Abstract
Major histocompatibility complex (MHC) class II engagement by toxic shock syndrome toxin 1 (TSST-1) transduces signals leading to proinflammatory cytokine gene expression (tumor necrosis factor alpha [TNF-α]) in human monocytes. To study the proinflammatory role of MHC class II molecules expressed by bronchial epithelial cells (BEC), primary human BEC were isolated from surgical bronchial samples, expanded in vitro, and cultured in the presence or absence of gamma interferon (IFN-γ) for 48 h. 125I-TSST-1 binding to BEC pretreated with IFN-γ was inhibited up to 97% by anti-MHC class II monoclonal antibody 3B12, indicating that in BEC also MHC class II molecules were targets for the staphylococcal exotoxin. As analyzed by a quantitative reverse transcriptase PCR, a 1-h stimulation of BEC with TSST-1 resulted in a vigorous expression of TNF-α and interleukin-8 (IL-8) genes. TNF-α and IL-8 expression was optimal in BEC pretreated with 50 IU of IFN-γ/ml, whereas TSST-1 stimulation of BEC pretreated with 200 IU of IFN-γ/ml failed to enhance either TNF-α or IL-8 transcripts. In a time course study, peak expression of TNF-α and IL-8 mRNA was reached 6 h after TSST-1 stimulation. These results demonstrate that bacterial superantigen TSST-1 binds to MHC molecules on BEC and induces TNF-α and IL-8 gene expression upon engagement of MHC class II molecules on BEC, thus contributing to the perpetuation of bronchial mucosa inflammation via chemokine or cytokine gene expression.
Human bronchial epithelial cells (BEC) have a major role in the lower-airway mucosal immune or inflammatory response (29). They not only provide a physiological barrier against pathogens but also take part in the recruitment of immune effector cells such as neutrophils and eosinophils (7, 27). Furthermore, BEC express and secrete proinflammatory cytokines (interleukin-1 [IL-1], IL-6, tumor necrosis factor alpha [TNF-α], granulocyte-macrophage colony-stimulating factor [GM-CSF]) or chemokines (eotaxin, MCP-1, IL-8, RANTES) under various in vitro and in vivo proinflammatory conditions (4, 10, 12, 13, 17, 23, 28). TNF-α appears to play a critical role in the induction of the inflammatory response against pathogens and modulates neutrophil chemotaxis and BEC adhesion molecule expression (7). IL-8 is a potent chemotactic factor for neutrophils and eosinophils (9, 22). Its expression in bronchial epithelial cells is enhanced by asthma (13) or during bacterial or viral infections (14).
Gamma interferon (IFN-γ) induces major histocompatibility complex (MHC) class II molecule expression on BEC in vitro (20, 21). In vivo, the expression of MHC class II molecules is enhanced by asthma and lung neoplasia, allowing BEC to function as antigen-presenting cells and to interact with T cells (8, 19, 21). MHC class II engagement by the staphylococcal superantigen toxic shock syndrome toxin 1 (TSST-1) or by specific monoclonal antibodies (MAbs) generates intracellular signals leading to the expression of proinflammatory cytokine genes in human primary T cells and monocytes (6, 24, 25, 30). Since upper-airway epithelial cells express MHC class II molecules in conditions of acute or chronic inflammation, we have examined here whether exposure of MHC class II+ BEC to the staphylococcal exotoxin TSST-1 may further enhance BEC proinflammatory response.
MATERIALS AND METHODS
Reagents.
Recombinant human IFN-γ and dispase I were purchased from Boehringer Mannheim, Rotkreuz, Switzerland; TSST-1 was purchased from Toxin Technology (Sarasota, Fla.); penicillin-streptomycin, trypsin, Hanks' balanced salt solution (HBSS), bovine fibronectin, bovine collagen I, and amphotericin B were purchased from Gibco-BRL, Gaithersburg, Md.; bovine serum albumin and digitonin were purchased from Fluka, Buchs, Switzerland; LHC basal medium, LHC8 medium, retinoic acid, and epinephrine were purchased from Biofluids Inc., Rockville, Md.; DNase I was purchased from Sigma (St. Louis, Mo.); fluorescein isothiocyanate (FITC)-conjugated anti-cytokeratin MAb was purchased from DAKO, Zug, Switzerland; and phycoerythrin (PE)-conjugated anti-DR MAb was purchased from Becton Dickinson (San Jose, Calif.). Human IL-8 plasmid was a gift from J.-J. Mermoud, Glaxo IMB, Geneva, Switzerland.
Isolation of primary human bronchial epithelial cells.
Tumor-free bronchial samples were obtained from three patients undergoing pneumonectomy for bronchial cell carcinoma. The research protocol was approved by the Hospital Review Board, and informed consent was obtained from all subjects before tissue sampling. Bronchial mucosa was first excised, trimmed, and extensively washed in HBSS containing penicillin (100 U/ml)-streptomycin (100 μg/ml) and amphotericin B (100 U/ml). To dissociate BEC, a piece (4 by 4 mm) of bronchial tissue was incubated for 3 h in 0.5 ml of LHC basal medium supplemented with penicillin-streptomycin, 30 U of dispase I/ml, and 2 μg of DNase I/ml in a six-well culture plate (Nunc, Roskilde, Denmark) pretreated with coating solution consisting of LHC basal medium containing bovine fibronectin (10 μg/ml), bovine collagen I (30 μg/ml), and bovine serum albumin (10 μg/ml). Epithelial cells were scraped, harvested, and centrifuged at 400 × g for 5 min at 4°C. Then they were washed in HBSS and resuspended in LHC9 medium (LHC8 medium supplemented with 0.33 nM retinoic acid and 0.5 μg of epinephrine/ml) in a six-well plate pretreated with coating solution. LHC9 culture medium was changed after 48 h and twice a week thereafter. At confluence, BEC were washed twice in Ca2+- and Mg2+-free HBSS and detached from plastic by being incubated for 15 min at 37°C in EGTA-trypsin (0.025%). After two washes in HBSS, cells were plated onto a 24-well culture plate in LHC9 medium (106 cells/ml) for another 48 h. BEC were at this step preincubated with IFN-γ and then with TSST-1, or left untreated, as described in Results. MHC class II and cytokeratin expression on BEC was assessed by flow cytometry (EPICS II; Coulter, Hialeah, Fla.) or alternatively by direct immunofluorescence with a fluorescence microscope (Wild Leitz, Wetzlar, Germany). For cytokeratin expression, BEC were first fixed in 1% paraformaldehyde for 30 min at 4°C and then permeabilized for 10 min at room temperature with digitonin (0.1 mg/ml) prior to incubation with 0.1 ml of FITC-conjugated anti-cytokeratin MAb.
TSST-1 labeling and binding assay.
TSST-1 (10 μg) was labeled with 40 μCi of 125I (>7.4 GBq/ml; Hartmann Analytica, Braunschweig, Germany) by using chloramine-T and purified from free 125I on a Sephadex G-25 column (NAP-5; Pharmacia, Uppsala, Sweden). For binding assays, 5 × 105 BEC pretreated for 48 h with 200 U of IFN-γ/ml (or left untreated for control BEC) were incubated for 60 min in 24-well culture plates with 125I-labeled TSST-1 (50,000 cpm). In competition experiments, BEC were preincubated for 30 min with anti-MHC class II MAb 3B12 (immunoglobulin G1 [IgG1]) (24), with control anti-MHC class I MAb BBM1 (American Type Culture Collection, Manassas, Va.), or with isotype control myeloma protein MOPC21 (IgG1; Cappel, Aurora, Ohio) prior to incubation with 125I-TSST-1. After five washes in LHC9 medium, BEC were detached from plastic by being incubated for 15 min at 37°C in EGTA-trypsin (0.025%) and precipitated in 10% trichloroacetic acid. After centrifugation, pellet radioactivity was measured in a scintillation γ counter. Nonspecific binding was determined from parallel tubes containing a 1,000-fold excess of unlabeled toxin.
Quantitative IL-8 RT-PCR.
A quantitative reverse-transcriptase PCR (RT-PCR) assay was carried out as described previously (31). Total cellular RNA was isolated as described by Chomczynski and Sacchi (3). The sample concentration was measured by spectrophotometry at 260 nm, and RNA quality was checked on a 1% agarose gel. The RT reaction mixture (1 μl of 3′ primer [50 pmol/ml], 1 μl of avian myeloblastosis virus [AMV] buffer [500 mM Tris-HCl, pH 8.3, 500 mM KCl, 50 mM MgCl2], 0.8 ml of 25 mM dithiothreitol, 0.8 μl of 10 mM deoxynucleoside triphosphate, 8 U of RNasin, and 0.3 U of AMV RT), 1 μl of total cellular RNA, 1 μl of internal standard (PSSB plasmid), and up to 20 μl of H2O were incubated at 42°C for 60 min, heated at 95°C for 2 min, and then quickly chilled on ice. PCR amplification was carried out in a 50-μl final volume by mixing 20 μl of RT mixture with 30 μl of PCR buffer (500 mM Tris-HCL [pH 8.3], 500 mM KCl) containing 50 pmol of 5′ primers, 0.25 μCi of [α-32P]dCTP, and 0.5 U of Taq DNA polymerase (Perkin-Elmer, Branchburg, N.J.). After 3 min of denaturation at 94°C, samples were subjected to 25 amplification cycles. The PCR conditions involved denaturation at 95°C for 1 min, annealing at 54°C for 40 s, and extension at 72°C for 1 min. PCR products from PSSB RNA amplified with IL-8 primers were 284 bp long and were designed to be longer than PCR products from target mRNA (263 bp). PCR products were denatured and separated on a sequencing gel. mRNA-derived signals were quantified on a gamma counter (Instant Imager; Packard Instruments, Meriden, Conn.). The amount of specific mRNA, expressed as copies per microliter, was calculated from the following formula: a × r/b where a corresponds to the number of copies of the internal standard, b corresponds to the concentration of the RNA sample, and r corresponds to the ratio between TNF-α- or IL-8-specific mRNA and internal standard signals in counts per minute. Sample results were finally expressed as the fold induction over that of the control sample.
Quantitative TNF-α RT-PCR.
The assay was carried out as described previously (6, 31). PCR products from pAW109 RNA (Perkin-Elmer) amplified with TNF-α primers were 301 bp long and were designed to be shorter than PCR products from target mRNA (325 bp). PCR product processing and result analysis were performed as described above for the IL-8 RT-PCR assay.
RESULTS
IFN-γ induces HLA-DR expression on primary bronchial epithelial cells.
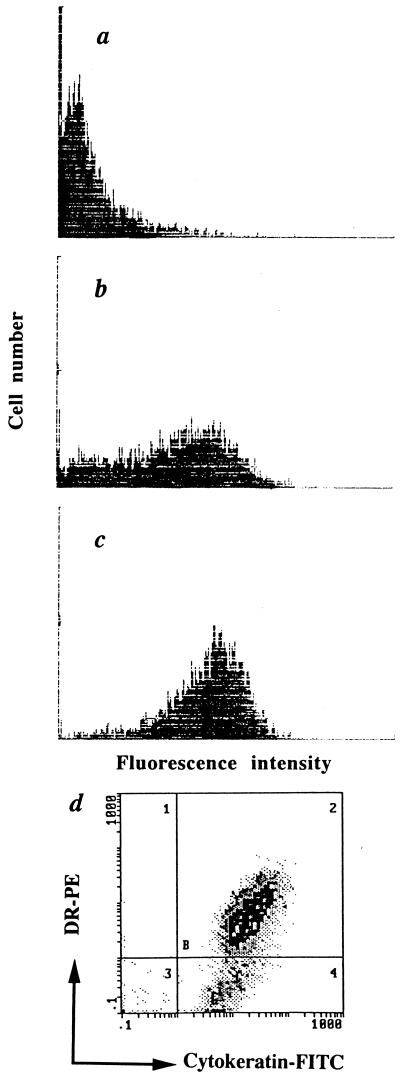
BEC were expanded for 2 weeks as described in Materials and Methods (in the absence of fetal calf serum to impair fibroblast growth) and, as indicated (see Fig. 1 legend), were pretreated with IFN-γ for 48 h or left untreated. To assess the epithelial origin of cells isolated from surgical samples as well as HLA-DR expression, cells were permeabilized and stained with anti-cytokeratin and anti-DR MAb (Fig. 1). More than 95% of the BEC were cytokeratin bright, and <1% of the cells incubated with IFN-γ expressed HLA-DR in the absence of simultaneous expression of cytokeratin, indicating that culture contamination by mucosal fibroblasts was negligible. IFN-γ-induced HLA-DR expression was dose dependent. In the absence of IFN-γ, less than 5% of the BEC expressed MHC class II molecules. A 48-h incubation with 50 IU of IFN-γ/ml resulted in HLA-DR expression in 68% of the BEC; expression peaked at 92% of the BEC in the presence of 200 IU of IFN-γ/ml.
FIG. 1.
IFN-γ induces a dose-dependent expression of HLA-DR. Untreated BEC (a) or BEC preincubated for 48 h with IFN-γ at 50 U/ml (b) or 200 U/ml (c and d) are shown. HLA-DR expression was analyzed by single (a, b, and c) or double color flow cytometry (d; HLA-DR–PE versus cytokeratin-FITC). Following IFN-γ exposure, HLA-DR was expressed by 68 (b) and 92% (c) of BEC.
TSST-1 binds to MHC class II molecules on BEC.
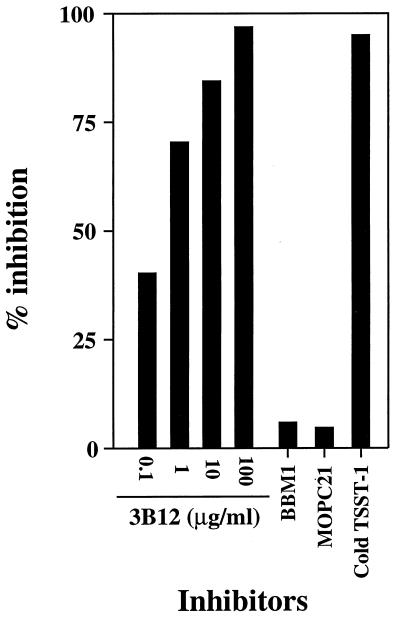
BEC pretreated for 48 h with 200 U of IFN-γ/ml (or left untreated for control BEC) were incubated as described in Materials and Methods with 125I-TSST-1. 125I-TSST-1 binding to IFN-γ-pretreated BEC was inhibited up to 97% by preincubation with increasing concentrations of anti-DR, -DP, and -DQ MAb 3B12 (Fig. 2). In contrast, incubation with anti-class I MAb BBM1 (IgG1; 100 μg) or with isotype control myeloma protein MOPC21 (IgG1; 100 μg) did not result in more than 6% binding inhibition, comparable to a 5% nonspecific inhibition deduced from incubation with cold TSST-1. In absence of BEC pretreatment with IFN-γ, only 8% of the total 125I-TSST-1 bound to control BEC. This strongly suggested that MHC class II molecules were unique binding sites for TSST-1 on BEC and made unlikely the presence of another receptor.
FIG. 2.
TSST-1 binds to MHC class II molecules on BEC. BEC pretreated for 48 h with IFN-γ at 200 U/ml were incubated with 125I-labeled TSST-1 alone or with anti-class II MAb 3B12, anti-class I MAb BBM1 (100 μg/ml), and isotype control myeloma protein MOPC21 (100 μg/ml) as competitors. Nonspecific binding was assessed from competition with cold TSST-1 (10 μg) in excess. Results from duplicate samples are expressed as percent inhibition of 125I-labeled toxin binding to IFN-γ-pretreated BEC.
Engagement of MHC class II by TSST-1 induces TNF-α and IL-8 mRNA expression.
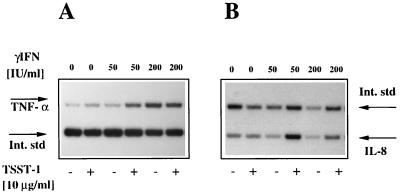
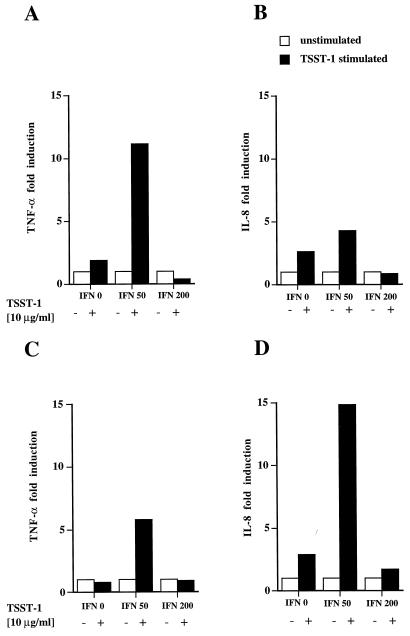
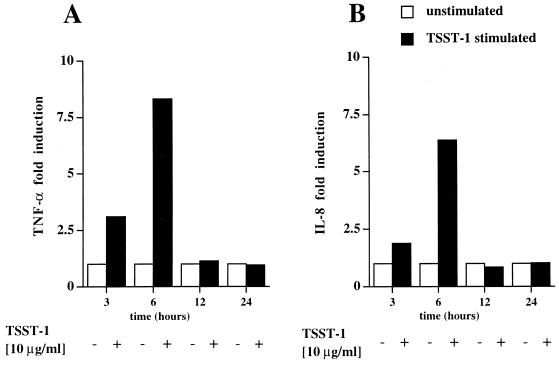
Human macrophages express HLA-DR and secrete TNF-α and IL-1 upon engagement of HLA-DR molecules by TSST-1 (6, 30). To determine whether HLA-DR expression induced by IFN-γ on primary BEC could also result in the production of the proinflammatory cytokine TNF-α or chemokine IL-8 upon MHC class II molecule engagement by TSST-1, BEC isolated from surgical samples from two patients were incubated with TSST-1 (10 μg/ml) for 1 h, with or without pretreatment with increasing concentrations of IFN-γ. After extraction (3), total RNA was analyzed by quantitative RT-PCR assay for TNF-α and IL-8 transcripts (Fig. 3). Induction of TNF-α transcripts upon TSST-1 stimulation, calculated as described in Materials and Methods, was the most vigorous in cells preincubated with IFN-γ (50 IU/ml) and was enhanced, in comparison to that for unstimulated cells, by 11.1-fold and 5.8-fold in patients 1 and 2, respectively (Fig. 4A and C). IL-8 gene expression followed a similar IFN-γ dose dependence (4.3-fold and 14.8-fold induction over that for unstimulated cells) (Fig. 4B and D). A dose-dependent effect of IFN-γ alone on cytokine gene expression was present and was more pronounced on mRNA expression of TNF-α than on that of IL-8 (Fig. 3). However, when BEC were stimulated with 200 IU of IFN-γ/ml, TNF-α or IL-8 mRNA was not further enhanced by additional stimulation with TSST-1. In contrast, even in the absence of IFN-γ pretreatment, a small but reproducible induction in IL-8 gene expression was detectable upon TSST-1 stimulation. In a time course study, a primary bronchial epithelial cell line isolated from patient 3 was preincubated with the optimal concentration of 50 IU of IFN-γ/ml for 48 h and then was stimulated with TSST-1 (10 μg/ml) for the indicated periods of time (Fig. 5). TNF-α mRNA induction was detectable after 3 h (3.1-fold induction), peaked at 6 h (8.2-fold induction), and decreased rapidly thereafter. IL-8 transcript induction followed a parallel pattern of expression (respectively, 1.9- and 6.4-fold induction).
FIG. 3.
Engagement of MHC class II molecules by TSST-1 on BEC induces TNF-α and IL-8 gene expression. Results of a representative experiment showing expression of TNF-α (A) and IL-8 mRNA (B) by BEC as measured by quantitative RT-PCR are presented. BEC isolated from patient 2 were pretreated with IFN-γ for 48 h, as indicated, and then were incubated for 1 h with medium alone (unstimulated) or TSST-1 (10 μg/ml). Int. std., internal standard.
FIG. 4.
Quantitative RT-PCR analysis of TNF-α (A and C) and IL-8 mRNA (B and D) expression by MHC class II+ BEC upon TSST-1 exposure. BEC lines were derived from patients 1 (A and B) and 2 (C and D). Sample results were compared to the internal standard, corrected for RNA concentration, and expressed as fold induction over that of TSST-1-unstimulated but IFN-γ-pretreated BEC (see Materials and Methods).
FIG. 5.
Time-course analysis of TNF-α and IL-8 mRNA expression upon BEC stimulation by TSST-1. After exposure to IFN-γ (50 U/ml) for 48 h, BEC isolated from patient 3 were incubated for 3, 6, 12, and 24 h with medium alone (unstimulated) or with TSST-1 (10 μg/ml). Samples were analyzed as described for Fig. 3.
DISCUSSION
We have shown in this paper that MHC class II molecules bind TSST-1 and that their engagement by TSST-1 on BEC pretreated with IFN-γ at the optimal concentration of 50 IU/ml induced a vigorous expression of TNF-α and IL-8 genes, which peaked about 6 h after the initial stimulation. Several studies have demonstrated that bronchial epithelial cells are potential sources of proinflammatory mediators such as IL-1β, IL-8, TNF-α, and GM-CSF (4, 10, 12, 13, 17, 23, 28). These cytokines are synthesized and constitutively released from bronchial epithelial cell cultures, and their production is enhanced by TNF-α or IL-1β (1, 5). In vivo, MHC class II molecules are expressed by epithelial cells of the upper and lower respiratory airways in inflammatory conditions including asthma, chronic inflammatory lung disease, lung carcinoma, and allergic rhinitis (8, 18, 19, 21). In nonallergic subjects, MHC class II molecule expression by nasal epithelial cells was directly correlated with the number of neutrophils and lymphocytes in the nasal brushing (18). In vitro, nonprofessional antigen-presenting cells, such as epithelial cells from the airways or from the gut, are able to express MHC class II molecules upon incubation with IFN-γ and as such acquire the capacity to present antigen to T cells or to induce T-cell tolerance (8, 11, 20). We demonstrate here, furthermore, that MHC class II molecules upregulated on IFN-γ-pretreated BEC are the only significant targets of TSST-1, in view of a 97% specific inhibition obtained by BEC preincubation with anti-MHC class II MAb 3B12, and with regard also to a 5% nonspecific binding (Fig. 2). This makes highly unlikely the existence on BEC of a second receptor for TSST-1 whose engagement would lead to signal transduction.
Our data show that BEC preincubated with IFN-γ can further enhance their proinflammatory activity upon engagement of MHC class II molecules by bacterial superantigens. IFN-γ-induced MHC class II expression was clearly dose dependent (Fig. 1). However, optimal induction of TNF-α and IL-8 genes occurred in TSST-1-stimulated BEC pretreated with 50 IU of IFN-γ/ml, a concentration which does not correspond to the peak of MHC class II molecule expression obtained with concentrations of from 200 to 400 IU of IFN-γ/ml. This was mainly due to an IFN-γ dose-dependent TNF-α and IL-8 mRNA expression which could not be further enhanced by stimulation with TSST-1 (Fig. 3). This may have resulted also, in part, at least, for IL-8 gene expression, from an inhibitory effect of IFN-γ at high concentration, as previously documented (2). We thus demonstrate that MHC class II molecules on BEC not only may contribute to antigen presentation (8, 19–21), but also may transduce signals, leading to the generation of proinflammatory mediators. Over the past years, several studies have clearly demonstrated that MHC class II molecules engaged by TSST-1 on monocytes (16), B cells (6a), NK cells (25), and activated human T cells (25) were able to transduce signals leading to early and late activation events. BEC should be added to the list. In monocytes, engagement of MHC class II molecules by TSST-1 not only induced a transcriptional activation of the TNF-α gene but also strongly stimulated TNF-α translation and secretion (6). In human activated T cells, MHC class II molecule cross-linking by anti-DR antibodies resulted in the production of IL-2, IFN-γ, IL-3, and TNF-α mRNA transcripts (24). The present data suggest that IL-8 gene expression in BEC was also the consequence of a direct transcriptional and/or posttranscriptional regulation of the IL-8 gene by signals transduced via MHC class II molecules. Indeed, since IL-8 and TNF-α transcripts both peaked 6 h after BEC stimulation by TSST-1, it seems unlikely that IL-8 gene expression merely resulted from autocrine TNF-α stimulation (10, 17, 26). Our observations on BEC are reminiscent of recent findings showing that engagement of MHC class II molecules on IFN-γ-treated fibroblast-like synoviocytes by staphylococcal enterotoxin A selectively induced the production of interstitial collagenase (15). We here show furthermore that ligation of MHC class II molecules by bacterial superantigens in an open, nonsterile environment such as the lower respiratory tract may lead to the amplification of a local preexisting inflammatory response.
Taken together, we demonstrate in this paper not only that BEC may play the role of nonprofessional antigen-presenting cells (8, 19–21) but also that the expression of MHC class II molecules may contribute to sustaining the proinflammatory activity of the epithelial lining. Since numerous inflammatory conditions such as bronchial asthma and neoplasia may enhance MHC class II molecule expression by epithelial cells, interaction with bacterial pathogens able to secrete superantigens may be of clinical relevance and may contribute to the induction of a cascade of proinflammatory mediators and to neutrophil or eosinophil chemotaxis.
ACKNOWLEDGMENTS
We thank Christine de Gunten for excellent secretarial help.
This work was supported by Swiss National Fund for Scientific Research grant no. 32432.91/1,2 and 049678.96.
REFERENCES
- 1.Abdelaziz M M, Devalia J L, Khair O A, Calderon M, Sapsford R J, Davies R J. The effect of conditioned medium from cultured human bronchial epithelial cells on eosinophil and neutrophil chemotaxis and adherence in vitro. Am J Respir Cell Mol Biol. 1995;13:728–737. doi: 10.1165/ajrcmb.13.6.7576711. [DOI] [PubMed] [Google Scholar]
- 2.Cassatella M A, Guasparri I, Ceska M, Bazzoni F, Rossi F. Interferon-gamma inhibits interleukin-8 production by human polymorphonuclear leucocytes. Immunology. 1993;78:177–184. [PMC free article] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Churchill L, Friedman B, Schleimer R P, Proud D. Production of granulocyte-macrophage colony-stimulating factor by cultured human tracheal epithelial cells. Immunology. 1992;75:189–195. [PMC free article] [PubMed] [Google Scholar]
- 5.Cromwell O, Hamid Q, Corrigan C J, Barkans J, Meng Q, Collins P D, Kay A B. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology. 1992;77:330–337. [PMC free article] [PubMed] [Google Scholar]
- 6.Espel E, Garcia-Sanz J A, Aubert V, Menoud V, Sperisen P, Fernandez N, Spertini F. Transcriptional and translational control of TNF-α gene expression in human monocytes by MHC class II ligands. Eur J Immunol. 1996;26:2417–2424. doi: 10.1002/eji.1830261023. [DOI] [PubMed] [Google Scholar]
- 6a.Fuleihan R, Spertini F, Geha R S, Chatila T. Role of protein kinase activation in the induction of B cell adhesion by MHC class II ligands. J Immunol. 1992;149:1853–1858. [PubMed] [Google Scholar]
- 7.Godding V, Stark J M, Sedgwick J B, Busse W W. Adhesion of activated eosinophils to respiratory epithelial cells is enhanced by tumor necrosis factor-α and interleukin-1β. Am J Resp Cell Mol Biol. 1995;13:555–562. doi: 10.1165/ajrcmb.13.5.7576691. [DOI] [PubMed] [Google Scholar]
- 8.Kalb T H, Chuang M T, Marom Z, Mayer L. Evidence for accessory cell function by class II MHC antigen-expressing airway epithelial cells. Am J Respir Cell Mol Biol. 1991;4:320–329. doi: 10.1165/ajrcmb/4.4.320. [DOI] [PubMed] [Google Scholar]
- 9.Kunkel S L, Standiford T, Kasahara K, Strieter R M. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp Lung Res. 1991;17:17–23. doi: 10.3109/01902149109063278. [DOI] [PubMed] [Google Scholar]
- 10.Kwon O J, Au B T, Collins P D, Adcock I M, Mak J C, Robbins R R, Chung K F, Barnes P J. Tumor necrosis factor-induced interleukin-8 expression in cultured human airway epithelial cells. Am J Physiol. 1994;267:L398–L405. doi: 10.1152/ajplung.1994.267.4.L398. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Yio X Y, Mayer L. Human intestinal epithelial cell-induced CD8+ T cell activation is mediated through CD8 and the activation of CD8-associated p56lck. J Exp Med. 1995;182:1079–1088. doi: 10.1084/jem.182.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lilly C M, Nakamura H, Kesselman H, Nagler-Anderson C, Asano K, Garcia-Zepeda E A, Rothenberg M E, Drazen J M, Luster A D. Expression of eotaxin by human lung epithelial cells. J Clin Investig. 1997;99:1767–1773. doi: 10.1172/JCI119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marini M, Vittori E, Hollemborg J, Mattoli S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol. 1992;89:1001–1009. doi: 10.1016/0091-6749(92)90223-o. [DOI] [PubMed] [Google Scholar]
- 14.Martich G D, Danner R L, Ceska M, Suffredini A F. Detection of interleukin-8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J Exp Med. 1991;173:1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehindate K, Thibodeau J, Dohlsten M, Kalland T, Sekaly R P, Mourad W. Cross-linking of major histocompatibility complex class II molecules by staphylococcal enterotoxin A superantigen is a requirement for inflammatory cytokine gene expression. J Exp Med. 1995;182:1573–1577. doi: 10.1084/jem.182.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morio T, Geha R S, Chatila T A. Engagement of MHC class II molecules by staphylococcal superantigens activates src-type protein tyrosine kinases. Eur J Immunol. 1994;24:651–658. doi: 10.1002/eji.1830240325. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura H, Yoshimura K, Jaffe H A, Crystal R G. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991;266:19611–19617. [PubMed] [Google Scholar]
- 18.Nonaka M, Nonaka R, Jordana M, Dolovich J. GM-CSF, IL-8, IL-1R, TNF-αR, and HLA-DR in nasal epithelial cells in allergic rhinitis. Am J Respir Crit Care Med. 1996;153:1675–1681. doi: 10.1164/ajrccm.153.5.8630619. [DOI] [PubMed] [Google Scholar]
- 19.Rossi G A, Sacco O, Balbi B, Oddera S, Mattioni T, Corte G, Ravazzoni C, Allegra L. Human ciliated bronchial epithelial cells: expression of the HLA-DR antigens and of the HLA-DR alpha gene, modulation of HLA-DR antigens by gamma-interferon and antigen-presenting function in the mixed leukocyte reaction. Am J Respir Cell Mol Biol. 1990;3:431–439. doi: 10.1165/ajrcmb/3.5.431. [DOI] [PubMed] [Google Scholar]
- 20.Salik E, Tyorkin M, Mohan S, George I, Becker K, Oei E, Kalb T, Sperber K. Antigen trafficking and accessory cell function in respiratory epithelial cells. Am J Respir Cell Mol Biol. 1999;21:365–379. doi: 10.1165/ajrcmb.21.3.3529. [DOI] [PubMed] [Google Scholar]
- 21.Saunders N A, Smith R J, Jetten A M. Differential responsiveness of human bronchial epithelial cells, lung carcinoma cells, and bronchial fibroblasts to interferon-γ in vitro. Am J Respir Cell Mol Biol. 1994;11:147–152. doi: 10.1165/ajrcmb.11.2.8049075. [DOI] [PubMed] [Google Scholar]
- 22.Sehmi R, Cromwell O, Warlaw A J, Moqbel R, Kay A B. Interleukin-8 is a chemoatractant for eosinophils purified from subjects with blood eosinophilia but not from normal healthy subjects. Clin Exp Allergy. 1993;23:1027–1036. doi: 10.1111/j.1365-2222.1993.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 23.Sousa A R, Lane S J, Nakhosteen J A, Yoshimura T, Lee T H, Poston R N. Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am J Respir Cell Mol Biol. 1994;10:142–147. doi: 10.1165/ajrcmb.10.2.8110469. [DOI] [PubMed] [Google Scholar]
- 24.Spertini F, Chatila T, Geha R S. Signals delivered via MHC Class II molecules synergize with signals delivered via TCR/CD3 to cause proliferation and cytokine gene expression in T cells. J Immunol. 1992;149:65–70. [PubMed] [Google Scholar]
- 25.Spertini F, Spits H, Geha R S. Staphylococcal exotoxins deliver activation signals to human T-cell clones via major histocompatibility complex class II molecules. Proc Natl Acad Sci USA. 1991;88:7533–7537. doi: 10.1073/pnas.88.17.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Standiford T J, Kunkel S L, Basha M A, Chensue S W, Lynch III J P, Toews G B, Westwick J, Strieter R M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Investig. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stark J M, Godding V, Sedgwick J B, Busse W W. Respiratory syncytial virus infection enhances neutrophil and eosinophil adhesion to cultured respiratory epithelial cells. J Immunol. 1996;156:4774–4782. [PubMed] [Google Scholar]
- 28.Stellato C, Collins P, Ponath P D, Soler D, Newman W, LaRosa G, Li H D, White J, Schwiebert L M, Bickel C, Liu M, Bochner B S, Williams T, Schleimer R P. Production of the novel C-C chemokine MCP-4 by airway cells and comparison of its biological activity to other C-C chemokines. J Clin Investig. 1997;99:926–936. doi: 10.1172/JCI119257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson A B. Immunological functions of the pulmonary epithelium. Eur Respir J. 1995;8:127–169. doi: 10.1183/09031936.95.08010127. [DOI] [PubMed] [Google Scholar]
- 30.Trede N S, Geha R S, Chatila T. Transcriptional activation of IL-1β and tumor necrosis factor-α genes by MHC Class II ligands. J Immunol. 1991;146:2310–2315. [PubMed] [Google Scholar]
- 31.Wang A M, Doyle M V, Mark D F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86:9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]