Abstract
Alpha-hemolysin (Hly) is a common exotoxin produced by Escherichia coli that enhances virulence in a number of clinical infections. The addition of hemolysin production to laboratory bacterial strains is known to increase the lethality of E. coli peritonitis. However, the mechanisms involved have not been determined and the contribution of hemolysin to the alterations in the host intraperitoneal environment and the leukocyte response is not known. Utilizing a rat peritonitis model, we show that wild-type hemolytic E. coli strains have a significant competitive advantage over nonhemolytic strains within the peritoneum. To examine the specific contribution of Hly to E. coli-induced virulence and alterations within the peritoneum, a mixed peritonitis model of E. coli, Bacteroides fragilis, and sterile fecal adjuvant was used. Three transformed E. coli strains were utilized: one strongly secretes active hemolysin (WAF 270), a second secretes active hemolysin but a reduced amount (WAF 260), and the third does not produce hemolysin (WAF 108). After an equal inoculum of each of the three strains, WAF 270 produced a markedly increased lethality and an increased recovery of both E. coli and B. fragilis from the host relative to the other strains. Changes in the intraperitoneal pH, degree of erythrocyte lysis, and recruitment and viability of leukocytes within the peritoneum following the induction of peritonitis differed significantly between the strongly hemolytic and nonhemolytic strains. Induction of peritonitis with WAF 270 caused a pronounced decrease in intraperitoneal pH, lysis of most of the intraperitoneal erythrocytes, and a marked decrease in recoverable viable leukocytes compared to WAF 108. Thus, hemolysin production by E. coli within the peritoneum may alter not only the host's ability to control the hemolytic strain itself but also other organisms.
Intraperitoneal infections continue to cause significant morbidity and mortality despite significant advances in critical care and antimicrobial therapy (10, 13, 19, 40). Most intraperitoneal infections result from a breach in the integrity of the gastrointestinal system, allowing the introduction of both aerobic and anaerobic bacteria into the peritoneum. Escherichia coli emerges as the predominate pathogen in 60% of intraperitoneal infections (18, 24). The characteristics that allow E. coli to adapt to the peritoneal environment and establish infection are not clearly understood.
Pathogenic E. coli may produce a variety of virulence factors that, for a particular infection site, may promote colonization, enhance growth, cause tissue invasion or destruction, or alter the host inflammatory response. Isolated pathogens frequently express combinations of these virulence factors that presumably provide a competitive advantage over other bacteria (29–33, 35, 37). One known E. coli virulence factor is the potent exotoxin alpha-hemolysin (Hly). Approximately 50% of E. coli isolates causing extraintestinal infections in humans are hemolytic (6, 8, 9, 12, 28). Hly is the prototype of the RTX cytolysins. It is a 107-kDa protein that was originally characterized by its ability to lyse erythrocytes (7, 20, 26). Toxicity to erythrocytes is related to the formation of transmembrane pores in the lipid bilayers of erythrocyte membranes and is calcium dependent (2, 4, 5, 11, 25). Since the characterization of its toxicity to erythrocytes, Hly has been shown to be toxic to a wide variety of mammalian cells over a broad range of concentrations and to have stimulatory effects on inflammatory cells at very low concentrations (3, 14, 17, 22, 23, 36).
We sought to determine the influence of Hly as a virulence factor in intraperitoneal infections. First, we demonstrated a significant competitive advantage of wild-type hemolytic strains over nonhemolytic strains in rat peritonitis. Whether or not this advantage resulted from Hly production or from the expression of other virulence factors in these wild-type strains is not clear. To examine the specific contribution of Hly to enhanced virulence, genetically modified E. coli strains that differ only in the ability to produce this exotoxin were utilized (hemolytic, Hly+; nonhemolytic, Hly−). We examined the influence of Hly on mortality, bacterial recovery, and abscess formation with these strains in a rat peritonitis model. The alterations in the intraperitoneal environment after the induction of peritonitis with either the Hly+ or Hly− strain were examined, and we demonstrate that the enhanced mortality of the Hly+ strain can be blocked by preimmunization with Hly+ supernatant.
MATERIALS AND METHODS
General experimental design.
First, pooled rat cecal contents were used to induce peritonitis in animals, and the ratios of wild-type Hly+ to Hly− E. coli within the inoculum and within the peritoneum during early (6-h) and late (24-h) peritonitis and during the abscess phase (10 days) were determined. Blood samples were also obtained at the early and late peritonitis phases, and the Hly+/Hly− ratios were determined. Next, the influence of Hly in peritonitis was examined by comparing the responses to three genetically modified E. coli strains (WAF 270, WAF 260, and WAF 108), which differ only in the ability to produce Hly. A sterile fecal adjuvant, Bacteroides fragilis, and one of the E. coli strains were combined to produce the inoculum used to induce peritonitis. The influences of Hly on mortality, on bacterial recovery from the blood and peritoneum, and on abscess production were analyzed. Subsequently, the alterations produced by the Hly+ and Hly− isogenic strains in intraperitoneal pH, leukocyte recovery, leukocyte viability, and leukocyte degranulation were determined. The degree of intraperitoneal lysis of erythrocytes (RBC) and the influence of free hemoglobin on in vivo growth kinetics was also determined. Finally, we examined the ability of preimmunization to protect the animals against Hly+ E. coli-induced mortality.
Animals.
All animals used both for collection of cecal contents and for individual experiments were female Sprague-Dawley rats (Hilltop Lab Animals, Inc., Scottsdale, Pa.) weighing 180 to 220 g; they were housed for at least 5 days prior to each experiment. Animals were maintained according to National Research Council standards.
Shift to hemolytic E. coli populations in peritonitis. (i) Preparation of standard fecal inoculum.
Cecal contents from rats were collected, pooled, and immediately refrigerated. Cecal material was combined with an equal volume of 0.9% saline for intraperitoneal injection.
(ii) Induction of peritonitis.
Halothane-anesthetized rats were inoculated by percutaneous intraperitoneal injection of 1 ml of fecal inoculum, and animals were immediately given 10 ml of 0.9% saline subcutaneously. After inoculation, animals were allowed food and water ad libitum and observed for mortality or sacrificed at predetermined time intervals.
(iii) Analysis of Hly+/Hly− ratios.
Animals were sacrificed at 6 and 24 h and at 10 days. Analysis at 6 and 24 hours consisted of quantitative blood and peritoneal cultures. Blood (1 ml) was collected by cardiac puncture with heparinized syringes, diluted with 1 ml of saline, and quantitatively cultured. Results are expressed as log10 CFU per milliliter of blood. Quantitative peritoneal cultures were performed by lavage with 10 ml of saline. Animals analyzed at 10 days underwent laparotomy for collection of intraperitoneal abscesses. Abscesses were weighed, homogenized in 4.5 ml of Hanks' balanced salt solution (HBSS), and quantitatively cultured.
Identification and quantification of E. coli in all samples (stool, blood, peritoneal fluid, abscess) were performed by serial dilution of samples and plate enumeration. All E. coli organisms were confirmed by API testing (API Laboratory Products, Rayleigh, Essex, United Kingdom). Hemolytic activity was determined by aerobic culture on 5% sheep's blood agar, and the ratio of Hly+ to Hly− was determined.
Determination of virulence using isogenic Hly+/Hly− strains. (i) Preparation of sterile fecal adjuvant.
Cecal contents of rats were collected, pooled, and combined with prereduced HBSS (Sigma Chemical Co., St. Louis, Mo.), glycerol, and brain heart infusion broth (BHI) (BBL, Cockeysville, Md.) to a final concentration of 25% (wt/vol). The resulting mixture was strained through four single layers of surgical mesh gauze to remove large particulate matter, sterilized by autoclaving for 40 min, and subsequently frozen until use.
(ii) Bacterial strains, culture conditions, and enumeration.
Three genetically modified E. coli strains, WAF 270, WAF 260, and WAF 108, were kindly provided by R. A. Welch and were stored at −85°C until use. Their characterization, DNA fragment isolation, and the recombinant methodology used in their construction have been described previously (16, 41–43). The genetic characteristics of the host E. coli strain of the hemolysin determinant and of the parent strain of the hemolysin determinant are briefly described.
Each of the three recombinant strains was constructed by transformation of E. coli J198 with one of three recombinant plasmids containing the hemolysin determinant: pSF4000, pSF4000:Tn1, or pAN202-312. E. coli J198 is a nonpathogenic human fecal isolate (O22 ColV− Hly−). WAF 270 and WAF 108 were derived by transformation with plasmids pSF4000 and pSF4000:Tn1, respectively. The complete plasmid containing the Hly determinant and a chloramphenicol resistance gene (pSF4000, extracted from E. coli J96; both plasmids contain a chromosomally derived hemolysin determinant from J96 [O4 K6 ColV+ Hly+], a clinically pathogenic isolate) was electroporated into J198 to form WAF 270. Plasmid pSF4000:Tn1 was derived from pSF4000 insertion of an ampicillin resistance transposon, Tn1, which prevents transcription of the Hly gene. The pSF4000:Tn1 plasmid was electroporated into J198 to form WAF 108, which produces no Hly. WAF 260 contains plasmid pAN202-312, which contains the hemolysin determinant from a hemolytic strain isolated from mouse feces. Plasmid pSF4000 produces roughly 50 times the amount of hemolysin as does pAN202-312.
E. coli strains were grown in BHI with appropriate antibiotic selection (20 μg of chloramphenicol per ml for WAF 270 and WAF 260, 20 μg of chloramphenicol per ml and 100 μg of ampicillin per ml for WAF 108) overnight at 37°C. Bacteria were washed and quantified by plate enumeration. B. fragilis (ATCC 23745) was grown anaerobically in a mixture containing 37 g of BHI, 5 g of yeast extract, 1 mg of vitamin K1, 5 mg of hemin, 1 mg of resazurin, and 0.5 g of cysteine (Carr-Scarborough Microbiologicals, Decatur, Ga.) for 24 h, collected by centrifugation, washed twice in HBSS, and quantified by plate enumeration.
(iii) Induction of peritonitis.
Mixed intraperitoneal infections were induced by intraperitoneal injection of 1 ml containing sterile fecal adjuvant, 106 CFU of E. coli (either WAF 108, WAF 260, or WAF 270), and 108 CFU of B. fragilis. Animals were resuscitated with 10 ml of 0.9% saline by subcutaneous injection and observed for mortality or sacrificed at predetermined time intervals.
(iv) Bacterial and abscess recovery from animals at 6, 15, and 24 h and at 10 days.
Rats were sacrificed after induction of peritonitis at 24 h or 10 days. Blood (1 ml) was collected by cardiac puncture with heparinized syringes and quantitatively cultured by plate enumeration on sheep blood agar (aerobically) and brucella agar (anaerobically). Quantitative peritoneal cultures were performed by lavage with 10 ml of saline. Animals analyzed at 10 days underwent laparotomy for collection of intraperitoneal abscesses. Abscesses were weighed, homogenized in 4.5 ml of HBSS, and quantitatively cultured.
Additional animals were inoculated and analyzed at 6, 15, and 24 h. The blood, peritoneum, liver, spleen, and lungs were quantitatively cultured. Results are expressed as log10 CFU per gram of tissue or per milliliter.
(v) Hemolysin-induced alterations of intraperitoneal pH, erythrocyte integrity, and leukocyte recruitment, viability, and degranulation.
Alterations in the intraperitoneal environment were assessed after the induction of peritonitis. After induction, animals were analyzed at 2, 6, and 9 h by intraperitoneal placement of a pH probe via a small laparotomy incision and, after measurement of pH, underwent peritoneal lavage with 10 ml of normal saline. The laparotomy incisions were subsequently temporarily closed with clips, and the abdominal contents were agitated for 30 seconds. Peritoneal lavage fluid was then collected; 1 ml was used for quantitative culture by plate enumeration on sheep blood agar, and the remaining fluid was divided into aliquots.
Quantitation of the degree of intraperitoneal RBC lysis following induction of peritonitis was determined by hemolysis assay. Intact cells were removed from samples of lavage fluid by low-speed centrifugation, and the absorbance of the supernatant was determined at 543 nm (Asupernatant). Alternatively, a commercial lytic reagent (Zap-oglobin II; Coulter Diagnostics, Hialeah, Fla.) was added to lavage fluid and complete lysis of RBC was confirmed by microscopic examination (hemocytometer; Hausser Scientific). Absorbance of the lysed sample was then determined at 543 nm (Alysed). The percent intraperitoneal lysis of RBC was determined as Asupernatant/Alysed × 100.
The recruitment of intraperitoneal leukocytes, leukocyte viability, and leukocyte degranulation in response to peritonitis induced with either of the two E. coli strains was determined. RBC within the lavage fluid samples were lysed, and the numbers of leukocytes were determined by a cell counter (Coulter Electronics, Inc., Hialeah, Fla.) and by hemocytometer.
Separate samples of peritoneal lavage fluid were used to assay leukocyte viability. After low-speed centrifugation, the remaining cell suspension was stained for cell viability by trypan blue exclusion (Sigma). The number of nonviable cells (stained) per 100 leukocytes was determined.
Neutrophil primary and secondary granule release (either by neutrophil degranulation or by neutrophil lysis) within the peritoneal cavity was assayed by using the Micrococcus lysodeikticus assay. Briefly, 0.1 ml of cell-free peritoneal lavage fluid was added to 0.25 mg of M. lysodeikticus (M3770; Sigma) per ml in 0.1 M potassium phosphate buffer, and the loss of absorbance was determined over 2 min by spectrophotometer at 450 nm and 25°C. Lysozyme standard (L6876; Sigma) was used to calculate the linear regression for rates of loss of optical density of the M. lysodeikticus suspension, and unknown values were determined with the resulting equation.
(vi) Preimmunization of rats with partially purified hemolysin supernatant.
To investigate whether preexposure of rats to hemolysin could abrogate mortality resulting from hemolytic E. coli-induced peritonitis, rats were preimmunized with hemolysin-rich WAF 270 supernatant, hemolysin-deficient supernatant of WAF 108, or neither prior to induction of peritonitis with WAF 270. Supernatants for preimmunization of animals were prepared by 6 h of growth of WAF 108 and WAF 270 in RPM1 1640 supplemented with 20 μg of chloramphenicol per ml (± ampicillin [100 mg/ml]) and 1% (wt/vol) bovine albumin (Sigma) at 37°C with agitation. Bacteria were separated from supernatants by centrifugation at 3,500 × g for 10 min at 4°C, and supernatants were subsequently filter sterilized with 0.2-μm-pore-size filters coated with 5% Tween 80 (J. T. Baker Chemical Co., Phillipsburg, N.J.). Sterility was confirmed by culture of the supernatants. Assays of the hemolytic activity of the resulting supernatants were then performed as described below.
Groups of 15 rats were immunized by four repeated intraperitoneal injections of supernatant from either WAF 108 or WAF 270. On day 1, animals were given 0.5 ml of the designated supernatant followed by 1, 3, and 3 ml, each at 4- to 5-day intervals. Immediately after each immunization, animals were resuscitated with 10 ml of 0.9% saline subcutaneously. Four days after the final inoculation, peritonitis was induced by intraperitoneal injection of 1.8 × 109 CFU of live WAF 270 with standard sterile stool adjuvant. For positive controls, peritonitis was induced in nine nonimmunized rats with 4.5 × 108 CFU of live WAF 270 with sterile stool adjuvant. All animals were resuscitated with 10 ml of 0.9% saline subcutaneously and observed for 10 days.
(vii) Hemolytic activity of culture supernatants.
Quantitation of the hemolytic activity of the supernatants from WAF 108 and WAF 270 was performed by a method adapted from Mackman and Holland (26). Briefly, 100 μl of sheep RBC was centrifuged in microcentrifuge tubes at 3,500 × g for 1 min. The pellet was resuspended in 700 μl of 0.9% saline and 25 mM CaCl2. Serial dilutions (400 μl) of the hemolysin-rich or hemolysin-deficient supernatants were combined with the RBC suspension and incubated at 37°C for 30 min. Unlysed cells were removed by centrifugation at 3,500 × g for 2 min, and 100 μl of the cell-free fluid was diluted with 900 μl of 0.9% saline. Absorbances were determined at 543 nm. Titers were arbitrarily defined as the inverse of the greatest dilution that resulted in 100% hemolysis of the RBC. The standard for 100% lysis was obtained by the addition of 400 μl of H2O in place of the diluted supernatant in the above assay. The standard for 0% lysis of RBC was obtained by substitution of 400 μl of 0.9% saline for the supernatant, and the resulting value was used to zero the scale of absorbance. Supernatant from WAF 108 resulted in no lysis of cells, while that from WAF 270 produced titers of 32 (100% lysis of RBC in an assay at a 1:32 dilution).
Statistics.
Dichotomous variables were analyzed with a chi-square test, and means (expressed ± standard errors of the means [SEM]) were compared with an unpaired Student's t test.
RESULTS
Hly+ E. coli strains demonstrate a competitive advantage in peritonitis.
Normal rat stool contains a large number and array of bacteria; however, following the induction of peritonitis, E. coli emerges as the predominant enteric pathogen. The percentage of Hly+ E. coli strains present in rat stool is variable but is consistently the minority of the total E. coli population (data not shown). To determine if wild-type Hly+ strains have a competitive advantage over Hly− strains, the ratio of Hly+/Hly− strains was determined in our standard fecal inoculum. Subsequently, this ratio was determined in the blood and peritoneal fluid at 6 and 24 h after the induction of peritonitis and in abscesses at 10 days after induction.
The standard fecal inoculum prepared from rats given a grain-based diet contains roughly 9.01 log10 CFU of total bacteria, of which 6.94 log10 CFU are Enterobacteriaceae and 6.06 ± 0.07 log10 CFU are E. coli. The percentages of E. coli strains that were Hly+ within the inoculum and within the rat peritoneum blood stream at each time point are shown in Table 1. Only 7% of the E. coli strains in the fecal inoculum were Hly+, but this percentage increased during the early (24% at 6 h) and late (71% at 24 h) peritonitis stages. This shift was most pronounced in the blood samples obtained at 24 h from induction of peritonitis, when 98% of the E. coli strains isolated were Hly+.
TABLE 1.
Shift toward hemolytic E. coli strains after intraperitoneal injection of standard stool inoculum
| Time | % Hemolytic E. coli ± SEM in:
|
Rat data
|
|||||
|---|---|---|---|---|---|---|---|
| Inoculum | Peritoneum | Blood | Abscess | No. inoculated | No. analyzed | % Mortality | |
| 0 h | 7 ± 1a | ||||||
| 6 h | 24 ± 7 | 54 ± 7 | 20 | 20 | 0 | ||
| 24 h | 71 ± 13 | 98 ± 3 | 13 | 7 | 46 | ||
| 10 days | 50 ± 11 | 15 | 11 | 27 | |||
P < 0.05 versus all other time points.
Influence of hemolysin on mortality from peritonitis in rats.
To examine the specific influence of Hly in peritonitis, we used the isogenic strains WAF 108, WAF 260, and WAF 270 in our fecal peritonitis model. Animals were injected with either 6.44 ± 0.05 log10 CFU of WAF 270 (strongly hemolytic), 6.44 ± 0.24 log10 CFU of WAF 260 (weakly hemolytic), or 6.55 ± 0.04 log10 CFU of WAF 108 (nonhemolytic) in a standard sterile stool adjuvant with 8.13 ± 0.07 log10 CFU of B. fragilis. Mortality at 24 h and at 10 days was recorded. Animals inoculated with WAF 270 had 32% mortality (10 of 31), versus no mortality in either the WAF 260 group (0 of 20) or the WAF 108 group (0 of 26) (P < 0.01). Animals in the WAF 270 group appeared ill, with blood-tinged nostrils and piloerection for greater than 24 h. The majority of deaths occurred within 24 h. All animals in the WAF 260 and WAF 108 groups appeared healthy at 24 h. The difference in mortality between the strongly hemolytic and nonhemolytic strains was even more pronounced in animals inoculated with 108 CFU of each: 8.45 ± 0.06 log10 CFU of WAF 270 produced 100% mortality (10 of 10), while 8.30 ± 0.01 log10 CFU of WAF 108 produced no mortality (0 of 10 animals). All deaths occurred within 24 h in the WAF 270 group. Finally, equal numbers of WAF 270 and WAF 108 (6.53 ± 0.26 log10 CFU total of E. coli) were combined with 8.13 ± 0.07 log10 CFU of B. fragilis in sterile stool adjuvant, and mortality was analyzed. This mixed inoculum of WAF 108 and WAF 270 produced an intermediate mortality of 25%, with two of eight animals dying.
Influence of hemolysin production on bacterial recovery after induction of peritonitis.
Groups of animals underwent induction of peritonitis with standard sterile stool adjuvant, 8.13 ± 0.07 log10 CFU of B. fragilis, and 6.44 ± 0.05 log10 CFU of WAF 270 or 6.55 ± 0.04 log10 CFU of WAF 108. Blood, peritoneum, spleen, liver, and lungs were cultured at 6, 15, and 24 h. Results are shown in Table 2. At the 6-h analysis, there were no significant differences between WAF 108 and WAF 270 except for an increase in the number of WAF 270 CFU per gram of spleen (7.88 ± 0.10 versus 6.52 ± 0.27 [P < 0.01). At 15 h, bacterial recovery from the peritoneum and spleen of animals injected with WAF 270 was significantly greater than that from animals inoculated with WAF 108. Induction of peritonitis with the hemolytic strain (WAF 270) resulted in increased recovery of E. coli and B. fragilis from the peritoneum at 24 h compared to the group that received the nonhemolytic strain (WAF 108). The addition of WAF 270 to the inoculum allowed greater recovery of B. fragilis from the blood than did the addition of WAF 108. Differences in bacterial recovery from the spleen at 24 h did not reach significance.
TABLE 2.
Influence of Hly on bacterial recovery following induction of rat peritonitis
| Organism, location, and strain | Log10 CFU ± SEM isolated ata:
|
||
|---|---|---|---|
| 6 h (n = 3) | 15 h (n = 3) | 24 h (n) | |
| E. coli | |||
| Peritoneum | |||
| WAF 270 | 5.83 ± 0.50 | 7.09 ± 0.48† | 6.38 ± 0.41† (6) |
| WAF 108 | 6.01 ± 0.09 | 4.52 ± 0.77 | 5.37 ± 0.24 (11) |
| Blood | |||
| WAF 270 | BDL | BDL | BDL (6) |
| WAF 108 | BDL | BDL | BDL (11) |
| Spleen | |||
| WAF 270 | 7.88 ± 0.10* | 8.92 ± 0.05† | 8.19 ± 0.13 (3) |
| WAF 108 | 6.52 ± 0.27 | 6.69 ± 0.57 | 7.43 ± 0.54 (3) |
| B. fragilis | |||
| Peritoneum | |||
| WAF 270 | 7.37 ± 0.31 | 6.62 ± 0.45 | 5.60 ± 0.35† (6) |
| WAF 108 | 7.89 ± 0.02 | 5.73 ± 0.17 | 4.66 ± 0.17 (11) |
| Blood | |||
| WAF 270 | 3.16 ± 0.17 | 1.43 ± 0.30 | 2.84 ± 0.31* (6) |
| WAF 108 | 3.59 ± 0.03 | 1.26 ± 0.14 | 1.63 ± 0.22 (11) |
| Spleen | |||
| WAF 270 | 6.10 ± 0.19 | 7.68 ± 0.23† | 7.66 ± 0.16 (3) |
| WAF 108 | 5.99 ± 0.08 | 5.94 ± 0.53 | 6.37 ± 0.67 (3) |
*,P < 0.01 versus WAF 108; †, P < 0.05 versus WAF 108; BDL, below detectable limits.
To characterize the influence of various amounts of hemolysin production on bacterial recovery after the induction of peritonitis, separate cohorts of animals were inoculated with 106 CFU of either WAF 270 (strongly hemolytic), an equal mixture of WAF 270 and WAF 108 (strongly hemolytic and nonhemolytic), WAF 260 (weakly hemolytic), or WAF 108 (non-hemolytic) in the same model as described above. Animals were analyzed at 24 h after the induction of peritonitis, and results are shown in Table 3. Results demonstrate a gradual decline in the recovery of E. coli from the peritoneum and of B. fragilis from both the peritoneum and the blood as the overall hemolysin secretion potential declines in the inoculum.
TABLE 3.
Influence of various levels of Hly production on bacterial recovery at 24 h after induction of rat peritonitis
| Organism and location | Log10 CFU ± SEMa
|
|||
|---|---|---|---|---|
| WAF 270 | WAF 270/108 | WAF 260 | WAF 108 | |
| E. coli | ||||
| Peritoneum | 6.94 ± 0.50* | 6.45 ± 0.41 | 6.34 ± 0.22 | 5.76 ± 0.17 |
| Blood | BDL | BDL | BDL | BDL |
| B. fragilis | ||||
| Peritoneum | 6.15 ± 0.34* | 5.71 ± 0.38* | 5.42 ± 0.18 | 4.91 ± 0.17 |
| Blood | 2.62 ± 0.41*† | 2.12 ± 0.28* | 1.65 ± 0.15 | 1.25 ± 0.13 |
*, P < 0.05 versus WAF 108; †, P < 0.05 versus WAF 260; BDL, below detectable limits.
Influence of hemolysin production on abscess recovery after induction of peritonitis.
Analysis of intraperitoneal abscesses at 10 days after the induction of peritonitis revealed a graded response in the number of abscesses and the abscess weight per animal (Table 4). Inoculation with the strongly hemolytic strain WAF 270 produced a greater number of abscesses and a greater total abscess weight per animal than any of the other inocula. In addition, WAF 270 allowed a significantly greater number of E. coli and B. fragilis organisms per animal to be recovered at the abscess stage than did the nonhemolytic strain, again showing a gradual decrease in recovery as the hemolysin secretion potential was diminished.
TABLE 4.
Influence of Hly production on abscess formation in the rat peritonitis model
| Strain | Mean ± SEMa
|
|||
|---|---|---|---|---|
| Abscesses/rat (n) | Abscess wt (mg)/rat (n) | Log10 CFU/rat (n = 5)
|
||
| E. coli | B. fragilis | |||
| WAF 270 | 5.0 ± 0.7*‡ (15) | 1,030 ± 310† (15) | 7.43 ± 0.19* | 8.45 ± 0.20* |
| WAF 270/108 | 2.5 ± 0.5 (10) | 431 ± 103 (10) | 6.68 ± 0.30 | 7.63 ± 0.38† |
| WAF 260 | 3.6 ± 0.5† (10) | 485 ± 65 (10) | 6.70 ± 0.28 | 8.01 ± 0.50† |
| WAF 108 | 1.9 ± 0.3 (15) | 320 ± 70 (15) | 5.71 ± 0.38 | 6.47 ± 0.52 |
*, P < 0.01 versus WAF 108; †, P < 0.05 versus WAF 108; ‡, P < 0.05 versus WAF 270/108.
Recovery of hemolytic and nonhemolytic E. coli after induction of peritonitis with a mixed inoculum of WAF 270-WAF 108.
Animals that were inoculated with equal numbers of WAF 270 and WAF 108 (6.53 ± 0.26 log10 CFU total of E. coli) were analyzed at 24 h and at 10 days, and the percentage of hemolytic versus nonhemolytic strains was determined. At 24 h, the hemolytic strains outnumbered the nonhemolytic strains and accounted for 72% of the E. coli strains isolated. However, by 10 days, the nonhemolytic E. coli strains greatly outnumbered the hemolytic E. coli strains, comprising 89% of the total isolated. This shift back toward nonhemolytic strains at 10 days parallels the results seen after induction of peritonitis with the rat fecal inoculum containing native flora. Additionally, the selective pressure to maintain the hemolysin determinate appears to also diminish, as some of the isolates represented E. coli strains that had lost their plasmids (as determined by antibiotic resistance).
Alterations in the intraperitoneal environment and inflammatory response after induction of peritonitis with WAF 108 and WAF 270.
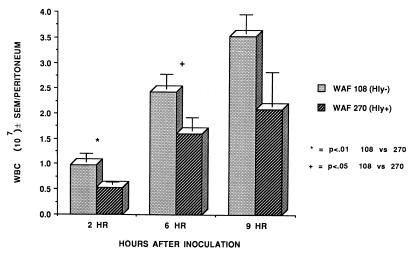
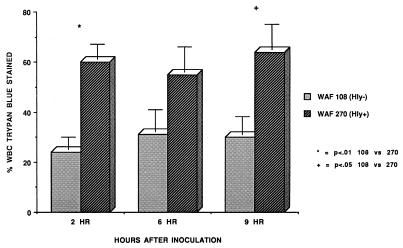
To examine the influence of Hly production by E. coli on the intraperitoneal environment following the induction of peritonitis, rats were inoculated with 108 CFU of either WAF 108 (8.68 ± 0.04 log10 CFU) or WAF 270 (8.63 ± 0.03 log10 CFU) and standard sterile stool adjuvant. The degree of intraperitoneal RBC lysis, the intraperitoneal pH, the numbers of intraperitoneal leukocytes, leukocyte viability, and leukocyte degranulation were assayed. Quantitative bacterial cultures were obtained at 2, 6, and 9 h. The inoculum with WAF 270 produced 100% mortality, while the inoculum with WAF 108 produced no mortality. Results are displayed in Fig. 1 through 6.
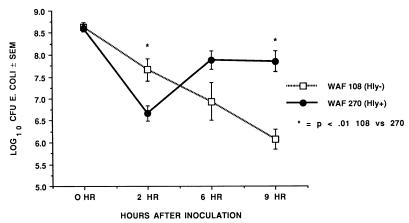
FIG. 1.
Recovery of E. coli from the peritoneum at 2, 6, and 9 h after induction of peritonitis with either WAF 270 or WAF 108 and sterile fecal adjuvant. Values are mean CFU of each strain and standard error expressed in log10. Numbers of animals assayed equal 16 for the 2- and 6-h time points and 9 for WAF 108 and 11 for WAF 270 at 9 h.
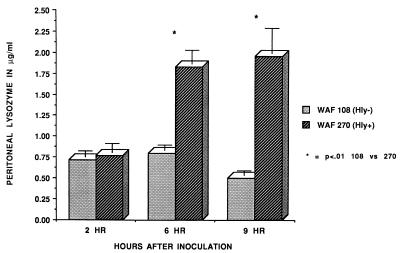
FIG. 6.
Peritoneal lysozyme activity at 2, 6, and 9 h after induction of peritonitis with either WAF 108 or WAF 270 and sterile fecal adjuvant. Activity was determined by the M. lysodeikticus assay, as described in the text. Values are means and standard errors for five animals in each group.
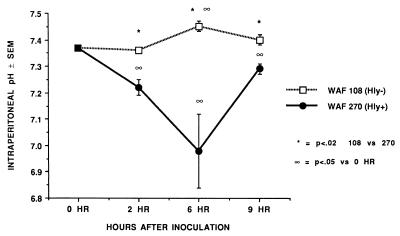
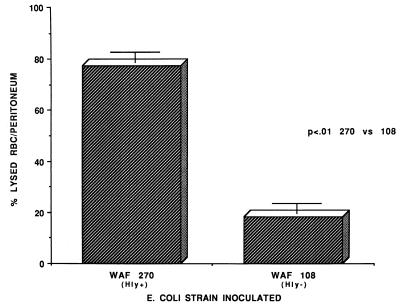
At 2 h after inoculation, the numbers of recoverable hemolytic E. coli strains decreased to levels that were significantly below the numbers of recoverable nonhemolytic E. coli strains. However, by 9 h WAF 270 significantly outnumbered WAF 108, by nearly 100-fold (Fig. 1). The intraperitoneal pH fell dramatically and remained low in rats injected with WAF 270 but was relatively unaffected by WAF 108 in the other group (Fig. 2). Induction of peritonitis with the hemolytic strain also had profound effects on intraperitoneal RBC and leukocytes. Although the total amount of hemoglobin within the peritoneum (free and within RBC) did not differ between the two groups (data not shown), the percentage of hemoglobin from lysed RBC (free hemoglobin) was significantly greater at each time point in animals inoculated with WAF 270 (Fig. 3). In addition, the leukocyte response to peritonitis was significantly different between the two groups. Recruitment of peritoneal leukocytes in animals that received WAF 270 was significantly less than in those that received WAF 108 (Fig. 4). A much larger percentage of leukocytes isolated from the peritoneum of the WAF 270 animals were nonviable, as indicated by their inability to exclude vital dye (Fig. 5). Moreover, WAF 270 caused significantly increased lysozyme activity at 6 and 9 h compared to WAF 108 (Fig. 6).
FIG. 2.
Intraperitoneal pH at 2, 6, and 9 h after induction of peritonitis with either WAF 270 or WAF 108 and sterile fecal adjuvant. Values mean and standard error for five animals within each group at each time point.
FIG. 3.
Degree of intraperitoneal RBC lysis produced by either WAF 270 or WAF 108 during peritonitis. The percent lysed RBC was determined by absorbance at 543 nm of free hemoglobin within either (i) cell-free lavage fluid after removing intact cells by low-speed centrifugation or (ii) lavage fluid following complete lysis of intact RBC with a commercial lytic reagent (Zap-oglobin II; Coulter Diagnostics). The percentage was determined as absorbance (i)/absorbance (ii) × 100. Values are mean percentages and standard errors for 14 animals in each group.
FIG. 4.
Number of leukocytes (WBC) recovered from the peritoneal cavity after induction of peritonitis with either WAF 108 or WAF 270 and sterile stool adjuvant. Values are means and standard errors for 13 animals in each group at 2 and 6 h and 9 animals in the WAF 108 group and 11 in the WAF 270 group at 9 h.
FIG. 5.
Nonviability of peritoneal leukocytes (WBC) recovered after induction of peritonitis with either WAF 108 or WAF 270 and sterile stool adjuvant, expressed as the number of trypan blue-stained cells per 100 leukocytes. Three or four animals were analyzed per group.
Preimmunization protects against hemolysin-induced lethality.
Animals were immunized with sterile supernatants from a 6-h growth of WAF 270 in RPM1 1640. Hemolytic titers of the supernatants were verified prior to each immunization. Control animals were immunized with WAF 108 supernatant or were not immunized at all. After induction of peritonitis with 1.8 × 109 WAF 270 organisms and standard fecal adjuvant (previously established 100% mortality in naïve rats), all of the nonimmunized animals died (five of five). Immunization with WAF 108 supernatant offered some protection, with 8 of 15 animals surviving at 10 days (P < 0.05 [by chi-square test, versus nonimmune animals]). These animals appeared ill, with blood-tinged nostrils, anorexia, and piloerection. Immunization with WAF 270 supernatant completely protected the rats from this lethal inoculum. All 15 animals survived the inoculum (P < 0.01 [by chi-square test, WAF 270 immune versus WAF 108 immune animals]), and these animals appeared much less ill than the others.
DISCUSSION
The E. coli exotoxin alpha-hemolysin is thought to be a significant virulence factor in several clinical infections, such as pyelonephritis and septicemia (6, 9, 19, 21, 30, 31, 37–39). Data obtained from both in vivo and in vitro infection models demonstrate hemolysin's toxicity to a wide variety of mammalian cells and organs. However, the specific role of this potent exotoxin in intraperitoneal infections is not clearly established.
Most intraperitoneal infections result from a breach in the integrity of the gastrointestinal tract, causing the introduction of a broad mixture of both aerobic and anaerobic bacteria into the peritoneal cavity. Of the numerous aerobic bacteria isolated from peritoneal infections, E. coli emerges as the predominate pathogen in over 60% of cases (18, 24). E. coli seems particularly adept at making the transition from the environment of the gastrointestinal tract to that of the peritoneum. The specific characteristics that allow this organism to so effectively evade host defenses, proliferate, and establish infection within the peritoneum are not clear. We postulate that Hly plays a key role in facilitating the pathogenicity of E. coli within the peritoneum. Welch et. al, using genetically modified hemolytic and nonhemolytic E. coli strains, demonstrated that changing a nonhemolytic E. coli strain to a hemolytic strain greatly reduced the 50% lethal dose of that strain in an experimental rat peritonitis model (41). The mechanisms for the increase in lethality were not established in that study. Our laboratory has previously demonstrated that two E. coli strains that differ only in the ability to produce Hly have markedly different lethality profiles (27). For example, an inoculum of 108 CFU of the Hly+ variant (WAF 270) was 100% lethal, while the same-size inoculum of the Hly− variant (WAF 108) was nonlethal. Additionally, although the hemolytic strain induces a significant interleukin 1 (IL-1) surge at 5 h after inoculation that is not demonstrable by the nonhemolytic strain, we have demonstrated that WAF 270-induced lethality is independent of both IL-1 and tumor necrosis factor (16).
In the present study, we have shown that wild-type hemolytic strains demonstrate a significant competitive advantage over nonhemolytic strains in a rat peritonitis model. Only 7% of the E. coli strains within the inoculum (pooled rat cecal contents) were hemolytic, but at 24 h after inoculation greater than 70% of the E. coli strains recovered from the peritoneum and nearly 100% recovered from the blood were hemolytic. In the chronic (abscess) phase of infection, the percentage that were hemolytic decreased somewhat, to approximately 50%, but still remained significantly greater than the percentage of the original inoculum. Thus, once hemolytic strains are introduced into the peritoneum, they appear to possess characteristics that allow them to survive and proliferate more effectively than nonhemolytic strains during periods of rapid proliferation. During the chronic, abscess phase of infection, this advantage seems to diminish considerably. The specific contribution of hemolysin to the competitive advantage cannot be established from this data. Therefore, we used the isogenic E. coli strains WAF 108, WAF 260, and WAF 270 to determine the specific contribution of hemolysin production in establishing peritonitis.
The otherwise genetically identical strains WAF 108 and WAF 270 differ only in the fact that the Hly determinant-carrying plasmid in WAF 108 contains a transposon (Tn1) which prevents Hly transcription, while WAF 270 does not. WAF 260 carries a different plasmid that produces hemolysin but at a level that is roughly 50-fold lower than that of WAF 270. The strong production of hemolysin by WAF 270 greatly increased the lethality after induction of peritonitis, as well as increasing both E. coli and B. fragilis recovery from the animals by 15 h after the induction of peritonitis and continuing through the abscess phase at 10 days. The increase in bacterial recovery generally decreased as the hemolysin-secreting potential within the inoculum decreased. The increased B. fragilis recovery in this model demonstrates that production of this exotoxin not only alters the fate of E. coli itself but also alters either the host's ability to eliminate other bacteria or the growth kinetics of B. fragilis. In either case, hemolysin production in clinical peritonitis likely alters the host-pathogen relationship for bacteria other than E. coli.
Hemolysin is known to be toxic to a wide variety of mammalian cells, and its production within the peritoneum may significantly alter both the host's intraperitoneal environment and the local inflammatory response. Indeed, this was shown to be the case in this model. Soon after the induction of peritonitis with either E. coli WAF 270 or WAF 108, significant differences in bacterial recovery, intraperitoneal pH, leukocyte response, and lysis of RBC developed. The induction of peritonitis with the nonhemolytic strain WAF 108 was followed by a near linear decrease in organisms recovered from the peritoneal cavity concomitant with a significant increase in the intraperitoneal pH in the first 9 h. This pattern is distinctly different than that produced by the hemolytic strain WAF 270. Bacterial recovery fell significantly more rapidly with WAF 270 than WAF 108 over the first 2 h, but WAF 270 recovery increased dramatically over the next several hours. Moreover, the intraperitoneal pH fell significantly over the first 6 h before it began to return toward baseline. Infection with WAF 270 caused intraperitoneal hemolysis and leukocyte nonviability after the induction of peritonitis. Total leukocyte recovery from the peritoneum was significantly less following induction of peritonitis with the hemolytic strain than the nonhemolytic strain, and the percentage of leukocytes that were viable was significantly less. WAF 270 also affected leukocyte function in vivo. Intraperitoneal lysozyme activity, a measure of leukocyte activation, was greater after inoculation with WAF 270 than with WAF 108. These findings are consistent with in vitro studies that demonstrate that hemolysin induces leukocyte degranulation followed by cell death as concentrations of hemolysin increase (3, 7, 14).
The clinical significance of these intraperitoneal changes and their role in the pathogenicity of peritonitis cannot be assessed from this study. However, clinical relevance may be postulated. The increase in free intraperitoneal hemoglobin may be of clinical significance since hemoglobin is a potent inhibitor of nitric oxide activity, and nitric oxide production by leukocytes is one of the host's defenses against bacterial infection. Additionally, both iron and hemoglobin are recognized as adjuvants for bacterial growth. Whether or not the intraperitoneal pH difference between the two organisms has a role in the pathogenesis of peritonitis is not known. However, reductions in extracellular pH have been shown to alter leukocyte activity in other models and to potentiate the impairment of neutrophil function produced by B. fragilis byproducts (1, 15, 34, 44). This in vivo data also demonstrates that hemolysin production within the peritoneal cavity results in a reduction both in leukocyte recruitment and in leukocyte viability. Yet the release of leukocyte products following induction of peritonitis with the hemolytic strain was actually increased relative to the nonhemolytic strain, suggesting an alteration in leukocyte function as well. These findings, coupled with those published previously regarding the pronounced alterations in cytokine production induced by the in vivo production of hemolysin, suggest that hemolysin secretion during clinical peritonitis may profoundly alter the host's inflammatory response (27). Thus, this may alter the ability to clear not only hemolysin-secreting strains but also other organisms as well.
The deleterious effects of hemolysin secretion were abrogated by preimmunization with hemolysin-rich supernatant, demonstrating that the host's susceptibility to hemolysin can be altered. The altered susceptibility is presumably an anamnestic response to hemolysin and lipopolysaccharide, as evidenced by the partial protection offered by WAF 108 supernatant and the total protection by WAF 270 supernatant, and given the nature of the immunization schedule.
In conclusion, hemolysin production during peritonitis increases the lethality of infection in this model. Its production increases the bacterial burden in the host, significantly alters the peritoneal environment, and alters leukocyte recoverability, viability, and function. The mechanisms of these changes are currently being investigated. We have previously shown significant alterations in the levels of IL-1 produced by Hly in vivo (27). Studies investigating whether or not other cytokine alterations contribute to the changes induced by Hly in vivo are ongoing. The possibility that Hly may alter the peritoneal mesothelium, altering the trafficking of leukocytes, is also being investigated.
ACKNOWLEDGMENTS
A. K. May was a recipient of the Surgical Infection Society/3M Fellowship Award. This work was supported by University of Virginia research and development grant 5-507-RR05431-28 and by NIH grant 29A128954-01.
E. coli WAF 108 and WAF 270 were kindly provided by R. A. Welch.
REFERENCES
- 1.Allen D B, Maguire J J, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, Chang M, Le A X, Hopf H W, Hunt T K. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;139:991–996. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 2.Bhakdi S, Mackman N, Nicaud J-M, Holland I B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986;52:63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakdi S, Muhly M, Korom S, Schmidt G. Effects of Escherichia coli hemolysin on human monocytes. J Clin Investig. 1990;85:1746–1753. doi: 10.1172/JCI114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehm D F, Welch R A, Snyder I S. Calcium is required for binding of Escherichia coli hemolysin (HlyA) to erythrocyte membranes. Infect Immun. 1990;58:1951–1958. doi: 10.1128/iai.58.6.1951-1958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehm D F, Welch R A, Synder I S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990;58:1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalieri S J, Bohach G A, Snyder I S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984;48:326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalieri S J, Snyder I S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte viability in vitro. Infect Immun. 1982;36:455–461. doi: 10.1128/iai.36.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooke E M. Properties of strains of Escherichia coli isolated from faeces of patients with ulcerative colitis, patients with acute diarrhea and normal persons. J Pathol Bacteriol. 1968;95:101–113. doi: 10.1002/path.1700950112. [DOI] [PubMed] [Google Scholar]
- 9.Cooke E M, Ewins S P. Properties of strains of Escherichia coli isolated from a variety of sources. J Med Microbiol. 1975;8:107–111. doi: 10.1099/00222615-8-1-107. [DOI] [PubMed] [Google Scholar]
- 10.Dellinger E P, Wertz M J, Meakins J L, et al. Surgical infection stratification system for intraabdominal infection. Arch Surg. 1985;120:21–27. doi: 10.1001/archsurg.1985.01390250015003. [DOI] [PubMed] [Google Scholar]
- 11.Eberspacher B H, Ferdinand, Bhakdi S. Quantitative study of the binding and hemolytic efficiency of Escherichia coli hemolysin. Infect Immun. 1989;57:983–988. doi: 10.1128/iai.57.3.983-988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans D J, Evans D G, Hohne C, Noble M A, Haldane E V, Lior H, Young L S. Hemolysin and K antigens in relation to serotype and hemagglutination type of Escherichia coli isolated from extraintestinal infections. J Clin Microbiol. 1981;13:171–178. doi: 10.1128/jcm.13.1.171-178.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farthmann E H, Schoffel U. Principles and limitations of operative management of intraabdominal infections. World J Surg. 1990;14:210–218. doi: 10.1007/BF01664875. [DOI] [PubMed] [Google Scholar]
- 14.Gadeberg O V, Orskov I, Rhodes K M. Cytotoxic effect of an alpha-hemolytic Escherichia coli strain on human blood monocytes and granulocytes in vitro. Infect Immun. 1983;41:358–364. doi: 10.1128/iai.41.1.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargan R A, Hamilton-Miller J M, Brumfitt W. Effect of pH and osmolality on in vitro phagocytosis and killing by neutrophils in urine. Infect Immun. 1993;61:8–12. doi: 10.1128/iai.61.1.8-12.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gleason T G, Houlgrave C W, May A K, Crabtree T D, Sawyer R G, Denham W, Norman J G, Pruett T L. Hemolytically active (acylated) alpha-hemolysin elicits interleukin-1β (IL-1β) but augments the lethality of Escherichia coli by an IL-1- and tumor necrosis factor-independent mechanism. Infect Immun. 1998;66:4215–4221. doi: 10.1128/iai.66.9.4215-4221.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimminger F, Sibelius U, Bhakdi S, Suttrop N, Seeger W. Escherichia coli hemolysin is a potent inductor of phosphoinositide hydrolysis and related metabolic responses in human neutrophils. J Clin Investig. 1991;88:1531–1539. doi: 10.1172/JCI115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hau T. Bacteria, toxins, and the peritoneum. World J Surg. 1990;14:167–175. doi: 10.1007/BF01664869. [DOI] [PubMed] [Google Scholar]
- 19.Hunt J L. Generalized peritonitis: to irrigate or not to irrigate the abdominal cavity. Arch Surg. 1982;117:209–212. doi: 10.1001/archsurg.1982.01380260075013. [DOI] [PubMed] [Google Scholar]
- 20.Jorgenson S E, Short E C, Kurtz H J, Mussen H K, Wu G K. Studies on the origin of the alpha haemolysin produced by Escherichia coli. J Med Microbiol. 1975;9:173–189. doi: 10.1099/00222615-9-2-173. [DOI] [PubMed] [Google Scholar]
- 21.Keane W F, Welch R, Gekker G, Peterson P K. Mechanism of Escherichia coli alpha-hemolysin induced injury to isolated renal tubular cells. Am J Pathol. 1987;126:350–357. [PMC free article] [PubMed] [Google Scholar]
- 22.Konig B, Konig W, Scheffer J, Hacker J, Goebel W. Role of Escherichia coli alpha-hemolysin and bacterial adherence in infection: requirement for release of inflammatory mediators from granulocytes and mast cells. Infect Immun. 1986;54:886–892. doi: 10.1128/iai.54.3.886-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konig B, Schonfeld W, Scheffer J, Konig W. Signal transduction in human platelets and inflammatory mediator release induced by genetically cloned hemolysin-positive and -negative Escherichia coli strains. Infect Immun. 1990;58:1591–1599. doi: 10.1128/iai.58.6.1591-1599.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorber B, Swenson R M. The bacteriology of intraabdominal infections. Surg Clin North Am. 1975;55:1349–1354. doi: 10.1016/s0039-6109(16)40792-9. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig A, Schmid A, Benz R, Goebel W. Mutations affecting pore formation by hemolysin from Escherichia coli. Mol Gen Genet. 1991;226:198–208. doi: 10.1007/BF00273604. [DOI] [PubMed] [Google Scholar]
- 26.Mackman N, Holland I B. Functional characterization of a cloned haemolysin determinant from E. coli of human origin, encoding information for the secretion of a 107L polypeptide. Mol Gen Genet. 1984;320:123–134. doi: 10.1007/BF00334104. [DOI] [PubMed] [Google Scholar]
- 27.May A K, Sawyer R G, Gleason T, Whitworth A, Pruett T L. In vivo cytokine response to Escherichia coli alpha-hemolysin utilizing genetically engineered hemolytic and nonhemolytic E. coli variants. Infect Immun. 1996;64:2167–2171. doi: 10.1128/iai.64.6.2167-2171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minshew B H, Jorgenson J, Counts G W, Falkow S. Association of hemolysin productions, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978;20:50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minshew B H, Jorgenson J, Swanstrum M, Grootes-Reuvecamp G A, Falkow S. Some characteristics of Escherichia coli strains from extraintestinal infections in humans. J Infect Dis. 1978;137:648–654. doi: 10.1093/infdis/137.5.648. [DOI] [PubMed] [Google Scholar]
- 30.Mobley H L, Green D H, Trifillis A L, Johnson D E, Chippendale G R, Lockatell C V, Jones B D, Warren J W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Hanley P, Lalonde G, Ji G. Alpha-hemolysin contributes to the pathogenicity of piliated diagalactoside-binding Escherichia coli in the kidney: efficacy of an alpha-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect Immun. 1991;59:1153–1161. doi: 10.1128/iai.59.3.1153-1161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Opal S M, Cross A S, Sadoff J C, Collins H H, Kelly N M. Efficacy of antilipopolysaccharide and anti-tumor necrosis factor monoclonal antibodies in a neutropenic rat model of Pseudomonas sepsis. J Clin Investig. 1991;88:885–890. doi: 10.1172/JCI115390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orskov F. Virulence factors of the bacterial cell surface. J Infect Dis. 1978;137:630–633. doi: 10.1093/infdis/137.5.630. [DOI] [PubMed] [Google Scholar]
- 34.Rotstein O D. Interactions between leukocytes and anaerobic bacteria in polymicrobial surgical infections. Clin Infect Dis. 1993;16(Suppl. 4):S190–S194. doi: 10.1093/clinids/16.supplement_4.s190. [DOI] [PubMed] [Google Scholar]
- 35.Sparling P F. Bacterial virulence and pathogenesis: an overview. Rev Infect Dis. 1983;5:S637–S646. doi: 10.1093/clinids/5.supplement_4.s637. [DOI] [PubMed] [Google Scholar]
- 36.Suttrop N, Floer B, Schnittler H, Seeger W, Bhakdi S. Effects of Escherichia coli hemolysin on endothelial cell function. Infect Immun. 1990;58:3796–3801. doi: 10.1128/iai.58.11.3796-3801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svanborg Eden C, de Man P. Bacterial virulence in urinary tract infection. Infect Dis Clin North Am. 1987;1:731–750. [PubMed] [Google Scholar]
- 38.Van den Bosch J F, Postma P, De Graaff J, MacLaren D M. Hemolysis by urinary Escherichia coli and virulence in mice. J Med Microbiol. 1981;14:321–333. doi: 10.1099/00222615-14-3-321. [DOI] [PubMed] [Google Scholar]
- 39.Waalwijk C, MacLaren D M, De Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983;42:245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh G L, Chiasson P, Hedderich G, Wexler M J, Meakins J L. The open abdomen. The Marlex mesh and zipper technique: a method of managing intraperitoneal infection. Surg Clin North Am. 1988;68:25–40. doi: 10.1016/s0039-6109(16)44430-0. [DOI] [PubMed] [Google Scholar]
- 41.Welch R A, Dellinger E P, Minshew B, Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981;294:665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- 42.Welch R A, Falkow S. Characterization of Escherichia coli hemolysins conferring quantitative differences in virulence. Infect Immun. 1984;43:156–160. doi: 10.1128/iai.43.1.156-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch R A, Hull R, Falkow S. Molecular cloning and physical characterization of a chromosomal hemolysin from Escherichia coli. Infect Immun. 1983;42:178–186. doi: 10.1128/iai.42.1.178-186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westman J A. Influence of pH and temperature on the luminol-dependent chemiluminescence of human polymorphonuclear leucocytes. Scand J Clin Lab Investig. 1986;46:427–434. doi: 10.3109/00365518609083694. [DOI] [PubMed] [Google Scholar]