Abstract
β-Keto radicals can be readily generated from single-electron oxidation and ring opening of cyclopropanols. Herein, we report new ways of trapping β-keto radicals derived from Mn(III)-mediated oxidative cyclopropanol ring opening with biaryl isonitriles and N-aryl acrylamides derived from anilines. Through tandem radical cyclization processes, substituted phenanthridines and oxindoles can be synthesized in one step and good to excellent yield. These new synthetic methods feature broad substrate scope and mild reaction conditions, efficiently form two carbon-carbon bonds, and use cheap and commercially available manganese salts as oxidants. Concomitant installation of ketone functionality in the final products provides a handle for further functionalization of these important and biologically relevant scaffolds.
Keywords: cyclopropanol, radical, phenanthridine, oxindole, manganese
Graphical Abstract
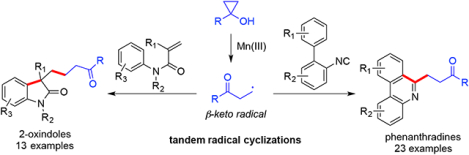
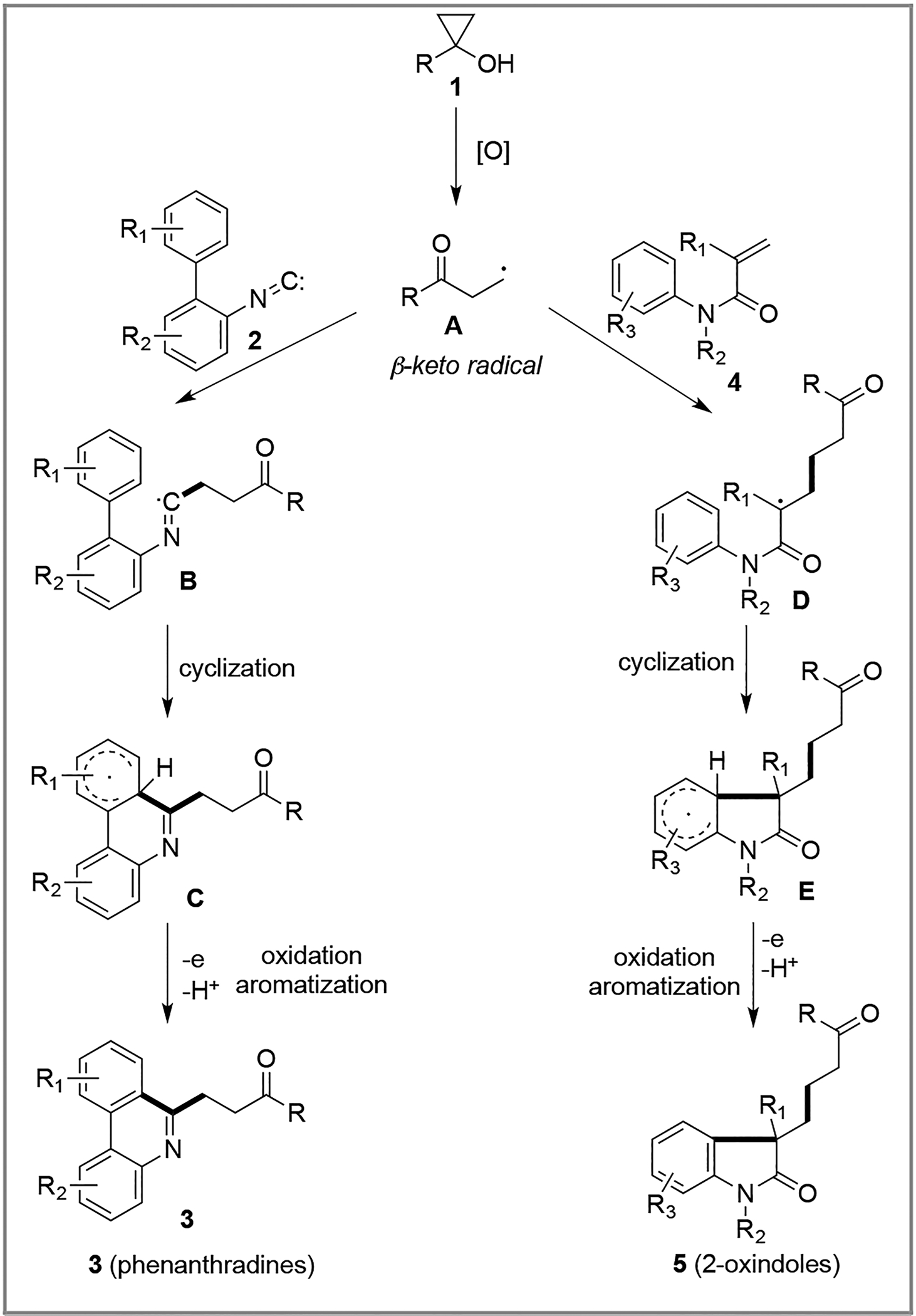
Our recent interest1 in harnessing the inherent ring-strain and unique reactivity of cyclopropanols2 for efficient carbon-carbon and carbon-heteroatom bond forming reactions prompted us to develop new ways of trapping the β-keto alkyl radicals derived from oxidative cyclopropanol ring opening reactions to synthesize medicinally useful chemical scaffolds. Cyclopropanols are readily available from the corresponding esters or lactones with the Kulinkovich or Simmons-Smith protocols. Under various oxidative conditions, they can be converted to highly reactive β-carbonyl radicals, which often undergo reaction pathways such as oxidations, dimerizations, and additions to unsaturated systems.3–6 We envisioned the possibility of trapping the β-keto alkyl radicals derived from cyclopropanols with biaryl isonitriles7 and N-aryl acrylamides8 (Scheme 1), both of which are effective radical acceptors. After the initial radical reaction with the β-keto alkyl radicals, the newly generated radicals B/D are expected to cyclize with the intramolecularly tethered aromatic ring to lead to 6-ketoalkylated phenanthridines 3 and 3-ketoalkylated 2-oxindoles 5 after further oxidation and aromatization. Substituted phenanthridines9 and 2-oxindoles10 are both important motifs in bioactive alkaloid natural products and drug molecules.
Scheme 1.
Reaction design.
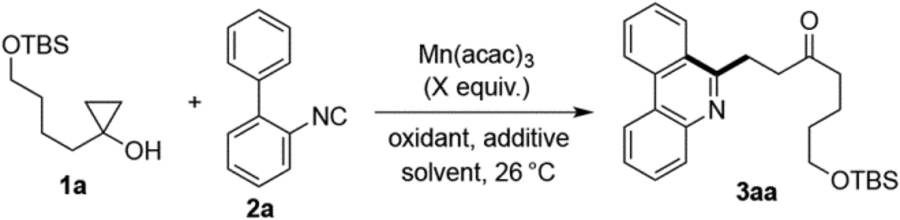
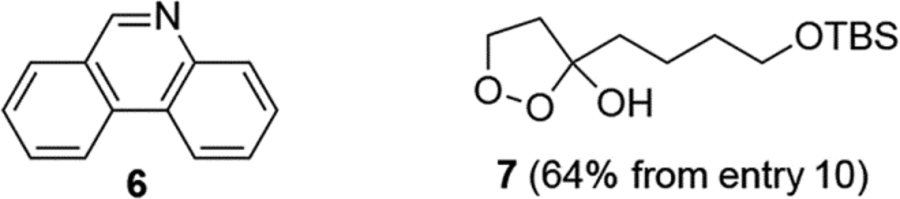
We began our studies by investigating the reaction of cyclopropanol 1a with 2-isocyanobiphenyl 2a under oxidative conditions. After addition of 1.7 equivalents of Mn(acac)3 to a stirred solution of 1a and 2a in MeOH, we observed rapid consumption of the cyclopropanol (<5 min) but low conversion of 2a. Gratifyingly, however, the corresponding phenanthridine derivative 3a was observed and isolated in 19% yield (entry 1). Reasoning that the reactive β-keto alkyl radical intermediate may have competitive self-coupling side reactions, we decided to use a 2-fold excess of 1a added slowly to the mixture of 2a and Mn(acac)3 and improved the yield to 56% (entry 2). After a brief optimization study summarized in table 1, we found that the cyclization could be most efficiently carried out using 2.2 equivalents of Mn(acac)3 and tert-butyl alcohol as solvent. The only side product observed in the reaction mixture was phenanthridine 6.11 Using either the cyclopropanol 1a or aryl isonitrile 2a as the excess reagent did not greatly affect the yield (entries 6 and 7), but around 50% of the aryl isonitrile 2a can be recovered when it is used in excess. Notably, we also found that Mn(acac)3 could be used in catalytic amounts in the presence of tert-butyl hydroperoxide (TBHP) as co-oxidant to give the product in 67% yield (entry 9). Since TBHP is an exceptionally dangerous chemical and more expensive than Mn(acac)3, we decided not to further optimize this catalytic condition. Interestingly, when oxygen was used as oxidant, no desired product was formed. Instead, a significant amount of cyclic peroxide 7 was formed (64% yield), presumably from trapping the β-keto alkyl radical derived from 1a by oxygen followed by cyclization on the newly generated ketone. A similar process has been reported by Kulinkovich et al.12
Table 1.
Phenanthridine synthesis optimization.a
| |||||
|---|---|---|---|---|---|
| entry | 1a/2a | X | solvent (conc.) | additive (equiv.) | yieldb |
| 1c | 1/1.1 | 1.7 | MeOH (0.01 M) | -- | 19% |
| 2c | 2/1 | 2.2 | MeOH (0.01 M) | -- | 56% |
| 3 | 1/2 | 1.5 | MeOH (0.01 M) | -- | 57% |
| 4 | 1/2 | 2.2 | MeOH (0.01 M) | -- | 70% |
| 5 | 1/2 | 2.2 | iPrOH (0.01 M) | -- | 67% |
| 6 | 1/2 | 2.2 | tBuOH (0.01 M) | -- | 83% |
| 7 | 2/1 | 2.2 | tBuOH (0.01 M) | -- | 86% |
| 8 | 2/1 | 2.2 | tBuOH (0.1 M) | -- | 65% |
| 9 | 2/1 | 0.1 | tBuOH (0.01 M) | TBHP (2.2) | 67% |
| 10 | 2/1 | 0.1 | tBuOH (0.01 M) | O2 (balloon) | 0% |
| |||||
Reactions conducted using 0.125 mmol of the limiting reagent (1a or 2a), with a solution of 1a added slowly to the solution of 2a and oxidant over 2 h under argon atmosphere at room temperature (RT).
Isolated yields following flash chromatography.
Oxidant added in one portion to mixture of 1a and 2a.
In order to probe the scope and generality of this new phenanthridine synthesis method, a collection of cyclopropanols and biaryl isonitriles were prepared and subjected to the optimized conditions (Figure 1). In general, variously substituted aryl and alkyl cyclopropanols underwent the reaction smoothly to obtain the products in moderate to excellent yields. Halogens including fluorine and bromine were tolerated, as well as methyl and silyl ethers (3aa-3ac, 3ja). Alkenes were also tolerated, with alkenes distal to the formed radical centers leading to significantly higher yields than substrates containing proximal alkenes (cf. 3ga vs 3ha). Aryl isonitriles with both electron-donating tert-butyl and electron-withdrawing trifluoromethyl groups on the 4’-position reacted well under these conditions. Napthyl-derived isonitrile reacted with high regioselectivity to form 3ed and 3gd (7:1 and 11:1 regiomeric ratio respectively). A larger scale reaction using 3.3 mmol of cyclopropanol 2e and 6.6 mmol of isonitrile 1a successfully gave compound 3ea in 62% yield, demonstrating the scalability of this process.
Having demonstrated the utility of trapping the β-keto alkyl radicals derived from cyclopropanols by aryl isonitriles in the synthesis of substituted phenanthridines, we reasoned that a similar process could take place if N-aryl acrylamides derived from anilines were used as the radical acceptors, which would provide efficient access to ketoalkylated 2-oxindole derivatives. 2-Oxindoles are privileged structural motifs found in many alkaloid natural products with medicinal importance as well as lifesaving pharmaceutical compounds.10
Starting from the reaction conditions established for the phenanthridine synthesis, we were able to quickly identify optimized conditions to unite readily available cyclopropanols and aniline-derived N-aryl acrylamides to 3,3-disubstituted 2-oxindoles (Table 3). Similarly to the phenanthridine synthesis, various cyclopropanols containing halogen, alkene, methyl ether and silyl ether functionalities were well tolerated in the reaction. Variation of N-substitution on the amide gave good yields in the case of N-methyl and N-benzyl amides (5ba and 5bb). Substitution on the aniline ring was found to have little effect on the reaction, with methyl (5bc) chloro (5bf), trifluoromethyl (5bd), and methoxy (5be, 5bg) substituents all giving good to excellent yields of the corresponding 2-oxindole products.
Table 3.
Mn(III)-mediated oxindole formation substrate scope.
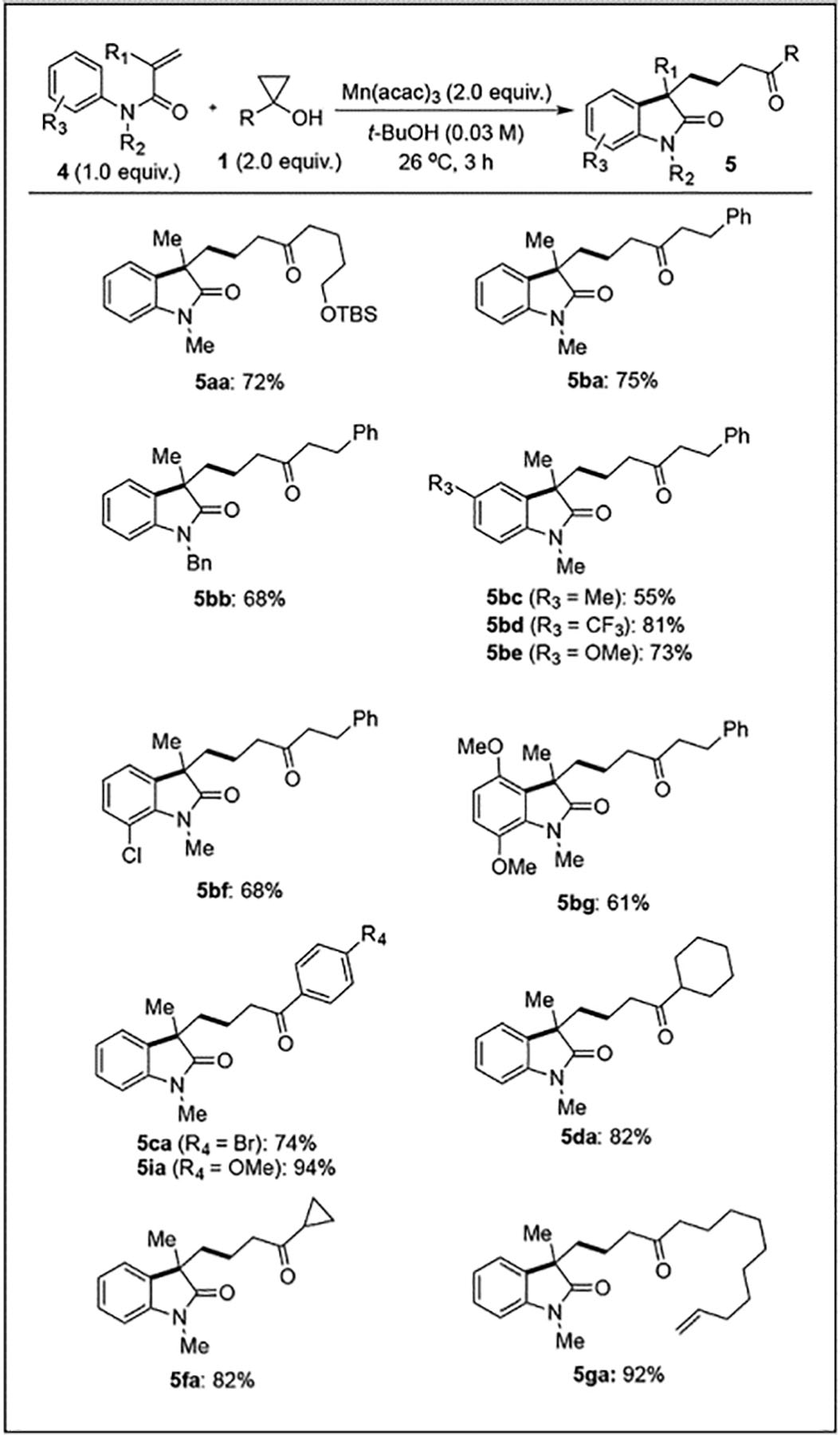
|
Mechanistically, the formation of both the phenanthridine and 2-oxindole derivatives is proposed to start with hydrogen atom abstraction and single electron oxidation of cyclopropanol 1 by Mn(acac)3 to produce an oxy-radical, which subsequently rearranges to β-keto alkyl radical A (Scheme 1). The β-keto radical then adds intermolecularly to either the aryl isonitrile 2 or the α,β-unsaturated amide 4 to produce an imidoyl radical B or an α-amido radical D. Subsequent homolytic aromatic substitution leads to the cyclohexadienyl radicals C and E. Oxidation and rearomatization of these cyclohexadienyl radicals with the second equivalent of Mn(acac)3 then leads to formation of the phenanthridine (3) or 2-oxindole (5) products. The fact that oxygen inhibits the conversion of 1 to 3 and the formation of peroxide 7 support the proposed radical process.
In summary, we have developed two novel applications for β-keto radicals derived from readily available cyclopropanols towards the synthesis of phenanthridine13 and 2-oxindole derivatives.14 These reactions showcase the applicability of cyclopropanols as a source of easily-generated alkyl radicals that can be integrated into heterocyclic scaffolds. The reactions are fast, efficient and take place under mild oxidative conditions to generate two carbon-carbon bonds in a radical cascade sequence. The resulting ketone functionality in the products can serve as a point of further diversification for complex molecule synthesis.
Supplementary Material
Table 2.
Mn(III)-mediated phenanthridine formation.
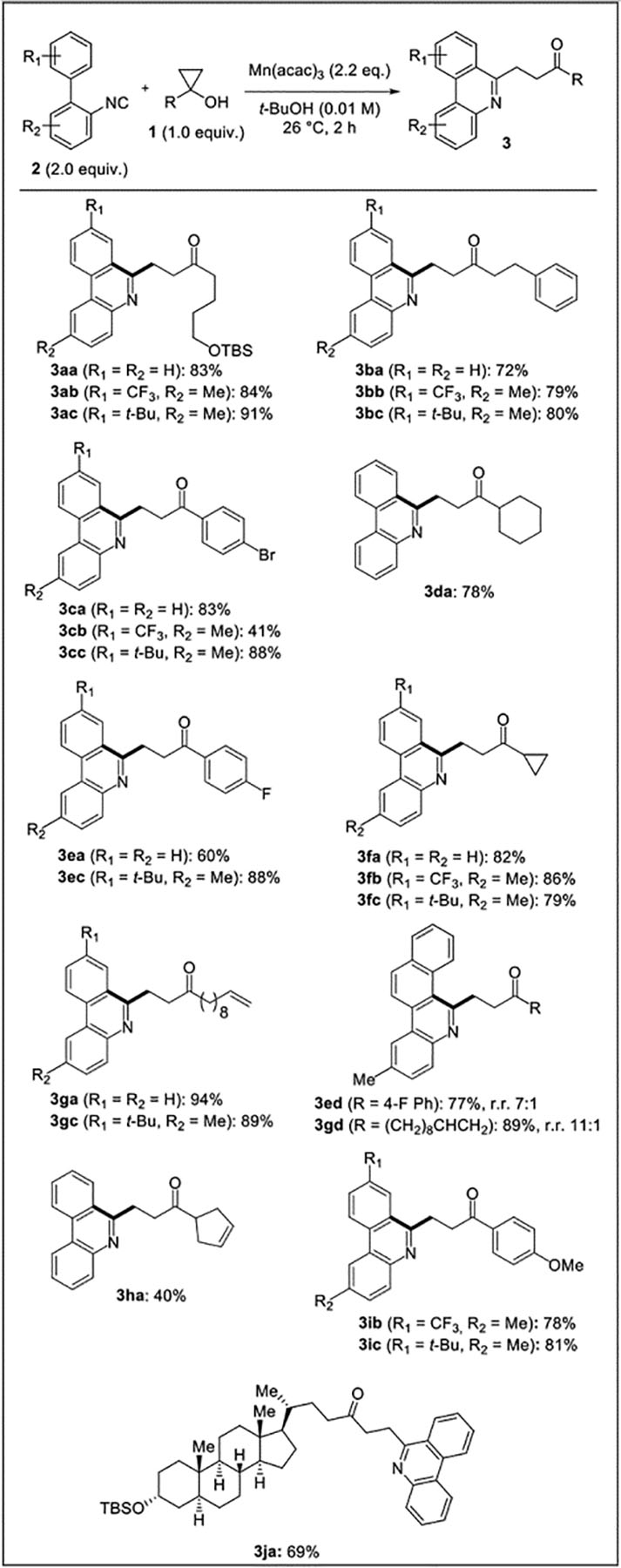
|
Acknowledgment
We thank NSF (CAREER 1553820) and the ACS petroleum research foundation (PRF# 54896-DNI1) for financial support and the NIH P30CA023168 for supporting shared NMR resources to Purdue Center for Cancer Research.
References and Notes
- (1).(a) Davis DC; Walker KL; Hu C; Zare RN; Waymouth RM; Dai MJ J. Am. Chem. Soc 2016, 138, 10693. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li Y; Ye Z; Bellman TM; Chi T; Dai M Org. Lett 2015, 17, 2186. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ye Z; Dai M Org. Lett 2015, 17, 2190. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ye Z; Gettys KE; Shen X; Dai M Org. Lett 2015, 17, 6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).For reviews:; (a) Kulinkovich OG; de Meijere A, Chem. Rev 2000, 100, 2789. [DOI] [PubMed] [Google Scholar]; (b) Rosa D; Nikolaev A; Nithiy N; Orellana A Synlett 2015, 26, 441. [Google Scholar]; (c) Nikolaev A; Orellana A Synthesis 2016, 48, 1741. [Google Scholar]
- (3).(a) Hasegawa E; Nemoto K; Nagumo R; Tayama E; Iwamoto H J. Org. Chem 2016, 81, 2692. [DOI] [PubMed] [Google Scholar]; (b) Ito Y; Fujii S; Saegusa T J. Org. Chem 1976, 41, 2073. [Google Scholar]; (c) U JS; Lee UJ; Cha JK Tetrahedron Lett 1997, 38, 5233. [Google Scholar]; (d) Hasegawa E; Tateyama M; Nagumo R; Tayama E; Iwamoto H Beilstein J. Org. Chem 2013, 9, 1397. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Snider BB; Kwon T J. Org. Chem 1992, 57, 2399 [Google Scholar]
- (4).(a) Wang Y-F; Chiba S J. Am. Chem. Soc 2009, 131, 12570. [DOI] [PubMed] [Google Scholar]; (b) Wang Y-F; Toh KK; Ng EPJ; Chiba S J. Am. Chem. Soc 2011, 133, 6411. [DOI] [PubMed] [Google Scholar]; (c) Iwasawa N; Hayakawa S; Funahashi M; Isobe K; Narasaka K Bull. Chem. Soc. Jpn 1993, 66, 819. [Google Scholar]; (d) Chiba S; Cao Z; El Bialy SAA; Narasaka K Chem. Lett 2006, 35, 18. [Google Scholar]; (e) Ilangovan A; Saravanakumar S; Malayappasamy S Org. Lett 2013, 15, 4968. [DOI] [PubMed] [Google Scholar]
- (5).(a) Wang S; Guo L-N; Wang H; Duan X-H Org. Lett 2015, 17, 4798. [DOI] [PubMed] [Google Scholar]; (b) Kananovich DG; Konik YA; Zubrytski DM; Jӓrving I; Lopp M Chem. Commun 2015, 51, 8349. [DOI] [PubMed] [Google Scholar]
- (6).(a) Jiao J; Nguyen LX; Patterson DR; Flowers RA II. Org. Lett 2007, 9, 1323. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao H; Fan X; Yu J; Zhu C J. Am. Chem. Soc 2015, 137, 3490. [DOI] [PubMed] [Google Scholar]; (c) Bloom S; Bume DD; Pitts CR; Lectka T Chem. – Eur. J 2015, 13, 8060. [DOI] [PubMed] [Google Scholar]; (d) Ren S; Feng C; Loh T-P Org. Biomol. Chem 2015, 13, 5105. [DOI] [PubMed] [Google Scholar]; (e) Huang F-Q; Xie J; Sun J-G; Wang Y-W; Dong X; Qi L-W; Zhang B Org. Lett 2016, 18, 684. [DOI] [PubMed] [Google Scholar]; (f) Bume DD; Pitts CR; Lectka T Eur. J. Org 2016, 26. [Google Scholar]
- (7).(a) Curran DP; Liu H J. Am. Chem. Soc 1992, 114, 5863. [Google Scholar]; (b) Curran DP; Ko S-B; Josien H Angew. Chem. Int. Ed 1996, 34, 2683. [Google Scholar]; (c) Nanni D; Pareschi P; Rizzoli C; Sgarabotto P; Tundo T Tetrahedron 1995, 51, 9045. [Google Scholar]; (d) Yamago S; Miyazoe H; Coto R; Hashidume M; Sawazaki T; Yoshida I J. Am. Chem. Soc 2001, 123, 3697. [DOI] [PubMed] [Google Scholar]; (e) Janza B; Studer A Org. Lett 2006, 8, 1875. [DOI] [PubMed] [Google Scholar]; (f) Mitamura T; Iwata K; Ogawa A J. Org. Chem 2011, 76, 3880. [DOI] [PubMed] [Google Scholar]; (g) Tobisu M; Koh K; Furukawa T; Chatani N Angew. Chem. Int. Ed 2012, 51, 11363. [DOI] [PubMed] [Google Scholar]; (h) Wang Q; Dong X; Xiao T; Zhou L Org. Lett 2013, 15, 4846. [DOI] [PubMed] [Google Scholar]; (i) Zhang B; Mück-Lichtenfeld C; Daniliuc CG; Studer A Angew. Chem. Int. Ed 2013, 52, 10792. [DOI] [PubMed] [Google Scholar]; (j) Leifert D; Daniliuc CG; Studer A Org. Lett 2013, 15, 6286. [DOI] [PubMed] [Google Scholar]; (k) Wang Q; Dong X; Xiao T; Zhou L Org. Lett 2013, 15, 4846. [DOI] [PubMed] [Google Scholar]; (l) Jiang H; Cheng YZ; Wang RZ; Zheng MM; Zhang Y; Yu SY Angew. Chem. Int. Ed 2013, 52, 13289. [DOI] [PubMed] [Google Scholar]; (m) Cao JJ; Wang X; Wang SY; Ji SJ Chem. Commun 2014, 50, 4643. [DOI] [PubMed] [Google Scholar]; (n) Gu LJ; Jin C; Liu JY; Ding HY; Fan BM Chem. Commun 2014, 50, 4643. [DOI] [PubMed] [Google Scholar]; (o) Xia ZH; Huang JB; He YM; Zhao JJ; Lei J; Zhu Q Org. Lett 2014, 16, 2546. [DOI] [PubMed] [Google Scholar]; (p) Xiao TB; Li LY; Lin GL; Wang QL; Zhang P; Mao ZW; Zhou L Green. Chem 2014, 16, 2418. [Google Scholar]; (q) Zhang B; Daniliuc CG; Studer A Org. Lett 2014, 16, 250. [DOI] [PubMed] [Google Scholar]; (r) Cao JJ; Zhu TH; Wang SY; Gu ZY; Wang X; Ji S Chem. Commun 2014, 50, 6439. [DOI] [PubMed] [Google Scholar]; (s) Wang L; Sha WX; Dai Q; Feng XM; Wu WT; Peng HB; Chen B; Cheng J Org. Lett 2014, 16, 2088. [DOI] [PubMed] [Google Scholar]; (t) Li ZJ; Fan FH; Yang J; Liu ZQ Org. Lett 2014, 16, 3396. [DOI] [PubMed] [Google Scholar]; (u) Sha WX; Yu JT; Jiang Y; Yang HT; Cheng J Chem. Commun 2014, 50, 9179. [DOI] [PubMed] [Google Scholar]; (v) Zhu ZQ; Wang TT; Bai P; Huang ZZ Org. Biomol. Chem 2014, 12, 5839. [DOI] [PubMed] [Google Scholar]; (w) Lu S; Gong Y; Zhou D J. Org. Chem 2015, 80, 9336. [DOI] [PubMed] [Google Scholar]; (x) Pan C; Zhang H; Han J; Cheng Y; Zhu C Chem. Commun 2015, 51, 3786. [DOI] [PubMed] [Google Scholar]; (y) Zhou Y; Wu C; Dong X; Qu J J. Org. Chem 2016, 81, 5202. [DOI] [PubMed] [Google Scholar]
- (8).For reviews, see:; (a) Chen J-R; Yu X-Y; Xiao W-J Synthesis 2015, 47, 604. [Google Scholar]; (b) Yu J-T; Pan C Chem. Commun 2016, 52, 2220–2236. [DOI] [PubMed] [Google Scholar]; (c) Song R-J; Liu Y; Xie Y-X; Li J-H Synthesis, 2015, 47, 1195. [Google Scholar]; For selected examples:; (e) Wei W-T; Zhou M-B; Fan J-H; Liu W; Song R-J; Liu Y; Hu M; Xie P; Li J-H Angew. Chem. Int. Ed 2013, 52, 3638. [DOI] [PubMed] [Google Scholar]; (f) Biswas P; Paul S; Guin J Angew. Chem. Int. Ed 2016, 55, 7756. [DOI] [PubMed] [Google Scholar]; (g) Kong W; Casimiro M; Fuentes N; Merino E; Nevado C Angew. Chem. Int. Ed 2013, 52, 13086. [DOI] [PubMed] [Google Scholar]; (h) Wang H; Guo L-N; Duan X-H Org. Lett 2013, 15, 5254. [DOI] [PubMed] [Google Scholar]; (i) Li Z; Zhang Y; Zhang L; Liu Z-Q Org. Lett 2014, 16, 382. [DOI] [PubMed] [Google Scholar]; (j) Xu Z; Yan C; Liu Z-Q Org. Lett 2014, 16, 5670. [DOI] [PubMed] [Google Scholar]; (k) Pan C; Zhang H; Zhu C Org. Biomol. Chem 2015, 13, 361. [DOI] [PubMed] [Google Scholar]; (l) Qiu J-K; Jiang B; Zhu Y-L; Hao W-J; Wang D-C; Sun J; Wei P; Tu S-J; Li G J. Am. Chem. Soc 2015, 137, 8928. [DOI] [PubMed] [Google Scholar]
- (9).(a) Ali AA; El Sayed HM; Abdallah OM; Steglich W Phytochemistry, 1986, 25, 2399. [Google Scholar]; (b) Viladomat F; Bastida J; Tribo G; Codina C; Rubiralta M Phytochemistry, 1990, 1307. [Google Scholar]; (c) Ishikawa T Med. Res. Rev 2001, 21, 61. [DOI] [PubMed] [Google Scholar]; (d) Simeon S; Rios JL; Villar A Pharmazie 1989, 44, 593. [PubMed] [Google Scholar]; (e) Phillips SD; Castle RN J. Heterocycl. Chem 1981, 18, 223. [Google Scholar]; (f) Abdel-Halim OB; Morikawa T; Ando S; Matsuda H; Yoshikawa M J. Nat. Prod 2004, 67, 1119. [DOI] [PubMed] [Google Scholar]; (g) Sripada L; Teske JA; Deiters A Org. Biomol. Chem 2008, 6, 263. [DOI] [PubMed] [Google Scholar]; (h) Nakanishi T; Suzuki M Org. Lett 1999, 1, 985. [DOI] [PubMed] [Google Scholar]; (i) Zuo GY; MEng FY; Hao XY; Zhang YL; Wang GC; Xu GL J. Pharm. Pharm. Sci 2008, 11, 90. [DOI] [PubMed] [Google Scholar]; (j) Su G; Pan C; Feng K; Dai Z; Qin J; Tang H; Liu H CN102887904A, 2013. [Google Scholar]; (k) Buyanov VN; Barberkina YP; Samoilova MY; Akhvlediani RN; Frolova YP; Kurkovskaya LN; Yershova YA; Safonova TS; Korovin BV; Suvorov NN Khim.-Farm. Zh 1994, 28 (1), 10–14. [Google Scholar]; (l) Tumir L-M; Piantanida I; Juranović I; Meić Z; Tomić S; Žinić M Chem. Commun 2005, 2561. [DOI] [PubMed] [Google Scholar]; (m) Dukši M; Baretić D; Čaplar V; Piantanida I Eur. J. Med. Chem 2010, 45, 2671. [DOI] [PubMed] [Google Scholar]; (n) Stevens N; O’Connor N; Vishwasrao H; Samaroo D; Kandel ER; Akins DL; Drain CM; Turro NJ J. Am. Chem. Soc 2008, 130, 7182. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Stojković MR; Miljanić S; Mišković K; Glavaš-Obrovac L; Piantanida I Mol. BioSyst 2011, 7, 1753. [DOI] [PubMed] [Google Scholar]
- (10).(a) Taber DF; Tirunahari PK Tetrahedron 2011, 67, 7195. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Badillo JJ; Hanhan NV; Franz AK Curr. Opin. Drug Discovery Dev 2010, 13, 758. [PubMed] [Google Scholar]; (c) Fensome A; Adams WR; Adams AL; Berrodin TJ; Cohen J; Huselton C; Illenberger A; Kern JC; Hudak VA; Marella MA; Melenski EG; McComas CC; Mugford CA; Slayden OD; Yudt M; Zhang Z; Zhang P; Zhu Y; Winneker RC; Wrobel JE J. Med. Chem 2008, 51, 1861. [DOI] [PubMed] [Google Scholar]; (d) Kumari G; Nutan; Modi M; Gupta SK; Singh RK Eur. J. Med. Chem 2011, 46, 1181. [DOI] [PubMed] [Google Scholar]; (e) Lo MM-C; Newmann CS; Nagayams S; Perlstein EO; Schreiber SL J. Am. Chem. Soc 2004, 126, 16077. [DOI] [PubMed] [Google Scholar]; (f) Ding K; Lu Y; Nikolovska-Coleska Z; Qui S; Ding Y; Gao W; Stuckey J; Krajewski K; Roller PP; Tomita Y; Parrish DA; Deschamps JR; Wang S J. Am. Chem. Soc 2005, 127, 10130. [DOI] [PubMed] [Google Scholar]; (g) Vintonyak VV; Warburg K; Kruse H; Grimme S; Hübel K; Rauh D; Waldmann H Angew. Chem. Int. Ed 2010, 49, 5902. [DOI] [PubMed] [Google Scholar]; (h) Yeung BKS; Zou B; Rottmann M; Lakshmninarayana SB; Ang SH; Leong SY; Tan J; Wong J; Keller-Maerki S; Fischli C; Goh A; Schmitt EK; Krastel P; Francotte E; Kuhen K; Plouffe D; Henson K; Wagner T; Winzeler EA; Petersen F; Brun R; Dartois V; Diagana TT; Keller TH J. Med. Chem 2010, 53, 5155. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Ding K; Lu Y; Nikolovska-Coleska Z; Wang G; Qiu S; Shangary S; Gao W; Qin D; Stukey J; Krajewski K; Roller PP; Wang S J. Med. Chem 2006, 49, 3432. [DOI] [PubMed] [Google Scholar]; (j) James MN; Williams GJB Can. J. Chem 1972, 25, 1473. [Google Scholar]; (k) Jossang A; Jossang P; Hamid A; Sevenet T; Bodo B J. Org. Chem 1991, 56, 6527. [Google Scholar]; (l) Anderton N; Cockrum PA; Cologate SM; Edgar JA; Flower K; Vit I; Willing RI Phytochemistry 1998, 48, 437. [Google Scholar]; (m) Shi J-S; Yu J-X; Chen X-P; Xu R-X Acta. Pharm. Sin 2003, 24, 97. [Google Scholar]; (n) Zhou J; Zhou S J. Ethnopharmacol 2010, 132, 15. [DOI] [PubMed] [Google Scholar]; (o) Chou C-H; Gong C-L; Chao CC; Lin C-H; Kwan C-Y; Hsieh C-L; Leung YM J. Nat. Prod 2009, 72, 830. [DOI] [PubMed] [Google Scholar]; (p) Galliford CV; Scheidt KA Angew. Chem. Int. Ed 2007, 46, 8748. [DOI] [PubMed] [Google Scholar]
- (11).Formation of phenanthridine was also noted by Chatani, who found that its formation could also be promoted by AlCl3, suggesting a Lewis acid-based mechanism. See; Tobisu M; Koh K; Furukawa T; Chatani N Angew. Chem., Int. Ed 2012, 51 (45), 11363–11366. [DOI] [PubMed] [Google Scholar]
- (12).Kulinkovich OG; Astashko DA; Tyvorskii VI; Ilyina NA Synthesis 2001, 10, 1453–1455. [Google Scholar]
- (13). Representative procedure for the phenanthridine synthesis: An argon-degassed solution of cyclopropanol 1 (1 equiv.) in tert-butyl alcohol (0.02 M) was added dropwise over two hours to an argon-degassed, stirred solution of 2-isocyano biphenyl 2 (2 equiv.) and manganese (III) acetylacetonate (2.2 equiv.) in tert-butyl alcohol (0.042 M) at 26 °C. The resulting solution (0.01 M with respect to the cyclopropanol) was stirred for an additional five minutes before the solvent was removed under reduced pressure and the resulting residue purified by flash chromatography to yield the desired 2-substituted phenanthradine 3. 3aa was prepared according to the above procedure using 1a (0.125 mmol, 31 mg) and 2a (0.25 mmol, 45 mg) and isolated as a yellow solid (43 mg, 83%) following flash chromatography (hexanes:ethyl acetate = 10:1). 1H NMR (500 MHz, CDCl3) δ 8.62 (d, J = 8.3 Hz, 1H), 8.52 (d, J = 8.0 Hz, 1H), 8.27 (d, J = 8.2 Hz, 1H), 8.05 (d, J = 7.5 Hz, 1H), 7.82 (t, J = 7.5 Hz, 1H), 7.69 (t, J = 7.85 Hz, 2H), 7.61 (t, J = 7.35 Hz, 1H), 3.69 (t, J = 6.8 Hz, 2H), 3.64 (t, J = 6.4 Hz, 2H), 3.16 (t, J = 6.9 Hz, 2H), 2.70 (t, J = 7.4 Hz, 2H), 1.73 (quin, J = 7.7 Hz, 2H), 1.58 (quin, J = 6.9 Hz, 2H), 0.90 (s, 9H), 0.06 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 210.9, 159.7, 143.7, 132.8, 130.5, 129.7, 128.7, 127.6, 126.5, 125.9, 125.6, 123.9, 122.6, 122.1, 63.1, 43.3, 39.5, 32.6, 29.2, 26.2, 20.6, 18.5, −5.1; IR (neat, cm−1): 2964, 2903, 1666, 1578, 1393, 1238, cm−1; MS (ESI): m/z = 422.24 calcd. for C26H25NO2Si [M+H]+, found 422.2.
- (14). Representative procedure for the 2-oxindole synthesis: An argon-degassed solution of cyclopropanol 1 (2 equiv.) in tert-butyl alcohol (0.0625 M) was added dropwise over three hours to an argon-degassed, stirred solution of acrylamide 4 (1 equiv.) and manganese (III) acetylacetonate (2.2 equiv.) in tert-butyl alcohol (0.114 M) at 26° C. The resulting solution (0.03 M with respect to the cyclopropanol) was stirred for an additional five minutes before the solvent was removed under reduced pressure and the resulting residue purified by flash chromatography to yield the desired oxindole. 5aa was prepared according to the above procedure using 4a (0.125 mmol, 22 mg) and 1a (0.25 mmol, 62 mg) and isolated as a yellow oil (38 mg, 72%) following flash chromatography (hexanes:ethyl acetate = 6:1). 1H NMR (400 MHz, CDCl3) δ 7.25 (t, J = 7.7 Hz, 1H), 7.17 (d, J = 7.3 Hz, 1H), 7.05 (t, J = 8.2 Hz, 1H), 6.83 (d, J =7.8 Hz, 1H), 3.56 (t, J = 6.2 Hz, 2H), 3.20 (s, 3H), 2.30 (t, J = 7.5 Hz, 2H), 2.26 (q, v = 7.2 Hz, 2H), 1.70–1.86 (m, 2H), 1.49–1.57 (m, 2H), 1.40–1.47 (m, 2H), 1.33 (s, 3H), 1.14–1.22 (m, 2H), 0.86 (s, 9H), 0.02 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 210.3, 180.4, 143.1, 133.6, 127.7, 126.4, 122.5, 107.9, 62.7, 48.2, 42.3, 23.2, 37.6, 32.1, 26.0, 25.8, 23.8, 20.1, 18.7, 18.2, 5.4; IR (neat, cm−1) 2951, 2928, 2856, 1714, 1613, 1493, 1253, 1100, 835; MS: m/z 418.28 calc. for C24H39NO3Si[M+H]+, found 418.2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


