Abstract
Background & aims
Emergency measures in the face of the recent COVID-19 pandemic have been different among countries, although most have opted for confinement and restrictions on social contact. These measures have generated lifestyle changes with potential effects on individuals' health. The disturbances in daily routines due to confinement and remote work have impacted circadian rhythms and energy balance; however, the consequences of these disruptions have not been studied in depth. The objective was to evaluate the impact of 12-week confinement on body weight, considering changes in several external synchronizers of the biological clock.
Methods
The participants, 521 university students (16–35 years), responded to 52 questions oriented to determine light exposure, sleep patterns, sedentary lifestyle, and eating times.
Results
We found a reduction in sunlight exposure and sleep duration, an increment in sedentarism and screen exposure, and a delay in the timing of the main meals and sleep in the whole cohort. These behavioral changes were associated with a twofold increase in obesity. Subjects who increased their sedentary hours and shortened their sleep to a higher degree were those who gained more bodyweight. The most influential factors in body weight variation during confinement were sleep duration, physical activity (sedentarism), and light (timing of screen exposure). The mediation model explained 6% of the total body weight variation.
Conclusions
Results support a significant impact of confinement on several external synchronizers of the biological clock and on body weight. Health-related recommendations during the pandemic must include behavioral recommendations to mitigate the adverse effects on the biological clock.
Keywords: Covid-19, Confinement, Biological clocks, External synchronizer, Obesity
1. Introduction
June 2021, eigthteen months after the first reported case of severe acute respiratory syndrome coronavirus 2 (SARS Cov-2) infection, the COVID-19 pandemic has affected more than 171 million people, with over 3,5 million deaths globally [1]. The measures taken by governments have dramatically changed our lifestyle, which is now characterized by confinement and social isolation. While needed to prevent contagion, these measures appear to have also negative health consequences [[2], [3], [4]]. Thus, the decades-long obesity pandemic may have worsened due to the measures imposed by the COVID-19 pandemic. Although the etiology of obesity identifies excessive energy intake as the main factor (positive energy balance), there are other critical factors involved, such as sedentarism, genetics, altered microbiota and sleep deprivation [[5], [6], [7], [8]]. Furthermore, obesity has been shown as the main modifiable risk factor for severity and mortality in COVID-19 [9], which is of particular concern at the public health level [10].
The internal clock of mammals has rhythmicity of approximately 24 h. It controls physical activity patterns, endocrine functions and multiple physiological processes, and it is synchronized by internal and external regulators [11]. Examples of external synchronizers are the changes in sleep/wake cycles, eating/fasting, or light/darkness. Alterations in these external synchronizers, as happens during shift work, social jet lag, or light pollution, can desynchronize our biological clock [12]. In connection with this, mandatory confinement has been shown to alter the sleep/wake patterns [13] and to decrease the light time exposure [14], desynchronizing the internal clocks, mainly due to sleep deprivation and increased sedentary lifestyle, which has been shown to increase the risk of metabolic diseases, such as obesity [15], triggering a vicious cycle.
The current study aimed to evaluate the impact of 12-week confinement on body weight, considering changes in several external synchronizers of the biological clock in university students.
2. Methods details
2.1. Population and study design
A descriptive exploratory study was carried out in 2020, between June 13 and 20, in the province of Lima, Perú, after 12-weeks of confinement. During the confinement period, the right to free transit was restricted. Only those who worked in essential services such as electricity, water, food, and health services could go out from their houses. Furthermore, only one person per family was allowed to go out to buy necessities like food or medicine. These restrictions were continuously prolonged and slightly modified by the emergency decree of the Peruvian state [16].
The information was collected using a questionnaire developed in Google Forms that was sent to the students' institutional mail of San Ignacio de Loyola University. Initially, 820 subjects responded to the questionnaire; the sample included adolescents and young adults (up to 35 years old). Postgraduate students were excluded from the survey because they were not full-time students and their schedules were significantly different from undergraduates'. Indeed, they usually had an external job and this would have differentially influenced the time of exposure to screens and sleep habits. In total, those students who were older than 35 years, postgraduate and those who had eating disorders or morbid obesity were also excluded (n = 40). After reviewing for quality control, 259 were excluded due to wrong or incomplete answers.
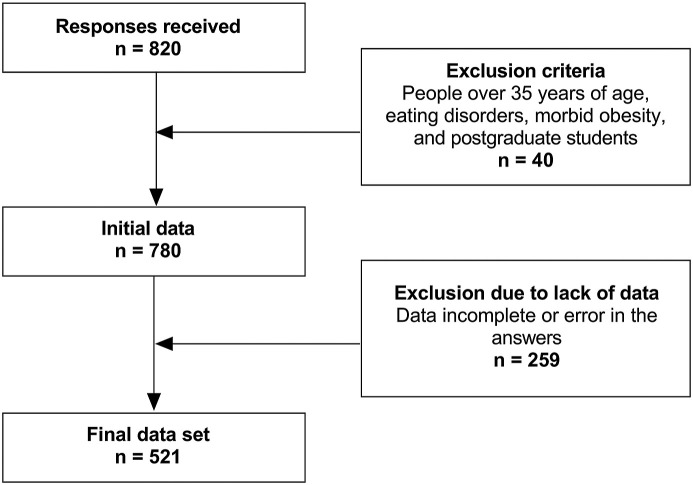
The final sample of the study was of 521 subjects (Fig. 1 ) (female = 63.72%, male = 36.28%, age = 20.93 ± 3.20 years, range = 16–35 years). Participants declared their willingness to participate in the study through informed consent. The study was conducted following the standards of the Declaration of Helsinki and was approved by the institutional review board of San Ignacio de Loyola University.
Fig. 1.
Sample collection chart.
2.2. Questionnaire
The questionnaire (supplemental material 1), which the research group developed, reflected the status of the participants before and after the 12-weeks of confinement. It consisted of 52 questions (4 questions about light exposure time, 6 questions about sleep patterns, 2 questions about sedentary lifestyle, and 6 about food timing, the remaining questions were not taken into account for this manuscript).
Two investigators reviewed the initial data to identify illogical values in the responses. For example: a. durations longer than 24 h per day (such as a sleep duration of 12 h and a screen exposure time of 16 h), b. incongruences in the timing of activities (such as reporting falling asleep at 21 h and having dinner at 23 h, or waking up at 10 h and having breakfast at 8 h). Illogical or incomplete responses on any of the variables ruled out participation.
2.2.1. Body weight variables
To estimate the body weight change during confinement, 3 questions were asked: The first question was about the self-reported body weight; the second was related to whether they had changed their body weight during confinement (with two options, yes or not). If the answer was yes, the last question was to what extent they changed their body weight (with the option of positive and negative values). Finally, only completed answers were considered. If the person was not sure about the variation of its weight and left that answer blank, the participation was suspended. The body mass index was calculated by dividing body weight by squared height and classified according to the World Health Organization (WHO) [17].
2.2.2. Chronotype and sleep habits
Individuals' chronotypes were assessed by the Munich Chronotype Questionnaire [18]; the midpoint of sleep was calculated as the midpoint between time falling asleep and rise time. Chronotypes were classified into morning, intermediate and evening types by dividing the sample of 521 students according to their midpoint of sleep, into tertiles, with the first tertile defining the morning type (midpoint of sleep < 3:45 h), the second tertile as intermediate (3:45 h - 4:52 h) and the third tertile as evening type (>4:52 h).
2.2.3. Screen exposure time
Screen exposure time (duration) was obtained through open questions such as: “During the first week of confinement, on average, how many hours per day did you spend in front of a screen?”
2.2.4. Sedentarism
To determine the sedentary time, the following question was formulated “During the first week of confinement, on average, how many hours did you sit per day?”
2.2.5. Food timing and midpoint of intake
The timing of the three main meals of the day, such as breakfast, lunch and dinner, were obtained through questions such as “Currently, on average, at what time do you have lunch?” or “During the first week of confinement, on average, at what time did you eat dinner?” The midpoint of intake was estimated by subtracting the last intake and the first intake, the result was divided by 2, and the first intake was added to that result, as previously reported by Lopez-Miguez et al. [19].
2.2.6. Fasting at night determination
Night fasting duration was calculated by subtracting to the timing of breakfast, the timing of the day before dinner.
2.3. Statistical analysis
The normality of the distribution of the variables was assessed using the Shapiro–Wilk Test. For data without a normal distribution, the median was compared using the Mann–Whitney U test. Median comparisons were used to determine the change in body mass index, sedentarism, sleep duration, external synchronizers and meal timing between the thirteenth week and the first week of confinement. In contrast, a comparison of proportions was used to evaluate the change in the body mass index classification and the chronotype classification. The One-Way ANOVA Test was used to evaluate differences between groups, and Tukey's Post Hoc Test to compare groups.
The statistical package used was STATA v16. The mediation analysis was performed with the IBM SPSS Amos program. A significance of 95% was used in all statistical analyses.
3. Results
We have evaluated the impact of 12-week confinement on body mass index (BMI) and several circadian synchronizers. Daily habits, sleep habits and feeding times in college students are presented in Table 1 .
Table 1.
General characteristics of college student at first and thirteenth week of confinement.
| N | First week of confinement |
Thirteenth week of confinement |
p value |
|---|---|---|---|
| 521 | 521 | ||
| Age, years | 21 (19–23) | ||
| Women, % (n) | 63.72 (332) | ||
| BMI, kg/m2 | 23.61 (23.33–23.90) | 24.17 (23.85–24.49) | <0.001 |
| Daily habits | |||
| Sunlight exposure duration, h | 2:06 (1:55–2:17) | 1:27 (1:13–1:36) | <0.001 |
| Sedentarism duration, h | 7:06 (6:49–7:24) | 9:34 (9:16–9:52) | <0.001 |
| Screen exposure time, h | 7:15 (6:59–7:32) | 9:49 (9:31–10:06) | <0.001 |
| Sleep habits | |||
| Ready for sleeping, h | 23:42 (23:34–23:50) | 00:34 (0:25–0:42) | <0.001 |
| Duration of sleep latency, h | 0:44 (0:40–0:48) | 0:43 (0:40–0:47) | 0.387 |
| Sleep time, h | 0:25 (0:16–0:35) | 1:17 (1:08–1:27) | <0.001 |
| Time to wake up, h | 8:34 (8:25–8:42) | 8:19 (8:11–8:28) | 0.029 |
| Sleep duration, h | 8:06 (7:58–8:14) | 7:01 (6:53–7:09) | <0.001 |
| MPS, h | 4:30 (4:22–4:38) | 4:49 (4:42–4:57) | <0.001 |
| Feeding time | |||
| Breakfast, h | 9:16 (9:09–9:24) | 9:29 (9:21–9:36) | 0.006 |
| Lunch, h | 13:55 (13:49–14:01) | 14:13 (14:07–14:19) | <0.001 |
| Dinner, h | 20:07 (20:00–20:13) | 20:28 (20:21–20:34) | <0.001 |
| MPI, h | 14:41 (14:35–14:46) | 14:58 (14:52–15:04) | <0.001 |
| Night fasting duration, h | 13:10 (13:03–13:18) | 13:00 (12:52–13:08) | 0.073 |
BMI: Body mass index; MPS: Midpoint of sleep; MPI: Midpoint of intake.
Values were mean (CI 95%), p value represent the delta of both periods derived from U-The Man Whitney test.
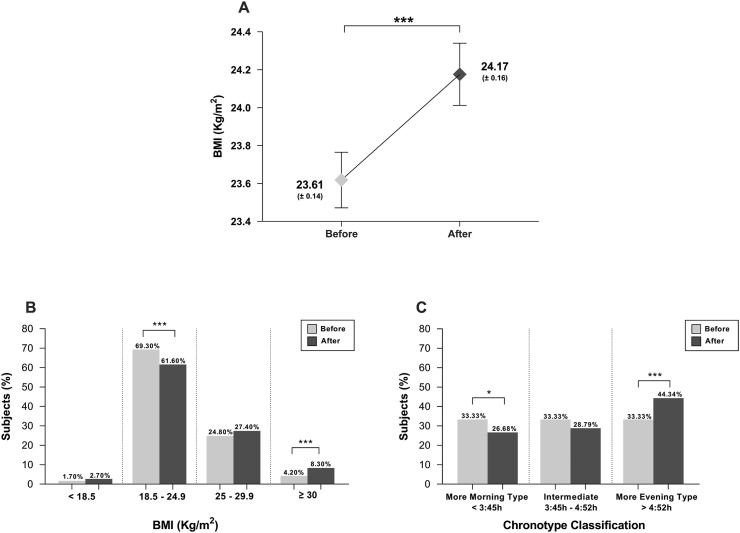
Overall, we observed statistically significant increases in BMI and a shift towards an evening chronotype (Fig. 2 ). The percentage of subjects below 18.5 (kg/m2) and between 25 and 29.9 (kg/m2) increased significantly (p < 0.001) (Fig. 2B) while the proportion of morning chronotypes decreased (p = 0.02), resulting in a significantly increased number of subjects in the evening chronotype (p < 0.001) (Fig. 2C).
Fig. 2.
Variation of main outcomes. A) Variation of BMI, B) Variation of BMI classification and C) Variation of chronotype classification. A shows the mean, and the dispersion is expressed as the Standard Error of the Mean and the U-The Man Whitney test was used. For B and C comparison of proportions was used. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05. BMI: Body mass index.
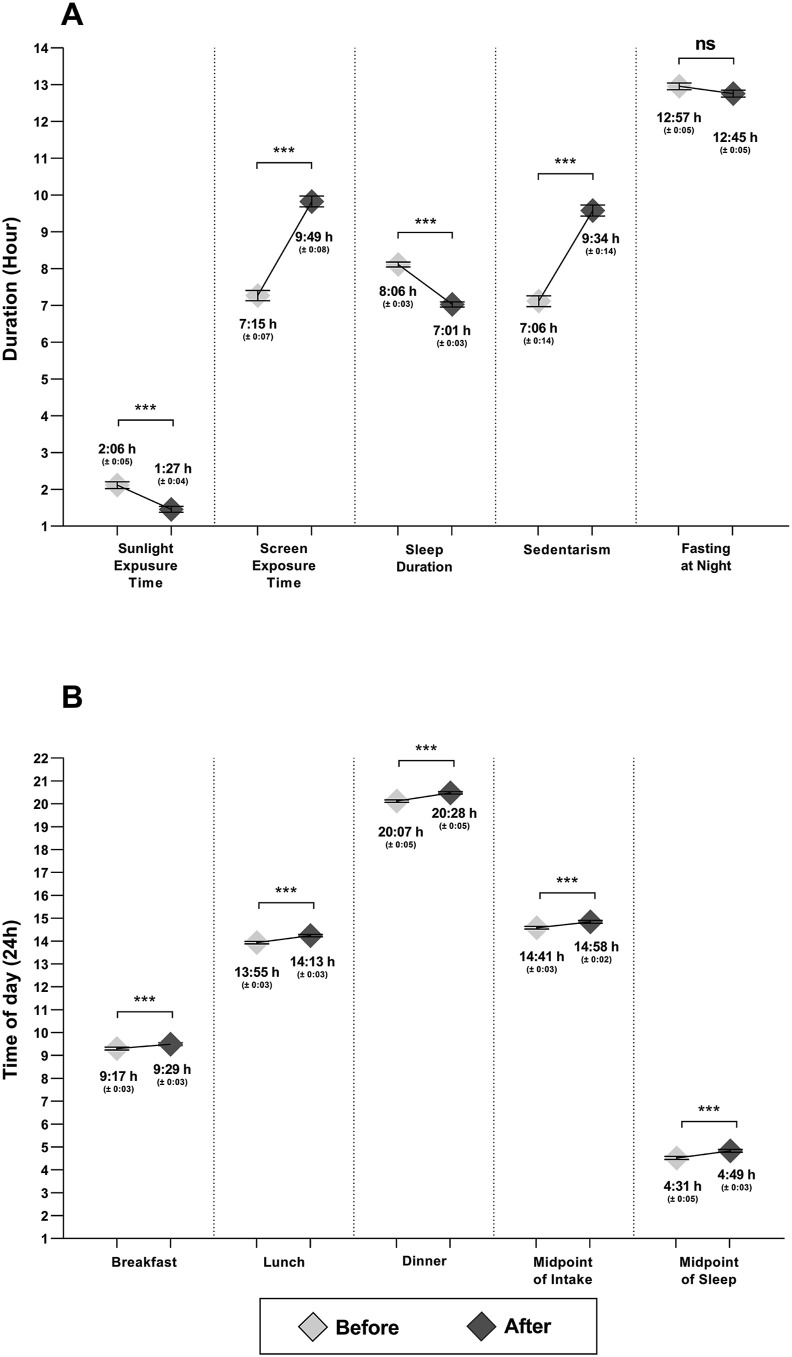
These changes in BMI and chronotype paralleled changes in factors known to synchronize the biological clock (i.e., external synchronizers) (Fig. 3 ). We found significant decreases in the duration of sunlight exposure (Δ −0:39h [−0:53h, −0:24h]) and of sleep (Δ −1:04h [−1:16h, −0:53h]) and significant increases in the duration of screen exposure (Δ 2:33h [2:09h, 2:57h]), and sedentarism (Δ 2:27h [IC 2:03h, 2:52h]) and a trend was found for decreases in night fasting duration (Δ −0:11h [−0:27h, −0:03h]) (p = 0.07) (Fig. 3A). Likewise, we found significant delays in food timing (a synchronizer of peripheral clocks) such as breakfast timing (Δ 0:12h [0:01h, 0:22h]), lunch timing (Δ 0:19h [0:11h, 0:27h]) and dinner timing (Δ 0:22h [0:12h, 0:31h]), and consequently in the midpoint of intake (Δ 0:16h [0:09h, 0:25h]). We also found a delay in the midpoint of sleep (Δ 0:18h [0:07h, 0:29h]) (p < 0.05) (Fig. 3B). All these external synchronizers changed significantly with confinement (p < 0.001), except for night fasting duration that only showed a trend (p = 0.07).
Fig. 3.
Changes in external synchronizers with confinement. Y axis represents duration in Figure A and timing in Figure B. Mean (SEM). The U-The Man Whitney test was used. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05, ns: not significant.
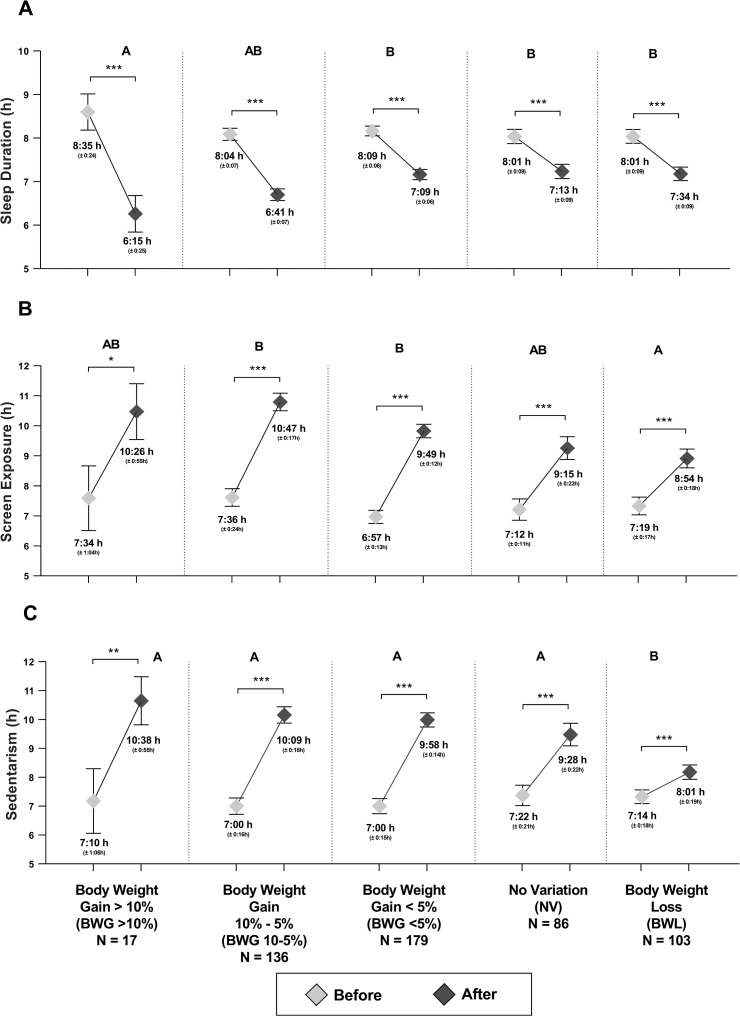
Changes in body weight were classified into five categories (Fig. 4 ), a) Body weight gain: greater than 10% (BWG >10%), between 10 and 5% (BWG 10-5%), less than 5% (BWG <5%); b) no variation (NV) and c) body weight loss (BWL). The percentage of people who gained body weight was large (64% of the total population, n = 332 from the total n = 521). From this population, a 3.3% gained more than the 10% of their initial body weight and a 26%, gained more than the 5% of their initial body weight.
Fig. 4.
External synchronizers and bodyweight variation during confinement. A) Sleep duration, B) Screen exposure time and C) Sedentarism. The capital letter represents the difference in the groups. Mean (SEM). The U-The Man Whitney test was used. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
Those subjects who gained more body weight (BWG >10% group) decreased their sleep duration to a greater extent (Δ −2:19h [−3:32h, −1:07h]) than those who gained less body weight or who even lost body weight during confinement (Fig. 4A). Furthermore, BWG 10-5% group showed a greater increase in screen exposure time (Δ 3:10h [2:21h, 4:00h]) compared to BWL (Δ 1:34h [0:43h, 2:25h]) (Fig. 4B). Also, the BWL group had a lower increase in sedentary hours (Δ 0:46h [−0:04h, 1:39h]) compared to the other groups (Fig. 4C).
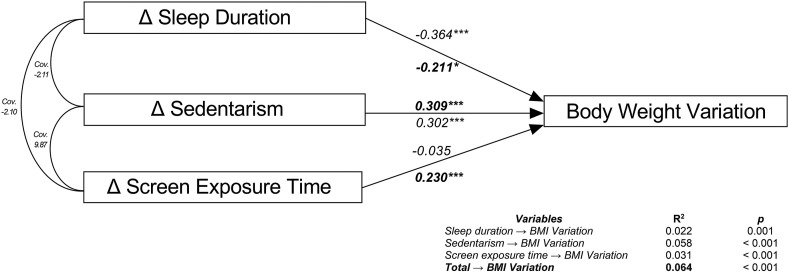
Mediation models for the external synchronizers' changes with confinement (Fig. 5 ) showed a direct effect of sedentarism, sleep duration, and screen exposure duration on body weight variation (BWV). The model was able to predict the variation in body weight in 6% (R2 = 0.064), showing a positive correlation between sedentarism and BWV (β = 0.302) and a negative correlation between sleep duration and BWV (β = −0.211). For every 1 h of increase in sedentarism, there was a 0.3% increase in body weight, and for every 1 h of increase in sleep duration, there was a 0.2% decrease in body weight (Fig. 5).
Fig. 5.
Mediation models of external synchronizerson body weight variation. Body weight variation (BWV) as the dependent variable, Δ sleep duration, Δ sedentarism, Δ screen exposure time as independent variables. Δ was calculated by subtracting the variables from the thirteenth week to the first week of confinement. The straight arrows represent the correlation between variables of interest; the curved arrows denote the covariance among the three represented external synchronizers. The beta coefficients are shown in italic letter while beta coefficients of the adjusted model are shown in bold letter. Cov. = Covariance. BMI = Body Mass Index. Δ = Delta. ∗∗∗p < 0.001, ∗p < 0.05.
Regarding meal timing, the model explained 0.6% (R2 = 0.006) of the variation in body weight, which suggests that the influence of meal timing on the BWV was low (data not shown). The coefficients of determination showed that sleep duration was the most influential variable on BWV when the model was not adjusted by the other external synchronizers (R2 = 0.022, p = 0.001); In contrast, after adjusting for external synchronizers, the most influential variable on BWV was sedentarism (R2 = 0.058, p < 0.001), followed by screen exposure time (R2 = −0.031, p < 0.001), and finally by sleep duration (R2 = 0.022, p < 0.001). Overall, the adjusted model predicted a 6% of the BWV (R2 = 0.064, p < 0.001).
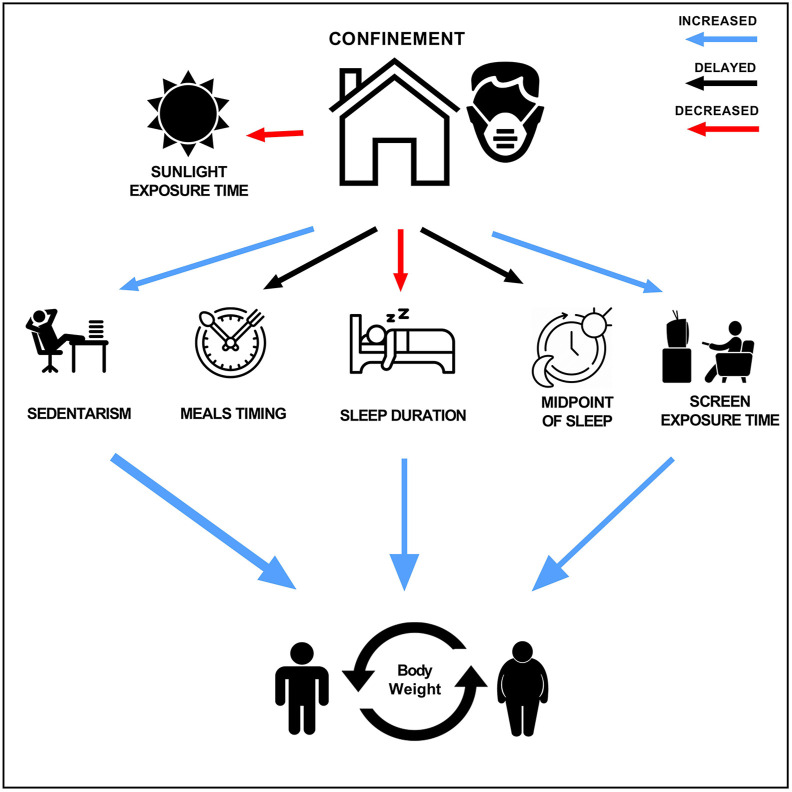
Figure 6 represents a summary of the impact of 12-week confinement on body weight, considering changes in several external synchronizers of the biological clock in these university students.
Fig. 6.
Changes after twelve weeks of confinement on biological clock synchronizers. Sunlight exposure time and sleep duration decreased. Sedentarism, screen exposure time and body weight increased while meals timing and midpoint of sleep were delayed. All changes were statistically significant p < 0.05.
4. Discussion
In this study, including 521 young university students, we found a significant increase in BMI and body weight together with variations in the duration and timing of various external synchronizers of the biological clock after 12-weeks of confinement. This confinement was part of the emergency measures implemented by the Peruvian Government against COVID-19 [16]. These changes included a twofold increase in the prevalence of obesity together with decreases in sunlight exposure and sleep duration. Moreover, confinement was associated with an increase in the screen exposure time and in sedentarism and a delay in the timing of the main meals and in the midpoint of sleep. Mediation analyses suggested that from these various external synchronizers, the most influential factors in the body weight variation with confinement were sleep (duration), physical activity (sedentarism), and light (timing of screen exposure).
Our data showed an overall increase in BMI both in men and women, with no significant sex interaction. In addition, according to BMI classification, the percentage of obesity doubled and the percentage of normal BMI decreased (~10%). When the population was classified according to the variation in body weight with confinement, our data showed that changes in body weight were mediated by three factors: sleep duration (R2 = 0.02), sedentarism (R2 = 0.06), and screen exposure (R2 = 0.03). The regression model explained a 6% of the total body weight variation; while this number does not seem to be relevant, it is higher than other classical known factors influencing obesity such as genetics, which has been shown to account for a ~2.7% of the variation in BMI [20].
Our results are in agreement with the body weight gain shown in other studies examining shorter lockdowns [21]. Furthermore, in the current study, subjects who gained more body weight (>10%) had a more significant increase in sedentarism (Δ 3:28h) vs. those who lost body weight (Δ 0:46h) during the 12 weeks of confinement. This increase would be related to a more positive energy balance due to a more sedentary lifestyle. Nevertheless, other circadian system alterations could also be involved, such as the more prolonged exposure to screens, the shorter exposure to sunlight, and the delay in the timing of sleep and food intake, which may be influencing the changes in chronotype towards a more evening-type. Unlike genetics, these factors are modifiable and should be considered a fundamental component in the recommendations to prevent the adverse effects associated with obesity.
The recent confinement has affected sunlight exposure, known as the strongest synchronizer of the circadian system [22]. In our study, the time of exposure to sunlight decreased by more than half an hour with confinement (Δ −0:39h). Conversely, we observed an increase of more than two hours in screen exposure (Δ 2:33h) in agreement with other studies examining the effects of confinement [23]. These changes may be related to variations in the study and work habits due to confinement, which demands greater use of electronics and increased exposure to digital media [24]. Decreased sunlight, together with continuous exposure to screens and delayed meals and sleep timings, affects the circadian system and may alter the synchronization between the central and peripheral clocks and between the biological clocks and the environment, causing chronodisruption and subsequently obesity [25].
It has been described that, in addition to light, which synchronizes our central clock, there are other external synchronizers directed towards specific peripheral clocks, such as those in adipose tissue [26]. Among these peripheral synchronizers, examples are related to changes in activity and resting and in eating and fasting. In addition, sleep can be understood as a result of- or a synchronizer of-the circadian system [27]. Circadian system alterations have been related to changes in adipose tissue metabolism [28], obesity [7,8] and cardiovascular risk [[29], [30], [31]]. Therefore, and considering that confinement has affected several known external synchronizers of the biological clock, we would expect a significant change in body weight as the one observed in our study.
There is concern about sleep habit disturbances that may have arisen during the pandemic [[32], [33], [34]]. We found an overall reduction in sleep duration of approximately one hour (Δ −1:04) in both genders, in agreement with other studies [13]. Our results show that those who shortened their sleep with confinement to a higher degree, more than two hours, (Δ −2:19h) were those who gained more body weight (11.9%). Furthermore, the significant delays observed for sleep timing in combination with the delay in the timing of the main meals: breakfast (Δ 0:12h), lunch (Δ 0:19h), and dinner (Δ 0:22h), explained the increase in the proportion of evening-types: 11% of the students became evening-types during the 12 weeks of confinement. Our results are consistent with other studies that show that confinement represents a parenthesis in sleep habits according to the natural changes in society [35]. In this context, it is now a challenge to maintain adequate metabolic health [36] with current and upcoming measures regarding confinement and social isolation.
Furthermore, confinement within homes, mobility restrictions, and social isolation rules have been traduced to less accessibility to fitness centers and public spaces for physical activity. In accordance, our results show an increase in sedentarism, similar to previous reports [21,23,37], although one study showed a slight increase in physical activity, especially for body weight training [38]. These results show a lower energy expenditure due to physical activity, one of the factors of body weight gain, with a probable loss of muscle mass and increased fat accumulation. Furthermore, sedentarism may lead to a flattening of the daily activity rhythms, resulting in a weakening of the signals to the internal clock, and therefore, chronodisruption.
Although the etiology of obesity recognizes dietary habits as the main factor with a positive energy balance, there are other intervening factors such as physical activity, genetics, microbiota, and sleep habits [[5], [6], [7], [8]]. Therefore, the obesity pandemic that has been going on for decades has found itself in the worst scenario with this new pandemic.
Considering that, in the short term, there is still uncertainty about the duration of the state of emergency, we contemplate that it is important to monitor changes in behavior and a good alternative is the use of smartphones and other wearable devices [39].
The current study has strengths and limitations. This is the first study that considers global changes in several external synchronizers of the circadian clock during confinement by using a comprehensive questionnaire consisted of 52 questions that addressed several aspects related to the circadian system such as exposure to sunlight, exposure to screens, sleeping habits, sedentary lifestyle, and timing of food intake, together with body weight changes. A relevant aspect is that due to the low variation of daylight length in Lima, sun hours were practically the same at the beginning and at the end of confinement during the 12 weeks of the study. Therefore, changes in sun exposure during the study were not affected by the shortening of days [40]. There are limitations associated with our study; first, the data are based on self-completed questionnaires. Moreover, we have no information about the students' dietary habits, apart from food intake timing. The results and conclusions apply to the specific group under investigation (young students). Nevertheless, data support a significant impact of confinement on several external synchronizers and body weight change in this population.
5. Conclusion
This study analyses how acute changes in our biological clock's external synchronizers may affect body weight in an unusual real-life situation. During confinement, there was an increase in BMI and in the proportion of obese students. Sleep patterns and daily habits were altered, finding a decrease in sleep duration and sunlight exposure time and an increase in the duration of exposure to screens. In addition, there was a delay in feeding schedules and in the midpoint of sleep. Finally, sedentarism seemed to be more relevant in body weight variation than sleep duration and screen exposure time.
As long as the vaccine for SARS-CoV-2 is not widely available, there is great concern about the possible impact of confinements. Healthier lifestyle changes appear to be more urgent now than ever. This study has shown the association between changes in the external circadian rhythm synchronizers and body weight gain.
Funding statement
This work has been supported in part by The Spanish Government of Investigation, Development and Innovation (SAF2017-84135-R) including FEDER co-funding; The Autonomous Community of the Region of Murcia through the Seneca Foundation (20795/PI/18) and NIDDK R01DK105072 granted to M. Garaulet. In addition, P. González-Muniesa has received support from the CIBER Physiopathology of Obesity and Nutrition (CIBERobn), Carlos III Health Research Institute (CB12/03/30002).
Author contributions
Baquerizo-Sedano L, designed the study, analyzed and interpreted the data and wrote the first draft of the manuscript. Chaquila JA, analyzed and interpreted the data and wrote the first draft of the manuscript. Aguilar L, designed the study and contributed to data collection. Ordovás JM, contributed to data interpretation and review of the manuscript. González-Muniesa P, analyzed and interpreted the data and reviewed the manuscript. Garaulet M, analyzed and interpreted the data and reviewed the manuscript. All authors edited the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We are grateful to all the students of the San Ignacio de Loyola University who responded to the survey. We acknowledge the contribution of our colleagues from the San Ignacio de Loyola University: Hans Donayre, Melanie Agustin and Ariana Espino (Faculty of Health Sciences) in carrying out this study. And a special thanks to the director of the Nutrition and Dietetics career, Dayana Barriga, for her unconditional support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2021.06.019.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.COVID-19 Map - Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html n.d.
- 2.Jia P., Liu L., Xie X., Yuan C., Chen H., Guo B., et al. Impacts of COVID-19 lockdown on diet patterns among youths in China: the COVID-19 Impact on Lifestyle Change Survey (COINLICS) Appetite. 2020;158:105015. doi: 10.1038/s41366-020-00710-4. [DOI] [PubMed] [Google Scholar]
- 3.Galandra C., Cerami C., Santi G.C., Dodich A., Cappa S.F., Vecchi T., et al. Job loss and health threatening events modulate risk - taking behaviours in the Covid - 19 emergency. Sci Rep. 2020:1–10. doi: 10.1038/s41598-020-78992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Violant-Holz V., Gallego-Jiménez M.G., González-González C.S., Muñoz-Violant S., Rodríguez M.J., Sansano-Nadal O., et al. Psychological health and physical activity levels during the covid-19 pandemic: a systematic review. Int J Environ Res Publ Health. 2020;17:1–19. doi: 10.3390/ijerph17249419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González-Muniesa P., Mártinez-González M.-A., Hu F.B., Després J.-P., Matsuzawa Y., Loos R.J.F., et al. Obesity. Nat Rev Dis Prim. 2017;3:17034. doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 6.San-Cristobal R., Navas-Carretero S., Martínez-González M.Á., Ordovas J.M., Martínez J.A. Contribution of macronutrients to obesity: implications for precision nutrition. Nat Rev Endocrinol. 2020;16:305–320. doi: 10.1038/s41574-020-0346-8. [DOI] [PubMed] [Google Scholar]
- 7.Garaulet M., Ordovás J.M., Madrid J.A. The chronobiology, etiology and pathophysiology of obesity. Int J Obes (Lond) 2010;34:1667–1683. doi: 10.1038/ijo.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garaulet M., Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. doi: 10.1016/j.physbeh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y., Lu Y., Huang Y.M., Wang M., Ling W., Sui Y., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuschieri S., Grech S. Obesity population at risk of COVID-19 complications. Glob Heal Epidemiol Genom. 2020:4–9. doi: 10.1017/gheg.2020.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris C.J., Aeschbach D., Scheer F.A.J.L. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349:91–104. doi: 10.1016/j.mce.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Minguez J., Gómez-Abellán P., Garaulet M. Circadian rhythms, food timing and obesity. Proc Nutr Soc. 2016;75:501–511. doi: 10.1017/S0029665116000628. [DOI] [PubMed] [Google Scholar]
- 13.Pinto J., van Zeller M., Amorim P., Pimentel A., Dantas P., Eusébio E., et al. Sleep quality in times of Covid-19 pandemic. Sleep Med. 2020;74:81–85. doi: 10.1016/j.sleep.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Górnicka M., Drywień M.E., Zielinska M.A., Hamułka J. Dietary and lifestyle changes during COVID-19 and the subsequent lockdowns among polish adults: a cross-sectional online survey PLifeCOVID-19 study. Nutrients. 2020;12 doi: 10.3390/nu12082324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garaulet M., Gómez-Abellán P. Chronobiology and obesity. Nutr Hosp. 2013;28(Suppl 5):114–120. doi: 10.3305/nh.2013.28.sup5.6926. [DOI] [PubMed] [Google Scholar]
- 16.Gobierno del Perú Normativa sobre estado de emergencia por coronavirus. Compendio. 2021 https://www.gob.pe/institucion/pcm/colecciones/787-normativa-sobre-estado-de-emergencia-por-coronavirus [Google Scholar]
- 17.Weir C.B., Jan A. 2020. BMI classification percentile and cut off points. Treasure Island (FL) [PubMed] [Google Scholar]
- 18.Roenneberg T., Wirz-Justice A., Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythm. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Minguez J., Dashti H.S., Madrid-Valero J.J., Madrid J.A., Saxena R., Scheer F.A.J.L., et al. Heritability of the timing of food intake. Clin Nutr. 2019;38:767–773. doi: 10.1016/j.clnu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locke A.E., Kahali B., Berndt S.I., Justice A.E., Pers T.H., Day F.R., et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrea L., Pugliese G., Framondi L., Di Matteo R., Laudisio D., Savastano S., et al. Does Sars-Cov-2 threaten our dreams? Effect of quarantine on sleep quality and body mass index. J Transl Med. 2020;18:1–11. doi: 10.1186/s12967-020-02465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legates T.A., Fernandez D.C., Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15:443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Górnicka M., Drywień M.E., Zielinska M.A., Hamułka J. Dietary and lifestyle changes during COVID-19 and the subsequent lockdowns among polish Adults : PLifeCOVID-19 study. Nutrients. 2020;12:2324. doi: 10.3390/nu12082324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cellini N., Canale N., Mioni G., Costa S. 2020. Changes in sleep pattern, sense of time, and digital media use during COVID-19 lockdown in Italy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gómez-Abellán P., Madrid J.A., Ordovás J.M., Garaulet M. [Chronobiological aspects of obesity and metabolic syndrome] Endocrinol y Nutr organo la Soc Esp Endocrinol y Nutr. 2012;59:50–61. doi: 10.1016/j.endonu.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Johnston J.D., Ordovás J.M., Scheer F.A., Turek F.W. Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Adv Nutr. 2016;7:399–406. doi: 10.3945/an.115.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis P., Oster H., Korf H.W., Foster R.G., Erren T.C. Food as a circadian time cue — evidence from human studies. Nat Rev Endocrinol. 2020;16:213–223. doi: 10.1038/s41574-020-0318-z. [DOI] [PubMed] [Google Scholar]
- 28.Froy O., Garaulet M. The circadian clock in white and Brown adipose tissue: mechanistic, endocrine, and clinical aspects. Endocr Rev. 2018;39:261–273. doi: 10.1210/er.2017-00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Minguez J., Gómez-Abellán P., Garaulet M. Timing of breakfast, lunch, and dinner. Effects on obesity and metabolic risk. Nutrients. 2019;11:2624. doi: 10.3390/nu11112624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheer F.A.J.L., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potter G.D.M., Skene D.J., Arendt J., Cade J.E., Grant P.J., Hardie L.J. Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev. 2016;37:584–608. doi: 10.1210/er.2016-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casagrande M., Favieri F., Tambelli R., Forte G. The enemy who sealed the world: effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. 2020;75:12–20. doi: 10.1016/j.sleep.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramar K. 2020. The COVID-19 pandemic: reflections for the fi eld of sleep medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou S.-J., Wang L.-L., Yang R., Yang X.-J., Zhang L.-G., Guo Z.-C., et al. Sleep problems among Chinese adolescents and young adults during the coronavirus-2019 pandemic. Sleep Med. 2020;74:39–47. doi: 10.1016/j.sleep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright K.P., Linton S.K., Withrow D., Casiraghi L., Lanza S.M., de la Iglesia H., et al. Sleep in university students prior to and during COVID-19 Stay-at-Home orders. Curr Biol. 2020;30:R797–R798. doi: 10.1016/j.cub.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King A.J., Burke L.M., Halson S.L., Hawley J.A. The challenge of maintaining metabolic health during a global pandemic. Sports Med. 2020;50:1233–1241. doi: 10.1007/s40279-020-01295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C., Yang L., Liu S., Ma S., Wang Y., Cai Z., et al. Survey of insomnia and related social psychological factors among medical staff involved in the 2019 novel coronavirus disease outbreak. Front Psychiatr. 2020;11 doi: 10.3389/fpsyt.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Renzo L., Gualtieri P., Pivari F., Soldati L., Attinà A., Cinelli G., et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18:229. doi: 10.1186/s12967-020-02399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun S., Folarin A.A., Ranjan Y., Rashid Z., Conde P., Stewart C., et al. Using smartphones and wearable devices to monitor behavioral changes during COVID-19. J Med Internet Res. 2020;22 doi: 10.2196/19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calendario Solar Año 2020 Perú 2021. https://www.vercalendario.info/es/sol/peru-ano-calendario-2020.html%3E
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.