% CR 15% median DOI 4.8 mo | ORR 53% CR 18% | 1 (5.0%) | Median PFS 3.2; median OS 9.4 |
| Venetoclax monotherapy | 69 | Retrospective single center | 24§ | 69 | 67% | 5 (1-11) | “66% BTKi resistant” | ORR 50% CR 21% | — | Median PFS 8; median OS 13.5 |
| R-BAC | 71 | Retrospective multicenter | 36 | 66 | 58% | 2 (1-6) | ORR 68% CR 32%; median PFS 9.2 mo | ORR 83% CR 60% | 12 (33.3%) | Median PFS 10.1; median OS 12.5 |
| Brexucabtagene autoleucel | 77 | Phase 2 | 74 | 65 | NA | 3 (1-5) | ORR 38% | ORR 93% CR 67% | — | 1-y PFS 61% 1-y OS 83% |
| Lisocabtagene maraleucel | 53 | Phase 1 | 41 | 67 | NA | 3 (1-7) | ORR 66% | ORR 84% CR 59% | — | NA |
| Pirtobrutinib | 85 | Phase 1/2 | 61 | 69 | NA | 3 (2-4) | NA | ORR 52% CR 25% | NA | NA |
| Zilovertamab vedotin | 86 | Phase 1 | 15 | 70|| | NA | 4 (1-24)|| | NA | ORR 47% CR 13% | NA | NA |
DOI, duration of ibrutinib; DOR, duration of response; NA, not available.
Defined by Mantle cell lymphoma international prognostic index (MIPI) or sMIPI (simplified MIPI).
Survival time taken from date of last ibrutinib treatment, not the start of the next-line therapy.
All but 1 patient enrolled retrospectively.
22/24 BTKi exposed.
For all patients in the study; MCL subset was not reported separately.
Venetoclax is a potent oral inhibitor targeting the antiapoptotic BCL-2 protein. Eyre et al described the outcomes of venetoclax monotherapy as part of a compassionate access program in the United Kingdom.68 Twenty heavily pretreated covalent BTKi-resistant patients with MCL (n = 18 ibrutinib), who had an ORR to BTKi of 55%, received venetoclax monotherapy. Four patients had blastoid histology and most discontinued BTKi because of progressive disease. Most patients achieved a target dose of 800 to 1200 mg. Laboratory tumor lysis syndrome was observed in 4 (20%) patients, but clinical tumor lysis syndrome was not observed. The ORR was 53%; however, the median PFS was a disappointing 3.2 months, with median OS 9.4 months. Zhao et al69 also reported outcomes to venetoclax in 24 patients with R/R MCL after 5 prior therapies, among whom 67% were refractory to BTKi. ORR was 50% (CR, 21%) and the PFS was 8 months. Whole-exome sequencing noted BCL-2 mutations in only one-third of patients. Mutations in SMARCA4, TP53, CDKN2A, KMT2D, CELSR3, CCND1, and NOTCH2 were found in samples after progression on venetoclax. Newer generations of agents targeting BCL-2 are in rapid clinical development.70 The limited durability of responses suggests that the role of BCL2i in MCL is likely to be as part of multiagent combinations.
The most active chemoimmunotherapy regimen in covalent BTKi-resistant MCL is R-BAC. In a United Kingdom-Italian retrospective study, McCulloch et al described the outcomes of 36 patients with covalent BTK-resistant MCL (86% ibrutinib).71 Most patients (median, 2 prior lines; median age, 66 years) received the R-BAC500 protocol,72 with the intention of delivering 4 to 6 cycles. Dose reductions related to toxicity were required in 56% of the patients overall, and 90% were in ≥70 years. Hospitalization occurred in 50%, mostly because of neutropenic sepsis. Despite the considerable toxicity, the ORR was 83% (CR, 60%) with median PFS 10.1 months and OS 12.5 months. Selection bias and retrospective study design notwithstanding, R-BAC appears an encouraging and widely applicable option in this setting. Favorable outcomes were observed among the 11 patients in whom allo-SCT was performed as consolidation, with a 1-year PFS in 76%. Allo-SCT appears to be a highly active and potentially curative intervention in patients with MCL experiencing covalent BTKi failure; however, the optimal bridging strategy remains undefined.73, 74
CAR T-cell therapy
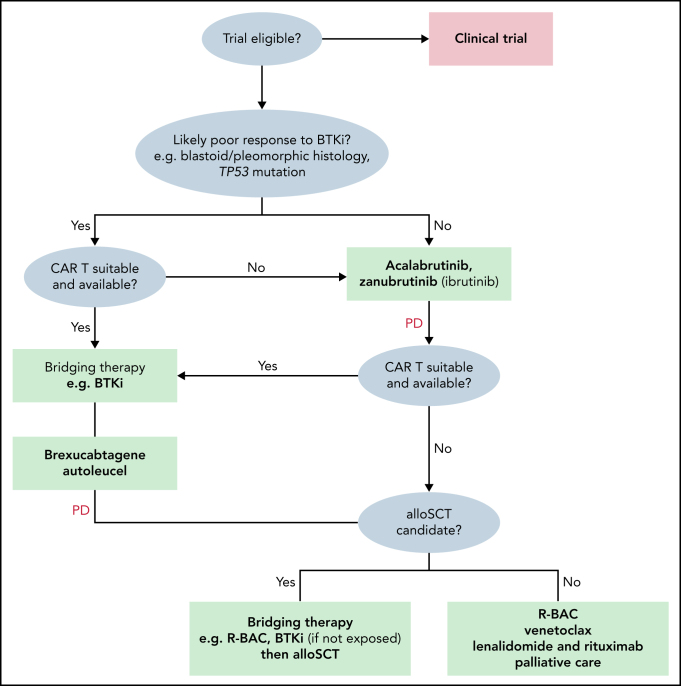
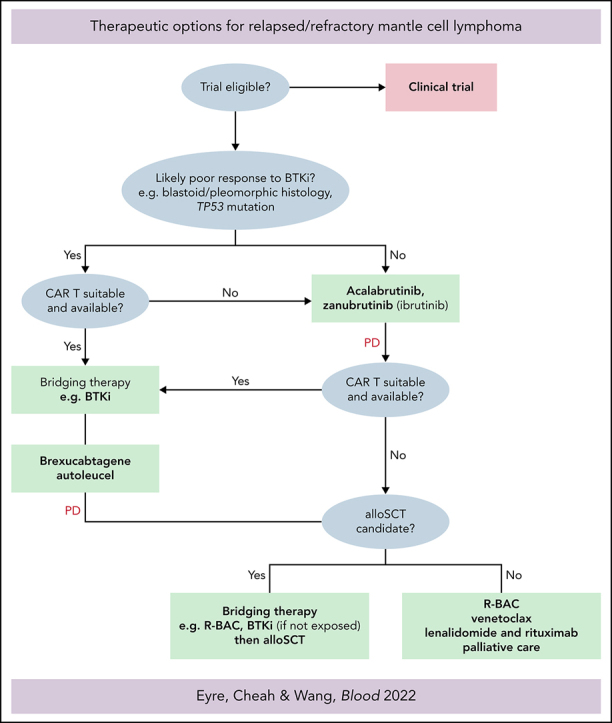
CAR T-cell therapy represents a major advance in malignant hematology. Mature data from a large, heavily pretreated DLBCL population from ZUMA-1 suggests very durable CRs in 30% to 40%.75 Brexucabtagene autoleucel (Tecartus, KTE-X19) is an anti-CD19 CAR T-cell therapy (CD28 costimulatory domain) approved by the FDA in July 2020 for all patients with R/R MCL and by the European Medical Agency in January 2021 for R/R MCL after ≥2 prior lines including a covalent BTKi. The international, multicenter, phase 2 ZUMA-2 trial76 enrolled patients with MCL after ≥3 prior lines including a prior covalent BTKi. Patients underwent leukapheresis, lymphocyte-depleting chemotherapy, and a single IV infusion of 2 × 106 CAR T cells per kilogram. Among 68 patients, 88% (n = 60) patients were R/R after ibrutinib or acalabrutinib or both, 25% (n = 17) had blastoid morphology, 47% (n = 32) had Ki67 ≥50%, and 9% (n = 6) had a TP53 mutation. The ORR was 93% (CR 67%) and 57% remained in remission after 12 months' follow-up. Grade ≥3 cytokine release syndrome (CRS), neurotoxicity, and infections were 15%, 31%, and 32%, respectively, and there were 2 grade 5 infections. With recently reported follow-up of 17.5 months, substantial and durable benefit of KTE-X19 was recorded. ORR was 92% (67% CR), with ongoing durable responses in 48% of all efficacy-evaluable patients at the data cutoff; 70% of those who achieved CR continue in response.77 An analysis in ZUMA-2 has also been performed according to patient POD24 status. Although ORR, CR rates, and safety end points were equivalent in POD24 and non-POD24 cohorts, the median PFS was substantially shorter in the POD24 group (11 vs 29 months), and CAR T-cell expansion was reduced. Explanation of these findings, including resistance mechanisms to anti-CD19 CAR T cells in R/R MCL remain to be fully determined.78 All 6 R/R MCL patients with aberrant TP53 who were efficacy-evaluable in ZUMA-2 achieved CR with CAR T-cell therapy.78 Given the difference in EMA and FDA labels, KTE-X19 may be used in a slightly different manner, according to the label. Whereas the label is broader in R/R MCL, it is reasonable to consider KTE-X19 in covalent BTKi-naive patients with high-risk features including blastoid morphology, high Ki-67% and TP53 mutation (Figure 1).
Figure 1.
Treatment algorithm for patients with R/R MCL.
Lisocabgene maraleucel (Liso-cel) is an anti-CD19 CAR T-cell therapy that involves 4-1BB as costimulatory molecule. In the ongoing TRANSCEND NHL001 study,53 Liso-cel has demonstrated promising clinical activity and a notably low incidence of grade ≥3 CRS and neurotoxicity. Among 32 patients with R/R MCL with a follow-up of 6 months, liso-cel resulted in an ORR of 84% (CR, 59%). The median duration of response was not reached. Grade ≥3 CRS and neurotoxicity events in 3% and 12.5%, respectively. Episodes of prolonged grade ≥3 cytopenias and grade ≥3 infections were relatively few, occurring in 34% and 16%, respectively.
The optimum therapeutic pathway in patients experiencing relapse after CAR T-cell therapy is currently unclear and under active investigation. Sustained cytopenias, poor performance status, and rapid disease kinetics may limit the ability to perform clinical trials in this area, and the evidence base at the moment remains limited.
MCL bridging to CAR T-cell therapy
High CAR T-cell preinfusion tumor volume is associated with reduced responses and early relapse after CAR T-cell therapy in NHL. The optimum bridging strategies remain undetermined in R/R MCL and require further study. Patients may benefit from continuing covalent BTKi beyond disease progression, to avoid disease flare. Other options may include radiation of localized disease bulk, non–cross-reactive immunochemotherapy or other experimental nonchemotherapeutic approaches. Although R-BAC is a valuable third-line option after covalent BTKi, use of bendamustine before apheresis is generally discouraged.
Allo-SCT vs CAR T cell
Whether allo-SCT after covalent BTKi bridging or CAR T-cell therapy is used first remains a subject of contention. Data supporting allo-SCT in this setting are based on a small contemporary European Society for Blood and Marrow Transplantation (EBMT) cohort79 (n = 22) that demonstrated an impressive 1-year PFS of 76% and a low (5%) nonrelapse mortality (NRM). EBMT data80 from the pre-BTKi era from 324 patients with R/R MCL treated with allo-SCT described a 1-year NRM of 24%, and a 4-year PFS of only 31% with a 1-year cumulative incidence of chronic GVHD of >40%. Allo-SCT may also have a role in TP53-mutated MCL. A small series (n = 42) suggested that survival outcomes were broadly equivalent in patients with TP53 mutations who have patients with R/R MCL and undergo allo-SCT, compared with those without TP53 mutation.81
Advantages of allo-SCT remain the long-term follow-up data and clear evidence of graft-versus-lymphoma effect. Advantages of CAR T-cell therapy are the demonstrable efficacy in resistant and active high-risk MCL, the lack of need for an allogenic donor, the low NRM, and the lower longer-term toxicity. Recent expert international consensus82 from the American Society for Transplantation and Cellular Therapy (ASTCT), Center for International Blood and Marrow Transplant Research (CIBMTR), and EBMT recommend offering CAR T-cell therapy before allo-SCT, when available (Figure 1). The efficacy and deliverability of allo-SCT after the failure of CAR T-cell therapy in R/R MCL is currently unknown.
Novel agents in development
Patients who develop disease progression or primary resistance to covalent BTKi remain a group with a significant unmet need. Numerous ongoing preclinical and clinical studies are actively investigating approaches in this area. A summary of some of the most advanced clinical developments follows.
Pirtobrutinib
Pirtobrutinib, formally LOXO-305, is a first in-class noncovalent, reversible, highly specific BTKi designed to be active in patients with mutations associated with covalent BTKi resistance, such as the C481S BTK binding site mutation.83 In the phase 1/2 BRUIN trial84, 85 of advanced B-cell malignancies, no dose-limiting toxicities were observed, and the maximum tolerated dose was not reached. The recommended phase 2 dose was 200 mg once daily. Tolerability across all 323 patients studied to date was excellent, albeit with relatively short follow-up. The most common AEs observed to date are fatigue (grade 1-2, 19%; grade 3, 1%), diarrhea (grade 1-2, 17% only), contusion (grade 1-2, 14% only), and neutropenia (grade 1-2, 3%; grade 3-4 10%). The rate of adverse events of special interest associated with covalent BTKi are also minimal. For example, atrial fibrillation has occurred in 2 patients (both grade 2, <1%), any grade hypertension in 5% (grade 3, 1%), rash in 11% (all grade 1-2), and arthralgia in 11% (all grade 1-2). The overall discontinuation rate related to treatment-related AEs is 1.5%.
In 56 efficacy-evaluable patients with R/R MCL after a median of 3 prior therapies, the ORR was 52% (CR, 25%). Among 52 patients who were exposed to a covalent BTKi, also after a median 3 prior therapies, the ORR was also 52%. Responses were observed in 2 patients after CAR T-cell therapy. Follow-up remains relatively short and needs to mature before a full understanding of the durability of benefit is reached. These data indicate that pirtobrutinib can overcome covalent BTKi resistance. The mechanisms by which this occurs remains incompletely understood and are under current investigation. Investigator's choice covalent BTKi monotherapy (ibrutinib, zanubrutinib, or acalabrutinib) is currently being compared with pirtobrutinib monotherapy in the randomized phase 3 superiority trial BRUIN-MCL321 (NCT04662255).
Zilovertamab vedotin
Receptor tyrosine kinase-like orphan receptor 1 (ROR1) is an oncoprotein that is physiologically expressed during embryogenesis, but is absent in normal human adult tissue. ROR1 is then pathologically reexpressed on several solid cancer and lymphoma tissues, including MCL. Zilovertamab (ZV) is an antibody-drug conjugate, that has an mAb that specifically binds to ROR1, a cleavable linker, and the antimicrotubule cytotoxin, monomethyl auristatin E (MMAE). ZV was administered IV every 3 weeks in a phase 1 first-in-human trial.86 Thirty-two patients with B-cell malignancies after a median of 4 prior therapies were enrolled. As expected with MMAE, adverse events included neutropenia and cumulative peripheral neuropathy. In 15 patients with R/R MCL with prior covalent BTKi exposure enrolled, the ORR was 47% (CR 13%), providing an early and promising proof-of-concept of a novel target in R/R MCL that will be further studied.
Bispecific antibodies
Anti-CD20/CD3 bispecific antibodies are in rapid clinical development. Several slightly different molecules are in advanced stages of phase 1 to phase 3 clinical trials, as monotherapy and in combinations. The major focus in the initial phases of development has been in R/R DLBCL and follicular lymphoma, with early impressive efficacy obtained with glofitamab,87 odronextamab,88 mosunetuzumab,89, 90 and epcoritamab.91 In contrast, outcomes in relatively small samples of treated patients with R/R MCL have been presented within larger cohorts with aggressive B-cell NHL (glofitamab, n = 6, all complete molecular remission; odronextamab, n = 6, ORR 67%, CR 33%; mosunetuzumab n = 3, ORR not reported; and epcoritamab n = 4, ORR 50%, CR 25%). Early efficacy in these small cohorts is encouraging, however; immune-related toxicities such as CRS and neurotoxicity are relatively low compared with CAR T-cell therapy. It is likely that further clinical development in R/R MCL will follow.
Final perspective
Despite the durable remissions observed after chemoimmunotherapy with or without auto-SCT, MCL relapse appears unavoidable. Although covalent BTKis provide excellent disease control for most patients, a high-risk subset characterized by POD24, TP53 aberrations, and blastoid histology experience limited benefit. Further, many patients with progression after covalent BTKis also have poor outcomes. For younger, fitter patients, anti-CD19 CAR T-cell therapy appears likely to replace allo-SCT. However, the toxicities of the current generation of agents are considerable and limit their application in a significant proportion of the older, frailer population. The development of agents such as noncovalent BTKis, BCL2is, CD20/CD3 bispecific antibodies, and novel antibody-drug conjugates, either alone or in combination, are likely to provide useful options for patients with R/R MCL. Given the rarity of the disease, we strongly encourage enrollment in clinical trials evaluating novel agents wherever possible.
Acknowledgments
T.A.E. recognizes support from the Oxford National Institutes for Health Research (NIHR) Biomedical Research Centre.
Footnotes
Eyre et al review the current state of the art of the treatment of mantle cell lymphoma that relapses after immunochemotherapy and autologous stem cell transplantation. They discuss Bruton tyrosine kinase (BTK) inhibitors and the mechanisms of BTK inhibitor resistance, chimeric antigen receptor T-cell therapy, and subsequent novel therapies.
Contributor Information
Chan Y. Cheah, Email: chan.cheah@health.wa.gov.au.
Michael L. Wang, Email: miwang@mdanderson.org.
Authorship
Contribution: T.A.E., C.Y.C., and M.L.W. all cowrote and edited the manuscript.
Conflict-of-interest disclosure: T.A.E. has received honoraria and/or advisory board honoraria from Roche, Gilead, KITE, Janssen, AbbVie, AstraZeneca, LOXO Oncology, Beigene, Incyte, and Secura Bio, receives research support from Gilead, Beigene, and AstraZeneca, has received travel support from Gilead, Takeda, and AbbVie, and served on a trial steering committee for LOXO Oncology. C.Y.C. has received consulting and/or advisory board honoraria from Roche, Janssen, MSD, Gilead, Ascentage Pharma, AstraZeneca, Lilly, TG therapeutics, Beigene, Novartis, and BMS; receives research support from BMS, Roche, AbbVie, and MSD; has received travel expenses from Roche and served on a trial steering committee for LOXO Oncology. M.L.W. has received consulting and/or advisory board honoraria from AstraZeneca, Bayer Healthcare, Beigene, CSTone DTRM Biopharma, (Cayman) Limited, Epizyme, Genentech, InnoCare, Janssen, Juno, Kite Pharma, Loxo Oncology, Miltenyi Biomedicine GmbH Oncternal, Pharmacyclics, VelosBio, Acerta Pharma, Anticancer Association, CAHON, Chinese Medical Association, Clinical Care Options, Dava Oncology, Hebei Cancer Prevention Federation, Imedex, Janssen, Moffit Cancer Center, Mumbai Hematology Group, Newbridge Pharmaceuticals, OMI, Physicians Education Resources (PER), Scripps, The First Afflicted Hospital of Zhejiang University, and BGICS. M.L.W. receives research support from Acerta Pharma, AstraZeneca, Beigene, BioInvent, Celgene, Innocare, Janssen, Juno, Kite Pharma, Lilly, Loxo Oncology, Molecular Templates, Oncternal, Pharmacyclics, and VelosBio.
REFERENCES
- 1.Geisler CH, Kolstad A, Laurell A, et al. Nordic Lymphoma Group Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Gouill S, Thieblemont C, Oberic L, et al. LYSA Group Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377(13):1250–1260. doi: 10.1056/NEJMoa1701769. [DOI] [PubMed] [Google Scholar]
- 3.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520–531. doi: 10.1056/NEJMoa1200920. [DOI] [PubMed] [Google Scholar]
- 4.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle cell lymphoma (MCL): long-term follow-up of the randomized European MCL Elderly Trial. J Clin Oncol. 2020;38(3):248–256. doi: 10.1200/JCO.19.01294. [DOI] [PubMed] [Google Scholar]
- 5.Rummel MJ, Niederle N, Maschmeyer G, et al. Study group indolent Lymphomas (StiL) Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 6.Robak T, Huang H, Jin J, et al. LYM-3002 Investigators Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372(10):944–953. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- 7.Robak T, Jin J, Pylypenko H, et al. LYM-3002 investigators Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(11):1449–1458. doi: 10.1016/S1470-2045(18)30685-5. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Salles G, Kumar A, et al. Role of maintenance rituximab after first-line bendamustine + rituximab or R-CHOP in patients with mantle cell lymphoma from a large US real-world cohort [abstract] Hematol Oncol. 2021;39 S2 Abstract hon.61_2880. [Google Scholar]
- 9.Y, amshon S, Martin P, Shah B, et al. Initial treatment with lenalidomide plus rituximab for mantle cell lymphoma (MCL): 7-year analysis from a multi-center phase 2 study [oral abstract] Blood. 2020;136:45–46. suppl 1. [Google Scholar]
- 10.Ruan J, Martin P, Shah B, et al. Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. N Engl J Med. 2015;373(19):1835–1844. doi: 10.1056/NEJMoa1505237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain P, Lee HJ, Steiner RE, et al. Frontline treatment with ibrutinib with rituximab (IR) combination is highly effective in elderly (≥65 years) patients with mantle cell lymphoma (MCL) - results from a phase 2 trial [abstract] Blood. 2019;134 suppl_1. Abstract 3988. [Google Scholar]
- 12.Phillips TJ, Bond D, Devata S, et al. The combination of venetoclax, lenalidomide and rituximab in patients with newly diagnosed mantle cell lymphoma induces high response rates and MRD undetectability [abstract] Hematol Oncol. 2021;39 S2. Abstract hon.61_2879. [Google Scholar]
- 13.Le Gouill S, Morschhauser F, Chiron D, et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial. Blood. 2021;137(7):877–887. doi: 10.1182/blood.2020008727. [DOI] [PubMed] [Google Scholar]
- 14.Visco C, Di Rocco A, Evangelista A, et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: results from the MANTLE-FIRST study [published correction appears in Leukemia. 2021;35(3):932] Leukemia. 2021;35(3):787–795. doi: 10.1038/s41375-020-01013-3. [DOI] [PubMed] [Google Scholar]
- 15.Rampotas A, Wilson MR, Lomas O, et al. Treatment patterns and outcomes of unfit and elderly patients with Mantle cell lymphoma unfit for standard immunochemotherapy: a UK and Ireland analysis. Br J Haematol. 2021;194(2):365–377. doi: 10.1111/bjh.17513. [DOI] [PubMed] [Google Scholar]
- 16.Visco C, Tisi MC, Evangelista A, et al. Fondazione Italiana Linfomi and the Mantle Cell Lymphoma Network Time to progression of mantle cell lymphoma after high-dose cytarabine-based regimens defines patients risk for death. Br J Haematol. 2019;185(5):940–944. doi: 10.1111/bjh.15643. [DOI] [PubMed] [Google Scholar]
- 17.McCulloch R, Lewis D, Crosbie N, et al. Ibrutinib for mantle cell lymphoma at first relapse: a United Kingdom real-world analysis of outcomes in 211 patients. Br J Haematol. 2021;193(2):290–298. doi: 10.1111/bjh.17363. [DOI] [PubMed] [Google Scholar]
- 18.Halldórsdóttir AM, Lundin A, Murray F, et al. Impact of TP53 mutation and 17p deletion in mantle cell lymphoma. Leukemia. 2011;25(12):1904–1908. doi: 10.1038/leu.2011.162. [DOI] [PubMed] [Google Scholar]
- 19.Rule S, Dreyling M, Goy A, et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica. 2019;104(5):e211–e214. doi: 10.3324/haematol.2018.205229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903–1910. doi: 10.1182/blood-2017-04-779736. [DOI] [PubMed] [Google Scholar]
- 21.Geisler CH, Kolstad A, Laurell A, et al. Nordic Lymphoma Group Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol. 2012;158(3):355–362. doi: 10.1111/j.1365-2141.2012.09174.x. [DOI] [PubMed] [Google Scholar]
- 22.Greenwell IB, Staton AD, Lee MJ, et al. Complex karyotype in patients with mantle cell lymphoma predicts inferior survival and poor response to intensive induction therapy. Cancer. 2018;124(11):2306–2315. doi: 10.1002/cncr.31328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiestner A. Targeting B-Cell receptor signaling for anticancer therapy: the Bruton's tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31(1):128–130. doi: 10.1200/JCO.2012.44.4281. [DOI] [PubMed] [Google Scholar]
- 24.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–778. doi: 10.1016/S0140-6736(15)00667-4. [DOI] [PubMed] [Google Scholar]
- 26.Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):48–56. doi: 10.1016/S1470-2045(15)00438-6. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391(10121):659–667. doi: 10.1016/S0140-6736(17)33108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Rule S, Zinzani PL, et al. Acalabrutinib monotherapy in patients with relapsed/refractory mantle cell lymphoma: final results from a phase 2 study [abstract] Hematol Oncol. 2021;39:2880. S2. [Google Scholar]
- 29.Furman RR, Byrd JC, Owen RG, et al. Pooled analysis of safety data from clinical trials evaluating acalabrutinib monotherapy in mature B-cell malignancies. Leukemia. 2021;35(11):3201–3211. doi: 10.1038/s41375-021-01252-y. [DOI] [PubMed] [Google Scholar]
- 30.So, ng Y, Zhou K, Zou D, et al. Treatment of patients with relapsed or refractory mantle-cell lymphoma with zanubrutinib, a selective inhibitor of Bruton's tyrosine kinase. Clin Cancer Res. 2020;26(16):4216–4224. doi: 10.1158/1078-0432.CCR-19-3703. [DOI] [PubMed] [Google Scholar]
- 31.Song Y, Zhou K, Zou D, et al. Zanubrutinib (Zanu) in patients (Pts) with relapsed/refractory (R/R) mantle cell lymphoma (Mcl): long-term efficacy and safety results from a phase 2 study [abstract] Eur Hematol Assoc. 2021 Abstract EP789. [Google Scholar]
- 32.Tam CS, Opat S, Simpson D, et al. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2021;5(12):2577–2585. doi: 10.1182/bloodadvances.2020004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillmen P, Byrd JC, Ghia P, et al. First results of a head-to-head trial of acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia [abstract] Hematol Oncol. 2021;39 doi: 10.1200/JCO.21.01210. S2. Abstract hon.33_2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam CS, Opat S, D'Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136(18):2038–2050. doi: 10.1182/blood.2020006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillmen P, Eichhorst B, Brown JR, et al. First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma [abstract] Eur Hematol Assoc. 2021 Abstract LB1900. [Google Scholar]
- 36.Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol. 2018;5(3):e109–e116. doi: 10.1016/S2352-3026(18)30018-8. [DOI] [PubMed] [Google Scholar]
- 37.Novak U, Fehr M, Schär S, et al. SAKK 36/13 – ibrutinib plus bortezomib and ibrutinib maintenance for relapsed and refractory mantle cell lymphoma: final report of a Phase I/II trial of the European MCL Network. Hematol Oncol. 2021;39:2879. S2. [Google Scholar]
- 38.Davids MS, Kim HT, Nicotra A, et al. Blood Cancer Research Partnership of the Leukemia and Lymphoma Society Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: a multicentre phase 1-1b study. Lancet Haematol. 2019;6(1):e38–e47. doi: 10.1016/S2352-3026(18)30196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin P, Bartlett NL, Blum KA, et al. A phase 1 trial of ibrutinib plus palbociclib in previously treated mantle cell lymphoma [published correction appears in Blood. 2019;134(11):908] Blood. 2019;133(11):1201–1204. doi: 10.1182/blood-2018-11-886457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378(13):1211–1223. doi: 10.1056/NEJMoa1715519. [DOI] [PubMed] [Google Scholar]
- 41.Trněný M, Lamy T, Walewski J, et al. SPRINT trial investigators and in collaboration with the European Mantle Cell Lymphoma Network Lenalidomide versus investigator's choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncol. 2016;17(3):319–331. doi: 10.1016/S1470-2045(15)00559-8. [DOI] [PubMed] [Google Scholar]
- 42.Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20(3):520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hess G, Herbrecht R, Romaguera J, et al. Phase 3 study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. 2009;27(23):3822-3829. [DOI] [PubMed]
- 44.Cheah CY, George A, Giné E, et al. European Mantle Cell Lymphoma Network Central nervous system involvement in mantle cell lymphoma: clinical features, prognostic factors and outcomes from the European Mantle Cell Lymphoma Network. Ann Oncol. 2013;24(8):2119–2123. doi: 10.1093/annonc/mdt139. [DOI] [PubMed] [Google Scholar]
- 45.Conconi A, Franceschetti S, Lobetti-Bodoni C, et al. Risk factors of central nervous system relapse in mantle cell lymphoma. Leuk Lymphoma. 2013;54(9):1908–1914. doi: 10.3109/10428194.2013.767454. [DOI] [PubMed] [Google Scholar]
- 46.Chihara D, Asano N, Ohmachi K, et al. Ki-67 is a strong predictor of central nervous system relapse in patients with mantle cell lymphoma (MCL) Ann Oncol. 2015;26(5):966–973. doi: 10.1093/annonc/mdv074. [DOI] [PubMed] [Google Scholar]
- 47.Ghesquieres H, Chevrier M, Laadhari M, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective ‘proof of concept' phase 2 study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA) Ann Oncol. 2019;30(4):621–628. doi: 10.1093/annonc/mdz032. [DOI] [PubMed] [Google Scholar]
- 48.Grommes C, Tang SS, Wolfe J, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood. 2019;133(5):436–445. doi: 10.1182/blood-2018-09-875732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the phase 2 ‘proof-of-concept' iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–130. doi: 10.1016/j.ejca.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 50.Rusconi C, Cheah CY, Tucker D, et al. Ibrutinib compared to immuno-chemotherapy for central nervous system relapse of mantle cell lymphoma: a report from Fondazione Italiana Linfomi (FIL) and European Mantle Cell Lymphoma Network (EMCLN) [abstract] Eur Hematol Assoc. 2020 Abstract S229. [Google Scholar]
- 51.Ahmed G, Hamadani M, Shah NN. CAR T-cell therapy for secondary CNS DLBCL [published online ahead of print 22 September 2021] Blood Adv. 2021 doi: 10.1182/bloodadvances.2021005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siddiqi T, Wang X, Blanchard MS, et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv. 2021;5(20):4059–4063. doi: 10.1182/bloodadvances.2020004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palomba ML, Gordon LI, Siddiqi T, et al. Safety and preliminary efficacy in patients with relapsed/refractory mantle cell lymphoma receiving lisocabtagene maraleucel in transcend NHL 001. Blood. 2020;136:10–11. suppl 1. [Google Scholar]
- 54.Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127(12):1559–1563. doi: 10.1182/blood-2015-10-673145. [DOI] [PubMed] [Google Scholar]
- 55.Cheah CY, Chihara D, Romaguera JE, et al. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol. 2015;26(6):1175–1179. doi: 10.1093/annonc/mdv111. [DOI] [PubMed] [Google Scholar]
- 56.Epperla N, Hamadani M, Cashen AF, et al. Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma-a “real world” study. Hematol Oncol. 2017;35(4):528–535. doi: 10.1002/hon.2380. [DOI] [PubMed] [Google Scholar]
- 57.Tucker D, Morley N, MacLean P, et al. The 5-year follow-up of a real-world observational study of patients in the United Kingdom and Ireland receiving ibrutinib for relapsed/refractory mantle cell lymphoma. Br J Haematol. 2021;192(6):1035–1038. doi: 10.1111/bjh.16739. [DOI] [PubMed] [Google Scholar]
- 58.Wang M, Rule S, Zinzani PL, et al. Durable response with single-agent acalabrutinib in patients with relapsed or refractory mantle cell lymphoma. Leukemia. 2019;33(11):2762–2766. doi: 10.1038/s41375-019-0575-9. [DOI] [PubMed] [Google Scholar]
- 59.Woyach JA, Furman RR, Liu T-M, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370(24):2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahal R, Frick M, Romero R, et al. Pharmacological and genomic profiling identifies NF-κB-targeted treatment strategies for mantle cell lymphoma. Nat Med. 2014;20(1):87–92. doi: 10.1038/nm.3435. [DOI] [PubMed] [Google Scholar]
- 61.Wu C, de Miranda NF, Chen L, et al. Genetic heterogeneity in primary and relapsed mantle cell lymphomas: impact of recurrent CARD11 mutations. Oncotarget. 2016;7(25):38180–38190. doi: 10.18632/oncotarget.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohanty A, Sandoval N, Das M, et al. CCND1 mutations increase protein stability and promote ibrutinib resistance in mantle cell lymphoma. Oncotarget. 2016;7(45):73558–73572. doi: 10.18632/oncotarget.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balsas P, Palomero J, Eguileor Á, et al. SOX11 promotes tumor protective microenvironment interactions through CXCR4 and FAK regulation in mantle cell lymphoma. Blood. 2017;130(4):501–513. doi: 10.1182/blood-2017-04-776740. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Yao Y, Zhang S, et al. Metabolic reprogramming toward oxidative phosphorylation identifies a therapeutic target for mantle cell lymphoma. Sci Transl Med. 2019;11(491):eaau1167. doi: 10.1126/scitranslmed.aau1167. [DOI] [PubMed] [Google Scholar]
- 65.Zhao X, Wang MY, Jiang H, et al. Transcriptional programming drives Ibrutinib-resistance evolution in mantle cell lymphoma. Cell Rep. 2021;34(11):108870. doi: 10.1016/j.celrep.2021.108870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang S, Jiang VC, Han G, et al. Longitudinal single-cell profiling reveals molecular heterogeneity and tumor-immune evolution in refractory mantle cell lymphoma. Nat Commun. 2021;12(1):2877. doi: 10.1038/s41467-021-22872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang M, Schuster SJ, Phillips T, et al. Observational study of lenalidomide in patients with mantle cell lymphoma who relapsed/progressed after or were refractory/intolerant to ibrutinib (MCL-004) J Hematol Oncol. 2017;10(1):171. doi: 10.1186/s13045-017-0537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eyre TA, Walter HS, Iyengar S, et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica. 2019;104(2):e68–e71. doi: 10.3324/haematol.2018.198812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao S, Kanagal-Shamanna R, Navsaria L, et al. Efficacy of venetoclax in high risk relapsed mantle cell lymphoma (MCL) – outcomes and mutation profile from venetoclax resistant MCL patients. Am J Hematol. 2020;95(6):623–629. doi: 10.1002/ajh.25796. [DOI] [PubMed] [Google Scholar]
- 70.Cheah CY, Verner E, Tam CS, et al. Preliminary safety data from patients (pts) with relapsed/refractory (R/R) B-cell malignancies treated with the novel B-cell lymphoma 2 (bcl2) inhibitor BGB-11417 [abstract] Hematol Oncol. 2021;39 S2. Abstract hon.85_2881. [Google Scholar]
- 71.McCulloch R, Visco C, Eyre TA, et al. Efficacy of R-BAC in relapsed, refractory mantle cell lymphoma post BTK inhibitor therapy. Br J Haematol. 2020;189(4):684–688. doi: 10.1111/bjh.16416. [DOI] [PubMed] [Google Scholar]
- 72.Visco C, Chiappella A, Nassi L, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol. 2017;4(1):e15–e23. doi: 10.1016/S2352-3026(16)30185-5. [DOI] [PubMed] [Google Scholar]
- 73.Lewis D, McCulloch R, Eyre T, et al. Allogeneic stem cell transplantation can be successfully delivered following BTKI therapy failure following R-bac Re-induction chemotherapy in patients with mantle cell lymphoma. Bone Marrow Transplant. 2020;55:514–515. suppl 1. [Google Scholar]
- 74.Marangon M, Visco C, Barbui AM, et al. Allogeneic stem cell transplantation in mantle cell lymphoma in the era of new drugs and CAR-T cell therapy. Cancers (Basel). 2021;13(2):291. doi: 10.3390/cancers13020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang M, Munoz J, Goy AH, et al. One-year follow-up of ZUMA-2, the multicenter, registrational study of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma [poster abstract] Blood. 2020;136:20–22. suppl 1. [Google Scholar]
- 78.Wang M, Munoz J, Goy A, et al. Outcomes with KTE-X19 in patients (pts) with relapsed/refractory (R/R) mantle cell lymphoma (MCL) in ZUMA-2 who had progression of disease within 24 months of diagnosis (POD24) J Clin Oncol. 2021;39:7547. 15 suppl. [Google Scholar]
- 79.Dreger P, Michallet M, Bosman P, et al. Ibrutinib for bridging to allogeneic hematopoietic cell transplantation in patients with chronic lymphocytic leukemia or mantle cell lymphoma: a study by the EBMT Chronic Malignancies and Lymphoma Working Parties. Bone Marrow Transplant. 2019;54(1):44–52. doi: 10.1038/s41409-018-0207-4. [DOI] [PubMed] [Google Scholar]
- 80.Robinson SP, Boumendil A, Finel H, et al. Long-term outcome analysis of reduced-intensity allogeneic stem cell transplantation in patients with mantle cell lymphoma: a retrospective study from the EBMT Lymphoma Working Party. Bone Marrow Transplant. 2018;53(5):617–624. doi: 10.1038/s41409-017-0067-3. [DOI] [PubMed] [Google Scholar]
- 81.Lin RJ, Ho C, Hilden PD, et al. Allogeneic haematopoietic cell transplantation impacts on outcomes of mantle cell lymphoma with TP53 alterations. Br J Haematol. 2019;184(6):1006–1010. doi: 10.1111/bjh.15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Munshi PN, Hamadani M, Kumar A, et al. ASTCT, CIBMTR, and EBMT clinical practice recommendations for transplant and cellular therapies in mantle cell lymphoma. Bone Marrow Transplant. 2021 doi: 10.1038/s41409-021-01288-9. https://doi.org/10.1038/s41409-021-01288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Woyach JA, Ruppert AS, Guinn D, et al. BTKC481S-Mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol. 2017;35(13):1437–1443. doi: 10.1200/JCO.2016.70.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang M, Shah NN, Alencar AJ, et al. LOXO-305, a next generation, highly selective, non-covalent BTK inhibitor in previously treated mantle cell lymphoma, Waldenström's macroglobulinemia, and other non-Hodgkin lymphomas: results from the phase 1/2 BRUIN study [oral abstract] Blood. 2020;136:8–10. suppl 1. [Google Scholar]
- 85.Mato AR, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892–901. doi: 10.1016/S0140-6736(21)00224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang M, Barrientos JC, Furman RR, et al. VLS-101, a ROR1-targeting antibody-drug conjugate, demonstrates a predictable safety profile and clinical efficacy in patients with heavily pretreated mantle cell lymphoma and diffuse large B-cell lymphoma [oral abstract] Blood. 2020;136:13–14. suppl 1. [Google Scholar]
- 87.Hutchings M, Morschhauser F, Iacoboni G, et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol. 2021;39(18):1959–1970. doi: 10.1200/JCO.20.03175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bannerji R, Allan JN, Arnason JE, et al. Odronextamab (REGN1979), a human CD20 x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-Hodgkin lymphoma, including patients refractory to CAR T therapy [oral abstract] Blood. 2020;136:42–43. suppl 1. [Google Scholar]
- 89.Budde LE, Sehn LH, Assouline S, et al. Mosunetuzumab, a full-length bispecific CD20/CD3 antibody, displays clinical activity in relapsed/refractory B-cell non-Hodgkin lymphoma (NHL): interim safety and efficacy results from a phase 1 study [abstract] Blood. 2018;132 suppl 1. Abstract LP-399. [Google Scholar]
- 90.Schuster SJ, Bartlett NL, Assouline S, et al. Mosunetuzumab induces complete remissions in poor prognosis non-Hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, and is active in treatment through multiple lines [abstract] Blood. 2019;134 suppl_1. Abstract 6. [Google Scholar]
- 91.Clausen MR, Lugtenburg P, Hutchings M, et al. Subcutaneous epcoritamab in patients with relapsed/refractory B-cell non-Hodgkin lymphoma: safety profile and antitumor activity [abstract] J Clin Oncol. 2021;39 15 suppl. Abstract 7518. [Google Scholar]