Abstract
Treatment of patients with patent Wuchereria bancrofti infection results in an acute clinical reaction and peripheral eosinophilia. To investigate the dynamics of the eosinophil response, changes in eosinophil activation and degranulation and plasma levels of eosinophil-active chemokines and cytokines were studied in 15 microfilaremic individuals in south India by sequential blood sampling before and after administration of 300 mg of diethylcarbamazine (DEC). Clinical symptoms occurred within 24 h. Plasma interleukin-5 (IL-5) and RANTES levels peaked 1 to 2 days posttreatment, preceding a peak peripheral eosinophil count at day 4. Major basic protein secretion from eosinophils paralleled IL-5 secretion, while levels of eosinophil-derived neurotoxin peaked at day 13 after treatment. Expression of the activation markers HLA-DR and CD25 on eosinophils rose markedly immediately after treatment, while expression of VLA-4 and α4β7 showed an early peak within 24 h and a second peak at day 13. Thus, the posttreatment reactions seen in filarial infections can be divided into an early phase with killing of microfilariae, clinical symptomatology, increases in plasma IL-5 and RANTES levels, and eosinophil activation and degranulation and a later phase with expression of surface integrins on eosinophils, recruitment of eosinophils from the bone marrow to tissues, and clearance of parasite antigen.
The current view of eosinophil production, migration, activation, and degranulation after a perturbation of the normal homeostatic environment suggests that eosinophils, in response to a primarily tissue-based signal such as a chemokine, increase the expression of β1- and β2-integrins and other adhesion molecules (e.g., CD44) to allow for receptor-mediated transendothelial migration (TEM). During their migration to sites of allergic or parasitic inflammation, eosinophils become activated, upregulate the surface expression of HLA-DR, CD25, and CD69, and degranulate when appropriately stimulated. The degranulation products, major basic protein (MBP), eosinophil-derived neurotoxin (EDN), eosinophil cationic protein (ECP), and eosinophil peroxidase (EPO), along with cytokines such as interleukin-5 (IL-5), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, and IL-10, act locally as well as at distant sites to promote growth and differentiation of eosinophil precursors.
Much of the information to date on eosinophil behavior and degranulation has been obtained in vitro. The eosinophilia that occurs following treatment of helminth infections (13, 17, 25, 27, 31) provides a unique physiologic system in which to study an evolving eosinophil response, the kinetics of secretion of eosinophil-active cytokines and degranulation products, and the expression of cell surface molecules such as integrins in relation to observed clinical and physiological events. In the present study, we sought to formulate a comprehensive picture of the various clinical and immunologic events that occur following treatment of bancroftian filariasis with diethylcarbamazine (DEC), focusing on eosinophil surface activation and degranulation and the kinetics of a variety of eosinophil-active cytokines such as GM-CSF and IL-5, inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and IL-6, and the chemokines important in eosinophil regulation, eotaxin and RANTES.
MATERIALS AND METHODS
Patients.
The study was carried out in and around Madras, India, an area highly endemic for Wuchereria bancrofti. Fifteen male microfilaremic patients were identified from the records of the municipal corporation filariasis control program, and informed consent was obtained prior to enrollment in the study. The patients ranged in age from 21 to 45 years and were healthy by history and physical examination at the time of the study. None had been previously diagnosed with filarial infection, and none had received antifilarial treatment. Four uninfected healthy individuals (three female and one male) from the same geographic area, ranging in age from 22 to 35 years, were used as controls.
Study design.
Informed consent was obtained from all patients; the study protocol was approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes Health, Bethesda, Md., and consent of the ethics committees of the participating institutes in India was obtained.
Prior to drug administration, blood was drawn between 9:00 and 10:00 p.m. on two consecutive nights, and 1 ml on each night was filtered through a 2-μm-pore-size Nuclepore filter to obtain a microfilarial count. After a history, physical examination, and baseline blood work on the day of treatment, a single dose of 300 mg of DEC was administered orally. Blood samples in EDTA were obtained at 1.5, 24, 48, and 96 h and at 6 and 13 days after DEC administration for complete blood count with differential and for flow cytometry for analysis of the surface phenotype of eosinophils. Plasma was collected at each time point and stored at −70°C until used. Control individuals were studied by using the same protocol except for night blood assays. Absence of filarial infection in control subjects was confirmed by a negative immunochromatographic filarial antigen test (ICT Diagnostics, Townsville, Queensland, Australia). Posttreatment adverse effects were graded as described previously (23). Briefly, each symptom was assigned a score between 0 and 3 depending on occurrence and severity, with 0 indicating absence and 3 denoting the greatest severity.
Measurement of cytokines, chemokines, and eosinophil degranulation products.
The measurements were performed by enzyme-linked immunosorbent assay (ELISA). The IL-5, RANTES, IL-10, IL-4, gamma interferon (IFN-γ), and GM-CSF assays used antibody pairs and techniques described previously (35). TNF-α levels were measured by using a commercially available high-sensitivity kit (Endogen; Cambridge, Mass.). Eotaxin levels were kindly assayed by ELISA by Adele Hartnell at the Heart, Lung and Blood Institute (London, England). MBP and EDN levels were assayed by a sandwich ELISA developed in the laboratory (unpublished data), using monoclonal and polyclonal antibodies raised against eosinophil granule proteins. EDN and MBP were isolated from a patient with hypereosinophilia and purified according to a technique described previously (2). Monoclonal antibodies (MAbs) were raised in mice, and purified polyclonal antibodies from immunized rabbits were used in the detection step. All proteins and MAbs were tested to ensure specificity, and all were monospecific. Briefly, for MBP, 96-well U-bottom Immulon plates were coated with an anti-MBP MAb at 2.5 μg/ml in phosphate-buffered saline (PBS) and left at 4°C overnight. After six washes with PBS–0.025% Tween, plates were blocked with 1× PBS with 0.05% Tween 20 and 5% BSA for 1 h at 37°C. After a further wash, plasma samples were placed on the plate in duplicate at dilutions of 1:10 and 1:50 for each sample. Purified MBP was used to construct the standard curve. After an overnight incubation at 4°C, rabbit anti-human MBP was used at a dilution of 1:5,000 and incubated at 37°C for 2 h. Following another wash, goat anti-rabbit alkaline phosphatase was added at 1:1,000 for 1 h at 37°C, and the plates were developed with p-nitrophenyl phosphate substrate and read on a kinetic microplate reader (Molecular Devices, Sunnyvale, Calif.). The sensitivity of the assay was 1.9 ng/ml.
The procedure for EDN was as described above except that an EDN-specific MAb was used to coat plates and purified EDN diluted in ELISA diluent was used for the standard curve. Rabbit anti-human EDN was used as the secondary antibody, and the level of detection was 7.6 ng/ml.
Flow cytometry sample preparation.
The relevant MAbs (5 to 10 μl) were added to prewetted Falcon 2054 tubes followed by 50 μl of whole blood and 50 μl of Hanks balanced salt solution (HBSS) (without Ca2+, Mg2+, or phenol red) with 0.2% BSA and 0.1% NaN3. The tubes were then vortexed and incubated in the dark for 30 min at 4°C. Following a wash with HBSS, 1 ml of FACS (fluorescence-activated cell sorting) lysing solution was added to each tube. The samples were vortexed vigorously and then incubated in the dark at room temperature for 15 min. When necessary, this lysis step was repeated for 5 min to completely clear the erythrocyte pellet. Following two more washes with HBSS, the cell pellet was resuspended in 250 μl of HBSS with 1% paraformaldehyde and stored at 4°C until analysis. All steps except the erythrocyte lysis were carried out on ice.
Flow cytometry sample analysis.
Samples were analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.). Isotype controls (Caltag, South San Francisco, Calif.) were used to set quadrants, and compensation was adjusted with single-color controls: anti-CD3 conjugated with fluorescein isothiocyanate (FITC) and phycoerythrin (PE) (clone UCHT1; Immunotech, Westbrook, Maine) and anti-CD3-Tricolor (clone S4.1; Caltag). Eosinophils were gated on a plot of side scatter (x axis) versus anti-CD16-PE (3G8; Caltag) (y axis), a method that has been found (15) to unequivocally delineate the eosinophils from the neutrophil cluster. Anti-CD16-PE or anti-CD16-FITC (3G8) was used in all relevant tubes to define eosinophils that are uniformly CD16 negative. The following panel of MAbs against surface activation markers and adhesion molecules was used to study eosinophils: anti-HLA-DR-PE (TU 36; Caltag), anti-CD25-FITC (CD25-3G10; Caltag), anti-CD11a-FITC (25.3; Immunotech), anti-CD11b-FITC (Bear1; Immunotech), anti-VLA-4-FITC (HP2.1; Immunotech), anti-CD23-FITC (TU 1; Caltag), anti-CD69-FITC (L78; Becton Dickinson), anti-CD44-FITC (Caltag), and anti-α4β7 (act-1; kindly supplied by Leukosite [Cambridge, Mass.], conjugated with FITC by Molecular Probes [Eugene, Oreg.]). Quadrants were drawn by applying gates for the eosinophil population to a plot of the isotype control. Data were analyzed with CELLQuest software, and both percent positivity and the mean fluorescence intensities of all markers were calculated.
Statistics.
Statistical analyses were performed by the Mann-Whitney test, Wilcoxon test, and Spearman rank correlation where appropriate.
RESULTS
Study population.
The patients in this study ranged in age from 21 to 40 years. The initial microfilarial counts ranged from 15 to 1,000/ml, with a geometric mean (GM) of 250/ml; 11 of 15 had initial microfilarial counts greater than 100/ml. The single dose of DEC resulted in a 97.7% drop in microfilarial count by 24 h, followed by a rebound and then a slower decline. By day 6, the microfilarial count had dropped to 19.5% of the pretreatment value (data not shown).
Following treatment, the most common symptoms were fever, headache, lethargy, and myalgias, which began within 4 h after DEC administration, peaked within 24 h, and rarely lasted beyond 48 h. There was a highly significant correlation between initial parasite levels and symptom severity at 12 h (P = 0.0025) and 24 h (P = 0.0019) posttreatment. Uninfected control individuals had no clinical reactions following DEC administration.
Eosinophil responses to treatment.
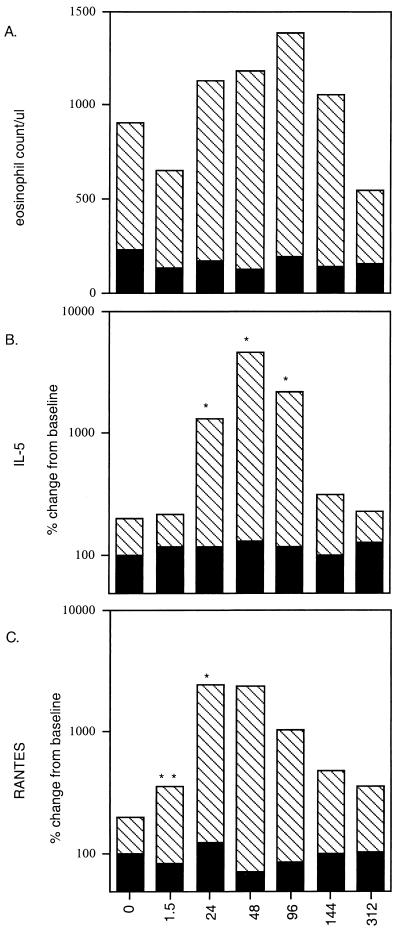
Baseline absolute eosinophil numbers ranged between 100 and 5,310/μl, with a GM of 678/μl, but there was no correlation between initial eosinophil percentage or absolute eosinophil counts and initial microfilarial counts. Within 1.5 h after DEC administration, there was a 24% drop in the GM of the peripheral eosinophil count. Following this initial drop in circulating eosinophils, there was a progressive rise in number, reaching a peak (maximum 200% of baseline) by day 3 or 4 after treatment (Fig. 1A). Eosinophil counts then declined to baseline between days 6 and 13. Healthy uninfected controls did not have elevated eosinophil counts at baseline, nor did the counts change significantly following DEC administration.
FIG. 1.
Kinetics of the peripheral eosinophil count and levels of plasma IL-5 and RANTES after treatment. Eosinophil counts expressed as the GM (A), plasma IL-5 levels (B), and RANTES levels (C) in study patients as a function of hours posttreatment (horizontal axes) are shown as hatched bars, while corresponding levels in controls are shown as solid bars. ∗, P < 0.01; ∗∗, P < 0.03.
Cytokine and chemokine response to treatment.
To study the kinetics of various cytokines and chemokines known to affect eosinophil production, activation, and migration, plasma levels of IL-5, GM-CSF, RANTES, and eotaxin were measured before and after treatment. In addition, plasma levels of cytokines important in filarial infections, i.e., IL-4, IL-10, and IFN-γ, were assayed at the same time points. Thirteen of 15 patients had no measurable plasma IL-5 at baseline, while 2 patients had IL-5 levels of 29.8 and 31 pg/ml at time zero. Following DEC administration, there was a significant rise (900% increase over baseline; GM, 1,182 pg/ml [P < 0.01]) in serum IL-5 levels in all patients by 24 h after treatment, which peaked at 48 h (1,700% over baseline; P < 0.01) at a GM level of 4,490 pg/ml (Fig. 1B). Levels were still elevated significantly at day 4 (GM, 2,074 pg/ml; P < 0.01) but then declined quickly to baseline by a week after DEC administration (Fig. 1B). The rise and peak of serum IL-5 predated the peak of eosinophilia by 2 to 3 days, suggesting that the early surge of IL-5 in the circulation is crucial to the maturation of eosinophils from bone marrow precursors and to their recruitment into the circulation.
GM-CSF, another eosinophilopoietic cytokine, was detected variably in 4 of 15 patients, but there were no observed patterns of secretion (data for this and the following cytokines are not shown). Similarly, TNF-α, IL-6, IFN-γ, IL-4, and IL-10 were rarely detected and, when found, were not related to either the eosinophil counts or the clinical reactions. There was, however, a significant amount of RANTES (GM, 13,936 pg/ml in patients) in the circulation at baseline. RANTES levels began rising significantly within 1.5 h (Fig. 1C) following treatment (P = 0.03), and by 24 h, there was a 2,304% (GM) increase over baseline (P < 0.01). This elevation was maintained until 48 h after treatment and then declined toward baseline by days 6 to 13, closely paralleling the secretion kinetics of IL-5. Levels of eotaxin in patient plasma, by contrast, remained unchanged after treatment (data not shown) and bore no relation to the eosinophil count.
Assessment of eosinophil degranulation products MBP and EDN.
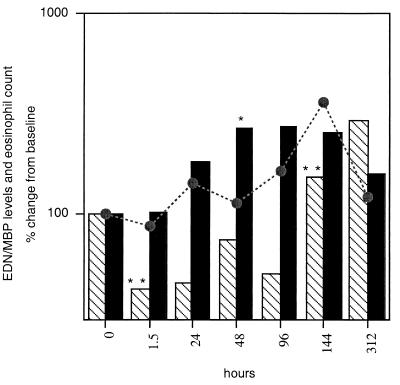
Measurement of eosinophil degranulation products in plasma indicated that eosinophils were actively degranulating, presumably as part of the process of microfilarial killing (Fig. 2). MBP was detected in the plasma at baseline (GM, 134.6 pg/ml) and started rising within 24 h after treatment. The kinetics of secretion of MBP closely paralleled that of IL-5, suggesting coordinated secretion of both IL-5 and MBP.
FIG. 2.
Kinetics of MBP (solid bars) and EDN (hatched bars) relative to the eosinophil count (broken line) as a function of hours after treatment. ∗, P < 0.003; ∗∗, P < 0.05.
A 264% increase in plasma levels of MBP was observed at 48 h (P < 0.003), prior to the greatest change in eosinophil count (Fig. 2), and levels remained elevated until day 6. EDN, however, appeared to be released and regulated differently (Fig. 2). Although the level of this degranulation product at baseline was comparable to MBP levels (135 pg/ml), a significant drop was observed within the first 24 h after treatment, beginning at 1.5 h (P < 0.05). Thereafter, a significant rise in EDN plasma levels appeared only at day 6 (152%; P < 0.05), just after the peak in eosinophil count (360% of baseline [Fig. 1]), suggesting that EDN was being secreted by the newly matured population of eosinophils entering the circulation from the bone marrow.
Flow cytometry.
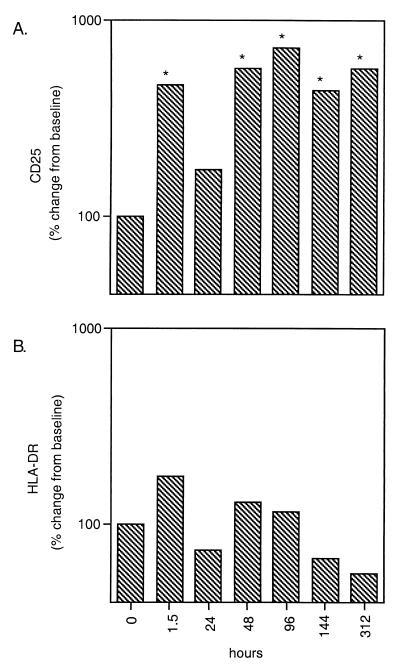
Expression of the activation markers CD25 and HLA-DR on eosinophils from the patient group as a whole (Fig. 3) was assessed. A very rapid increase in percent expression of HLA-DR (173% of baseline) and CD25, the IL-2 receptor (465.3% of baseline; P = 0.01), was observed as early as 1.5 h after treatment. Following a dip at 24 h, expression of CD25 remained significantly higher than baseline for the next 13 days (P < 0.05 for all time points; maximum, 722% of baseline); the percent expression of HLA-DR, while elevated for the first 4 days, did not show a statistically significant difference from baseline (maximum, 173%). Expression of the early leukocyte activation marker, CD69, was also elevated 24 to 48 h after treatment, but the change was not statistically significant (data not shown).
FIG. 3.
Expression of CD25 (A) and HLA-DR (B) on eosinophils as a function of hours posttreatment. ∗, P < 0.05.
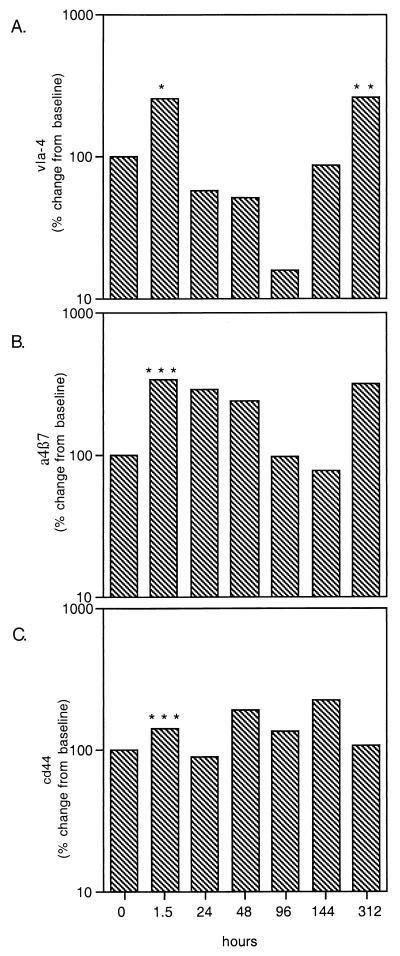
As the β1-integrins VLA-4 and α4β7 are known to play a crucial role in eosinophil tethering to, and trafficking through, the endothelial lining, their expression—along with the expression of the hyaluronate receptor CD44—was examined on the surface of eosinophils before and after treatment. Surface expression of the integrins displayed a bimodal pattern (Fig. 4A and B) with early upregulation starting 1.5 h after treatment (P < 0.04 for both), subsequent decline in expression, and a second peak at day 13. The initial increase in expression of VLA-4 was 256% of baseline (P = 0.01), while α4β7 was 334% of baseline at that time point. Surface expression of both markers declined until day 4 for VLA-4 and day 6 for α4β7; by day 13, a significant increase was again noted (257% of baseline for VLA-4 [P = 0.05] and 312% of baseline for α4β7). CD44 expression increased in the first 1.5 h to 139% of baseline (Fig. 4C; P = 0.04) and remained variably elevated for the first 7 days (maximum, 220%).
FIG. 4.
Expression of integrins VLA-4 (A), α4β7 (B), and CD44 (C) on eosinophils as a function of hours posttreatment. ∗, P = 0.01; ∗∗, P = 0.05; ∗∗∗, P < 0.04.
Expression of the β2-integrins CD11a and CD11b was also studied because of their important role in eosinophil adhesion, degranulation, and trafficking. No change was observed in expression of CD11b on the eosinophil surface at any time following DEC (data not shown), while there was a significant decline in expression of CD11a (74% of baseline at 1.5 h; P = 0.04) after treatment, with the nadir observed at day 4 (12% of baseline), followed by a return to baseline levels by day 13 (data not shown). CD23 (the low-affinity IgE receptor) expression varied after treatment, with an early significant increase (239%; P = 0.01) but no significant changes from baseline thereafter (data not shown).
Control group.
To ascertain that the surface changes on eosinophils were not caused by a nonspecific effect of DEC, we repeated the complete blood counts and analysis of surface changes on eosinophils by flow cytometry at identical time points in four uninfected healthy individuals following administration of a single dose of 300 mg of DEC. As mentioned earlier, no clinical symptoms occurred. There was a small, nonsignificant drop in eosinophil count at 1.5 h after DEC administration in all four individuals, but no further alteration in eosinophil count occurred. In addition, plasma levels of IL-5, RANTES (Fig. 1), MBP, and EDN (data not shown) did not vary appreciably from baseline at any time point. Similarly, we observed no consistent patterns or significant differences in surface receptor expression on eosinophils or their mean fluorescence intensities (data not shown).
DISCUSSION
The generally quiescent relationship that normally exists between the immune systems of chronically microfilaremic individuals and the filarial parasites they harbor is reflected by a lack of clinical symptomatology, absence of significant peripheral blood eosinophilia, and relatively low levels of filaria-specific immunoglobulin G (IgG). This tolerance is maintained at the T-cell level, as evidenced by the limited production of IL-2 and IFN-γ in response to specific filarial antigens in microfilaremic individuals. With treatment, however, this equilibrium is perturbed such that significant clinical reactions and eosinophilia characteristically occur. In this study, we focused on the eosinophilia that occurs following treatment of patent W. bancrofti infection to study the regulatory events underlying eosinophil activation, trafficking, and degranulation in a physiologic system in vivo.
Reflective of many similar findings in the literature (10, 21, 30), the peripheral microfilarial count fell precipitously at 24 h following treatment, followed by an increase in their number and a subsequent slower decline.
Administration of DEC, a micro- and macrofilaricidal drug, and/or ivermectin, a microfilaricidal drug, leads to rapid killing of microfilariae in the circulation and an immediate inflammatory response characterized in most patients by a brief serum sickness-like illness and eosinophilia. In our study, clinical symptoms occurred very quickly after treatment and peaked within 24 h, correlating with the onset of microfilarial killing in the circulation.
Eosinophilia was not prominent prior to treatment, occurring in only 3 of 15 patients. Following treatment, as has been observed in other studies (9, 25), there was an immediate drop in peripheral eosinophil count at 1.5 h, which likely reflects the immediate migration of eosinophils into tissues or, possibly, their adhesion to dying microfilariae within the circulation (7). The upregulation of surface integrins on eosinophils in this same time period (Fig. 4) lends additional support to these hypotheses.
The kinetics of the increase in peripheral eosinophil number and the peak occurring 4 days after treatment parallel, almost exactly, observations made in previous studies (1, 25, 27). It is now well established that IL-5 is a crucial factor in the recruitment of eosinophils into the circulation or tissues (8, 17, 24, 26, 29, 36), with important effects on the maturation and release of eosinophils from precursor cells in bone marrow and protection from apoptosis (34); we confirmed the definite relationship that exists between the peak of IL-5 secretion at 48 h following treatment and the recruitment and subsequent peak of eosinophils in the circulation. The fact that the eosinophil peak occurred at day 4 rather than at day 6 or 7 as reported in the literature could be reflective of the synergistic effect of RANTES on the eosinophil response or of the recruitment of eosinophils from the tissues into the circulation.
RANTES, a powerful chemoattractant for eosinophils (12, 33), is released from multiple sources including platelets, T cells, and monocytes. It stimulates eosinophil cationic protein release from eosinophils (36) and an increase in their TEM by inducing the expression of surface adhesion molecules (12). In our study, RANTES secretion paralleled the secretion of IL-5, suggesting that this chemokine was also instrumental in recruitment of eosinophils into the circulation, expression of adhesion receptors, and, perhaps more important, recruitment of eosinophils to sites of inflammation (36). Plasma eotaxin levels did not change after treatment; however, this may not necessarily reflect its importance in eosinophil recruitment at local sites of inflammation. Studies in mice indicate that systemic administration of eotaxin induced a sequestration of eosinophils from the tissues into the blood, while its subcutaneous injection induced a local tissue eosinophilia; IL-5, not eotaxin, mobilized the bone marrow pool of eosinophils (29).
Eosinophil degranulation, the release of toxic cationic proteins from secondary granules within the cell, is one of the major effector functions of eosinophils. Although the toxicity of these proteins varies (2, 18), MBP produces most helminthotoxic activity (2, 6, 18, 40) by virtue of its relative abundance in the eosinophil granule. Secretion of all four granule proteins is stimulated by the cytokines IL-3, IL-5, and GM-CSF (20). IL-5, however, though a strong secretagogue for human eosinophils, appears to need a secondary event, e.g., adhesion via β2 integrins, for degranulation to occur (14).
In studies of the Mazzotti reaction following treatment of onchocerciasis with DEC (3, 22) or amocarzine (16), levels of both EDN and MBP increased and peaked within the first 5 days, with EDN appearing first (within 8 h). In association with eosinopenia, there is an accumulation of eosinophils and deposition of MBP around dying microfilariae in the skin (16, 22) and possibly an amplification of the inflammatory reaction via eosinophil granule-mediated mast cell degranulation (3). In a study of bancroftian filariasis following treatment with DEC for 14 days (1), the peak in mean MBP levels occurred at day 8. Stimulation of granule protein secretion does, however, occur variably in other conditions such as cancer (39) and may even occur spontaneously, depending on incubation temperatures and time before centrifugation and recovery of serum during processing of blood samples (32).
In the present study, MBP was detected at baseline prior to treatment (mean, 134.6 pg/ml). Baseline MBP levels in a variety of parasitic infections (38) such as onchocerciasis, bancroftian filariasis, and schistosomiasis tend to be higher than in other protozoal infections such as malaria. Levels of MBP began to rise within 24 h, and the kinetics of its secretion closely paralleled that of IL-5. Previous evidence indicates that IL-5 is secreted by eosinophils (11) and that IL-5 and GM-CSF colocalize to MBP-positive granules (28); it is plausible to speculate that both IL-5 and MBP are secreted from eosinophils in the circulation or that IL-5 stimulated the release of MBP from eosinophils. By 48 h following treatment, levels of MBP had risen 264% from baseline and remained elevated for 7 days, reflecting delayed clearance of MBP from the circulation, further secretion from newly recruited eosinophils at later time points, or secretion of MBP from cells in tissues or in bone marrow (1). Our results indicate that the kinetics of secretion of EDN were quite different: levels actually fell from a baseline of 135 pg/ml and began to rise again only around day 7. Since EDN levels began to rise coincident with the peak of eosinophilia, it seems likely that the source was the newly recruited eosinophils or immature cells still developing in the bone marrow. Although the differential secretion of EDN and MBP has not been noted previously, there is precedence for the differential secretion of eosinophil granule proteins in bacterial and viral infections (19). EDN levels measured in other parasitic diseases seem to correlate with the number of peripheral blood eosinophils (38). Further, EDN, as an RNase, may be initially consumed during the migration of eosinophils into tissues and initial killing of microfilariae, accounting for its decline within the first few days after treatment.
Eosinophils ordinarily circulate in the bloodstream in an inactivated state. During inflammation or sudden antigen release, mediators such as histamine, leukotrienes, prostaglandins, and cytokines are released locally and can act as chemoattractants or cell adhesion molecule-inducing agents. The process of TEM is initiated with eosinophil rolling on the endothelial cell surface, an interaction mediated by L-selectins on the eosinophil and E- and P-selectins on the endothelium. Activation of β2-integrins occurs during this step and triggers a conformational change in the integrin heterodimer, resulting in greater ligand affinity and firm, shear-resistant, integrin-mediated adhesion. Eosinophils bind via LFA-1 and Mac-1 to ICAM-1 and via VLA-4 to VCAM-1, a process upregulated by IL-5, IL-3, GM-CSF, and platelet-activating factor. Movement of the eosinophil from the apical to the basolateral surface of endothelial cells, the process known as diapedesis or TEM, then occurs.
In our system, the dynamics of integrin expression on eosinophils appeared to follow a bimodal pattern. Both VLA-4 and α4β7 were upregulated within 1.5 h after treatment, coincident with eosinopenia, presumably mediating either shear-resistant adhesion to endothelial molecules or clumping of eosinophils around damaged or dying microfilariae, an effect that is clearly demonstrable in vitro (unpublished data). The subsequent decline in integrin expression may reflect the preponderance of inactivated eosinophils demarginating or being recruited into the circulation over the next few days. These then encounter dying microfilariae, are activated, and subsequently migrate into the lymphoid tissues in the process of clearance. The second peak of integrin expression occurred just after the peak of eosinophilia; the eosinophil count subsequently fell, likely reflecting the mass migration of eosinophils into the tissues mediated by both β1- and β2-integrins.
Expression of the β2-integrin CD11b remained at a high level before and after treatment, a finding supported by studies in onchocerciasis, a related filarial infection (4). Since β2-integrin engagement appears to be integral to degranulation induced by cytokines as well as by TEM, the constantly high level of expression may reflect its multiplicity of functions. Expression of CD11a, however, decreased initially, followed by a subsequent increase to baseline levels. The reasons for this are unclear. Surface expression on eosinophils of the activation markers HLA-DR and CD25 (the IL-2 receptor) increased significantly in the first hours after treatment; the former may reflect the recently discovered capability of eosinophils to present antigen (37), while the latter would presumably be important in the generalized activation of these cells. Finally, CD44, the hyaluronic acid receptor, underwent a significant increase in expression in the first hours after treatment and reached a peak at day 7, before the second peak in expression of VLA-4 and α4β7. This again serves to underscore the likelihood of a second wave of migration of eosinophils into the tissues at that time period. In addition to their all-important role in cell trafficking into the tissues, integrins may also play a role in adherence of eosinophils to parasites themselves. The deposition of fibronectin on the surface of infective larvae of Onchocerca volvulus has recently been demonstrated (N. W. Brattig, unpublished data). Eosinophils preferentially attach to parasites, initially through Fc receptors on the cell surface and then by an irreversible adhesion that induces degranulation (4). Homotypic (eosinophil-eosinophil) adhesion reactions and heterotypic (eosinophil-ECM) reactions then occur; these reactions serve presumably to amplify the direct cellular reaction with parasite surfaces, resulting in the accumulation of degranulation products and other toxic molecules as well as in paralysis of the larvae. Once released, degranulation products such as MBP serve to increase the adherence of eosinophils and neutrophils (5).
Based on our data, the immunologic events following treatment of microfilaremia due to W. bancrofti can be thought to occur in two phases. In the first, there is killing of microfilariae within hours after administration of DEC, with massive intravascular release of antigen accompanied by clinical symptoms. Following an initial eosinopenia and upregulation of surface integrins, perhaps reflective of the adherence of these cells to dying microfilariae, there is a marked rise in plasma levels of IL-5 and RANTES that induces the maturation and release of eosinophils from bone marrow and subsequent migration into tissues. Of importance, the eosinophil peak was temporally distant from clinical symptoms, emphasizing the fact that these cells do not appear to cause clinical reactions in the posttreatment setting. In the second phase of the posttreatment reaction, another peak of surface integrin expression occurs, preceding migration of newly released eosinophils into the tissues and clearance of antigen. Although our work and other studies demonstrate that the decline in microfilarial count occurs over 1 year or more following a single dose of DEC, clinical symptoms disappear quickly. This is explained by the fact that after the initial massive release of antigen, the decline in microfilarial count is gradual, with presumably constant low-level release of antigen, a process unlikely to be significant in the production of symptoms. Since eosinophils are involved in acute inflammation and situations in which antigen is released in large amounts acutely, the ongoing low-level release of antigen following the initial massive release may account for the lack of a continued eosinophil response. It is likely that eosinophils, though initially involved in the inflammation following massive killing of microfilariae, do not play a central role in the subsequent slow decline in parasite count.
In summary, the study of posttreatment eosinophilia in filarial infections provides a physiologic human system in which to focus, in particular, on sequential events in eosinophil behavior and activation. This system allows the kinetics of surface receptor molecules on eosinophils to be detailed, a dynamic process that underscores the complex interplay of eosinophil-active cytokines and chemokines and surface adhesion molecules in eosinophil activation and trafficking in vivo.
ACKNOWLEDGMENTS
This work was supported in part by the UNDP/World Bank/WHO Programme for Research and Training in Tropical Diseases.
We thank the staff and students of the Tuberculosis Research Centre in Madras, India, for generously sharing their work space and Brenda Rae Marshall for editorial assistance.
REFERENCES
- 1.Ackerman S J, Gleich G J, Weller P F, Ottesen E A. Eosinophilia and elevated serum levels of eosinophil major basic protein and Charcot-Leyden crystal protein (lysophospholipase) after treatment of patients with Bancroft's filariasis. J Immunol. 1981;127:1093–1098. [PubMed] [Google Scholar]
- 2.Ackerman S J, Gleich G J, Loegering D A, Richardson B A, Butterworth A E. Comparative toxicity of purified human eosinophil granule proteins for schistosomula of Schistosoma mansoni. Am J Trop Med Hyg. 1985;34:735–745. doi: 10.4269/ajtmh.1985.34.735. [DOI] [PubMed] [Google Scholar]
- 3.Ackerman S J, Kephart G M, Francis H, Awadzi K, Gleich G J, Ottesen E A. Eosinophil degranulation. An immunologic determinant in the pathogenesis of the Mazzotti reaction in human onchocerciasis. J Immunol. 1990;144:3961–3969. [PubMed] [Google Scholar]
- 4.Brattig N W, Abakar A Z, Geisinger F, Kruppa T F. Cell-adhesion molecules expressed by activated eosinophils in Onchocerca volvulus infection. Parasitol Res. 1995;81:398–402. doi: 10.1007/BF00931501. [DOI] [PubMed] [Google Scholar]
- 5.Butterworth A E, Vadas M A, Wassom D L, Dessein A, Hogan M, Sherry B, Gleich G J, David J R. Interactions between human eosinophils and schistosomula of Schistosoma mansoni. II. The mechanism of irreversible eosinophil adherence. J Exp Med. 1979;150:1456–1471. doi: 10.1084/jem.150.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butterworth A E, Wassom D L, Gleich G J, Loegering D A, David J R. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979;122:221–229. [PubMed] [Google Scholar]
- 7.Chandrashekar R, Rao U R, Subrahmanyam D. Effect of diethylcarbamazine on serum dependent cell-mediated immune reactions to microfilariae in vitro. Tropenmed Parasitol. 1984;35:177–182. [PubMed] [Google Scholar]
- 8.Coffman R L, Seymour B W, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 9.Cooper P J, Guderian R H, Prakash D, Remick D G, Espinel I, Nutman T B, Taylor D W, Griffin G E. RANTES in onchocerciasis: changes with ivermectin treatment. Clin Exp Immunol. 1996;106:462–467. doi: 10.1046/j.1365-2249.1996.d01-868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreyer G, Coutinho A, Miranda D, Noroes J, Rizzo J, Galdino E, Rocha A, Medeiros Z, Andrade L D, Santos A, Figueredo-Silva J, Ottesen E A. Treatment of bancroftian filariasis in Recife, Brazil: a two-year comparative study of the efficacy of single treatments with ivermectin or diethylcarbamazine. Trans R Soc Trop Med Hyg. 1995;89:98–102. doi: 10.1016/0035-9203(95)90674-6. [DOI] [PubMed] [Google Scholar]
- 11.Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J Exp Med. 1994;179:703–708. doi: 10.1084/jem.179.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebisawa M, Yamada T, Bickel C, Klunk D, Schleimer R P. Eosinophil transendothelial migration induced by cytokines. III. Effect of the chemokine RANTES. J Immunol. 1994;153:2153–2160. [PubMed] [Google Scholar]
- 13.Francis H, Awadzi K, Ottesen E A. The Mazzotti reaction following treatment of onchocerciasis with diethylcarbamazine: clinical severity as a function of infection intensity. Am J Trop Med Hyg. 1985;34:529–536. doi: 10.4269/ajtmh.1985.34.529. [DOI] [PubMed] [Google Scholar]
- 14.Fujisawa T, Terada A, Atsuta J, Iguchi K, Kamiya H, Sakurai M. IL-5 as a strong secretagogue for human eosinophils. Int Arch Allergy Immunol. 1997;114(Suppl. 1):81–83. doi: 10.1159/000237726. [DOI] [PubMed] [Google Scholar]
- 15.Gopinath R, Nutman T B. Identification of eosinophils in lysed whole blood using side scatter and CD16 negativity. Commun Clin Cytometry. 1997;30:313–316. [PubMed] [Google Scholar]
- 16.Gutierrez-Pena E J, Knab J, Buttner D W. Immunoelectron microscopic evidence for release of eosinophil granule matrix protein onto microfilariae of Onchocerca volvulus in the skin after exposure to amocarzine. Parasitol Res. 1998;84:607–615. doi: 10.1007/s004360050459. [DOI] [PubMed] [Google Scholar]
- 17.Hagan J B, Bartemes K R, Kita H, Ottesen E A, Awadzi K, Nutman T B, Gleich G J. Elevations in granulocyte-macrophage colony-stimulating factor and interleukin-5 levels precede posttreatment eosinophilia in onchocerciasis. J Infect Dis. 1996;173:1277–1280. doi: 10.1093/infdis/173.5.1277. [DOI] [PubMed] [Google Scholar]
- 18.Hamann K J, Gleich G J, Checkel D A, Loegering D A, McCall J W, Barker R L. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J Immunol. 1990;144:3166–3173. [PubMed] [Google Scholar]
- 19.Karawajczyk M, Pauksen L, Peterson C G, Eklund E, Venge P. The differential release of eosinophil granule proteins. Studies on patients with acute bacterial and viral infections. Clin Exp Immunol. 1995;25:713–719. doi: 10.1111/j.1365-2222.1995.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 20.Kato M, Kita H, Morikawa A. Role of tyrosine kinases in human eosinophil degranulation. Int Arch Allergy Immunol. 1997;114(Suppl. 1):14–17. doi: 10.1159/000237710. [DOI] [PubMed] [Google Scholar]
- 21.Kazura J, Greenberg J, Perry R, Weil G, Day K, Alpers M. Comparison of single-dose diethylcarbamazine and ivermectin for the treatment of bancroftian filariasis in Papua New Guinea. Am J Trop Med Hyg. 1993;49:804–811. doi: 10.4269/ajtmh.1993.49.804. [DOI] [PubMed] [Google Scholar]
- 22.Kephart G M, Gleich G J, Connor D H, Gibson D W, Ackerman S J. Deposition of eosinophil granule major basic protein onto microfilariae of Onchocerca volvulus in the skin of patients treated with diethylcarbamazine. Lab Investig. 1984;50:51–61. [PubMed] [Google Scholar]
- 23.Kumaraswami V, Ottesen E A, Vijayasekaran V, Devi U, Swaminathan M, Aziz M A, Sarma G R, Prabhakar R, Tripathy S P. Ivermectin for the treatment of Wuchereria bancrofti filariasis: efficacy and adverse reactions. JAMA. 1988;259:3150–3153. [PubMed] [Google Scholar]
- 24.Lee N A, McGarry M P, Larson K A, Horton M A, Kristensen A B, Lee J J. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158:1332–1344. [PubMed] [Google Scholar]
- 25.Limaye A P, Abrams J S, Silver J E, Awadzi K, Francis H F, Ottesen E A, Nutman T B. Interleukin-5 and the posttreatment eosinophilia in patients with onchocerciasis. J Clin Investig. 1991;88:1418–1421. doi: 10.1172/JCI115449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limaye A P, Abrams H S, Silver J E, Ottesen E A, Nutman T B. Regulation of parasite-induced eosinophilia: selectively increased interleukin 5 production in helminth-infected patients. J Exp Med. 1990;172:399–402. doi: 10.1084/jem.172.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limaye A P, Ottesen E A, Kumaraswami V, Abrams J S, Regunathan J, Vijayasekaran V, Jayaraman K, Nutman T B. Kinetics of serum and cellular interleukin-5 in posttreatment eosinophilia of patients with lymphatic filariasis. J Infect Dis. 1993;167:1396–1400. doi: 10.1093/infdis/167.6.1396. [DOI] [PubMed] [Google Scholar]
- 28.Moqbel R, Levi-Schaffer F, Kay A B. Cytokine generation by eosinophils. J Allergy Clin Immunol. 1994;94:1183–1188. doi: 10.1016/0091-6749(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 29.Mould A W, Matthaei K I, Young I G, Foster P S. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Investig. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moulia-Pelat J-P, Nguyen L N, Glaziou P, Chanteau S, Ottesen E A, Cardines R, Martin P-M V, Cartel J-L L. Ivermectin plus diethylcarbamazine: an additive effect on early microfilarial clearance. Am J Trop Med Hyg. 1994;50:206–209. doi: 10.4269/ajtmh.1994.50.206. [DOI] [PubMed] [Google Scholar]
- 31.Ottesen E A, Weller P F. Eosinophilia following treatment of patients with schistosomiasis mansoni and Bancroft's filariasis. J Infect Dis. 1979;139:343–347. doi: 10.1093/infdis/139.3.343. [DOI] [PubMed] [Google Scholar]
- 32.Reimert C M, Poulsen L K, Bindslev-Jensen C, Kharazmi A, Bendtzen K. Measurement of eosinophil cationic protein (ECP) and eosinophil protein X/eosinophil derived neurotoxin (EPX/EDN). Time and temperature dependent spontaneous release in vitro demands standardized sample processing. J Immunol Methods. 1993;166:183–190. doi: 10.1016/0022-1759(93)90359-f. [DOI] [PubMed] [Google Scholar]
- 33.Rot A, Krieger M, Bunner T, Bischoff S C, Schall T J, Dahinden C A. RANTES and macrophage inflammatory protein 1 induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon H U. Molecular mechanisms of defective eosinophil apoptosis in diseases associated with eosinophilia. Int Arch Allergy Immunol. 1997;113:206–208. doi: 10.1159/000237548. [DOI] [PubMed] [Google Scholar]
- 35.Steel C, Nutman T B. Helminth antigens selectively differentiate unsensitized CD45RA+CD4+ human T cells in vitro. J Immunol. 1998;160:351–360. [PubMed] [Google Scholar]
- 36.Sur S, Kita H, Gleich G J, Chenler T C, Hunt L W. Eosinophil recruitment is associated with IL-5, but not with RANTES, twenty-four hours after allergen challenge. J Allergy Clin Immunol. 1996;97:1272–1278. doi: 10.1016/s0091-6749(96)70195-1. [DOI] [PubMed] [Google Scholar]
- 37.Tamura N, Ishii N, Nakazawa M, Nagaya M, Yoshinari M, Amano T, Nakazima H, Minami M. Requirement of CD80 and CD86 molecules for antigen presentation by eosinophils. Scand J Immunol. 1996;44:229–238. doi: 10.1046/j.1365-3083.1996.d01-303.x. [DOI] [PubMed] [Google Scholar]
- 38.Tischendorf F W, Brattig N W, Buttner D W, Pieper A, Lintzel M. Serum levels of eosinophil cationic protein, eosinophil-derived neurotoxin and myeloperoxidase in infections with filariae and schistosomes. Acta Trop. 1996;62:171–182. doi: 10.1016/s0001-706x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- 39.Trulson A, Nilsson S, Venge P. The eosinophil granule proteins in serum, but not the oxidative metabolism of the blood eosinophils, are increased in cancer. Br J Haematol. 1997;98:312–314. doi: 10.1046/j.1365-2141.1997.2203035.x. [DOI] [PubMed] [Google Scholar]
- 40.Wassom D L, Gleich G L. Damage to Trichinella spiralis newborn larvae by eosinophil major basic protein. Am J Trop Med Hyg. 1979;28:860–863. [PubMed] [Google Scholar]