Abstract
Group II phospholipase A2 (PLA2) is a newly recognized antibacterial acute-phase protein. Recently we observed that transgenic mice expressing group II PLA2 (PLA2+ mice) were able to resist experimental Staphylococcus aureus infection by killing the bacteria, as indicated by improved survival and by the small numbers of live bacteria in their tissues (V. J. O. Laine, D. S. Grass, and T. J. Nevalainen, J. Immunol. 162:7402–7408, 1999). To establish the role of group II PLA2 in Escherichia coli infection, the host responses of PLA2+ mice and their PLA2-deficient C57BL/6J littermates (PLA2− mice) were studied after intraperitoneal administration of E. coli. The levels of group II PLA2 in sera of PLA2+ mice increased after the administration of E. coli, and the concentration of group II PLA2 correlated significantly with the catalytic activity of PLA2 in serum. PLA2+ mice showed lower rates of mortality and less bacterial growth in peritoneal lavage fluid, blood, and spleen and liver tissues than PLA2− mice. Unlike the observations with staphylococcal infection, serum and peritoneal lavage fluid did not inhibit the growth of E. coli in vitro. The results indicate that expression of the group II PLA2 transgene improves the host defense of mice against E. coli infection.
Phospholipase A2 (PLA2; EC 3.1.1.4) catalyzes the hydrolysis of the sn-2 fatty acyl bond of phospholipids and produces free fatty acids and lysophospholipids. Activation of PLA2 has been implicated as an early event in the inflammatory pathway (30). The PLA2s found in human serum (20) are group I PLA2 (pancreatic) and group II PLA2, which are both 14-kDa secretory proteins (4). Group II PLA2 was originally purified from synovial fluid (9) and blood platelets (15). This enzyme is present in numerous human tissues and body fluids (11). The concentration of group II PLA2 in serum increases during severe acute inflammatory diseases (20) and after surgery (7, 17). The catalytic activity of PLA2 found in blood plasma is due to the presence of group II PLA2 during various pathological conditions, including sepsis (6), bacterial infections (29), arthritis (14), acute pancreatitis (21), and multiple organ failure (25). It has been suggested that group II PLA2 is an acute-phase protein (26) produced by hepatocytes (3, 22). Increased concentrations of this enzyme in serum are indicative of a poor prognosis for patients with severe diseases such as sepsis (8) and multiple organ failure (25).
Inflammatory mediators, such as prostaglandins, have been proposed to play a crucial role in the development of multiple organ failure. The rate-limiting step in the generation of these mediators is the release of arachidonic acid by PLA2 (1). Thus, inhibition of PLA2 has been proposed as a possible means of intervention in preventing symptoms of severe inflammatory diseases (1). However, the exact role of group II PLA2 in inflammation remains unknown.
C57BL/6J mice lack endogenous group II PLA2 production because of a mutation in the gene encoding this enzyme (PLA2− mice) (12). Recently, transgenic C57BL/6J mice expressing the human group II PLA2 gene were generated (5). Human group II PLA2-transgenic mice (PLA2+ mice) have alopecia and epidermal and cutaneous adnexal hyperplasia but no other abnormality in phenotype, whereas PLA2− mice have a normal phenotype (5). In PLA2+ mice, expression of group II PLA2 has been observed in hepatocytes, epidermal keratinocytes, fibroblast-like cells of the dermis, connective tissue fibroblasts, epithelial and smooth muscle cells of the urinary bladder, and cells of Bowman's capsule (24).
Recently, we found that the PLA2+ mice were resistant to experimental Staphylococcus aureus infection (16). The resistance was presumably due to killing of the bacteria by group II PLA2, as indicated by improved clearance of S. aureus from the tissues of the PLA2+ mice compared to that of their PLA2− littermates. Moreover, group II PLA2 was responsible for killing S. aureus incubated in serum of PLA2+ mice in vitro (16). Earlier, purified group II PLA2 was shown to be bactericidal against gram-positive organisms in vitro (32). However, the bactericidal potency of group II PLA2 against Escherichia coli and other gram-negative microbes in vitro is minimal (32) and requires the action of other proteins, e.g., bactericidal/permeability-increasing protein (33) and complement (19).
The present study was aimed at finding out whether the overexpression and high concentrations of human group II PLA2 in sera of PLA2+ mice would be harmful or beneficial to the host during severe E. coli infection. To address this issue, host defense of both PLA2+ and PLA2− mice during experimental E. coli peritonitis was studied. Our results show that PLA2+ mice have a higher level of resistance to E. coli infection than PLA2− mice.
MATERIALS AND METHODS
Experimental animals.
The production of transgenic mice expressing human group II PLA2 is described in detail elsewhere (5). Of the several transgenic mouse lines characterized, one with high-level catalytic activity of PLA2 in serum was chosen for the production of PLA2+ and PLA2− mice for the present experiments (5). The animals were kept under pathogen-free conditions as described previously (16). Male mice were used throughout the present experiments. PLA2+ mice were identified by their specific phenotype (5) and by the presence of high concentrations of human group II PLA2 in their sera as measured by time-resolved fluoroimmunoassay (23). Nontransgenic male C57BL/6J littermates of the same age (8 to 10 weeks) and approximately the same weight were used as PLA2− control animals.
Bacteria and infection protocol.
E. coli bacteria (ATCC 25922; American Type Culture Collection, Manassas, Va.) were stored in water with 20% glycerol at −70°C before the onset of experiments. The bacteria were cultured on brain heart infusion agar (BHIA; Life Technologies, Paisley, United Kingdom) for 18 to 24 h. Three colonies of bacteria were suspended in 10 ml of brain heart infusion broth (Life Technologies) and was shaken (240 rpm) for 1 h at 37°C. The volume was adjusted to 200 ml with BHIB, and the suspension was shaken (240 rpm) for 2 h 15 min at 37°C and then centrifuged for 10 min at 3,000 × g and 4°C. The pellet was resuspended in 10 ml of sterile saline and centrifuged for 10 min at 3,000 × g and 4°C. The washing steps were repeated three times. The optical density at 650 nm of the final bacterial suspension was measured with an Ultrospec III densitometer (Pharmacia LKB, Uppsala, Sweden) and adjusted with sterile saline to 1.10. The bacterial suspensions were further adjusted to the desired concentrations by dilution with sterile saline. The bacterial suspensions, diluted to deliver specific numbers of CFU, were injected intraperitoneally (i.p.) in 0.33-ml volumes in all experiments. For example, a 1:20 dilution of bacterial suspension (optical density at 650 nm, 1.10) contained 4.8 × 107 CFU of E. coli bacteria/ml of suspension. A 0.33-ml aliquot of this suspension contained 1.6 × 107 CFU of E. coli. To confirm the number of bacteria used in the in vivo and in vitro experiments, a series of 10-fold dilutions of the suspension were plated on BHIA for the measurement of bacterial growth.
The complement sensitivity of E. coli was tested by mixing 20 μl of a solution containing 10 mmol of HEPES buffer (pH 7.4)/liter, 1.0 × 105 CFU of E. coli/ml, 10 mg of bovine serum albumin/ml, and 2 mmol of CaCl2/liter with 20 μl of human serum and shaking (240 rpm) for 2 h at 37°C. The human serum used in this experiment contained 0.77 mg of C3 and 0.14 mg of C4/liter. For control experiments, complement was inactivated by incubating the serum for 30 min at 56°C.
Concentration of human group II PLA2 and catalytic activity of PLA2 in serum.
Time-related changes in the concentration of group II PLA2 in sera of PLA2+ mice were determined 3, 6, 12, 24, 48, 168, and 336 h after the administration of 5.0 × 106 CFU of E. coli. Mice were anesthesized mildly with diethyl ether, and blood was collected from their tails into sterile tubes (Nunc CryoTube; InterMed, Copenhagen, Denmark) before the administration of E. coli and at various time points after infection. The animals were sacrificed by cervical dislocation, and samples for histological analysis were obtained immediately after blood sampling. Blood specimens were kept at room temperature for 2 to 3 h. Serum was separated by centrifugation at 2,400 × g and 4°C for 15 min and stored at −70°C for the biochemical measurements. The concentration of human group II PLA2 in serum was measured by time-resolved fluoroimmunoassay as described previously (23). The immunochemically determined concentrations were then compared to pretreatment values obtained for the same animals. The catalytic activity of PLA2 in serum was measured by using radioactive mixed micelles as a substrate as described previously (18).
Histological examinations.
Samples for histological studies were obtained from the internal organs of the experimental animals, fixed in 10% phosphate-buffered formalin, and embedded in paraffin. Five-micrometer-thick sections were stained with hematoxylin and eosin.
Survival of E. coli-inoculated mice.
Based on preliminary results of studies using a series of dilutions of E. coli suspension (three to six animals/group), 8.0 × 106 CFU/animal (corresponding to an approximate 30% lethal dose [LD30] in PLA2− mice), 1.6 × 107 CFU/animal (LD70), and 3.0 × 107 CFU/animal (LD85) were chosen for the evaluation of the differences in mortality between PLA2+ and PLA2− mice. Mortality and clinical status of the mice were registered 6, 12, and 24 h after the administration of E. coli and, thereafter, every 24 h up to 4 days. Samples for histological analysis were obtained from mice found dead and from surviving mice sacrificed by cervical dislocation 96 h after the administration of E. coli. Groups of PLA2+ and PLA2− mice (n = 10/group) were observed for 3 weeks after the administration of 1.6 × 107 CFU of E. coli/animal to confirm that there was no mortality after 96 h. To study the effect of bacterial viability on mouse mortality, E. coli bacteria were killed by heating suspensions at 100°C for 10 min and then injected into the peritoneal cavities of PLA2− and PLA2+ mice at a dose corresponding to 1.6 × 107 CFU (the LD70 for PLA2− mice) of E. coli/animal.
Bacterial growth in the peritoneal cavity, blood, and internal organs of mice after administration of E. coli.
To study bacterial growth in the peritoneal cavity, blood, spleen, and liver, blood samples were obtained from the tail and animals were killed 3, 6, 12, and 24 h after the administration of 5.0 × 106 CFU of E. coli. The peritoneal cavity was lavaged with 2 ml of sterile saline. Approximately 0.1-g samples of the spleen and liver tissues were washed with sterile saline and homogenized separately in 0.4 ml of ice-cold sterile saline (Ultra-Turrax homogenizer; IKA, Staufen, Germany). A series of 10-fold dilutions of blood, tissue homogenates, and peritoneal lavage fluid (PLF) were plated on BHIA for the measurement of bacterial growth.
Effect of serum and PLF on growth of E. coli in vitro.
To obtain serum for in vitro experiments, the animals were anesthetized lightly with ether and their tails and abdominal skin were sterilized with 70% ethanol. A 0.2-ml blood sample was collected from the tip of the tail into a sterile tube (Nunc CryoTube). The samples were kept at room temperature for 2 to 3 h, centrifuged for 15 min at 2,400 × g and 4°C, and stored at −70°C. Serum and PLF were obtained from mice before and 18 h after the administration of E. coli. The bactericidal activity of serum in vitro was measured as described previously (32), with slight modifications. Briefly, 20 μl of HEPES buffer (10 mmol/liter, pH 7.4) containing 1.0 × 105 CFU of E. coli/ml, 10 mg of bovine serum albumin/ml, and 2 mmol of CaCl2/liter was mixed with 20 μl of serum or PLF and shaken at (240 rpm) for 2 h at 37°C. After the incubation, the number of live E. coli was measured on BHIA plates as described above.
Statistical analyses.
One-way analysis of variance (ANOVA) was used to study the significance of the increase in the concentration of group II PLA2 in serum after the administration of E. coli. Pearson's linear regression analysis was used to study the correlation between the catalytic activity of PLA2 and the concentration of human group II PLA2 in sera of PLA2+ mice. Kaplan-Meier plots were constructed and the log-rank test was used to test the differences in mortality between the PLA2+ and PLA2− mice. Kruskal-Wallis nonparametric analysis and the Mann-Whitney U test were used to test the significance of the differences between the groups in the numbers of live bacteria in the PLF, spleen, liver, and blood. One-way ANOVA was used to study the significance of the differences between the groups in the numbers of live bacteria in serum and PLF in vitro. Values are expressed as means ± standard errors of the means (SEM). All statistical calculations were performed with Statistica software (StatSoft, Tulsa, Okla.).
RESULTS
Concentration of human group II PLA2 and catalytic activity of PLA2 in serum.
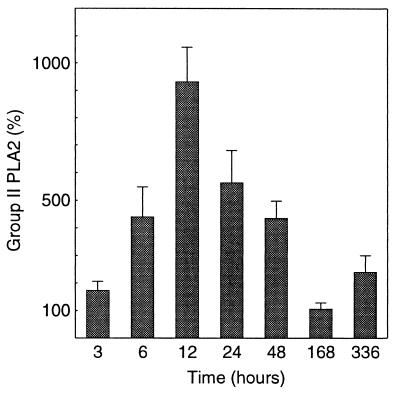
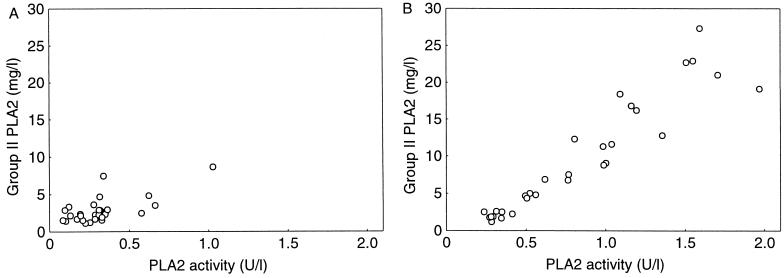
To evaluate the systemic PLA2 responses in PLA2+ and PLA2− mice, we measured the concentration of group II PLA2 and the catalytic activity of PLA2 in serum at various time points after the administration of E. coli. The concentration of human group II PLA2 in the sera of PLA2+ mice increased eightfold after the administration of 5.0 × 106 CFU of E. coli, with a peak at 12 h postinoculation, and decreased to the pretreatment level in 168 h. Thereafter, the levels of group II PLA2 in serum remained constant (Fig. 1). The concentration of group II PLA2 in serum correlated with the catalytic activity of PLA2 in both unchallenged and E. coli-infected PLA2+ mice (Fig. 2). Neither human group II PLA2 nor PLA2 catalytic activity was found in sera of PLA2− mice.
FIG. 1.
Concentrations of group II PLA2 in sera of PLA2+ mice after i.p. injection of 5.0 × 106 CFU of E. coli (P = 0.004, one-way ANOVA). The preinjection level of group II PLA2 was set at 100%, and the values are mean concentrations, relative to preinjection values, ± SEMS. n = 3 to 5 at 3, 168, and 336 h and n = 7 to 12 at 6 to 48 h for each group.
FIG. 2.
Correlation between the catalytic activity of PLA2 and the concentration of immunoreactive group II PLA2 in sera of unchallenged PLA2+ mice of both sexes (n = 26, r = 0.67; P = 0.0001, Pearson's linear regression analysis) (A) and of PLA2+ mice 12 to 48 h after i.p. injection of 5.0 × 106 CFU of E. coli (n = 30, r = 0.94; P < 0.0001) (B).
Clinical symptoms and histological changes during E. coli infection.
To study the possible role of the group II PLA2 transgene in the development of symptoms and signs of inflammation, the clinical status and histological changes in internal organs of the experimental animals were observed after the administration of lethal doses of E. coli. All unchallenged PLA2+ mice exhibited alopecia and cutaneous adnexal hyperplasia. The PLA2+ mice and their PLA2− littermates behaved normally before the administration of E. coli. During the first day after the administration of E. coli, both PLA2+ and PLA2− mice developed symptoms typical of endotoxemia, including diarrhea, anorexia, shivering, lethargy, and lacrimation. The symptoms were more severe in the PLA2− mice. The PLA2+ mice grew some hair 2 to 4 days after the administration of bacteria. The numbers of neutrophil polymorphonuclear leukocytes (PMNs) in the peritoneum and lung parenchyma increased, and occasional small inflammatory cell infiltrates appeared in the peritoneal loose connective tissue and fat of both PLA2+ and PLA2− mice 6 to 48 h after the administration of bacteria.
Survival of mice after E. coli infection.
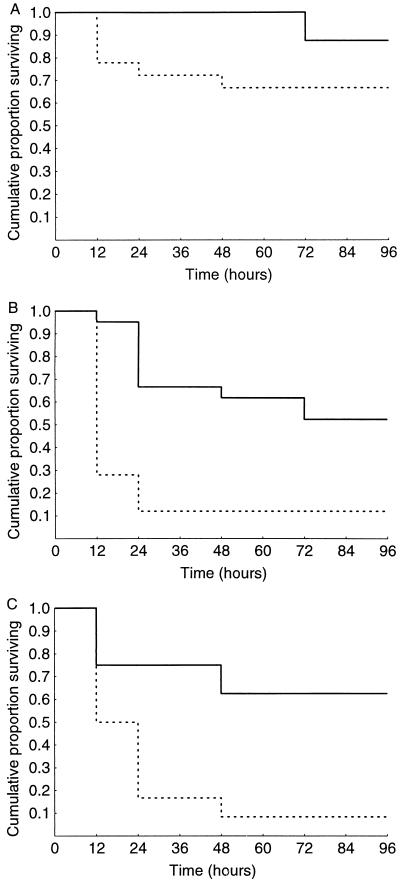
To evaluate the possible role of group II PLA2 in the outcome of experimental E. coli infection, mortality of PLA2+ and PLA2− mice was studied after the administration of E. coli at doses corresponding to the LD30, LD70, and LD85 for PLA2− mice. It appeared that PLA2+ mice showed a lower mortality rate than their PLA2− littermates (Fig. 3). At the LD30, however, the difference was not significant (Fig. 3A). With higher doses of E. coli, the mortality rate of PLA2− mice was much higher and the protection against death caused by the E. coli was statistically significant (Fig. 3B and C). Heat-killed bacteria administered at a dose corresponding to the LD70 for PLA2− mice did not cause clinical symptoms or any deaths in the PLA2+ or PLA2− mice (n = 5 for both groups).
FIG. 3.
(A) Survival analysis of PLA2+ mice (solid lines) (n = 8) and PLA2− mice (dotted lines) (n = 18) after i.p. injection of 8.0 × 106 CFU of E. coli; P = 0.24 between the groups (log-rank test). (B) Survival analysis of PLA2+ mice (n = 21) and PLA2− mice (n = 25) after i.p. injection of 1.6 × 107 CFU of E. coli; P = 0.00016. (C) Survival analysis of PLA2+ mice (n = 8) and PLA2− mice (n = 12) after i.p. injection of 3.0 × 107 CFU of E. coli; P = 0.014.
Extent of E. coli infection.
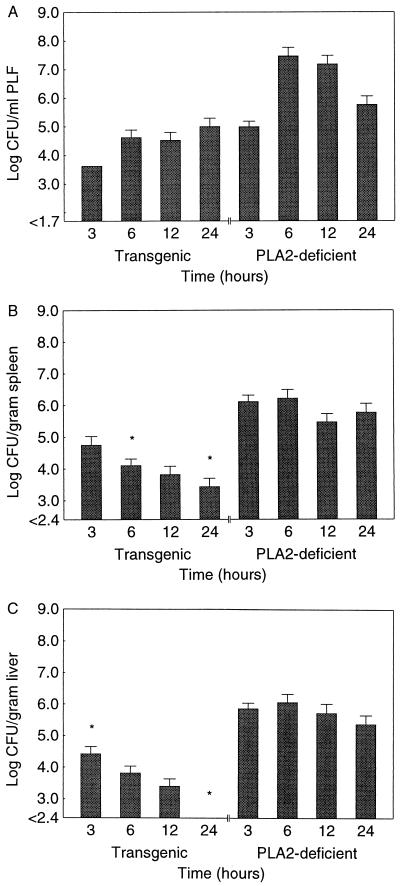
Recently we observed that the transgenic mice used in the present study showed increased clearance of bacteria from their organs after experimental S. aureus infection (16). Thus, we tested the hypothesis that group II PLA2 has a role in the clearance of E. coli from the host. The numbers of live E. coli in the PLF, spleen, and liver of PLA2+ and PLA2− mice were measured 3, 6, 12, and 24 h after the administration of a sublethal dose (5.0 × 106 CFU) of E. coli (Fig. 4). The numbers of live E. coli in the spleen and liver were smaller in PLA2+ than in PLA2− mice. A dramatic decrease was observed in the liver 24 h after the administration of bacteria. Statistically significant differences between the groups' PLF bacterial counts were not observed. There were no live E. coli in the blood of PLA2+ mice (n = 19) during the experiment, while bacteremia developed in 7 of 20 PLA2− mice within 24 h after the administration of E. coli (9.5 × 104 ± 5.5 × 104 CFU/ml of blood; P = 0.005).
FIG. 4.
Numbers of live E. coli in PLF (A), spleen (B), and liver (C) of PLA2+ and PLA2− mice 3, 6, 12, and 24 h after administration of 5.0 × 106 CFU of E. coli. The values are means ± SEMs; n = 4 at 3 h, and n = 7 to 12 at 6, 12, and 24 h. P = 0.32 (PLF), 0.022 (spleen), and 0.00041 (liver) (Kruskal-Wallis ANOVA). ∗, P < 0.05 (Mann-Whitney U test for comparison of values for PLA2+ and PLA2− mice at the same time points). Sensitivities of the assay were 1.7 log CFU for PLF and 2.4 log CFU for tissue homogenates.
Effect of serum, PLF, and complement on growth of E. coli in vitro.
Since the clearance of E. coli was more efficient in PLA2+ mice than in PLA2− mice, we tested the bactericidal potency of serum and PLF from these mice against E. coli in vitro. It appeared that there were no significant differences between PLA2+ and PLA2− mice in terms of growth of E. coli in sera. Moreover, PLF of neither group of mice inhibited the growth of E. coli in vitro (Table 1). Since complement is known to effect the lysis of some strains of E. coli, and since this could affect the results of group II PLA2-dependent lysis, we examined the killing of our E. coli strain in normal and heat-inactivated sera. The growth of E. coli in vitro in normal human serum with intact complement was similar to that in serum with heat-inactivated complement (5.3 × 105 and 6.8 × 105 CFU/ml, respectively).
TABLE 1.
Growth of E. coli incubated in vitro in the presence of serum and PLF of PLA2+ and PLA2− micea
| Specimen and study group | E. coli concn (CFU [106]/ml) | n | P |
|---|---|---|---|
| Serum | |||
| PLA2− mice | 1.21 ± 0.11 | 7 | |
| PLA2+ mice | 1.02 ± 0.15 | 7 | 0.36b |
| PLF | |||
| PLA2− mice | 1.02 ± 0.11 | 4 | |
| PLA2+ mice | 1.32 ± 0.22 | 4 | 0.20c |
Samples of serum and PLF were incubated with E. coli as described in Materials and Methods. Values are means ± SEM.
Versus value for PLA2− mouse serum; one-way ANOVA.
Versus value for PLA2− mouse PLF; one-way ANOVA.
DISCUSSION
To investigate the role of group II PLA2 in E. coli infection, we compared the host defenses of human group II PLA2 transgenic mice and their group II PLA2-deficient littermates after i.p. administration of live E. coli. The concentration of group II PLA2 in sera of PLA2+ mice was somewhat higher (approximately 2,500 μg/liter) (16) than that found in humans with severe infectious diseases. These diseases include bacterial infections (240 μg/liter) (29), sepsis (880 μg/liter) (25), peritonitis (440 μg/liter) (25), and typhoid fever (1,440 μg/liter) (13). The PLA2+ mice used in the present study showed no clinical symptoms of inflammation and behaved normally despite the exceedingly high PLA2 catalytic activity (up to 100-fold higher than in PLA2− mice) and concentration of group II PLA2 in their sera.
In the present study, we showed that the concentration of group II PLA2 increases in sera of PLA2+ mice, after the administration of E. coli, with a peak at 12 h. The catalytic activity of PLA2 in the sera of PLA2+ mice correlated significantly with the concentration of group II PLA2 in the same sera. These results suggest that the PLA2 activity of PLA2+ mouse serum is derived solely from human group II PLA2 and not from other (inherited mouse) PLA2s. The group II PLA2 response in sera of PLA2+ mice appears earlier than that observed in human patients after surgery (17). However, the increase in concentration of group II PLA2 in sera of PLA2+ mice after the injection of E. coli follows the same time schedule as that seen in sera of healthy volunteers after the administration of endotoxin (27). The results suggest that bacteria and bacterial products induce a rapid systematic increase in the concentration of group II PLA2 in vivo. A more delayed release of the enzyme, perhaps due to secondary endotoxemia, may be responsible for the increased levels of PLA2 in sera of patients who have undergone surgery.
On the one hand, the symptoms of sepsis after the administration of E. coli were less severe in PLA2+ than in PLA2− mice. On the other hand, the concentration of group II PLA2 in sera of PLA2+ mice increased eightfold. Therefore, the increased amount of group II PLA2 in serum was not responsible for the septic symptoms of E. coli infection in the experimental animals of the present study. On the contrary, PLA2+ mice showed a lower mortality rate than PLA2− mice after being given large doses of live E. coli, and the bacteria were more effectively cleared from the tissues and body fluids of the PLA2+ mice. Thus, expression of human group II PLA2 appears to protect PLA2+ mice against E. coli administered in amounts that are lethal to their PLA2− littermates. These data indicate that group II PLA2 has an important role in the host defense against E. coli infection.
We observed previously that the PLA2+ mice used in the present study were highly resistant to S. aureus infection (16). Human and rabbit group II PLA2s are capable of killing staphylococci and other gram-positive bacteria in vitro (28, 32). Moreover, the bactericidal activity against S. aureus and the concentration of group II PLA2 in plasma increased in parallel during experimental E. coli infection of baboons (31). It has been hypothesized that group II PLA2 alone is sufficient to kill gram-positive bacteria in vitro (28, 32). In contrast, the bactericidal mechanism of group II PLA2 against E. coli and other gram-negative bacteria requires the presence of the bactericidal/permeability-increasing protein of PMNs (33) and/or components of complement that further potentiate the bactericidal effect (19). The host resistance of the PLA2+ mice against S. aureus was effected by direct bacterial killing by group II PLA2 present in their body fluids (16).
To evaluate mechanisms involved in the protection by group II PLA2 against E. coli infection, we studied bacterial killing in PLA2+ mice after E. coli infection. The clearance of bacteria from internal organs was faster in PLA2+ mice than in PLA2− mice. However, neither serum nor PLF of PLA2+ mice was bactericidal against E. coli in vitro. These results suggest that group II PLA2 alone is not able to kill E. coli in mouse body fluids but presumably requires the coaction of another agent(s) present in inflammatory and/or tissue cells of PLA2+ mice to act against E. coli. Because the E. coli strain used in the present study is resistant to complement, it is also unlikely that the increased clearance of E. coli in the PLA2+ mice is due to an inflammation-mediated increase in the activity of complement. It is thus possible that there are group II PLA2-dependent mechanisms other than direct bacterial killing that improve host resistance to gram-negative bacterial infection.
E. coli lipopolysaccharide (LPS; endotoxin) causes an increase in the level of group II PLA2 in sera of human volunteers (27). In the present experiment, doses corresponding to the LD70 of heat-killed E. coli did not cause clinical symptoms or deaths in mice, but live bacteria were needed for these effects. Therefore, LPS released from the E. coli strain used in the present study obviously played only a minor role in the mortality of the animals. In another set of experiments, we injected large doses of E. coli LPS (5 and 20 mg/kg) into PLA2+ and PLA2− animals and found that the expression of human group II PLA2 did not markedly influence the course of E. coli LPS-induced shock and mortality (unpublished observations). Group II PLA2 seems to decrease the viability of E. coli or its ability to spread in the host rather than attenuate the symptoms caused by E. coli endotoxin.
Group II PLA2 is involved in the production of bioactive molecules, such as prostaglandins and leukotrienes, which in turn are important mediators of the inflammatory response (30). Group II PLA2 was found to propagate the inflammatory reaction by activating T lymphocytes (2), and it has been hypothesized that the action of PLA2 is important in the adhesion of PMNs to tissues (10). Thus, the improved host response shown by the PLA2+ mice may result from an increased inflammatory reaction or improved action of leukocytes. The effects of group II PLA2 on the systemic inflammatory reaction and activation of leukocytes in internal organs are under investigation. It seems that the PLA2+ mice have a better systemic inflammatory cell response than their group II PLA2− littermates (unpublished data). On the other hand, the abnormal phenotype (adnexal and epidermal hyperplasia) of the PLA2+ mice does not involve an increase of the inflammatory infiltrate in the skin (5).
Taken together, the results presented here show that the expression of human group II PLA2 in transgenic mice improves their host defense against experimental E. coli infection. The detailed mechanisms involved remain to be established.
ACKNOWLEDGMENTS
This work was supported by the Turku University Foundation, Turku University Hospital, the Finnish Medical Foundation, the Emil and Blida Maunula Foundation, and the Maud Kuistila Foundation.
We thank Anne Jokilammi-Siltanen, Kati Talvinen, and Heikki Peuravuori for skillful technical assistance and Klaus Elenius and Mikael Skurnik for valuable advice.
REFERENCES
- 1.Anderson B O, Moore E E, Banerjee A. Phospholipase A2 regulates critical inflammatory mediators of multiple organ failure. J Surg Res. 1994;56:199–205. doi: 10.1006/jsre.1994.1032. [DOI] [PubMed] [Google Scholar]
- 2.Asaoka Y, Yoshida K, Sasaki Y, Nishizuka Y, Murakami M, Kudo I, Inoue K. Possible role of mammalian secretory group II phospholipase A2 in T-lymphocyte activation: implication in propagation of inflammatory reaction. Proc Natl Acad Sci USA. 1993;90:716–719. doi: 10.1073/pnas.90.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowl R M, Stoller T J, Conroy R R, Stoner C R. Induction of phospholipase A2 gene expression in human hepatoma cells by mediators of the acute phase response. J Biol Chem. 1991;266:2647–2651. [PubMed] [Google Scholar]
- 4.Dennis E A. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci. 1997;22:1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- 5.Grass D S, Felkner R H, Chiang M Y, Wallace R E, Nevalainen T J, Bennett C F, Swanson M E. Expression of human group II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J Clin Investig. 1996;97:2233–2241. doi: 10.1172/JCI118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green J A, Smith G M, Buchta R, Lee R, Ho K Y, Rajkovic I A, Scott K F. Circulating phospholipase A2 activity associated with sepsis and septic shock is indistinguishable from that associated with rheumatoid arthritis. Inflammation. 1991;15:355–367. doi: 10.1007/BF00917352. [DOI] [PubMed] [Google Scholar]
- 7.Grönroos J M, Kuttila K, Nevalainen T J. Group II phospholipase A2 in serum in critically ill surgical patients. Crit Care Med. 1994;22:956–959. doi: 10.1097/00003246-199406000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Guidet B, Piot O, Masliah J, Barakett V, Maury E, Bereziat G, Offenstadt G. Secretory non-pancreatic phospholipase A2 in severe sepsis: relation to endotoxin, cytokines and thromboxane B2. Infection. 1996;24:103–108. doi: 10.1007/BF01713312. [DOI] [PubMed] [Google Scholar]
- 9.Hara S, Kudo I, Chang H W, Matsuta K, Miyamoto T, Inoue K. Purification and characterization of extracellular phospholipase A2 from human synovial fluid in rheumatoid arthritis. J Biochem (Tokyo) 1989;105:395–399. doi: 10.1093/oxfordjournals.jbchem.a122675. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson P B, Schrier D J. Regulation of CD11b/CD18 expression in human neutrophils by phospholipase A2. J Immunol. 1993;151:5639–5652. [PubMed] [Google Scholar]
- 11.Kallajoki M, Nevalainen T J. Expression of group II phospholipase A2 in human tissues. In: Uhl W, Nevalainen T J, Büchler M W, editors. Phospholipase A2. Basic and clinical aspects in inflammatory diseases. Vol. 24. Basel, Switzerland: Karger AG; 1997. pp. 8–16. [Google Scholar]
- 12.Kennedy B P, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt D E, Cromlish W A. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J Biol Chem. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- 13.Keuter M, Dharmana E, Kullberg B J, Schalkwijk C, Gasem M H, Seuren L, Djokomoeljanto R, Dolmans W M, van den Bosch H, van der Meer J W. Phospholipase A2 is a circulating mediator in typhoid fever. J Infect Dis. 1995;172:305–308. doi: 10.1093/infdis/172.1.305. [DOI] [PubMed] [Google Scholar]
- 14.Kortekangas P, Aro H T, Nevalainen T J. Group II phospholipase A2 in synovial fluid and serum in acute arthritis. Scand J Rheumatol. 1994;23:68–72. doi: 10.3109/03009749409103030. [DOI] [PubMed] [Google Scholar]
- 15.Kramer R M, Hession C, Johansen B, Hayes G, McGray P, Chow E P, Tizard R, Pepinsky R B. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989;264:5768–5775. [PubMed] [Google Scholar]
- 16.Laine V J O, Grass D S, Nevalainen T J. Protection by group II phospholipase A2 against S. aureus. J Immunol. 1999;162:7402–7408. [PubMed] [Google Scholar]
- 17.Laine V J O, Grönroos J M, Nevalainen T J. Serum phospholipase A2 in patients after splenectomy. Eur J Clin Chem Clin Biochem. 1996;34:419–422. [PubMed] [Google Scholar]
- 18.Laine V J O, Nyman K M, Peuravuori H J, Henriksen K, Parvinen M, Nevalainen T J. Lipopolysaccharide induced apoptosis of rat pancreatic acinar cells. Gut. 1996;38:747–752. doi: 10.1136/gut.38.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen L M, Inada M, Weiss J. Determinants of activation by complement of group II phospholipase A2 acting against Escherichia coli. Infect Immun. 1996;64:2425–2430. doi: 10.1128/iai.64.7.2425-2430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nevalainen T J. Serum phospholipases A2 in inflammatory diseases. Clin Chem. 1993;39:2453–2459. [PubMed] [Google Scholar]
- 21.Nevalainen T J, Grönroos J M, Kortesuo P T. Pancreatic and synovial type phospholipases A2 in serum samples from patients with severe acute pancreatitis. Gut. 1993;34:1133–1136. doi: 10.1136/gut.34.8.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nevalainen T J, Kallajoki M, Pesonen E, Andersson S, Kärkkäinen P, Höckerstedt K. Origin of circulating group II phospholipase A2 in hepatocytes in a patient with epitheloid hemangioendothelioma of the liver. Lab Investig. 1996;74:585–591. [PubMed] [Google Scholar]
- 23.Nevalainen T J, Kortesuo P T, Rintala E, Märki F. Immunochemical detection of group I and group II phospholipases A2 in human serum. Clin Chem. 1992;38:1824–1829. [PubMed] [Google Scholar]
- 24.Nevalainen T J, Laine V J O, Grass D S. Expression of human group II phospholipase A2 in transgenic mice. J Histochem Cytochem. 1997;45:1109–1119. doi: 10.1177/002215549704500808. [DOI] [PubMed] [Google Scholar]
- 25.Nyman K M, Uhl W, Forsström J, Büchler M, Beger H G, Nevalainen T J. Serum phospholipase A2 in patients with multiple organ failure. J Surg Res. 1996;60:7–14. doi: 10.1006/jsre.1996.0003. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa M, Arakawa H, Yamashita S, Sakamoto K, Ikei S. Postoperative elevations of serum interleukin 6 and group II phospholipase A2: group II phospholipase A2 in serum is an acute phase reactant. Res Commun Chem Pathol Pharmacol. 1992;75:109–115. [PubMed] [Google Scholar]
- 27.Pruzanski W, Wilmore D W, Suffredini A, Martich G D, Hoffman A G, Browning J L, Stefanski E, Sternby B, Vadas P. Hyperphospholipasemia A2 in human volunteers challenged with intravenous endotoxin. Inflammation. 1992;16:561–570. doi: 10.1007/BF00918980. [DOI] [PubMed] [Google Scholar]
- 28.Qu X D, Lehrer R I. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Neurochem Res. 1998;23:807–814. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rintala E M, Nevalainen T J. Group II phospholipase A2 in sera of febrile patients with microbiologically or clinically documented infections. Clin Infect Dis. 1993;17:864–870. doi: 10.1093/clinids/17.5.864. [DOI] [PubMed] [Google Scholar]
- 30.Vadas P, Pruzanski W, Stefanski E, Ellies L G, Aubin J E, Sos A, Melcher A. Extracellular phospholipase A2 secretion is a common effector pathway of interleukin-1 and tumour necrosis factor action. Immunol Lett. 1991;28:187–193. doi: 10.1016/0165-2478(91)90002-r. [DOI] [PubMed] [Google Scholar]
- 31.Weinrauch Y, Abad C, Liang N-S, Lowry S F, Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. J Clin Investig. 1998;102:633–638. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinrauch Y, Elsbach P, Madsen L M, Foreman A, Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Investig. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss J, Inada M, Elsbach P, Crowl R M. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J Biol Chem. 1994;269:26331–26337. [PubMed] [Google Scholar]