Abstract
Background:
Pediatric obsessive–compulsive disorder (OCD) has been associated with poorer planning in laboratory, school and home settings. It is unclear whether this impairment is a standalone cognitive issue or the result of OCD symptoms. No study has examined the influence of provoked distress on planning performance and neural correlates in pediatric OCD.
Methods:
Before and after a symptom provocation task, youth with OCD (n = 23; 9 boys; mean age ± standard deviation 15.1 ± 2.6 years) and matched healthy controls (n = 23) completed the Tower of London task during functional MRI scanning.
Results:
During planning, participants with OCD recruited the left superior frontal gyrus to a greater extent than healthy controls after symptom provocation (group × time point interaction; t44 = 5.22, p < 0.001). In a seeded, region of interest–constrained, functional connectivity analysis, we identified greater connectivity between the left superior frontal gyrus and the right middle frontal gyrus, left precuneus and left inferior parietal lobule in participants with OCD than healthy controls. We also identified greater connectivity between the right amygdala and right medial frontal gyrus in patients with OCD than healthy controls, but only before symptom provocation.
Limitations:
The fixed-order design of the study and the number of participants taking medication (n = 20) should be noted.
Conclusion:
Participants with OCD demonstrated greater amygdalar–cortical connectivity before symptom provocation, while sustaining greater recruitment and connectivity of task-related planning areas throughout the task. These results suggest that brain activity and connectivity is altered after symptom provocation, in the absence of impaired planning performance.
Introduction
Pediatric obsessive–compulsive disorder (OCD) is a common neurodevelopmental illness (0.25% to 2.9% prevalence1,2) characterized by distressing thoughts and repetitive behaviours.3 Neuroimaging studies have associated OCD with altered function of distinct cortico–striato–thalamo–cortical (CSTC) circuits,4 including the sensorimotor CSTC circuit (sensory motor area, posterior putamen and thalamus); the dorsal cognitive CSTC circuit (pre–sensory motor area, dorsolateral prefrontal cortex [PFC], dorsomedial PFC, dorsal caudate and thalamus); the ventral cognitive CSTC circuit (inferior frontal gyrus, ventrolateral PFC, ventral caudate and thalamus); the affective CSTC circuit (orbital frontal cortex, nucleus accumbens and thalamus); and the frontolimbic circuit (ventromedial PFC and amygdala). Furthermore, many recent neurocircuit-based models of OCD suggest that cognitive CSTC circuits regulate the affective and frontolimbic circuits.4,5 Few adult OCD studies and no pediatric studies have directly tested cognitive–affective interactions6 and frontolimbic connectivity.7,8
Behavioural studies have demonstrated significant deficits in executive function across domains in adults with OCD,9–12 but such deficits appear to be less widespread in children and youth with OCD.13 Our recent study suggested that, among a variety of executive-function tasks (e.g., flexible thinking, working memory, planning and response inhibition), planning was affected in at-risk siblings and children with OCD,14 hinting at its role as an underlying trait marker. This deficit in planning continues throughout the lifetime: in Tower of London15 tasks, adults with OCD have shown decreased accuracy16–20 or slower reaction times16–20 compared to healthy controls.
Some researchers have postulated that these deficits occur because the Tower of London task recruits many brain regions that are directly implicated in OCD, including areas involved in the dorsal cognitive CSTC circuit (e.g., premotor cortex, Brodmann area [BA] 6; supplementary motor area, BA 8/32; dorsolateral PFC, BA 9/46), the ventral cognitive CSTC circuit (e.g., rostrolateral PFC, BA 10) and frontoparietal areas (e.g., inferior parietal lobule, BA 40; precuneus, BA 7).21,22
Alternatively, OCD-related affective interference might impact planning indirectly: neuroimaging studies have shown that poor task accuracy is related to increased amygdalar recruitment and decreased cortical (e.g., precuneus, premotor cortex, sensory motor area and anterior cingulate cortex) and subcortical (thalamus and caudate nucleus) recruitment.6 To our knowledge, no study has directly tested the effect of triggering OCD symptoms on planning performance in either children or adults, and few studies have examined limbic interference in people with OCD during cognitive tasks, although research has found that state anxiety can influence brain activity related to the Tower of London task.8
Symptom provocation tasks (SPTs) have been used for decades to trigger symptoms in adults with OCD.23 In the present study, we assessed performance on the Tower of London task before and after a validated SPT, while recording functional MRI (fMRI) data, to determine how provoked distress affected planning in pediatric patients with OCD.
We hypothesized that patients with OCD would demonstrate worse response time or accuracy on the Tower of London task, less frontal and parietal brain area recruitment, and enhanced limbic network recruitment compared to matched healthy controls. We also hypothesized that after symptom provocation we would see even greater behavioural differences, even less task-related brain area recruitment and (per previous research8) increased connectivity between cognitive and limbic brain areas (e.g., the amygdala), suggesting limbic interference.
Methods
Participants
We recruited children and youth diagnosed with OCD (n = 23; 9 boys and 14 girls; 9.8–18.8 years old; mean age ± standard deviation [SD] 15.1 ± 2.6 years) from a hospital clinic’s cognitive behavioural therapy program. We recruited healthy controls (n = 23) from the community via advertisements.
We excluded any participant with a major medical condition, a history of head trauma, a contraindication for MRI (e.g., magnetic implant or claustrophobia) or a history of substance dependence or abuse.
We excluded any healthy control who reported a history of mental illness or a first-degree relative with OCD or Tourette syndrome, or who self-reported symptoms of OCD (i.e., Children’s Yale–Brown Obsessive–Compulsive Scale self-report version [CY-BOCS-SR] score ≥ 8)24 on scanning day. Four healthy controls who reported subthreshold symptoms (i.e., CY-BOCS-SR score 1–7) on scanning day were excluded only in sensitivity analyses.
We excluded patients with OCD who had a history of bipolar disorder, psychosis, intellectual disability or pervasive developmental disorder (e.g., autism spectrum disorder). We permitted a lifetime history of anxiety disorder, depression, attention-deficit/hyperactivity disorder or tic disorder symptoms, because these are common comorbidities of OCD.25 We excluded 1 OCD participant from the entire study because they reported no OCD symptoms on scanning day (this person was not included in the OCD group total of 23 participants). Five participants with OCD who reported subthreshold symptoms (i.e., CY-BOCS-SR score 1–7) on scanning day were excluded only in sensitivity analyses. For lifetime diagnoses among patients with OCD, see Appendix 1, Table S1, available online at www.jpn.ca/lookup/doi/10.1503/jpn.220064/tab-related-content).
Parents or guardians and all study participants provided informed consent or assent before beginning the study. All aspects of the study were approved by our institutional ethics committee.
Clinical measures
During study enrolment, research staff administered a phone interview to parents to obtain information about age, sex, handedness, eyeglass or contact lens prescription, current medications, lifetime diagnoses and MRI contraindications. For healthy controls, the Anxiety Disorder Interview Schedule for Children IV (ADIS-P)26 and the Children’s Yale–Brown Obsessive–Compulsive Scale clinician report version (CY-BOCS-CR)27 were administered by trained clinical masters-level research assistants, supervised by clinical psychologists. For patients with OCD, the ADIS-P and CY-BOCS-CR were administered at clinic intake by clinical psychologists to establish the presence of lifetime psychiatric diagnoses.
After scanning (with parental help as needed) participants completed the CY-BOCS-SR24 and a modified version of the Florida Obsessive–Compulsive Inventory (FOCI)28 to identify symptoms of OCD (current, previous or never experienced) and the extent to which current symptoms induced distress (rating from 0 to 8). Finally, participants provided a self-report of their Tanner pubertal stage based on visual diagrams.29,30
Study protocol
Tower of London task
The Tower of London task (described in depth in previous papers31,32) measures executive function — particularly planning performance. During each trial, participants mentally move beads between rods to match a particular pattern, with up to 5 moves needed. In the control condition, participants count beads (e.g., all the blue beads).
The task was explained outside of the scanner using a wooden model, and then practised using a computerized version31 with verbal feedback after each trial. Participants practised until they reached 80% success. During scanning, participants received no feedback. The Tower of London task was administered before and after the SPT (see next section). In the scanner, stimuli were presented using E-prime (version 2.0; Psychological Software Tools) and a PowerLite Home Cinema 5010 3LCD (Epson) projector viewed through a mirror attached to the head coil. Participants responded using the right index and middle fingers. A crescent-shaped response box relayed the responses by fibre optic cable (Photon Control) to the stimulus computer. If a participant’s vision was not 20/20 and was not corrected with contact lenses, we used their prescription to select MRI-compatible eyeglasses.
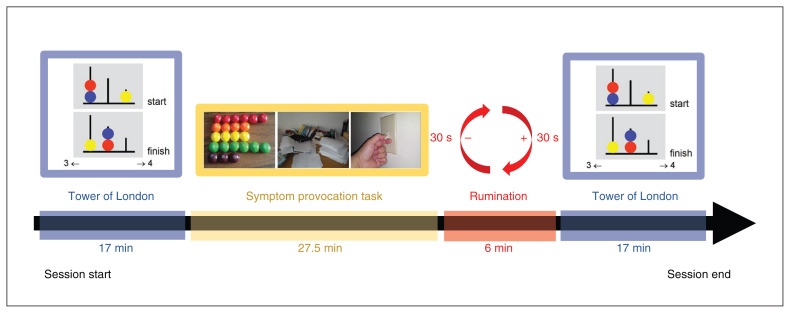
In the present study, 2 fMRI runs occurred before the SPT, and 2 occurred after. Previous studies have used 1 run instead of 2,19,32 but using 2 runs reduced the time pediatric patients were required to remain completely still. Each run sampled sequentially from a randomized trial list and took 8.5 min to complete. In keeping with previous Tower of London imaging studies19,32 and to reduce carryover effects, all 3-, 4- and 5-move trials were followed by a counting trial. Four events (10 s) occurred at the end of each run, each displaying a fixation cross in the middle of the screen. Because of the self-paced nature of the task, the number of trials differed across participants, consistent with other studies that have used this paradigm.19,31,32 For a diagram of task order, see Figure 1.
Figure 1.
Order of tasks in each MRI recording session. Each session began with 2 runs (8.5 min each) of the Tower of London task (17 min total). This was followed by the symptom provocation task, which ran for 27.5 min. Participants were then asked to ruminate on an image from the symptom provocation task that they found particularly distressing (6 min). Two more 8.5-min runs of the Tower of London task completed the paradigm.
Symptom provocation task
In the SPT (described in a previous article33), participants were exposed to alternating blocks of standardized visual stimuli aimed at provoking OCD symptoms (e.g., pictures from the cleaning or contamination, bad thoughts, and symmetry or ”just right” OCD symptom dimensions34), as well as blocks of fear, neutral and rest (e.g., fixation) stimuli. After each block, participants were asked to rate how “bothered” the images made them feel on a scale of 1 to 3. After the SPT, participants were asked to remember and imagine the stimulus they found most bothersome over a period of 6 min (alternating “imagine” and “rest” blocks, 30 s per block) to encourage rumination.
Responses to the neutral condition were subtracted from the cleaning or contamination, bad thoughts, and symmetry or ”just right” conditions to form symptom provocation distress scores for each dimension. We did not use the fear condition in the current analysis, because our previous work found no significant group differences.33
Links to previously published studies
Although the SPT neuroimaging data have been published previously,33 the Tower of London neuroimaging data presented below are original. We published a paper that used the neuroimaging Tower of London paradigm35 in monozygotic twins discordant for OCD, but the twin data are not included in the present study sample. Approximately half of the present sample (13 participants with OCD and 9 healthy controls) participated in our previous study of neurocognitive OCD risk markers.14
Image acquisition
The following sequences were collected on a 3 T Discovery MR750 MRI scanner (GE Medical Systems) with a 12-channel head coil: a 3-dimensional fast spoiled gradient echo imaging structural scan (repetition time 8.184 ms, echo time 3.192 ms, matrix 256 × 256 mm, 182 slices, voxel size 1 × 1 × 1 mm); a field map to account for B0 distortions; and functional gradient echo-planar images (repetition time 2000 ms, echo time 25 ms, field of view = 256, matrix 96 × 96, 41–43 interleaved slices per volume, 3 × 3 mm in-plane resolution, slice thickness 3 mm, interslice gap 1 mm). The number of volumes for each Tower of London run was 260, for a total of 520 volumes pre-SPT and 520 volumes post-SPT. We used sagittal acquisition for all scans because it displayed the lowest amount of signal dropout over ventromedial prefrontal cortical structures during piloting. A pediatric neuroradiologist found no clinically relevant incidental findings in the structural scans.
Image preprocessing
We used SPM12 (www.fil.ion.ucl.ac.uk/spm) and the Field Map toolbox36 for image analysis. Functional images were reoriented, slice-time-corrected, unwrapped and realigned, and coregistered. Data were then normalized to the Montreal Neurological Institute T1 template and resliced to a 3 × 3 × 3 resolution, before spatially smoothing with an 8 mm full width at half maximum Gaussian kernel.
For each participant, we created first-level general linear model design matrices by modelling the onset and duration of all correctly answered trials (counting, 1-move, 2-move, 3-move, 4-move and 5-move) with δ functions convolved with the hemodynamic response function. Incorrect trials were modelled using 1 nuance regressor, and motion parameters were included as regressors of no interest. Runs with motion greater than 3 mm or 3° of rotation were excluded entirely. We found no significant difference in movement between the groups (Appendix 1, Table S2). Fixation trials were assigned to the implicit baseline. To remove low-frequency noise, we applied the default high-pass filter (128 s cut-off).
Next, we pooled the 2 pre-SPT runs and the 2 post-SPT runs. The primary first-level contrasts of interest were “planning” (i.e., −5, 1, 1, 1, 1, 1) and “task load” (i.e., 0, −2.5, −1.5, −0.5, 1, 3.5). We then brought the first-level contrast images forward to second-level group analyses.
Statistical analyses
Behavioural analyses
We analyzed behavioural responses in R (version 4.0.2; www.r-project.org). Given the presence of extreme scores in selected outcome measures, we applied a Winsorizing technique: scores below the first percentile or above the 99th percentile were set to equal those at the first and 99th percentiles, respectively. Results using this technique are presented here. Similar findings emerged without Winsorization.
We used the nlme package (CRAN.R-project.org/package=nlme) to evaluate performance (accuracy and response time) and change (post-SPT minus pre-SPT) at each level of task load (counting, 1-, 2-, 3-, 4- or 5-move) using linear mixed effects models. We entered all variables and interactions among variables as fixed effects. We included a random intercept, and we allowed heteroskedasticity in residuals across the Tower of London task load, which resulted in substantially improved model fit compared to a model without such heteroskedasticity for accuracy and response time (likelihood ratio test p < 0.0001 for each outcome). We set the statistical threshold at α = 0.05 (Bonferroni-corrected) to account for comparison of all 6 task load conditions.
Associations between changes in Tower of London performance across time points and OCD characteristics
We used additional analyses to determine whether observed changes in Tower of London performance were associated with OCD-specific characteristics, including level of SPT-induced distress (in all participants) and reported OCD severity (in patients with OCD only). For all models, we specified random intercepts and allowed heteroskedasticity across Tower of London conditions. We considered an interaction between task load and OCD-specific characteristics but dropped it from all models because improvement to model fit did not justify the added complexity (likelihood ratio test p < 0.05, family-wise error [FWE], voxel-based correction).
Neuroimaging analyses
We conducted activation and connectivity analyses by running second-level analyses in the multivariate and repeated-measure (MRM) toolbox.37 The MRM toolbox overcomes known issues in SPM12 by selecting the correct F-ratio error terms for group-level repeated-measures statistics.38
Brain activity analyses
We conducted exploratory whole-brain analyses at pFWE < 0.05 (voxel-based correction) for comparisons with previous studies. We also tested a priori hypotheses in a combined set of regions of interest (ROIs) selected from relevant OCD meta-analyses.22,23,39 We used MarsBaR (marsbar.sourceforge.net) to place 10 mm (unilateral) or 20 mm (bilateral) spherical ROIs around peak voxel coordinates in regions relevant to emotional processing: the dorsolateral PFC (BA 9), the parietal cortex (BA 40 and 7) and the premotor areas (BA 6/8), covering brain areas known to be recruited during the Tower of London task; the orbital frontal cortex (BA 11); the inferior occipital gyrus (BA 19); the middle temporal gyrus (BA 21); and the amygdala.39 We selected the temporal pole (BA 38) because it has been implicated previously during symptom provocation.33 Spheres were combined into a single ROI mask. See Appendix 1, Table S3, for more details, including a full list of ROIs in the combined mask. See Appendix 1, Figure S2, for a diagram.
For whole-brain and a priori analyses, we used repeated-measures analyses of variance to explore the main effects of task, group, time point and their interaction. We used the default Pillai trace statistic, with 5000 permutations and a statistical threshold of pFWE < 0.05 (voxel-based correction). To describe the direction of significant findings, we extracted the peak voxel activity of significant clusters via MarsBaR.
Associations between brain recruitment across time points and OCD characteristics
We used the β values extracted from peak voxel activity to create change in β value scores, which we then used to determine whether there was a relationship between brain activity, group, SPT distress rating and OCD severity.
Connectivity analysis
We used the generalized psychophysiological interaction toolbox (www.nitrc.org/projects/gppi) to model task-related functional connectivity during correctly answered trials. We used a total of 3 seeds: a left superior frontal gyrus (SFG) seed based on brain activity results, and left (x, y, z = −23, −2, −16) and right amygdala (x, y, z = 23, 0, −16) seeds based on previous studies of frontolimbic connectivity during emotional and cognitive paradigms in patients with OCD and their unaffected siblings.7,8,40 All connectivity analyses used 6 mm spherical seed regions. Psychophysiological interaction models included the 5 task regressors, 5 psychophysiological interaction regressors, the time point course of the seed region and 6 motion parameters.
Like previous studies,40 we conducted separate analyses for each seed region in MRM based on the connectivity results, submitting the planning versus counting contrast to an repeated-measures analysis of variance exploring the influence of group (OCD v. healthy controls), time point (pre-SPT v. post-SPT) and their interaction. Analyses were confined to the combined ROI mask. We used the Pillai trace statistic with 5000 permutations and a statistical threshold of pFWE < 0.05. Permutation-based tests are robust to violated statistical assumptions.41 We extracted values from significant clusters using MarsBaR to describe the direction of findings and run post hoc between-group analyses.
Results
Participants
The total sample consisted of 23 patients with OCD and 23 healthy controls (Table 1). Healthy controls were well matched in terms of age, sex, handedness, IQ and Tanner pubertal stage.29,30
Table 1.
Participant demographic characteristics at the time of scanning
| Patients with OCD* n = 23 |
Healthy controls n = 23 |
p value† | |
|---|---|---|---|
| Age, yr, mean ± SD | 15.1 ± 2.6 | 14.2 ± 3.1 | 0.28 |
| Male, n (%) | 9 (39%) | 7 (30%) | 0.76 |
| Right-handed, n (%) | 22 (96%) | 20 (87%) | 0.61 |
| IQ, points, mean ± SD‡ | 105.38 ± 9.42 | 114.11 ± 13.98 | 0.09 |
| Tanner stage, mean ± SD§ | |||
| Primary | 3.5 ± 1.2 | 3.2 ± 1.2 | 0.49 |
| Secondary | 3.6 ± 1.2 | 3.1 ± 1.5 | 0.29 |
OCD = obsessive–compulsive disorder; SD = standard deviation.
Among the participants with OCD, 11 described themselves as “white,” 1 participant described themselves as “Aboriginal” and 1 described themselves as “other”; the remaining participants chose not to provide their race or ethnicity. Racial or ethic information was not captured for the healthy control group.
Independent-samples t test (2-tailed) for continuous measures. We assumed equal variances, because Levene’s test was not significant in any instance (p > 0.05). Fisher exact test (2-tailed) for dichotomous measures.
Data available for 13 patients with OCD and 9 healthy controls.
Data available for 21 patients with OCD and 22 healthy controls.
Among the patients with OCD, the reported average age of onset of OCD symptoms (± SD) was 9.3 ± 3.1 years, and symptom severity ratings at intake were in the severe range, based on the CY-BOCS-CR (mean ± SD 24.3 ± 8.4).27 Patients reported milder symptoms on the day of MRI scanning using the CY-BOCS-SR (mean ± SD 13.5 ± 6.0).24 On average, scanning occurred 1.7 years after patients’ initial clinic assessment and diagnosis (range 23 d to 4.5 yr). Most patients with OCD (17 of 23) had completed at least 1 full round of the clinic’s group-based family cognitive behavioural therapy between the initial assessment and the scan day.
According to parent reports, 20 of 23 patients with OCD had begun taking at least 1 psychotropic medication on scanning day: selective serotonin reuptake inhibitors (n = 19), benzodiazepines (n = 2), stimulants (n = 2), tricyclic antidepressants (n = 2), atypical antipsychotics (n = 1), α2-adrenergic agonists (n = 1) and L-tryptophan (n = 1).
At initial clinic intake, approximately half (57 %; 13/23) of patients with OCD had current comorbid diagnoses and symptoms, including anxiety disorders (25%; 6/23), major depression (4%; 1/23), attention-deficit/hyperactivity disorder (17 %; 4/23), Tourette syndrome (8 %; 2/23) and tics (8 %; 2/23).
Behavioural results
As reported in an earlier paper,33 different levels of distress were elicited across OCD symptom dimensions; the symmetry or ”just right” condition robustly discriminated between groups (mean scores: patients with OCD 1.56/3.00; healthy controls 1.30/3.00; p < 0.006). The other dimensions did not show significant group effects (Appendix 1, Figure S3A, and associated text).
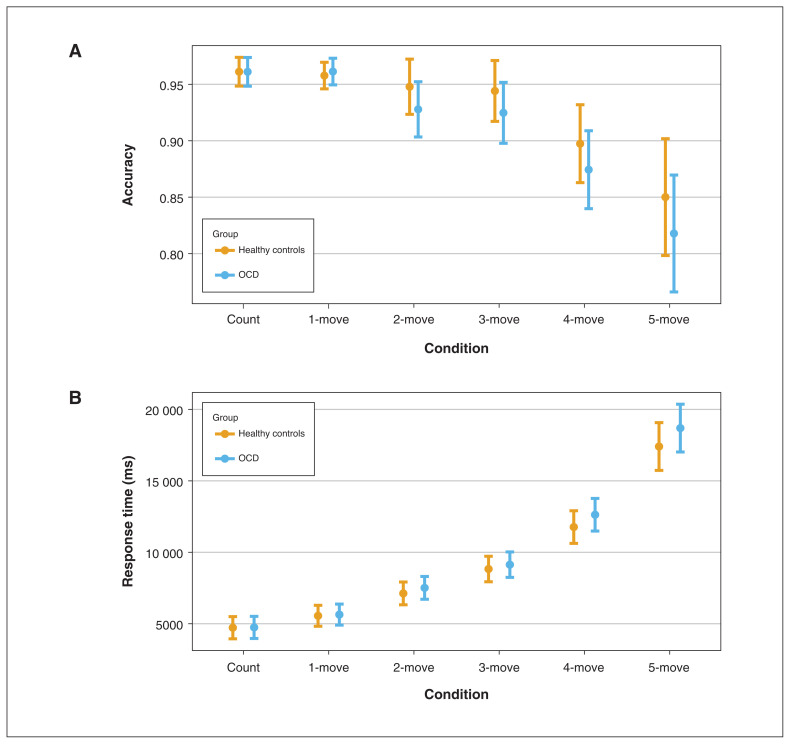
We analyzed Tower of London performance (accuracy and response time) as a function of group, time point (pre- and post-SPT) and task load. We found no significant main effect of group on accuracy (χ21 = 0.02; p = 0.89) or response time (χ21 = 0.07; p = 0.79), and no interaction between group and performance (Figure 2) or group and time point. Additional analyses are reported in Appendix 1, Figures S3B and S4).
Figure 2.
Tower of London performance in patients with obsessive–compulsive disorder (OCD; n = 23) and healthy controls (n = 23) by task load (i.e., condition). Main effect of task load on (A) accuracy (marginal pseudo-R2 = 0.17, p < 0.001) and (B) response time (marginal pseudo-R2 = 0.63, p < 0.001). We found no significant main effects or interactions with group type.
We observed no significant relationships between changes in Tower of London performance (accuracy or response time) and SPT-induced distress scores (Appendix 1, Figure S5). We observed no significant relationships between performance and OCD severity.
Neuroimaging findings
Combined ROI analysis
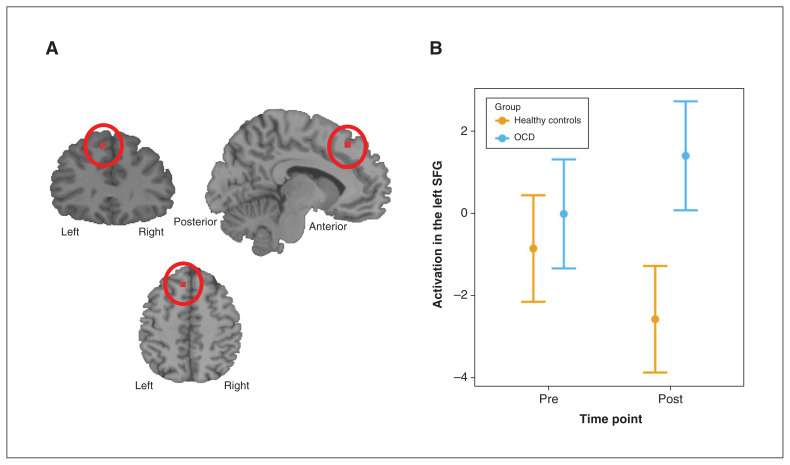
An a priori, ROI-constrained analysis (pFWE < 0.05, voxel-based correction) of the planning contrast resulted in a significant effect for the group × time point interaction: patients with OCD exhibited greater recruitment of the left SFG in the presupplementary motor area (BA 8; see Appendix 1, Table S3, for all ROIs used in the analysis) than the healthy controls (who exhibited a potential reduction) during the post-SPT Tower of London task compared to the pre-SPT Tower of London task (peak voxel F = 22.96; pFWE = 0.005, voxel-based correction; x, y, z = −9, 32, 50; BA 8; cluster extent = 7; Figure 3; standardized mean difference at post-SPT of 1.3 [95% confidence interval 0.7–1.9]). We found no significant results for the main effect of group. Results for the overall task and time point contrasts are reported in Appendix 1, Tables S4 to S6.
Figure 3.
Neuroimaging results for patients with obsessive–compulsive disorder (OCD; n = 23) versus healthy controls (n = 23), before and after after the symptom provocation task (SPT). (A) We found a significant group × time point interaction in a cluster around the left superior frontal gyrus (SFG; x, y, z = −9, 32, 50). (B) The extracted β values for peak activation of this cluster are presented graphically. Patients with OCD exhibited greater recruitment of the left SFG during planning post-SPT compared to pre-SPT. Left SFG recruitment appeared to decrease in the healthy controls from pre- to post-SPT, but this finding was nonsignificant.
Association analyses
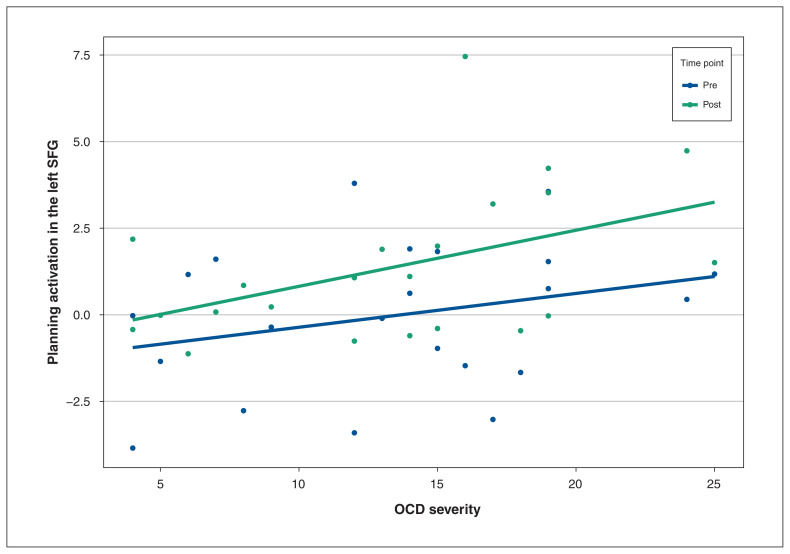
Severity of OCD was associated with left SFG recruitment at both time points (χ21 = 6.47; p = 0.011), such that greater OCD severity was associated with greater left SFG activation during planning overall (r = 0.35; Figure 4) after collapsing across the pre-SPT and post-SPT time points. OCD severity was not associated with change in left SFG activation from pre-SPT to post-SPT.
Figure 4.
Activation of the left superior frontal gyrus (SFG), pre- and post-symptom provocation task (SPT), by obsessive–compulsive disorder (OCD) severity. Greater OCD severity (patients with OCD; n = 23) was associated with greater recruitment of the left SFG both pre- and post-SPT (r = 0.35, p = 0.011).
We found no evidence of an association between self-reported OCD severity on scanning day and change from pre-SPT to post-SPT Tower of London task performance for either response time (χ21 = 0.21; p = 0.65) or accuracy (χ21 = 1.23; p = 0.27). We found no association between symptom provocation distress score and change in left SFG activation (all p values > 0.40; Appendix 1, Figure S2).
We performed a sensitivity analysis (removing participants with subclinical OCD symptoms) and recreated all figures (Appendix 1, Figures S6 to S10). We found no notable change in the pattern of results with the smaller sample.
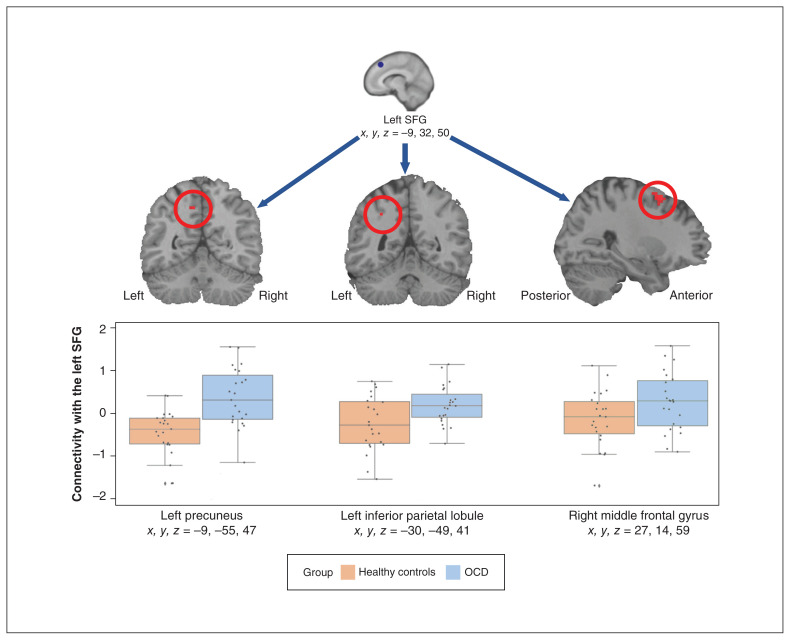
Seed-based connectivity analysis
We found a significant effect of group, indicating that patients with OCD (n = 23), compared to healthy controls (n = 23), showed significantly greater connectivity during planning than counting conditions between the left SFG seed (x, y, z = −9, 32, 50) and the left precuneus (x, y, z = −9, −55, 47), right middle frontal gyrus (x, y, z = 27, 14, 59) and left inferior parietal lobule (x, y, z = −30, −49, 41) in the combined ROI mask, and collapsing across time points (Figure 5 and Appendix 1, Table S7). Time point was not significant, but because patients with OCD always exhibited greater connectivity than healthy controls, the following should be noted: t tests conducted on the extracted connectivity values around the middle frontal gyrus, precuneus and inferior parietal lobule peaks showed significant differences both pre-SPT (p < 0.01) and post-SPT (p < 0.02) for the precuneus, but only pre-SPT (p < 0.01) and not post-SPT (p = 0.75) for the middle frontal gyrus. Extracted values for the left inferior parietal lobule reached significance only when time point was collapsed (p > 0.05), and not during individual time points.
Figure 5.
Results of the left superior frontal gyrus (SFG) seeded connectivity analysis. Using the planning versus counting condition contrast and a 6 mm seed in the left SFG, the connectivity analysis (patients with obsessive–compulsive disorder [OCD] v. healthy controls) showed significantly greater connectivity with the left precuneus, left inferior parietal lobule and right middle frontal gyrus (results were restricted to the combined region of interest mask). We found no interaction with time point and as a result, time point is collapsed in the figure. SPT = symptom provocation task.
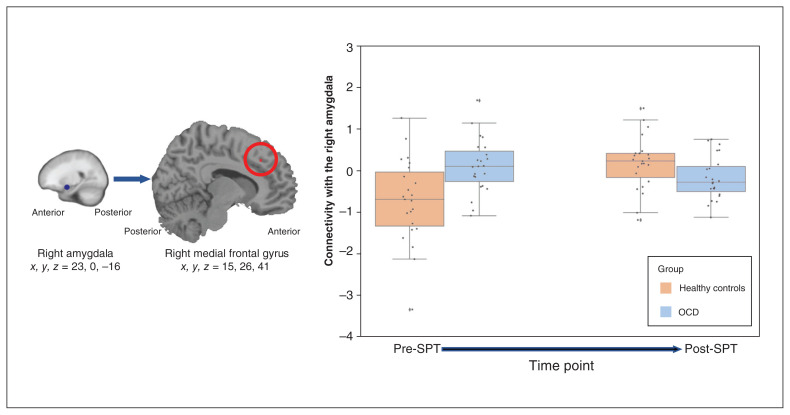
In contrast, connectivity results for the seed placed in the right amygdala (x, y, z = 23, 0, −16) was sensitive to time point and group (Figure 6) during planning versus counting conditions: in the combined ROI mask, the connectivity analysis found a significant 2-way interaction (group × time point) between the right amygdala and the right medial frontal gyrus (x, y, z = 15, 26, 41; BA 46; voxels = 5; F = 23.831; p = 0.018). Pre-SPT, patients with OCD showed greater connectivity between right amygdala and right middle frontal gyrus than healthy controls (p = 0.002). Post-SPT, this group difference disappeared (p = 0.08). We found no significant connectivity effects for the left amygdala.
Figure 6.
Results of the right amygdala seeded connectivity analysis. (A) Using the planning versus counting condition contrast and a 6 mm seed in the right amygdala, the connectivity analysis showed a significant relationship with the right medial frontal gyrus that changed over time. (B) The extracted connectivity values revealed that connectivity decreased for patients with obsessive–compulsive disorder (OCD; n = 23) and increased for healthy controls (n = 23) post-symptom provocation task (SPT) versus pre-SPT.
Whole-brain analysis
We conducted exploratory whole-brain analyses at pFWE < 0.05 (voxel-based correction) and found no significant main or interaction effects of group for the planning or task load contrasts. Time point (pre-/post-SPT) also did not discriminate between groups (Appendix 1, Tables S8 to S10).
Discussion
This was, to our knowledge, the first study to explore the effect of symptom provocation on planning performance in a pediatric sample of patients with OCD. Although children and youth with OCD in the present study did not demonstrate planning performance deficits or differences in whole-brain fMRI analyses, an ROI-restricted fMRI analysis found that patients with OCD exhibited greater recruitment of the left SFG (BA 8) following provoked distress compared to healthy controls. As well, left SFG activity was related to increased symptom severity, regardless of SPT-related distress. Furthermore, when we used the left SFG as a seed for functional connectivity analyses, we found that planning (versus counting) was related to increased connectivity with the left precuneus, the right middle frontal gyrus and the left inferior parietal lobule. These regions have previously been implicated in the successful completion of the Tower of London task.22 Our results hint that pediatric patients may over-recruit these areas to perform at par with healthy controls. Despite an absence of behavioural performance differences in working memory at low task loads, previous research in adults with OCD7 identified compensatory frontoparietal brain activity in patients with OCD compared to healthy controls.
A recently published review highlights the importance of the PFC in the etiology of OCD symptoms.42 One theory is that deficits in a PFC cognitive control network (such as the dorsal cognitive CSTC circuit described in previous neurocircuit-based models of OCD4,5,43) could lead directly to compulsions, because hypoactivity could cause impairments in inhibitory control and automatic engagement in compulsive behaviours, particularly in stressful circumstances. Hyperactivity in PFC regions at baseline and during symptom provocation is thought to reflect compensatory responses that boost performance of critical PFC executive control functions, including decision-making and goal-directed planning.42
In the results of the connectivity analysis, we also observed increased connectivity in patients with OCD compared to healthy controls between the amygdala and PFC pre-SPT, but not post-SPT. This finding was in contrast our hypothesis that symptom provocation would increase interference from affect-related brain areas, but was in line with our previous work (which did not find strong recruitment of the limbic network during symptom provocation in pediatric OCD33), and with a meta-analysis suggesting that amygdala hypoactivity is common in pediatric OCD,44 compared to the amygdala hyperactivity seen in adults with OCD.4 We have suggested31 that children may be particularly adept at regulating their emotional response to triggering events after medical and cognitive behavioural therapy. The mechanism may be hyperactivation of the medial frontal cortex, an adaptive response that reduces limbic interference described in other studies of medical intervention in patients with OCD.39,45 Future studies should use samples consisting of both medicated and nonmedicated patients with OCD to explore this mechanism further.
Only 1 other Tower of London neuroimaging study (and its follow-up) has been reported in pediatric OCD.31,46 Huyser and colleagues31 found a behavioural effect: pediatric patients with OCD (n = 25) versus healthy controls (n = 25) exhibited slower response times at baseline. Imaging revealed that patients with OCD were less likely to recruit frontal (BA 6/8/9) and parietal (BA 2/40) regions than healthy controls. These differences dissipated after cognitive behavioural therapy and remained absent at the 2-year follow-up (n = 15 per group) by van der Straten and colleagues.46 In the present study, most participants had begun pharmacological treatment and had completed at least 1 full round of group-based family cognitive behavioural therapy by the day of scanning; they may have been more like participants in the follow-up study.
Although our ROI analyses used similar coordinates and sphere sizes for dorsolateral and rostrolateral prefrontal brain areas, the study by van der Straten and colleagues46 did not include an ROI that captured activity in the left SFG, and thus could not find this effect.
Future studies should include ROIs related to the dorsal cognitive CSTC circuit (including the dorsal aspects of the PFC), given the current neurocircuit-based models of OCD4,5,43 and this circuit’s implication in executive function, such as working memory and planning, as well as emotion regulation.4
Limitations
Given the fixed-order design of the study, our findings of a group × time point interaction should be interpreted with caution. It is possible that increased left SFG recruitment post-SPT could be attributed to order effects such as learning, boredom or underlying changes in anxiety and self-monitoring as the session progressed. However, we found no significant left SFG main effect for time point (Appendix 1, Table S5).
The sample size in this study was also a limitation. Future mega-analyses, such as those conducted by the ENIGMAOCD working group,47 will pool raw, task-based fMRI data from multiple smaller studies such as this one. Although these mega-analyses will be unable to answer the specific question of how symptom provocation might directly influence planning, they will be able to look more broadly at questions related to affective and cognitive processing in patients with OCD across the lifespan. They will also be able to address the potential effect of biological sex.
Another limitation was that participants with common OCD comorbidities (anxiety, depression, attention-deficit/hyperactivity disorder and tic disorders) were included to improve the generalizability of our findings. Previous work39 found that these comorbidities were associated with decreased amygdalar activity in comparisons of patients with OCD versus healthy controls. Future studies should include measures such as the State–Trait Anxiety Inventory48 to better quantify these factors on the day of scanning.
Conclusion
The present study found alterations in activation and connectivity during planning after symptom provocation in children and youth with OCD versus healthy controls, in the absence of impaired planning performance. Future studies should use samples consisting of both medicated and nonmedicated patients to explore this idea further.
Supplementary Material
Acknowledgements
This work was supported by the British Columbia Children’s Hospital (BCCH), the Michael Smith Foundation for Health Research, the British Columbia Provincial Health Services Authority and the Canadian Institutes of Health Research. We are grateful to the clinical psychologists of the BCCH Provincial Obsessive–Compulsive Disorders Program, who administered and scored clinical measures at intake: Katherine McKenney, PhD, Annie Simpson, PhD, Robert Selles, PhD, and David Schuberth, PhD. We also thank colleagues who provided input on previous drafts (Todd Woodward, PhD, and Tamara Vanerwal, MD) and our research assistants and volunteers (Laura Belschner, MSc, Diana Franco Yamin, MA, Yayuk Joffres, MSc, Rachel Ho, BSc, Heping Sheng, MD, and Ryan Lim, BSc). We thank Donna Lang, PhD, and Manraj K.S. Heran, MD, for reviewing scans for incidental findings. Finally, we appreciate the time and effort given by our participants and their families.
Footnotes
Competing interests: F. Jaspers-Fayer and E. Stewart received support from the British Columbia Children’s Hospital Research Institute and the Michael Smith Foundation for Health Research. O.A. van den Heuvel has received grants from the Netherlands Organization for Health Research (project number: 91717306) and the National Institute of Mental Health (awarded to Blair Simpson with O.A. van den Heuvel as co-applicant; project number MH113250); consultation fees from Lundbeck and honoraria from Benecke de Tijdstroom; and has participated in an advisory board for the International OCD Foundation. E. Stewart received BC Provincial Health Services Authority research funding; and served on the scientific and clinical advisory board of the International OCD Foundation, the medical advisory board of the International Tourette Association of America, the Youth Development Instrument Provincial Practice and Policy Advisory Board and the scientific advisory committee for Anxiety Canada. No other competing interests declared.
Contributors: F. Jaspers-Fayer, J. Negreiros, E. Chan, O.A. van den Heuvel and E. Stewart designed the study. F. Jaspers-Fayer, S. Yao Lin, E. Chan, R. Ellwyn, B. Lin and E. Stewart acquired the data, which F. Jaspers-Fayer, S. Yao Lin, J.R. Best, A. Lillevik Thorsen, S. de Wit, O.A. van den Heuvel and E. Stewart analyzed. F. Jaspers-Fayer, J.R. Best, A. Lillevik Thorsen, B. Lin and E. Stewart wrote the article, which F. Jaspers-Fayer, S. Yao Lin, J.R. Best, A. Lillevik Thorsen, J. Negreiros, E. Chan, R. Ellwyn, S. de Wit, O.A. van den Heuvel and E. Stewart reviewed. All authors approved the final version to be published, agreed to be accountable for all aspects of the work and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Heyman I, Fombonne E, Simmons H, et al. Prevalence of obsessive–compulsive disorder in the British nationwide survey of child mental health. Br J Psychiatry 2001;179:324–9. [DOI] [PubMed] [Google Scholar]
- 2.Valleni-Basile LA, Garrison CZ, Jackson KL, et al. Frequency of obsessive–compulsive disorder in a community sample of young adolescents. J Am Acad Child Adolesc Psychiatry 1994;33:782–91. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth edition. Arlington (VA): American Psychiatric Association Publishing; 2013. [Google Scholar]
- 4.van den Heuvel OA, van Wingen G, Soriano-Mas C, et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol 2016;26:810–27. [DOI] [PubMed] [Google Scholar]
- 5.Milad MR, Rauch SL. Obsessive–compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci 2012; 16:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Heuvel OA, Matrix-Cols D, Zwitser G, et al. Common limbic and frontal-striatal disturbances in patients with obsessive compulsive disorder, panic disorder and hypochondriasis. Psychol Med 2011;41:2399–410. [DOI] [PubMed] [Google Scholar]
- 7.de Vries FE, de Wit SJ, Cath DC, et al. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive–compulsive disorder. Biol Psychiatry 2014;76:878–87. [DOI] [PubMed] [Google Scholar]
- 8.van Velzen LS, de Wit SJ, Curcic-Blake B, et al. Altered inhibitionrelated frontolimbic connectivity in obsessive–compulsive disorder. Hum Brain Mapp 2015;36:4064–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashyap H, Abramovitch A. Neuropsychological research in obsessive–compulsive disorder: current status and future directions. Front Psychiatry 2021;12:721601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder HR, Kaiser RH, Warren SL, et al. Obsessive–compulsive disorder is associated with broad impairments in executive function: a meta-analysis. Clin Psychol Sci 2015;3:301–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin NY, Lee TY, Kim W, et al. Cognitive functioning in obsessive–compulsive disorder: a meta-analysis. Psychol Med 2014;44:1121–30. [DOI] [PubMed] [Google Scholar]
- 12.Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin Psychol Rev 2013;33:1163–71. [DOI] [PubMed] [Google Scholar]
- 13.Abramovitch A, Abramowitz JS, Mittelman A, et al. Neuropsychological test performance in pediatric obsessive–compulsive disorder— a meta-analysis. J Child Psychol Psychiatry 2015;56:837–47. [DOI] [PubMed] [Google Scholar]
- 14.Negreiros J, Belschner L, Best J, et al. Neurocognitive risk markers in pediatric obsessive–compulsive disorder. J Child Psychol Psychiatry 2020;61:605–13. [DOI] [PubMed] [Google Scholar]
- 15.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 1982;298:199–209. [DOI] [PubMed] [Google Scholar]
- 16.Bey K, Kaufmann C, Lennertz L, et al. Impaired planning in patients with obsessive compulsive disorder and unaffected first degree relatives: evidence for a cognitive endophenotype. J Anxiety Disord 2018;57:24–30. [DOI] [PubMed] [Google Scholar]
- 17.Katrin Kuelz A, Riemann D, Halsband U, et al. Neuropsychological impairment in obsessive-compulsive disorder—improvement over the course of cognitive behavioral treatment. J Clin Exp Neuropsychol 2006;28:1273–87. [DOI] [PubMed] [Google Scholar]
- 18.Nielen MMA, den Boer JA. Neuropsychiological performance of OCD patients before and after treatment with fluoxetine: evidence for persistent cognitive deficits. Psychol Med 2003;33:917–25. [DOI] [PubMed] [Google Scholar]
- 19.van den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Frontalstriatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry 2005;62:301–9. [DOI] [PubMed] [Google Scholar]
- 20.Vaghi MM, Hampshire A, Fineberg NA, et al. Hypoactivation and dysconnectivity of a frontostriatal circuit during goal-directed planning as an endophenotype for obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 2017;2:655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell R, Maruff P, Kyrios M, et al. Cognitive deficits in obsessive–compulsive disorder on tests of frontal-striatal function. Biol Psychiatry 1998;43:348–57. [DOI] [PubMed] [Google Scholar]
- 22.Nitschke K, Kostering L, Finkel L, et al. A meta-analysis on the neural basis of planning: activation likelihood estimation of functional brain imaging results in the Tower of London tasks. Hum Brain Mapp 2017;38:396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotge JY, Guehl D, Dilharreguy B, et al. Provocation of obsessive–compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J Psychiatry Neurosci 2008;33:405–12. [PMC free article] [PubMed] [Google Scholar]
- 24.Storch EA, Murphy T, Adkins J, et al. The Children’s Yale–Brown Obsessive–Compulsive Scale: psychometric properties of child-and parent-report formats. J Anxiety Disord 2006;20:1055–70. [DOI] [PubMed] [Google Scholar]
- 25.Ivarsson T, Melin K, Wallin L. Categorical and dimensional aspects of co-morbidity in obsessive-compulsive disorder (OCD). Eur Child Adolesc Psychiatry 2008;17:20–31. [DOI] [PubMed] [Google Scholar]
- 26.Silverman W, Albano A. The anxiety disorders interview schedule for children–IV (child and parent versions). New York: Psychological Corporation; 1996. [Google Scholar]
- 27.Scahill L, Riddle M, McSwiggin-Hardin M, et al. Children’s Yale–Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 1997;36:844–52. [DOI] [PubMed] [Google Scholar]
- 28.Storch EA, Kaufman DAS, Bagner D, et al. Florida obsessive–compulsive inventory: development, reliability, and validity. J Clin Psychol 2007;63:851–9. [DOI] [PubMed] [Google Scholar]
- 29.Marshall WA, Tanner J. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall WA, Tanner J. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huyser C, Veltman D, Wolters L, et al. Functional magnetic resonance imaging during planning before and after cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 2010;49:1238–48. [DOI] [PubMed] [Google Scholar]
- 32.van den Heuvel OA, Groenewegen HJ, Barkhof F, et al. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of the Tower of London task. Neuroimage 2003;18:367–74. [DOI] [PubMed] [Google Scholar]
- 33.Jaspers-Fayer F, Lin SY, Chan E, et al. Neural correlates of symptom provocation in pediatric obsessive–compulsive disorder. Neuroimage Clin 2019;24:102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bloch MH, Landeros-Weisenberger A, Rosario M, et al. Meta-analysis of symptom structure of obsessive–compulsive disorder. Am J Psychiatry 2008;165:1532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaspers-Fayer F, Negreiros J, Lin SY, et al. Cognitive planning neural correlates in a pediatric monozygotic twin pair discordant for obsessive-compulsive disorder: exploring potential application in precision medicine. J Clin Psychiatry 2017;78:e1320. [DOI] [PubMed] [Google Scholar]
- 36.Andersson JLR, Hutton C, Ashburner J, et al. Modeling geometric deformations in EPI time series. Neuroimage 2001;13:903–19. [DOI] [PubMed] [Google Scholar]
- 37.McFarquhar M, McKie A, Emsley R, et al. Multivariate and repeated measures (MRM): a new toolbox for dependent and multimodal group-level neuroimaing data. Neuroimage 2016; 132:373–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFarquhar M. Modeling group-level repeated measurements of neuroimaging data using the univariate general linear model. Front Neurosci 2019;13:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorsen AL, Hagland P, Radua J, et al. Emotional processing in obsessive-compulsive disorder: a systematic review and meta-analysis of 25 functional neuroimaging studies. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lillevik Thorson A, de Wit SJ, Hagland P, et al. Stable inhibition-related inferior frontal hypoactivation and fronto-limbic hyperconnectivity in obsessive–compulsive disorder after concentrated exposure therapy. Neuroimage Clin 2020;28:102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. Neuroimage 2014;92:381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmari SE, Rauch SL. The prefrontal cortex and OCD. Neuropsychopharmacology 2022;47:211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shephard E, Batistuzzo MC, Hoexter MQ, et al. Neurocircuit models of obsessive-compulsive disorder: limitations and future directions for research. Braz J Psychiatry 2022;44:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brem S, Hauser TU, Iannaaone R, et al. Neuroimaging of cognitive brain function in paediatric obsessive-compulsive disorder: a review of the literature and preliminary meta-analysis. J Neural Transm (Vienna) 2012;119:1425–48. [DOI] [PubMed] [Google Scholar]
- 45.van den Heuvel OA, de Wit SJ. Medial frontal hyperactivation in the developing obsessive-compulsive disorder brain: an adaptive response rescued by medication related reduction of limbic interference. J Am Acad Child Adolesc Psychiatry 2018;57:368–9. [DOI] [PubMed] [Google Scholar]
- 46.van der Straten A, Huyser C, Wolters L, et al. Long-term effects of cognitive behavioral therapy on planning and prefrontal cortex function in pediatric obsessive–compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3:320–8. [DOI] [PubMed] [Google Scholar]
- 47.van den Heuvel OA, Boedhoe PSW, Bertolin S, et al. An overview of the first 5 years of the ENIGMA obsessive-compulsive disorder working group: the power of worldwide collaboration. Hum Brain Mapp 2022;43:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spielberger CD, Gorssuch RL, Lushen PR. Manual for the state-trait anxiety inventory. Sunnyvale (CA): Consulting Psychologists Press; 1983. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.