Abstract
Liver-stage antigen 1 (LSA-1) is a potential vaccine candidate against preerythrocytic stages of malaria. We report here the immunogenicity of linear synthetic constructs delineated as TH-cell determinants from the nonrepeat regions of Plasmodium falciparum LSA-1 in murine models and human subjects from areas where malaria is endemic in Rajasthan State, India. Seven peptide constructs (LS1.1 to LS1.7) corresponding to predicted T-cell sites from both the N- and C-terminal regions and peptide LS1R from a repeat region of PfLSA-1 were synthesized to analyze the cellular immune responses. These linear peptides were also tested for humoral responses in order to determine if there were any overlapping B-cell epitopes in the predicted T-cell sites. Most peptides induced cellular responses in peptide-immunized BALB/c and C57BL/6 mice as measured by proliferation and cytokine analysis. Cross-reactive T-cell recognition of P. falciparum-based peptides in Plasmodium berghei-immune animals was evaluated, but only one peptide, LS1.2 (amino acids 1742 to 1760) triggered T-cell proliferation and interleukin-2 and gamma interferon secretion in P. berghei-immune splenocytes of BALB/c and C57BL/6 mice as well as in Thamnomys gazellae (natural host of P. berghei ANKA). In an enzyme-linked immunosorbent assay with the peptides, only one peptide, LS1.1, was recognized by anti-P. berghei liver-stage serum. Three peptides (LS1.1, LS1.2, and LS1.3) of the eight peptides tested in this study were recognized by a relatively large percentage of P. falciparum-exposed human subjects; the reactivities ranged from ∼45% for LS1.3 to ∼60% for LS1.1 and LS1.2. Interestingly, all of the eight putative T-cell determinants were also recognized by the sera collected from malaria patients, although the response was variable in nature. These TH- and B-cell epitopes may be of potential value for preerythrocytic antigen-based malaria subunit vaccine formulations.
Malaria continues to be the major cause of mortality and morbidity in the most heavily populated area of the world. It is estimated that there are 300 to 500 million new Plasmodium infections and 2 to 5 million deaths annually due to malaria (42). In the past few years, due to the development of drug resistance in the malarial parasite and insecticide resistance in the vector, malaria control programs have been severely impeded. These factors call for the development of a vaccine against malaria for improving public health in the tropical world. Immunization studies using irradiated sporozoites have indicated that responses to preerythrocytic-stage antigens expressed by sporozoite and/or liver-stage parasites can provide complete immunity both in rodents and humans (6, 10, 16, 19, 26, 32, 36). Naive human volunteers immunized by irradiated sporozoites exhibit sterile immunity and develop a T-cell-proliferative response to several preerythrocytic and blood stage antigens (21). The complex life cycle of the malarial parasite and the extensive variability among strains of the same Plasmodium species dictate that an effective malaria vaccine will probably require inducing protective antibodies as well as effector CD4+ and CD8+ T lymphocytes specific for variants of multiple antigens expressed at different stages of the life cycle.
The Plasmodium falciparum liver stage antigen 1 (PfLSA-1) gene encodes a protein of ∼230 kDa and is expressed throughout the liver schizogony (15, 20, 43). The protein is localized as flocculent material within the parasitophorous vacuole, which forms a stroma in which hepatic merozoites are released and which may adhere to the merozoites. A homologue of PfLSA-1 in the Plasmodium berghei preerythrocyte parasite has been characterized; it has been suggested that this homologue is involved in the induction of protective immunity against murine malaria (2). The PfLSA-1 protein contains a large central repeat region that is polymorphic in length and that is flanked by relatively invariant nonrepeat N- and C-terminal regions (43). Mice immunized with a PfLSA-1 peptide (EQQSDLEQERLAKEKLQ) were protected against challenge with P. berghei sporozoites. The antipeptide antibodies recognized P. berghei LSA-2, and spleen cells from these protected mice killed P. berghei-infected hepatocytes in vitro (20). Studies by Hill et al. revealed the potential importance of PfLSA-1 as a vaccine candidate by showing that a peptide, corresponding to amino acids 1786 to 1794 of the PfLSA-1, bound to HLA B53, which was found to be associated with resistance to severe malaria in Gambians (17, 18). These and a few other studies have established that the T- and B-cell epitopes in PfLSA-1 protein are highly immunogenic in humans naturally exposed to malaria and that the protein may be an important vaccine target antigen (2, 11).
We have been interested in defining B- and T-cell epitopes of major malaria vaccine target antigens for their inclusion in subunit vaccine constructs (3, 7, 8, 9, 22, 23, 35). In the present study, we have attempted to characterize TH-cell epitopes in the nonrepeat portion of PfLSA-1 in mice and in humans. We have initially used mice to analyze the T-cell responses to these peptides in murine models; we then analyzed the cellular and humoral immune responses to these peptide epitopes in malaria-exposed human subjects from areas of Rajasthan State in India where malaria is endemic. One of the goals of the present study was also to inquire if the results of immune responses in murine models can serve as useful indicators for narrowing down the number of peptides that need to be tested in human studies.
MATERIALS AND METHODS
Experimental animals.
Eight- to 12-week-old female, inbred BALB/c (I-Ad I-Ed) and C57BL/6 (I-Ab I-Eo) mice were purchased from IFFA CREDO, Brussels, Belgium, and the small-animal facility of the National Institute of Immunology, New Delhi, India. Breeding and maintenance of the natural host of P. berghei ANKA, Thamnomys gazellae, were performed in the experimental animal house of Prince Leopold Institute of Tropical Medicine, Antwerp, Belgium. All the experimental animals were housed, fed, and used in the experiments in accordance with guidelines set forth in the National Institutes of Health manual “Guide for the Care and Use of Laboratory Animals” (29).
Synthetic peptides.
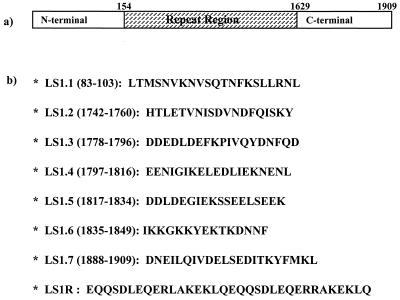
The procedures employed for synthesis, purification, and characterization of the synthetic constructs used in this study were essentially the same as those described in our earlier work (3, 7, 8, 9, 22, 23, 35). Briefly, peptides were synthesized by a stepwise solid-phase procedure (Boc-chemistry) using an automated peptide synthesizer (model 430A; Applied Biosystems, Foster City, Calif.). Peptides were purified by gel filtration with Sephadex G-25, followed by reverse-phase high-performance liquid chromatography on a μ-Bondapak C18 column (Waters Associates, Milford, Pa.). The identity and purity of peptides were confirmed by amino acid analysis and in some cases also by sequencing on an automated peptide sequencer (model 477A; Applied Biosystems). Lyophilized peptides were dissolved in ultrapure water and stored frozen at −20°C, and their serial dilutions in the culture medium were prepared immediately before each assay. The sequences of these peptide constructs are shown in Fig. 1.
FIG. 1.
(a) Schematic representation of P. falciparum LSA-1; (b) sequences of peptides used in this study. All the peptides represent amino acid sequences deduced from the genetic sequence of the P. falciparum NF54 isolate.
Immunization of experimental animals with synthetic peptides.
Groups of BALB/c and C57BL/6 mice were immunized once with 40 to 60 μg of peptide per mouse, emulsified 1:1 (vol/vol) in complete Freund's adjuvant (CFA) containing the bacterial strain H37Ra (Difco Laboratories, Detroit, Mich.) by injecting subcutaneously at the tail base and hind footpad. As a control, groups of naive and phosphate-buffered saline (PBS; emulsified 1:1 in CFA)-immunized mice were included in the study.
P. berghei ANKA-immune animals.
Immune C57BL/6 mice were obtained after three intravenous injections, spaced 14 days apart, of 30,000 γ-irradiated sporozoites each (12,000 rads, 60Co source). Immune BALB/c mice and T. gazellae animals were obtained by single injections of 10,000 γ-irradiated sporozoites each.
Cell culture medium.
For human peripheral blood mononuclear cell (PBMC) preparation, modified McCoy's 5A medium (Life Technologies, Inc., Grand Island, N.Y.) supplemented with 10% normal AB+ human serum (donors not previously exposed to malaria), 2 mM l-glutamine, 25 mM HEPES, 0.2% NaHCO3, 1% antibiotic/antimycotic solution (100×), and 0.1 mM minimal essential medium (MEM) nonessential amino acid solution was used. RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 50 mM β-mercaptoethanol, 2 mM l-glutamine, 25 mM HEPES, 0.2% NaHCO3, 1% antibiotic/antimycotic solution (100×), and 0.1 mM MEM nonessential amino acid solution was used for the mouse lymphocyte proliferation assay. For hepatocyte preparation, MEM supplemented with 5% FCS and 1% penicillin-streptomycin was used.
Generation of anti-P. berghei liver-stage sera.
A six-week-old BALB/c mouse previously irradiated at 800 rads from a 60Co source was infected with 2 million P. berghei sporozoites via the intravenous route in the tail vein. Twenty-four hours later, the liver was removed aseptically from the infected mouse and hepatocytes were prepared by collagenase perfusion. Liver fragments were perfused with 50 ml of 1 mM HEPES buffer, followed by 50 ml of the same buffer containing 0.05% collagenase (Sigma Chemical Co., St. Louis, Mo.) and 5 mM CaCl2. All solutions were maintained at 37°C during the perfusion process. The distended liver pieces were teased in HEPES buffer, and the released cells were filtered and then washed three times. The cell pellet was suspended in complete MEM. Hepatocyte viability was assessed by light microscopy.
One million infected hepatocytes were injected intravenously via the tail vein into 10 naive BALB/c mice. Two weeks later, sera were collected from all immunized mice, pooled together, aliquoted, and frozen at −20°C until further use.
Murine spleen and lymph node T-cell proliferation assay.
All immunized and control mice were sacrificed after 10 to 12 days of immunization by cervical dislocation. The spleen and lymph nodes (inguinal and popliteal) were aseptically removed, and cell suspensions were made. Cells were washed three times with serum-free RPMI 1640 medium and then cultured in a flat-bottom 96-well plate (Falcon; Becton Dickinson, Lincoln Park, N.J.) at a concentration of 5 × 105 cells per well in complete RPMI 1640 medium. Appropriate peptides were incubated with the seeded lymphocytes at different concentrations. All cultures were set up in quadruplicate, and the plates were incubated at 37°C and 5% CO2 in a humidified chamber. As a positive control concanavalin A (Sigma), at a previously determined optimal concentration of 5 μg/ml, was used as a nonspecific polyclonal mitogen. After 48 (concanavalin A) or 100 h (synthetic peptides), cultures were pulsed with 1 μCi of [methyl-3H]thymidine (Amersham International, Buckinghamshire, United Kingdom)/well for 12 h. The cells were then harvested onto glass fiber filters by using the PHD cell harvester (Cambridge Technology, Inc., Watertown, Mass.), and the [3H]thymidine incorporation was determined by β-emission liquid scintillation spectroscopy (Betaplate; Pharmacia, Uppsala, Sweden). The geometric mean of the counts per minute for each set of quadruplicate wells was calculated, and the stimulation index (SI) was determined as the geometric mean counts per minute of the peptide-stimulated culture divided by the geometric mean counts per minute of the unstimulated culture (background). The standard deviations in all cases never exceeded 20% of the means. An SI value of 2.0 was considered as the cutoff value.
Study sites and human subjects.
Two rural areas of high endemicity in the Rajasthan state were investigated: Jodhpur and Udaipur. Donors ranged in age from 12 to 57 years and were of both sexes. Informed consent from all the human subjects was obtained after explaining to them the objectives of the study in detail, particularly emphasizing the fact that the study was approved by the institutional Human Volunteers Research Ethical Committee of the International Centre for Genetic Engineering and Biotechnology, New Delhi, India.
(i) Sample collection for humoral responses.
A total of 61 serum samples were collected from P. falciparum-infected human subjects admitted to the local hospitals of rural areas of Jodhpur and Udaipur city. All these patients presented with characteristic symptoms of high fever, chill, and rigor. Blood for serum collection from these patients was generally obtained 2 weeks after the completion of drug treatment. For negative control, serum samples were also collected from 10 healthy individuals who had no known past history of malaria and who were negative for malaria by slide examination at the time of drawing blood. With the P. falciparum lysate used as the capture antigen, these sera (each diluted 1/200) yielded an average optical density at 490 nm (OD490) of 0.28 with a standard deviation (SD) of 0.036 by enzyme-linked immunosorbent assay (ELISA). With the synthetic peptide constructs in the same assay, these sera gave average OD490s (± SD) of 0.012 ± 0.013 (LS1.1), 0.010 ± 0.009 (LS1.2), 0.007 ± 0.005 (LS1.3), 0.007 ± 0.009 (LS1.4), 0.008 ± 0.008 (LS1.5), 0.016 ± 0.008 (LS1.6), 0.025 ± 0.020 (LS1.7), and 0.023 ± 0.011 (LS1R).
(ii) Sample collection for lymphocyte transformation assay.
For the analysis of T-cell responses, peripheral blood samples from 22 confirmed P. falciparum-infected patients who had recovered from their last malaria episodes about 10 to 14 weeks prior to study were collected under medical supervision. Samples of 10 to 15 ml of venous blood were obtained in sterile tubes containing citrate-phosphate-dextrose (CPD) with McCoy's 5A medium (GIBCO BRL, Grand Island, N.Y.). To determine whether PfLSA-1 peptides stimulated proliferation and cytokine production by lymphocytes from persons not sensitized to the malaria antigen, blood was also obtained from seven healthy donors who had never been exposed to malaria. All the blood samples from human volunteers were malaria parasite negative by slide examination at the time of sample collection, and the volunteers gave informed consent to participate in this study.
Preparation of human PBMC and lymphocyte transformation assay.
Blood samples were collected aseptically in CPD tubes in the field and transported in ice to the laboratory within 6 h. The PBMC were isolated on a density gradient (Histopaque-1077; Sigma Chemical Co.) by centrifugation. Briefly, a 50-ml centrifuge tube containing 10 ml of Histopaque was loaded with 10 to 15 ml of donor blood and centrifuged at 1,800 rpm (400 × g) for 20 min at room temperature. The PBMC at the interphase were collected, washed three times in 0.9% normal saline, and resuspended in complete McCoy's 5A culture medium. Viable PBMC counts were made under a phase-contrast microscope by the trypan blue dye exclusion test.
A total of 2 × 105 to 3 × 105 PBMC in 100 μl of culture medium were plated in a 96-well flat-bottom plate with various concentrations of PfLSA-1 synthetic constructs and cultured in triplicate wells for 6 days in a humidified atmosphere with 5% CO2 at 37°C. Control cultures received 100 μl of culture medium (background), 100 μl of control peptide (hen egg lysozyme [HEL]; 20 μg/ml), and 100 μl of phytohemagglutinin (PHA); 5 μg/ml; Sigma). After 96 h of stimulation, 100 μl of cell-free supernatant was removed from each well, saved for the estimation of gamma interferon (IFN-γ) concentration, and replaced with 100 μl of complete culture medium. In the 6 days of culture, the cells were pulsed with 1 μCi of [methyl-3H]thymidine (Amersham International)/well during the last 16 h. The cells were then harvested onto glass fiber filters by using the PHD cell harvester (Cambridge Technology, Inc.), and the [3H]thymidine incorporation was determined by β-emission liquid scintillation spectroscopy (Betaplate; Pharmacia). Results were expressed as SI, and a positive lymphoproliferative response was determined as being one in which SI was >2 (mean counts per minute of test cultures > mean counts per minute of control cultures + 2 SD of the control mean). As a control, PBMC from individuals with no history of exposure to malaria were included in each set of experiments.
Measurement of lymphokines in the culture supernatants.
For the analysis of lymphokines in the culture supernatants of in vitro-stimulated murine lymph node cells, culture supernatants were collected after 48 (for interleukin-2 [IL-2] analysis) and 96 h (for IFN-γ and IL-4 analysis) of antigen stimulation. In the similar way, culture supernatants from stimulated human PBMC were also collected and pooled for IFN-γ measurement after 96 h of stimulation. All these lymphokines were measured by using a murine and human cytokine immunoassay kit (Genzyme Corp., Cambridge, Mass.) following the procedure provided by the manufacturer. An anti-rat IFN-γ detection ELISA kit (Cell Biology Products, GIBCO BRL, Gaithersburg, Md.) was utilized for the detection of IFN-γ in the culture supernatants of P. berghei-immunized T. gazellae spleen cells stimulated with PfLSA-1 peptides.
Measurement of antibody responses by ELISA.
Antibody titers against PfLSA-1 peptides in mouse and human sera were determined by the standard ELISA method as described previously. Briefly, flat-bottom Immulon-2 ELISA plates (Dynatech Laboratories Inc., Chantilly, Va.) were coated at 4°C overnight with 100 μl of capture antigen (10 μg of synthetic peptide/ml) in PBS (0.01 M; pH 7.2) and then washed with PBS and 0.05% Tween-20 (PBS-T). The uncovered reactive sites in the wells were blocked by incubation with a 5% solution of non-fat dried milk powder in PBS. All sera to be tested were diluted 1/200 (human sera) and 1/100 to 1/12,800 (anti-liver-stage sera from BALB/c mouse) in PBS containing 0.1% nonfat dried milk powder and incubated in the capture antigen-coated wells for 4 h at room temperature in a humid chamber. The unbound antibodies were washed off with PBS-T. The horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) or goat anti-human IgG (Sigma) was used at a 1:1,000 dilution as the second antibody in the respective assays. The plates were incubated further for 90 min in a humid chamber and then washed with PBS-T. The enzyme reaction was developed with o-phenylenediamine dihydrochloride as the chromogen and hydrogen peroxide as the substrate. After the reaction was stopped with 8 N sulfuric acid, the OD490 of the reaction product in the wells was recorded by using a Microplate reader (Molecular Devices, Palo Alto, Calif.). The cutoff value for a positive response was defined as an OD greater than or equal to the mean plus 2 SD of sera from 10 human donors who had never been exposed to malaria.
RESULTS
Analysis of T-cell responses and cytokine profile in murine models.
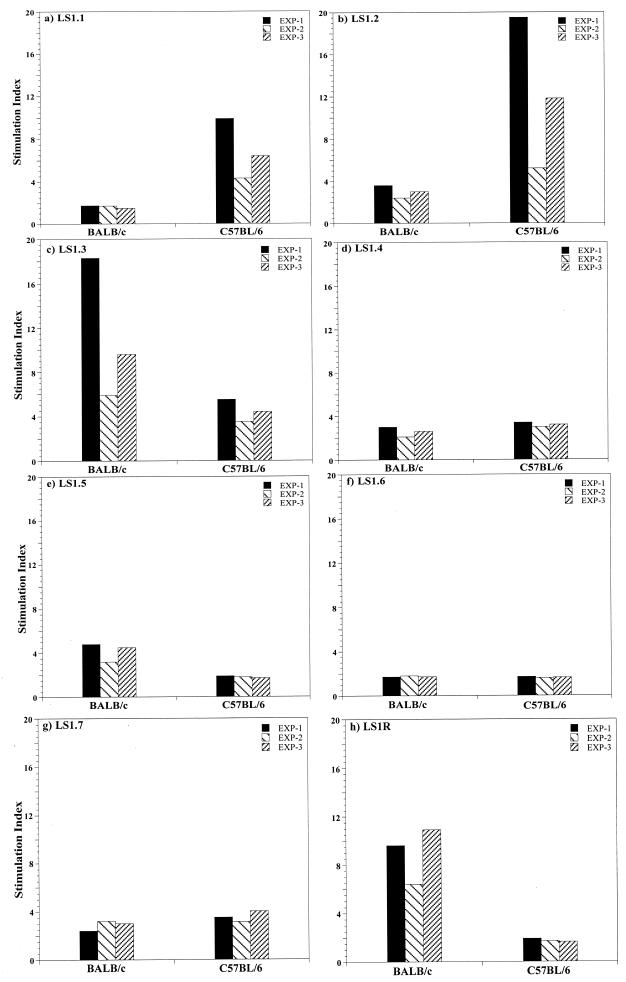
We analyzed the complete PfLSA-1 sequence for amphipathicity (25) and identified several sequences which may represent immunodominant TH-cell antigenic sites. Seven synthetic peptides from the nonrepeat N- and C-terminal regions of PfLSA-1 were selected and synthesized (Fig. 1). Peptide LS1R, representing the PfLSA-1 repeat sequence, was also synthesized. In order to determine the abilities of individual peptides to prime murine T cells, groups of BALB/c and C57BL/6 mice were immunized with each peptide emulsified with CFA. After 10 to 12 days, draining lymph node T cells were stimulated with the respective peptide at various concentrations. The results of T-cell proliferation are presented as the maximum SI obtained at a given peptide concentration (5 to 40 μg/ml) in three separate experiments. Cytokines (IL-2, IFN-γ, and IL-4) analysis was carried out with the pooled culture supernatants collected from all three separate experiments. We observed diverse T-cell proliferative responses and cytokine production levels for each peptide (Fig. 2 and Table 1). As shown in Fig. 2b to d, peptides LS1.2, LS1.3, and LS1.4 induced T-cell proliferation in both strains of mice, although the intensity of the immune response varied; peptide LS1.2 induced more proliferation in C57BL/6 (maximum SI: 19.5) than in BALB/c mice (maximum SI: 3.6), while LS1.3 induced more proliferation in BALB/c (maximum SI: 18.3) than in C57BL/6 mice (maximum SI: 5.5). Peptide LS1.4 induced similar levels of T-cell proliferation in both strains of mice (maximum SI: ∼3). The immune responses induced by some peptides were genetically restricted; peptides LS1.5 (Fig. 2e) and LS1R (Fig. 2h) induced T-cell responses only in BALB/c mice (maximum SIs: 4.8 and 10.9, respectively), whereas peptide LS1.1 (Fig. 2a) induced a strong T-cell response only in C57BL/6 mice (maximum SI: 9.9). Only peptide LS1.6 (Fig. 2f) did not induce any T-cell proliferation in either of the strains of mice (SI: <2). In control naive and adjuvant-immunized mice, no T-cell proliferation was observed at any concentration of the peptides, which ruled out the possibility of mitogenic activity or contamination in peptides (data not shown). Further, in order to determine the TH1/TH2 profile of the lymphocyte proliferation, levels of IL-2, IFN-γ, and IL-4 secretion in culture supernatants were analyzed with mouse cytokine ELISA kits (see Materials and Methods). The supernatants were collected from lymphocyte cultures after 48 (for IL-2 estimation) and 96 h (for IL-4 and IFN-γ estimation) of incubation following in vitro peptide stimulation. As shown in Table 1, only peptide LS1R induced the secretion of both TH1- (IL-2 and IFN-γ) and TH2 (IL-4)-derived cytokines in BALB/c mice. Peptides LS1.1, LS1.2, and LS1.3 induced a significant amount of IL-2 and IFN-γ in both BALB/c and C57BL/6 mice but no IL-4, indicating that a TH1-type subset is involved in the recognition of these peptides. Peptide LS1.4 induced a low level of IL-2 and IFN-γ secretion in both the strains of mice, while LS1.5 induced a low level of IL-2 and IFN-γ in only BALB/c mice. Stimulation of lymph node cells with peptides LS1.6 and LS1.7 did not induce secretion of any of the three cytokines at any concentration of the peptides (Table 1).
FIG. 2.
Lymph node T-cell proliferative responses in BALB/c and C57BL/6 mice immunized with PfLSA-1-based synthetic peptides. All mice were immunized with 60 to 70 μg of peptide in CFA, and 10 to 12 days later cells from the draining lymph nodes were cultured for 100 h with various concentrations of homologous peptides. Concanavalin A (5 μg/ml) was used as a positive control. All values are means of quadruplicate determinations with standard deviations not more than 20% of the means. The results are expressed as SIs, defined as counts per minute in stimulated cultures divided by counts per minute in unstimulated cultures. An SI value of more than 2.0 was scored as positive result. Background counts per minute ranged from 235 to 5,560 for BALB/c mice and from 160 to 6,446 for C57BL/6 mice in all cases. The results shown are from three experiments performed separately, and results are expressed as maximum SI obtained at a given peptide concentration.
TABLE 1.
Cytokine analysisa of culture supernatants of murine lymph node cells after in vitro stimulation with PfLSA-1-derived synthetic peptides
| Peptide | Peptide concn (μg/ml) | Secretion of
indicated cytokine (pg/ml) by:
|
|||||
|---|---|---|---|---|---|---|---|
| BALB/c
mice
|
C57BL/6 mice
|
||||||
| IL-2 | IFN-γ | IL-4 | IL-2 | IFN-γ | IL-4 | ||
| LS1.1 | 0 | <5 | 75 | <5 | 40 | <10 | <5 |
| 10 | 120 | 180 | <5 | 360 | 9,800 | <5 | |
| 20 | 120 | 360 | <5 | 260 | 5,600 | <5 | |
| 40 | 260 | 1,500 | <5 | 280 | 1,200 | <5 | |
| LS1.2 | 0 | 40 | 100 | <5 | 80 | 60 | <5 |
| 10 | 160 | 800 | <5 | 400 | 10,400 | <5 | |
| 20 | 180 | 660 | <5 | 360 | 8,800 | <5 | |
| 40 | 90 | 160 | <5 | 440 | 9,200 | <5 | |
| LS1.3 | 0 | 70 | 120 | <5 | 15 | <10 | <5 |
| 10 | 360 | 600 | <5 | 18 | 135 | <5 | |
| 20 | 180 | 3,000 | <5 | 30 | 540 | <5 | |
| 40 | 135 | 10,200 | <5 | 30 | 675 | <5 | |
| LS1.4 | 0 | 50 | 120 | <5 | 8 | <10 | <5 |
| 10 | 180 | 450 | <5 | 18 | 1,440 | <5 | |
| 20 | 165 | 600 | <5 | 30 | 600 | <5 | |
| 40 | 135 | 1,560 | <5 | 8 | 510 | <5 | |
| LS1.5 | 0 | 60 | <10 | <5 | 10 | <10 | <5 |
| 10 | 165 | 120 | <5 | 15 | <10 | <5 | |
| 20 | 225 | 180 | <5 | 18 | 45 | <5 | |
| 40 | 165 | 45 | <5 | 15 | 30 | <5 | |
| LS1.6 | 0 | <5 | <10 | <5 | <5 | <10 | <5 |
| 10 | <5 | <10 | <5 | <5 | <10 | <5 | |
| 20 | <5 | <10 | <5 | <5 | <10 | <5 | |
| 40 | <5 | <10 | <5 | <5 | <10 | <5 | |
| LS1.7 | 0 | <5 | <10 | <5 | <5 | <10 | <5 |
| 10 | <5 | <10 | <5 | <5 | <10 | <5 | |
| 20 | <5 | <10 | <5 | <5 | <10 | <5 | |
| 40 | <5 | <10 | <5 | <5 | <10 | <5 | |
| LS1R | 0 | <5 | <10 | <5 | <5 | <10 | <5 |
| 10 | 45 | 525 | 75 | <5 | <10 | <5 | |
| 20 | 60 | 705 | 85 | <5 | <10 | <5 | |
| 40 | 135 | 840 | 240 | <5 | <10 | <5 | |
Cytokine analysis was performed by using specific murine cytokine ELISA kits. Data analysis was done with the respective standard cytokine curves.
Since a PfLSA-1 homologue in P. berghei has been described (2), we also investigated if any of the PfLSA-1-derived peptides were able to cross-stimulate the T cells from P. berghei-exposed mice. A group of BALB/c and C57BL/6 mice were immunized by injecting γ-irradiated P. berghei ANKA sporozoites. As a natural host for P. berghei ANKA, T. gazellae mice were also included in this study. In a lymphoproliferation assay using splenocytes of P. berghei ANKA-immune mice, only peptide LS1.2 was able to induce a positive T-cell response (SI >2) in both the strains of mice (Fig. 3). Peptide LS1R also induced a positive proliferative response in P. berghei ANKA-immune splenocytes of BALB/c mice (maximum SI: 2.25) but not in C57BL/6 mice (Fig. 3). Likewise, immune T. gazellae splenocytes were able to recognize only the peptide LS1.2 (maximum SI: 4.3) in a T-cell proliferation assay (Table 2). In this experiment, peptide LS1.2 was able to induce a substantial amount of IFN-γ (28 U/ml) in the culture supernatants after 96 h of stimulation. On the other hand, peptide LS1R induced IFN-γ secretion without any T-cell proliferation. Splenocytes from the naive control animal did not proliferate or secrete IFN-γ when stimulated with any of the eight peptides (Table 2).
FIG. 3.
Proliferative responses in P. berghei-immune splenocytes of BALB/c and C57BL/6 mice. Immune mice were obtained after immunization with irradiated P. berghei sporozoites. Immune splenocytes were stimulated with PfLSA-1-based peptides ex vivo at a concentration of 20 μg/ml. The results were expressed as SIs, and a value of more than 2.0 was scored as a positive result. Background counts per minute were 5,336 and 3,136 for BALB/c and C57BL/6 mice, respectively. The results shown are from a representative experiment performed more than three times.
TABLE 2.
Lymphoproliferation and IFN-γ secretion by naive and P. berghei-immune T. gazellae splenocytes upon in vitro stimulation with PfLSA-1-derived peptides
| Stimulanta | SI for indicated
lymphocytes
|
IFN-γ secretion (U/ml)b | |
|---|---|---|---|
| Naive | Immune | ||
| LS1.1 | 0.6 | 1.2 | <5 |
| LS1.2 | 1.3 | 4.3 | 28 |
| LS1.3 | 0.8 | 1.2 | <5 |
| LS1.4 | 0.75 | 0.8 | <5 |
| LS1.5 | 0.6 | 0.75 | <5 |
| LS1.6 | 0.86 | 0.7 | <5 |
| LS1.7 | 1.4 | 0.75 | <5 |
| LS1R | 0.9 | 1.1 | 12 |
Splenocytes were stimulated with various peptides at the final concentration of 20 μg/ml.
IFN-γ analysis was done only with culture supernatants of P. berghei-immune splenocytes by using a rat IFN-γ ELISA kit. The IFN-γ concentration in the control culture (no antigen) supernatant was always <5 U/ml.
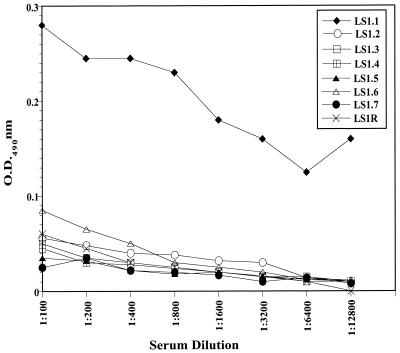
Recognition of PfLSA-1-derived peptides by anti P. berghei liver-stage sera.
T-cell epitope sequences in malaria antigens often overlap with B-cell determinants (3, 4, 7, 11, 13, 22, 35). In order to find out if any of the synthetic peptides represented cross-reactive B-cell epitopes in P. berghei infection, anti- P. berghei liver-stage serum was generated in BALB/c mice as described in Materials and Methods. Antibody reactivity to synthetic peptides was assessed by ELISA using serum dilutions from 1:100 to 1:12,800. As shown in Fig. 4, only peptide LS1.1 was recognized by anti-P. berghei liver-stage serum (OD490: 0.17 at 1:12,800). All the other seven PfLSA-1-based peptides did not show any significant reactivity with anti-P. berghei liver-stage serum.
FIG. 4.
Reactivity of anti-P. berghei liver-stage serum antibodies to PfLSA-1-based peptides in an ELISA. Anti-P. berghei liver-stage sera were generated by injecting 1 million infected hepatocytes intravenously into a group of 10 naive BALB/c mice. Two weeks later, sera were collected from all immunized mice and pooled together. Negative-control values were obtained by incubating peptides with normal mouse serum antibodies and did not exceed an OD of 0.02.
Analysis of T-cell responses and IFN-γ production in human subjects exposed and not exposed to P. falciparum.
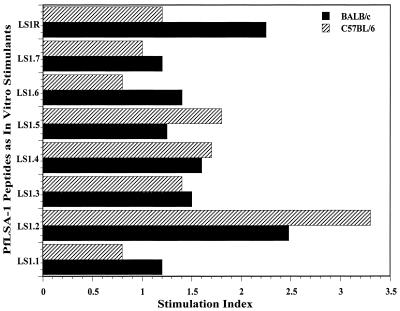
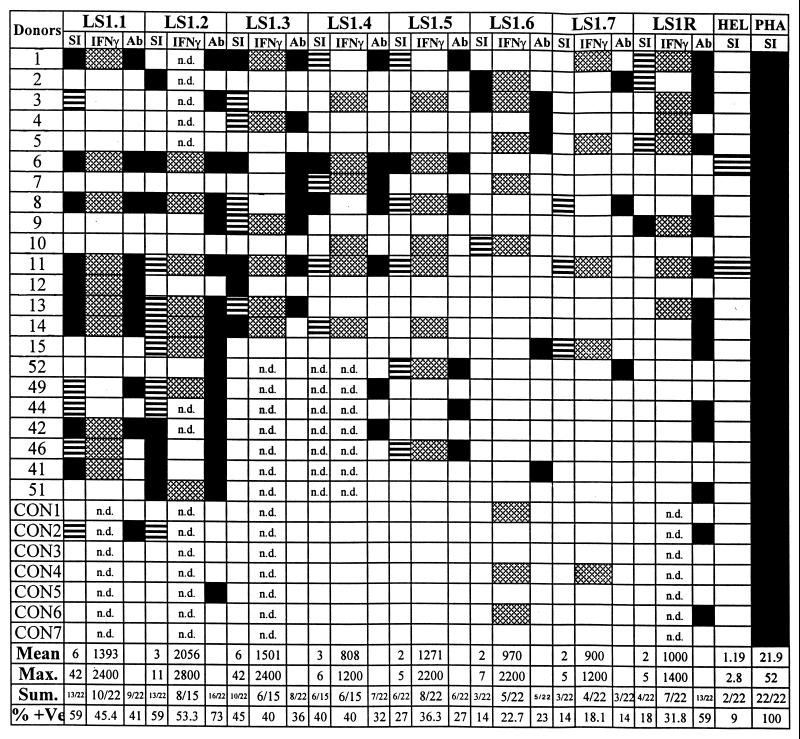
Proliferative responses of the malaria-exposed human T cells and IFN-γ secretion in response to each of the PfLSA-1-based synthetic peptides were studied. Blood samples from 22 P. falciparum-exposed human donors and seven nonexposed control donors were collected. In all the lymphoproliferation assays, subjects who showed SIs of >2 were considered positive responders. There were individual variations in the pattern of response to the different concentrations of peptides used. Some subjects responded at lower concentrations of peptides (2.5 and 5 μg/ml), while a higher concentration (10 or 20 μg/ml) was required to induce a maximal response in other donors. Therefore, the individuals who showed SIs >2 for at least one of the four concentrations of peptides were scored as positive responders. Nonspecific polyclonal T-cell mitogen PHA (5 μg/ml) and a synthetic peptide from HEL (HEL46–61; NTDGSTDYGILQINSR) were used as the positive and negative controls, respectively, in these experiments. All the donors tested in this study exhibited a positive response to PHA (mean SI: 21.9; maximum SI: 52). On the other hand, except for donors 6 (SI: 2.8) and 11 (SI: 2.2), the donors did not respond to the control peptide, HEL46–61. The results from these studies are summarized in Fig. 5. Specific lymphoproliferative responses were observed for all the PfLSA-1-derived peptides, but the extent of the response varied in different individuals. We found that peptides LS1.1 and LS1.2 represented the most widely recognized T-cell epitopes, both inducing a proliferative response in as many as 59% of the individuals (13 of 22 tested), followed by LS1.3, which was recognized by ∼45% of P. falciparum-exposed donors (10 of 22 tested). The mean SIs for LS1.1, LS1.2, and LS1.3 were 5.84, 3.17, and 5.54 respectively, while maximum SIs for these peptides were 42.0, 10.6, and 42.0, respectively. The PBMC of 40% of exposed donors were able to recognize peptide LS1.4, while peptide LS1.5 was recognized by only ∼27% of malaria-exposed donors. Peptides LS1.6, LS1.7, and LS1R were relatively poorly recognized; only ∼13 to 18% of donors responded to these peptides (Fig. 5). Although SIs for most of the positive responders were between 2 and 5, SIs for some donors were higher than 10 with peptides LS1.1, LS1.2, and LS1.3. Except for one control donor (CON2), who responded weakly to peptides LS1.1 (SI: 2.4) and LS1.2 (SI: 2.6), none of the nonexposed donors responded to any of the peptides.
FIG. 5.
PBMC proliferation and IFN-γ responses in P. falciparum-exposed human subjects and nonexposed control donors. Blood was collected during the convalescence period, since the in vitro cellular response to parasite antigens is diminished during acute infections (30, 33, 38). Results are expressed as SIs, and only the maximum SI obtained at any of the peptide concentrations used is taken into consideration. Proliferative responses were considered positive if the SI was more than 2. □, negative response (SI < 2); ▤, positive response with 2 < SI < 5; ■, positive response with SI > 5. For IFN-γ: □, negative response; ▩, positive response (IFN-γ concentration in culture supernatant > 400 pg/ml). Mean IFN-γ production in the culture supernatants of control cultures (without antigen) of all donors was 180 ± 20 pg/ml. For antibody response: □, negative; ■, positive. PBMC from control individuals with no known history of malaria were tested in parallel (CON1 to CON7). As negative and positive controls, an unrelated TH epitope from HEL and PHA, respectively, were included in the study at single concentrations of 20 and 5 μg/ml, respectively. Background count-per-minute ranges: for malaria exposed donors, 69 to 3,480 (geometric mean = 378 cpm); for control donors, 190 to 911 (geometric mean = 480 cpm). We summarized the results only from malaria-exposed individuals. n.d., not determined; Sum., summary; % + Ve, percent positive.
Due to the known susceptibility of the liver stages of the malaria parasite to IFN-γ (27), produced as a result of cellular responses to the parasite antigens, the ability of human PBMCs to secrete this cytokine in response to PfLSA-1-based peptide stimulation was also assessed. The supernatants were collected after 96 h of stimulation, and IFN-γ levels in the samples were measured at the antigen concentration at which the maximum proliferation was observed. The IFN-γ secretion was assessed by comparison with control culture supernatant without antigen (mean IFN-γ concentration: 180 ± 20 pg/ml). A value of >400 pg of IFN-γ secretion/ml was considered positive. Overall, positive responses were detected in a large proportion of infected individuals, ranging from a minimum of 18.1% for peptide LS1.7 to a maximum of 53.3% for peptide LS1.2. The highest mean IFN-γ production was 2,056 ± 566 pg/ml in response to peptide LS1.2, followed by 1,501 ± 513 pg/ml when stimulated with peptide LS1.1 (Fig. 5). In some cases, however, production of IFN-γ was observed even when no T-cell proliferation occurred. On the other hand, the reverse was also observed, i.e., T-cell proliferation followed by no IFN-γ production. Also, as shown in Fig. 5, few control donors' PBMC were able to secrete IFN-γ without any lymphoproliferation. This is in accordance with previously described studies with PfLSA-1-based peptides (11, 20).
Humoral responses in P. falciparum-exposed human donors.
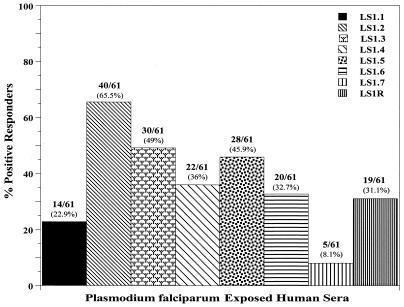
A total of 61 serum samples, including those collected from exposed donors which were used in T-cell proliferation experiments, were analyzed to assess antibody responses against PfLSA-1-based synthetic peptides. The positive responders were defined as those who showed an OD490 value in an ELISA above the mean + 2 SD of 10 normal control sera when reacted with PfLSA-1-based synthetic peptides (see Materials and Methods). The normal control sera were obtained from volunteers who had no history of malaria infection. We found that all the eight peptides were recognized, to various degrees, by circulating IgG antibodies in the sera of human subjects recovering from natural P. falciparum infection. As shown in Fig. 6, synthetic peptides LS1.2, LS1.3, and LS1.5 were more widely recognized (45 to 65% positive responders) than the others. Peptide LS1.7 was the most poorly recognized; only ∼8% of the malaria-exposed sera reacted with this peptide.
FIG. 6.
Antibody responses in human subjects exposed to P. falciparum malaria. Human serum samples were diluted to 1:200 and used in ELISA to detect antibody levels against PfLSA-1-derived peptides. The positive responders were defined as those who had ODs above the mean plus 2 SD of 10 normal control sera. The cutoff OD for each peptide is indicated in Materials and Methods.
A synoptic summary of the humoral and cellular immune responses in twenty-two malaria-exposed individuals against PfLSA-1-derived peptides is presented in Table 3. There seems to be a correlation between the humoral and cellular responses in malaria-exposed donors to most of the synthetic peptides. On the other hand, peptides LS1R and LS1.6 appear to be poor T-cell epitopes and reasonably good B-cell epitopes. The correlation between cellular and humoral immune responses for each malaria-exposed donor is also shown in Fig. 5. Most of the P. falciparum-exposed donors who showed positive cellular responses also exhibited a positive antibody response, indicating a noticeable correlation between B- and T-cell responses and IFN-γ secretion (r > 0.729).
TABLE 3.
Synoptic summary of immune responses in 22 P. falciparum-exposed human subjects against PfLSA-1-derived T- and B-cell epitopes
| PfLSA-1-derived peptide | % Positive responders
|
||
|---|---|---|---|
| B-cell response | T-cell response | IFN-γ secretion | |
| LS1.1 | 40.9 | 59.0 | 45.4 |
| LS1.2 | 72.7 | 59.0 | 53.3 |
| LS1.3 | 36.3 | 45.4 | 40.0 |
| LS1.4 | 31.8 | 40.0 | 40.0 |
| LS1.5 | 27.2 | 27.2 | 36.3 |
| LS1.6 | 22.7 | 13.6 | 22.7 |
| LS1.7 | 13.6 | 13.6 | 18.1 |
| LS1R | 59.0 | 18.1 | 31.8 |
DISCUSSION
T cells play a crucial role in malaria immunity both by regulating antibody production and mediating antibody-independent cellular effector mechanisms (28). Epidemiological studies in areas of endemicity are required to collect information about antibody and T-cell immune responses against defined parasite molecules during natural sensitization against malaria. In the present study, we have attempted to characterize T-cell determinants in the nonrepeat regions of PfLSA-1 by using synthetic peptides in mice and in P. falciparum-exposed human subjects living in an area in India where malaria is endemic. Results of T-cell proliferation studies with two different strains of mice showed that of the eight predicted T-cell epitope sequences, at least four peptides (LS1.2, LS1.3, LS1.4, and LS1.7) induced T-cell responses in both the strains of mice. Peptides LS1.5 and LS1R induced lymphoproliferation only in BALB/c mice, while peptide LS1.1 was able to induce substantial T-cell proliferation only in C57BL/6 mice. Results of measurements of cytokines released upon in vitro stimulation of peptide-primed T lymphocytes with homologous peptides by and large supported the proliferation data. Analysis of cytokines in the culture supernatants of peptide-induced lymphocytes demonstrated that most of the PfLSA-1-based peptides induced the TH1-type subset to secrete IL-2 and IFN-γ but did not activate the TH2-type subset to secrete IL-4. Only peptide LS1R induced both the TH1 and TH2 types of T cells. Similar observations were made by White et al., who found that immunization with P. berghei sporozoites induced T cells that secreted IL-2 and IFN-γ but not IL-4 (40). It has also been proposed that P. berghei sporozoite-induced TH1 cells play a central role in the induction of effector CD8+ T cells and possibly also in the regulation of TH2 cells secreting IL-4 (40). Thus, cytokines secreted by T cells after activation qualitatively influence the nature of the immune response. However, it is not clear from our experiments why mostly TH1-biased T-cell subsets are induced upon immunization with these peptides. There could be several factors responsible for the observed polarized TH1 response, which include the dose and nature of the antigen, antigen-presenting cells, antigen processing and presentation pathways, and costimulatory molecules involved in activation (34, 41). We did not explore this any further. Taken together, our results indicate that all PfLSA-1-based predicted T-cell epitopes, except LS1.6, on their own can generate memory T cells, which proliferate in vitro upon stimulation with the homologous peptide. However, at the same time it is clear that the cellular response to synthetic peptides is genetically restricted, at least to some extent, suggesting that using a single strain, for example BALB/c mice, as an initial screen for selecting T-cell sequences may not always be adequate.
Earlier studies have suggested the presence of a PfLSA-1 homologue in P. berghei, and mice immunized with PfLSA-1-based peptide sequences were protected against heterologous challenge with P. berghei sporozoites (2). It was thus appealing to probe whether any of the PfLSA-1-based synthetic peptides would cross-react with T lymphocytes from mice exposed to P. berghei sporozoites. We found that at least one of the peptides (LS1.2) triggered the stimulation of splenocytes obtained from mice immunized with irradiated P. berghei sporozoites, suggesting that during immunization, memory T cells cross-reacting with the PfLSA-1 peptide were generated; these T cells are later expanded upon restimulation with the specific LS1.2 sequence. The only other peptide that showed cross-reactivity at the T-cell level was the repeat-based peptide LS1R, but the response was genetically restricted to the BALB/c (IAd IEd) strain of mice. Essentially similar results were obtained when P. berghei-primed splenocytes from T. gazellae, a natural host of P. berghei, were stimulated in vitro with the synthetic peptides; only LS1.2 showed cross-reactivity in terms of lymphoproliferation and IFN-γ secretion in the culture supernatants. These results show that LS1.2 represents an immunodominant and cross-reactive T-cell epitope in the C-terminal region of PfLSA-1.
Since several T-cell determinants in malaria antigens are known to overlap with B-cell determinant sequences (3, 4, 11, 20), we wondered if any of the synthetic peptides in the present study also represented cross-reactive B-cell epitopes. Results of ELISA carried out with anti-P. berghei liver-stage serum revealed that only one peptide (LS1.1) represented a P. berghei ross-reactive B-cell epitope. Interestingly, peptide LS1.2, which showed cross-reactivity at the T-cell level, showed no reactivity with P. berghei-infected mouse serum. Likewise, the repeat-based peptide LS1R, which showed cross-reactivity at the T-cell level, also did not react with the anti-P. berghei liver-stage serum. The repeat-based peptide sequences have been described as B-cell epitopes in humans (20). These results suggest that peptides LS1.1 and LS1.2 may represent sequences that are similar to those in the putative P. berghei LSA-1 or even perhaps in some other P. berghei antigens. However, how these peptides with no apparent sequence homology can cross-react both at the T-cell and B-cell levels is well established (1, 21, 39).
In order to find out if PfLSA-1-based peptides that induced cellular immune responses in mice also represent T-cell determinants in humans exposed to P. falciparum infection, we analyzed blood samples from an area in India where malaria is endemic. Earlier studies have reported the presence of TH and cytotoxic T lymphocyte (CTL) epitopes in PfLSA-1 in malaria-exposed human subjects. For example, an immunodominant HLA B53-restricted CTL epitope, described by Hill et al., in a Gambian population was found to be associated with resistance to severe malaria (18). Other studies characterized several TH epitopes, mainly in the C-terminal domain of PfLSA-1, in the sporozoite-immunized human volunteers (24) and malaria-infected human subjects, including sequence overlapping LS1.1, and LS1.5 (11, 20). Results of the present study showed that the T cells from a large proportion of human donors exposed to malaria proliferated in response to PfLSA-1-based peptides. Similar to what was found in the murine model, peptides LS1.1, LS1.2, LS1.3, and LS1.4 represented immunodominant T-cell epitopes in humans. Although, we did not carry out the HLA typing of the infected donors, broad recognition of PfLSA-1 peptides, particularly with LS1.1, LS1.2, LS1.3, and LS1.4, may indicate a lack of genetic restriction and the presence of a universal character as already described for P. falciparum (5, 37) and tetanus toxin peptides (31). On the other hand, peptides LS1.6 and LS1.7 were poorly recognized in both mice and humans. Our results with LS1.7 in humans are in contrast with the earlier finding that LS1.7 peptide (amino acids 1888 to 1909) was recognized by more than 75% of human donor PBMC from an area of endemicity in Papua New Guinea (mean SI: 7.2) (11). This is surprising, and it is not clear to us if this is due to parasite strain variations or to variations in exposure to malaria and genetic background of the population in question.
IFN-γ is the most potent cytokine known to act upon P. falciparum liver schizogony in vitro (27). The inhibiting effect of IFN-γ on liver stages in both primate and rodent models has already been observed (12, 27). We also found that PfLSA-1-based peptides induce antigen-specific IFN-γ secretion by PBMC in a large proportion of individuals. However, here again the IFN-γ levels did not always correlate with the lymphocyte proliferation data. This lack of correlation between IFN-γ production and T-cell proliferation with malarial antigens has been observed earlier by us and several other workers (11, 14, 20, 22).
It is generally believed that identification of T-cell epitopes capable of eliciting an immune response in individuals of different genetic backgrounds would be necessary for designing a subunit vaccine. In the present study, we have defined immunodominant TH-cell epitopes commonly recognized by murine T cells and PBMC from a large proportion of malaria-exposed humans with diverse genetic backgrounds. Other studies have also shown that T-cell epitopes from vaccine target antigens of P. falciparum are commonly recognized by both murine and human T cells (5, 37). Given the large number of peptide sequences from different vaccine target antigens that may have to be assessed and the difficulties in collecting blood samples sufficient for these assays from the infected patients, this strategy may be an alternative even though there may be an inherent risk of missing some epitopes.
The antibody response to PfLSA-1 during the course of natural infection has been investigated earlier, and several B-cell epitopes have been characterized (11, 20). Our results of ELISA with synthetic peptides showed that most of the predicted T-cell epitope sequences also reacted with P. falciparum-exposed Indian sera. The greatest response was obtained with peptide LS1.2, which reacted with more than 60% of the serum samples, followed by LS1.3. Notably, LS1.2 and LS1.3 also represented dominant human T-cell epitopes, suggesting that such peptide sequences may be good candidates to be included in multiple-epitope-based polypeptide vaccine constructs. Likewise, our results have also suggested that peptides LS1.1, LS1.4, and LS1.5 represent reasonably dominant B- and T-cell epitopes in PfLSA-1. A sequence within peptide LS1.3 was found to be associated with resistance to severe malaria (18), and the sequences overlapping with the peptides LS1.1 and LS1.5 have also been defined as immunodominant T-cell epitopes in earlier studies (11, 24). The results of the present study have further supported these findings. On the other hand, our results have shown that peptides LS1.2 and LS1.4 represent newly identified TH epitopes, which have not been described earlier. The repeat-based peptide LS1R reacted to only 31.1% of malaria-exposed donors, in comparison to the results of a previous study, where this peptide was shown to be recognized by 75% of P. falciparum-exposed human sera (20).
In conclusion, our study has revealed the widespread immunogenicity of PfLSA-1-derived synthetic peptides in murine models and malaria-exposed human subjects and only a very limited cross-recognition of PfLSA-1-based peptides by P. berghei sporozoite-sensitized T cells in rodent malaria models. The ability of a peptide epitope to bind and to be presented in the context of several DR molecules reflects a broad major histocompatibility complex promiscuous binding pattern. The possibility of the presence of promiscuous epitopes in the conserved regions of the PfLSA-1 protein may be of the utmost importance in malaria vaccine development. Our results also indicate that synthetic peptides which represent T-cell epitopes in the murine model also by and large are responsive in human subjects as T-cell determinants. It may therefore be desirable or necessary to first assess the immunogenicities of synthetic peptides in murine models in order to narrow down the number of peptides to a more manageable size for studies in humans exposed to the infectious agents.
ACKNOWLEDGMENTS
We thank Ratanmani Joshi and Padma R. Suresh for critical reading of the manuscript and valuable suggestions. We are grateful to A. Mathur, V. Gulati, and S. Bist for helping in collection of human blood samples from areas of endemicity. We also thank Ratanmani Joshi for her help in synthesis and purification of peptides, and Narendra S. Negi for animal management and care. We are indebted to the patients and control donors who participated in this study.
REFERENCES
- 1.Akhiani A A, Nilsson L A, Ouchterlony O. Immunological cross-reactivity between Schistosoma mansoniand cholera toxin. Parasite Immunol (Oxford) 1997;19:355–361. doi: 10.1046/j.1365-3024.1997.d01-226.x. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson C T, Hollingdale M R, Aikawa M. Localization of a 230 kDa parasitophorous vacuole membrane antigen of Plasmodium bergheiexoerythrocytic schizonts (LSA-2) by immunoelectron and confocal laser scanning microscopy. Am J Trop Med Hyg. 1992;46:533–537. doi: 10.4269/ajtmh.1992.46.533. [DOI] [PubMed] [Google Scholar]
- 3.Bharadwaj A, Sharma P, Joshi S K, Singh B, Chauhan V S. Induction of protective immune responses by immunization with linear multiepitope peptides based on conserved sequences from Plasmodium falciparumantigens. Infect Immun. 1998;66:3232–3241. doi: 10.1128/iai.66.7.3232-3241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottius E, BenMohammed L, Brahimi K, Gras H, Lepers J P, Raharimalala L, Aikawa M, Meis J, Slierendregt B, Sartan A, Thomas A, Druilhe P. A novel Plasmodium falciparumsporozoite and liver stage antigen (SALSA) defines major B, T helper, and CTL epitopes. J Immunol. 1996;156:2874–2884. [PubMed] [Google Scholar]
- 5.Calvo-Calle J M, Hammer J, Sinigaglia F, Clavijo P, Moya-Castro Z R, Nardin E H. Binding of malaria T cell epitopes to DR and DQ molecules in vitro correlates with immunogenicity in vivo. J Immunol. 1997;159:1362–1373. [PubMed] [Google Scholar]
- 6.Chatterjee S, Francois G, Druilhe P, Timperman G, Wery M. Immunity to Plasmodium bergheiexoerythrocytic forms derived from irradiated sporozoites. Parasitol Res. 1996;82:297–303. doi: 10.1007/s004360050117. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee S, Sharma P, Kumar S, Chauhan V S. Fine specificity of immune responses to epitopic sequences in synthetic peptides containing B and T epitopes from the conserved Plasmodium falciparumblood-stage antigens. Vaccine. 1995;13:1474–1481. doi: 10.1016/0264-410x(94)00052-o. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee S, Wery M, Sharma P, Chauhan V S. A conserved peptide sequence of the Plasmodium falciparum circumsporozoite protein and antipeptide antibodies inhibit Plasmodium berghei sporozoite invasion of Hep-G2 cells and protect immunized mice against P. bergheisporozoite challenge. Infect Immun. 1995;63:4375–4381. doi: 10.1128/iai.63.11.4375-4381.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauhan V S, Chatterjee S, Johar P K. Synthetic peptides based on conserved Plasmodium falciparum antigens are immunogenic and protective against Plasmodium yoeliimalaria. Parasite Immunol (Oxford) 1993;15:239–242. doi: 10.1111/j.1365-3024.1993.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 10.Clyde D F, McCarthy V C, Miller R M, Woodward W E. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24:397–401. doi: 10.4269/ajtmh.1975.24.397. [DOI] [PubMed] [Google Scholar]
- 11.Connelly M, King C L, Bucci K, Walters S, Genton B, Alpers M P, Hollingdale M, Kazura J W. T-cell immunity to peptide epitopes of liver-stage antigen 1 in an area of Papua New Guinea in which malaria is holoendemic. Infect Immun. 1997;65:5082–5087. doi: 10.1128/iai.65.12.5082-5087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira A, Schofield L, Enea V, Schellekens H, Van der Meide P, Collins W E, Nussenzweig R S, Nussenzweig V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-IFN. Science. 1986;232:881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- 13.Fidock D A, Grans-Masse H, Lepers J P, Brahimi K, BenMohammed L, Mellouk S, Guerin-Marchand C, Lodono A, Raharimalala L, Meis J, Langsley G, Roussilhon C, Tartar A, Druilhe P. Plasmodium liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol. 1994;153:190–204. [PubMed] [Google Scholar]
- 14.Good M F, Pombo D, Quakyi I A, Riley E M, Houghten R A, Menon A, Alling D W, Berzofsky J A, Miller L H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci USA. 1988;85:1199–1203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerin-Marchand C, Druilhe P, Galey B, Londono A, Patarapotikul J, Beaudoin R L, Dubeaux C, Tartar A, Mercerear-Puijalon O, Langsley G. A liver-stage-specific antigen of Plasmodium falciparumcharacterized by gene cloning. Nature. 1987;329:164–167. doi: 10.1038/329164a0. [DOI] [PubMed] [Google Scholar]
- 16.Herrington D, Davis J, Nardin E, Beier M, Cortese J, Eddy H, Losonsky G, Hollingdale M, Sztein M, Levin M, Nussenzweig R S, Clyde D, Edelman R. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg. 1991;45:539–547. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- 17.Hill A V S, Elvin J, Willis A C, Aidoo M, Allsopp C E M, Gotch F M, Gao X M, Takiguchi M, Greenwood B M, Townsend A R M, McMichael A J, Whittle H C. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature. 1992;360:434–439. doi: 10.1038/360434a0. [DOI] [PubMed] [Google Scholar]
- 18.Hill A V S, Allsopp C E M, Kwiatkowski D, Anstey N M, Twumasi T, Rowe P A, Bennett S, Brewster D, McMichael A J, Greenwood B M. Common West African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman S L, Oster C N, Mason C, Bieir J C, Sherwood J A, Ballou W R, Mugambi M, Chulay J D. Human lymphocyte proliferative response to sporozoite T cell epitopes correlates with resistance to falciparum malaria. J Immunol. 1989;142:1299–1303. [PubMed] [Google Scholar]
- 20.Hollingdale M R, Aikawa M, Atkinson C T, Ballou W R, Chen G, Li J, Meis J F G M, Sina B, Wright C, Zhu J. Non-CS pre-erythrocytic protective antigens. Immunol Lett. 1990;25:71–76. doi: 10.1016/0165-2478(90)90094-7. [DOI] [PubMed] [Google Scholar]
- 21.Ignatowicz L, Rees W, Pacholczyk R, Ignatowicz H, Kushnir E, Kappler J, Marrack P. T cells can be activated by peptides that are unrelated in sequence to their selecting peptide. Immunity. 1997;7:179–186. doi: 10.1016/s1074-7613(00)80521-x. [DOI] [PubMed] [Google Scholar]
- 22.Kabilan L, Sharma V P, Kaur P, Ghosh S K, Yadav R S, Chauhan V S. Cellular and humoral immune responses to well defined blood stage antigens (major merozoite surface antigen) of Plasmodium falciparumin adults from an Indian zone where malaria is endemic. Infect Immun. 1994;62:685–691. doi: 10.1128/iai.62.2.685-691.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur P, Sharma P, Kumar A, Chauhan V S. Synthetic, immunological and structural studies on repeat unit peptides of Plasmodium falciparumantigens. Int J Pept Protein Res. 1990;36:515–521. doi: 10.1111/j.1399-3011.1990.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 24.Krzych U, Lyon J A, Jareed T, Schneider I, Hollingdale M R, Gordon D M, Ballou W R. Lymphocytes from volunteers immunized with irradiated Plasmodium falciparumsporozoites recognize liver and blood stage malaria antigens. J Immunol. 1995;155:4072–4081. [PubMed] [Google Scholar]
- 25.Margalit H, Cournette J L, Cease K B, Delisi C, Berzofsky J A. Prediction of immunodominant T cell antigenic sites from the primary sequence. J Immunol. 1987;138:2213–2229. [PubMed] [Google Scholar]
- 26.Mellouk S, Lunel F, Sedegah M, Beaudoin R L, Druilhe P. Protection against malaria induced by irradiated sporozoites. Lancet. 1990;335:721. doi: 10.1016/0140-6736(90)90832-p. [DOI] [PubMed] [Google Scholar]
- 27.Mellouk S, Maheshwari R K, Rhodes-Feiuillette A, Beaudoin R L, Berbiguier N, Matile H, Miltgen F, Landeau I, Pied S, Chigot J P, Friedman R M, Mazier D J. Inhibitory effects of IFNs and IL-1 on the development of Plasmodium falciparumin human hepatocyte cultures. J Immunol. 1987;139:4192–4195. [PubMed] [Google Scholar]
- 28.Nardin E H, Nussenzweig R S. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu Rev Immunol. 1993;11:687–693. doi: 10.1146/annurev.iy.11.040193.003351. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health. Guide for the care and use of laboratory animals. National Institutes of Health publication no. 86-23. U.S. Washington, D.C.: Department of Health and Human Services; 1985. [Google Scholar]
- 30.Otoo L N, Riley E M, Menon A, Byass P, Greenwood B M. Cellular immune responses to Plasmodium falciparumantigens in children receiving long term anti-malarial chemoprophylaxis. Trans R Soc Trop Med Hyg. 1989;83:778–782. doi: 10.1016/0035-9203(89)90324-6. [DOI] [PubMed] [Google Scholar]
- 31.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 32.Rieckmann K H, Beaudoin R L, Cassells J S, Sell K W. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull W H O. 1979;57(Suppl.1):261–265. [PMC free article] [PubMed] [Google Scholar]
- 33.Riley E M, MacLennan C, Wiatkowski D K, Greenwood B M. Suppression of in vitrolymphoproliferative responses in acute malaria patients can be partially reversed by indomethacin. Parasite Immunol (Oxford) 1989;11:509–517. doi: 10.1111/j.1365-3024.1989.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 34.Romagnani S, Kapsenberg M, Radbruch A, Adorini L. Th1 and Th2 cells. Res Immunol. 1998;149:871–873. doi: 10.1016/s0923-2494(99)80016-9. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P, Kumar A, Singh B, Bharadwaj A, Sailaja V N, Adak T, Kushwaha A, Malhotra P, Chauhan V S. Characterization of protective epitopes in a highly conserved Plasmodium falciparumantigenic protein containing repeats of acidic and basic residues. Infect Immun. 1998;66:2895–2904. doi: 10.1128/iai.66.6.2895-2904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sina B J, Do Rosario V E, Woollett G, Sakhuja K, Hollingdale M R. Plasmodium falciparum sporozoite immunization protects against Plasmodium bergheisporozoite infection. Exp Parasitol. 1993;77:129–135. doi: 10.1006/expr.1993.1069. [DOI] [PubMed] [Google Scholar]
- 37.Sinigaglia F, Guttinger M, Kilgus J, Doran D M, Matile H, Etlinger H, Trzeciak A, Gillessen D, Pink J R L. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988;336:778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- 38.Troye-Blomberg M, Romero P, Patarroyo M E. Regulation of the immune response in Plasmodium falciparum malaria. III. Proliferative response to antigen in vitroand subset composition of T cells from patients with acute infection or from immune donors. Clin Exp Immunol. 1984;58:380–387. [PMC free article] [PubMed] [Google Scholar]
- 39.Wahlin B, Sjolander A, Ahlborg N, Udomsangpetch R, Scherf A, Mattei D, Berzins K, Perlmann P. Involvement of Pf155/RESA and cross-reactive antigens in Plasmodium falciparummerozoite invasion in vitro. Infect Immun. 1992;60:443–449. doi: 10.1128/iai.60.2.443-449.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White K L, Jarboe D L, Krzych U. Immunization with irradiated Plasmodium bergheisporozoites induces IL-2 and IFN-γ but not IL-4. Parasite Immunol (Oxford) 1994;16:479–491. doi: 10.1111/j.1365-3024.1994.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 41.Windhagen A, Scolz C, Hollsberg P, Fukaura H, Sette A, Hafler D A. Modulation of cytokine patterns of human autoreactive T cell clones by a single amino acid substitution of their peptide ligand. Immunity. 1995;2:373–380. doi: 10.1016/1074-7613(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. World Health Organization Fact Sheet N94 (revised). Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 43.Zhu J, Hollingdale M R. Structure of Plasmodium falciparumliver stage antigen-1. Mol Biochem Parasitol. 1991;48:223–226. doi: 10.1016/0166-6851(91)90117-o. [DOI] [PubMed] [Google Scholar]