Clinical research in an era of pandemics
In addition to its overwhelming impacts on global health and health systems' resilience, the COVID-19 pandemic has elicited an unprecedented global scientific effort. Since early in the onset of the pandemic, numerous randomized clinical trials (RCTs) were set up to give a response to the unmet clinical needs [1]. Remarkably, platform trials (PTs) emerged as a largely employed appraisal in clinical research in the wake of COVID-19, e.g. Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia (REMAP-CAP), WHO Solidarity, RECOVERY, ATTACC, PRINCIPLE, AGILE, COPPS, and OPTIMISE C-19, among others. The results from some of these trials have led to the current standard of care recommendations issued in guidelines worldwide for the treatment of COVID-19. However, for most clinicians and investigators, the basics of PTs, as well as their constraints and advantages, remain largely unknown.
In this Commentary, we focus on the basic concepts and methodological keys of PTs using some of the recent developments in COVID-19 treatment to illustrate the potential of these trial designs.
Master protocols and PTs: the basics
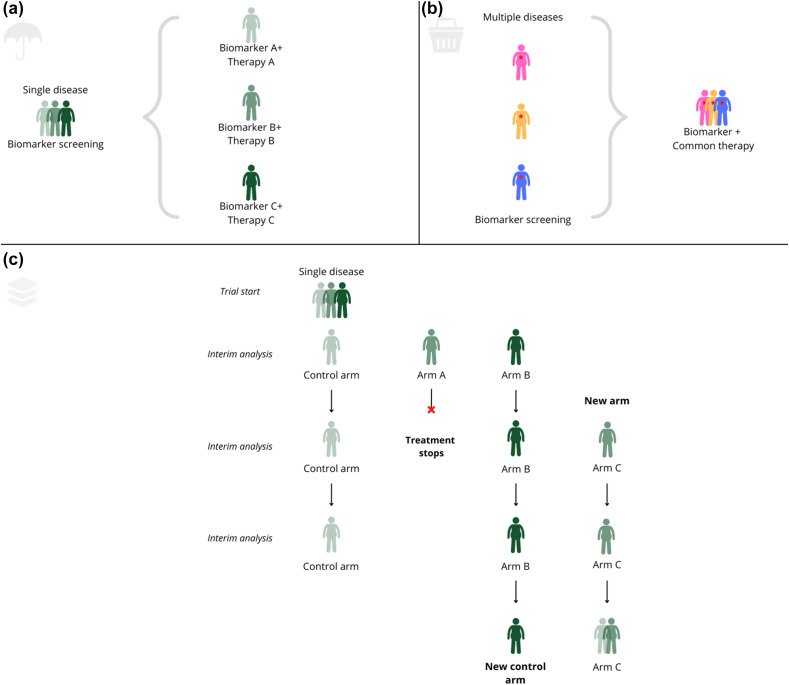
Alongside umbrella and basket trials, PTs are one of the possible designs of master protocols (MPs) (Fig. 1 and Glossary in Table 1 ). MPs allow testing for multiple hypotheses within a single protocol and stratification by (biomarker) subgroup, leading to a more personalized evaluation of therapies compared with traditional RCTs [[2], [3], [4]]. Whereas traditional trials are based on a “one population, one drug, one disease” strategy, PTs and umbrella and basket trials share key design components and operational aspects that aim to increase the efficiency (in both time and resources) of achieving high-quality evidence for medical interventions by evaluating multiple populations, multiple interventions, and/or multiple diseases at the same time [2].
Fig. 1.
Scheme of master protocols' design. (a) Umbrella trial, (b) Basket trial, and (c) Platform trial.
Table 1.
Glossary of basic terms related to platform trials
| Main definitions |
|---|
| Adaptive designs for clinical trials: an adaptive trial uses data that accumulates during the study to modify study elements in a flexible though prespecified manner. Elements that may be modified include the sample size, end points, eligible populations, randomization ratio, and interventions. |
| Master protocol: overarching trial characteristics and rules guiding several parallel subtrials (either concomitant or sequential) under the same protocol and governance structure. |
| Multiarm multistage (MAMS) trials: adaptive design and set-up that aims to answer multiple questions simultaneously under the same regulatory framework. Several different treatment options can be compared simultaneously, usually against a single control arm. Tested treatments can be different drugs at single dosages, a combination of drugs, or various doses of the same drug. Adaptation rules and interim analyses allow for changes in the subtrials/arms that constitute part of the trial. The master protocol of MAMS can be adopted in different forms. |
| Basket and umbrella trials: both are multiarm, often adaptive designs of randomized clinical trials under a master protocol. In the former, multiple diseases or phenotypes are treated with the same treatment, whereas in the latter, multiple treatments are tested against the same disease. |
| Platform trials: derivation of MAMS that consist of a prospective, disease-focused, adaptive, randomized clinical trial that compares multiple interventions against a single, constant control group. |
| Bayesian statistics: a statistical set of methods characterized by an approach in which probability expresses the degree of certainty of an event to happen within a timeframe. In platform trials, this allows us to decide whether to continue or discontinue an arm containing active treatment on the basis of interim analysis and the prediction that the drug may or may not achieve a certain threshold of efficacy (i.e. futility/efficacy stop rules). |
| Specific Appendices (Domain and Intervention specific): these appendices contain the specific details that concern each subtrial or arm that is incorporated into the platform trial, whereas the master protocol provides the overarching information. |
| Integrated Research Platform: the combination of the governing bodies, the network of clinical sites, the methodological tools including the master protocol, and the regulatory and legal aspects that allow setting up a platform trial. |
Before the outbreak of COVID-19, MPs were mostly used in oncology and haematology (e.g. I-SPY in breast cancer and GBM AGILE in brain tumours) and less frequently in mental illnesses (e.g. DIAN-TU in Alzheimer and CATIE in schizophrenia) and infectious disease (e.g. ADAPT for the treatment of multi-resistant pathogens, PREVAIL II in Ebola, and REMAP-CAP in pneumonia) [3]. MPs may be built leveraging the infrastructure and similarities of previous individual trials or be set up de novo, and they can both compare directly competing interventions and evaluate them in parallel with their respective controls.
Owing to PTs' adaptive design, new arms can be added along the way and those reaching a prespecified probability of failure or success can be dropped (and become the new standard of care in case of success) [4]. As non-efficacious compounds are dropped early, patients are at a lower risk of being exposed to these compounds and pharmaceutical companies can quickly move on to investigate novel compounds at lower costs. The use of a shared control arm for multiple active compounds is efficient, and the multifactorial design (allowing participants to receive a randomized allocation for more than one aspect of their treatment) adds even more efficiency. From the participant's perspective, this reduces the probability of receiving no active treatment at all.
The main advantages of PTs are the likely smaller sample size and shorter time to answer any given question compared with a fixed design. However, PTs are not without shortcomings, which might be more or less challenging depending on the context, e.g. during COVID-19, many of the steps involved were shortened. Among the potential disadvantages, the two most relevant ones refer, on the one hand, to the increased design and operational complexity, which is largely accompanied by an increased difficulty in explaining the concepts to funders, the general public, and the research community; on the other hand, the longer trial set up time including protocol writing, ethics, and institutional approval.
REMAP-CAP: an example of global multicollaborative success
REMAP-CAP was conceived after the Influenza A H1N1 (pdm2009) pandemic and was first funded in 2014 as a global trial simultaneously investigating multiple interventions for severe community-acquired pneumonia with the specific aim of being prepared for the next pandemic [5]. Even before the COVID-19 pandemic, a “sleeping” Pandemic Appendix to the Core Protocol was approved, which described potential adaptations applicable during a pandemic, e.g. primary end point, platform eligibility, and statistical model. The latter serves to enable a separate analysis of patients affected during pandemic and enrolment of patients in different disease severity states and to evaluate the differential efficacy of interventions depending on illness severity [5]. REMAP-CAP findings, alongside RECOVERY [6], WHO Solidarity [7], and other relevant trials, critically contributed to informing COVID-19 treatment guidelines.
The design approach of REMAP-CAP was specifically chosen for its value in future pandemics that involved hospitals and, specifically, intensive care units. The trial was set up in a modular way, with a Core Protocol describing all general aspects of the trial and Domain-specific Appendices for each of the therapeutic areas of interest (domains). Within each domain, two or more mutually exclusive interventions were compared. Patients could be eligible for one or more domains. The modular structure of the protocol allowed new interventions and domains to be submitted as substantial amendments without changing the Core Protocol. There are additional appendices for the statistical analysis, pandemics, and each region the trial is active in, to allow for regional differences in legislation (Region Specific Appendices). This modular structure is also reflected in the governance structure [5].
The statistical framework underpinning the REMAP design is Bayesian. There is one overarching Bayesian cumulative logistic model driving all adaptations, rules for statistical parametrization, and result summaries. The decision to use a Bayesian analysis was driven in part by the uncertainty of the extent of the pandemic. The sample size could be small or large, and there may be important external events, such as other trial results, that alter the design of REMAP-CAP.
Operationally, the challenges of running a global trial in the midst of a pandemic were substantial. Some challenges, such as limited availability of research personnel and other resources, were unavoidable. The trial design, embedding research in clinical care and aiming to optimize the trade-off between learning and doing, alleviated some of the operational challenges [8]. The biggest challenges, however, were not related to the design. The most important hurdles to delivering the trial during the pandemic were in facilitating collaboration, data collection and sharing, leveraging funding, and optimizing prioritization at a global level. These challenges cannot be solved by any individual trial but are extremely important for the global research community, funders, and regulators to address together [9,10].
Future perspectives
Boosting adaptive designs through reusable trial infrastructures such as PTs might help overcome some of the major challenges and limitations of standalone RCTs conducted in the field of infectious diseases, such as overly optimistic effect sizes; unnuanced conclusions based on dichotomization of results; limited focus on patient-centred outcomes other than mortality; lack of flexibility and ability to adapt, increasing the risk of inconclusive results and limiting knowledge gains before trial completion; and inefficiency due to lack of reuse of trial infrastructure [11]. However, clinical research and drug development through broader use of adaptive platform designs in COVID-19 specifically and in infectious diseases in general would require some large scientific, cultural, and infrastructural changes. Among some salient scientific challenges, there are still some gaps to be filled in COVID-19 (e.g. treatment of long/persistent COVID-19) and biomarkers need to be developed that will allow for stratification in other relevant infectious diseases, including neglected tropical diseases. From the cultural or educational perspective, further steps should be taken for achieving a scenario in which investigators, sponsors, and health authorities are familiar with the benefits of PTs and aligned in joint undertakings that expand their use. Moreover, infrastructures and collaborative pathways are to be built from which further PTs in infectious diseases might emerge. For instance, electronic health records can be embedded in trial design, with automatic integration leading to substantial logistic improvements regarding data collection, integration of randomization modules, and alerts about potentially eligible patients. The European Union Patient-centric Clinical Trial Platforms Consortium is creating such structure and framework for four diseases, including tuberculosis, relying on the concept of integrated research platforms [12]. The European Clinical Research Alliance on Infectious Diseases offers a coordinated approach that aims to build a permanent, not-for-profit, pan-European clinical research network capable of rapidly initiating and completing high-quality clinical studies with greater speed and efficiency [13].
In conclusion, MP and particularly adaptive PT designs constitute an innovative, flexible, multicollaborative, and efficient way forward to boost clinical research in infectious diseases that has already proved valuable in COVID-19.
Author contributions
Conceptualized by JMP, LD, and SB; Original draft written by JMP, LD, and SB; Review and editing done by JMP, LD, and SB; Final version approved by JMP, LD, and SB; Visualization, supervision, and administration performed by JMP.
Transparency declaration
The REMAP-CAP trial is funded by the Platform for European Preparedness Against Re-emerging Epidemics consortium by the European Union, FP7-HEALTH-2013-INNOVATION-1 (grant 602525), the Australian National Health and Medical Research Council (grant APP1101719), the New Zealand Health Research Council (grant 16/631), the Canadian Institute of Health Research Strategy for Patient-Oriented Research Innovative Clinical Trials Programme (grant 158584), the UK National Institute for Health Research (NIHR) and the NIHR Imperial Biomedical Research Centre, the Health Research Board of Ireland (grant CTN 2014-012), the UPMC Learning While Doing Programme, the Breast Cancer Research Foundation, the French Ministry of Health (grant PHRC-20-0147), and the Minderoo Foundation; EU Patient-centric Clinical Trial Platforms has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 853966-2. This joint undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA, Children's Tumor Foundation, Global Alliance for TB Drug Development non-profit organization, and Springworks Therapeutics Inc. This publication reflects only the authors' views. The joint undertaking is not responsible for any use that may be made of the information it contains.
Editor: L. Leibovici
References
- 1.Fragkou P.C., Belhadi D., Peiffer-Smadja N., Moschopoulos C.D., Lescure F.X., Janocha H., et al. Review of trials currently testing treatment and prevention of COVID-19. Clin Microbiol Infect. 2020;26:988–998. doi: 10.1016/j.cmi.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry S.M., Connor J.T., Lewis R.J. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA. 2015;313:1619–1620. doi: 10.1001/jama.2015.2316. [DOI] [PubMed] [Google Scholar]
- 3.Woodcock J., LaVange L.M. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377:62–70. doi: 10.1001/jama.2015.2316. [DOI] [PubMed] [Google Scholar]
- 4.Adaptive Platform Trials Coalition. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18:797–807. doi: 10.1038/s41573-019-0034-3. [DOI] [PubMed] [Google Scholar]
- 5.REMAP-CAP A randomised, embedded, multi-factorial, adaptive platform trial for community-acquired pneumonia. https://www.remapcap.org/ Last accessed 22 August 2022. Available from:
- 6.RECOVERY Randomized evaluation of COVID-19 therapy. https://www.recoverytrial.net Last accessed 22 August 2022. Available from:
- 7.WHO COVID-19 solidarity therapeutics trial. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments Last accessed 22 August 2022. Available from:
- 8.Angus D.C. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323:1895–1896. doi: 10.1001/jama.2020.4984. [DOI] [PubMed] [Google Scholar]
- 9.Goossens H., Derde L., Horby P., Bonten M. The European clinical research response to optimise treatment of patients with COVID-19: lessons learned, future perspective, and recommendations. Lancet Infect Dis. 2022;22:e153–e158. doi: 10.1016/S1473-3099(21)00705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Depoortere E., Sowinski S., van Hengel A., Kerstiëns B., Norstedt I. COVID-19 kick-starts a new era for clinical trials and pandemic preparedness in Europe. Lancet Infect Dis. 2022;22:315. doi: 10.1016/S1473-3099(22)00059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granholm A., Alhazzani W., Derde L.P.G., Angus D.C., Zampieri F.G., Hammond N.E., et al. Randomised clinical trials in critical care: past, present and future. Intensive Care Med. 2022;48:164–178. doi: 10.1007/s00134-021-06587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EU-PEARL European Union patient-centric clinical trial platforms. https://eu-pearl.eu/ Available from:
- 13.European clinical research alliance on infectious diseases. www.ecraid.eu Last accessed 23 August 2022. Available from: